Abstract
Objective
Lung transplantation is therapeutic for end-stage lung disease, but survival is limited due to bronchiolitis obliterans syndrome and restrictive chronic lung allograft dysfunction. We sought a common denominator in lung transplant recipients, analyzing risk factors that trigger immune responses that lead to bronchiolitis obliterans syndrome.
Methods
We collected blood from patients who underwent lung transplant at our institution. Exosomes were isolated from the sera of recipients with risk factors for chronic rejection and from stable recipients. Exosomes were analyzed with western blot, using antibodies to lung self-antigens K alpha 1 tubulin and collagen-V, costimulatory molecules (costimulatory molecule 80, costimulatory molecule 86), transcription factors (nuclear factor kappa-light-chain-enhancer of activated B cells, hypoxia-inducible factor 1α, Class II Major Histocompatibility Complex Transactivator), and 20S proteasome.
Results
Of the 90 patients included, we identified 5 with grade 3 primary graft dysfunction, 5 without, 15 with respiratory viral infection, 10 with acute rejection, 10 with donor-specific antibodies (DSA), 5 without DSA, and 10 who were stable for exosome isolation. Recipients with grade 3 primary graft dysfunction, respiratory viral infection, acute rejection, and DSA had exosomes containing self-antigens; exosomes from stable recipients did not. Exosomes from recipients with grade 3 primary graft dysfunction, acute rejection, and DSA also demonstrated costimulatory molecule 80, costimulatory molecule 86, major histocompatibility complex class II, transcription factor, and 20S proteasome.
Conclusions
Transplanted lungs with grade 3 primary graft dysfunction, symptomatic respiratory viral infection, acute rejection, and immune responses induce exosomes that contain self-antigens, costimulatory molecules, major histocompatibility complex class II, transcription factors, and 20S proteasome. Release of circulating exosomes post-transplant from the aforementioned stress-inducing insults augment immunity and may play an important role in the pathogenesis of bronchiolitis obliterans syndrome.
Key Words: lung transplant, circulatory exosomes, rejection, primary graft dysfunction, novel mechanism, acute rejection, DSA
Abbreviations and Acronyms: Abs, antibodies; AEC, airway epithelial cell line; AR, acute rejection; BALF, bronchoalveolar lavage fluid; BOS, bronchiolitis obliterans syndrome; CD80, costimulatory molecule 80; CD86, costimulatory molecule 86; CIITA, Class II Major Histocompatibility Complex Transactivator; CLAD, chronic lung allograft dysfunction; Col-V, collagen V; CR, chronic rejection; DSA, donor-specific antibodies; HIF-1α, hypoxia-inducible factor 1α; HLA, human leukocyte antigen; ICAM, intercellular adhesion molecule; Kα1T, K alpha 1 tubulin; LTx, lung transplantation; LTxRs, lung transplant recipients; MHC, major histocompatibility complex; miRNA, microRNA; NF-κβ, nuclear factor kappa-light-chain-enhancer of activated B cells; PBS, phosphate-buffered saline; PGD, primary graft dysfunction; RVI, respiratory viral infection; SAgs, self-antigens; VCAM, vascular cell adhesion molecule

Induction of exosomes may be a mechanism for development of chronic allograft rejection.
Central Message.
Risk for bronchiolitis obliterans syndrome after lung transplant by primary graft dysfunction, viral infections, and allo-immune responses may be mediated by exosomes with allo and lung antigens.
Perspective.
This provides a mechanism by which primary graft dysfunction, respiratory viral infection, acute rejection, and anti-HLA leads to bronchiolitis obliterans syndrome (BOS) post-lung transplant. All can lead to induction, release of exosomes with donor HLA, and lung antigens resulting in increased immune responses. Monitoring circulating exosomes can be a potential biomarker for patients at risk for BOS.
See Commentary on page 2107.
Human lung transplantation (LTx) is a viable treatment option for patients with end-stage lung diseases such as idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, and cystic fibrosis.1, 2 Short-term survival has improved, but acute rejection (AR) and chronic rejection (CR) in the form of chronic lung allograft dysfunction (CLAD) remain obstacles to long-term allograft function. CLAD currently includes both restrictive airway disease and an obstructive form of pathology called bronchiolitis obliterans syndrome (BOS). Roughly 50% of LTx recipients (LTxRs) develop BOS within 5 years of LTx.3 Both AR and BOS are considered primarily due to immunologic insults to the transplanted lungs. Some have reported a significant correlation between BOS and de novo development of antibodies (Abs) to mismatched donor human leukocyte antigen (HLA) (donor-specific antibodies [DSA]) and Abs to lung-associated self-antigens (SAgs), K alpha 1 tubulin (Kα1T), and collagen V (Col-V) after LTx.4, 5 In addition, primary graft dysfunction (PGD),6 respiratory viral infections (RVIs),7, 8, 9 and number of AR episodes10, 11 are thought to increase the risk for development of BOS.
The mechanisms by which all of the aforementioned risk factors lead to BOS remain largely unclear. We hypothesized that PGD, AR, DSA, and/or BOS can induce and release of exosomes into circulation, which may play a role in inducing rejection (Figure 1 ). In this communication, we will provide evidence for circulating exosomes originating from the transplanted lungs and will describe how these exosomes play a vital role in activating and perpetuating immune responses leading to BOS (Video 1).
Video 1.

Exosomes in lung trasplant outcomes: Circulatory exosomes with lung-associated antigens are present in primary graft dysfunction, before development of donor-specific antibodies, acute rejection, and chronic rejection. Therefore, circulatory exosomes with lung-associated antigens are a novel mechanism of inducing immune responses leading to rejection of graft after lung transplantation. Video available at: https://www.jtcvs.org/article/S0022-5223(19)30343-5/fulltext.
Figure 1.
Induction of exosomes may be a mechanism for development of chronic lung allograft rejection. Primary graft dysfunction (PGD), respiratory viral infection (RVI), donor-specific antibodies (DSA), and antibodies to self-antigens (Anti-SAgs) triggers stress on the endoplasmic reticulum, which releases the exosomes containing lung self-antigens and donor HLA. The persistence of exosomes, DSA, and antibodies to lung self-antigens leads release of more exosomes and activate the immune response, which may lead development of chronic rejection (bronchiolitis obliterans syndrome [BOS]). The transient exosomes do not activate immune responses, leading to rejection.
Exosomes are membrane vesicles (40-100 nm in diameter) produced by endocytic pathways and secreted into body fluids. They contain membrane and cytosolic proteins and molecules essential for exosome biogenesis. Exosomes also have cell-specific components including proteins, messenger RNA, and microRNA (miRNA; Figure 2 ).12 Exosome-specific proteins include CD9, CD63, CD81, and CD82; proteins involved in exosome biogenesis include actin, annexins, tumor susceptibility gene 101, fibronectin-1, and vesicle-associated membrane protein 8.13 Exosomes derived from cancer cells harbor dicer AGO2 and TRBP proteins, which can convert pre-miRNA into mature miRNA, leading to silencing of messenger RNA of target cells and initiate tumors. Hence, it has been proposed that differentially expressed protein and miRNA in cancer-derived exosomes may serve as potential biomarkers.14
Figure 2.
Exosome release and its composition following lung transplantation. Exosomes are microvesicles produced by budding in early endosomes. These microvesicles either get degraded by lysosomes or release from the cells. Exosomes released after lung transplant contains lung self-antigens collagen type V and K alpha 1 tubulin, co-stimulatory molecules (CD80 and 86), exosomes markers (CD9, CD63 and CD81), MHC molecules, adhesion molecules, as well as messenger RNA (mRNA) and microRNA (miRNA). CD80 and 86, Costimulatory molecules 80 and 86; CD9, costimulatory molecule 9; CD63, costimulatory molecule 63; CD81, costimulatory molecule 81; MHC, major histocompatibility complex; miRNA, microRNA; mRNA, messenger RNA.
Few reports describe the characteristics of exosomes found in bronchoalveolar lavage fluid (BALF) and serum samples after LTx.15, 16 Gregson and colleagues15 analyzed exosomal RNA collected from BALF following LTx and demonstrated that exosomes isolated from patients with acute cellular rejection are distinct, with their molecular signatures primarily involving immune activation in comparison with stable LTxRs. Recently, we demonstrated that exosomes isolated from sera and BALF from LTxRs diagnosed with AR or CR contain not only DSA but also lung SAgs Col-V and Kα1T16 and also presented electron microscopic evidence for the expression of lung SAgs on the surface of exosomes. Exosomes isolated from stable LTxRs did not contain lung SAgs.16 These results suggest that exosomes induced during rejection of the transplanted lungs can induce immune responses, thus increasing the risk for development of BOS.
Methods
Patient Selection
In 2017, 90 patients underwent LTx at Norton Thoracic Institute in Phoenix, Ariz. For this preliminary analysis, we identified 5 LTxRs diagnosed with PGD grade 3, 5 LTxRs with PGD grade 0, 15 LTxRs with symptomatic RVIs requiring intervention, 15 LTxRs without RVI and in stable condition, 10 LTxRs with AR, 10 without AR, 5 with de novo DSA, 5 without DSA, 10 diagnosed with BOS, and 10 stable LTxRs. All LTxRs selected for this study had undergone bilateral LTx and had no significant differences in demographics (eg, sex, age, reason for transplant, HLA matching, etc [Table 1 ]). For each group (ie, PGD, RVI, AR, DSA, and BOS) we identified time-matched controls for analysis. This study was approved by the human studies institutional review board of St Joseph's Hospital and Medical Center IRB committee (approval number PHXB-16-0027-10-18; date February 27, 2018).
Table 1.
Clinical and demographics data of lung transplant recipients
| Clinical features | Groups |
|||||
|---|---|---|---|---|---|---|
| Group 1 (n = 45) |
Group 2 (n = 5) |
Group 3 (n = 10) |
Group 4 (n = 5) |
Group 5 (n = 10) |
Group 6 (n = 15) |
|
| Stable/control | DSA patients | BOS patients | PGD3 | Acute rejection | RVI | |
| Age, y | 51 ± 13 | 51 ± 19 | 54 ± 15 | 44 ± 19 | 58 ± 13 | 57 ± 12 |
| Sex | ||||||
| Male | 35 | 3 | 6 | 3 | 6 | 9 |
| Female | 10 | 2 | 4 | 2 | 4 | 6 |
| Race | ||||||
| White | 40 | 5 | 8 | 5 | 9 | 15 |
| American Asian | 5 | 0 | 2 | 0 | 1 | 0 |
| End-stage disease | ||||||
| COPD | 14 | 1 | 3 | 2 | 5 | 5 |
| IPF | 7 | 2 | 3 | 1 | 2 | 3 |
| CF | 6 | 2 | 2 | 0 | 2 | 2 |
| Pulmonary fibrosis | 5 | 0 | 1 | 1 | 1 | 2 |
| ILD | 3 | 0 | 1 | 1 | 0 | 1 |
| others | 10 | 0 | 0 | 0 | 0 | 2 |
| Mean time of sampling from LTx, mo | 28 ± 8 | 16 ± 8 | 32 ± 10 | 38 ± 27 hr | 16 ± 10 | 18 ± 12 |
| Mismatch HLA | ||||||
| A | 1.8 ± 0.5 | 2.1 ± 0.5 | 1.8 ± 0.5 | 1.4 ± 0.6 | 1.8 ± 0.5 | 1.7 ± 0.5 |
| B | 2.2 ± 0.8 | 1.7 ± 0.8 | 1.5 ± 0.5 | 1.3 ± 0.8 | 1.8 ± 0.8 | 1.2 ± 0.8 |
| C | 1.9 ± 0.9 | 1.5 ± 0.4 | 1.4 ± 0.3 | 1.6 ± 0.8 | 1.7 ± 0.5 | 1.5 ± 0.3 |
| PGD | ||||||
| Grade 1 | – | – | – | – | – | – |
| Grade 2 | – | – | – | – | – | – |
| Grade 3 | – | – | – | 5 | – | – |
| Acute rejection, n | ||||||
| A1 | – | – | – | – | 2 | – |
| A2 | – | – | – | – | 8 | – |
| Viral infection, n | ||||||
| RSV | – | – | – | – | – | 6 |
| Rhino virus | – | – | – | – | – | 4 |
| Corona virus | – | – | – | – | – | 4 |
| Parainfluenza | – | – | – | – | – | 1 |
| BOS | ||||||
| Grade 1 | – | – | 2 | – | – | – |
| Grade 2 | – | – | 2 | – | – | – |
| Grade 3 | – | – | 6 | – | – | – |
DSA, Donor-specific antibodies; BOS, bronchiolitis obliterans syndrome; PGD, primary graft dysfunction; RVI, symptomatic respiratory viral infection; COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; CF, cystic fibrosis; ILD, Interstitial lung disease; LTx, lung transplant; HLA, human leukocyte antigen; –, not applicable; RSV, respiratory syncytial virus.
Cell Culture and Treatment
The KCC-266 airway epithelial cell line (AEC) was developed in our laboratory from a lung airway biopsy, immortalized by transfection with the pRSV-Tag plasmid, and cultured in RPMI-1640 medium supplemented with bovine serum albumin (2%), L-glutamine (2 mM), nonessential amino acids (100 μM), HEPES (25 mM), sodium pyruvate (1 mM), penicillin (100 U/mL), and streptomycin (0.1 mg/mL) as previously described.17 The AECs (1 × 106 cells) were cultured for 24 hours in the presence of the anti-HLA (Hi PRA, 1:50 dilution), and Abs to lung SAgs (Col-V and Kα1T) at 20 μg/mL. Cell supernatant was collected from KCC-266 after 24 hours for exosome isolation. Cells without treatment were used as control.
Exosome Isolation
Circulating exosomes from LTxRs were isolated using the Total Exosome Isolation Kit (Invitrogen by Thermo Fisher Scientific, Waltham, Mass) as per manufacturer protocol. To summarize in brief, plasma was centrifuged at 2000g and 10,000g for 20 minutes to remove debris. Plasma was diluted with phosphate-buffered saline (PBS, 0.5 volume) and exosomes precipitation solution (0.2 volume), incubated for 10 minutes, and then centrifuged at 10,000g for 5 minutes at room temperature. The exosome pellet was dissolved in PBS and used for all analysis.
Exosomes from culture media were isolated using ultracentrifugation. In brief; cell supernatant was centrifuged for 30 minutes at 3000g and 10,000g to remove cells and debris. The supernatant was then centrifuged at 100,000g for 120 minutes to isolate exosomes. Exosome pellets were stored at −80°C for experiments.
Enzyme-Linked Immunosorbent Assay for HLA
We performed enzyme-linked immunosorbent assay to detect donor HLA in exosomes as described by Logozzi and colleagues18 with minor modifications. A 96-well plate was coated with anti-CD9 Ab (BioLegend, San Diego, Calif) and incubated overnight at 4°C. The plate was washed with PBS and blocked with 0.5% bovine serum albumin in PBS (blocking buffer) and incubated with exosomes (1 mg/mL) isolated from the plasma of LTxRs overnight at 37°C. Murine mAbs to HLA2 or A3 were added and incubated for 2 hours at room temperature, followed by secondary Ab goat anti-mouse conjugated with horseradish peroxidase. After 3 washes with PBS, a reaction was developed with chemiluminescent reagent (MilliporeSigma, Burlington, Mass) and the reaction was stopped with 0.1 N HCl. Optical density was measured at 450 nm.
Western Blot Analysis
Isolated exosomes were used to analyze the expression of lung-associated SAgs (Col-V and Kα1T), costimulatory molecules (costimulatory molecule 80 [CD80] and costimulatory molecule 86 [CD86]), transcription factor (nuclear factor kappa-light-chain-enhancer of activated B cells [NF-kB], hypoxia-inducible factor 1α [HIF-1α], and Class II Major Histocompatibility Complex Transactivator [CIITA]), major histocompatibility complex (MHC) class II, adhesion molecules (intercellular adhesion molecule [ICAM], vascular cell adhesion molecule [VCAM]), and 20S Proteasome using western blot with specific Abs. Loading control CD9 was used. Protein (10 μg) was resolved and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with blocking buffer. Specific primary Abs and corresponding secondary Abs goat anti-rabbit and mouse conjugated with horseradish peroxidase were used to detect expression of proteins. Enhanced chemiluminescent substrate (MilliporeSigma) was used to develop blots, and imaging was done using Odyssey CLx imaging system (LI-COR Biosciences, Lincoln, Neb). Band intensity was quantified by Image J Software (National Institutes of Health, Bethesda, Md).
To determine lung SAgs Col-V and Kα1T by western blot, rabbit anti-Col-V (Abcam, Inc, Cambridge, United Kingdom) and mouse anti-Kα1T (Santa Cruz Biotechnology, Inc, Santa Cruz, Calif) Abs were used.
MHC class II molecules were detected by western blot using Abs to MHC class II (Abcam, Inc). Costimulatory molecules, CD80 and CD86, were detected using rabbit anti, CD 80 and 86 (Abcam, Inc).
Transcription factors NF-KB, HIF-1α, and CIITA were identified by western blot using Abs specific to NF-kB (Cell Signaling Technology, Inc, Danvers, Mass), HIF-1α, and CIITA (Abcam, Inc). Adhesion molecules ICAM and VCAM were detected by western blot using mouse anti Abs to ICAM and VCAM (BioLegend). 20S proteasome, α3 subunit in the exosomes were detected using anti-mouse to α3 subunit 20s proteasomes (Santa Cruz Biotechnology). ER stress markers (Eif2α and PERK) were analyzed by immunoblot using Abs specific to the Eif2α and PERK.
Statistical Analysis
Statistical analysis was calculated with GraphPad Prism 6 (GraphPad Software Inc, La Jolla, Calif). Statistical data were expressed as mean ± standard deviation. We used t-test 2-tailed nonparametric (Mann–Whitney U test) to detect statistical significance. P values less than .05 were considered statistically significant in each analysis. Image J software was used to quantify optical density of blot. Fold changes are based on experiment versus control of each group.
Results
Severe PGD3 After LTx Induces Exosomes From the Transplanted Organ, Which Are Released Into the Circulation and Contain Lung SAgs Kα1T and Col-V
Exosomes were isolated from the plasma of 5 LTxRs with PGD3 and 5 LTxRs without PGD (ie, PGD0) diagnosed according to the International Society for Heart and Lung Transplantation guidelines. The results, presented in Figure 3 , A, demonstrate exosomes present in the circulation with specific marker CD9, although the quantity is greater in the plasma of LTxRs with PGD3 than in the patients with PGD0. However, only LTxRs diagnosed with PGD3 induced circulating exosomes with lung SAgs Kα1T and Col-V (illustration of 2/5 patient data). Semiquantitative analysis by densitometry, shown in Figure 3, B, demonstrates that exosomes isolated from LTxRs with PGD3 have significantly greater levels of both lung SAgs Col-V (2.29-fold increase, P = .0087) and Kα1T (3.25-fold increase, P = .0087) than patients with PGD0.
Figure 3.
PGD3 exosomes contain Col-V and Kα1T. Exosomes were isolated from the plasma of LTxRs with PGD3 (n = 5) and LTxRs without PGD (PGD0, n = 5). Exosomes were subjected to immunoblot. A, Western blot shows that exosomes from LTxRs with PGD3 contain lung SAgs Col-V and Kα1T but not with PGD0. B, Densitometry analysis showed significant increases in Col-V (P = .0087) and Kα1T (P = .0087) levels in PGD3 patients. CD9 was used as loading control marker. Data represented as box-whisker graph (Col-V: PGD3: median 1.028-fold change, interquartile range 0.954- to 2.111-fold change, PGD0: median 0.683-fold change, interquartile range 0.209- to 0.953-fold change; Kα1T: PGD3: median 1.507-fold change, interquartile range 0.543- to 2.497-fold change; PGD0: median 0.453-fold change, interquartile range 0.322- to 0.716-fold change). The experiment was done twice. PGD, Primary graft dysfunction; Col-V, collagen V; Kα1T, K alpha 1 tubulin; CD9, costimulatory molecule 9.
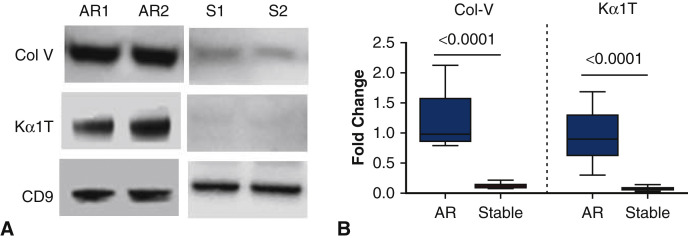
LTxRs Diagnosed With AR Induce Exosomes With Lung SAgs That Are Detectable in the Circulation
To demonstrate that AR may also induce exosomes from the transplanted organ, we isolated exosomes from 10 LTxRs diagnosed with AR and time-matched 10 stable LTxRs. Exosomes with specific marker CD9 were present in similar quantities both in LTxRs with rejection (representative 2/10 patients’ data, Figure 4 , A) and in those without rejection (representative of 2/10 stable patients), only the LTxRs diagnosed with AR demonstrated the presence of lung SAgs Col-V and Kα1T (Figure 4, A). Figure 4, B, represents the densitometry results of 10 LTxRs diagnosed with rejection and 10 stable LTxRs; the exosomes isolated from LTxRs with AR had significantly greater levels of both lung SAgs Col-V (9.46-fold increase, P < .0001) and Kα1T (12.6-fold increase, P < .0001) than those isolated from stable LTxRs.
Figure 4.
Exosomes from LTxRs with AR show lung SAgs Col-V and Kα1T. Exosomes were isolated from plasma of LTxRs with AR (n = 10) and from stable LTxRs (n = 10) and were subjected to immunoblot. A, Western blot demonstrates that exosomes from LTxRs with AR contain lung SAgs Col-V and Kα1T, but these SAgs were not present in stable LTxRs. B, Densitometry analysis shows significant increase of Col-V (P ≤ .0001) and Kα1T (P ≤ .0001) in exosomes isolated from LTxRs with AR. CD9 was used as loading control marker. Box-whisker graph signified Col-V: AR median: 0.979-fold change (interquartile range, 0.797- to 2.125-fold change), median of stable, 0.114-fold change (interquartile range 0.078- to 0.221-fold change); Kα1T: AR: median 0.900-fold change (interquartile range 0.302- to 1.691-fold change) S: median 0.075-fold change (interquartile range 0.028- to 0.138-fold change). The experiment was performed in duplicate. AR, Acute rejection; S, stable; Col-V, collagen V; Kα1T, K alpha 1 tubulin; CD9, costimulatory molecule 9.
Exosomes Isolated From LTxRs With Viral Infection Contain SAgs
To determine whether symptomatic RVI can also lead to exosome induction from the transplanted organ, we analyzed 15 patients diagnosed with symptomatic RVI and 15 stable patients with no clinical signs of RVI. As shown previously, both groups had similar levels of exosomes containing specific marker CD9; however, only those with symptomatic RVI demonstrated exosomes containing lung SAgs Col-V and Kα1T (Figure 5 , A). Figure 5, B, presents results of semiquantitation using densitometry of all patients (15 with symptomatic RVI and 15 stable without RVI); levels of exosomes with lung SAgs were significantly increased in patients with symptomatic RVI compared with stable patients (9.92-fold increase in lung SAg Col-V, P = .0079; 4-fold increase in Kα1T, P = .0079).
Figure 5.
Presence of lung SAgs in exosomes isolated from LTxRs with RVI. Exosomes were isolated from plasma of LTxRs diagnosed with RVI (n = 15) and from stable LTxRs (n = 15) and were subjected to immunoblot. A, Western blot demonstrates that exosomes from LTxRs with viral, symptomatic RVI have lung SAgs Col-V and Kα1T; exosomes isolated from stable LTxRs do not. B, Densitometry analysis showed significant increase in Col-V (P = .0079) and Kα1T (P = .0079) levels in LTxRs with RVI. CD9 was used as loading control marker. Box-whisker plot represents median value for each group Col V: RVI: median 1.364-fold change (interquartile range, 0.787- to 1.560-fold change): S: median 0.135-fold change (interquartile range, 0.076- to 0.169-fold); Kα1T: RVI: median 0.536-fold change (interquartile range, 0.337- to 0.838-fold): Stable median, 0.124-fold change (interquartile range, 0.094- to 0.200-fold). The experiment was done twice. RVI, Symptomatic respiratory viral infection; S, stable; Col-V, collagen V; Kα1T, K alpha 1 tubulin; CD9, costimulatory molecule 9.
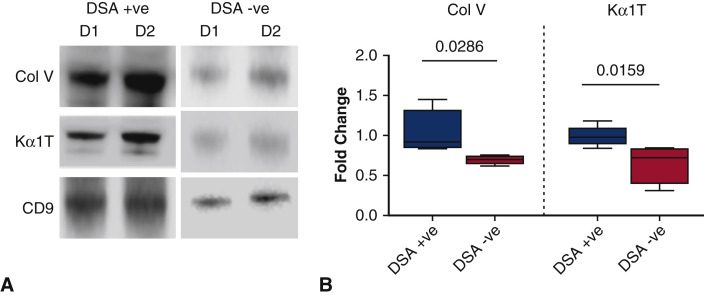
De Novo DSA Development Releases Exosomes With Lung SAgs
De novo development of Abs to DSA is generally thought to increase the risk for development of both AR and CR following LTx. To determine whether early development of DSA following LTx can induce circulating exosomes, we selected 5 LTxRs who developed DSA within 3 months of transplant and 5 LTxRs without DSA matched for duration following transplant. As shown in Figure 6 , A, exosomes isolated from LTxRs who developed de novo DSA contained greater levels of lung SAgs Col-V and Kα1T than LTxRs without demonstrable DSA. Semiquantitation by densitometry of 5 LTxRs with DSA and 5 without DSA (Figure 6, B) demonstrated significantly greater levels of exosomes containing lung SAgs in LTxRs with DSA than in those without (1.47-fold increase of lung SAg Col-V, P = .0286; 1.56-fold increase in Kα1T, P = .0159).
Figure 6.
DSA + LTxRs release lung SAgs containing exosomes. Exosomes were isolated from the plasma of LTxRs diagnosed with DSA (n = 5) and from stable LTxRs (n = 5) and were subjected to immunoblot. A, Western blot demonstrates that DSA + LTxRs release exosomes with lung SAgs Col-V and Kα1T, but stable LTxRs do not. B, Densitometry analysis shows significant increase in Col-V (P = .0286) and Kα1T (P = .0159). CD9 was used as loading control marker. Data indicated as box-whisker graph (Col-V: DSA+: median 0.922-fold change, interquartile range 0.832- to 1.454-fold; DSA–: median 0.709-fold change, interquartile range 0.625- to 0.767-fold. Kα1T: DSA+: median 1.005-fold change, interquartile range 0.844- to 1.185-fold; DSA–: median 0.779-fold change, interquartile range 0.313- to 0.849-fold). The experiment was repeated 2 times. DSA+, Donor HLA–specific antibodies positive; DSA−, donor HLA–specific antibodies negative; D, donor-specific antibodies; Col-V, collagen V; Kα1T, K alpha 1 tubulin; CD9, costimulatory molecule 9.
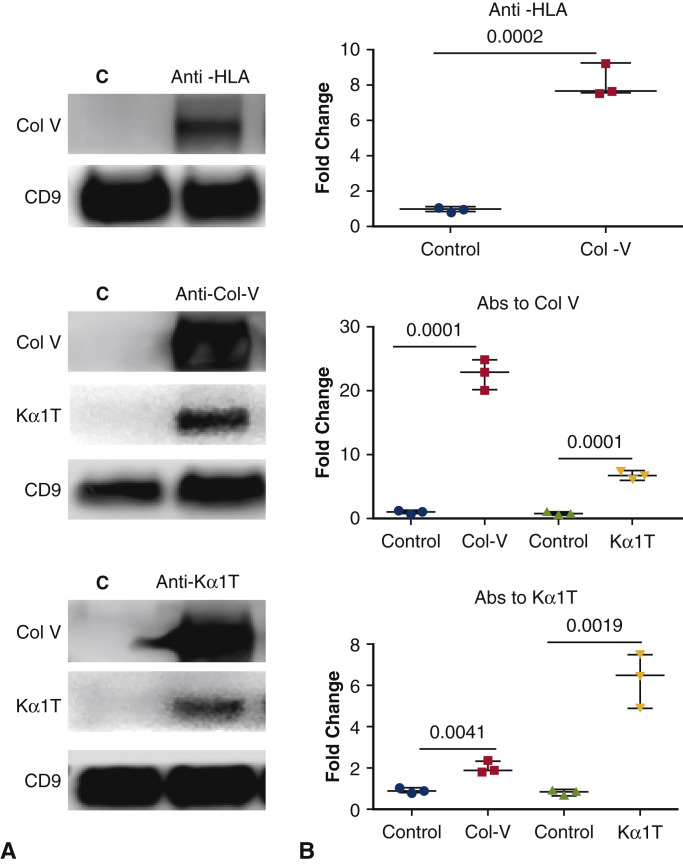
To determine whether binding of Abs to HLA or lung SAgs can induce exosomes in vitro, we incubated AECs with anti-HLA or anti-lung SAgs. Exosomes were isolated from the supernatant after overnight incubation with Abs. The exosomes contained lung SAgs, demonstrating that ligation by specific Abs can result in the induction and release of exosomes (Figure 7 ). Control cells without addition of antibodies did not induce exosomes containing lung self-antigens into the supernatants.
Figure 7.
Anti–HLA (HiPRA) or Abs to SAgs Col-V and Kα1T induce exosomes with lung SAgs. Exosome were isolated from AECs treated with anti–HLA or Abs to SAgs and protein was isolated and subjected to western blot. A, Western blot data show that treatment with anti-HLA and Abs to SAgs induced release of exosomes containing SAgs Col-V and Kα1T. CD9 was used as loading control. B, Densitometry analysis shows significant increase in Col-V expression after treatment with HLA antibodies and antibodies to SAgs. The experiment was done twice. HLA, Human leukocyte antigen; Col-V, collagen V; CD9, costimulatory molecule 9; Kα1T, K alpha 1 tubulin; Abs, antibodies.
Release of Lung SAgs Containing Exosomes in LTxRs Diagnosed With BOS
To determine whether LTxRs diagnosed with BOS also induce circulatory exosomes with lung Sags, we identified 10 LTxRs diagnosed with BOS as per International Society for Heart and Lung Transplantation guidelines and 10 stable, time-matched LTxRs. Circulating exosomes were isolated from the plasma at or around the time of diagnosis. Purified exosomes were subjected to western blot for presence of lung SAgs. As shown in Figure 8 , A, exosomes isolated from LTxRs diagnosed with BOS contained lung SAgs. Densitometry analysis demonstrated significantly greater levels of exosomes with lung SAgs Col-V and Kα1T in the exosomes isolated from LTxRs with BOS than in those isolated from stable LTxRs (2.08-fold change in the levels of Col-V, P = .0043; 2.8-fold change in Kα1T, P = .0173; Figure 8, B).
Figure 8.
Exosomes isolated from LTxRs with bronchiolitis obliterans syndrome contain lung SAgs Col-V and Kα1T. Exosomes were isolated from plasma of LTxRs with bronchiolitis obliterans syndrome (n = 10) and from stable LTxRs (n = 10) and were subjected to immunoblot. A, Western blot demonstrates that exosomes from LTxRs with bronchiolitis obliterans syndrome contain lung SAgs Col-V and Kα1T, but exosomes from stable LTxRs do not. B, Densitometry analysis shows significant increase in Col-V (P = .043) and Kα1T (P = .0173) in exosomes from LTxRs with bronchiolitis obliterans syndrome but not in exosomes from stable LTxRs. CD9 was used as loading control marker. Box-whisker graph signified median of each group with lower to higher range: Col-V: bronchiolitis obliterans syndrome: median 1.511-fold change (interquartile range 0.962- to 2.111-fold change), stable median, 0.765-fold change (interquartile range, 0.628- to 0.953-fold change). Kα1T: bronchiolitis obliterans syndrome: median 1.507-fold change (interquartile range 0.543- to 2.497-fold change), stable median, 0.557-fold change (interquartile range 0.429- to 0.716-fold change). The experiment was carried out in duplicate. B, Bronchiolitis obliterans syndrome; S, stable; Col-V, collagen V; Kα1T, K alpha 1 tubulin; CD9, costimulatory molecule 9; BOS, bronchiolitis obliterans syndrome.
Exosomes Isolated From LTxRs With PGD, Symptomatic RVI, AR, DSA, and BOS Contain Not Only Allo- and Lung SAgs but Also Contain Immunoregulatory Molecules, Proinflammatory Transcription Factors, Proteasome, and Stress Markers
Circulating exosomes isolated from LTxRs with PGD3, PGD0, AR, DSA+, DSA−, BOS, and from stable LTxRs were analyzed by western blot using specific Abs to MHC class II molecule; co-stimulatory molecules CD80 and CD86; transcription factors NF-κB, CIITA, and HIFα; 20S proteasome; and stress markers eIF2alpha and PERK. The results, which are presented in Table 1, summarize our findings: namely, that exosomes from all of the aforementioned post-LTx clinical conditions contain not only allo- and lung SAgs, but also important immunoregulatory molecules and stress markers. It is of interest that costimulatory molecules CD80 and CD86 were notably absent in the exosomes isolated from patients with symptomatic RVI. Overall, data presented in Table 2 suggest that exosomes present in the circulation have many of the molecules necessary to augment immunogenicity, which can lead to CLAD.
Table 2.
Characteristics of exosomes isolated from PGD3 symptomatic respiratory viral infection, acute rejection, BOS, and DSA lung transplant recipients
| Costimulatory molecules |
Transcription factor |
Adhesion molecules |
20S proteasome |
ER stress markers |
Donor HLA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHC II | CD80 | CD86 | CIITA | NF-κB | HIF-1α | ICAM | VCAM | α-subunit | eIF2α | PERK | ||
| PGD-3 | + | + | + | + | + | + | + | + | + | ND | ND | + |
| Symptomatic respiratory viral infection | + | – | – | + | + | + | + | + | + | ND | ND | + |
| Acute rejection | + | + | + | + | ND | ND | + | + | + | + | + | + |
| BOS | + | + | + | + | + | + | + | + | + | + | + | + |
| DSA | + | ND | ND | + | + | ND | ND | ND | + | ND | ND | + |
ER, Endoplasmic reticulum; HLA, human leukocyte antigen; MHC-II, major histocompatibility complex–class II molecule; CD80, costimulatory molecule 80; CD86, costimulatory molecule 86; CIITA, Class II Major Histocompatibility Complex Transactivator; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; HIF-1α, hypoxia-inducible factor 1-alpha; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; eIF-2α, eukaryotic Initiation Factor 2 alpha; PERK, protein kinase R–like endoplasmic reticulum kinase; PGD, Primary graft dysfunction; +, present; ND, not done; –, absent; BOS, bronchiolitis obliterans syndrome; DSA, donor-specific antibodies.
Discussion
Risk factors associated with CLAD include PGD, RVI, AR, and de novo development of Abs to DSA. Our goal was to define a common denominator in LTxRs diagnosed with PGD, RVI, AR, or DSA that triggers immune responses that can lead to BOS. In this communication, we present a unifying, biologically plausible mechanistic link between PGD, RVI, and alloimmune responses that can result in CLAD (ie, induction and release of circulating exosomes with lung SAgs, allo-antigens, and immune-stimulatory molecules).
Gregson and colleagues15 analyzed exosomal RNA from BALF following LTx and demonstrated that exosomes isolated from patients with acute cellular rejection are distinct, with their molecular signatures primarily involving immune activation in comparison with stable LTxRs. BALF cell pellet and exosome transcriptome in patients with AR and CR after LTx have demonstrated molecular signatures primarily involving innate and adaptive cytotoxic immune responses.19 Recently, we demonstrated that exosomes isolated from sera and BALF of LTxRs diagnosed with AR or CR contains not only DSA but also the lung SAgs Col-V and Kα1T. These results suggest that exosomes induced during rejection of the transplanted lungs can induce immune responses, thereby increasing the risk of development of BOS.16 Heart and skin transplant models in mice have shown that donor-derived exosomes containing donor MHC can cross-dress recipient antigen-presenting cells, leading to activation and proliferation of alloreactive T cells via the semi-direct pathway of allorecognition.20, 21
Previous studies from our laboratory have shown that Abs to HLA and/or tissue-restricted SAgs develop months before lung allograft rejection, suggesting a pathogenic role for Abs in the development of BOS following LTx.20 Furthermore, in most patients who develop either Abs to HLA or Abs to lung SAgs have a greater chance of developing Abs to each other, suggesting spreading of immune responses resulting in CLAD. However, the mechanism leading to the spreading was unclear, as HLA molecules and lung SAgs are encoded by different genes in different chromosomes. The results presented here and studies in murine models strongly support an important role for exosomes in inducing and spreading of immune responses leading to CLAD.22, 23
In this study, we demonstrated that increased expression of Col-V and Kα1T in circulating exosomes isolated from LTxRs developed PGD, RVI, AR, DSA, and BOS. The exosomes also contained MHC class II molecules, its transcription factor CIITA, and proinflammatory transcription factors such as NF-κβ and HIFα and 20S proteosome. Furthermore, exosomes from LTxRs with PGD3, AR, and BOS contained co-stimulatory molecules (CD80, 86), supporting the theory that the exosomes can be highly immunogenic. An important role for 20S proteasome in augmenting immune responses by exosomes was recently presented by Dieude and colleagues.24 They showed that humoral immune responses in mice to the kidney SAg perlecan are induced by the presence of 20s proteasomes within the exosomes, and removal of the activity of proteasome abrogated the immune responses to perlecan.24 We demonstrated that mice immunized with exosomes isolated from LTxRs diagnosed with BOS induced development of both humoral and cellular immune responses to Col-V and Kα1T, but not in stable patients.16 In addition, distinct differences were noted in exosomes between BOS and stable LTxRs with respect to their immunogenicity.22
Using a syngeneic cardiac transplant model, Sharma and colleagues23 demonstrated that Abs to cardiac myosin administered immediately following syngeneic murine heterotopic cardiac transplant results in graft failure, and the released exosomes contain cardiac myosin and vimentin. Further, this study demonstrated an important role for exosomes in graft failure, as immunization with exosomes isolated from sera following graft failure also developed Abs to cardiac myosin and vimentin and resulted in graft loss 8 days after syngeneic cardiac transplant.23 Following human renal transplantation, there is evidence for release of increased levels of circulating C4d+ plasma endothelial microvesicles that correlated with antibody-mediated AR.25 This indicates that Abs developed de novo can induce exosomes and may participate in antibody-mediated rejection.25
One of the major limitations of this study is relatively small number of patients analyzed for each clinical condition and not analyzing various sub categories. Future studies are needed to with exosomes isolated from different grades of PGD, AR, as well as BOS.
In conclusion, our results demonstrated that circulating exosomes are induced and can be easily isolated from LTxRs diagnosed with PGD, RVI, AR, DSA, and BOS. These circulating exosomes are distinct in their properties compared with stable LTxRs with respect to the presence of tissue-associated lung SAgs Col-V and Kα1T, co-stimulatory molecules, MHC class II, its transcription factor CIITA, proinflammatory transcription factors including NF-κB, HIFα, and 20S proteasome. We propose that the induction of circulating exosomes is an important unifying mechanistic link between PGD, RVI, and alloimmune responses that increase the risk for the development of CLAD following LTx.
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
Acknowledgments
We thank Clare Sonntag and Billie Glasscock for their assistance in submitting this paper.
Footnotes
This work was supported by grants from the National Institutes of HealthR21AI123034, HL092514, and HL056643 (to T.M.).
Supplementary Data
Exosomes in lung trasplant outcomes: Circulatory exosomes with lung-associated antigens are present in primary graft dysfunction, before development of donor-specific antibodies, acute rejection, and chronic rejection. Therefore, circulatory exosomes with lung-associated antigens are a novel mechanism of inducing immune responses leading to rejection of graft after lung transplantation. Video available at: https://www.jtcvs.org/article/S0022-5223(19)30343-5/fulltext.
References
- 1.Arcasoy S.M., Kotloff R.M. Lung transplantation. N Eng J Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 2.Trulock E.P., Edwards L.B., Taylor D.O., Boucek M.M., Keck B.M., Hertz M.I. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart-lung transplantation report—2006. J Heart Lung Transplant. 2006;25:880–892. doi: 10.1016/j.healun.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Estenne M., Hertz M.I. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2003;166:440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 4.Jaramillo A., Smith M.A., Phelan D., Sundaresan S., Trulock E.P., Lynch J.P. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67:1155–1161. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 5.Hachem R.R., Yusen R.D., Meyers B.F., Aloush A.A., Mohanakumar T., Patterson G.A. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973–980. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharat A., Saini D., Steward N., Hachem R., Trulock E.P., Patterson G.A. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharat A., Kuo E., Saini D., Steward N., Hachem R., Trulock E.P. Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg. 2010;90:1637–1644. doi: 10.1016/j.athoracsur.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almaghrabi R.S., Omrani A.S., Memish Z.A. Cytomegalovirus infection in lung transplant recipients. Exp Rev Respir Med. 2017;11:377–383. doi: 10.1080/17476348.2017.1317596. [DOI] [PubMed] [Google Scholar]
- 9.Fisher C.E., Mohanakumar T., Limaye A.P. Respiratory virus infections and chronic lung allograft dysfunction: assessment of virology determinants. J Heart Lung Transplant. 2016;35:946–947. doi: 10.1016/j.healun.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar P.R., Carpenter D., Ritter J., Yusen R.D., Witt C.A., Byers D.E. The role of C4d deposition in the diagnosis of antibody-mediated rejection after lung transplantation. Am J Transplant. 2018;18:936–944. doi: 10.1111/ajt.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z., Yang W., Steward N., Sweet S.C., Danziger-Isakov L., Heeger P.S. Role of circulating microRNAs in the immunopathogenesis of rejection after pediatric lung transplantation. Transplantation. 2017;101:2461–2468. doi: 10.1097/TP.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson R.J., Jensen S.S., Lim J.W.E. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 13.Raimondo F., Morosi L., Chinello C., Magni F., Pitto M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics. 2011;11:709–720. doi: 10.1002/pmic.201000422. [DOI] [PubMed] [Google Scholar]
- 14.Melo S.A., Sugimoto H., O'Connell J.T., Kato N., Villanueva A., Vidal A. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregson A.L., Hoji A., Injean P., Poynter S.T., Briones C., Palchevskiy V. Altered exosomal RNA profiles in bronchoalveolar lavage from lung transplants with acute rejection. Am J Respir Crit Care Med. 2015;192:1490–1503. doi: 10.1164/rccm.201503-0558OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunasekaran M., Xu Z., Nayak D.K., Sharma M., Hachem R., Walia R. Donor-derived exosomes with lung self-antigens in human lung allograft rejection. Am J Transplant. 2017;17:474–484. doi: 10.1111/ajt.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaramillo A., Smith C.R., Maruyama T., Zhang L., Patterson G.A., Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64:521–529. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 18.Logozzi M., Angelini D.F., Iessi E., Mizzoni D., DiRaimo R., Federic C. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017;403:318–329. doi: 10.1016/j.canlet.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 19.Weigt S.S., Wang X., Palchevsky V., Gregson A.L., Patel N., DerHovanessian A. Gene expression profiling of bronchoalveolar lavage cells preceding a clinical diagnosis of chronic lung allograft dysfunction. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169894. e0169894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morelli A.E., Bracamonte-Baran W., Burlingham W.J. Donor-derived exosomes: the trick behind the semidirect pathway of allorecognition. Curr Opin Organ Transplant. 2017;22:46–54. doi: 10.1097/MOT.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q., Rojas-Canales D.M., Divito S.J., Shufesky W.J., Stolz D.B., Erdos G. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest. 2016;126:2805–2820. doi: 10.1172/JCI84577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunasekaran M., Sharma M., Hachem R., Bremner R., Smith M.A., Mohanakumar T. Circulating exosomes with distinct properties during chronic lung allograft rejection. J Immunol. 2018;200:2535–2541. doi: 10.4049/jimmunol.1701587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma M., Liu W., Perincheri S., Gunasekaran M., Mohanakumar T. Exosomes expressing the self-antigens myosin and vimentin play an important role in syngeneic cardiac transplant rejection induced by antibodies to cardiac myosin. Am J Transplant. 2018;18:1626–1635. doi: 10.1111/ajt.14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieude M., Bell C., Turgeon J., Beillevaire D., Pomerleau L., Yang B. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerated rejection. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aac9816. 318ra200. [DOI] [PubMed] [Google Scholar]
- 25.Tower C.M., Reyes M., Nelson K., Leca N., Kieran N., Muczynski K. Plasma C4d+ endothelial microvesicles increase in acute antibody-mediated rejection. Transplantation. 2017;101:2235–2243. doi: 10.1097/TP.0000000000001572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exosomes in lung trasplant outcomes: Circulatory exosomes with lung-associated antigens are present in primary graft dysfunction, before development of donor-specific antibodies, acute rejection, and chronic rejection. Therefore, circulatory exosomes with lung-associated antigens are a novel mechanism of inducing immune responses leading to rejection of graft after lung transplantation. Video available at: https://www.jtcvs.org/article/S0022-5223(19)30343-5/fulltext.