Abstract
Interleukin (IL)-12 modulates the generation and function of various immune cells and plays a vital role in the pathogenesis of Sjögren's syndrome (SS). Myeloid-derived suppressor cells (MDSCs) are involved in autoimmune diseases by regulating various immune responses. However, it has not been confirmed whether inflammatory IL-12 participates in the progression of SS via regulating MSDCs. In the present study, the plasma levels of IL-12 were detected by ELISA in SS-like non-obese diabetic (NOD) mice. The mice were treated by intraperitoneal injection of IL-12 and anti-IL-12 antibody, respectively, and then the salivary flow rate was detected. The pathology of submandibular glands was evaluated in tissue sections stained with hematoxylin and eosin. The proportion of MDSCs was assessed by flow cytometry. The results showed that plasma IL-12 was significantly increased in the SS-like NOD mice comparing with that noted in the control mice. The exogenous IL-12 exacerbated SS-like symptoms of NOD mice and promoted the generation of both bone marrow (BM) and splenic MDSCs in the SS-like NOD mice. Of note, anti-IL-12 alleviated SS-like symptoms of NOD mice and inhibited the generation of BM and splenic MDSCs. Moreover, the generation of MDSCs was crippled in the IL-12-deficient C57BL/6 (Il-12−/− B6) mice. Our findings suggest that aggravation of SS-like symptoms by IL-12 in NOD mice may be attributed to its promotion of MDSC development.
Keywords: Sjögren's syndrome, myeloid-derived suppressor cells, interleukin-12
Introduction
Sjögren's syndrome (SS), a common chronic systemic autoimmune disease with mononuclear infiltration in exocrine glands, is typically characterized by dry mouth and eyes (1–3). Patients suffer from lack of effective treatment for the complex pathogenesis of SS (4). High levels of pro-inflammatory cytokines, such as interleukin (IL)-12, IL-6, interferon (IFN)α and IFNγ, tumor necrosis factor (TNF)-α, serum autoantibodies, including antinuclear antibodies, antibodies against Ro/SSA and La/SSB, rheumatoid factor (RF) and serious infiltration of T and B cells are considered as the key factors leading to exocrine gland dysfunction (5–7). Targeting of the disordered cytokine network has shown promise for SS treatment (8).
Interleukin (IL)-12, consisting of two subunits, namely IL-12p40 and IL-12p35, has a powerful impact on the T cell responses in inflammation (9). IL-12 has been recognized to be produced by inflammatory myeloid cells and is involved in various autoimmune inflammatory conditions by linking innate and adaptive immunity (10). Studies have shown that IL-12 is increased in the salivary glands of SS patients and MRL-lpr mice during the course of murine SS (11–13), and IL-12-transgenic mice develop SS-like symptoms (14). Currently, biologics that block IL-12 and its family member IL-23, considered to be effective therapeutic targets, have been used in clinical testing for a variety of autoimmune diseases including SS (8,15). Yet, the effects and mechanisms of IL-12 on the immunity system in different disorders are far from clear.
Myeloid-derived suppressor cells (MDSCs) were initially described as a heterogeneous population of immature myeloid cells with immune-regulating activity (16,17) and consist of two subsets: Granulocytic MDSCs (G-MDSCs) and monocytic MDSCs (M-MDSCs) (18). MDSCs were firstly identified in tumor infiltration and were found to increase and support tumor growth and development (19,20). Recent studies have demonstrated that MDSCs also play an important role in pathological conditions including autoimmune diseases. Vlachou and colleagues demonstrated that MDSCs showed impaired expansion and function in NZB/W F1 lupus-prone mice (21). Our previous studies demonstrated that MDSCs exacerbate SS by inhibiting Th2 cells (22) and have a crucial role in systemic lupus erythematosus (SLE) by regulating the balance of Th17/Treg cells (23), while the mechanisms of MDSC accumulation in SS are largely unknown. In addition, MDSCs were found to aggravate rheumatoid arthritis (RA) by promoting Th17 cell differentiation (24) and were found to show aberrant function in experimental inflammatory colitis (25). However, the regulation of MDSCs in SS warrants further investigation.
Previous studies have demonstrated that IL-12 plays a pro-inflammatory role in the pathogenesis of SS. IL-12 was reported to promote the recruitment of MDSCs and impair their suppressive function (26–28). Therefore, the present study aimed to ascertain whether IL-12 aggravates SS by modulating the expansion and function of MDSCs.
In the present study, increased plasma IL-12 was demonstrated in NOD mice which is one of the most accurate models in deciphering the pathologic mechanisms of SS (29,30). In vivo, exogenous IL-12 aggravated SS-like disease by promoting the expansion of bone marrow (BM) and splenic MDSCs, while anti-IL-12 showed protective function for SS-like disease by inhibiting the development of BM and splenic MDSCs in NOD mice. In addition, IL-12 deficiency decreased the generation of MDSCs. These results indicated that IL-12 aggravates SS by promoting the expansion of MDSCs.
Materials and methods
Mice
In total, five female NOD mice (weight, 15–17 g; age, 3 weeks), 35 female NOD mice (weight, 19–22 g; age, 7 weeks), five female ICR mice (weight, 22–25 g; age, 3 weeks), five female C57BL/6 (B6) mice (weight, 22–25 g; age, 3 weeks) and 25 female Il-12−/− B6 mice (weight, 22–25 g; age, 3 weeks) were purchased from the Model Animal Research Center of Nanjing University, and were housed in the animal center of the Affiliated Drum Tower Hospital of Nanjing University Medical School under pathogen-free conditions at 22±2°C with a relative humidity of 55±15% under a 12-h light/dark cycle. All animals had free access to water and food. Il-12−/- B6 mice also known as IL-12p40 knockout (KO) B6 mice were obtained from The Jackson Laboratory. All animal experiments conformed to the Regulation of Animal Care Management of the Ministry of Public Health, China and were approved by the Ethics Committee of the Medical School of Nanjing University (Nanjing, China). Mice in each group were anesthetized by intraperitoneal injection of 5% pentobarbital sodium (50 mg/kg). Under anesthesia, mice were exsanguinated and the submandibular glands and spleen were immediately collected.
Non-obese diabetic (NOD) mouse as an appropriate model of Sjögren's syndrome (SS) has biochemical and immunological similarities with human SS (31). Previous studies including ours suggested that 4-week old female NOD mice showed no lymphocyte infiltration in lacrimal glands and submandibular glands. However, the infiltrating cells appeared in the lacrimal glands and submandibular glands in 6-week old mice (32). Therefore, we selected 4-week-old NOD mice as control mice without SS-like symptoms and 8-week-old NOD mice with SS-like symptoms.
Salivary flow rate
The salivary flow rate of mice was measured as previously described (33). Briefly, the mice were anesthetized with 5% pentobarbital sodium (50 mg/kg) and stimulated with pilocarpine (0.1 mg/kg) intraperitoneally (i.p.). The saliva was collected for a 15-min period from the oral cavity by micropipette, and the volume of saliva was determined gravimetrically.
Histological analysis
For histological analysis, submandibular glands (SGs) were fixed with 4% paraformaldehyde at 4°C for 24 h, embedded in paraffin and cut into 3-µm-thick sections. Tissue sections were stained with hematoxylin and eosin (H&E). Each staining step was performed at room temperature. Images were captured using a light microscope (Olympus FSX100; Olympus Corporation; magnification, ×10).
Flow cytometric analysis
Spleen single-cell suspensions were prepared after lysing red blood cells. The appropriate number of cells was pre-incubated with antibodies (eBioscience) in optimal concentration. For analysis of MDSCs, cells were pre-incubated with surface marker antibodies, including anti-mouse CD11b-APC and anti-mouse Gr1-PE and then analyzed on a FACS Calibur flow cytometer (BD Biosciences, Mountain View, CA, USA).
For analysis of the CD3+IFNγ+ T cells, the cells were incubated with 20 ng/ml phorbol myristate acetate (PMA) plus 1 µg/ml ionomycin with 5 µg/ml of brefeldin A (Enzo Life Sciences, Inc., East Farmingdale, NY, USA) at 37°C for 4 h. First, surface CD3 with anti-mouse CD3-APC was stained. Subsequently, cells were fixed with Cytofix/Cytoperm solution (BD Pharmingen), and then incubated with anti-mouse IFN-γ-PerCP-Cyanine 5.5 (eBioscience; clone no. XMG1.2; cat. no. 16-7311-81) and analyzed on a FACSCalibur flow cytometer (BD Biosciences).
MDSCs suppress T cell proliferation
According to the manufacturer's instructions (Invitrogen; Thermo Fisher Scientific, Inc.), cells from the spleen of 12-week old female NOD mice were labeled with CFSE. CFSE-labeled splenocytes were co-cultured without or with MDSCs at ratios of 2:1, 4:1 and 8:1 in 96-well round-bottom plates and were stimulated with the anti-CD3/CD28 antibody for 3 days. CFSE-labeled CD3+ T cells were analyzed using a FACSCalibur flow cytometer.
IL-12 and anti-IL-12 treatment of the mice
In the IL-12 treatment experiments, female 11-week-old NOD mice were injected with 40 µg/kg IL-12 (BioLegend) once a day for a total of 7 times or with the same volume of PBS.
In the anti-IL-12 treatment experiments, female 11-week-old NOD mice were injected with 4 mg/kg anti-IL-12 (BioLegend) once intraperitoneally (i.p.) and with commensurable Rat IgG2a isotype.
ELISA assay
IL-12 consists of two subunits, IL-12p40 and IL-12p35, to the formation of the biologically active p70 compound. In our study, plasma IL-12p70 was assessed by a standard mouse sandwich ELISA kit (R&D Systems) according to the manufacturer's instructions.
Statistical analysis
The results of 3 independent experiments are presented as mean ± SEM. The two experimental groups were analyzed using Student's t-test. One-way ANOVA followed by the Least Significant Difference post hoc test were used to account for multiple comparisons. A P-value <0.05 was considered to indicate statistical significance.
Results
Increased IL-12 in the Sjögren's syndrome (SS) mice
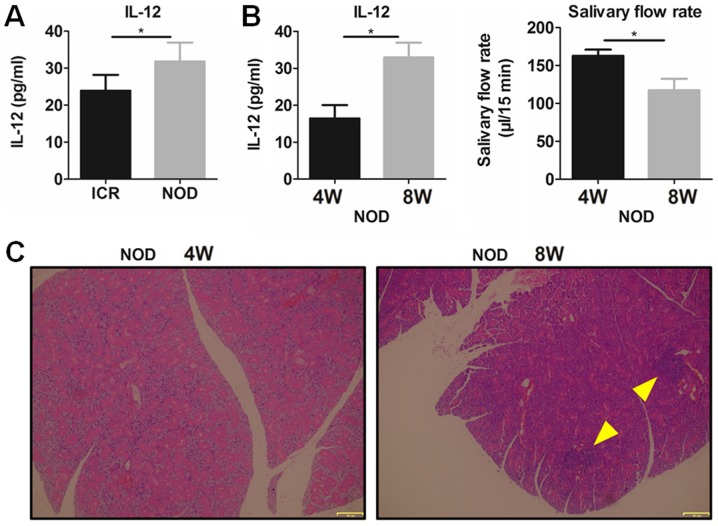
To confirm the role of IL-12 in SS, plasma IL-12 was detected in the NOD mice and ICR control mice. The results showed that plasma IL-12 was significantly increased in the NOD mice with SS-like symptoms comparing with that noted in the ICR mice (Fig. 1A). It was found that 8-week-old NOD mice had a significantly higher plasma IL-12 and a significantly lower salivary flow rate (Fig. 1B) and more serious lymphocytic infiltration in submandibular glands (SG) (Fig. 1C) than these parameters in the 4-week NOD mice, which indicated that SS-like symptoms existed in the 8-week-old NOD mice but not in the 4-week NOD mice. These results suggest that increased IL-12 participates in the pathogenesis of SS-like disease.
Figure 1.
Plasma IL-12 is increased in NOD mice with SS-like symptoms. (A) Plasma levels of IL-12 in 12-week-old NOD and ICR mice were detected by ELISA. (B) Plasma IL-12 and the saliva flow rate in 4- and 8-week-old NOD mice were detected. *P<0.05, n=5. Error bars indicate SEM. (C) H&E staining of SGs for histological analysis (magnification, ×100). Arrows indicate the locus of lymphocytic infiltration in SGs. IL-12, interleukin-12; SS, Sjögren's syndrome; SGs, submandibular glands; H&E, hematoxylin and eosin; W, week.
IL-12 increases MDSCs and exacerbates SS in NOD mice
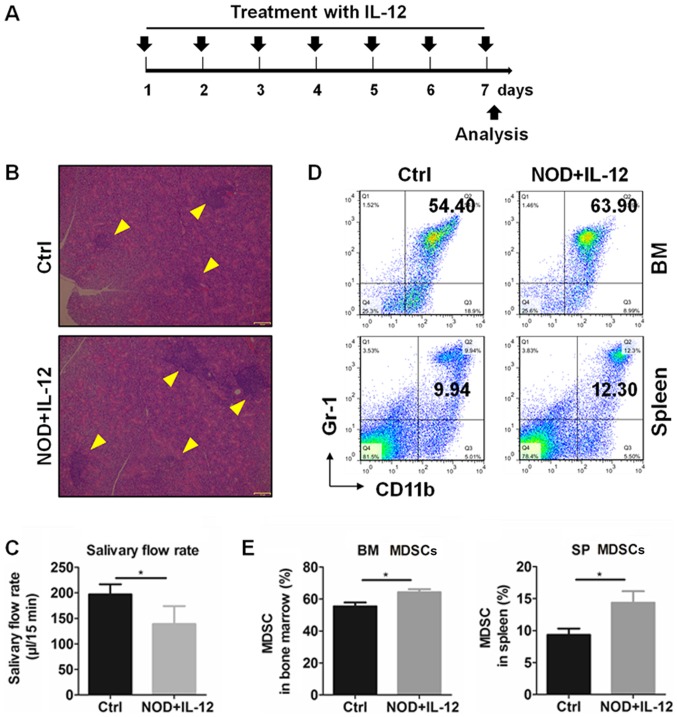
Previous studies have reported that MDSCs are significantly involved in autoimmune diseases by regulating various immune responses (34), and IL-12 could modulate the number and function of MDSCs (28). Therefore, we subsequently determined the relationship between IL-12 and MDSCs in the SS mice. Exogenous IL-12 was injected into 11-week-old NOD mice according to a treatment schedule (Fig. 2A) and mice were sacrificed at day 7. According to results of the histologic and salivary flow rate from mice, IL-12 aggravated the inflammation in the SGs (Fig. 2B) and reduced the secretion function of the exocrine glands (Fig. 2C). The percentages of MDSCs in the bone marrow (BM) and spleen (SP) were detected by flow cytometry (Fig. 2D) and the results showed that treatment with IL-12 significantly increased the percentage of the BM and SP MDSCs (Fig. 2E). These findings suggest that IL-12 promotes the generation of MDSCs and exacerbates SS.
Figure 2.
IL-12 promotes the development of MDSCs in bone marrow (BM) and spleen (SP) of NOD mice. Eleven-week-old NOD mice were intraperitoneally treated with PBS (Ctrl) or IL-12 (NOD+IL-12). (A) IL-12 treatment schedule. (B) Lymphocytic infiltration in SGs from mice. Arrows indicate the locus of lymphocytic infiltration in SGs. (C) Salivary flow rate of mice. (D) Representative flow cytometry graphs of BM and SP MDSCs (CD11b+Gr-1+). (E) Summary of flow cytometry results of BM and SP MDSCs. Error bars indicate SEM. *P<0.05, n=5. IL-12, interleukin-12; SS, Sjögren's syndrome; MDSCs, myeloid-derived suppressor cells; SGs, submandibular glands.
Anti-IL-12 decreases the generation of MDSCs and improves SS in NOD mice
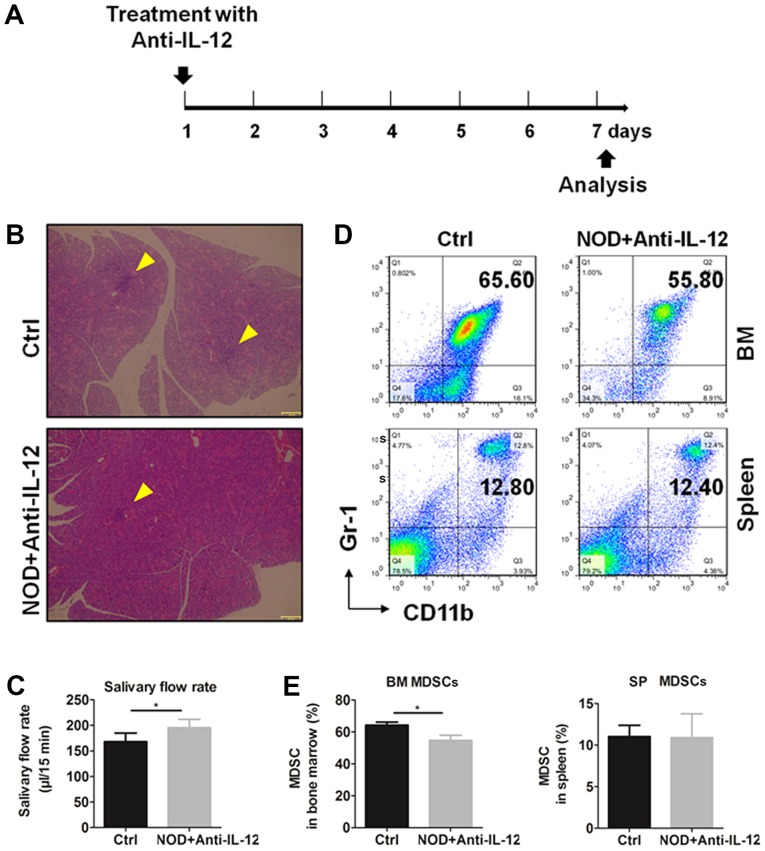
To verify the effects of IL-12 on the generation of MDSCs, NOD mice were treated i.p. with or without anti-IL-12 antibody, respectively, according to the treatment schedule (Fig. 3A) and mice were sacrificed at day 7. Histologic results and salivary flow rate from mice showed that anti-IL-12 alleviated the inflammation in SGs (Fig. 3B) and partially restored the secretion function of exocrine glands (Fig. 3C). The percentages of MDSCs in bone marrow and spleen were detected by flow cytometry (Fig. 3D) and the results showed that anti-IL-12 significantly increased the percentages of BM and SP MDSCs (Fig. 3E). These results showed that anti-IL-12 decreased MDSCs and improved SS-like syndrome.
Figure 3.
Anti-IL-12 alleviates SS and decreases the percentage of MDSCs in BM and SP of NOD mice. Eleven-week-old NOD mice were intraperitoneally treated with (NOD+Anti-IL-12) or without (Ctrl) anti-IL-12. (A) Anti-IL-12 treatment schedule. (B) Lymphocytic infiltration in SGs from mice. Arrows indicate the locus of lymphocytic infiltration in SGs. (C) Salivary flow rate of mice. (D) Representative flow cytometry graphs of BM and SP MDSCs. (E) Summary of flow cytometry results of BM and SP MDSCs. Error bars indicate SEM. *P<0.05, n=5. IL-12, interleukin-12; SS, Sjögren's syndrome; MDSCs, myeloid-derived suppressor cells; SGs, submandibular glands; BM, bone marrow; SP, spleen.
IL-12 deficiency impairs the generation of MDSCs
To determine the regulatory effects of IL-12 on MDSCs, the percentages of MDSCs were detected in IL-12-deficient C57BL/6 (Il-12−/− B6) mice. Compared with the B6 mice, Il-12−/- B6 mice had a significantly lower percentage of SP MDSCs (Fig. 4A). In addition, the percentage of SP MDSCs decreased with age from 4 to 10 weeks in the Il-12−/ B6 mice (Fig. 4B). These findings suggest that IL-12 induces the generation of MDSCs.
Figure 4.
IL-12 deficiency impairs generation of MDSCs in C57BL/6 mice. (A) SP MDSCs were detected by flow cytometry in 8-week-old C57BL/6 (B6) and Il-12−/- B6 mice. (B) Kinetic changes of SP MDSCs at 4, 6, 8 and 10 weeks of age in the Il-12−/− B6 mice. Error bars indicate SEM. *P<0.05, n=5. W, week; IL-12, interleukin-12; MDSCs, myeloid-derived suppressor cells; SP, spleen.
MDSCs display immunosuppressive activity in SS mice
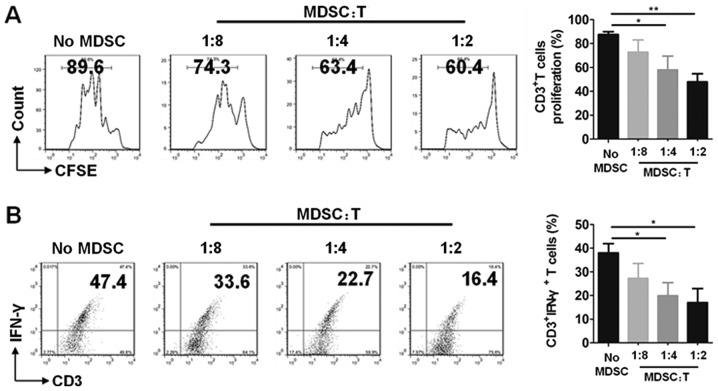
The immunosuppressive function is an important characteristic of MDSCs. To confirm that CD11b+Gr-1+ cells that were determined are MDSCs in this study, T cells were co-cultured without or with MDSCs at the different ratios of 8:1, 4:1 and 2:1, respectively. The results showed that MDSCs had significant suppressive ability on T cell proliferation (Fig. 5A) and decreased the percentages of CD3+IFNγ+ T cells in ratios of 4:1 and 2:1 (Fig. 5B). These results demonstrated that CD11b+Gr1+ MDSCs exhibited immunosuppressive activity in our study.
Figure 5.
MDSCs display T cell suppressive activity in SS mice. Cells from spleen of 12-week-old NOD mice were co-cultured without or with MDSCs in different ratios (MDSC:T) at 8:1, 4:1 and 2:1, respectively for 3 days. (A) Representative flow cytometry graphs (left) and the summary of flow cytometry results (right) of T cell proliferation. (B) Representative flow cytometry graphs (left) and the summary of flow cytometry results (right) of CD3+IFNγ+ T cells. Error bars indicate SEM. *P<0.05 and **P<0.01, n=5. SS, Sjögren's syndrome; MDSCs, myeloid-derived suppressor cells.
Discussion
In the present study, it was demonstrated that IL-12 and MDSCs are involved in the disease progression of Sjögren's syndrome (SS). Plasma IL-12 was elevated in NOD mice, and IL-12 enhanced the expansion of MDSCs in the NOD mice. Anti-IL-12 decreased the percentages of MDSCs in the NOD mice, and IL-12 deficiency impaired the generation of MDSCs gradually with age. All of these results suggest a pathogenic role of IL-12 in SS by promoting the expansion of MDSCs.
IL-12 is known as an inflammatory cytokine involved in various autoimmune diseases (35–38). According to major advances in the last few years, dysregulation of plasma cytokines has been demonstrated to play a significant role in contributing to the dysfunction of exocrine glands in SS, and IL-12 has been considered as one of the main pathogenic factors as well as IL-17 and IL-23 (8,39). However, IL-12 has been mainly studied in terms of lymphocyte responses and the exact mechanisms of IL-12 in the regulation of myeloid cells in SS are not fully understood.
MDSCs, possessing a prominent capacity to suppress T cell activation, have been regarded as a potent inducer for tumor immune escape. The immunosuppressive function is an important characteristic of MDSCs. T cells were co-cultured with MDSCs and the results showed that MDSCs had significant suppressive ability on T cell proliferation and decreased the percentages of CD3+IFNγ+ T cells. The expression of IFNγ indicates the activation level of T cells (23,24). Thus, changes in the CD3+IFNγ+ T cells in T cells co-cultured with CD11b+Gr-1+ cells represented the suppressive effects of the CD11b+Gr-1+ cells, which demonstrated that the purified CD11b+Gr-1+ cells were MDSCs in this study. In addition, functional molecules Arg1, iNOS in splenic G-MDSCs and M-MDSCs were determined by qPCR in our previous study (23). The abnormal expansion of MDSCs has also been studied in inflammatory conditions including SLE mice and lupus patients (23), collagen-induced arthritis mice and RA patients (24,40), inflammatory bowel diseases (41) and experimental autoimmune encephalitis (EAE) (42,43). The percentages and function of MDSCs, G-MDSCs and M-MDSCs have been demonstrated to play a significant role in immune disorders. In the present study, it was demonstrated that, compared to 12-week-old ICR mice, MDSCs were increased significantly in the bone marrow and spleen of the age-matched NOD mice (Fig. S1). Our previous research demonstrated that accumulative MDSCs exacerbate SS by inhibiting the Th2 cell response (22). However, the cause of MDSCs and these subset accumulations in SS are largely unknown.
In the present study, our results demonstrated that IL-12 aggravated SS-like disease, while anti-IL-12 attenuated SS-like disease in NOD mice. Moreover, increased IL-12 and a higher percentage of MDSCs were associated with SS pathogenesis. Increased percentages of MDSCs in IL-12-treated NOD mice, decreased percentages of MDSCs in anti-IL-12-treated NOD mice and gradually reduced percentages of MDSCs in Il-12−/- B6 mice with age suggested that IL-12 and the regulation of MDSCs may be important in SS pathogenesis. Anti-IL-12 treatment resulted in the amelioration of SS-like symptoms in the NOD mice accompanied with an approximately 10% decreased MDSCs in the bone marrow (BM) but not in the spleen (SP) in the present study. The reason for this phenomenon may due to the fact that anti-IL-12 mainly ameliorates SS-like disease in NOD mice by inhibiting the generation of BM MDSCs and improving the suppressive function of MDSCs, but not by inhibiting the expansion of SP MDSCs. IL-12 is a critical cytokine that bridges innate and adaptive immunity and can increase an antitumor immune response by inducing the production of IFNγ (44,45). MDSCs were firstly identified as being involved in tumor infiltration and increased and supported tumor growth and development (19,20). Studies found that IL-12 promotes the recruitment of MDSCs and impairs their suppressive function in tumors and infection (26–28). In our previous study, the number of MDSCs were found to be increased in SS and MDSC transfer exacerbated the SS-like symptoms in NOD mice (22). In the present study, it was found that IL-12 upregulated BM and SP MDSCs and aggravated SS in NOD mice. The mechanisms of MDSCs involved in SS remain largely unknown. Our findings indicated that the number of MDSCs was increased following IL-12 treatment, while the suppressive function did not increase accordingly. Therefore, our results suggest that IL-12 impairs the suppressive function of MDSCs in NOD mice. This is in line with the previous study that IL-12 can promote the recruitment of MDSCs and impair their suppressive function. Our findings indicated that MDSCs under SS conditions are functionally similar to those associated with tumors and infections in response to IL-12.
MDSCs consist of two subsets: Granulocytic MDSCs (G-MDSCs) and monocytic MDSCs (M-MDSCs) (18). The percentages and function of MDSCs, G-MDSCs and M-MDSCs are important in the pathogenesis of immune disorders. The changes in G-MDSCs and M-MDSCs were determined in the present study. It was found that IL-12 and anti-IL-12 significantly altered the percentages of total MDSCs and the percentages of G-MDSCs and M-MDSCs slightly in the BM and SP (Fig. S2). SP G-MDSCs were significantly decreased in 10-week-old M-MDSCs and were significantly decreased in the 8-week and 10-week-old Il-12−/− B6 mice (Fig. S3). Previous studies indicated that macrophages, dendritic cells (DCs) and regulatory T cells participate in the pathogenesis of autoimmune diseases, including SS. The role of regulatory T cells (Treg), as one of the important immunosuppressive cells, in SS pathogenesis is still controversial due to its complex pathogeny (46,47). In the present study, IL-12 and anti-IL-12-treatment showed almost no effects on changes in Treg cells (Fig. S4A). Exogenous IL-12 slightly increased the percentage of spleen macrophages, while anti-IL-12-treatment significantly decreased the percentage of macrophages in the NOD mice (Fig. S4B). The contribution of DCs in SS remains to be evaluated. This is partly due to the low frequency of DCs in the circulation and inflamed tissues. DCs were not detected in response to IL-12 in this study. However, previous studies indicated that DCs migrated from the peripheral blood into the exocrine glands to initiate and perpetuate autoimmune response (48,49). In summary, these findings indicted that the changes were not specific to MDSCs in response to IL-12 in SS.
The development and expansion of MDSCs are regulated by multiple factors which is a complex and gradual phenomenon. Among the related signaling groups, the inflammatory cytokines and damage-associated molecular patterns are important (17). Previous studies demonstrated that IL-12 treatment impaired the suppressive function of MDSCs by upregulating surface markers and decreasing nitric oxide synthase and IFNγ mRNA in the tumor microenvironment (28). Furthermore, transfer of IL-12-producing cells significantly decreased the number of MDSCs in tumors but not in the spleen of C57BL/6 mice and promoted G-MDSCs in tumors to secrete IFNγ to induce tumor regression (27). Moreover, one study found that pro-inflammatory signals induce MDSC recruitment and both p40 and p35 (two subunits of IL-12) knockout mice showed decreased MDSC recruitment and increased monocyte and neutrophil influx (26). In the present study increased IL-12 was found to be an important pro-inflammatory cytokine, promoted MDSC development to accelerate SS progression. However, the exact mechanisms of IL-12 involved in the promotion of MDSCs and the inflammatory role of MDSCs in SS warrant further investigation.
In conclusion, collectively, it was revealed that IL-12 deteriorates SS-like disease by enhancing MDSC expansion. These results provide new insight into the pathogenetic mechanisms and elucidate a potential novel therapy for SS.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (NSFC) (grant nos. 81571583 and 81770061 to GY; 31872732 to YH).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YH and GY initiated, designed and supervised the project. JQ and DL carried out the experiments. XZ, YP and TW contributed to the animal experiments such as breeding procedures and tissue collection. GS and HD performed the flow cytometric analysis and conducted the data analyses. All authors were involved in writing the paper and approved the submitted version.
Ethics approval and consent to participate
All animal experiments conformed to the Regulation of Animal Care Management of the Ministry of Public Health, P.R. China and were approved by the Ethics Committee of the Medical School of Nanjing University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
- 1.Soliotis FC, Moutsopoulos HM. Sjogren's syndrome. Autoimmunity. 2004;37:305–307. doi: 10.1080/08916930410001708715. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Jin L, Kong F, Zhang H, Fang X, Chen Z, Wang G, Li X, Li X. Clinical and immunological consequences of total glucosides of paeony treatment in Sjögren's syndrome: A randomized controlled pilot trial. Int Immunopharmacol. 2016;39:314–319. doi: 10.1016/j.intimp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Mavragani CP, Moutsopoulos HM. Sjögren's syndrome. Annu Rev Pathol. 2014;9:273–285. doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzioufas AG, Kapsogeorgou EK, Moutsopoulos HM. Pathogenesis of Sjögren's syndrome: What we know and what we should learn. J Autoimmun. 2012;39:4–8. doi: 10.1016/j.jaut.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Moriyama M, Hayashida JN, Toyoshima T, Ohyama Y, Shinozaki S, Tanaka A, Maehara T, Nakamura S. Cytokine/chemokine profiles contribute to understanding the pathogenesis and diagnosis of primary Sjögren's syndrome. Clin Exp Immunol. 2012;169:17–26. doi: 10.1111/j.1365-2249.2012.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavragani CP. Mechanisms and new strategies for primary Sjögren's syndrome. Annu Rev Med. 2017;68:331–343. doi: 10.1146/annurev-med-043015-123313. [DOI] [PubMed] [Google Scholar]
- 8.Roescher N, Tak PP, Illei GG. Cytokines in Sjogren's syndrome: Potential therapeutic targets. Ann Rheum Dis. 2010;69:945–948. doi: 10.1136/ard.2009.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vignali DA, Kuchroo VK. IL-12 family cytokines: Immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 11.Bikker A, van Woerkom JM, Kruize AA, Wenting-van Wijk M, de Jager W, Bijlsma JW, Lafeber FP, van Roon JA. Increased expression of interleukin-7 in labial salivary glands of patients with primary Sjögren's syndrome correlates with increased inflammation. Arthritis Rheum. 2010;62:969–977. doi: 10.1002/art.27318. [DOI] [PubMed] [Google Scholar]
- 12.Manoussakis MN, Boiu S, Korkolopoulou P, Kapsogeorgou EK, Kavantzas N, Ziakas P, Patsouris E, Moutsopoulos HM. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren's syndrome: Correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–3988. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi Y, Haneji N, Hamano H. Cytokine gene expression and autoantibody production in Sjögren's syndrome of MRL/lpr mice. Autoimmunity. 1996;23:269–277. doi: 10.3109/08916939608995349. [DOI] [PubMed] [Google Scholar]
- 14.Vosters JL, Landek-Salgado MA, Yin H, Swaim WD, Kimura H, Tak PP, Caturegli P, Chiorini JA. Interleukin-12 induces salivary gland dysfunction in transgenic mice, providing a new model of Sjögren's syndrome. Arthritis Rheum. 2009;60:3633–3641. doi: 10.1002/art.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, Cua DJ. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: Critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younos I, Donkor M, Hoke T, Dafferner A, Samson H, Westphal S, Talmadge J. Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int Immunopharmacol. 2011;11:816–826. doi: 10.1016/j.intimp.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Vlachou K, Mintzas K, Glymenaki M, Ioannou M, Papadaki G, Bertsias GK, Sidiropoulos P, Boumpas DT, Verginis P. Elimination of granulocytic myeloid-derived suppressor cells in lupus-prone mice linked to reactive oxygen species-dependent extracellular trap formation. Arthritis Rheumatol. 2016;68:449–461. doi: 10.1002/art.39441. [DOI] [PubMed] [Google Scholar]
- 22.Qi J, Li D, Shi G, Zhang X, Pan Y, Dou H, Yao G, Hou Y. Myeloid-derived suppressor cells exacerbate Sjögren's syndrome by inhibiting Th2 immune responses. Mol Immunol. 2018;101:251–258. doi: 10.1016/j.molimm.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Ji J, Xu J, Zhao S, Liu F, Qi J, Song Y, Ren J, Wang T, Dou H, Hou Y. Myeloid-derived suppressor cells contribute to systemic lupus erythaematosus by regulating differentiation of Th17 cells and Tregs. Clin Sci (Lond) 2016;130:1453–1467. doi: 10.1042/CS20160311. [DOI] [PubMed] [Google Scholar]
- 24.Guo C, Hu F, Yi H, Feng Z, Li C, Shi L, Li Y, Liu H, Yu X, Wang H, et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann Rheum Dis. 2016;75:278–285. doi: 10.1136/annrheumdis-2014-205508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontaki E, Boumpas DT, Tzardi M, Mouzas IA, Papadakis KA, Verginis P. Aberrant function of myeloid-derived suppressor cells (MDSCs) in experimental colitis and in inflammatory bowel disease (IBD) immune responses. Autoimmunity. 2017;50:170–181. doi: 10.1080/08916934.2017.1283405. [DOI] [PubMed] [Google Scholar]
- 26.Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, Kielian T. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J Immunol. 2015;194:3861–3872. doi: 10.4049/jimmunol.1402689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011;133:221–238. doi: 10.1111/j.1365-2567.2011.03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Stern ME, Pflugfelder SC. Desiccating environmental stress exacerbates autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. J Autoimmun. 2008;30:212–221. doi: 10.1016/j.jaut.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah M, Edman MC, Janga SR, Shi P, Dhandhukia J, Liu S, Louie SG, Rodgers K, Mackay JA, Hamm-Alvarez SF. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjögren's syndrome. J Control Release. 2013;171:269–279. doi: 10.1016/j.jconrel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cha S, Peck AB, Humphreys-Beher MG. Progress in understanding autoimmune exocrinopathy using the non-obese diabetic mouse: An update. Crit Rev Oral Biol Med. 2002;13:5–16. doi: 10.1177/154411130201300103. [DOI] [PubMed] [Google Scholar]
- 32.Hunger RE, Carnaud C, Vogt I, Mueller C. Male gonadal environment paradoxically promotes dacryoadenitis in nonobese diabetic mice. J Clin Invest. 1998;101:1300–1309. doi: 10.1172/JCI1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin X, Rui K, Deng J, Tian J, Wang X, Wang S, Ko KH, Jiao Z, Chan VS, Lau CS, et al. Th17 cells play a critical role in the development of experimental Sjögren's syndrome. Ann Rheum Dis. 2015;74:1302–1310. doi: 10.1136/annrheumdis-2013-204584. [DOI] [PubMed] [Google Scholar]
- 34.Crook KR, Liu P. Role of myeloid-derived suppressor cells in autoimmune disease. World J Immunol. 2014;4:26–33. doi: 10.5411/wji.v4.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 36.Hagberg N, Joelsson M, Leonard D, Reid S, Eloranta ML, Mo J, Nilsson MK, Syvänen AC, Bryceson YT, Rönnblom L. The STAT4 SLE risk allele rs7574865[T] is associated with increased IL-12-induced IFN-γ production in T cells from patients with SLE. Ann Rheum Dis. 2018;77:1070–1077. doi: 10.1136/annrheumdis-2017-212794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson C, Davies R, Choy E. Anti cytokine therapy in chronic inflammatory arthritis. Cytokine. 2016;86:92–99. doi: 10.1016/j.cyto.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Rostami A, Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J Neurol Sci. 2013;333:76–87. doi: 10.1016/j.jns.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat Rev Rheumatol. 2013;9:544–556. doi: 10.1038/nrrheum.2013.110. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Wang S, Huang Y, Wang H, Zhao J, Gaskin F, Yang N, Fu SM. Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation. Clin Immunol. 2015;157:175–186. doi: 10.1016/j.clim.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haile LA, von Wasielewski R, Gamrekelashvili J, Krüger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. Myeloid-derived suppressor cells in inflammatory bowel disease: A new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. doi: 10.1053/j.gastro.2008.06.032. 881.e1-e5. [DOI] [PubMed] [Google Scholar]
- 42.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189:4295–4304. doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldner MJ, Neurath MF. Gene therapy using IL 12 family members in infection, auto immunity, and cancer. Curr Gene Ther. 2009;9:239–247. doi: 10.2174/156652309788921099. [DOI] [PubMed] [Google Scholar]
- 45.Zundler S, Neurath MF. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015;26:559–568. doi: 10.1016/j.cytogfr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos NM, Moutsopoulos HM. Foxp3+ T-regulatory cells in Sjogren's syndrome: Correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am J Pathol. 2008;173:1389–1396. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottenberg JE, Lavie F, Abbed K, Gasnault J, Le Nevot E, Delfraissy JF, Taoufik Y, Mariette X. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjögren's syndrome. J Autoimmun. 2005;24:235–242. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Ozaki Y, Ito T, Son Y, Amuro H, Shimamoto K, Sugimoto H, Katashiba Y, Ogata M, Miyamoto R, Murakami N, et al. Decrease of blood dendritic cells and increase of tissue-infiltrating dendritic cells are involved in the induction of Sjögren's syndrome but not in the maintenance. Clin Exp Immunol. 2010;159:315–326. doi: 10.1111/j.1365-2249.2009.04071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Blokland SC, van Helden-Meeuwsen CG, Wierenga-Wolf AF, Drexhage HA, Hooijkaas H, van de Merwe JP, Versnel MA. Two different types of sialoadenitis in the NOD- and MRL/lpr mouse models for Sjögren's syndrome: A differential role for dendritic cells in the initiation of sialoadenitis? Lab Invest. 2000;80:575–585. doi: 10.1038/labinvest.3780062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.