Abstract
Electroacupuncture (EA), a traditional Chinese therapeutic technique, is considered an effective method for treating certain painful neuropathies induced by various neuropathological damage. The current study investigated the effect of EA on intrathecal (IT) morphine-induced hyperalgesia (MIH) and examined the hypothesis that activation of cannabinoid receptor 1 (CB1) could enhance the antinociceptive effect of EA on MIH via regulation of the extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway. Using a rat model of IT MIH, mechanical and thermal hyperalgesia were evaluated by an electronic von Frey filament and hotplate at baseline (1 day before IT administration) and at days 1, 3, 5 and 7 after IT administration. Rats received IT normal saline, IT morphine or IT morphine + EA at ST36-GB34. The protein levels of ERK1/2, phosphorylated (p)-ERK1/2 and CB1 in the spinal cord were assayed by western blotting. Furthermore, the effect of IT injection of the CB1 agonist WIN 55,212-2 and the CB1 antagonist SR141716 on the antinociceptive effect of EA in rats with MIH was investigated. Nociceptive behavior and ERK1/2, phosphorylated (p)-ERK1/2 and CB1 protein levels were evaluated as mentioned above. The results revealed that chronic IT injections of morphine induced a significant decrease in mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) accompanied with remarkable upregulation of p-ERK1/2 in the spinal cord, which could be attenuated by EA at the ST36-GB34 acupoints. In the rat model of MIH, IT injection of WIN 55,212-2 combined with EA induced a significant increase in MWT and TWL accompanied with a significant decrease in p-ERK1/2 and a significant increase in CB1 protein level compared with EA alone, while SR141716 induced the opposite results. The present study suggests that EA alleviates hyperalgesia induced by IT injection of morphine partially through the inhibition of ERK1/2 activation. Activation of the CB1 receptor enhances the antinociceptive effect of EA in rats with MIH partly through the regulation of the spinal CB1-ERK1/2 signaling pathway.
Keywords: electroacupuncture, morphine-induced hyperalgesia, cannabinoid receptor 1, extracellular signal-regulated kinase 1/2
Introduction
Morphine-induced hyperalgesia (MIH) is a type of classic opioid-induced hyperalgesia (OIH) that is characterized by increased sensitivity to noxious stimuli or even a painful response to previously non-noxious stimuli (allodynia) induced by long-time use of morphine (1). It was suggested that paradoxical pain can be elicited by chronic opioid exposure in humans and in animal models (2). Certain neuroplastic adaptations, including increased expression of calcitonin gene related peptide, substance P and various nociceptive receptors, were deemed to be the possible mechanism underlying OIH (3,4). The activation of mitogen-activated protein kinase (MAPK) in the central and peripheral nervous systems was indicated to be a possible signaling pathway in morphine-induced neuroplastic adaptations by a series of findings from different laboratories (4). Extracellular signal-regulated protein kinase 1/2 (ERK1/2), a member of the MAPK family, serves an important role in OIH by being activated by self-phosphorylation and mediating the synthesis and expression of downstream neuropeptides. The phosphorylated form of ERK1/2 (p-ERK1/2) can cause the activation of ERK1/2 and transfer the electrical signal to the nucleus to cause cell damage (5–7).
Cannabinoid receptors, which comprise two subtypes, including CB1 and CB2, belong to the G protein-coupled receptor superfamily and are involved in the modulation of pain sensation (8–10). CB1, which is mainly expressed in the central nervous system (CNS), is considered to mediate the pain sensation of the CNS (11). CB1-knockout mice exhibited reduced locomotor activity and hypoalgesia in hot plate and formalin tests (12). In addition, upregulation of CB1 receptor primarily within the ipsilateral superficial spinal cord dorsal horn was revealed in sciatic nerve injury (chronic constriction injury)-induced hyperalgesia model in rats, which was partially attributed to ERK activation (13). A previous study suggested that the CB1-selective cannabinoid receptor antagonist AM251 completely reversed the peripheral antinociception induced by the µ-opioid receptor agonist morphine but not by agonists of δ- or κ-opioid receptors, which indicated that CB1 is involved in the analgesic mechanism of morphine (14). The CB2 receptor is expressed mainly in the immune system and in hematopoietic cells (15). A previous study indicated that co-administration of a selective CB2 agonist (AM 1241) attenuates chronic intraperitoneal morphine exposure-mediated thermal hyperalgesia and tactile allodynia in rats, which is partially due to attenuated immunoreactivity of the spinal astrocyte and microglial marker and pro-inflammatory mediators interleukin-1β and tumor necrosis factor-α (16). However, the exact association between MIH and CB1/2 in CNS is poorly understood.
Electroacupuncture (EA) has been demonstrated to effectively mitigate hyperalgesia induced by chronic constriction injury (CCI) and cancer pain caused by intraplantar injection of Walker 256 carcinoma cells in rats (17,18). In addition, EA combined with a sub-threshold dose of morphine (2.5 mg/kg) enhanced the anti-inflammatory hyperalgesia effect compared with that produced by each component alone in rats, which indicated that there is a synergistic association between EA and morphine in this regard (19). However, whether EA can attenuate the hyperalgesia induced by chronic morphine exposure is still unknown. Another study revealed that EA inhibited zymosan-induced hypernociception in rats. The CB1-selective antagonist AM251 and the CB2-selective antagonist AM630 significantly reversed the antinociceptive and anti-inflammatory effects of EA separately, suggesting that CB1 and CB2 are involved in the mechanism of EA (20).
As mentioned above, ERK1/2, a classic member of the MAPK family, is involved in the mechanism of OIH. However, ERK1/2 is also considered as a mediator between EA effects and CBs (21). In the Freund's complete adjuvant-induced hind paw pain model in rats, thermal hyperalgesia and ERK phosphorylation in the ipsilateral dorsal horn of L4-5 segments were inhibited by EA stimulation (22). Notably, nocifensive behavior and activation of ERK1/2 in the lumbar dorsal spinal cord were also observed following intrathecal (IT) injection of a CB1 receptor antagonist, namely AM251, which were both inhibited by IT injection of a MAPK/ERK kinase inhibitor, namely U0126 (23). However, it is unknown whether ERK1/2 is involved in mediating EA's effect through CB1/2 in the spinal cord following MIH.
The present study hypothesized that EA could ameliorate MIH and that this effect was partially mediated by CBs via the ERK1/2 signaling pathway. The present study aimed to evaluate the effect of EA on nociceptive behavior, as well as the activation of ERK1/2 and to investigate the effect of CB1 activation or inhibition in regulating the effect of EA via the ERK1/2 activated state in rats undergoing MIH.
Materials and methods
Animals
All experimental protocols were approved by the Animal Experimental Ethics Committee of Tianjin Medical University General Hospital (Tianjin, China). A total of 128 adult male Sprague-Dawley rats, weighing 240±20 g each, were obtained from the Laboratory Animal Center of the Military Medical Science Academy (Beijing, China). For 1 week before the experiments, all animals were housed in cages (5 rats per cage) at room temperature (20–22°C) with 30–70% humidity on a 12 h light-dark cycle, were fed a standard diet and had access to water.
IT morphine delivery
The rats were anesthetized with 3% sevoflurane plus 60% oxygen and catheterizations of the rat spinal subarachnoid space were performed on anesthetized rats 3 days before morphine administration, as described by Yaksh and Rudy (24). Briefly, rats were implanted with a PE-10 polyethylene catheter (8 cm) the lumbar subarachnoid space. Rats with no postoperative neurological deficits following surgery were kept for the experiments. Animals showing neurological dysfunction such as paralysis postoperatively were immediately sacrificed using carbon dioxide. Upon surgery, rats were kept in individual cages for 3 days before morphine administration.
EA
Rats received EA stimulation (16 rats/group) (2 Hz, 1.5 mA, 30 min) at 20 min after each administration, as described by Yu et al (18). Briefly, rats without any anesthetic drug received acupuncture with two pairs of stainless acupuncture needles connected to two pairs of electrodes. Each pair of needles was inserted perpendicularly ~6 mm into the ipsilateral acupoints on the hind legs of the rats. Acupoints were located according to the Zusanli (ST36) and Yanglingquan (GB34) acupoints in humans. In rats, ST36 is located at the proximal 1/5 point on the line from the depression lateral to the patella ligament, while GB34 is located at the depression anterior and inferior to the fibular head (19). A total of 2 non-acupoints were located 0.5 cm horizontal and lateral to the ST36 and GB34 acupoints, respectively, at non-meridian points. A constant electronic pulse (2 Hz, 1.5 mA) was administered by an electroacupuncture stimulator (SDZ-II; Suzhou Medical Appliance Factory, Suzhou, China) which was connected to the other end of the electrodes. When the EA was starting, the current was stimulating from one acupoint (ST36 or GB34) to another nonacupoint. Rats in the control group (n=16) received the acupuncture needles at the same points as the rats in the EA group but without EA treatment. These rats were kept in tubular acrylic holders for 30 min as served as controls.
Experimental protocol
Experiment 1: Effects of EA on MIH
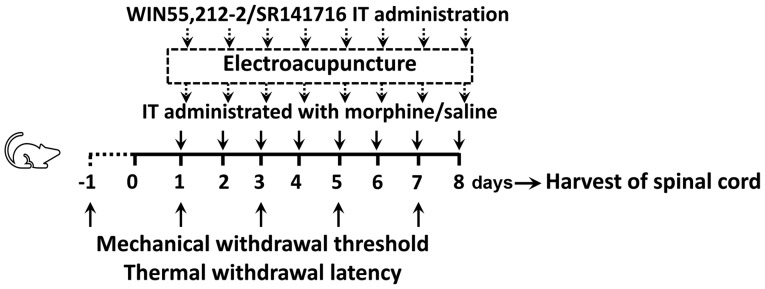
The animals were randomly divided into 3 groups (n=16 rats/group): The control group (C); the chronic morphine group (M); and the morphine + EA at ST36-GB34 group (ME). Animals in the M and ME groups were IT administrated twice with 15 µg (10 µl) morphine at 8 am and 6 pm daily for 8 days. Animals in the C group were IT treated daily with 10 µl saline at the same time as the M group for 8 days. The animals in the ME group received EA stimulation (2 Hz, 1.5 mA, 30 min, two times/day) at the Zusanli-Yanglingquan acupoints (ST36-GB34) 20 min after morphine or saline administration every day. The mechanical withdrawal threshold (MWT; n=8 rats/group) and thermal withdrawal latency (TWL; n=8 rats/group) were determined at baseline (24 h before IT administration, day-1) and at the same time following the second treatment on days 1, 3, 5 and 7 after drug administration. On the 8th day after drug administration, randomized selecting of 6 rats in each group to collect the L4-6 segments of the spinal cord for determination of the levels of ERK1/2, p-ERK1/2 and CB1 in the intumescentia lumbalis of the spinal cord (as shown in Fig. 1).
Figure 1.
Experimental design. Male Sprague-Dawley rats (weighing 240±20 g each) randomly received C, M or ME treatment. Animals in the M and ME groups were IT administered with 15 µg (10 µl) morphine twice daily at 8 am and 6 pm for 8 days, while animals in the C group were IT treated with 10 µl saline at the same time as the M group daily for 8 days. Furthermore, the animals in the ME group also received EA stimulation (2 Hz, 1.5 mA, 30 min) at Zusanli-Yanglingquan acupoints (ST36-GB34), 20 min after morphine or saline administration every day. In order to verify the key role of CB1, the CB1 agonist (WIN 55,212-2) and antagonist (SR141716) were IT administered, respectively following morphine, while animals in the other groups were injected with the same volume of saline. Mechanical withdrawal threshold and thermal withdrawal latency were determined at baseline (24 h before IT administration, day-1) and at the same time after the second treatment on days 1, 3, 5 and 7 after administration. On the 8th day after administration, the L4-6 segments of the spinal cord were collected for determining the levels of ERK1/2, phosphorylated ERK1/2 and CB1 in the intumescentia lumbalis of the spinal cord. IT, intrathecally; EA, electroacupuncture; CB1, cannabinoid receptor 1; ERK1/2, extracellular signal-regulated kinase 1/2; C, control; M, chronic morphine; ME, morphine + EA at ST36-GB34.
Experiment 2: Role of CB1 on the protective effects of EA against MIH
The CB1 agonist WIN 55,212-2 and antagonist SR141716 were used in this experiment. The animals were randomly divided into 5 groups (n=16 rats/group): i) The C group; ii) the M group; ii) the ME group; iv) the morphine + EA treatment + CB1 agonist WIN 55,212-2 group (MEW); and v) the morphine + EA treatment + CB1 antagonist SR141716 group (MES). Saline, morphine and EA treatment were administered as in experiment 1. WIN 55,212-2 (Cayman Chemical Company, Ann Arbor, MI, USA; 30 µg) (25) and SR141716 (Cayman Chemical Company; 30 µg) (26), which were dissolved in 5% dimethyl sulfoxide (10 µl; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were IT administrated to the MEW and MES groups, respectively, upon morphine administration, followed by 10 µl normal saline. The animals in the C, M and EA groups received the same volume of vehicle in identical conditions. MWT (n=8 rats/group) and TWL (n=8 rats/group) were determined at baseline (24 h before IT administration) and at the same time after the second treatment on days 1, 3, 5 and 7 post-administration. On day 8 after administration, randomized selecting of 6 rats in each group to collect the L4-6 segments of the spinal cord to detect the levels of ERK1/2, p-ERK1/2 and CB1 in the intumescentia lumbalis of the spinal cord was performed (as shown in Fig. 1).
Mechanical hyperalgesia
On days 1, 3, 5 and 7 post-administration, 8 rats per group were chosen for the mechanical hyperalgesia test. Mechanical hyperalgesia was assessed using an electronic von Frey filament (BSEVF3; Harvard Apparatus, Holliston, MA, USA), as described previously (18). Animals were placed in individual wire cages (20×20×30 cm) and allowed to acclimatize for 1 h before testing. Mechanical allodynia was determined by calculating the mean value of 3 MWT measurements with an interval of 5 min between each measurement. A cut-off pressure of 60 g was used to prevent tissue damage.
Thermal hyperalgesia
On days 1, 3, 5 and 7 post-administration, another 8 rats per group (not the same rats that were used to do the mechanical hyperalgesia test) were chosen to do the thermal hyperalgesia test. Thermal hyperalgesia was determined with Intelligence Hot plate equipment (YLS-6B; Zhenghua Biologic Apparatus Facilities Ltd., Co., Hefei, China), as described previously (18). Animals were allowed to habituate to the environment for 1 h before testing. Animals were placed on the hot plate (50°C) until a positive response (a clear paw withdrawal) was observed. The time was then recorded as the TWL. The mean TWL was obtained from the mean value of the 3 measurements of TWL with an interval of 5 min between each. A cut-off time of 30 sec was used to prevent tissue damage.
Protein analysis by western blotting
Tissues from the lumbar spinal cord (n=6 rats/group) were quickly removed under anesthesia on day 8 after administration and immediately frozen in liquid nitrogen at −196°C. Tissues were homogenized in immunoprecipitation buffer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing Protease Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA) and centrifuged at 15,000 × g for 5 min at 4°C. Protein concentration was determined by the bicinchoninic acid assay method (Pierce; Thermo Fisher Scientific, Inc.). Equal quantities of protein (30 µg) were used to determine the protein expression of ERK1/2, p-ERK1/2, CB1 and β-actin. Samples were separated using SDS-PAGE (8–10% gradient gels) and then transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat milk for 1 h at room temperature and incubated with anti-ERK1/2 (1:500; cat. no. 4696; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-p-ERK1/2 antibody (1:500; cat. no. 5726; Cell Signaling Technology, Inc.) or anti-CB1 (1:100; cat. no. ab167366; Abcam, Cambridge, UK) or anti-β-actin antibodies (1:5,000; cat no. A5441; Sigma-Aldrich; Merck KGaA) overnight at 4°C. The membranes were washed with 1X TBS-Tween-20 (TBST) buffer for 30 min and incubated with an horseradish peroxidase conjugated rabbit anti-mouse secondary antibody (1:2,000; cat. no. 58802; Cell Signaling Technology, Inc.) for 2 h at room temperature. The membranes were washed with TBST buffer for additional 30 min and visualized using Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA, USA) for 1 min, followed by film exposure for 30 sec to 2 min. The results were analyzed using Quantity One analysis software (version 4.6.7; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data are reported as the mean ± standard deviation. An unpaired Student's t test was used if the values had a Gaussian distribution, while Mann-Whitney test was used if values did not have such a distribution, to analyze differences between 2 groups and one-way analysis of variance with Bonferroni comparison was employed to analyze interactions among various groups. P<0.05 was considered to indicate a statistically significant difference. Significance testing was 2-tailed. Statistical analysis was performed using GraphPad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA) and SPSS statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA).
Results
EA at ST36-GB34 acupoints attenuates IT MIH
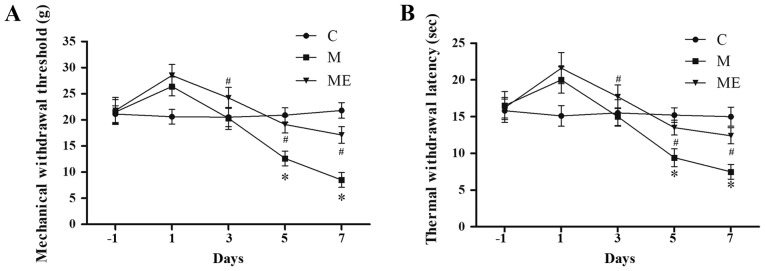
Compared with the control animals (group C), IT administration of morphine (group M) significantly decreased MWT and TWL on days 5 and 7 (P<0.05; Fig. 2A and B), which indicated that the animal model of IT morphine-induced hyperalgesia was successfully established. In addition, compared with the M group, combined EA at ST36-GB34 acupoints (group ME) induced a significant increase in MWT and TWL on days 5 and 7 (P<0.05; Fig. 2A and B), which indicated that EA at ST36-GB34 acupoints significantly reduced the mechanical and thermal hyperalgesia induced by IT administration of morphine.
Figure 2.
Effects of EA on the behavioral tests of morphine-induced hyperalgesia. On day-1 (1 day before IT administration) and in days 1, 3, 5 and 7 after drug administration, rats received IT normal saline, IT morphine and IT morphine + EA at ST36-GB34. Next, (A) mechanical hyperalgesia and (B) thermal hyperalgesia were evaluated by electronic von Frey filament and hot plate, respectively. Data are expressed as the mean ± standard deviation (n=8 rats/group for mechanical hyperalgesia test and n=8 rats/group for thermal hyperalgesia test). *P<0.05 vs. the C group, #P<0.05 vs. the M group. IT, intrathecal; EA, electroacupuncture; C, control; M, chronic morphine; ME, morphine + EA at ST36-GB34.
Inhibition of the spinal cord ERK1/2 activation and increased expression of CB1 caused by EA at the ST36-GB34 acupoints may be involved in the protective effects of EA against MIH
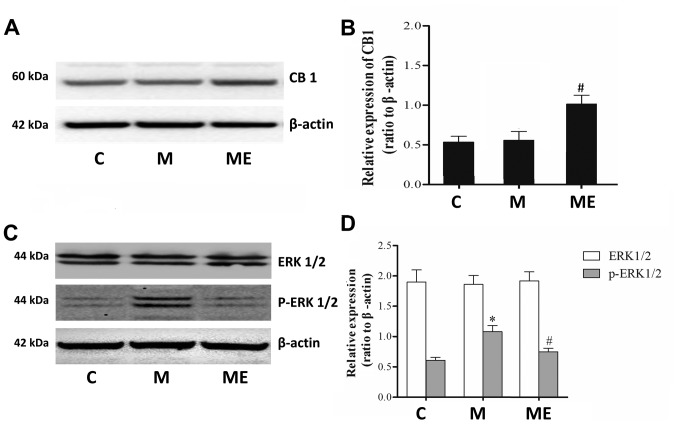
Compared with the control group (C), repeated IT treatment with morphine did not affect the protein levels of the CB1 receptor in the spinal cord. However, in comparison with the repeated administration of morphine group (M), repeated morphine plus EA raised the expression of the CB1 receptor in the spinal cord (P<0.05; Fig. 3A and B). Furthermore, there was also a significant increase in p-ERK1/2 (P<0.05; Fig. 3C and D) but not in ERK1/2 (P>0.05; Fig. 3C and D) levels in the spinal cord of animals in the chronic morphine (M) group compared with the C group. EA at ST36-GB34 acupoints (ME) induced a significant decrease in p-ERK1/2 (P<0.05; Fig. 3C and D) but not ERK1/2 (P>0.05; Fig. 3C and D) levels compared with the ME group, which indicated that EA stimulation at acupoints attenuated the level of ERK1/2 activation caused by chronic IT administration of morphine.
Figure 3.
Effects of EA on CB1, ERK1/2 and p-ERK1/2 expression in morphine-induced hyperalgesia. Spinal cord tissue from different groups was collected 8 days after intrathecal treatment with saline, morphine and EA. (A) CB1, (C) ERK1/2 and p-ERK1/2 levels were detected by western blotting. Quantitative analysis of (B) CB1, (D) ERK1/2 and p-ERK1/2 are shown as the ratio of protein relative density to β-actin. Data are expressed as the mean ± standard deviation (n=6 rats/group). *P<0.05 vs. the C group, #P<0.05 vs. the M group. EA, electroacupuncture; CB1, cannabinoid receptor 1; ERK1/2, extracellular signal-regulated kinase 1/2; p, phosphorylated; C, control; M, chronic morphine; ME, morphine + EA at ST36-GB34.
IT administration of WIN 55,212-2/SR141716 enhances/attenuates the inhibitory effects of EA on MIH at the ST36-GB34 acupoints
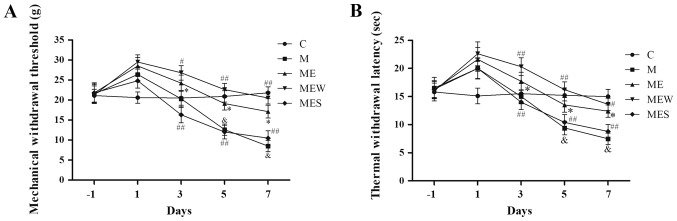
As mentioned above, IT administration of morphine significantly decreased MWT and TWL (P<0.05 in group M vs. group C; Fig. 4A and B). EA at the ST36-GB34 acupoints induced a significant increase in MWT and TWL (P<0.05 in the ME group vs. the M group; Fig. 4A and B). However, compared with the ME group, IT administration of WIN 55,212-2 (group MEW) significantly increased MWT and TWL on days 3–7 (P<0.05 for MWT on day 3 and TWL on day 7; P<0.01 for MWT on days 5 and 7 and for TWL on days 3 and 5; Fig. 4A and B). Compared with the ME group, IT administration of SR141716 (group MES) significantly decreased MWT and TWL on days 3 to 7 (P<0.01; Fig. 4A and B). These results demonstrated that activation of CB1 significantly enhanced the inhibitory effect of EA on IT morphine-induced mechanical and thermal hyperalgesia at the ST36-GB34 acupoints, while inhibition of CB1 attenuated the inhibitory effect of EA.
Figure 4.
Effects of EA upon administration of WIN 55,212-2 or SR141716 on the behavioral tests of morphine-induced hyperalgesia. On day-1 (1 day before IT administration) and on days 1, 3, 5 and 7 after drug administration in rats that received IT normal saline, IT morphine, IT morphine + EA at ST36-GB34, IT morphine + EA treatment + CB1 agonist WIN 55,212-2 or IT morphine + EA treatment + CB1 antagonist SR141716, (A) mechanical hyperalgesia and (B) thermal hyperalgesia were assessed by electronic von Frey filament and hot plate, respectively. Data are expressed as the mean ± standard deviation (n=8 rats/group for mechanical hyperalgesia test and n=8 rats/group for thermal hyperalgesia test). *P<0.05 vs. the C group, #P<0.05 vs. the M group, ##P<0.01 vs. the M group, &P<0.05 vs. the ME group. IT, intrathecal; EA, electroacupuncture; CB1, cannabinoid receptor 1; C, control; M, chronic morphine; ME, morphine + EA at ST36-GB34; MEW, the morphine + EA treatment + CB1 agonist WIN 55,212-2 group; MES, the morphine + EA treatment + CB1 antagonist SR141716 group.
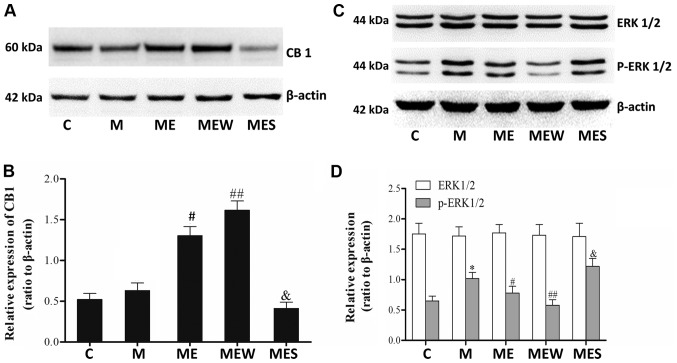
Inhibition of ERK1/2 activation induced CB1 overexpression may enhance the inhibitory effects of EA on MIH at the ST36-GB34 acupoints
There were significant increases in p-ERK1/2 levels in the spinal cord of the animals in the chronic morphine (M) group compared with those in the C group (P<0.05 M vs. C; Fig. 5C and D). These increases were significantly attenuated by EA at the ST36-GB34 acupoints (group ME; P<0.05; Fig. 5C and D). Compared with the ME group, IT administration of the CB1 agonist WIN 55,212-2 combined with EA significantly increased the CB1 levels but decreased the p-ERK1/2 levels in the spinal cord of rats with IT MIH (P<0.01; Fig. 5). On the contrary, there was a significant decrease in CB1 protein level and a significant increase in p-ERK1/2 level in the spinal cord of rats that received IT administration of the CB1 antagonist SR141716 combined with EA (P<0.05 compared with the ME group; Fig. 5A). There was no significant difference in total ERK1/2 levels across all groups (P>0.05; Fig. 5C and D). These results indicated that EA at the ST36-GB34 acupoints may have a protective effect against MIH through upregulating CB1 and downregulating ERK1/2 activation.
Figure 5.
Effects of EA upon administration of WIN 55,212-2 or SR141716 on CB1, ERK1/2 and p-ERK1/2 expression in morphine-induced hyperalgesia. Spinal cord tissue from different groups was collected 8 days after intrathecal treatment with saline, morphine, EA, WIN 55,212-2 and SR141716. (A) CB1, (C) ERK1/2 and p-ERK1/2 were detected by western blotting. Quantitative analysis of (B) CB1, (D) ERK1/2 and p-ERK1/2 are shown as the ratio of protein relative density to β-actin. Data are expressed as the mean ± standard deviation (n=6 rats/group). *P<0.05 vs. the C group, #P<0.05 vs. the M group, ##P<0.01 vs. the M group, &P<0.05 vs. the ME group. EA, electroacupuncture; CB1, cannabinoid receptor 1; ERK1/2, extracellular signal-regulated kinase 1/2; p, phosphorylated; C, control; M, chronic morphine; ME, morphine + EA at ST36-GB34; MEW, the morphine + EA treatment + CB1 agonist WIN 55,212-2 group; MES, the morphine + EA treatment + CB1 antagonist SR141716 group.
Discussion
The present study used a rat model of chronic MIH to investigate the antinociceptive mechanism of EA and to explore the effect of CB1 in this mechanism. The results of the present study indicated that 2-Hz EA at the ST36-GB34 acupoints attenuated MIH, which was accompanied by an increase in CB1 levels and a decrease in p-ERK1/2 levels. The present results also revealed that IT administration of the CB1 agonist WIN 55,212-2 combined with EA at the above acupoints enhanced the antinociceptive effect of EA on MIH and induced an increase in CB1 levels and a decrease in p-ERK1/2 levels compared with administration of EA alone, while the CB1 antagonist SR141716 had the opposite effect. These data indicated that EA at the ST36-GB34 acupoints may have a protective effect against MIH through upregulating CB1 and downregulating ERK1/2 activation.
As a type of Chinese traditional therapy, EA is used to treat various diseases. There is increasing evidence that EA may have clinical potential in attenuating certain types of chronic pain, including adjuvant arthritis, CCI and cancer-associated pain (17,18,27). Frequency is regarded as an important parameter in EA treatment, with 2, 15 and 100 Hz being the most commonly used frequencies for analgesic therapy. Among these frequencies, 2-Hz EA has been demonstrated to have a better analgesic effect for neuropathic pain; thus, this frequency was selected in the present study (18). The data from the present study revealed that 2-Hz EA at the ST36-GB34 acupoints but not at non-acupoints attenuated mechanical and thermal hyperalgesia caused by IT administration of morphine, which is similar to the findings of previous studies regarding the antinociceptive effect of EA at the ST36 and GB34 acupoints (17,18,28).
Activation of ERK1/2 within spinal neurons by various peripheral noxious stimulation has been reported to be involved in generating pain hypersensitivity (29). Activation of ERK1/2 induced short-term functional changes by non-transcriptional processing and long-term neuronal plastic changes by increasing the gene transcription of hyperalgesia-associated downstream neuropeptides (30). Furthermore, there are accumulating data about the roles of ERK in mediating the neuronal plasticity that contributes to MIH (4). Previous evidence has suggested that activation of ERK in the spinal cord is implicated in the formation of MIH (7,31). It was reported that IT injection of morphine for 7 days induced a remarkable increase in p-ERK1/2 levels in the spinal cord of rats, which contributed to morphine tolerance and associated hyperalgesia (7,32). Inhibition of ERK1/2 activation by IT injection of the ERK1/2 inhibitor U0126 or knockdown of spinal ERK1/2 by antisense oligonucleotides attenuated withdrawal-induced mechanical allodynia in rats (31,33). Consistent with previous studies, the results of the present study indicated that the p-ERK1/2 levels in the spinal cord significantly increased by IT injection of morphine (15 µg, twice a day) for 7 days in rats with MIH. Recent studies suggested that EA at acupoints attenuated hyperalgesia caused by peripheral noxious stimulation and decreased the activation of ERK1/2 (18,28). The results of the present study revealed that, accompanied by attenuated mechanical and thermal hyperalgesia, EA at the ST36-GB34 acupoints induced a decrease in p-ERK1/2 levels in the spinal cord of rats that received IT morphine for 7 days. These data demonstrated that inhibition of ERK1/2 activation is at least partially involved in the EA treatment of MIH.
CB1 is highly expressed in regions involved in pain transmission and modulation, including the majority (76–83%) of nociceptive neurons of dorsal root ganglions, the dorsal horn of the spinal cord, the thalamus and the periaqueductal grey (34,35). In the spinal cord, results revealed that CB1 levels were a slightly increased by IT morphine and EA administration could greatly increase the expression of CB1 under IT morphine administration. Upregulation of CB1 partially participated in the antinociceptive effect of EA and CB1 may serve a major role in this process at the level of the spinal cord, which is in agreement with a previous study (36).
As aforementioned, upregulation of the CB1 receptor in the spinal cord was observed in a sciatic nerve injury-induced hyperalgesia model in rats (13). Another study suggested that the CB1 antagonist AM251 completely reversed the peripheral antinociception induced by morphine, which demonstrated that CB1 is involved in the analgesic mechanism of morphine (14). Consistent with earlier studies (13,14), the results of the present study revealed that chronic IT injection of morphine significantly increases CB1 protein levels along with the onset of hyperalgesia. In a pain model of L5 spinal nerve ligation, intraperitoneal injection of the CB1 agonist WIN 55,212-2 significantly attenuated mechanical hyperalgesia and thermal allodynia, while co-administration of the CB1 antagonist SR141716 but not the CB2 antagonist SR144528 prevented this effect, suggesting that this effect of WIN 55,212-2 is mediated via the CB1 receptor (37). Additionally, CB1 was also considered to be involved in the mechanism of EA treatment. The CB1 selective antagonist AM251 significantly reversed the antinociceptive and anti-inflammatory effects of EA in a rat model of zymosan-induced hypernociception (20). The results of the present study revealed that IT injection of the CB1 agonist WIN 55,212-2 enhanced the antinociceptive effect of EA and induced a significant increase in CB1 protein levels in a rat model of MIH, while IT injection of the CB1 antagonist SR141716 induced the opposite results. These data demonstrated that the CB1 receptor system was partially involved in the mechanism of EA treatment. Various studies have suggested that the ERK signaling pathway is involved in the antinociceptive mechanism of the CB1 receptor system (23,38). Katsuyama et al (23) demonstrated that IT injection of the CB1 antagonist AM251 induced a remarkable activation of ERK1/2 in the spinal cord along with nocifensive behavior in mice, while the CB1 agonist ACEA and the MAPK/ERK inhibitor U0126 reversed these results. A previous study suggested that the ERK1/2 signaling pathway may be involved in EA pretreatment-induced cerebral ischemic tolerance via the cannabinoid CB1 receptor in rats (21). The results of the present study revealed that the CB1 agonist WIN 55,212-2 combined with EA decreased the p-ERK1/2 levels compared with EA treatment alone, while the CB1 antagonist SR141716 induced the opposite results. These data demonstrated that the enhancement produced by the CB1 agonist WIN 55,212-2 on the effect of EA in attenuating MIH was partially mediated by inhibition of ERK1/2 activation. However, the results of the current study revealed that EA treatment alone increased the CB1 protein levels and decreased the p-ERK1/2 levels in rats with MIH, which indicated that other mechanisms probably participated in the inhibition of ERK1/2 activation.
In conclusion, the present study suggests that EA produces an antinociceptive effect on IT injection of morphine-induced hyperalgesia partially through the inhibition of ERK1/2 activation. Activation of the CB1 receptor induced an enhancement of the EA-mediated antinociceptive effect on MIH partially through regulation of the spinal CB1-ERK1/2 signaling pathway. The current study may contribute to the understanding of the antinociceptive mechanism of EA and developing novel methods for the treatment of MIH.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Fund of China (grant no. 81671888 awarded to Dr Yonghao Yu; and grant nos. 81772043 and 81471842 awarded to Dr Keliang Xie).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YZ, GW, KX and YoY conceived and designed the study. YZ, YaY, YuY, YC and CW performed the experiments. YZ and YaY wrote the manuscript. KX and YoY reviewed and edited the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
All experimental protocols were approved by the Animal Experimental Ethics Committee of Tianjin Medical University General Hospital (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Ossipov MH, Lai J, Vanderah TW, Porreca F. Induction of pain facilitation by sustained opioid exposure: Relationship to opioid antinociceptive tolerance. Life Sci. 2003;73:783–800. doi: 10.1016/S0024-3205(03)00410-7. [DOI] [PubMed] [Google Scholar]
- 3.Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–324. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol. 2009;40:101–107. doi: 10.1007/s12035-009-8074-z. [DOI] [PubMed] [Google Scholar]
- 5.Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: An in vitro and in vivo study. Eur J Neurosci. 2001;14:1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- 6.Durham PL, Russo AF. Serotonergic repression of mitogen-activated protein kinase control of the calcitonin gene-related peptide enhancer. Mol Endocrinol. 1998;12:1002–1009. doi: 10.1210/mend.12.7.0135. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23:2576–2586. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- 8.Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE. The role of endocannabinoids in pain modulation. Fundam Clin Pharmacol. 2013;27:64–80. doi: 10.1111/fcp.12008. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 10.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 12.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim G, Sung B, Ji RR, Mao J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain. 2003;105:275–283. doi: 10.1016/S0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 14.da Fonseca Pacheco D, Klein A, de Castro Perez A, da Fonseca Pacheco CM, de Francischi JN, Duarte ID. The mu-opioid receptor agonist morphine, but not agonists at delta- or kappa-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br J Pharmacol. 2008;154:1143–1149. doi: 10.1038/bjp.2008.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system. Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tumati S, Largent-Milnes TM, Keresztes A, Ren J, Roeske WR, Vanderah TW, Varga EV. Repeated morphine treatment-mediated hyperalgesia, allodynia and spinal glial activation are blocked by co-administration of a selective cannabinoid receptor type-2 agonist. J Neuroimmunol. 2012;244:23–31. doi: 10.1016/j.jneuroim.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Wang C, Gu G, Li H, Zhao H, Wang K, Han F, Wang G. The effects of electroacupuncture at the ST36 (Zusanli) acupoint on cancer pain and transient receptor potential vanilloid subfamily 1 expression in Walker 256 tumor-bearing rats. Anesth Analg. 2012;114:879–885. doi: 10.1213/ANE.0b013e318246536d. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Zhao C, Luo X. The effects of electroacupuncture on the extracellular signal-regulated kinase 1/2/P2X3 signal pathway in the spinal cord of rats with chronic constriction injury. Anesth Analg. 2013;116:239–246. doi: 10.1213/ANE.0b013e31826f0a4a. [DOI] [PubMed] [Google Scholar]
- 19.Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG. A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci. 2008;84:159–165. doi: 10.1016/j.rvsc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Gondim DV, Araújo JC, Cavalcante AL, Havt A, Quetz Jda S, Brito GA, Ribeiro Rde A, Lima Vale M. CB1 and CB2 contribute to antinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular joint. Can J Physiol Pharmacol. 2012;90:1479–1489. doi: 10.1139/y2012-130. [DOI] [PubMed] [Google Scholar]
- 21.Du J, Wang Q, Hu B, Peng Z, Zhao Y, Ma L, Xiong L, Lu Y, Zhu X, Chen S. Involvement of ERK 1/2 activation in electroacupuncture pretreatment via cannabinoid CB1 receptor in rats. Brain Res. 2010;1360:1–7. doi: 10.1016/j.brainres.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Jang JY, Kim HN, Koo ST, Shin HK, Choe ES, Choi BT. Synergistic antinociceptive effects of N-methyl-D-aspartate receptor antagonist and electroacupuncture in the complete Freund's adjuvant-induced pain model. Int J Mol Med. 2011;28:669–675. doi: 10.3892/ijmm.2011.728. [DOI] [PubMed] [Google Scholar]
- 23.Katsuyama S, Mizoguchi H, Komatsu T, Nagaoka K, Sakurada S, Sakurada T. The cannabinoid 1 receptor antagonist AM251 produces nocifensive behavior via activation of ERK signaling pathway. Neuropharmacology. 2010;59:534–541. doi: 10.1016/j.neuropharm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 25.Cui JH, Kim WM, Lee HG, Kim YO, Kim CM, Yoon MH. Antinociceptive effect of intrathecal cannabinoid receptor agonist WIN 55,212-2 in a rat bone tumor pain model. Neurosci Lett. 2011;493:67–71. doi: 10.1016/j.neulet.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 26.Johanek LM, Heitmiller DR, Turner M, Nader N, Hodges J, Simone DA. Cannabinoids attenuate capsaicin-evoked hyperalgesia through spinal and peripheral mechanisms. Pain. 2001;93:303–315. doi: 10.1016/S0304-3959(01)00336-0. [DOI] [PubMed] [Google Scholar]
- 27.Shou Y, Yang Y, Xu MS, Zhao YQ, Ge LB, Zhang BM. Electroacupuncture inhibition of hyperalgesia in rats with adjuvant arthritis: Involvement of cannabinoid receptor 1 and dopamine receptor subtypes in striatum. Evid Based Complement Alternat Med. 2013;2013:393460. doi: 10.1155/2013/393460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JY, Park JJ, Jeon S, Doo AR, Kim SN, Lee H, Chae Y, Maixner W, Lee H, Park HJ. From peripheral to central: The role of ERK signaling pathway in acupuncture analgesia. J Pain. 2014;15:535–549. doi: 10.1016/j.jpain.2014.01.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 30.Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Cao JL, Liu HL, Wang JK, Zeng YM. Cross talk between nitric oxide and ERK1/2 signaling pathway in the spinal cord mediates naloxone-precipitated withdrawal in morphine-dependent rats. Neuropharmacology. 2006;51:315–326. doi: 10.1016/j.neuropharm.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance: Involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci. 2008;28:5836–5845. doi: 10.1523/JNEUROSCI.4170-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao JL, He JH, Ding HL, Zeng YM. Activation of the spinal ERK signaling pathway contributes naloxone-precipitated withdrawal in morphine-dependent rats. Pain. 2005;118:336–349. doi: 10.1016/j.pain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Rani Sagar D, Burston JJ, Woodhams SG, Chapman V. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos Trans R Soc Lond B Biol Sci. 2012;367:3300–3311. doi: 10.1098/rstb.2011.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svízenská I, Dubový P, Sulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures-a short review. Pharmacol Biochem Behav. 2008;90:501–511. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Wei H, Yao X, Yang L, Wang S, Guo F, Zhou H, Marsicano G, Wang Q, Xiong L. Glycogen synthase kinase-3β is involved in electroacupuncture pretreatment via the cannabinoid CB1 receptor in ischemic stroke. Mol Neurobiol. 2014;49:326–336. doi: 10.1007/s12035-013-8524-5. [DOI] [PubMed] [Google Scholar]
- 37.Bridges D, Ahmad K, Rice AS. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro R, Wen J, Li S, Zhang Y. Involvement of ERK1/2, cPLA2 and NF-κB in microglia suppression by cannabinoid receptor agonists and antagonists. Prostaglandins Other Lipid Mediat. 2013;100-101:1–14. doi: 10.1016/j.prostaglandins.2012.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.