Abstract
Numerous behavioral observations and brain function studies have demonstrated that neurological differences exist between East Asians and Westerners. However, the extent to which these factors relate to differences in brain structure is still not clear. As the basis of brain functions, the anatomical differences in brain structure play a primary and critical role in the origination of functional and behavior differences. To investigate the underlying differences in brain structure between the two cultural/ethnic groups, we conducted a comparative study on education‐matched right‐handed young male adults (age = 22–29 years) from two cohorts, Han Chinese (n = 45) and Caucasians (n = 45), using high‐dimensional structural magnetic resonance imaging (MRI) data. Using two well‐validated imaging analysis techniques, surface‐based morphometry (SBM) and voxel‐based morphometry (VBM), we performed a comprehensive vertex‐wise morphometric analysis of the brain structures between Chinese and Caucasian cohorts. We identified consistent significant between‐group differences in cortical thickness, volume, and surface area in the frontal, temporal, parietal, occipital, and insular lobes as well as the cingulate cortices. The SBM analyses revealed that compared with Caucasians, the Chinese population showed larger cortical structures in the temporal and cingulate regions, and smaller structural measures in the frontal and parietal cortices. The VBM data of the same sample was well‐aligned with the SBM findings. Our findings systematically revealed comprehensive brain structural differences between young male Chinese and Caucasians, and provided new neuroanatomical insights to the behavioral and functional distinctions in the two cultural/ethnic populations.
Keywords: brain structure, brain mapping, cultural difference, morphometry, MRI, neuroimaging
1. INTRODUCTION
Differences in genetics, culture, and environmental exposures may lead to brain structure and function differences between various populations. There is extensive evidence suggesting that culture and sustained experiences could affect brain and behavior. East Asian and Western cultures have different biases for information processing due to different experiences and cultural values (Nisbett & Masuda, 2003; Nisbett & Miyamoto, 2005; Nisbett, Peng, Choi, & Norenzayan, 2001; Peng & Nisbett, 2000). In general, East Asians tend to consider themselves as part of a whole based on their collectivist culture, so that have a holistic information‐processing bias, that is, they encode information of context and objects together and prioritize relational information over categorical information. Whereas, Westerners trend to use rules and categories to process central objects and organize information, because of the individualistic nature of their culture.
Recent neuroimaging studies have revealed cultural differences in neural correlation of cognition and behavior (Ames & Fiske, 2010; Chiao, Cheon, Pornpattanangkul, Mrazek, & Blizinsky, 2013; Han & Northoff, 2008; Han et al., 2013). These studies, comparing functional magnetic resonance imaging (fMRI) results of East Asians and Westerners, found stable differences in attention, categorization, and contextual processing between the two groups. Compared with Westerners, East Asians were found more likely to detect changes in contextual information in a scene (Masuda & Nisbett, 2006). In addition, people's perception of the visual world may be affected by culture. East Asians, compared with Westerners, process visual information in a more holistic way considering the relationship between objects and context, whereas Westerners focus on salient objects independent of the context in an analytical style (Goh et al., 2010; Ji, Peng, & Nisbett, 2000; Kitayama, Duffy, Kawamura, & Larsen, 2003; Masuda, Gonzalez, Kwan, & Nisbett, 2008; Nisbett & Masuda, 2003; Nisbett & Miyamoto, 2005; Nisbett et al., 2001). Cultural differences have also been linked to variations in processing facial stimuli (Blais, Jack, Scheepers, Fiset, & Caldara, 2008; Jack, Blais, Scheepers, Schyns, & Caldara, 2009; Tardif et al., 2017).
Cultural differences have been shown to influence linguistic functions of the brain (Xu, Baldauf, Chang, Desimone, & Tan, 2017). Tan et al. (Siok, Perfetti, Jin, & Tan, 2004; Siok, Niu, Jin, Perfetti, & Tan, 2008) explored the structural‐functional basis of development dyslexia in Chinese readers using functional MRI, and found that the biological basis of impaired reading was dependent on culture. For readers of alphabetic writing systems (e.g., English), dyslexia was associated with dysfunction of the left temporoparietal and occipitotemporal regions (Aylward et al., 2003; Horwitz, Rumsey, & Donohue, 1998; Johansson, 2006; Shaywitz et al., 1998; Sun, Lee, & Kirby, 2010; Temple et al., 2003). For readers of logographic writing systems (e.g., Chinese), impaired reading was associated with structural and functional abnormalities of the left middle frontal gyrus.
Culture has also been shown to impact nonlinguistic neural functioning. Social cognition studies found that people from East Asian cultural contexts performed better on tasks with interdependent demands than on those with independent demands, whereas Westerners displayed an opposite pattern (Hedden, Ketay, Aron, Markus, & Gabrieli, 2008; Kitayama et al., 2003). In a recent quantitative meta‐analysis, Han and Ma (2014) classified 35 functional MRI studies that compared participants from East Asian (Chinese, Japanese, and Korean) and Western (American and European) societies into three domains of social cognitive, social affective, and nonsocial cognitive tasks. They investigated cultural differences in brain activity underlying social and nonsocial processes and found that cultural differences in the two processes were mediated by distinct neural networks. East Asians showed increased neural activity in the brain regions related to mentalizing and emotion regulation, whereas Western culture were associated with increased neural activity in the brain areas related to self‐reflection and emotional responses (Han & Ma, 2014).
Although a great number of behavioral observations and fMRI studies suggested neural differences between cultures, possible corresponding differences in brain structure have not been adequately investigated (Park & Huang, 2010). To date, only a few structural imaging studies have explored the brain structural differences between East Asians and Westerners. Zilles, Kawashima, Dabringhaus, Fukuda, and Schormann (2001) examined differences in brain hemispheric shape across Japanese and European subjects using MRI and three‐dimensional reconstruction, and reported shorter and wider hemispheres in Japanese subjects compared with Europeans. Kochunov et al. used deformation field morphometry to detect differences in brain shape between English‐speaking Caucasians and Chinese‐speaking Asians. They attributed the differences in the gyri of the frontal, temporal, and parietal lobes to the learned cognitive strategy of native language, which altered systems‐level organization of linguistic functions, ultimately affecting anatomical plasticity (Kochunov et al., 2003). A voxel‐based morphometry (VBM) analysis of monolingual and multilingual speakers showed greater gray matter density in the left superior and middle temporal gyri, as well as the right superior temporal and left inferior frontal gyri in Chinese‐language speakers, regardless of whether they were Chinese or European (Green, Crinion, & Price, 2007). In one of our previous works (Tang et al., 2010), we performed a morphometric comparison study using structural MRI data and found that anatomical brain measurements including whole brain shape, size, and regional brain structure volumes were significantly different between Chinese and Caucasian populations. In order to assess whether cognitive differences between Asians and Westerners were accompanied by differences in brain structure, Chee, Zheng, Goh, Park, and Sutton (2011) conducted a comparative study of structural volume and cortical thickness on cognitively matched young and older Chinese Singaporean and non‐Asian American adults. The results showed that non‐Asian American young adults had higher cortical thickness in frontal, parietal, and medial‐temporal polymodal association areas in both hemispheres.
Structural MRI is an integral part of assessing functional and behavioral differences. Morphometry is a valuable tool for studying human brain plasticity in vivo, and anatomical brain differences play a primary and critical role in the origination of functional and behavioral differences. Although the above structural imaging studies have examined differences in brain structure between East Asians and Westerners, the locations and extent of the differences between these two cohorts are still poorly understood. The contradictory findings among these structural and functional imaging studies may be caused by reliance on a single brain morphological measurement (Green et al., 2007; Tang et al., 2010; Zilles et al., 2001), or by the narrow focus of the brain areas (Green et al., 2007; Kochunov et al., 2003), brain features (Chee et al., 2011; Green et al., 2007; Kochunov et al., 2003; Tang et al., 2010; Zilles et al., 2001), and subjects examined (Chee et al., 2011). In the present study, we explored the cultural/ethnicity‐based brain differences with high quality structural MRI data from comparatively large samples of education‐matched right‐handed young Chinese and Caucasian cohorts. Comprehensive surface‐based morphometry analyses were conducted across the brain for multiple structural measurements: cortical thickness, cortical volumes, and surface area. Furthermore, the VBM analysis was used to investigate differences in gray matter intensity and volume features. Our findings will provide new neuroanatomical insights to the behavioral and functional distinctions in the two populations.
2. MATERIALS AND METHODS
2.1. Subjects
About 59 normal right‐handed Chinese male volunteers, without a history of any neurological, psychiatric, or medical illness, were recruited from the local community through the research center for sectional and imaging anatomy at Shandong University Cheeloo College of Medicine. All participants were examined by two experienced neurosurgeons to exclude prior neurological diseases. Handedness test was assessed by the Edinburgh handedness inventory (Oldfield, 1971). Based on the quality of imaging data, 45 subjects (age = 23.51 ± 1.14 years, education = 15.84 ± 0.37 years) were used in the final analysis. The local ethics committee at Shandong University Cheeloo College of Medicine approved the study, and all participants provided written informed consent before participating.
About 45 gender and education‐level (15.53 ± 1.06 years) matched right‐handed normal Caucasian (age = 25.87 ± 2.20 years) subjects were selected from the Human Connectome Project (HCP) datasets (Van Essen et al., 2012). The demographic features for the two groups are presented in Table 1.
Table 1.
Demographic details of the subjects used in this study
| Group details | Chinese | Caucasian |
|---|---|---|
| Number of subjects | 45 | 45 |
| Gender | Male | Male |
| Handedness | Right‐handed | Right‐handed |
| Age (years) | 23.51 (1.14) | 25.87 (2.20) |
| Education (years) | 15.84 (0.37) | 15.53 (1.06) |
| Mean (SD) | Mean (SD) |
2.2. Data acquisition and preparation
The 45 Caucasian subjects selected from the HCP database were scanned in a Siemens 3.0T Skyra MRI scanner (Siemens AG, Erlangen, Germany) using a 32‐channel head coil with a customized protocol (Glasser et al., 2013). For each subject, three‐dimensional (3D) T1‐weighted MRI images were acquired using a MP‐RAGE sequence with 0.7 mm isotropic resolution. The typical acquisition parameters were echo time (TE) of 2.14 ms, repetition time (TR) of 2,400 ms, inversion time (TI) of 1,000 ms, flip angle of 8°, and 224 mm of field of view. The acquisition matrix was 224 × 224 × 256 in the x‐, y‐, and z‐dimensions.
For the 45 Chinese volunteers, high resolution MR images were also acquired on a Siemens 3.0T Skyra MRI scanner using a 32‐channel head coil. The sequence relevant to this study was a T1‐weighted 3D MP‐RAGE optimized for gray–white matter contrast, and the similar imaging parameters used in this study were as follows: TE = 2.96 mm, TR = 2,300 ms, TI = 900 ms, flip angle = 9° and field of view = 256 × 256. Matrix = 256 × 256 and each volume consisted of 192 slices with voxel sizes of 1 × 1 × 1 mm3.
All data were converted to NIFTI format for analysis using the MRIcron software, and all brain images were globally aligned to the ICBM152 brain template using a six‐parameter linear transformation to obtain common coordinates and spatial resolution. A quality control (QC) procedure was carried out by one rater on all raw T1‐weighted images to ensure no visible motion artefacts or poor resolution of gray/white matter contrast.
2.3. Corticometric and morphometric analysis
2.3.1. Image processing
All the MR images were processed with the FreeSurfer software (version5.3.0, https://surfer.nmr.mgh.harvard.edu) to reconstruct and co‐register the cortical surfaces and estimate brain structural features including cortical thickness, cortical volume, and surface area. The stream of processing for each MR image included removal of nonbrain tissue using a hybrid watershed algorithm (Segonne et al., 2004), bias field correction, automated Talairach transformation, segmentation of subcortical white matter and deep gray matter structures (Fischl et al., 2002, 2004), intensity normalization, tessellation of the gray/white matter boundary, and gray/cerebrospinal fluid (CSF) boundary, automated topology correction (Fischl, Liu, & Dale, 2001; Segonne et al., 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/CSF borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Fischl & Dale, 2000). On completion of the cortical models, individual cortical folding patterns were then registered to a spherical atlas in order to match cortical geometry across subjects. Thickness was calculated at each location of the cortex as the distance between the white matter and pial surface (Fischl & Dale, 2000). Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) as well as manual measurements (Kuperberg et al., 2003). All data were smoothed with a 20‐mm full width half maximum (FWHM) Gaussian kernel. The cerebral cortex was then parcellated based on gyral and sulcal information derived from manually traced brains (Desikan et al., 2006; Fischl et al., 2004). Morphometric evaluation of each hemisphere was conducted independently. The pial surface and gray–white matter junction meshes were carefully reviewed and edited manually as necessary by a single neuroanatomist (Tang), and all morphometric results were verified with the assistance of quality control processes.
2.4. Surface‐based morphometric analysis
Statistical analyses on vertex‐wise were implemented using the SurfStat toolbox (http://www.math.mcgill/keith/surfstat/), which is a Matlab toolbox for the statistical analysis of surface data using linear mixed effects models. Between‐group comparisons were conducted between the Chinese and Caucasian groups with respect to cortical volume, cortical surface area and cortical thickness using one‐way ANOVA, controlling for age, education‐level and the total cortical volume, surface area, and mean cortical thickness across the whole cortex. Significance values were corrected by false discovery rate (FDR) with a level of 0.05.
2.5. Voxel‐based morphometry (VBM) analysis
A long‐standing issue with brain morphometry studies is the concern that results acquired from different methods may be difficult to compare (Chee et al., 2011; Klauschen, Goldman, Barra, Meyer‐Lindenberg, & Lundervold, 2009). To address this concern, we also processed the same image data with Voxel‐based morphometry (VBM) (Ashburner & Friston, 2000; Good et al., 2001), which has been widely used in both cross‐sectional and longitudinal studies for detecting group anatomical differences throughout the brain and allowing the production of maps of density, volume, or other features of the brain tissue on a voxel‐by‐voxel basis. In the current study, we performed a VBM analysis using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8/) developed by Christian Gaser within the Statistical Parametric Mapping 12 software (SPM12) to confirm the cortical differences between the Chinese and Caucasian cohorts.
All the brain images from Chinese and Caucasian cohorts were spatially normalized to the ICBM space template using the high‐dimensional diffeomorphic anatomical registration using exponentiated Lie algebra (DARTEL) algorithm and segmented into gray matter (GM), white matter (WM), and Cerebrospinal fluid (CSF). Then a data quality assessment procedure was used to verify the correction of output files. In order to compensate for the inexact nature of the anatomical standardization and render the data more normally distributed, the optimally standardized GM images were smoothed by convolving with an isotropic Gaussian kernel of 8mm full width at half maximum (FWHM). Finally, voxel‐wise parametric statistical tests, which compare the smoothed gray matter images from the two groups, were performed. For statistical map generation, a height threshold of uncorrected p < .001 was utilized, clusters less than 50 voxels were not reported.
3. RESULTS
3.1. Cortical morphometric analysis
The cortical morphometric analysis showed structural differences between Chinese and Caucasian cohorts in distributed brain regions, such as the frontal, temporal, parietal, occipital, insular, and cingulate cortices, which were consistent for the cortical volume, cortical surface area, and cortical thickness.
3.2. Between‐group differences in cortical volume
Significant vertex‐wise differences between Chinese and Caucasian groups in cortical volume are illustrated in Figure 1. Compared with the Caucasian group, the Chinese group showed a larger volume in the bilateral middle and inferior temporal gyri, the bilateral middle cingulate and para‐cingulate gyri, the bilateral olfactory gyri, the bilateral para‐hippocampal gyri, the bilateral middle occipital gyri, the bilateral gyrus rectus, the right fusiform gyri, and the left angular gyrus. The Caucasian subjects showed a larger volume in the bilateral medial prefrontal lobes, the bilateral superior frontal gyri, the bilateral orbitofrontal gyri, the right precentral gyrus, and left paracentral lobule.
Figure 1.
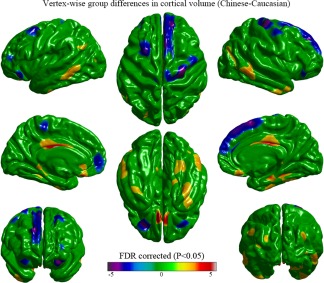
Vertex‐wise group differences in cortical volume removing the effects of age, education‐level, and total cortical volume (T‐map, thresholded at FDR corrected p < .05). The Chinese group had a larger volume in broad cortical regions including the bilateral middle and inferior temporal gyri, the bilateral middle cingulate and para‐cingulate gyri, the bilateral olfactory gyri, the bilateral para‐hippocampal gyri, the bilateral middle occipital gyri, the bilateral gyrus rectus, the right fusiform gyri, and the left angular gyrus. The Caucasian group had a larger volume in the bilateral medial prefrontal lobes, the bilateral superior frontal gyri, the bilateral orbitofrontal gyri, the right precentral gyrus, and left paracentral lobule [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Between‐group differences in cortical surface area
Significant vertex‐wise differences between Chinese and Caucasian groups in cortical surface area are shown as Figure 2. The Chinese subjects had a larger surface area in the left middle temporal gyrus, angular gyrus and gyrus rectus, and the right middle occipital gyrus. The surface area in the bilateral superior frontal gyri, the bilateral medial prefrontal lobes, the bilateral orbitofrontal gyri, the bilateral insula lobes, and right precentral gyrus was larger in the Caucasian group.
Figure 2.
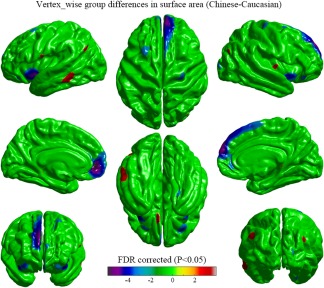
Vertex‐wise group differences in cortical surface area removing the effects of age, education‐level, and total surface area (T‐map, thresholded at FDR corrected p < .05). The Chinese subjects had an average larger surface area in the cortical regions including the left middle temporal gyrus, angular gyrus and gyrus rectus, and the right middle occipital gyrus. The surface area in the bilateral superior frontal gyri, the bilateral medial prefrontal lobes, the bilateral orbitofrontal gyri, the bilateral insula lobes, and right precentral gyrus was larger in the Caucasian group [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Between‐group differences in cortical thickness
Significant differences in cortical thickness between the two groups are illustrated in Figure 3. The Chinese subjects showed larger cortical thickness in the bilateral middle and inferior temporal gyri, the bilateral para‐hippocampal gyri, the bilateral fusiform gyri, the bilateral anterior and middle cingulate and para‐cingulate gyri, the bilateral middle occipital gyri, the bilateral insular lobes, the left precuneus and superior frontal gyrus, the left cortical region surrounding the calcarine fissure, the left orbitofrontal gyrus, and gyrus rectus. The Caucasian subjects showed larger cortical thickness in the bilateral motor speech area (Broca's area), the bilateral precentral and postcentral gyri, the bilateral middle frontal gyri, the bilateral orbitofrontal gyri, the right angular gyrus and supra‐marginal gyrus, the left paracentral lobule, and the right precuneus.
Figure 3.
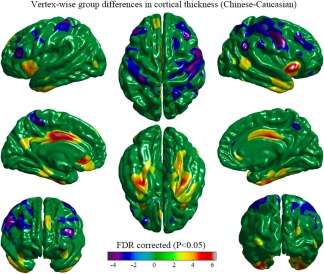
Vertex‐wise group differences in cortical thickness removing the effects of age, education‐level, and mean cortical thickness (T‐map, thresholded at FDR corrected p < .05). The Chinese group showed a thicker cortex in the bilateral middle and inferior temporal gyri, the bilateral para‐hippocampal gyri, the bilateral fusiform gyri, the bilateral anterior and middle cingulate and para‐cingulate gyri, the bilateral middle occipital gyri, the bilateral insular lobes, the left precuneus and superior frontal gyrus, the left cortical region surrounding the calcarine fissure, the left orbitofrontal gyrus, and gyrus rectus. The Caucasian group showed a thicker cortex in the motor speech area (Broca's area), the precentral and postcentral gyri, the middle frontal gyri and the orbitofrontal gyri bilaterally, the right angular gyrus and supra‐marginal gyrus, the right precuneus, and the left paracentral lobule [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5. VBM analysis
We investigated the gray matter intensity and volume differences between Chinese and Caucasian groups using an uncorrected p < .001 with an extent threshold of 50 voxels in VBM and found that the results were consistent with the SBM analysis. The Chinese cohort showed greater regional density and volume in the bilateral middle and inferior temporal gyri, the bilateral olfactory gyri, the bilateral para‐hippocampal gyri, the bilateral gyrus rectus, the right fusiform gyri, and the right cuneus. The Caucasian group showed greater density and volume in the medial prefrontal lobes, the superior and middle frontal gyri, the motor speech area (Broca's area), the precentral and postcentral gyri, the angular gyri and the orbitofrontal gyri bilaterally, the left inferior occipital gyrus, and the left paracentral lobule (Figure 4).
Figure 4.
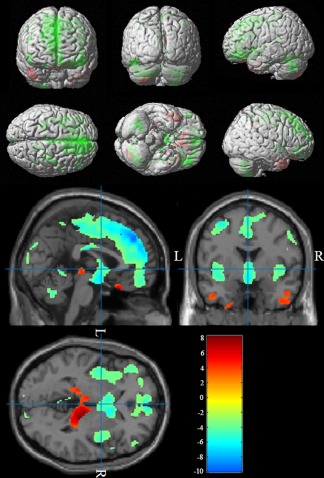
VBM‐identified differences in gray matter density between Chinese and Caucasian cohorts (Chinese‐Caucasian). The Chinese cohort showed greater density and volume in the bilateral middle and inferior temporal gyri, the bilateral olfactory gyri, the bilateral para‐hippocampal gyri, the bilateral gyrus rectus, the right fusiform gyri, and the right cuneus. The Caucasian group showed greater density and volume in the medial prefrontal lobes, the superior and middle frontal gyri, the motor speech area (Broca's area), the precentral and postcentral gyri, the angular gyri and the orbitofrontal gyri bilaterally, the left inferior occipital gyrus, and the left paracentral lobule [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
A rapidly growing body of imaging studies have identified consistent behavior and functional differences between Chinese and Caucasian populations (Goh & Park, 2009; Han & Ma, 2014; Han & Northoff, 2008). Not all regions showing functional differences between the two populations were seen to have structural differences. The areas showing neuroanatomical differences, however, have shown robust and highly reproducible functional differences between East Asian and Westerners (Kochunov et al., 2003). It is still unclear how the brain structural differences are associated with culture or ethnicity (Park & Huang, 2010). In this study, we selected two comparable samples (45 subjects for each group) from the Chinese and Caucasian young populations that are well matched for gender, handedness and education to explore the structural differences between Chinese and Caucasian brains. We performed comprehensive SBM and VBM analyses of multiple brain structural features of each cohort, and identified significant between‐group differences in cortical thickness, cortical volume, and cortical surface area, as well as gray matter intensity consistently in distributed brain regions, including the frontal, temporal, parietal, occipital, insular, and cingulate cortices (see Figures 1, 2, 3, 4 and Supporting Information). The Chinese population showed greater structural features in the temporal lobe and cingulate gyrus, and smaller features in the frontal lobe and parietal lobe, compared with the Caucasian cohort.
Compared with previous studies comparing brain differences of different ethnic groups, there are two main strength in the current study. First, we used data from homogeneous populations and MRI acquisition protocols. For the Chinese cohort, we only enrolled native Chinese‐speaking subjects of the Han ethnicity. Similar population homogeneity was achieved in the Caucasian group by only including native English speaking HCP subjects. Both cohorts were scanned on the same model of Siemens 3.0T Skyra MRI scanner with a 32‐channel head coil and 3D MP‐RAGE sequence with similar parameters that ensure the imaging data are highly comparable. Second, we applied both SBM and VBM analysis to study the group differences, and obtained consistent findings across multiple morphometry measures and brain regions. For example, while there was no significant difference in the surface area of the left Broca's area between the two groups, the Caucasian group exhibited greater cortical thickness and volume than the Chinese group. These findings were also verified with the VBM analysis.
Our findings were consistent with the existing findings on the behavioral and brain functional differences between English‐speaking Caucasian and Chinese‐speaking Asians from a comprehensive anatomical perspective. The medial prefrontal cortex plays an important role in the cognition processes of memory and decision making (Euston, Gruber, & McNaughton, 2012), including conflict monitoring (Botvinick, Cohen, & Carter, 2004), error detection (Holroyd, Coles, & Nieuwenhuis, 2002), executive control (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004), and reward‐guided learning (Rushworth, Noonan, Boorman, Walton, & Behrens, 2011). A meta‐analysis suggested that the Westerners had stronger activity in the ventral medial prefrontal cortex (Han & Ma, 2014), the results of the present study showed greater surface area and cortical volume in the medial prefrontal cortex in Caucasians, which had not been reported by the previous structural imaging studies.
The fusiform gyrus plays a key role in face detection and identification (Gold et al., 2014; Gold, Mundy, & Tjan, 2012; Liu, Harris, & Kanwisher, 2010; McCarthy, Puce, Gore, & Allison, 1997). The differences between Oriental and Occidental groups in the processing of facial stimuli have been widely reported (Blais et al., 2008; Jack et al., 2009; Kim et al., 2006; Lee & Ham, 2008). It has been found that East Asians focused on a single central region when viewing faces, whereas Westerners scanned more broadly and focus on both eyes and mouth (Blais et al., 2008). In another study focusing on cultural differences in processing emotional faces, Jack et al. (2009) found that culture played an important role in modulating perceptions of emotion. Consistently with these functional data, we found thicker cortex and larger cortical volume in the fusiform gyrus in the Chinese group compared with the Caucasian group.
The middle temporal gyrus is thought to participate in short‐term verbal memory, verbal fluency and word generation (Petersen, Fox, Posner, Mintun, & Raichle, 1988), and has been shown to play a significant role in the processing of sounds with complex spectral and temporal properties (Mirz et al., 1999; Zahn et al., 2000). The Chinese language is both more structurally complex and more aurally complex than English and other European languages (Kochunov et al., 2003). Our results showed greater cortical thickness and gray matter density in the bilateral middle temporal gyri and right anterior temporal lobe in the Chinese population, which may be explained by the greater demand on these regions by the Chinese language (Crinion et al., 2009). With respect to the language area, we also found significantly larger cortical thickness and cortical volume as well as gray matter density in the bilateral inferior frontal gyrus, which is consistent with one current study (Chee et al., 2011). However, it is dissimilar to a previous study (Siok et al., 2004). This may be explained by the fact that university students in China commonly study and practice English, and therefore all of the Chinese participants in the study were bilingual.
The findings of the current and previous studies regarding brain structural differences in different ethnic groups can provide a foundation for more in‐depth brain mapping research and disease studies. The morphometry differences that we found using MP‐RAGE scans can motivate connectome imaging studies to further investigate the brain connectivity differences of these affected brain regions. Both tractography‐based and functional MRI‐based methods can be used to push forward the research along this direction. A thorough understanding of the structural and functional differences of the human brains across different ethnic groups will be crucial for studying various neurological disorders. It can lead to the development of ethnic‐specific atlas construction, and customized clinical trial for each ethnic group. These research efforts will also provide the biological basis for our understanding of ethnic and cultural differences among different groups.
There are several limitations in the current study. One is the sample size and gender of the selected subjects, each cohort group only comprised 45 male subjects. Although the sample size is relatively large compared with previous studies, a total of 90 subjects may be not enough for an imaging study, even if our results based on various morphological measurements were consistent. Therefore, we will continue to add more subjects, especially female and aged subjects in subsequent brain structure comparison studies to validate our findings. The other limitation is the data modality, only structural MRI data were used in the present study. Multi‐modal imaging data, including diffusion MRI and fMRI, and behavior data should also be examined in future studies to find out the brain structure and function differences between these two groups, for example, the between‐group differences of white matter.
5. CONCLUSION
Here we performed a comprehensive vertex‐wise morphometric analysis of the brain structures between Chinese and Caucasian cohorts and identified consistent significant between‐group differences in cortical thickness, volume and surface area in the frontal, temporal, parietal, occipital, and insular lobes as well as the cingulate cortices. These findings systematically revealed comprehensive brain structural differences between young male Chinese and Caucasians, and provided new neuroanatomical insights to the behavioral and functional distinctions in the two cultural/ethnic populations.
The authors declare that they have no conflicts of interest to disclose. All authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
We thank Nan Zhang, Xiaoxiao Yang, Ruilin Jin, Yong Wu, and Yan Shi (Shandong University, China) for data acquisition for Chinese subjects. Data of Caucasian cohorts were provided by the Human Connectome Project, WU‐Minn Consortium (Principal Investigator: David Van Essen and Kamil Ugurbil, 1U54MH091657).
Tang Y, Zhao L, Lou Y, et al. Brain structure differences between Chinese and Caucasian cohorts: A comprehensive morphometry study. Hum Brain Mapp. 2018;39:2147–2155. 10.1002/hbm.23994
Funding information National Natural Science Foundation of China, Grant/Award Number: 81301280; China Scholarship Council, Grant/Award Number: 201506225068; Specialized Research Fund for the Doctoral Program of Higher Education of China, Grant/Award Number: 20120131120043; Promotive Research Fund for Excellent Young and Middle‐aged Scientists of Shandong Province, Grant/Award Number: BS2011YY035; Major Scientific and Technological Innovation Project of Shandong Province, Grant/Award Number: 2017CXGC1501; The Fundamental Research Funds of Shandong University, Grant/Award Number: 2015JC009
Contributor Information
Yuchun Tang, Email: yuchuntang@gmail.com.
Shuwei Liu, Email: lshw@sdu.edu.cn.
REFERENCES
- Ames, D. L. , & Fiske, S. T. (2010). Cultural neuroscience. Asian Journal of Social Psychology, 13, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2000) Voxel‐based morphometry–the methods. Neuroimage, 11:805–21. [DOI] [PubMed] [Google Scholar]
- Aylward, E. H. , Richards, T. L. , Berninger, V. W. , Nagy, W. E. , Field, K. M. , Grimme, A. C. , … Cramer, S. C. (2003). Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology, 61, 212–219. [DOI] [PubMed] [Google Scholar]
- Blais, C. , Jack, R. E. , Scheepers, C. , Fiset, D. , & Caldara, R. (2008). Culture shapes how we look at faces. PLoS One, 3, e3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick, M. M. , Cohen, J. D. , & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8, 539–546. [DOI] [PubMed] [Google Scholar]
- Chee, M. W. , Zheng, H. , Goh, J. O. , Park, D. , & Sutton, B. P. (2011). Brain structure in young and old East Asians and Westerners: Comparisons of structural volume and cortical thickness. Journal of Cognitive Neuroscience, 23, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao, J. Y. , Cheon, B. K. , Pornpattanangkul, N. , Mrazek, A. J. , & Blizinsky, K. D. (2013). Cultural neuroscience: Progress and promise. Psychological Inquiry, 24, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion, J. T. , Green, D. W. , Chung, R. , Ali, N. , Grogan, A. , Price, G. R. , … Price, C. J. (2009). Neuroanatomical markers of speaking Chinese. Human Brain Mapping, 30, 4108–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R. S. , Segonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Killiany, R. J. (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31:968–80. [DOI] [PubMed] [Google Scholar]
- Euston, D. R. , Gruber, A. J. , & McNaughton, B. L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , & Dale, A. M. (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A, 97:11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Liu, A. , & Dale, A. M. (2001) Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging, 20:70–80. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33:341–55. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , van der Kouwe, A. J. , Makris, N. , Segonne, F. , Quinn, B. T. , & Dale, A. M. (2004) Sequence‐independent segmentation of magnetic resonance images. Neuroimage, 23 Suppl 1:S69–84. [DOI] [PubMed] [Google Scholar]
- Glasser, M. F. , Sotiropoulos, S. N. , Wilson, J. A. , Coalson, T. S. , Fischl, B. , Andersson, J. L. , … Consortium, W. U.‐M. H. (2013). The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage, 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, C. D. , Johnsrude, I. S. , Ashburner, J. , Henson, R. N. , Friston, K. J. , & Frackowiak, R.S. (2001) A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage, 14:21–36. [DOI] [PubMed] [Google Scholar]
- Goh, J. O. , Leshikar, E. D. , Sutton, B. P. , Tan, J. C. , Sim, S. K. , Hebrank, A. C. , & Park, D. C. (2010). Culture differences in neural processing of faces and houses in the ventral visual cortex. Social Cognitive and Affective Neuroscience, 5, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh, J. O. , & Park, D. C. (2009). Culture sculpts the perceptual brain. Progress in Brain Research, 178, 95–111. [DOI] [PubMed] [Google Scholar]
- Gold, J. M. , Barker, J. D. , Barr, S. , Bittner, J. L. , Bratch, A. , Bromfield, W. D. , … Srinath, A. (2014). The perception of a familiar face is no more than the sum of its parts. Psychonomic Bulletin & Review, 21, 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, J. M. , Mundy, P. J. , & Tjan, B. S. (2012). The perception of a face is no more than the sum of its parts. Psychological Science, 23, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D. W. , Crinion, J. , & Price, C. J. (2007). Exploring cross‐linguistic vocabulary effects on brain structures using voxel‐based morphometry. Bilingualism: Language and Cognition, 10, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. , & Ma, Y. (2014). Cultural differences in human brain activity: A quantitative meta‐analysis. Neuroimage, 99, 293–300. [DOI] [PubMed] [Google Scholar]
- Han, S. , & Northoff, G. (2008). Culture‐sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nature Reviews Neuroscience, 9, 646–654. [DOI] [PubMed] [Google Scholar]
- Han, S. , Northoff, G. , Vogeley, K. , Wexler, B. E. , Kitayama, S. , & Varnum, M. E. (2013). A cultural neuroscience approach to the biosocial nature of the human brain. Annual Review of Psychology, 64, 335–359. [DOI] [PubMed] [Google Scholar]
- Hedden, T. , Ketay, S. , Aron, A. , Markus, H. R. , & Gabrieli, J. D. (2008). Cultural influences on neural substrates of attentional control. Psychological Science, 19, 12–17. [DOI] [PubMed] [Google Scholar]
- Holroyd, C. B. , Coles, M. G. , & Nieuwenhuis, S. (2002). Medial prefrontal cortex and error potentials. Science (New York, N.Y.), 296, 1610‐1 author reply 1610‐1. [DOI] [PubMed] [Google Scholar]
- Horwitz, B. , Rumsey, J. M. , & Donohue, B. C. (1998). Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences of the United States of America, 95, 8939–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, R. E. , Blais, C. , Scheepers, C. , Schyns, P. G. , & Caldara, R. (2009). Cultural confusions show that facial expressions are not universal. Current Biology : Cb, 19, 1543–1548. [DOI] [PubMed] [Google Scholar]
- Ji, L. J. , Peng, K. , & Nisbett, R. E. (2000). Culture, control, and perception of relationships in the environment. Journal of Personality and Social Psychology, 78, 943–955. [DOI] [PubMed] [Google Scholar]
- Johansson, B. B. (2006). Cultural and linguistic influence on brain organization for language and possible consequences for dyslexia: A review. Annals of Dyslexia, 56, 13–50. [DOI] [PubMed] [Google Scholar]
- Kim, J. S. , Yoon, H. W. , Kim, B. S. , Jeun, S. S. , Jung, S. L. , & Choe, B. Y. (2006). Racial distinction of the unknown facial identity recognition mechanism by event‐related fMRI. Neuroscience Letters, 397, 279–284. [DOI] [PubMed] [Google Scholar]
- Kitayama, S. , Duffy, S. , Kawamura, T. , & Larsen, J. T. (2003). Perceiving an object and its context in different cultures: A cultural look at new look. Psychological Science, 14, 201–206. [DOI] [PubMed] [Google Scholar]
- Klauschen, F. , Goldman, A. , Barra, V. , Meyer‐Lindenberg, A. , & Lundervold, A. (2009) Evaluation of automated brain MR image segmentation and volumetry methods. Hum Brain Mapp, 30:1310–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov, P. , Fox, P. , Lancaster, J. , Tan, L. H. , Amunts, K. , Zilles, K. , … Gao, J. H. (2003). Localized morphological brain differences between English‐speaking Caucasians and Chinese‐speaking Asians: New evidence of anatomical plasticity. Neuroreport, 14, 961–964. [DOI] [PubMed] [Google Scholar]
- Kuperberg, G. R. , Broome, M. R. , McGuire, P. K. , David, A. S. , Eddy, M. , Ozawa, F. , … Fischl, B. (2003) Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry, 60:878–88. [DOI] [PubMed] [Google Scholar]
- Lee, B. T. , & Ham, B. J. (2008). Serotonergic genes and amygdala activity in response to negative affective facial stimuli in Korean women. Genes, Brain and Behavior, 7, 899–905. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Harris, A. , & Kanwisher, N. (2010). Perception of face parts and face configurations: An FMRI study. Journal of Cognitive Neuroscience, 22, 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, T. , Gonzalez, R. , Kwan, L. , & Nisbett, R. E. (2008). Culture and aesthetic preference: Comparing the attention to context of East Asians and Americans. Personality and Social Psychology Bulletin, 34, 1260–1275. [DOI] [PubMed] [Google Scholar]
- Masuda, T. , & Nisbett, R. E. (2006). Culture and change blindness. Cognitive Science, 30, 381–399. [DOI] [PubMed] [Google Scholar]
- McCarthy, G. , Puce, A. , Gore, J. C. , & Allison, T. (1997). Face‐specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience, 9, 605–610. [DOI] [PubMed] [Google Scholar]
- Mirz, F. , Ovesen, T. , Ishizu, K. , Johannsen, P. , Madsen, S. , Gjedde, A. , & Pedersen, C. B. (1999). Stimulus‐dependent central processing of auditory stimuli: A PET study. Scandinavian Audiology, 28, 161–169. [DOI] [PubMed] [Google Scholar]
- Nisbett, R. E. , & Masuda, T. (2003). Culture and point of view. Proceedings of the National Academy of Sciences of the United States of America, 100, 11163–11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett, R. E. , & Miyamoto, Y. (2005). The influence of culture: Holistic versus analytic perception. Trends in Cognitive Sciences, 9, 467–473. [DOI] [PubMed] [Google Scholar]
- Nisbett, R. E. , Peng, K. , Choi, I. , & Norenzayan, A. (2001). Culture and systems of thought: Holistic versus analytic cognition. Psychological Review, 108, 291–310. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9:97–113. [DOI] [PubMed] [Google Scholar]
- Park, D. C. , & Huang, C. M. (2010). Culture wires the brain: A cognitive neuroscience. Perspectives on Psychological Science, 5, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, K. , & Nisbett, R. E. (2000). Dialectical responses to questions about dialectical thinking. American Psychologist, 55, 1067–1068. [PubMed] [Google Scholar]
- Petersen, S. E. , Fox, P. T. , Posner, M. I. , Mintun, M. , & Raichle, M. E. (1988). Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature, 331, 585–589. [DOI] [PubMed] [Google Scholar]
- Rosas, H. D. , Liu, A. K. , Hersch, S. , Glessner, M. , Ferrante, R. J. , Salat, D. H. , … Fischl, B. (2002) Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology, 58:695–701. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof, K. R. , Ullsperger, M. , Crone, E. A. , & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306, 443–447. [DOI] [PubMed] [Google Scholar]
- Rushworth, M. F. , Noonan, M. P. , Boorman, E. D. , Walton, M. E. , & Behrens, T. E. (2011). Frontal cortex and reward‐guided learning and decision‐making. Neuron, 70, 1054–1069. [DOI] [PubMed] [Google Scholar]
- Segonne, F. , Dale, A. M. , Busa, E. , Glessner, M. , Salat, D. , Hahn, H. K. , & Fischl, B. (2004) A hybrid approach to the skull stripping problem in MRI. Neuroimage, 22:1060–75. [DOI] [PubMed] [Google Scholar]
- Shaywitz, S. E. , Shaywitz, B. A. , Pugh, K. R. , Fulbright, R. K. , Constable, R. T. , Mencl, W. E. , … Gore, J. C. (1998). Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences of the United States of America, 95, 2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok, W. T. , Perfetti, C. A. , Jin, Z. , & Tan, L. H. (2004). Biological abnormality of impaired reading is constrained by culture. Nature, 431, 71–76. [DOI] [PubMed] [Google Scholar]
- Siok, W. T. , Niu, Z. , Jin, Z. , Perfetti, C. A. , & Tan, L. H. (2008) A structural‐functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci U S A, 105:5561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. F. , Lee, J. S. , & Kirby, R. (2010). Brain imaging findings in dyslexia. Pediatrics and Neonatology, 51, 89–96. [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Hojatkashani, C. , Dinov, I. D. , Sun, B. , Fan, L. , Lin, X. , … Toga, A. W. (2010). The construction of a Chinese MRI brain atlas: A morphometric comparison study between Chinese and Caucasian cohorts. Neuroimage, 51, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif, J. , Fiset, D. , Zhang, Y. , Estephan, A. , Cai, Q. , Luo, C. , … Blais, C. (2017). Culture shapes spatial frequency tuning for face identification. The Journal of Experimental Psychology: Human Perception and Performance, 43, 294–306. [DOI] [PubMed] [Google Scholar]
- Temple, E. , Deutsch, G. K. , Poldrack, R. A. , Miller, S. L. , Tallal, P. , Merzenich, M. M. , & Gabrieli, J. D. (2003). Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 100(5), 2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen, D. C. , Ugurbil, K. , Auerbach, E. , Barch, D. , Behrens, T. E. , Bucholz, R. , … Consortium, W. U.‐M. H. (2012) The Human Connectome Project: a data acquisition perspective. Neuroimage, 62:2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Baldauf, D. , Chang, C. Q. , Desimone, R. , & Tan, L. H. (2017). Distinct distributed patterns of neural activity are associated with two languages in the bilingual brain. Science Advances, 3, e1603309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn, R. , Huber, W. , Drews, E. , Erberich, S. , Krings, T. , Willmes, K. , & Schwarz, M. (2000). Hemispheric lateralization at different levels of human auditory word processing: A functional magnetic resonance imaging study. Neuroscience Letters, 287, 195–198. [DOI] [PubMed] [Google Scholar]
- Zilles, K. , Kawashima, R. , Dabringhaus, A. , Fukuda, H. , & Schormann, T. (2001). Hemispheric shape of European and Japanese brains: 3‐D MRI analysis of intersubject variability, ethnical, and gender differences. Neuroimage, 13, 262–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


