Abstract
In order to examine the inhibitory effects of microRNA (miR)-26b-5p on thyroid cancer (TC), the clinicopathological features and pathological tissues of 67 patients were collected. The expression levels of miR-26b-5p were detected in TC and paracarcinoma tissues by quantitative polymerase chain reaction, and the association between miR-26b-5p expression and the clinicopathological features of the patients was analyzed using t-test or one-way analysis of variance. In addition, B-CPAP TC cells were infected with a lentivirus to induce miR-26b-5p overexpression and proliferation was detected by Cell Counting kit-8. Subsequently, migration and invasion were detected by Transwell and Matrigel assays, respectively, and the molecular mechanism of action was investigated by western blotting. The results demonstrated that the expression levels of miR-26b-5p were significantly lower in TC tissues compared with paracarcinoma tissues (P<0.01), and miR-26b-5p was associated with lymph node metastasis (P<0.05). In addition, overexpression of miR-26b-5p inhibited the proliferation, invasion and migration of B-CPAP cells. Western blot analysis demonstrated that the protein expression levels of phosphorylated glycogen synthase kinase-3β (pGsk-3β) were decreased, and the expression of β-catenin was decreased in B-CPAP cells overexpressing miR-26b-5p. These results demonstrated that miR-26b-5p may exert antitumor activity. In addition, at the molecular level, these effects may be associated with the Gsk-3β/β-catenin pathway.
Keywords: thyroid cancer, microRNA-26b-5p, lymph node metastasis, migration and invasion, glycogen synthase kinase-3β
Introduction
Thyroid cancer (TC) is the most common type of endocrine malignancy (1); ~90% of thyroid cancer cases are of poorly differentiated poorly differentiated thyroid carcinoma, the prevalence of which is increasing (2–4). Following treatment with specific methods, including surgery, radioactive iodine and thyroid-stimulating hormone inhibition, the majority of patients exhibit an improvement. However, there remains a risk of tumor recurrence and metastasis (5–7). Therefore, it is necessary to understand the occurrence and development of TC, in order to identify specific effective targets for diagnosis and treatment.
MicroRNAs (miRNAs/miRs) are non-coding single- stranded small molecule RNAs that exert their biological function by inhibiting the transcription and translation of target genes, via binding to the 3′ non-coding region of the target mRNA (8,9). Previous studies have demonstrated that miRNAs are closely associated with the occurrence and development of numerous tumors, including ovarian cancer, liver cancer, stomach cancer and breast cancer (10–13). Therefore, miRNAs have attracted increasing attention in tumor research.
Previous studies have reported that differentially expressed miRNAs in the tissues of patients with TC may be screened by gene chip technology (14,15). These findings indicated that, in the tissues of patients with TC, the expression levels of miR-26b-5p are significantly lower compared with in normal tissues; in addition, it was revealed that miR-26b-5p may be associated with the occurrence and development of TC. Therefore, it was be hypothesized that miR-26b-5p may be a clinically relevant target for the treatment of TC.
However, the molecular mechanism underlying the anti-TC effect of miR-26b-5p remains unclear. A previous study using TargetScan predicted that the target gene for miR-26b-5p is glycogen synthase kinase-3β (Gsk-3β) (16). It is well documented that the Gsk-3β/β-catenin pathway serves an important role in the development of TC (17,18).
In the present study, the expression levels of miR-26b-5p were detected in the tissues of patients with TC, and the association between miR-26b-5p and clinicopathological features was examined. Additionally, the inhibitory effect of miR-26b-5p on B-CPAP TC cells and its mechanism based on the Gsk-3β/β-catenin pathway were investigated. The results suggested that miR-26b-5p was downregulated in TC tissues and that miR-26b-5p expression may be associated with lymph node metastasis. In addition, miR-26b-5p was able to inhibit the proliferation, migration and invasion of TC, which may be associated with the Gsk-3β/β-catenin pathway.
Materials and methods
Chemicals and materials
A total of 67 patients with TC undergoing surgical treatment at the Provincial Hospital of Shandong University were enrolled between February 2017 and September 2017. TC and corresponding paracarcinoma tissues were collected. TC was confirmed by pathological diagnosis and written informed consent was obtained from all patients. The 67 samples were collected and stored in liquid nitrogen until further use. The samples were collected from 17 men and 50 women, with an average age of 45 years (age range, 18–75 years).
RNAiso reagent, Prime Script 1st Strand cDNA Synthesis kit for reverse transcription (RT) and SYBR Premix Ex Taq for quantitative PCR (qPCR) were purchased from Takara Bio, Inc. Fetal bovine serum (FBS) and RPMI-1640 medium were purchased from Thermo Fisher Scientific, Inc. The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo. Anti-GAPDH (cat. no. sc-32233), anti-Gsk-3β (cat. no. sc-71186), anti-phosphorylated (p) GSK-3β (cat. no. sc-81495) and anti-β-catenin (cat. no. sc-65480) were purchased from Santa Cruz Biotechnology, Inc. The lentiviral vectors were purchased from GeneChem, Inc. The Dual-Glo™ Luciferase Assay System and Lipofectamine 2000 were purchased from Promega Corporation.
Detection of miR-26b-5p expression
The expression levels of miR-26b-5p were detected in tissues from patients with TC by RT-qPCR, using the aforementioned materials. Total RNA was extracted from fresh frozen tissue using the RNAiso RNA extraction kit, according to the manufacturer's protocol, and total RNA concentration and purity were determined using a UV spectrophotometer. The primers were synthesized by Takara Bio, Inc. The miR-26b-5p primers were designed based on the sequence 5′-UCAAGUAAUUCAGGAUAGGU-3. U6 was used as an internal reference gene and its primer sequences were as follows: Forward 5′-GGAACGATACAGAGAAGATTAGC-3′; and reverse 5′-TGGAACGCTTCACGAATTTGCG-3′. cDNA synthesis was conducted using an RT kit in a 10 µl volume, according to the manufacturer's protocol. The RT temperature protocol was as follows: 37°C for 1 h and 85°C for 5 min. qPCR was performed on a Roche LightCycler 480 II instrument (Roche Molecular Diagnostics) as follows: denaturation (95°C, 30 sec), PCR reaction for 45 cycles (95°C for 5 sec, 60°C for 30 sec), melting (95°C for 5 sec, 60°C for 1 min), cooling (50°C, 30 sec). The final experimental data were standardized to the internal standard U6 and were analyzed using the 2−∆∆Cq method (19): ΔCq=CqmiRNA-Cqinternal reference, ΔΔCq=ΔCqSample-ΔCqMax. The maximum value of all data (ΔCqMax) was used to normalize and correct the samples.
Cell culture
The B-CPAP cell line was donated by Professor Kennichi Kakudo (The Human Pathology Laboratory of Wakayama Medical University (Wakayama, Japan) (20) and the 293 cell line was purchased from GeneChem, Inc. The B-CPAP cell line was verified using the short-tandem repeat (STR) profiling. Briefly, DNA was extracted using Axygen genomic extraction kit and amplified using 20-STR amplification scheme (Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd.). The test of STR loci and sex gene Amelogenin was performed using an ABI 3730XL analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.). The cells were cultured in RPMI-1640 (B-CPAP) or Dulbecco's modified Eagle's medium (Sigma-Aldrich; Merck KGaA) growth medium supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a thermostatic incubator containing 5% CO2. All experiments were performed on cells in the logarithmic growth phase.
Cell infection
miR-26b-5p mimics on a lentiviral vector were designed and synthesized by Shanghai GeneChem Co., Ltd., based on the following sequence: 5′-UUCAAGUAAUUCAGGAUAGGU-3′. Once the cells reached 80% confluence in 96-well plates, B-CPAP cells were infected with a miR-26b-5p-overexpressing lentivirus or an empty vector at a multiplicity of infection value of 50. After 12 h, the virus solution was aspirated and replaced with fresh medium. The fluorescence efficiency was observed after 48–72 h, and the infection efficiency was detected by RT-qPCR, and stably overexpressing cells and negative control (NC) were selected for subsequent experiments.
CCK-8 cell viability assay
Following digestion of miR-26b-5p-overexpressing cells and negative control cells with trypsin, the cells were adjusted to 5,000 cells/well and seeded in 96-well plates at 37°C in an incubator containing 5% CO2 saturated humidity. Cells were cultured for 24, 48, 72 and 96 h, and viability was detected using the CCK-8 assay. Briefly, 10 µl CCK-8 and 90 µl fresh medium were added to each well. After 90 min at 37°C, optical density values were determined at 450 nm using a microplate reader. The experiment was repeated at least three times.
Cell migration assay
Cell migration was detected using a Transwell assay. miR-26b-5p-overexpressing B-CPAP cells and negative control B-CPAP cells were re-suspended (1×105 cells/ml) in RPMI-1640 medium, and a 200 µl cell suspension was added to the upper chamber of Transwell plates. In addition, 600 µl complete medium containing 5% FBS was added to the lower chamber. After culturing for 18 h, the cells in the lower chamber were washed twice with PBS, fixed with 95% ethanol at 37°C for 15 min, and stained with hematoxylin at 37°C for 10 min. The cells were then washed three times with water, air-dried and observed using light microscopy (magnification, ×200).
Cell invasion assay
Cell invasion was detected using a Matrigel assay. Matrigel was thawed at 4°C overnight and thoroughly mixed with serum-free medium to obtain a 1:7 ratio on ice. Briefly, 70 µl diluted Matrigel was added to the Transwell plates and incubated at 37°C for 30 min, in order to polymerize the Matrigel. miR-26b-5p-overexpressing B-CPAP cells and NC B-CPAP cells were re-suspended (1×105 cells/ml) in RPMI-1640 medium. Briefly, a 200 µl cell suspension was added to the upper chamber and 600 µl complete medium containing 10% FBS was added to the lower chamber. After culturing for 18 h, the chamber was washed twice with PBS and the cells in the lower chamber were fixed with 95% ethanol at 37°C for 15 min. The cells were then stained with hematoxylin at 37°C for 10 min and the chamber was washed with water three times. After natural air-drying, the chamber membranes were sealed with slides, and observed under an inverted light-transmitting microscope (magnification, ×200). In total, five fields were randomly selected to count the number of cells.
Dual-luciferase enzyme assay
TargetScan (release no. 3.1; Whitehead Institute for Biomedical Research) was used to predict target genes of miR-26b-5p. The results revealed Gsk-3β to be a potential target, and this was verified via a dual-luciferase enzyme assay. Briefly, 293 cells (at a density of 4×105 cells/ml) were seeded in 24-well plates and cultured for 24 h. The luciferase reporter plasmids containing wild-type or mutant 3′-untranslated regions (UTRs) of Gsk-3β (Promega Corporation) were transfected into cells alongside miR-26b-5p mimics using Lipofectamine 2000 (transfection of 1 µg plasmid) at 37°C. After ~48 h incubation at 37°C, firefly and Renilla luciferase activities were assessed. Normalized relative light units represent firefly luciferase activity/Renilla luciferase activity.
Western blot analysis
The protein expression levels of Gsk-3β, p-Gsk-3β and β-catenin in both miR-26b-5p-overexpressing and NC B-CPAP cells were detected via western blotting. Cellular proteins were extracted by using RIPA (Beijing Solarbio Science and Technology Co., Ltd.) containing phosphorylase inhibitors, and protein quantification was performed using a bicinchoninic acid kit. Subsequently, equal amounts of proteins (30 µg) were separated using 10% SDS-PAGE, and were transferred to polyvinylidene difluoride membranes (EMD Millipore), which were blocked with 5% milk at 37°C for 1 h. The membranes were then incubated overnight at 4°C with the following primary polyclonal antibodies: anti-GAPDH (1:1,000), anti-Gsk-3β (1:1,000), anti-p-GSK-3β (1:1,000) and anti-β-catenin (1:1,000). Membranes were then incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no. abs20002; Absin Bioscience, Inc.; 1:5,000) at 37°C for 1 h. The membranes were imaged using enhanced chemiluminescence (ProteinSimple) and analyzed using Photoshop 6.0 (Adobe Systems, Inc.) and protein expression was normalized to GAPDH (dilution, 1:1,000).
Statistical analysis
All experiment was repeated at least 3 times, and data were analyzed using SPSS 22.0 statistical software (IBM Corp.), and a receiver operating characteristic (ROC) curve was analyzed and drawn using Prism 7 (GraphPad Software, Inc.). Measurement results are expressed as the mean ± standard deviation. Student's t-test was used to compare two groups, whereas Spearman's correlation analysis was used to analyze the correlation between tumor stage and miR-26b-5p expression. P<0.05 was considered to indicate a statistically significant difference.
Results
Association between miR-26b-5p expression and clinicopathological features in TC
As shown in Table I, no significant differences in miR-26b-5p expression levels were identified between patients of different ages and sexes, nor was it associated with tumor size. In addition, Spearman's correlation analysis identified no significant correlation between miR-26b-5p expression and Tumor-Node-Metastasis (TNM) stage (21). The relative expression levels of miR-26b-5p in TC tissues (5.18±2.92) were lower compared with in paracarcinoma tissues (7.15±2.99) in the 67 patients (P<0.05; Table I). Additionally, the expression levels of miR-26b-5p were significantly lower in patients with lymph node metastasis compared with in patients without lymph node metastasis (P<0.05).
Table I.
Expression levels of miR-26b-5p in patients with thyroid cancer.
| Parameters | Cases | Expression of miR-26b-5p (mean ± SD) | P-value |
|---|---|---|---|
| Sex | 0.439 | ||
| Male | 17 | 4.70±2.48 | |
| Female | 50 | 5.34±3.06 | |
| Age (years) | 0.473 | ||
| ≥45 | 37 | 5.41±3.56 | |
| <45 | 30 | 4.89±3.20 | |
| Tumor size (cm) | 0.916 | ||
| ≥1 | 48 | 5.20±3.03 | |
| <1 | 19 | 5.12±2.72 | |
| Lymph node metastasis | 0.015 | ||
| Yes | 39 | 4.45±1.92 | |
| No | 28 | 6.19±3.72 | |
| TNM stagea | 0.473 | ||
| I | 35 | 4.37±0.80 | |
| II | 13 | 3.87±0.80 | |
| III | 19 | 4.60±0.49 | |
| Tissue | <0.001 | ||
| Cancer | 67 | 5.18±2.92 | |
| Normal | 67 | 7.15±2.99 |
As determined using The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM (21). AJCC, American Joint Committee on Cancer; miR-26b-56p, microRNA-26b-5p; SD, standard deviation; TNM, Tumor-Node-Metastasis.
As presented in Fig. 1, the area under the curve value was 0.7287 (95% confidence interval: 0.6422–0.8152; specificity value: 65.67%; sensitivity value: 76.12%), which suggested that miR-26b-5p has high specificity and sensitivity in the clinical diagnosis of TC.
Figure 1.
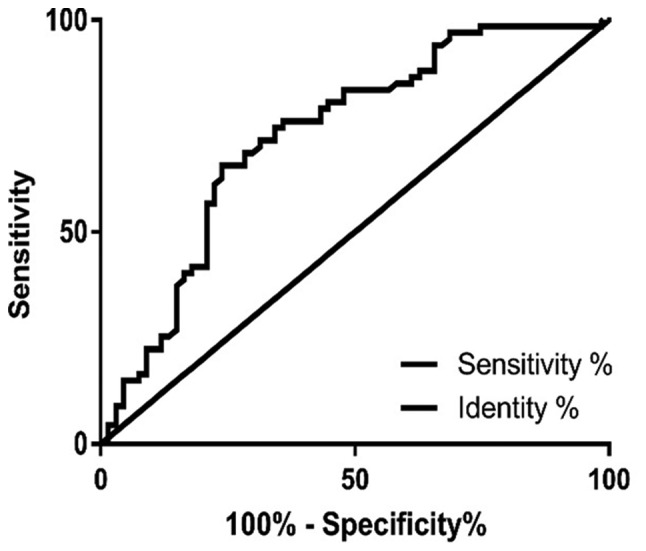
Receiver operating characteristic curve analysis of microRNA- 26b-5p between thyroid carcinoma tissues and paracarcinoma tissues.
miR-26b-5p overexpression inhibits B-CPAP cell proliferation
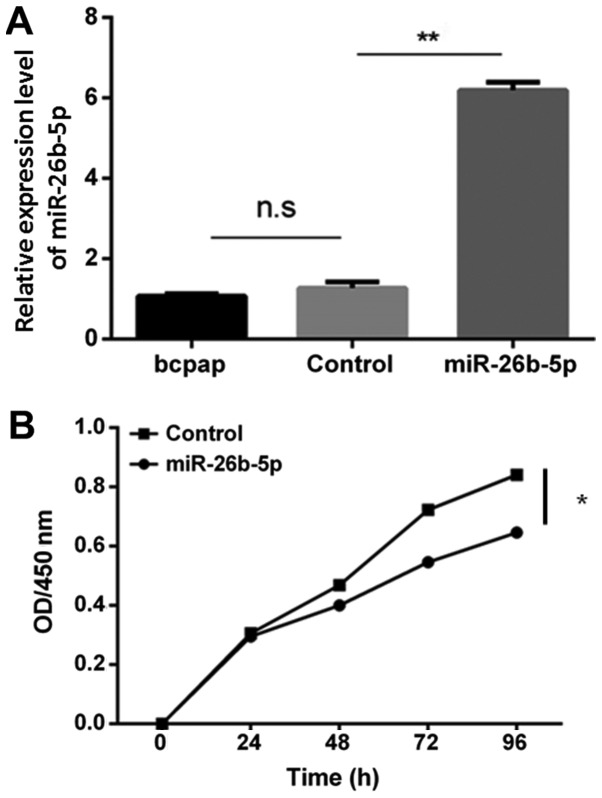
Successful cell transfection was confirmed using RT-qPCR (Fig. 2A); no significant difference was detected in the expression levels of miR-26b-5p between the blank control group and the empty vector control group (P>0.05). Conversely, the expression levels of miR-26b-5p were significantly increased in the miR-26b-5p-overexpressing cells compared with in the blank control and empty vector control groups (P<0.05). Furthermore, after 24 h, cell proliferation was reduced in the miR-26b-5p overexpression group compared with in the control group, thus suggesting that miR-26b-5p inhibited B-CPAP cell proliferation (P<0.05; Fig. 2B).
Figure 2.
miR-26b-5p overexpression inhibits BCPAP cell proliferation. (A) Relative expression levels of miR-26b-5p in BCPAP cells. (B) Cell Counting Kit-8 assay was used to detect proliferation of cells with or without miR-26b-5p overexpression. *P<0.05 and **P<0.01. miR-26b-5p, microRNA-26b-5p; ns, not significant; OD, optical density.
miR-26b-5p overexpression inhibits B-CPAP cell migration and invasion
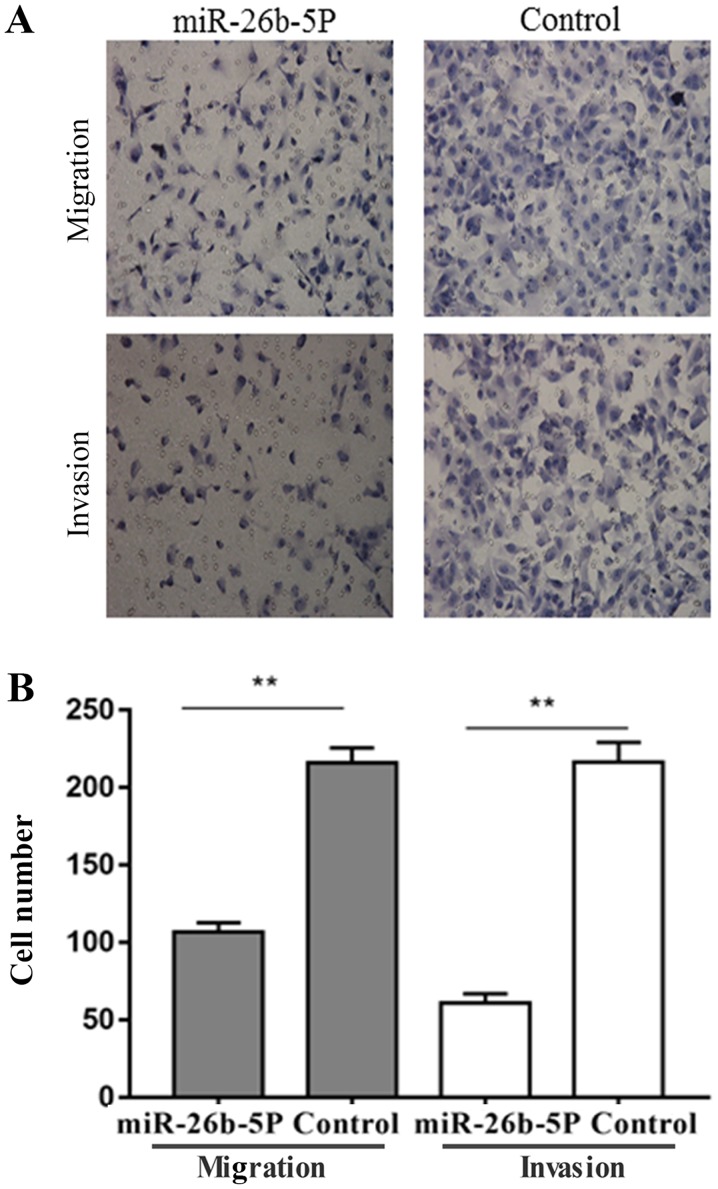
The results of the migration and invasion assays are presented in Fig. 3. Briefly, the number of migratory and invasive B-CPAP cells in the control group was 216±5.6 and 216.3±7.5, respectively. However, the number of migratory and invasive B-CPAP cells was decreased in the miR-26b-5p overexpression group compared with in the control group, to 106.7±3.5 and 61±3.5, respectively (P<0.001). These results suggested that miR-26b-5p overexpression inhibited the migration and invasion of B-CPAP cells.
Figure 3.
miR-26b-5p overexpression inhibits migration and invasion of B-CPAP cells. (A) Transwell and Matrigel assays were conducted to detect migration and invasion of B-CPAP cells with or without miR-26b-5p overexpression (magnification, ×200). (B) Number of migratory and invasive B-CPAP cells with or without miR-26b-5p overexpression. **P<0.01. miR-26b-5p, microRNA-26b-5p.
miR-26b-5p directly targets Gsk-3β
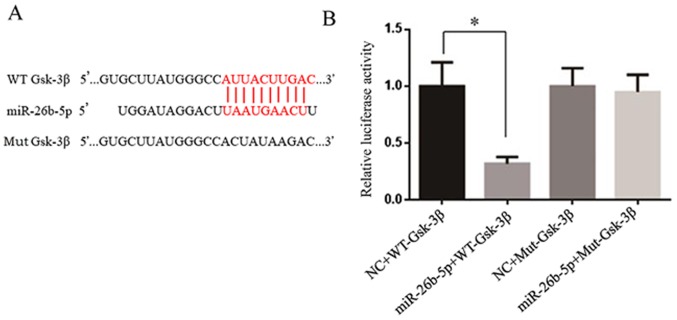
TargetScan was used to predict target genes of miR-26b-5p; Gsk-3β was predicted as a target gene, since the 3′-UTR of Gsk-3β contains numerous binding sites for miR-26b-5p (Fig. 4A). As presented in Fig. 4B, the luciferase reporter assay demonstrated that miR-26b-5p significantly inhibited the luciferase activity in the wild-type Gsk-3β group in vitro. Conversely, mutations in the binding site restored luciferase activity.
Figure 4.
Gsk-3β is a target gene of miR-26b-5p. (A) Comparison between miR-26b-5p and 3′-UTR of Gsk-3β; red indicates the complementary site. (B) Dual-luciferase reporter assay; miR-26b inhibited the luciferase activity of WT Gsk-3β, whereas it had no effect on Mut Gsk-3β. *P<0.05. Gsk-3β, glycogen synthase kinase-3β; miR-26b-5p, microRNA-26b-5p; Mut, mutant; NC, negative control; WT, wild-type.
miR-26b-5p downregulates the protein expression levels of pGsk-3β and β-catenin in B-CPAP cells
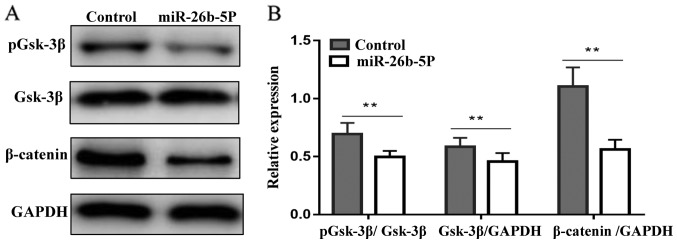
The results of western blotting are presented in Fig. 5. In the control group, β-catenin was highly expressed, whereas, in the miR-26b-5p overexpression group, the protein expression levels of β-catenin were significantly decreased (P<0.01). In addition, pGsk-3β in the miR-26b-5p overexpression group was decreased compared with in the control group (P<0.01). These results indicated that miR-26b-5p may exert antitumor activity by downregulating the expression of β-catenin and pGsk-3β proteins in B-CPAP cells.
Figure 5.
miR-26b-5p downregulates the protein expression levels of pGsk-3β and β-catenin in BCPAP cells. (A) Protein expression levels of Gsk-3β, pGsk-3β and β-catenin. (B) Relative expression levels of Gsk-3β, pGsk-3β and β-catenin. **P<0.01. Gsk-3β, glycogen synthase kinase-3β; miR-26b-5p, microRNA-26b-5p; ns, not significant; p, phosphorylated.
Discussion
miRNAs may bind to specific target genes, and are involved in the regulation of tumor growth, differentiation, apoptosis and other processes (22,23). Numerous miRNAs have been reported to be involved in the development of thyroid cancer, including miR-21, miR-338-3p, miR-221 and miR-146b-5p (24–27). Liu et al (28) revealed that miR-363-3p inhibits the proliferation, migration and invasion of TC by downregulating the expression of the target gene phosphatidylinositol-4,5- biphosphate 3-kinase catalytic subunit α. Zhang et al (29) suggested that miR-146a may inhibit the expression of target gene protein kinase Cε, and upregulated miR-146a expression promotes the apoptosis of TC cells. In addition, Yin et al (30) demonstrated that miR-195 may inhibit the proliferation and migration of B-CPAP and K1 TC cells by downregulating the expression of target genes cyclin D1 and fibroblast growth factor 2. Cheng et al (31) observed that overexpression of miR-150 inhibits the proliferation and invasion of tumor cells, and proposed that its target gene is Rho-associated coiled-coil containing protein kinase 1. These results suggested that certain miRNAs are expressed at low levels in the majority of tumors and under physiological conditions, and may serve a role in inhibiting tumor proliferation and migration. Therefore, the overexpression of miRNAs may provide a novel strategy for the clinical treatment and diagnosis of thyroid cancer.
In the present study, the expression levels of miR-26b-5p were significantly downregulated in TC compared with in adjacent tissues. This result suggested that detecting the expression levels of miR-26b-5p may be used to distinguish cancer from normal thyroid tissue. In addition, the present results demonstrated that pre-operative detection of miR-26b-5p expression levels combined with biopsy results may be helpful in the diagnosis of TC. There was no significant association between miR-26b-5p expression and the sex, age, tumor size and TNM stage of patients; however, the expression levels of miR-26b-5p were significantly higher in TC tissues without lymph node metastasis compared with in tissues with lymph node metastasis.
To further examine the biological role of miR-26b-5p in TC, miR-26b-5p was overexpressed in the TC cell line B-CPAP. Cytological experiments demonstrated that miR-26b-5p overexpression may inhibit the proliferation, migration and invasion of B-CPAP cells, thus suggesting that miR-26b-5p may serve a negative regulatory role in the development of TC, which is consistent with previous studies. For example, Wu et al (16) reported that miR-26b-5p is downregulated in lung cancer, and overexpression of miR-26b-5p promotes tumor proliferation and inhibits apoptosis. Kato et al (32) demonstrated that miR-26a/b inhibits prostate cancer migration and invasion by regulating La-related protein 1. Kozubek et al (33) revealed that miR-26b-5p expression is downregulated in melanoma, which may affect the progression of melanoma by regulating the mitogen-activated protein kinase pathway.
However, the molecular mechanism underlying the inhibitory effects of miR-26b-5p on TC remains unclear. TargetScan was used to predict the target gene of miR-26b-5p; Gsk-3β was identified as a potential target gene. The dual-luciferase enzyme assay confirmed that Gsk-3β may be a target of miR-26b-5p. Gsk-3β is an evolutionarily conserved serine/threonine kinase, which acts on numerous signaling proteins, structural proteins and transcription factors to regulate cell differentiation, proliferation, survival and apoptosis (34). Phosphorylation at different sites can regulate the activity of Gsk-3β: Phosphorylation at the 9th serine can inhibit the activity of Gsk-3β, and tyrosine phosphorylation at position 216 can enhance its activity (35). Gsk-3β substrates are widely involved in various physiological cell processes, and Gsk-3β serves a tumor-suppressing role in numerous types of cancer. Farago et al (36) demonstrated that following inhibition of Gsk-3β activity, the Wnt pathway is activated to promote breast cancer cell proliferation. Jiang et al (37) demonstrated that the expression levels of pGsk-3βSer9 are increased in lung cancer tissues, and that the expression levels of pGsk-3βSer9 are negatively correlated with lung cancer prognosis. Gsk-3β is a key kinase that determines the phosphorylation of β-catenin. An increase in Gsk-3β activity may promote the phosphorylation of β-catenin, which is degraded by the proteasome, resulting in antitumor effects. The anticancer effect of Gsk-3β is associated with obstruction of the Wnt signaling pathway, which is important for cell proliferation, differentiation and apoptosis (38). Further studies have demonstrated that the Wnt signaling pathway is involved in cell migration and invasion, and its regulation is associated with matrix metalloproteinase-7 (39), C-X-C motif chemokine receptor 4b and 7b (40). In the present study, the results demonstrated that the protein expression levels of pGsk-3βSer9 and β-catenin were significantly decreased in the miR-26b-5p overexpression group compared with in the empty vector group, so decreased phosphorylation could increase its activation. These findings suggested that miR-26b-5p may inhibit the proliferation, migration and invasion of B-CPAP cells via the Gsk-3β/β-catenin pathway. Lv et al (41) demonstrated that miR-26a is consistently downregulated in TC specimens, whereas the upregulation of miR-26a in TC cells lines induces G2 phase cell cycle arrest by targeting and downregulating CDC28 protein kinase regulatory subunit 2 (CKS2), further research indicated that the negative regulatory effects are related to the CKS2 downstream genes cyclin A, Bcl2 like 1 and AKT. Lin et al (42) demonstrated that CKS2 regulates AKT and Gsk-3β phosphorylation. The present study suggested that miR-26b-5p may directly regulate Gsk-3β; this may be associated with the mechanism underlying the effects of miR-26.
In conclusion, the present results suggested that miR-26b-5p was downregulated in TC tissues and was associated with lymph node metastasis. In addition, miR-26b-5p overexpression was able to inhibit the proliferation, migration and invasion of TC cells; these effects may be associated with the Gsk-3β/β-catenin pathway. The present results further demonstrated that miR-26b-5p may provide a novel basis for the auxiliary diagnosis of TC, providing better individualized treatment. However, the mechanism by which miR-26b-5p is involved in tumorigenesis and development remains unclear and requires further study.
Acknowledgements
Not applicable.
Funding
This work was supported financially by the Shandong Key Research and Development Plan (grant no. 2017GSF18120) and the Jinan Science and Technology Development Plan (grant no. 201704105).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AZ, TW, and LL conceived and designed the project and prepared the manuscript. AZ, GC, XC, CZ and HX conducted the cell experiments. MQ and XC analyzed the data. All authors read and approved the manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The Shandong Provincial Hospital Affiliated to Shandong University, and all participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Randle RW, Bushman NM, Orne J, Balentine CJ, Wendt E, Saucke M, Pitt SC, Macdonald CL, Connor NP, Sippel RS. Papillary thyroid cancer: The good and bad of the ‘Good Cancer’. Thyroid. 2017;27:902–907. doi: 10.1089/thy.2016.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garau LM, Rubello D, Ferretti A, Boni G, Volterrani D, Manca G. Sentinel lymph node biopsy in small papillary thyroid cancer. A review on novel surgical techniques. Endocrine. 2018;62:340–350. doi: 10.1007/s12020-018-1658-5. [DOI] [PubMed] [Google Scholar]
- 3.Yan S, Zhao W, Wang B, Zhang L. Preoperative injection of carbon nanoparticles is beneficial to the patients with thyroid papillary carcinoma: From a prospective study of 102 cases. Medicine (Baltimore) 2018;97:e11364. doi: 10.1097/MD.0000000000011364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulsson JO, Zedenius J, Juhlin CC. Papillary thyroid carcinoma with pleomorphic tumor giant cells in a pregnant woman-a case report. BMC Endocr Disord. 2018;18:46. doi: 10.1186/s12902-018-0275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yongfu Z, Ziyu L, Chen L, Jingchao X. Cervical mass as the initial manifestation of occult papillary thyroid carcinoma: Report of three cases. J Cancer Res Ther. 2018;14(Suppl):S544–S548. doi: 10.4103/0973-1482.204892. [DOI] [PubMed] [Google Scholar]
- 6.Hardin H, Helein H, Meyer K, Robertson S, Zhang R, Zhong W, Lloyd R. Thyroid cancer stem-like cell exosomes: Regulation of EMT via transfer of lncRNAs. Lab Invest. 2018;98:1133–1142. doi: 10.1038/s41374-018-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma B, Wei W, Xu W, Wang Y, Guan H, Fan J, Zhao Z, Wen D, Yang S, Wang Y, et al. Surgical confirmation of incomplete treatment for primary papillary thyroid carcinoma by percutaneous thermal ablation: A retrospective case review and literature review. Thyroid. 2018;28:1134–1142. doi: 10.1089/thy.2017.0558. [DOI] [PubMed] [Google Scholar]
- 8.Henn D, Abu-Halima M, Falkner F, Wermke D, Meme LG, Kühner C, Keller A, Kneser U, Meese E, Schmidt V, et al. Micro-RNA regulated pro-angiogenic signaling in arteriovenous loops in patients with combined vascular and soft tissue reconstructions-reisiting the nutrient flap concept. Plast. Reconstr. Surg. 2018;142:1. doi: 10.1097/PRS.0000000000004750. [DOI] [PubMed] [Google Scholar]
- 9.Gomes A, da Silva IV, Rodrigues CMP, Castro RE, Soveral G. The emerging role of microRNAs in aquaporin regulation. Front Chem. 2018;6:238. doi: 10.3389/fchem.2018.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigita G, Distefano R, Veneziano D, Romano G, Rahman M, Wang K, Pass H, Croce CM, Acunzo M, Nana-Sinkam P. Tissue and exosomal miRNA editing in non-small cell lung cancer. Sci Rep. 2018;8:10222. doi: 10.1038/s41598-018-28528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uen Y, Wang J, Wang C, Jhang Y, Chung J, Tseng T, Sheu M, Lee S. Mining of potential microRNAs with clinical correlation-regulation of syndecan-1 expression by miR-122-5p altered mobility of breast cancer cells and possible correlation with liver injury. Oncotarget. 2018;9:28165–28175. doi: 10.18632/oncotarget.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu DT, Yao HR, Li YY, Song YY, Su MY. MicroRNA-19b promotes the migration and invasion of ovarian cancer cells by inhibiting the PTEN/AKT signaling pathway. Oncol Lett. 2018;16:559–565. doi: 10.3892/ol.2018.8695. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Hersi HM, Raulf N, Gaken J, Folarin N, Tavassoli M. MicroRNA-9 inhibits growth and invasion of head and neck cancer cells and is a predictive biomarker of response to plerixafor, an inhibitor of its target CXCR4. Mol Oncol. 2018;12:2023–2041. doi: 10.1002/1878-0261.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, He M, Hou Y, Liang B, Zhao L, Ma S, Yu Y, Liu X. Expression profiles of microRNAs and their target genes in papillary thyroid carcinoma. Oncol Rep. 2013;29:1415–1420. doi: 10.3892/or.2013.2263. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Yuan Z, Fan Y, Deng X, Zheng Q. Integrated analyses of microRNA and mRNA expression profiles in aggressive papillary thyroid carcinoma. Mol Med Rep. 2013;8:1353–1358. doi: 10.3892/mmr.2013.1699. [DOI] [PubMed] [Google Scholar]
- 16.Wu T, Chen W, Liu S, Lu H, Wang H, Kong D, Huang X, Kong Q, Ning Y, Lu Z. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. FEBS Lett. 2014;588:2107–2114. doi: 10.1016/j.febslet.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 17.Mei JY, Zhang MJ, Wang YY, Liu YH. The positive clinical therapeutically effects of Escin on advanced thyroid cancer. Cancer Med. 2017;6:937–943. doi: 10.1002/cam4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbosh PH, Nephew KP. Multiple signaling pathways converge on beta-catenin in thyroid cancer. Thyroid. 2005;15:551–561. doi: 10.1089/thy.2005.15.551. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Wakasa T, Li Y, Bai Y, Liu Z, Ozaki T, Mori I, Miyauchi A, Kakudo K, Nakamura M. Up-regulation of urinary-type plasminogen activator correlates with high-risk papillary thyroid carcinoma with BRAF(V600E) mutation and its possible molecular mechanism. Pathol Res Pract. 2014;210:733–738. doi: 10.1016/j.prp.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manualand the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 22.Ambros V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/S0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 23.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon MP, Khan A. Micro-RNAs in thyroid neoplasms: molecular, diagnostic and therapeutic implications. J Clin Pathol. 2009;62:978–985. doi: 10.1136/jcp.2008.063909. [DOI] [PubMed] [Google Scholar]
- 25.Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V, Borbone E, Petrocca F, Alder H, Croce CM, Fusco A. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer. 2007;14:791–798. doi: 10.1677/ERC-07-0129. [DOI] [PubMed] [Google Scholar]
- 26.Sui GQ, Fei D, Guo F, Zhen X, Luo Q, Yin S, Wang H. MicroRNA-338-3p inhibits thyroid cancer progression through targeting AKT3. Am J Cancer Res. 2017;7:1177–1178. [PMC free article] [PubMed] [Google Scholar]
- 27.Lima CR, Geraldo MV, Fuziwara CS, Kimura ET, Santos MF. miRNA-146b-5p upregulates migration and invasion of different Papillary Thyroid Carcinoma cells. BMC Cancer. 2016;16:108. doi: 10.1186/s12885-016-2146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Li Q, Li R, Ren P, Dong S. MicroRNA-363-3p inhibits papillary thyroid carcinoma progression by targeting PIK3CA. Am J Cancer Res. 2017;7:148–158. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Zhang X, Li D, Li M, Ye M, Ding L, Cai H, Fu D, Lv Z. MicroRNA-146a targets PRKCE to modulate papillary thyroid tumor development. Int J Cancer. 2014;134:257–267. doi: 10.1002/ijc.28141. [DOI] [PubMed] [Google Scholar]
- 30.Yin Y, Hong S, Yu S, Huang Y, Chen S, Liu Y, Zhang Q, Li Y, Xiao H. miR-195 inhibits tumor growth and metastasis in papillary thyroid carcinoma cell lines by targeting CCND1 and FGF2. Int J Endocrinol. 2017;2017:6180425. doi: 10.1155/2017/6180425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng L, Zhou R, Chen M, Feng L, Li H. MicroRNA-150 targets Rho-associated protein kinase 1 to inhibit cell proliferation, migration and invasion in papillary thyroid carcinoma. Mol Med Rep. 2017;16:2217–2224. doi: 10.3892/mmr.2017.6842. [DOI] [PubMed] [Google Scholar]
- 32.Kato M, Goto Y, Matsushita R, Kurozumi A, Fukumoto I, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M, Ichikawa T, Seki N. MicroRNA-26a/b directly regulate La-related protein 1 and inhibit cancer cell invasion in prostate cancer. Int J Oncol. 2015;47:710–718. doi: 10.3892/ijo.2015.3043. [DOI] [PubMed] [Google Scholar]
- 33.Kozubek J, Ma Z, Fleming E, Duggan T, Wu R, Shin DG, Dadras SS. In-depth characterization of microRNA transcriptome in melanoma. PLoS One. 2013;8:e72699. doi: 10.1371/journal.pone.0072699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forde JE, Dale TC. Glycogen synthase kinase 3: A key regulator of cellular fate. Cell Mol Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farago M, Dominguez I, Landesman-Bollag E, Xu X, Rosner A, Cardiff RD, Seldin DC. Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res. 2005;65:5792–5801. doi: 10.1158/0008-5472.CAN-05-1021. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y, Miao J, Wang D, Zhou J, Liu B, Jiao F, Liang J, Wang Y, Fan C, Zhang Q. MAP30 promotes apoptosis of U251 and U87 cells by suppressing the LGR5 and Wnt/β-catenin signaling pathway, and enhancing Smac expression. Oncol Lett. 2018;15:5833–5840. doi: 10.3892/ol.2018.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Chen XJ, Meng J, Yue W, Yu J, Yang J, Yao Z, Zhang L. Fibulin-3 suppresses Wnt/β-catenin signaling and lung cancer invasion. Carcinogenesis. 2014;35:1707–1716. doi: 10.1093/carcin/bgu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aman A, Piotrowski T. Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell. 2008;15:749–761. doi: 10.1016/j.devcel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Lv M, Zhang X, Li M, Chen Q, Ye M, Liang W, Ding L, Cai H, Fu D, Lv Z. miR-26a and its target CKS2 modulate cell growth and tumorigenesis of papillary thyroid carcinoma. PLoS One. 2013;8:e67591. doi: 10.1371/journal.pone.0067591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Lin L, Fang Z, Lin H, Lin H, You H, Wang J, SU H, Wang F, Zhang ZY. Depletion of Cks1 and Cks2 expression compromises cell proliferation and enhance chemotherapy-induced apoptosis in HepG2 cells. Oncol Rep. 2016;35:26–32. doi: 10.3892/or.2015.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.