Abstract
Diabetic cardiomyopathy (DCM) is a major complication of diabetes and myocardial fibrosis is its major pathological feature. Calcium-sensing receptor (CaSR) is a G protein-coupled receptor and participates in the regulation of calcium homeostasis; it is implicated in a range of diseases, including myocardial ischemia/reperfusion injury, myocardial infarction and pulmonary hypertension. However, whether CaSR is associated with myocardial fibrosis in DCM has remained elusive. In the present study, type 1 diabetic (T1D) rats and primary neonatal rat cardiac fibroblasts (CFs) were used to observe changes in CaSR to assess its potential as an indicator of myocardial fibrosis. The in vivo experiments revealed that in the T1D and CaSR agonist (R568) groups, evident collagen (Col)-I and -III deposition was present after 12 weeks. Furthermore, the in vitro experiment indicated that the levels of transforming growth factor (TGF)-β1, phosphorylated (p-) protein kinase C, p-p38, p-Smad2, TβRI, TβRII, along with the intracellular Ca2+ levels and the content of TGF-β1 in the culture medium were significantly increased in a high-glucose (HG) group and an R568-treated group. Treatment with the CaSR inhibitor Calhex231 significantly inhibited the abovementioned changes. Collectively, the results indicated that the increase of CaSR expression in CFs may induce intracellular Ca2+ increases and the activation of TGF-β1/Smads, and enhance the proliferation of CFs, along with the excessive deposition of Col, resulting in myocardial fibrosis. The present results indicate an important novel mechanism for HG-induced myocardial fibrosis and suggest that CaSR may be a promising potential therapeutic target for DCM.
Keywords: diabetic cardiomyopathy, myocardial fibrosis, calcium sensitive receptor, fibroblasts, TGF-β1/Smads
Introduction
In recent years, the incidence of diabetes and associated mortalities have exhibited a trend of rapid growth worldwide (1). Diabetic cardiomyopathy (DCM) is a major complication of diabetes. The major pathological changes involved are myocardial hypertrophy, apoptosis and myocardial interstitial fibrosis (2). Of these, myocardial fibrosis is the major pathological feature, and may eventually induce cardiac remodeling, cardiac dilatation, cardiac dysfunction, arrhythmia and congestive heart failure (3). According to recent data (4), cardiac fibroblasts (CFs) are highly activated during diabetes, which results in a disorder of the dynamic balance of cardiac extracellular matrix synthesis and deposition, along with the excessive deposition of collagen (Col), thus leading to myocardial fibrosis and cardiac dysfunction (5,6). However, the exact mechanisms underlying myocardial fibrosis in DCM remain elusive.
The calcium-sensitive receptor (CaSR) is a member of the C family of the G protein coupling receptor superfamily and is widely expressed in prokaryotic and eukaryotic cells. CaSR is involved in regulating the homeostasis of calcium and other metal ions, proliferation, differentiation, chemotaxis, apoptosis, gene expression, membrane potential, and aging (7,8).
Studies by our group and other research groups have demonstrated that the CaSR is involved in myocardial ischemia/reperfusion injury, myocardial infarction and pulmonary hypertension (9,10). A recent study by our group indicated that the expression of CaSR in the myocardium tissues of diabetic rats, and CFs treated with high concentrations of glucose was significantly increased (11). However, whether CaSR participates in diabetic myocardial fibrosis has remained elusive.
In the present study, a rat model of type 1 diabetes (T1D) and CFs cultured under high-glucose (HG) conditions were subjected to treatment with a CaSR agonist or inhibitor in order to explore the functional role of CaSR in diabetic myocardial fibrosis and the underlying mechanisms.
Materials and methods
Animal experimental protocol
Male Wistar rats (weight, 250±50 g; age, 8 weeks) were provided by the Animal Research Institute of Harbin Medical University (HMU; Harbin, China). The study was approved by the HMU Medical Science Ethics Committee. All rats were maintained under a 12-h light/dark cycle and fed with standard chow and clean water ad libitum. The rats were randomly divided into four groups (n=8/group): i) control group: age-matched non-diabetic Wistar rats were injected with citric acid-citrate sodium buffer; ii) T1D group: intraperitoneal injection of streptozotocin (STZ; 60 mg/kg; Sigma-Aldrich; Merck KGaA) (12); iii) T1D+R568 group: T1D rats were subcutaneously injected with R568 (10 µmol/kg/day in saline); iv) T1D+Calhex231 group: T1D rats were subcutaneously injected with Calhex231 (10 µmol/kg/day in saline). Rats in the four groups were sacrificed after 12 weeks and a range of indices were assessed.
Isolation and incubation of neonatal rat CFs
Neonatal rat CFs were isolated from the hearts of 1–3-day-old Wistar rats. In brief, the heart was quickly removed, immediately placed in D-Hank's solution, cut into pieces (0.5 mm3) and digested with trypsin (Beyotime Institute of Biotechnology) for 8 min. The digestion was then terminated by adding Dulbecco's modified Eagle's medium (DMEM). After the same process had been repeated 8 times, cells were collected by centrifugation at 800 × g for 10 min and a temperature of 4°C. After 2 h of incubation, the unattached cells were discarded; the attached cells (CFs) were plated in a petri dish and maintained at 37°C in a 5% CO2 humidified incubator in DMEM containing 10% fetal bovine serum and 1% penicillin-streptomycin. The media was changed two times/week. To ensure the purity of the CFs, the cells were passaged for three generations and then CFs were treated with HG (40 mM), the CaSR agonist R568 (5 µM), the CaSR inhibitor Calhex231 (3 µM), the transforming growth factor (TGF)-β-type I/II receptor (TβRI/II) kinase inhibitor LY2109761 (20 µM) or TGF-β1 (5, 10, 20 ng/ml; Shanghai San Shu Biotechnology, Ltd.).
Analysis of serum and culture media
Blood samples obtained from the aorta were centrifuged at 1,200 × g for 20 min and serum was stored at −80°C until assayed. Random serum insulin levels were determined using a commercially available ultrasensitive ELISA kit (cat. no. 201804A; Beyotime Institute of Biotechnology). Serum levels of triacylglycerol (TG) and total cholesterol (TC) were analyzed using a standard biochemistry panel (cat. no. 201812AS/201812ED; Beyotime Institute of Biotechnology). Blood glucose in blood samples from the tail vein was measured using a blood glucose analyzer (ACCU-CHEK; Roche Diagnostics GmbH).
Culture media of the CFs were collected to determine TGF-β1, Col-I/III and matrix metallopeptidase (MMP)-2/9 levels (Wuhan Boster Biological Technology, Ltd.). These cytokines were determined by ELISA using commercially available detection kits (cat. nos. EK0513, EK0411, EK0424, EK0467, EK0459, EK0465), following the supplier's protocols.
Hematoxylin and eosin (H&E), Masson trichrome and Sirius red staining
The rats were sacrificed with 200 mg/kg pentobarbital sodium by intraperitoneal injection and the heart was quickly removed and washed with phosphate buffer. The cardiac tissue was fixed in 10% buffered formaldehyde at 4°C for 12 h, embedded in paraffin, sliced at 4 mm and used for morphological assessment. Pathological changes in cardiac tissues were observed by H&E, paraffin sections were stained with 0.5% hematoxylin at room temperature for 10 min and 0.5% eosin for 3 min. The extent of myocardial fibrosis was determined by Masson trichrome (1%) and Sirius red staining (0.5%); sections were stained for 10 min at 55°C and for 30 min at room temperature, respectively. Analysis was conducted with an optical microscopy (BX61; Olympus Corporation).
Western blot analysis
The rat hearts and CF cells were homogenized in 0.5 ml radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) prior to transfer to small tubes and rotation for 1 h at 4°C. Solubilized proteins were collected after centrifugation at 3,000 × g for 30 min at 4°C. The supernatant was then collected and stored at −80°C. The protein concentration of each sample was quantified using a Bicinchoninic Acid Protein Assay kit (Beyotime Institute of Biotechnology). Protein lysates (20 µg/lane) from cells of each group were separated by SDS-PAGE (12.5%) and electro-transferred onto polyvinylidene difluoride membranes (EMD Millipore). Non-specific proteins on membranes were blocked with 5% non-fat dried milk for 2 h at room temperature. Subsequently, the membranes were incubated overnight at 4°C with the following primary antibodies (1:1,000 dilution): CaSR (cat. no. sc-47741), TGF-β1 (cat. no. sc-130348); TβRI/II (cat. nos. sc-518045 and sc-17799); and Col-I/III (cat. nos. sc-59772 and sc-271249; all Santa Cruz Biotechnology, Inc.); Smad2 and phosphorylated (p)-Smad2 (cat. nos. 5339 and 18338; Cell Signaling Technology, Inc.); protein kinase C (PKC; cat. no. 2056); p-PKC (cat. no. 2060); p38 (cat. no. 9212); p-p38 (cat. no. 9215); and matrix metallopeptidase (MMP)2/9 (cat. nos. 4022 and 3852; all Cell Signaling Technology, Inc.); and β-actin (cat. no. sc-69879) and β-tubulin (cat. no. sc 55529; Santa Cruz Biotechnology, Inc.). The membranes were then incubated with horseradish peroxidase-conjugated anti-mouse/anti-rabbit immunoglobulin G antibodies (cat. nos. bs-0295M-HRP and bs-0296R-HRP; BIOSS) at a 1:5,000 dilution for 1 h at room temperature. The specific complex was visualized using an enhanced chemiluminescence plus western blot detection system (Immobilon Western HRP; EMD Millipore). The relative intensities of protein bands were finally quantified using a Bio-Rad Chemi EQ densitometer (Bio-Rad Laboratories, Inc.) and the band density was semi-quantified using AlphaView v3.2.2 software (ProteinSimple; Bio-Techne).
Detection of cell proliferation and migration by Cell Counting Kit (CCK)-8 and a scratch wound repair assay
CFs were plated onto 96-well plates at a density of 2×103 cells/well. After 12 h, the medium was replaced with an antibiotic-free medium and the wells were divided into four groups: control (5.5 mM), HG (40 mM), HG+R568 (40 mM + 5 µM), and HG+Calhex231 (40 mM + 3 µM), with five replications/group. Finally, cell proliferation was detected according to the CCK-8 assay kit (cat. no. AR1199, Wuhan Boster Biological Technology, Ltd.) instructions (absorbance at 450 nm).
Rat CFs treated with HG (40 mM), R568 (5 µM) or Calhex231 (3 µM) were then subjected to scratch assays as previously described (13). Images were captured at 0 and 24 h after scratching using phase-contrast microscopy.
Measurement of intracellular Ca2+ using Fluo-3/AM probes
CFs treated with HG (40 mM), R568 (5 µM) or Calhex231 (3 µM) were stained using 5 mM Fluo-3/AM (cat. no. ab145254; Abcam) for 30 min at 37°C in the dark. Subsequently, the cells were washed with Ca2+-free Tyrode's solution to remove residual dye. The fluorescence of Ca2+ was then measured by fluorescence microscopy (BX61; Olympus Corporation). The excitation wavelength was 488 nm and the emission wavelength was 530 nm.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
To determine the mRNA expression of Col-I, Col-III, MMP-2 and MMP-9, total RNA was extracted from CFs using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Complementary DNA was synthesized from 2 µg total RNA by using a Superscript Reverse Transcription kit (TaqMan™; cat. no. 4366596; Thermo Fisher Scientific, Inc.). For the quantification of the mRNA levels of Col-I/III and MMP-2/9, PCR was performed using a SYBR Green PCR Reagent kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) in a Light Cycler® 480 Sequence Detection System (Roche Applied Science). β-actin served as the internal control and the relative expression was determined. The primer sequences used in the present study were as follows: Col-I forward, 5′-ATGTTCAGCTTTGTGGAC-3′ and reverse, 5′-GGATGCCATCTTGTCCAG-3′; Col-III forward, 5′-CAAAGGAGAGCCAGGAGCAC-3′ and reverse, 5′-CTCCAGGCGAACCATCTTTG-3′; MMP-2 forward, 5′-TCAAATCGGACTGGCTGGGC-3′ and reverse, 5′-AACCAGGCCTCTTCACGTCC-3′; MMP-9 forward, 5′-GAGGGGGAGGAGCTAGTTTGCC-3′ and reverse, 5′-AAGGACAGCGTGCAGAGAGGG-3′; β-actin forward, 5′-CCGGCTTCGCGGGCGACG-3′ and reverse, 5′-TCCCGGCCAGCCAGGTCC-3′.
Statistical analyses
All experiments were performed at least three times independently. All data are expressed as the mean ± standard error of the mean. Statistical analysis was performed by a two-tailed Student's t-test or one-way analysis of variance, followed by the Bonferroni multiple comparisons test using SPSS 18.0 software (SPSS Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Successful modeling of T1D cardiomyopathy
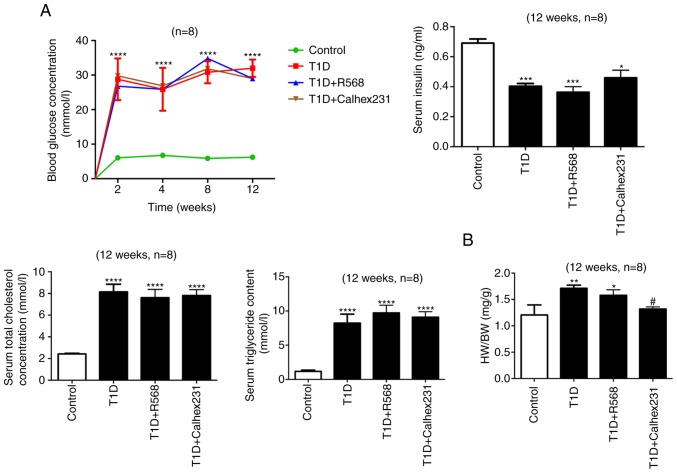
Blood glucose at weeks 2, 4, 8 and 12, along with insulin levels, the ratio of heart weight to body weight (HW/BW), as well as serum TGs and TCs on week 12 were determined. The results indicated that compared with the control group, the blood glucose levels at each time-point were higher, the insulin level was significantly decreased, and TG and TC were significantly increased in the T1D group, T1D+R568 group and T1D+Calhex231 group. Notably, compared with that in the control group, HW/BW was significantly increased in the T1D and T1D+R568 groups, but compared with that in the T1D group, it was significantly decreased in the T1D+Calhex231 group (Fig. 1).
Figure 1.
Successful modeling of type 1 diabetic cardiomyopathy. (A) Random blood glucose was assessed at weeks 2, 4, 8 and 12. Other indicators were analyzed at week 12 after successful modeling. Blood glucose, TG, TC and insulin levels in the serum. (B) HW/BW. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. the Control; #P<0.05 vs. T1D rats (n=8). TG, triacylglycerol; TC, total cholesterol; HW/BW, heart weight to body weight ratio; T1D, type 1 diabetes.
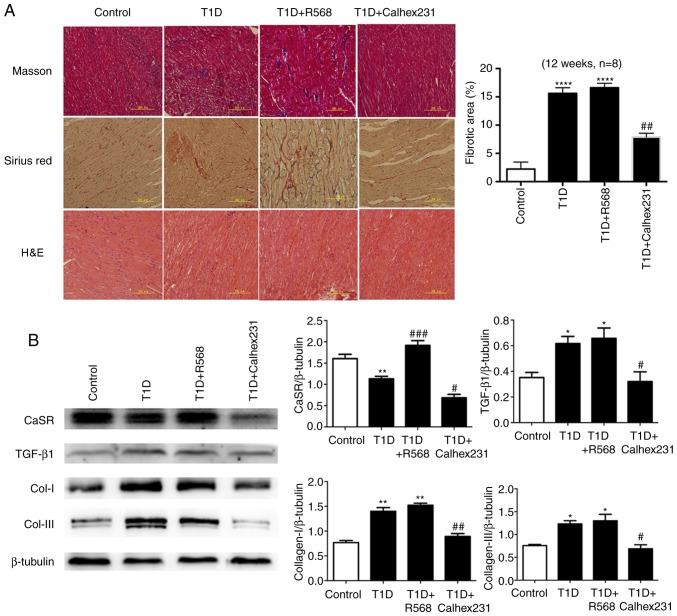
Masson staining and Sirius red staining revealed large amounts of collagen deposition in the interstitial and perivascular areas, particularly in denatured and necrotic areas; H&E staining further indicated that the cardiac myocytes were disordered and hypertrophic in the T1D group, T1D+R568 group and T1D+Calhex231 group (Fig. 2A).
Figure 2.
(A) Effects of CaSR on collagens and morphological changes of myocardial tissue in rats. Masson's trichrome staining (collagen deposition displayed in blue) and Sirius red staining (collagen fibers displayed in red). Representative images of H&E staining examined by transmission electron microscopy. (B) Representative western blots for the detection of CaSR, Col-I, Col-III and TGF-β1 in comparison with β-actin expression in rats. *P<0.05, **P<0.01, ****P<0.0001 vs. Controls; #P<0.05, ##P<0.01, ###P<0.001 vs. T1D rats (n=8). TGF, transforming growth factor; T1D, type 1 diabetes; CaSR, calcium-sensing receptor; Col, collagen.
Effects of CaSR on collagen in T1D rats
At week 12, western blot analysis of cardiac tissue homogenate indicated that compared with that in the control group, the expression of Col-I, Col-III and TGF-β1 was increased in the T1D group and T1D+R568 groups, and the expression of CaSR was significantly increased in the T1D+R568 group. However, the opposite results were observed in the T1D+Calhex231 group (Fig. 2B).
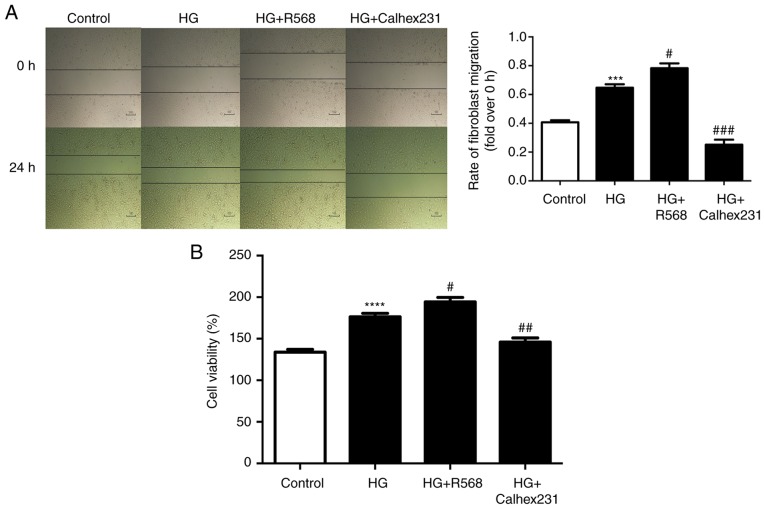
Effects of CaSR on proliferation and migration of CFs
The effect of osmotic control was detected using mannitol (40 mM), and the results revealed that mannitol had no effect on the viability of cardiac fibroblasts (data not shown), however, CFs treated with high glucose (40 mM) exhibited an increase in viability in a time-dependent manner (data not shown). These results indicated that the effect of high glucose on viability may not be attributed to high osmolarity and treatment with 40 mM glucose was considered as the high glucose group in subsequent experiments.
The results of the scratch wound repair assay indicated that the rate of CF migration was higher in the HG group and the HG+R568 group compared with that in the control group, while the migration was significantly reduced in the HG+Calhex231 group (Fig. 3A). The proliferation (cell viability) of CFs at 24 h was detected using CCK-8 assays. Compared with that in the control group, the cell proliferation was greater in the HG group and the HG+R568 group, but was significantly decreased in the HG+Calhex231 group (Fig. 3B).
Figure 3.
Proliferation and migration of diabetic CFs. CFs were cultured for 24 h in a control group (5.5 mM), HG group (40 mM), HG+R568 (5 µM) group and HG+Calhex231 (3 µM) group. CF migration and proliferation (cell viability) were detected by (A) scratch wound repair assays, as well as (B) a CCK-8 assay, respectively. ***P<0.001, ****P<0.0001 vs. the Control; #P<0.05, ##P<0.01, ###P<0.001 vs. HG (n≥16). HG, high glucose; CFs, cardiac fibroblasts.
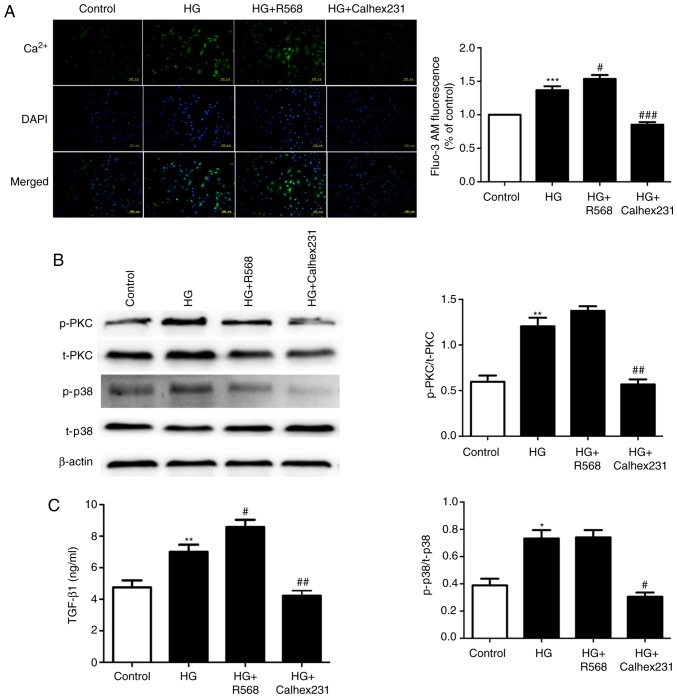
Effects of CaSR on intracellular calcium concentration and the amount of TGF-β1 secreted by CFs
To further study the mechanism of diabetic myocardial fibrosis, cytosolic Ca2+ was determined with Fluo-3/AM staining and assessment of the fluorescence intensity. The results indicated that the fluorescence intensity was higher in the HG and HG+R568 groups, and was lower in the HG+Calhex231 group (Fig. 4A). The protein levels of p-PKC and p-P38 were significantly increased in the HG and HG+R568 groups. However, the opposite result was obtained in the Calhex231 group (Fig. 4B). The expression of TGF-β1 was higher in the HG and HG+R568 groups but was significantly lower in the HG+Calhex231 group (Fig. 4C).
Figure 4.
Measurement of intracellular Ca2+ in CFs, the level of p-PKC/p38 and the expression of TGF-β1 in the culture medium. CFs were cultured for 24 h at 37°C in a control group (5.5 mM), HG group (40 mM), HG+R568 (5 µM) group and HG+Calhex231 (3 µM) group. (A) Cytosolic Ca2+ was stained with Fluo-3/AM and assessed by measuring the fluorescence intensity. (B) The protein levels of p-PKC and p-p38 were evaluated by western blot analysis. (C) The expression of TGF-β1 in the culture supernatants of the CFs was determined using ELISA. *P<0.05, **P<0.01, ***P<0.001 vs. the Control; #P<0.05, ##P<0.01, ###P<0.001 vs. HG (n≥16). TGF, transforming growth factor; p-PKC, phosphorylated protein kinase C; HG, high glucose; CFs, cardiac fibroblasts.
Signaling pathways associated with myocardial fibrosis
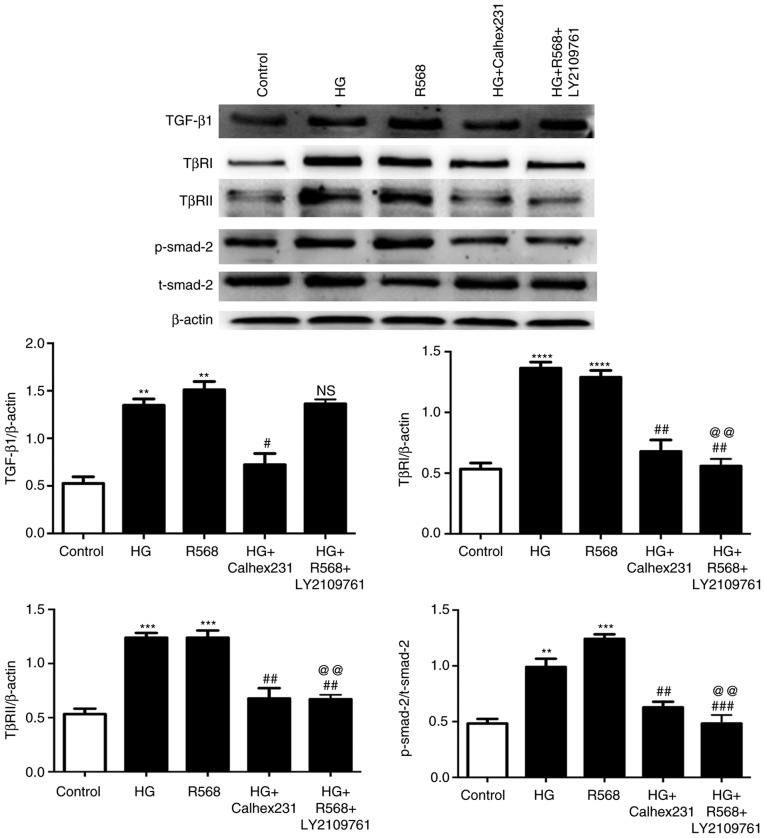
Western blot analysis indicated that the protein levels of TGF-β1, TβRI, TβRII and p-Smad2 were significantly upregulated in the HG and R568 groups, and were markedly downregulated in the HG+Calhex231 and HG+R568+LY2109761 groups compared to the HG and R568 groups, except for the expression of TGF-β1 in the LY2109761 group (Fig. 5).
Figure 5.
Effects of TβRI/II kinase inhibitor (LY2109761) on TGF-β1/Smads pathway in CFs. CFs were cultured for 48 h at 37°C in an HG group (40 mM), R568 (5 µM), HG+Calhex231 (3 µM) and HG+R568+LY2109761 (20 µM) group. Representative western blots of TGF-β1, TβRI, TβRII in comparison with β-actin expression and p-Smad2 in comparison with t-Smad2 expression in CFs are provided. **P<0.01, ***P<0.001, ****P<0.0001 vs. the Control; #P<0.05, ##P<0.01, ###P<0.001 vs. HG; @@P<0.01 vs. R568 (n≥3). HG, high glucose; p-/t-Smad, phosphorylated/total Smad; TGF, transforming growth factor; TβRI, TGF-β-type I receptor; CFs, cardiac fibroblasts.
Effects of TGF-β1 on extracellular matrix (ECM) of CFs
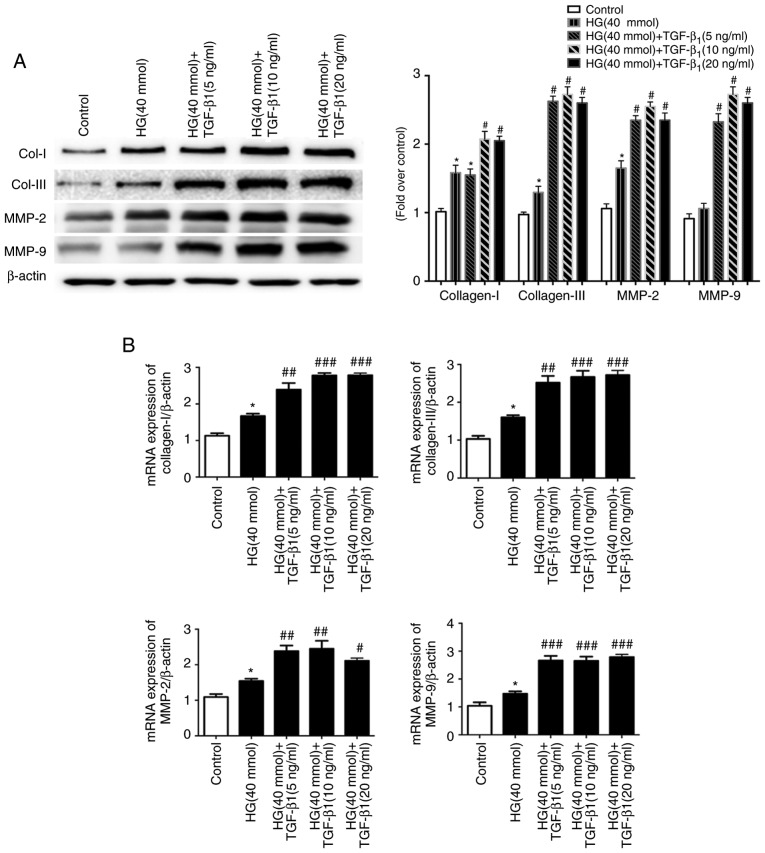
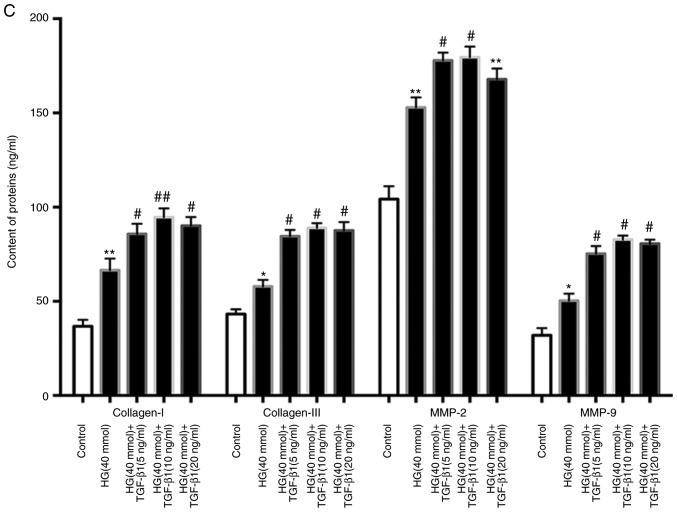
Since excessive ECM is the major cause of myocardial fibrosis, the changes in the mRNA and the protein levels of Col-I, Col-III, MMP-2 and MMP-9 after treatment with HG (40 mmol) and TGF-β1 (0, 5, 10 or 20 ng/ml) were assessed (Fig. 6A and B). Furthermore, the release of Col-I, Col-III, MMP-2 and MMP-9 was determined by ELISA. HG and TGF-β1 (5, 10 and 20 ng/ml) stimulation caused a significant increase in the production of these factors by CFs compared to the control and HG groups (Fig. 6C).
Figure 6.
Effects of TGF-β1 in culture medium on the changes of extracellular matrix components. Cardiac fibroblasts were treated with HG (40 mM) in the presence or absence of TGF-β1 (5, 10, 20 ng/ml) for 48 h at 37°C. (A) After different treatments, the protein expression of Col-I, Col-III, MMP-2 and MMP-9 was evaluated by western blot analysis. (B) The changes in the mRNA levels of Col-I, Col-III, MMP-2 and MMP-9 were measured by RT-qPCR, and the mRNA levels were normalized to β-actin, which was used as the housekeeping gene. (C) The cell supernatants were collected for the determination of Col-I, Col-III, MMP2 and MMP9 expression using ELISA. *P<0.05, **P<0.01 vs. the Control; #P<0.05, ##P<0.01, ###P<0.001 vs. HG (n≥8). Col, collagen; SMA, smooth muscle actin; MMP, matrix metallopeptidase; TGF, transforming growth factor; HG, high glucose.
Discussion
Diabetes is a metabolic disease characterized by hyperglycemia due to impaired insulin secretion or insulin resistance. Persistent hyperglycemia and metabolic disorders may lead to the impairment of tissues and organs, particularly the cardiovascular system, nervous system and kidneys. DCM is a heart disease independent of congenital heart disease, coronary heart disease, and valvar heart disease, and is also a significant cause of the increased mortality in patients with diabetes (14).
Myocardial tissue mainly consists of cardiomyocytes and non-cardiomyocytes. CFs account for 90% of the total non-cardiomyocytes and are not only the structural scaffolds of the heart, but also link myocardial cells, endothelial cells and blood vessels (15). CFs are involved in maintaining homeostasis and the remodeling of ECM, electrophysiological activity and the production of cell growth factors (16). Col-I and -III are two major components of the ECM, and have an important role in maintaining the structure and function of the heart (17).
According to the existing literature, HG levels may stimulate the proliferation of fibroblasts, promote myofibroblast trans-differentiation and activate the transcription and secretion of ECM proteins via the activation of angiotensin II, TGF-β, the extracellular signal-regulated kinase signaling pathway and reactive oxygen species production in vitro (18–20). However, these previous studies did not yield any conclusive evidence, and the precise mechanisms of hyperglycemia in the remodeling and fibrosis of the diabetic heart still remain elusive.
To further elucidate the role of CaSR in myocardial fibrosis in DCM, a rat model of T1D was generated. Polydipsia, polyuria, evident emaciation, increased blood glucose, TC and TG, and decreased insulin activity were observed in rats treated with STZ, and optionally with R568 or Calhex231, thus indicating that the T1D rat model had been successfully generated.
At 12 weeks after modeling, the HW/BW was significantly increased in the T1D group and the T1D+R568 group, which may have been associated with the weight loss and an increase in the myocardial ECM. This speculation is supported by the results of the analysis of cardiac morphology and determination of associated proteins. H&E staining indicated that the cardiac myocytes of T1D rats were disordered and hypertrophic. Masson staining and Sirius red staining revealed large amounts of Col deposition in the interstitial and perivascular areas, particularly in denatured and necrotic areas, while the CaSR agonist and the CaSR inhibitor respectively promoted and inhibited these changes. The expression of Col-I and Col-III proteins in the myocardial tissue was significantly increased in the T1D group and the T1D+R568 group, but was significantly decreased in the T1D+Calhex321 group. These results demonstrated that myocardial remodeling and myocardial fibrosis had clearly occurred in the T1D rats and CaSR may be associated with the increased ECM and deposition of Col.
It is well known that the proliferation and activation of CFs represent the major pathways for Col secretion and the increased ECM (21). A previous study by our group indicated that CaSR is expressed in CFs (22). However, the association of changes of CaSR expression in CFs in DCM has remained to be fully elucidated. To address this question, a series of experiments was performed.
In the present study, CCK-8 assays indicated that HG treatment increased the proliferation of CFs. It was also observed that R568 further promoted the proliferation of CFs; however, Calhex231 significantly inhibited these changes. This indicated that CaSR is closely associated with the proliferation of CFs.
The proliferation and activation of CFs, as well as the increased ECM, are important mechanisms of myocardial fibrosis (23). Intracellular calcium is an important second messenger and the driving force of CF activation (24,25). Studies by our group (26) and other research groups (12,27) have demonstrated that the increase or activation of CaSR expression increases intracellular calcium through the G protein/phospholipase C/inositol triphosphate pathway. To investigate the role of CaSR in the activation of CFs, the effect of HG treatment on intracellular calcium and a cell scratch assay were determined. The results of the Fluo-3/AM fluorescence probe analyses and cell scratch assay indicated that HG increased intracellular calcium release and the migration of CFs. Furthermore, R568 or Calhex231 promoted or inhibited these changes, respectively. It is therefore evident that CaSR activation in CFs promotes the proliferation and migration of CFs.
MMPs participate in the degradation of various protein components of the ECM. Different types of MMPs degrade different types of protein; MMP2 mainly degrades Col-IV, while MMP9 breaks down laminin and fibronectin (28). The present study indicated that HG conditions and exogenous TGF-β1 activated CFs and upregulated the expression of MMP2 and MMP9. The upregulated MMP2 and MMP9 provided additional ECM space for cell migration and the secretion of Col-I and Col-III through the degradation of laminin and fibronectin.
TGF-β1 is a potent cytokine with a driving role in development, fibrosis and cancer (29). It promotes differentiation of CFs and activation of the renin angiotensin aldosterone system, and causes an increased abundance of NADPH (30). Furthermore, accumulation of intracellular Ca2+, which promotes mitosis, induces cell proliferation (25,29). More importantly, it promotes the secretion of TGF-β1 modulated by mitogen-activated protein kinase family members in CFs (30,31). In the present study, HG and HG + R568 increased the levels of p-PKC, p-p38 and the content of TGF-β1 in CFs and their culture medium, while Calhex231 caused a significant reduction. To further verify the role of TGF-β1, CFs were treated with TβRI/II kinase inhibitor, and it was revealed that the expression of TβRI/II and Smad2 was downregulated, while TGF-β1 was not affected; this indicated the regulatory role of CaSR through alteration of the intracellular calcium concentration. The effect of increased TGF-β1 caused by CaSR was further assessed, and the results demonstrated increased mRNA levels of Col-I, Col-III, MMP-2 and MMP-9 and enhanced protein expression as well as release of the relevant proteins into the culture medium.
It has previously been reported that the occurrence and development of myocardial fibrosis is closely associated with the activation of the TGF-β1/Smad pathway (32). TGF-β1 is associated with its receptor TβRII, which activates TβRI kinase, causing the phosphorylation of Smad2/3, which then combines with Smad4 and forms a complex. This complex then translocates to the nucleus and regulates the transcription of target genes, including Smad7. Smad7 is an inhibitory Smad, which degrades Smad2, Smad3 and TGF-β1 via the ubiquitin protease degradation system (33). The present study indicated that HG levels and CaSR agonists significantly increased TGF-β1 and p-Smad2, while CaSR inhibitors exerted the opposite effects.
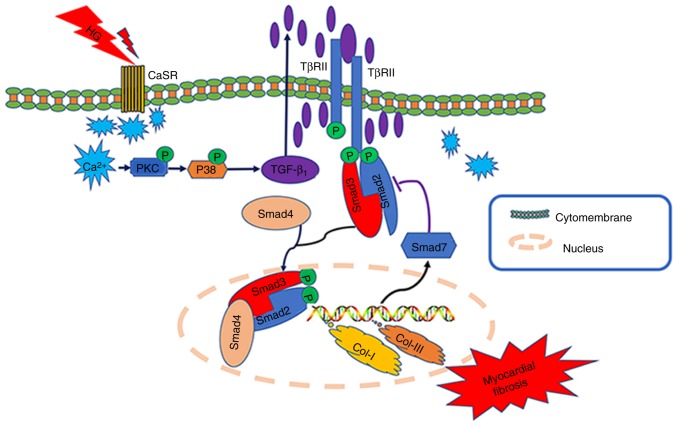
Collectively, based on the aforementioned experimental results and previous studies, it may be hypothesized that during diabetes (hyperglycemia), the upregulated expression of CaSR in the CFs may lead to increases in intracellular Ca2+ (a second messenger) (34), and further activate the TGF-β1/Smads pathway. This results in the proliferation and activation of fibroblasts, and eventually leads to myocardial fibrosis (Fig. 7). Future studies by our group will further clarify the role and specific mechanisms of CaSR in myocardial fibrosis, and provide novel targets and an experimental basis for the prevention and treatment of DCM.
Figure 7.
Schematic diagram illustrating the role of CaSR in the activation of high glucose-treated cardiac fibroblasts. CaSR, calcium-sensing receptor.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MF
myocardial fibrosis
- CaSR
calcium-sensing receptor
- DCM
diabetic cardiomyopathy
- HG
high glucose
- MMP2/9
matrix metalloproteinase2/9
- α-SMA
α-smooth muscle actin
- TGF-β1
transforming growth factor β1
- STZ
streptozotocin
- CFs
cardiac fibroblasts
- TC
total cholesterol
- H&E
hematoxylin and eosin
- DMEM
Dulbecco's modified Eagle's medium
- CCK-8
Cell Counting Kit-8
- EdU
5-ethynyl-2′-deoxyuridine
Funding
This study was supported by the National Natural Science Foundation of China (no. 81800260) and the Heilongjiang Postdoctoral Fund (no. LBH-Z17103).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CX and HL conceived and supervised the study. HY and YW designed experiments. HY, YF, BZ and YS performed experiments. TG, CW and HY analyzed the data. HY drafted the manuscript. All authors reviewed the results and approved the final version of the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The animal raising and handling procedures were performed in accordance with the Guide for the Care and Use of Laboratory The study was approved by the Harbin Medical University Medical Science Ethics Committee (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Echouffo-Tcheugui JB, Dagogo-Jack S. Preventing diabetes mellitus in developing countries. Nat Rev Endocrinol. 2012;8:557–562. doi: 10.1038/nrendo.2012.188. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, McLennan SV, Allen TJ, Tsoutsman T, Semsarian C, Twigg SM. Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. Am J Physiol Cell Physiol. 2009;297:C1490–C1500. doi: 10.1152/ajpcell.00049.2009. [DOI] [PubMed] [Google Scholar]
- 3.Westermeier F, Riquelme JA, Pavez M, Garrido V, Díaz A, Verdejo HE, Castro PF, García L, Lavandero S. New Molecular Insights of Insulin in Diabetic Cardiomyopathy. Front Physiol. 2016;7:125. doi: 10.3389/fphys.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowlkes V, Clark J, Fix C, Law BA, Morales MO, Qiao X, Ako-Asare K, Goldsmith JG, Carver W, Murray DB, et al. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci. 2013;92:669–676. doi: 10.1016/j.lfs.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: Pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res. 2014;164:323–335. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson KR, Lord CK, West TA, Stewart JA., Jr Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS One. 2013;8:e72080. doi: 10.1371/journal.pone.0072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tharmalingam S, Hampson DR. The Calcium-Sensing Receptor and Integrins in Cellular Differentiation and Migration. Front Physiol. 2016;7:190. doi: 10.3389/fphys.2016.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendy GN, Canaff L. Calcium-Sensing Receptor Gene: Regulation of Expression. Front Physiol. 2016;7:394. doi: 10.3389/fphys.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng X, Li HX, Shao HJ, Li GW, Sun J, Xi YH, Li HZ, Wang XY, Wang LN, Bai SZ, et al. Involvement of calcium-sensing receptors in hypoxia-induced vascular remodeling and pulmonary hypertension by promoting phenotypic modulation of small pulmonary arteries. Mol Cell Biochem. 2014;396:87–98. doi: 10.1007/s11010-014-2145-9. [DOI] [PubMed] [Google Scholar]
- 10.Xu C, Zhang W, Jiang C, Sun Y, Wang R. Involvement of calcium sensing receptor in myocardial ischemia/reperfusion injury and apoptosis. J Mol Cell Cardiol. 2007;42:S80–S81. doi: 10.1016/j.yjmcc.2007.03.741. [DOI] [Google Scholar]
- 11.Wang Y, Gao P, Wei C, Li H, Zhang L, Zhao Y, Wu B, Tian Y, Zhang W, Wu L, et al. Calcium sensing receptor protects high glucose-induced energy metabolism disorder via blocking gp78-ubiquitin proteasome pathway. Cell Death Dis. 2017;8:e2799. doi: 10.1038/cddis.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong S, Li G, Zheng D, Wu J, Sun D, Yang F, Yu X, Li T, Sun A, Liu J, et al. A novel role for the calcium sensing receptor in rat diabetic encephalopathy. Cell Physiol Biochem. 2015;35:38–50. doi: 10.1159/000369673. [DOI] [PubMed] [Google Scholar]
- 13.Liang CC, Park AY, Guan JL. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 14.Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: A short review. Mol Cell Biochem. 2004;261:187–191. doi: 10.1023/B:MCBI.0000028755.86521.11. [DOI] [PubMed] [Google Scholar]
- 15.Lam S, Verhagen NAM, Strutz F, van der Pijl JW, Daha MR, van Kooten C. Glucose-induced fibronectin and collagen type III expression in renal fibroblasts can occur independent of TGF-β1. Kidney Int. 2003;63:878–888. doi: 10.1046/j.1523-1755.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 16.Spector KS. Diabetic cardiomyopathy. Clin Cardiol. 1998;21:885–887. doi: 10.1002/clc.4960211205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehlet SN, Willumsen N, Armbrecht G, Dietzel R, Brix S, Henriksen K, Karsdal MA. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS One. 2018;13:e0194458. doi: 10.1371/journal.pone.0194458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-β1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediators Inflamm. 2016;2016:8319283. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao M, Wang X, Wang X, Zhang T, Chi Y, Gao F. The Notch pathway mediates the angiotensin II-induced synthesis of extracellular matrix components in podocytes. Int J Mol Med. 2015;36:294–300. doi: 10.3892/ijmm.2015.2193. [DOI] [PubMed] [Google Scholar]
- 21.Olson ER. Signaling mechanisms controlling the proliferation and differentiation of cardiac fibroblasts (unpublished PhD thesis) Kent State University, College of Biomedical Sciences. 2006 [Google Scholar]
- 22.Zhang X, Zhang T, Wu J, Yu X, Zheng D, Yang F, Li T, Wang L, Zhao Y, Dong S, et al. Calcium sensing receptor promotes cardiac fibroblast proliferation and extracellular matrix secretion. Cell Physiol Biochem. 2014;33:557–568. doi: 10.1159/000358634. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Ranjbarvaziri S, Lay FD, Zhao P, Miller MJ, Dhaliwal JS, Huertas-Vazquez A, Wu X, Qiao R, Soffer JM, et al. Genetic Regulation of Fibroblast Activation and Proliferation in Cardiac Fibrosis. Circulation. 2018;138:1224–1235. doi: 10.1161/CIRCULATIONAHA.118.035420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du G, Fischer BE, Voss KO, Becker G, Taucher-Scholz G, Kraft G, Thiel G. The absence of an early calcium response to heavy-ion radiation in Mammalian cells. Radiat Res. 2008;170:316–326. doi: 10.1667/RR1270.1. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Wang X, Mei Z, Gong J, Huang L, Gao X, Zhao Y, Ma J, Qian L. BNIP3L promotes cardiac fibrosis in cardiac fibroblasts through [Ca2+]i-TGF-β-Smad2/3 pathway. Sci Rep. 2017;7:1906. doi: 10.1038/s41598-017-01936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WH, Fu SB, Lu FH, Wu B, Gong DM, Pan ZW, Lv YJ, Zhao YJ, Li QF, Wang R, et al. Involvement of calcium-sensing receptor in ischemia/reperfusion-induced apoptosis in rat cardiomyocytes. Biochem Biophys Res Commun. 2006;347:872–881. doi: 10.1016/j.bbrc.2006.06.176. [DOI] [PubMed] [Google Scholar]
- 27.Li GW, Miao HZ, Bo L, Wang GZ, Jin L, Lin Y, Deng ZH, Xiao W. Calcium-sensing receptor modulates pulmonary artery tension through G-protein-PLC-IP_3 pathways. Chin J Pathophysiol. 2015;31 (In Chinese) [Google Scholar]
- 28.Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31(Suppl 1):177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 29.Nüchel J, Ghatak S, Zuk AV, Illerhaus A, Mörgelin M, Schönborn K, Blumbach K, Wickström SA, Krieg T, Sengle G, et al. TGFB1 is secreted through an unconventional pathway dependent on the autophagic machinery and cytoskeletal regulators. Autophagy. 2018;14:465–486. doi: 10.1080/15548627.2017.1422850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Meng T, Sun C. Protective effect of diltiazem on myocardial ischemic rats induced by isoproterenol. Mol Med Rep. 2018;17:495–501. doi: 10.3892/mmr.2017.7906. [DOI] [PubMed] [Google Scholar]
- 31.Ryu JM, Lee MY, Yun SP, Han HJ. High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-beta1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J Cell Physiol. 2010;224:59–70. doi: 10.1002/jcp.22091. [DOI] [PubMed] [Google Scholar]
- 32.Shi BH, Zong-Pei XU, Fan GW. The research progress on treatment of myocardial fibrosis by regulating TGF-β1/Smads pathway. Zhongguo Yaolixue Tongbao. (In Press) [Google Scholar]
- 33.Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duran J, Troncoso MF, Lagos D, Ramos S, Marin G, Estrada M. GDF11 Modulates Ca2+-Dependent Smad2/3 Signaling to Prevent Cardiomyocyte Hypertrophy. Int J Mol Sci. 2018;19:1333–1342. doi: 10.3390/ijms19051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.