Abstract
Serum interleukin (IL)-17A level is associated with higher microvessel density and poor prognosis in liver cancer. However, the specific mechanism underlying the role of IL-17A in liver cancer remains controversial. In the present study, the effect of IL-17A on liver cancer cells was examined. IL-17A had no evident impact on vascular endothelial growth factor A (VEGFA) production in HepG2 and Huh7.5 cells as determined by reverse transcription-quantitative PCR and ELISA, but it did stimulate angiogenic CXC chemokine secretion, including chemokine (C-X-C motif) ligand 1 (CXCL1), CXCL2, CXCL3, CXCL5, CXCL6 and CXCL8 in Huh7.5 cells and CXCL2 in HepG2 cells. In addition, the production of angiostatic chemokines such as CXCL10 was not affected. The supernatant of Huh7.5-IL17A cells promoted endothelial cell chemotaxis, which was attenuated by the C-X-C chemokine receptor type 2 (CXCR2) inhibitor SB225002. Although there was no role of IL-17A in promoting in vitro cell proliferation, IL-17A markedly increased the tumor growth of Huh7.5 cells in both subcutaneous and orthotopic xenograft models with increased vascularization. Taken together, these results demonstrated that IL-17A may stimulate chemokine-induced angiogenesis and promote tumor progression, independent of VEGF signaling. The CXCL-CXCR2 axis may be a novel target for the anti-angiogenesis treatment of liver cancer.
Keywords: liver cancer, angiogenesis, interleukin-17A, CXC chemokines, C-X-C chemokine receptor type 2
Introduction
The cytokine interleukin (IL)-17A, produced by T helper cells (Th17), CD8-positive T cells, neutrophils, γδT cells, and natural killer cells, plays an important role in host pathogen defense by activating the inflammatory response, which recruits neutrophils to fight infection (1). Notably, the chronic inflammatory microenvironment established by IL-17A and other inflammatory cytokines may facilitate chronic diseases in the liver, including non-alcoholic steatohepatitis (2), and hepatocellular carcinoma (HCC) initiation and progression (3,4). For example, IL-17A knockout significantly reduced tumor incidence from 65 to 20% in diethyl nitrosamine-induced HCC models (5). In addition to the role of tumor initiation, IL-17A may also have an impact on tumor proliferation, angiogenesis, metastasis and chemoresistance (6). IL-17A activates STAT3 and NF-κB signaling pathways, regulating the expression of a panel of genes and aggravating tumor malignancy (7). IL-17A not only promotes tumor growth in animal models (5,8), but also predicts poor prognosis in a large portion of clinical reports, including gastric, lung and breast cancers, and HCC (9–12). We have previously reported that elevated serum IL-17A levels were positively correlated with a larger tumor size and an increased risk of early recurrence of HCC after curative hepatectomy (13). The number of intratumoral Th17 cells positively correlates with microvessel density and poor survival, suggesting that IL-17A may stimulate tumor growth by promoting angiogenesis (14).
While IL-17A does not have a direct role on endothelial cell proliferation, it may stimulate the production and secretion of pro-angiogenic factors from both tumor cells and fibroblasts in some tumor types (15). IL-17A stimulates vascular endothelial growth factor A (VEGFA) production and promotes angiogenesis in non-small-cell lung cancer (16), gastric cancer (17) and colorectal cancer (18). In addition to VEGFA, some CXC chemokines have been shown to play important roles in promoting tumoral angiogenesis via the receptor C-X-C chemokine receptor type 2 (CXCR2). CXCR2 is a 7-transmembrane G protein-coupled receptor that mediates chemotaxis during an immune response. Upregulation of CXCR2 has been reported in inflammatory diseases including rheumatoid arthritis, psoriasis and atherosclerosis (19). In addition to expression on a subset of leukocytes, CXCR2 is also present on microvascular endothelial cells, and has been shown to be involved in endothelial cell chemotaxis and angiogenesis (20). The CXC chemokines with a glutamic acid-leucine-arginine (ELR) motif such as CXCL1/2/3/5/6/8 can bind to CXCR2 and are pro-angiogenic (21). The expression of IL-8 (CXCL8) is correlated with vascularity in gastric cancers (22), and CXCL1/3/5/8 augments angiogenesis and tumorigenesis in renal cell carcinoma (23). IL-8 secreted from activated stellate cells also promotes angiogenesis in HCC (24). In particular, IL-17A has been shown to promote the CXCR2-dependent angiogenesis and tumor growth of non-small cell lung cancer, while it has no effect on VEGFA secretion (25).
Since the IL-17A receptor is extensively expressed in liver cell types including liver cancer cells, it is of interest to determine the direct role of IL-17A in these cells. Previous studies have suggested that IL-17A may influence HCC cell survival and migration (26,27). However, it remains unclear whether IL-17A can regulate the production of VEGFA or other pro-angiogenic factors directly from liver cancer cells, thereby contributing to angiogenesis. The present study examined the effect of IL-17A on Huh7.5 and HepG2 cells. The results indicated that IL-17A stimulated the secretion of angiogenic CXC chemokines and promoted CXCR2-dependent endothelial chemotaxis.
Materials and methods
Cell cultures
The liver cancer cell line HepG2 (28,29) was supplied by American Type Culture Collection and Huh7.5 (30) cells were gifted by Dr. Aihua Zheng (Chinese Academy of Sciences). The endothelial cell HUVEC was a gift from Dr. Peigang Wang (Capital Medical University). The cell lines were authenticated by short tandem repeat profiling (Beijing Center for Physical and Chemical Analysis). These cells were cultured in Dulbecco's modified Eagle's medium (DMEM; high glucose; Hyclone; GE Healthcare Life Sciences) supplemented with 10% fetal bovine serum (FBS; Biological Industries) and 105 U/l penicillin and 100 mg/l streptomycin (Hyclone; GE Healthcare Life Sciences). When indicated, 100 ng/ml recombinant IL-17A (R&D Systems, Inc.) was added to the culture media.
For IL-17A or enhanced green fluorescent protein (EGFP) overexpressing cells, pcDH-EF1-IL17A-IRES-puromycin or pcDH-EF1-EGFP-IRES-puromycin were constructed using a pcDH vector (System Biosciences). Construction was performed by GenScript and was sequenced to be correct. For pseudovirus production, lentiviral vector, pLP1, pLP2 and pVSVG (System Biosciences) were co-transfected into 293T cells using X-tremeGENE HP (Roche Diagnostics). After 12 h, the media was removed and fresh media was added. The supernatants were collected at 48 h post-transfection and filtered through 0.45 µm filters to remove the cell debris. HepG2 or Huh7.5 cells were cultured with the pseudovirus-containing media with polybrene (8 µg/ml; Sigma Aldrich; Merck KGaA) for 2 days and the stably transduced cells were selected using 5 µg/ml puromycin (Shanghai Qcbio Science & Technologies Co., Ltd.).
ELISA
Cells (1×105) were cultured in 6-well plates for 24 h. The cell-free supernatant was collected and stored at −70°C. The concentrations of IL-17A, VEGFA, CXCL10 and CXCL2 were measured using commercially available ELISA kits for IL-17A (cat. no. CHE0054; Beijing 4A Biotech Co., Ltd.), VEGFA (cat. no. SEA143Hu; Cloud-Clone Corp.), CXCL10 (cat. no. SEA371Hu; Cloud-Clone Corp.) and CXCL2 (cat. no. SEB603Hu; Cloud-Clone Corp.).
Cell proliferation assay
Cell proliferation was measured using a Cell Counting Kit-8 (CCK-8) kit (Dojindo Molecular Technologies, Inc.). In this assay, cells were cultured in 96-well plates (2×103 cells/well) for 0, 1, 3, 5 or 7 days. CCK-8 (10 µl) was added to each well and the cells were cultured for 2 h at 37°C. Then, the absorbance was measured at 450 nm using a microplate reader (Thermo Electron Corporation). All tests were performed in triplicate.
RNA extraction and reverse transcription-quantitative PCR
Total RNA was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. cDNA was synthesized from 1 µg total RNA using FastKing RT kits (Tiangen Biotech Co., Ltd.), according to the manufacturer's instructions. Briefly, genomic DNA was removed using gDNA buffer at 42°C for 3 min and then reverse transcription was performed at 42°C for 15 min followed by 95°C for 3 min. RT-qPCR was performed on an ABI 7500 Real-Time PCR Systems (Applied Biosystems; Thermo Fisher Scientific, Inc.) using SYBR-Green (Toyobo Life Sciences). The PCR thermocycling conditions were as follows: 50°C incubation for 2 min, 95°C initial denaturation for 10 min, then 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. Fluorescence data were collected in the extension step (72°C for 30 sec). All reactions were run in triplicate. The housekeeping gene GAPDH served as an internal control. The 2−ΔΔCq method was used to determine expression levels (31). The primer sequences are as follows: CXCL1 forward (F), TCAATCCTGCATCCCCCATAGTTA and reverse, (R) GTGAGCTTCCTCCTCCCTTCT; CXCL2 F, CGCATCGCCCATGGTTA and R, ACAGCCACCAATAAGCTTCC; CXCL3 F, GCTTGTCTCAACCCCGCA and R, CACCCTGCAGGAAGTGTCAATG; CXCL4 F, ATCGCACTGAGCACTGAGATCC and R, GACCTGGGAGGTGGTCTTCAC; CXCL5 F, TTTACAGACCACGCAAGGAGT and R, TTTCCTTGTTTCCACCGTCCA; CXCL6 F, AGCAAGTTTGTCTGGACCCG and R, CAGAAAACTGCTCCGCTGAA; CXCL7 F, GGAAAGGAACCCATTGCAACC and R, TCTGGGTCCAGGCAGATTTT; CXCL8 F, TCCTGATTTCTGCAGCTCTGT and R, CCAGACAGAGCTCTCTTCCA; CXCL9 F, AAGCCCTTCCTGCGAGAAAA and R, TTCACATCTGCTGAATCTGGGT; CXCL10 F, AAGCCAATTTTGTCCACGTGTT and R, AGCACTGCATCGATTTTGCTC; CXCL11 F, TCGAAGCAAGCAAGGCTTAT and R, TTCAGATGCTCTTTTCCAGGACT; CXCL12 F, AGTGTGCATTGACCCGAAGC and R, GCAGGCCCTTCCCTAACAC; CXCL14 F, TGAAGCCAAAGTACCCGCAC and R, TGACCTCGGTACCTGGACAC; VEGFA F, CTACCTCCACCATGCCAAGT and R, GCAGTAGCTGCGCTGATAGA; VEGFR2 F, CAAGTGGCTAAGGGCATGGA and R, ATTTCAAAGGGAGGCGAGCA; IL17RA F, CTGATGGGGACCCAAACCAC and R, CCACAGGGTGAAGCTCACAC; and GAPDH F, CTGCACCACCAACTGCTTAG and R, GAGCTTCCCGTTCAGCTCAG.
Transwell assay
Chemotaxis of HUVECs was evaluated using a transwell assay. Briefly, HUVECs were cultured in DMEM with 2% FBS for 8 h. Following this, 1×104 cells in DMEM with 2% FBS were placed in the upper chamber with 8-µm pores (BD Biosciences) pre-coated with 100 µg/ml Matrigel (BD Biosciences) for 30 min at 37°C. Cell-free media from liver cancer cells cultured with 2% FBS for 24 h was added to the lower compartments of the chamber. For the inhibitory assay, 400 nM CXCR2 inhibitor SB225002 (MedChem Express) was added to the upper and lower compartments of the chamber. HUVECs were cultured overnight, and then the cells on the upper surface of the filters were wiped away using cotton swabs. The filters were fixed with 4% paraformaldehyde/PBS for 10 min at 4°C, permeabilized with 0.2% Triton X-100/PBS for 10 min at 4°C and stained with DAPI (Yeasen Shanghai Biotechnology Co., Ltd.) at room temperature for 5 min. The number of invasive cells was observed under a fluorescent microscope (BX51; Olympus Corporation) and quantified by counting cells in five randomly selected high-powered fields (magnification, ×400) in each well.
Xenograft tumor models
The Animal Studies Committee of China-Japan Friendship Hospital approved all of the animal experiments in the present study, which were performed according to the Principles of Laboratory Animal Care (32). For subcutaneous xenograft models, 6–8 week old female nude (nu/nu) mice (Charles River Laboratories, Inc.), weighing 21.0±0.82 g were used in the present study. Mice were maintained under specific-pathogen-free conditions and had access to food and water ad libitum. Mice were acclimatized to standardized laboratory conditions for 1 week prior to experimentation (24±2°C; 50±10% relative humidity; 12 h light/dark cycle). Mice in the control group were inoculated with 5×106 Huh7.5-EGFP cells and the experimental group were inoculated with Huh7.5-IL17A cells in 100 µl FBS-free culture medium in their backs. The subcutaneous tumor volume was monitored at day 8, 12, 16, 19, 22 and 26 by measuring the tumor diameters (mm) along the largest (a) and perpendicular (b) diameters, from which the tumor volume (V; mm3) was calculated according to the formula: V=0.5 × a × b2. For the liver metastasis model, the nude mice were anesthetized with 240 mg/kg avertin (intraperitoneal; Sigma-Aldrich; Merck KGaA). The skin was disinfected with 75% alcohol and an oblique incision was made on the left side to pull out the spleen. A total of 1×106 Huh7.5-EGFP cells in 25 µl FBS-free culture medium were injected into the spleen of the control group and in the experimental group an equal number of Huh7.5-IL17A cells in 25 µl FBS-free culture medium were injected into the spleen. After injection and needle withdrawal, a dry cotton swab was used for 2 min for hemostasis and cell leakage oppression. Then, the spleen was replaced into the abdominal cavity and the skin was sutured. The mice were euthanized and the tumors were excised for histological analysis 3 and 4 weeks later for the subcutaneous and liver metastasis models, respectively.
Histological analysis
Subcutaneous tumors or the livers were fixed in 4% paraformaldehyde/PBS overnight at 4°C, and then prepared into 5 µm paraffin sections for hematoxylin and eosin staining. The sections were stained with hematoxylin for 5 min and eosin for 1 min at room temperature. Immunohistochemistry was performed according to the standard procedure. Briefly, the tumor slides were dewaxed in xylene for 5 min twice, rehydrated in 100% ethanol for 5 min twice, 95% ethanol for 3 min twice and 70% ethanol for 1 min at room temperature, and were washed with tap water. The slides were subjected to antigen retrieval using 0.01 M citrate buffer (pH 6.0; Beyotime Institute of Biotechnology) and boiling in the microwave for 10 min, slides were then washed with tap water. The slides were incubated in 3% H2O2 for 10 min at room temperature and blocked in 3% goat serum (cat. no. ZLI-9022; OriGene Technologies, Inc.) for 1 h at room temperature. The primary rabbit anti-cluster of differentiation (CD)-31 antibody (1:500; ab182981; Abcam) was applied to the slides overnight at 4°C. Sections were then incubated with goat-anti-rabbit secondary antibody conjugated with horseradish peroxidase (ready to use; cat. no. PV-6001; ZSGB-Bio) at room temperature for 30 min. Finally, diaminobenzidine tetrachloride was used for color development and the slides were counterstained with hematoxylin for 5 min at room temperature. Slides were observed under a light microscope (BX53; Olympus Corporation) at ×400 magnification and imaged using Canon EOS600D camera (Canon, Inc.).
Statistical analysis
Sections showing positive staining for CD31 were assessed in 6 fields of view (magnification, ×400) and the percentage of positive staining was calculated with Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.). Student's t-test was used to compare the statistical difference between two groups. For data containing multiple groups, statistical analyses were performed using one-way analysis of variance followed by the Tukey's post hoc test using GraphPad Prism software (version 6.01; GraphPad Software, Inc.). Data are presented as the mean ± SD. P<0.05 was considered significant as indicated in the figure legends.
Results
Expression of IL17RA and VEGFR2 on liver cancer cells
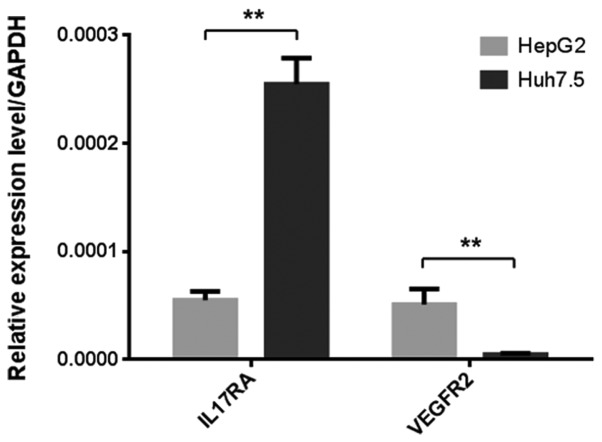
Since IL-17A has been shown previously to upregulate VEGFA in some tumor cells (16–18), it was originally hypothesized that the potentially increased level of VEGFA from liver cancer cells may act on its own VEGFR2 receptor to stimulate cell proliferation. Hence, the present study first examined the expression of IL17RA and VEGFR2 in Huh7.5 and HepG2 cells using RT-qPCR. IL17RA and VEGFR2 were expressed in both cell lines, which was consistent with previous reports (33,34). While VEGFR2 expression was relatively low in Huh7.5 cells, IL17RA expression was significantly higher in Huh7.5 cells than in HepG2 cells (Fig. 1).
Figure 1.
Expression of IL17RA and VEGFR2 in liver cancer-derived cell lines. The expression of IL17RA and VEGFR2 in HepG2 and Huh7.5 cells was examined by reverse transcription-quantitative PCR. GAPDH served as the internal control. **P<0.01. IL17RA, interleukin 17 receptor A; VEGFR2, vascular endothelial growth factor receptor 2.
Overexpression of IL-17A does not promote in vitro liver cancer cell proliferation
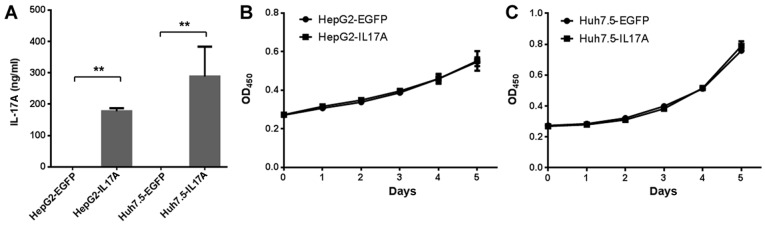
To study the effect of IL-17A on liver cancer cells, the present study constructed IL-17A overexpressing cells using lentiviral vectors. Although IL-17A is not naturally expressed in liver cancer cells, this method allows for the study of the role of IL-17 in the following in vivo tumor growth experiment, and it has been commonly used in previous IL-17A studies (25,35,36). IL-17A secretion was detected at >100 ng/ml in Huh7.5-IL17A and HepG2-IL17A cells but not Huh7.5-EGFP and HepG2-EGFP cells, when the cell confluency was ~20% (Fig. 2A). The effect of IL-17A on the proliferation of liver cancer cells was then determined. Using a CCK-8 assay, the overexpression of IL-17A did not significantly affect the cell proliferation rate of Huh7.5 or HepG2 cells (Fig. 2B and C).
Figure 2.
IL-17A has no effect on the proliferation of liver cancer cells. (A) HepG2 and Huh7.5 cells overexpressing EGFP or IL-17A were cultured in 6-well plates for 24 h. The concentration of IL-17A in the cell-free supernatants was measured by IL-17A ELISA. **P<0.01. The effect of IL-17A overexpression on the proliferation of (B) HepG2 and (C) Huh7.5 cells was determined by Cell Counting Kit-8 assays. Data derived from three independent experiments are presented as the mean ± SD. IL, interleukin; EGFP, enhanced green fluorescent protein; OD, optical density.
IL-17A upregulates the production of proangiogenic CXC chemokines but not VEGFA in liver cancer cells
Next, the present study examined the possibility that IL-17A may upregulate VEGFA expression in liver cancer cells, which may then in turn promote cell proliferation and angiogenesis. Notably, the results revealed that the VEGFA expression was not altered in IL-17A overexpressing cells, in either of the cell lines tested (Fig. 3A and B). The overexpression of IL-17A selectively and significantly upregulated the expression of pro-angiogenic CXC chemokines CXCL1, CXCL2, CXCL3, CXCL5, CXCL6 and CXCL8 in Huh7.5 cells, and CXCL2 in HepG2 cells, while the expression of the angiostatic chemokine CXCL10 was unchanged (Fig. 3A and B). Other CXC chemokines (data not shown) were expressed at extremely low levels or not detected by RT-qPCR. These results are consistent with those of recombinant IL-17A stimulation (Fig. 3C and D). The secretion of VEGFA, CXCL2 and CXCL10 were further confirmed by ELISA in the presence or absence of recombinant IL-17A, and in the IL-17A or EGFP overexpressing cells (Fig. 3E and F). These data suggest that the pro-angiogenic CXC chemokines upregulated by IL-17A may promote angiogenesis in liver cancer.
Figure 3.
IL-17A upregulates the production of pro-angiogenic CXC chemokines in liver cancer cells. The effect of IL-17A overexpression on the expression levels of angiogenic factors in (A) Huh7.5 and (B) HepG2 cells was determined by RT-qPCR. *P<0.05 and **P<0.01 vs. corresponding EGFP group. The effect of recombinant IL-17A (50 ng/ml) stimulation on the expression levels of angiogenic factors in (C) Huh7.5 and (D) HepG2 cells was determined by RT-qPCR. *P<0.05 and **P<0.01 vs. corresponding Huh7.5 or HepG2 only group. The effect of recombinant EGFP or IL-17A overexpression on the secretion of VEGFA, CXCL2 and CXCL10 in (E) Huh7.5 and (F) HepG2 cells was determined by ELISA. Data were derived from three independent experiments and are presented as the mean ± SD. IL17A was added to the culture medium where indicated (+ IL17A). Huh7.5/HepG2-EGFP cells stably expressed EGFP and Huh7.5/HepG2-IL17A cells stably expressed IL17A. *P<0.05 and **P<0.01. IL, interleukin; EGFP, enhanced green fluorescent protein; RT-qPCR, reverse transcription-quantitative PCR; VEGFA, vascular endothelial growth factor A; CXCL, chemokine (C-X-C motif) ligand.
IL-17A-expressing Huh7.5 cells promote endothelial chemotaxis in a CXCR2-dependent manner
As CXC chemokines do not directly stimulate endothelial proliferation, but increase endothelial cell invasion, the present study examined the chemotaxis effect of the supernatants from IL-17A overexpressing liver cancer cells on endothelial invasion using Transwell assays. The supernatant from Huh7.5-IL17A cells significantly promoted HUVEC invasion compared with Huh7.5-EGFP cells, and this promotion could be inhibited by IL-17RA antibody (Fig. 4A and C), indicating that the enhanced invasion of HUVECs was mediated by the IL-17A-IL-17RA interaction in liver cancer cells. Since the production of CXC chemokines did not respond to IL-17A in HepG2 cells as strongly as in Huh7.5 cells (Fig. 3A-D), it was unsurprising that the overexpression of IL-17A in HepG2 did not significantly promote HUVEC invasion in the same manner as observed in Huh7.5 cells (Fig. 4B and D). Next, the present study tested whether the CXCL-CXCR2 axis was responsible for the enhanced chemotaxis effect in Huh7.5 cells by using the CXCR2 inhibitor SB225002. The addition of SB225002 to the assay significantly suppressed HUVEC invasion of the supernatant from Huh7.5-IL17A cells, indicating that IL-17A-stimulated endothelial chemotaxis is dependent on CXCR2 signaling. At the same time, SB225002 also inhibited the endothelial invasion of the supernatant from Huh7.5-EGFP cells, indicating that the basal level of CXC chemokines secreted from Huh7.5 cells may also function through CXCR2 in the absence of IL-17A (Fig. 4E and F).
Figure 4.
Effect of IL-17A on liver cancer cell-induced endothelial cell chemotaxis. The chemotaxis effect of the supernatants from liver cancer cells on HUVEC invasion was examined by Transwell assays. The effect of IL-17A overexpression in (A and C) Huh7.5 or (B and D) HepG2 cells on endothelial cell chemotaxis. Antibody against IL17RA (5 µg/ml) was added during liver cancer cell culture. Chemokine (C-X-C motif) receptor 2 inhibitor SB225002 was added to the supernatants from Huh7.5-EGFP or Huh7.5-IL17A. (A, B and E) Representative 4′,6-diamidino-2-phenylindole staining images of migrated HUVEC are presented and (C, D and F) the calculated values are shown. Data were derived from three independent experiments. Five randomly selected images (magnification, ×400) were used in the calculation for each well. *P<0.05 and **P<0.01, as indicated. IL, interleukin; EGFP, enhanced green fluorescent protein; SB, SB225002; Ab, antibody.
IL-17A promotes the in vivo growth and angiogenesis of Huh7.5 cells in a mouse model
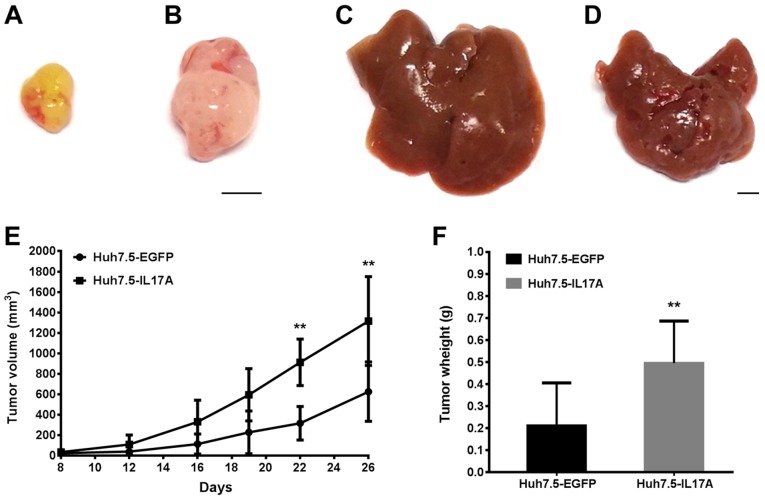
As Huh7.5 cells responded to IL-17A stimulation more so than HepG2 cells based on CXC chemokine production and endothelial cell chemotaxis, the present study performed the following experiments using Huh7.5 cells. To study the in vivo biological effect of IL-17A on liver cancer progression, the present study determined Huh7.5 tumor growth in both subcutaneous and liver xenograft models with or without IL17A overexpression. It has been shown previously that the mouse and human chemokine genes are closely related (37), with the human CXCR2 gene being functionally equivalent to mouse CXCR2 (19). Furthermore, mouse CXCR2 has been shown to respond to CXCL-chemokines from human cancer cell xenografts (25,38). Therefore, it is possible that IL-17A-stimulated CXC-chemokines from Huh7.5 cells could act through mouse CXCR2 to promote angiogenesis and tumor growth in a mouse model. Both the subcutaneous inoculation and intraspleen injection produced solid tumors of Huh7.5 under the skin and in the liver, respectively. Consistent with our hypothesis, the expression of IL-17A significantly promoted the growth of Huh7.5 tumors in both models compared with controls (Figs. 5, and 6A and B). The intensity of blood vessel (CD31) staining was higher in the IL-17A-expressing group than the EGFP-expressing group in the orthotopic models (Fig. 6C-E), and this result was similar in the subcutaneous models (data not shown). Since IL-17A did not directly augment cell proliferation in the in vitro assays, the in vivo data suggests that IL-17A in the tumor microenvironment promotes tumor growth by enhancing angiogenesis.
Figure 5.
Overexpression of IL-17A promotes Huh7.5 tumor growth in vivo. Representative tumors from a (A and B) subcutaneous model (n=6) and (C and D) liver model (n=5) are presented; (A and C) Huh7.5-EGFP and (Band D) Huh7.5-IL17A. Scale bar, 5 mm. (E) The tumor volumes of Huh7.5-EGFP and Huh7.5-IL17A tumors in the subcutaneous model were measured at the time-points indicated, and (F) the tumor weights are shown. Data are presented as the mean ± SD. **P<0.01 vs. Huh7.5-EGFP. IL, interleukin; EGFP, enhanced green fluorescent protein.
Figure 6.
Overexpression of IL-17A promotes growth and angiogenesis in Huh7.5 orthotopic tumors. The morphology of Huh7.5-EGFP- or Huh7.5-IL17A-derived liver tumors was examined by (A and B) hematoxylin and eosin staining, and (C and D) the blood vessels were observed via CD31 immunohistochemistry. Scale bar, 50 µm. (E) Quantitative analysis of the immunostaining-positive area in C&D. Data are presented as the mean ± SD. **P<0.01, as indicated. IL, interleukin; EGFP, enhanced green fluorescent protein; CD, cluster of differentiation.
Discussion
In the present study, it was demonstrated that IL-17A could stimulate the secretion of angiogenic CXC chemokines from liver cancer cells, which may recruit endothelial cells to the tumor cells in a CXCR2-dependent manner. Tumor angiogenesis was also promoted by IL-17A expression in vivo. The CXC chemokines can be classified as angiogenic or angiostatic predominantly based on the presence or absence of an ELR motif. The angiogenic CXC chemokines include CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8 and CXCL12, and the angiostatic chemokines include CXCL4, CXCL9, CXCL10, CXCL11 and CXCL14 (21). IL-17A was shown to increase the expression of CXCL1, CXCL2, CXCL3, CXCL5, CXCL6 and CXCL8 in Huh7.5 cells and upregulated CXCL2 in HepG2 cells. Additionally, angiostatic CXC chemokines were not affected by IL-17A in both cell lines. IL-17A has been reported to stimulate VEGFA production and promote angiogenesis in several cancer cell lines (16–18) and it has been shown previously that IL-17A does not affect VEGFA production but rather stimulates CXCL1, CXCL5, CXCL6 and CXCL8 production in non-small cell lung cancer cell lines (25). The results of the present study are consistent with those of Numasaki et al (25) in that IL-17A promoted angiogenesis and was more dependent on the CXCL-CXCR2 axis than on VEGFA. Taken together, this suggests that the effect of IL-17A on VEGFA production may be dependent on the cancer cell type.
Anti-angiogenesis therapy, especially anti-VEGF signaling drugs, has produced benefits in clinical trials for many types of cancers. However, they have also had limited benefits in patients with HCC (39,40), suggesting that another target may be required for HCC treatment. We previously reported that besides a bigger tumor size, elevated pre-therapy serum levels of IL-17 may serve as an additional risk factor for the early recurrence of HCC (13). Zhang et al (14) have demonstrated that the number of intratumoral Th17 cells positively correlates with microvessel density, suggesting that the accumulation of intratumoral IL-17-producing cells may promote tumor progression via the promotion of angiogenesis. These observations together are indicative of an important role for IL-17A in the progression of HCC and IL-17A-promoted angiogenesis may be a target for HCC therapy. In addition, the observation that IL-17A released in the tumor microenvironment in response to VEGF-blocking drugs triggers inflammatory and VEGF-independent angiogenesis that in turn induces drug resistance (41), further supports our finding that IL-17A may excite chemokine-induced angiogenesis independent of VEGF signaling.
CXCR2 is the putative receptor for pro-angiogenic CXC chemokines on endothelial cells (20). Previous studies have shown that in addition to mediating an inflammatory response, the CXC chemokine receptors CXCR1 and CXCR2 play important roles in tumor progression and metastasis; CXCR2 knockout mice significantly repress tumor growth and angiogenesis (38,42). In preclinical models of ovarian cancer, sorafenib treatment markedly elevated the expression of pro-angiogenic CXC chemokines, and CXCR2 inhibitor treatment stabilized the progression of sorafenib-resistant tumors (43), indicating that the CXCL-CXCR2 pathway may be important in compensating for the VEGF signaling pathway suggesting that the simultaneous blockage of these two pathways could improve the outcomes of anti-angiogenic tumor therapy. In the present study, blocking of CXCR2 significantly reduced endothelial chemotaxis to the Huh7.5 supernatant. Since CXC chemokines were produced at the basal level even without IL-17A stimulation, the CXCR2 inhibitor not only inhibited IL-17A-stimulated chemotaxis, but also chemotaxis in the absence of IL-17A, indicating that CXCR2 may be a potential target for anti-angiogenesis in liver cancer.
The present study also demonstrated that different liver cancer cell lines may respond differently to IL-17A. Although IL-17A did not affect the growth rate of Huh7.5 and HepG2 cells, the expression of CXC chemokines in Huh7.5 cells increased much more than in HepG2 cells in the presence of IL-17A, and the stimulatory effect of IL-17A on endothelial chemotaxis was stronger in Huh7.5 cells. Since the expression of IL17RA was much higher in Huh7.5 cells than in HepG2 cells, it would be of interest to investigate whether the increased response to IL-17A observed in Huh7.5 cells was due to the higher expression of the receptor. In addition, the expression levels of VEGFA and VEGFR2 were higher in HepG2 cells than Huh7.5 cells, and HepG2 exhibited slightly stronger endothelial cell chemotaxis without IL-17A stimulation. Whether the different expression ratio of IL17RA and VEGFR2 regulates angiogenesis and tumor growth in vitro and in vivo may possess further clinical implications, and may be investigated in future work.
Acknowledgements
The authors would like to thank Dr Aihua Zheng (Chinese Academy of Sciences, Beijing, China) for generously providing the Huh7.5 cells, and Dr Peigang Wang (Capital Medical University, Beijing, China) for providing the HUVEC cells.
Funding
The present study was supported by Youth Foundation of China-Japan Friendship Hospital (grant no. 2015-1-QN-19).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
LL, ZW and ZY designed the experiment. LL acquired the funding. LL and XL performed the animal experiments. HS and SW performed the cell experiments and analyzed the data. HT and YS performed the PCR experiments. SS and LX performed the ELISA experiments. JH and WZ performed the histological examinations. ZW and ZY supervised the research group. ZW wrote and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Animal Studies Committee of China-Japan Friendship Hospital approved all of the animal experiments in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marengo A, Rosso C, Bugianesi E. Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 2016;67:103–117. doi: 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- 3.Gomes AL, Teijeiro A, Buren S, Tummala KS, Yilmaz M, Waisman A, Theurillat JP, Perna C, Djouder N. Metabolic Inflammation-Associated IL-17A causes Non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2016;30:161–175. doi: 10.1016/j.ccell.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 4.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun C, Kono H, Furuya S, Hara M, Hirayama K, Akazawa Y, Nakata Y, Fujii H. Interleukin-17A plays a pivotal role in chemically induced hepatocellular carcinoma in mice. Dig Dis Sci. 2016;61:474–488. doi: 10.1007/s10620-015-3888-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang B, Kang H, Fung A, Zhao H, Wang T, Ma D. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediators Inflamm. 2014;2014:623759. doi: 10.1155/2014/623759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70:10112–10120. doi: 10.1158/0008-5472.CAN-10-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 10.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, Hao K, Yu L, Zhang X. Serum interleukin-17 as a diagnostic and prognostic marker for non-small cell lung cancer. Biomarkers. 2014;19:287–290. doi: 10.3109/1354750X.2014.908954. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Weng W, Xu W, Wang Y, Yu W, Tang X, Ma L, Pan Q, Wang J, Sun F. Prognostic significance of interleukin 17 in cancer: A meta-analysis. Int J Clin Exp Med. 2014;7:3258–3269. [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Du J, Liu L, Li Q, Rong W, Wang L, Wang Y, Zang M, Wu Z, Zhang Y, Qu C. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS One. 2012;7:e50035. doi: 10.1371/journal.pone.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 16.Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin S, Cao S, Che D, Liu F, Yu Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep. 2015;5:16053. doi: 10.1038/srep16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Yang T, Liu X, Guo JN, Xie T, Ding Y, Lin M, Yang H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumour Biol. 2016;37:5493–5501. doi: 10.1007/s13277-015-4372-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Mihara K, Smit MJ, Krajnc-Franken M, Gossen J, Rooseboom M, Dokter W. Human CXCR2 (hCXCR2) takes over functionalities of its murine homolog in hCXCR2 knockin mice. Eur J Immunol. 2005;35:2573–2582. doi: 10.1002/eji.200526021. [DOI] [PubMed] [Google Scholar]
- 20.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 21.Palacios-Arreola MI, Nava-Castro KE, Castro JI, Garcia-Zepeda E, Carrero JC, Morales-Montor J. The role of chemokines in breast cancer pathology and its possible use as therapeutic targets. J Immunol Res. 2014;2014:849720. doi: 10.1155/2014/849720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ, Tahara E. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93–100. [PMC free article] [PubMed] [Google Scholar]
- 23.Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol. 2005;175:5351–5357. doi: 10.4049/jimmunol.175.8.5351. [DOI] [PubMed] [Google Scholar]
- 24.Zhu B, Lin N, Zhang M, Zhu Y, Cheng H, Chen S, Ling Y, Pan W, Xu R. Activated hepatic stellate cells promote angiogenesis via interleukin-8 in hepatocellular carcinoma. J Transl Med. 2015;13:365. doi: 10.1186/s12967-015-0730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Wu PW, Yuan XW, Li J, Shi XL. Interleukin-17A inhibits cell autophagy under starvation and promotes cell migration via TAB2/TAB3-p38 mitogen-activated protein kinase pathways in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2016;20:250–263. [PubMed] [Google Scholar]
- 27.Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-κB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6:e21816. doi: 10.1371/journal.pone.0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Terrada D, Cheung SW, Finegold MJ, Knowles BB. Hep G2 is a hepatoblastoma-derived cell line. Hum Pathol. 2009;40:1512–1515. doi: 10.1016/j.humpath.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Sumpter R, Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M., Jr Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Eighth editor; Washington, D.C.: 2011. Guide for the care and use of Laboratory Animals. [Google Scholar]
- 33.Huang J, Zhang X, Tang Q, Zhang F, Li Y, Feng Z, Zhu J. Prognostic significance and potential therapeutic target of VEGFR2 in hepatocellular carcinoma. J Clin Pathol. 2011;64:343–348. doi: 10.1136/jcp.2010.085142. [DOI] [PubMed] [Google Scholar]
- 34.Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, Qiu SJ. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. doi: 10.1186/1756-9966-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 36.Hu J, Ye H, Zhang D, Liu W, Li M, Mao Y, Lu Y. U87MG glioma cells overexpressing IL-17 acclerate early-stage growth in vivo and cause a higher level of CD31 mRNA expression in tumor tissues. Oncol Lett. 2013;6:993–999. doi: 10.3892/ol.2013.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nomiyama H, Osada N, Yoshie O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Genes Cells. 2013;18:1–16. doi: 10.1111/gtc.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009;69:411–415. doi: 10.1158/0008-5472.CAN-08-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun W, Cabrera R. Systemic treatment of patients with advanced, unresectable hepatocellular carcinoma: Emergence of therapies. J Gastrointest Cancer. 2018;49:107–115. doi: 10.1007/s12029-018-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raoul JL, Gilabert M, Adhoute X, Edeline J. An in-depth review of chemical angiogenesis inhibitors for treating hepatocellular carcinoma. Expert Opin Pharmacother. 2017;18:1467–1476. doi: 10.1080/14656566.2017.1378346. [DOI] [PubMed] [Google Scholar]
- 41.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 42.Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2004;172:2853–2860. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- 43.Devapatla B, Sharma A, Woo S. CXCR2 Inhibition combined with sorafenib improved antitumor and antiangiogenic response in preclinical models of ovarian Cancer. PLoS One. 2015;10:e0139237. doi: 10.1371/journal.pone.0139237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.