Introduction
Asthma is a common chronic condition affecting both children and adults, and is characterized by chronic airway inflammation leading to bronchial hyper-responsiveness and mucous hypersecretion. Asthma can be triggered by a variety of stimuli, leading to recurrent and reversible episodes of wheezing, shortness of breath, chest tightness, and coughing[1]. Asthma prevalence has increased dramatically both in the United States (US) and worldwide. Recent statistics from the National Health Interview Surveys (NHIS) and the US Centers for Disease Control and Prevention (CDC) estimated that 26.5 million people in the US, including 6.1 million children, have asthma[2]. Globally, about 235 million people suffer from asthma[3]. Implicated in the development of asthma are respiratory viral infections and infections with atypical bacteria (such as mycoplasma and/or chlamydia). Infectious agents have not only been associated with the inception of disease in asthma, they have also been involved in its exacerbations. In fact, in 2015 more than 11.5 million people with asthma, including nearly 3 million children, had one or more exacerbations of their asthma[4]. The burden of asthma is significant, both in terms of financial expenses and lost productivity, and in many situations, it is an infectious agent that initiates an asthma exacerbation. This review will discuss the epidemiology of respiratory infections (figure 1) in the development of asthma, as well as potential mechanisms that may translate these respiratory infections into asthma.
Figure 1. Pathogens associated with asthma development and exacerbation.
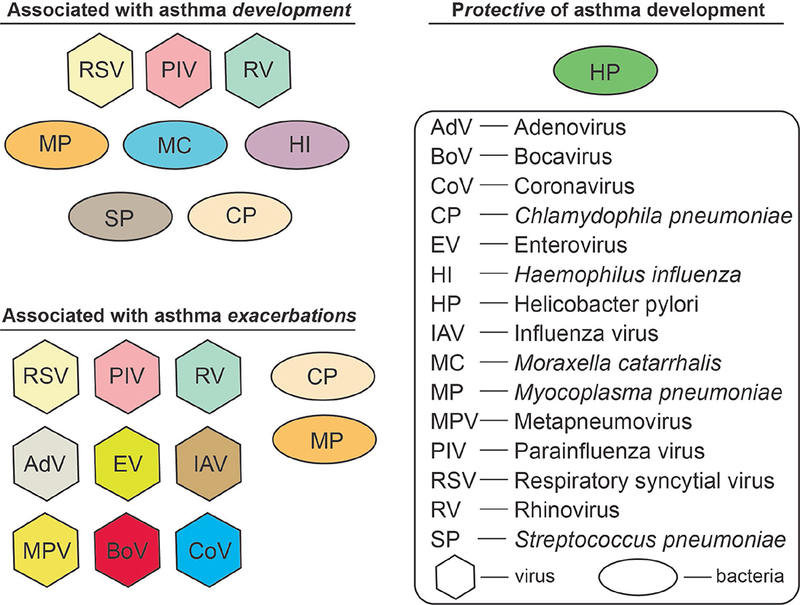
Early life infections play a role in the development of asthma and disease exacerbation. This figure lists the pathogens that have been associated with asthma development (upper left) and asthma exacerbations (bottom left). H. pylori has been shown to be protective of asthma development (upper right). Abbreviations are explained in the box; viruses are shown in hexagrams, while bacteria are in ovals. See text for more details.
Epidemiology of infections and asthma
The role of early-life infections in the development of asthma results from complex interactions between pathogens, genetics, and environmental factors such as tobacco smoke. Acute respiratory infections are common in asthmatic patients. While controversial, some investigators believe there may be an increased risk of infection among atopic patients caused by opportunistic infections, especially in patients with severe atopic diseases[5]. For instance, viral or bacterial infections were observed in 70% of adult inpatients with an asthma exacerbation[6] and clinical studies report that asthma onset after an acute respiratory illness is exceedingly common (up to 45% of adult-onset asthma cases)[7]. Of course, this just supports the idea that respiratory infections can exacerbate asthma, but does not prove that patients with asthma are more likely to have a respiratory infection. A study comparing over eleven thousand individuals, reported increased risk of developing asthma in individuals who had any infection independent of their smoking history[8]. Individuals with early asthma who never smoked had significantly increased risk of any infection (hazard ratio 1.65; 95% confidence interval 1.40–1.94), pneumonia (2.44; 1.92–3.11) or any non-respiratory tract infection (1.36; 1.11–1.67); results were similar in smokers with early asthma. The researchers also found that asthma history was not limited to early disease as individuals who never smoked but had any history of asthma during their lifetime also had significantly increased risk of infection (1.44; 1.24–1.66) and pneumonia (1.99; 1.62–2.44)[8]. However, there remains the issue of bias – those with asthma are more likely to have clinically significant infections. Finally, not surprisingly, early infections but not smoking history were best indicators for the development of asthma.
Viral infections and development of asthma
As mentioned, evidence supports the role of early-life viral infections in the development of asthma. Viral infections are common during early childhood development. Infant respiratory viral infection and childhood asthma are the most common acute and chronic diseases of childhood, respectively[9]. So, it is not surprising that these two illnesses overlap. Bronchiolitis and early wheezing are frequently seen in children, usually as a result of a viral infection (regardless of presence of asthma). Respiratory viruses often are detected in first-time wheezing children, and may associate with the development of atopic disease[10]. Studies have shown that one-third of all children suffer from infection-induced wheezing during the first 3 years of life[ 11, 12]. Up to half of all children have acute wheezing at least once before school age, and amongst these children, 30% to 40% develop recurrent wheezing[11]. Moreover, viral respiratory infections have been implicated in up to 80% of wheezing episodes and asthma exacerbations[13]. These studies demonstrate the relationship of viral infections and development or wheezing and/or exacerbation of asthma.
Respiratory syncytial virus (RSV), rhinovirus (RV) and parainfluenza viruses (parainfluenza virus 1 and 2) are most commonly detected in asthma exacerbations in children, but they have been associated as well in the development of the disease – especially with severe infections by these viruses early in life[14–17]. Wheezing illnesses in infancy and early childhood caused by viral infection of RV, RSV, and parainfluenza strongly correlate with asthma development later in life[18–20]. While RSV and RV appear to be the viruses most associated with asthma development, a larger group of pathogens have been identified in asthma exacerbations (Figure 1). Within the range of these respiratory viral pathogens, the most common viruses identified during an asthma exacerbation are RV (44-88%), RSV (2-20%), and PIV (2-11%)[21]. Other viruses associated with asthma exacerbations (usually less than 5% of the time) include Adenovirus, Enterovirus (non-RV), Influenza, Metapneumovirus, Bocavirus, and Coronovirus[22, 23]. It is also important to note that around 10% of these cases have a co-infection with more than one virus – usually with RV being the other virus identified[24].
RSV and development of asthma
The initial studies linking respiratory viral infections with development of asthma focused on RSV, which is one of the most common infections in children and infants. RSV is also known as one of the leading causes of severe respiratory infection in infants and several studies have strongly suggested a causal role for RSV in the development of asthma and allergen sensitization[24–27]. A cohort study by El Saleeby, et al, found that high levels of RSV titer were associated with more severe respiratory viral disease outcomes. In this study previously healthy RSV-infected children under 2 years old who had significantly higher viral titers had increased requirements for intensive medical care and were more prone to respiratory failure when compared to those with lower titers[28]. Asthma outcomes were similarly affected as demonstrated by a multi-center cohort study in the US and Finland conducted by Hasegawa, et al, determining the relationship between viral load in RSV infected children and clinical symptoms of asthma. Out of the 2,612 children in the study, 67% developed RSV bronchiolitis, but most interestingly, children with higher RSV titer had higher risk for severe bronchiolitis[29]. Taken together, these studies demonstrate the relevance of severe RSV infections in the development of asthma and asthma symptoms.
Enteroviruses and asthma
Enteroviruses (EV) are RNA viruses of the family Picornaviridae, of which the most common is RV, which has been associated with asthma development and exacerbation[30]. The Childhood Origins of ASThma (COAST) cohort study followed children from birth and identified RV as the viral etiology of 90% of wheezing illnesses by age 3 years; RV was identified as the major risk factor for asthma at 6 years of age[21]. While RV is associated with asthma development, the COAST investigators used mathematical modeling to demonstrate that RV infection likely followed the development of atopy. This raises a question as to whether respiratory RV infections drive development of asthma, or simply uncover an existing predisposition for the disease.
RV can be divided into three clades, RV-A, RV-B, and RV-C. Of these three, RV-C has been found to most strongly correlate with severity of asthma exacerbations[23]. A recent study from China further verified that RV-C is associated most often with both inpatient and outpatient asthma exacerbations, but also demonstrated that high titer of RV-A also could lead to significant exacerbation of disease[24]. Clearly RV is a major component of asthma disease burden, even if it is not causative of disease development.
In addition to RV, other EV have been identified in patients undergoing an asthma exacerbation, and these viruses may play a role in the inception of asthma. Many studies have reported an association with increased incidence of EV infections and asthma development[31–34]. One study retrospectively compared patients over a decade (January 2000-December 2011) and examined the relationship between EV infection and asthma. The incidence of asthma was 1.48-fold higher in patients (≤ 5 years old) who had an EV infection compared to those who did not[35]. Although the results were intriguing, it is important to note that this was a retrospective study depending upon claims data.
Influenza and asthma
Influenza circulates both as a seasonal infection and occasional pandemic. The seasonal variety clearly causes asthma exacerbations, but appears to be a minor contributor to the overall burden of asthma disease development. During the 2009 influenza pandemic (pH1N1), asthma was the most common co-morbidity among patients, accounting for 22-29% of all hospitalized patients with influenza[36–39]. Children with asthma accounted for 44% of hospitalized children with influenza although the major differences between the pandemic influenza and seasonal influenza was that pH1N1 was associated with higher incidence of pneumonia (46% versus 40%, pH1N1 versus seasonal, p = 0.04) and a greater need for intensive care (22% versus 16%, p = 0.01)[40]. Another study analyzed data from 12 different Canadian pediatrics hospitals during the pH1N1 pandemic and compared them with data from children hospitalized with seasonal influenza A. The results from that study revealed that pre-existing asthma was overrepresented in pH1N1 infected relative to seasonal influenza A infections[41]. Further, pH1N1 seemed to cause more disease in older children, with the median age for these pH1N1 patients being 4.8 years old, while the median age for seasonal influenza A patients was 1.7 years[41]. Data from 272 patients hospitalized for at least 24 hours with an influenza-like illness and a positive H1N1 polymerase chain reaction test, demonstrated that 73% of the patients had at least one underlying medical condition; these conditions included asthma, diabetes, pregnancy, and other heart, lung, and neurologic diseases[36]. Although asthma was over-represented in patients hospitalized with pH1N1, at least one study found that having asthma led to a more rapid recovery from the viral infection[42]. Supporting the idea that asthma might have a protective advantage in the recovery from pH1N1, a retrospective chart review of two case-series found that those with asthma who were hospitalized with pH1N1 were less likely to have pneumonia, need mechanical ventilation, or die compared to those admitted without asthma [42]. So, while influenza may be associated with asthma exacerbations, it also appears that asthma may protect against pandemic influenza mediated morbidity and mortality.
The mechanisms linking respiratory viral infections to development of asthma and its exacerbation are actively being studied. Some links relate to the role of innate lymphoid cells (ILCs) translating RSV infection into atopic disease. ILCs can be classified based on their transcriptional regulation and cytokine production into ILC1, ILC2, and ILC3 cells, which largely emulate the adaptive CD4+ T helper 1 (Th1), Th2, and Th17 cells, respectively. ILC2s produce high levels of interleukin 13 (IL-13) and interleukin 5 (IL-5), two cytokines known to play important roles in asthma. IL-5 is a required cytokine for eosinophil development, and several new asthma treatments have been developed to block its effects[43]. IL-13 has been implicated in IgE synthesis, mucus hypersecretion, airway hyperresponsiveness (AHR), and fibrosis[44]. In a murine model, RSV induced robust IL-13 production from ILC2 during the early phase of the infection[45]. This increased production of IL-13 was found to depend upon thymic stromal lymphopeitein (TSLP) signaling. Neutralizing TSLP resulted in significant reduction of IL-13, and the post-viral airway disease[45].
We have utilized the murine parainfluenza virus, Sendai virus (SeV), to explore the translation of a respiratory viral infection into asthma. Mice infected with SeV develop post-viral airway hyper-reactivity and mucous cell metaplasia after clearance of the virus. The mechanistic pathway depends upon the initial recruitment of a subset of CD49d expressing neutrophils, which require cysteinyl leukotrienes for their survival. These neutrophils induce expression of the high-affinity receptor for IgE, FcεRI, on lung conventional dendritic cells (DC). At the same time the mouse makes IgE against SeV, and this leads to crosslinking of FcεRI on the DC. This crosslinking of DC FcεRI induces production of CCL28, a chemokine that recruits IL-13 producing lymphocytes to the lung. The IL-13 then drives subsequent development of post-viral airway disease[46]. Interestingly, exposure to a non-viral antigen during the antiviral immune response is sufficient to drive allergic disease against the non-viral antigen. Thus, this model translates a respiratory viral infection into atopy and asthma. Importantly, components of this pathway are present in the human. We demonstrated CD49d expressing neutrophils in the nasal lavage of humans, and expression of the cysteinyl leukotriene receptor on these cells[47]. Human conventional DC express FcεRI, and the level of expression is increased during a respiratory viral infection[48]. Crosslinking DC FcεRI leads to release of CCL28, and humans make IgE against viruses, such as RSV and RV[49–54]. Whether this pathway does indeed translate a respiratory viral infection to asthma in human infants remains to be fully determined.
Atypical bacterial infection and development of asthma
Infections with atypical bacteria also appear to play a role in the induction and exacerbation of asthma in both children and adults. Several studies suggest that atypical respiratory pathogens such as Chlamydophila pneumoniae (CP) and Mycoplasma pneumoniae (MP) and fungi like Aspergillosis may contribute to the pathogenesis of asthma[55–59]. Chronic CP infections are more frequent in asthmatic patients and have been associated with poor asthma control[60, 61]. Von, et al, investigated the relationship between severity of asthma, CP titers, and antibodies specific for CP’s heat shock protein (chsp60), and their association with asthma. Patients (n = 116) were categorized into three groups based on the severity of their asthma (mild, moderate, or severe). Although antibodies against chsp60 were elevated in the asthmatic group compared to the controls, the difference did not reach statistical significance. However, severe and moderate asthma were significantly associated with the presence of elevated anti-chsp60 IgA, suggesting chronic infection in these more severe asthma patients[62]. An additional study reported that IgE against CP strongly and positively associated with asthma severity, suggesting a role of anti-CP IgE (and by extension, CP) in the pathogenesis of asthma[63]. While MP’s role in asthma has been less intensively investigated than CP, MP has been associated with recurrent wheeze and may be present as a co-infection with respiratory viruses. Children with asthma were more likely to have MP-specific IgM than those without asthma (39% vs 0%)[64], suggesting increased exposure/colonization of MP in those with asthma.
In addition to CP and MP, emerging data suggest species of the Streptococcus, Moraxella, and Haemophilus genera also associate with respiratory illnesses and asthma development[65, 66]. Hypopharyngeal colonization with S. pneumoniae, H. influenza, or M. catarrhalis in neonates was reported to increase the risk for recurrent wheeze and asthma early in life[67]. In neonates, colonization with H. influenza or M. catarrhalis (or both) significantly associated with persistent wheeze (hazard ratio, 2.40 (CI: 1.45 to 3.99)), acute severe exacerbation of wheeze (hazard ratio, 2.99 (CI: 1.66 to 5.39)), and hospitalization for wheeze (hazard ratio, 3.85 (CI: 1.90 to 7.79))[67]. In fact, children colonized by these bacteria as neonates had a higher prevalence of asthma by 5 years of age, as well as increased beta-agonist reversibility compared to those children not colonized as neonates with these organisms. Other bacteria such as Helicobacter pylori (H. pylori) and Bordetella pertussis (B. Pertussis) have been associated with asthma, as well. In subjects under 40 years of age, H. pylori infection (as documented by IgG against H. pylori in the peripheral blood) appears to protect against asthma (OR 0.503), but not other allergic diseases[68]. B. Pertussis has not been associated with development of asthma, but may infect asthma patients more frequently. Following a pertussis outbreak in California and Minnesota, Capili and colleagues conducted a population-based, case-control study to determine the prevalence of pertussis and its association with asthma[69]. They reported an increased risk of B. pertussis infection among subjects with asthma (adjusted OR for B. pertussis infection with pre-existing asthma, 1.73; (CI: 1.12 - 2.67); p = 0.013). Interestingly, the majority of this risk was attributable to children (OR 1.92 (1.2 – 3.09); p = 0.007) not adults (OR 1.14 (0.37 – 3.55); p = 0.820). These authors calculated the population attributable risk of asthma for pertussis infection at 17%[69]. Thus, bacterial infections can have implications beyond just the development and/or exacerbation of asthma.
Similar to viral infections, the mechanisms by which atypical bacterial drive inception of asthma are being studied. Early infection with CP or MP leads to a higher risk for asthma through induction of type 2 airway inflammation, mucus cell metaplasia, and airway hyperreactivity -- all hallmarks of asthma. For instance, CP infection was shown to induce a Th2 immune response, as well as both airway eosinophilia and neutrophilia leading to permanent alteration of lung structure and function[70]. Using a mouse model, it was shown that infection with a chlamydia species (C. muridarum) during allergen sensitization (using ovalbumin) led to a neutrophilic inflammatory response in the lung associated with a Th1/Th17 response, but an inhibited Th2 response[71]. This model is reminiscent of the human neutrophilic asthma phenotype. If CP and MP do cause asthma, then treatment with macrolide antibiotics (for which CP and MP are sensitive) should prevent or ameliorate asthma. In fact, treatment with a macrolide antibiotic in vitro, did block CP induced mucin production in cultured human airway cells[72]. A larger clinical study found macrolide treatment reduced the severity of respiratory tract infections, but had no impact on the symptom scores or the use of albuterol[73]. Therefore, it remains unclear how important atypical bacterial infections are to the pathogenesis of asthma.
Conclusion
This review has discussed the association between various respiratory pathogens and the development and exacerbation of asthma. Viral respiratory infections are common causes of acute illnesses in both adults and children, and are strongly associated with inception and exacerbation of asthma. As discussed, several other pathogens, such as atypical bacteria like CP and MP, also have been linked to the onset and exacerbation of asthma. Some microorganisms (like H. pylori) have a protective effect against the development of asthma. Further studies are required to better outline the complex interaction between human hosts and pathogens that lead to (or prevent) asthma development. These studies will require multidisciplinary approaches including epidemiological studies with longitudinal cohorts and mechanistic mouse models. These future investigations will increase our knowledge of the pathological process during infections that lead to the development of asthma, and will enable us to identify new therapeutic targets. These future interventions hopefully will reduce and maybe eliminate development and exacerbations of asthma.
Key points:
Respiratory RNA viral infections have been correlated with the development and exacerbation of asthma (and possibly allergies).
Atypical bacteria and some typical bacteria have also been associated with asthma development.
The mechanisms linking these organisms to development and exacerbation of asthma are areas actively being explored.
Synopsis.
Asthma and allergic diseases have become more prevalent, although the reasons for this increase in disease burden are not known. Understanding why these diseases have become more common requires knowledge of the disease pathogenesis. Multiple studies have identified respiratory viral infections and atypical bacteria as potential etiologic agents underlying the development of asthma (and possibly allergies). This review will discuss the epidemiology and potential mechanistic studies that provide links between these infectious agents and the development (and exacerbation) of asthma. These studies provide insight into the increase in disease prevalence and have identified potential targets for future therapeutic intervention.
Acknowledgments
Disclosures: JR has no disclosures; MHG is the editor of this edition of Immunology and Allergy Clinics, has been on the advisory board for AstraZeneca, has received research funds from the National Institutes of Health and the Research Institute at Nationwide Children’s Hospital, is an Associate Editor for the Annals of Allergy, Asthma, and Immunology, and is on the board of directors of the American Board of Allergy and Immunology, the Asthma and Allergy Foundation of America, and the American Academy of Allergy, Asthma, and Immunology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jenny Resiliac, Division of Allergy and Immunology; 700 Children’s Drive; Columbus, OH 43205; Biomedical Sciences Graduate Program, The Ohio State University College of Medicine; Center for Translational and Clinical Research, The Research Institute at Nationwide Children’s Hospital; Division of Allergy and Immunology, Department of Pediatrics, The Ohio State University College of Medicine.
Mitchell H. Grayson, Biomedical Sciences Graduate Program, The Ohio State University College of Medicine; Center for Translational and Clinical Research, The Research Institute at Nationwide Children’s Hospital; Division of Allergy and Immunology, Department of Pediatrics, The Ohio State University College of Medicine.
References
- 1.National Asthma E, Prevention P: National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics--2002. JAllergy Clin Immunol 2002, 110(5 Suppl):S141–219. [PubMed] [Google Scholar]
- 2.United States Center for Disease Control and Prevention. Most Recent Asthma Data [https://www.cdc.gov/asthma/most_recent_data.htm] Accessed 10/15/18.
- 3.World Health Organization. Asthma [http://www.who.int/news-room/fact-sheets/detail/asthma] Accessed 10/15/18.
- 4.United States Center for Disease Control and Prevention. 2015. National Health Interview Survey (NHIS) Data [https://www.cdc.gov/asthma/nhis/2015/table5-1.htm] Accessed 10/15/18.
- 5.Juhn YJ: Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? J Allergy Clin Immunol 2014, 134(2):247–257; quiz 258-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iikura M, Hojo M, Koketsu R, Watanabe S, Sato A, Chino H, Ro S, Masaki H, Hirashima J, Ishii S et al. : The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS One 2015, 10(4):e0123584.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn DL: Infectious asthma: a reemerging clinical entity? JFam Pract 1995, 41(2):153–157. [PubMed] [Google Scholar]
- 8.Helby J, Nordestgaard BG, Benfield T, Bojesen SE: Asthma, other atopic conditions and risk of infections in 105 519 general population never and ever smokers. J Intern Med 2017, 282(3):254–267. [DOI] [PubMed] [Google Scholar]
- 9.Busse WW, Lemanske RF Jr Gern JE: Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376(9743):826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turunen R, Koistinen A, Vuorinen T, Arku B, Soderlund-Venermo M, Ruuskanen O, Jartti T: The first wheezing episode: respiratory virus etiology, atopic characteristics, and illness severity. Pediatr Allergy Immunol 2014, 25(8):796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD: Tucson Children’s Respiratory Study: 1980 to present. JAllergy Clin Immunol 2003, 111(4):661–675; quiz 676. [DOI] [PubMed] [Google Scholar]
- 12.Piippo-Savolainen E, Korppi M: Long-term outcomes of early childhood wheezing. Curr Opin Allergy Clin Immunol 2009, 9(3): 190–196. [DOI] [PubMed] [Google Scholar]
- 13.Carroll KN, Hartert TV: The impact of respiratory viral infection on wheezing illnesses and asthma exacerbations. Immunol Allergy Clin North Am 2008, 28(3):539–561, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu P, Hartert TV: Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther 2011, 9(9):731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ: Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999, 282(15): 1440–1446. [DOI] [PubMed] [Google Scholar]
- 16.Wos M, Sanak M, Soja J, Olechnowicz H, Busse WW, Szczeklik A: The presence of rhinovirus in lower airways of patients with bronchial asthma. Am JRespir Crit Care Med 2008, 177(10):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF Jr,Shult PA, Gern JE: A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One 2007, 2(10):e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blomqvist S, Roivainen M, Puhakka T, Kleemola M, Hovi T: Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol 2002, 66(2):263–268. [DOI] [PubMed] [Google Scholar]
- 19.Rossi GA, Colin AA: Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J 2015, 45(3):774–789. [DOI] [PubMed] [Google Scholar]
- 20.Kneyber MCJ, Steyerberg EW, de Groot R, Moll HA: Long-term effects of respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a quantitative review. Acta Paediatr 2000, 89(6):654–660. [DOI] [PubMed] [Google Scholar]
- 21.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E et al. : Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008, 178(7):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tregoning JS, Schwarze J: Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010, 23(1):74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, Martin AC, Khoo SK, Cox DW, Geelhoed GC et al. : Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J 2011, 37(5):1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng SY, Wang LL, Ren L, Luo J, Liao W, Liu EM: Epidemiological analysis and follow-up of human rhinovirus infection in children with asthma exacerbation. J Med Virol 2018, 90(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B: Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 1995, 95(4):500–505. [PubMed] [Google Scholar]
- 26.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM: Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010, 65(12):1045–1052. [DOI] [PubMed] [Google Scholar]
- 27.Welliver RC: RSV and chronic asthma. Lancet 1995, 346(8978):789–790. [DOI] [PubMed] [Google Scholar]
- 28.El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP: Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. JInfectDis 2011, 204(7):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa K, Jartti T, Mansbach JM, Laham FR, Jewell AM, Espinola JA, Piedra PA, Camargo CA Jr.: Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis 2015, 211(10):1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemanske RF Jr., Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL et al. : Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005, 116(3): 571–577. [DOI] [PubMed] [Google Scholar]
- 31.Moss RB: Enterovirus 68 Infection-Association with Asthma. J Allergy Clin Immunol Pract 2016, 4(2):226–228. [DOI] [PubMed] [Google Scholar]
- 32.Foster CB, Coelho R, Brown PM, Wadhwa A, Dossul A, Gonzalez BE, Cardenas S, Sabella C, Kohn D, Vogel S et al. : A comparison of hospitalized children with enterovirus D68 to those with rhinovirus. Pediatr Pulmonol 2017, 52(6): 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyer K, Wang H, Salamon D, Leber A, Mejias A: Enterovirus D68 in Hospitalized Children: Sequence Variation, Viral Loads and Clinical Outcomes. PLoS One 2016, 11(n):e0167111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YC, Tsai CS, Yang YH, Huang KY, Hsieh WC, Kuo TY, Chin-Hung Chen V, Wong J, Ponton L, Wang TN: Association Between Enterovirus Infection and Asthma in Children: A 16-year Nationwide Population-based Cohort Study. Pediatr Infect Dis J 2018, 37(9):844–849. [DOI] [PubMed] [Google Scholar]
- 35.Yeh JJ, Lin CL, Hsu WH: Effect of enterovirus infections on asthma in young children: A national cohort study. Eur J Clin Invest 2017, 47(12). [DOI] [PubMed] [Google Scholar]
- 36.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R et al. : Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. NEngl J Med 2009, 361(20):1935–1944. [DOI] [PubMed] [Google Scholar]
- 37.O’Riordan S, Barton M, Yau Y, Read SE, Allen U, Tran D: Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ 2010, 182(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kloepfer KM, Olenec JP, Lee WM, Liu G, Vrtis RF, Roberg KA, Evans MD, Gangnon RE, Lemanske RF Jr., Gern JE: Increased H1N1 infection rate in children with asthma. Am JRespir Crit Care Med 2012, 185(12):1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myles P, Nguyen-Van-Tam JS, Semple MG, Brett SJ, Bannister B, Read RC, Taylor BL, McMenamin J, Enstone JE, Nicholson KG et al. : Differences between asthmatics and nonasthmatics hospitalised with influenza A infection. Eur Respir J 2013, 41(4):824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawood FS, Kamimoto L, D’Mello TA, Reingold A, Gershman K, Meek J, Arnold KE, Farley M, Ryan P, Lynfield R et al. : Children with asthma hospitalized with seasonal or pandemic influenza, 2003-2009. Pediatrics 2011, 128(1):e27–32. [DOI] [PubMed] [Google Scholar]
- 41.Tran D, Vaudry W, Moore DL, Bettinger JA, Halperin SA, Scheifele DW, Aziz S, investigators I: Comparison of children hospitalized with seasonal versus pandemic influenza A, 2004-2009. Pediatrics 2012, 130(3):397–406. [DOI] [PubMed] [Google Scholar]
- 42.McKenna JJ, Bramley AM, Skarbinski J, Fry AM, Finelli L, Jain S, Pandemic Influenza AVHIT: Asthma in patients hospitalized with pandemic influenza A(H1N1)pdm09 virus infection-United States, 2009. BMC Infect Dis 2013, 13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farne HA, Wilson A, Powell C, Bax L, Milan SJ: Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 2017, 9:CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME: Distinct roles for IL-13 and IL-4 via IL-13 receptor alphal and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A 2008, 105(20):7240–7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, Quitalig M, Hotard AL, Moore ML, Hartert TV et al. : Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol 2016, 138(3):814–824 e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheung DS, Ehlenbach SJ, Kitchens RT, Riley DA, Thomas LL, Holtzman MJ, Grayson MH: Cutting edge: CD49d+ neutrophils induce FcepsilonRI expression on lung dendritic cells in a mouse model of postviral asthma. J Immunol 2010, 185(9):4983–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sigua JA, Buelow B, Cheung DS, Buell E, Hunter D, Klancnik M, Grayson MH: CD49d-expressing neutrophils differentiate atopic from nonatopic individuals. J Allergy Clin Immunol 2014, 133(3):901–904 e905. [DOI] [PubMed] [Google Scholar]
- 48.Subrata LS, Bizzintino J, Mamessier E, Bosco A, McKenna KL, Wikstrom ME, Goldblatt J, Sly PD, Hales BJ, Thomas WR et al. : Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol 2009, 183(4):2793–2800. [DOI] [PubMed] [Google Scholar]
- 49.Khan SH, Grayson MH: Cross-linking IgE augments human conventional dendritic cell production of CC chemokine ligand 28. J Allergy Clin Immunol 2010, 125(1):265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam JS, Jackson WT, Hunter D, Proud D, Grayson MH: Rhinovirus specific IgE can be detected in human sera. J Allergy Clin Immunol 2013, 132(5):1241–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tam JS, Grayson MH: IgE and antiviral immune response in asthma. J Allergy Clin Immunol 2017, 139(5):1717. [DOI] [PubMed] [Google Scholar]
- 52.Bui RH, Molinaro GA, Kettering JD, Heiner DC, Imagawa DT, St Geme JW Jr.: Virus-specific IgE and IgG4 antibodies in serum of children infected with respiratory syncytial virus. J Pediatr 1987, 110(1):87–90. [DOI] [PubMed] [Google Scholar]
- 53.Rabatic S, Gagro A, Lokar-Kolbas R, Krsulovic-Hresic V, Vrtar Z, Popow-Kraupp T, Drazenovic V, Mlinaric-Galinovic G: Increase in CD23+ B cells in infants with bronchiolitis is accompanied by appearance of IgE and IgG4 antibodies specific for respiratory syncytial virus. J Infect Dis 1997, 175(1):32–37. [DOI] [PubMed] [Google Scholar]
- 54.Aberle JH, Aberle SW, Dworzak MN, Mandl CW, Rebhandl W, Vollnhofer G, Kundi M, Popow-Kraupp T: Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med 1999, 160(4):1263–1268. [DOI] [PubMed] [Google Scholar]
- 55.Metz G, Kraft M: Effects of atypical infections with Mycoplasma and Chlamydia on asthma. Immunol Allergy Clin North Am 2010, 30(4):575–585, vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juhn YJ, Kita H, Yawn BP, Boyce TG, Yoo KH, McGree ME, Weaver AL, Wollan P, Jacobson RM: Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol 2008, 122(4):719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehtinen P, Jartti T, Virkki R, Vuorinen T, Leinonen M, Peltola V, Ruohola A, Ruuskanen O: Bacterial coinfections in children with viral wheezing. Eur J Clin Microbiol Infect Dis 2006, 25(7):463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webley WC, Salva PS, Andrzejewski C, Cirino F, West CA, Tilahun Y, Stuart ES: The bronchial lavage of pediatric patients with asthma contains infectious Chlamydia. Am J Respir Crit Care Med 2005, 171(10):1083–1088. [DOI] [PubMed] [Google Scholar]
- 59.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM: The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 2006, 27(3):615–626. [DOI] [PubMed] [Google Scholar]
- 60.Specjalski K, Jassem E: Chlamydophila pneumoniae, Mycoplasma pneumoniae infections, and asthma control. Allergy Asthma Proc 2011, 32(2):9–17. [DOI] [PubMed] [Google Scholar]
- 61.Cunningham AF, Johnston SL, Julious SA, Lampe FC, Ward ME: Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J 1998, 11(2):345–349. [DOI] [PubMed] [Google Scholar]
- 62.Von HL, Vasankari T, Liippo K, Wahlstrom E, Puolakkainen M: Chlamydia pneumoniae and severity of asthma. Scand J Infect Dis 2002, 34(1):22–27. [DOI] [PubMed] [Google Scholar]
- 63.Hahn DL, Schure A, Patel K, Childs T, Drizik E, Webley W: Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One 2012, 7(4):e35945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith-Norowitz TA, Silverberg JI, Kusonruksa M, Weaver D, Ginsburg D, Norowitz KB, Durkin HG, Hammerschlag MR, Bluth MH, Kohlhoff SA: Asthmatic children have increased specific anti-Mycoplasma pneumoniae IgM but not IgG or IgE-values independent of history of respiratory tract infection. Pediatr Infect Dis J 2013, 32(6):599–603. [DOI] [PubMed] [Google Scholar]
- 65.Nagayama Y, Tsubaki T, Nakayama S, Sawada K, Taguchi K, Toba T, Kohno Y: Bacterial colonization in respiratory secretions from acute and recurrent wheezing infants and children. Pediatr Allergy Immunol 2007, 18(2): 110–117. [DOI] [PubMed] [Google Scholar]
- 66.Klemets P, Lyytikainen O, Ruutu P, Ollgren J, Kaijalainen T, Leinonen M, Nuorti JP: Risk of invasive pneumococcal infections among working age adults with asthma. Thorax 2010, 65(8):698–702. [DOI] [PubMed] [Google Scholar]
- 67.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV et al. : Childhood asthma after bacterial colonization of the airway in neonates. NEngl J Med 2007, 357(15):1487–1495. [DOI] [PubMed] [Google Scholar]
- 68.Lim JH, Kim N, Lim SH, Kwon JW, Shin CM, Chang YS, Kim JS, Jung HC, Cho SH: Inverse Relationship Between Helicobacter Pylori Infection and Asthma Among Adults Younger than 40 Years: A Cross-Sectional Study. Medicine (Baltimore) 2016, 95(8):e2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Capili CR, Hettinger A, Rigelman-Hedberg N, Fink L, Boyce T, Lahr B, Juhn YJ: Increased risk of pertussis in patients with asthma. JAllergy Clin Immunol 2012, 129(4):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel KK, Webley WC: Evidence of infectious asthma phenotype: Chlamydia-induced allergy and pathogen-specific IgE in a neonatal mouse model. PLoS One 2013, 8(12):e83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horvat JC, Starkey MR, Kim RY, Beagley KW, Preston JA, Gibson PG, Foster PS, Hansbro PM: Chlamydial respiratory infection during allergen sensitization drives neutrophilic allergic airways disease. J Immunol 2010, 184(8):4159–4169. [DOI] [PubMed] [Google Scholar]
- 72.Morinaga Y, Yanagihara K, Miyashita N, Seki M, Izumikawa K, Kakeya H, Yamamoto Y, Mukae H, Yamada Y, Kohno S et al. : Azithromycin, clarithromycin and telithromycin inhibit MUC5AC induction by Chlamydophila pneumoniae in airway epithelial cells. Pulm Pharmacol Ther 2009, 22(6):580–586. [DOI] [PubMed] [Google Scholar]
- 73.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, Jackson DJ, Baxi SN, Benson M, Burnham CD et al. : Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA 2015, 314(19):2034–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]


