Abstract
Mechanical forces drive the remodeling of tissues during morphogenesis. This relies on the transmission of forces between cells by cadherin-based adherens junctions, which couple the force-generating actomyosin cytoskeletons of neighboring cells. Moreover, components of cadherin adhesions adopt force-dependent conformations that induce changes in the composition of adherens junctions, enabling transduction of mechanical forces into an intracellular response. Cadherin mechanotransduction can mediate reinforcement of cell–cell adhesions to withstand forces but also induce biochemical signaling to regulate cell behavior or direct remodeling of cell–cell adhesions to enable cell rearrangements. By transmission and transduction of mechanical forces, cadherin adhesions coordinate cellular behaviors underlying morphogenetic processes of collective cell migration, cell division, and cell intercalation. Here, we review recent advances in our understanding of this central role of cadherin adhesions in force-dependent regulation of morphogenesis.
Keywords: E-cadherin, adherens junction, mechanical force, mechanotransduction, collective migration, spindle orientation, intercalation
Introduction
Morphogenesis comprises the collection of processes that shape tissues and organisms, which relies on the coordinated regulation of cell behavior. Though most apparent throughout embryonic development, morphogenetic processes continue to play a role in adult tissues (for instance, during tissue regeneration upon wounding as well as pathological conditions such as cancer invasion). Morphogenesis inherently is a mechanical process, as the coordinated generation of forces by cells is required to establish tissue shape 1, 2. In addition, forces exerted on cells by each other and their surroundings serve as instructive signals to which cells adapt their behavior. This depends on the ability of cells to sense and convert mechanical forces into a biochemical intracellular response (termed mechanotransduction), which is mediated by force-induced changes in the conformation of proteins and the composition of molecular complexes 3, 4. Mechanical forces typically originate from contraction of the actomyosin cytoskeleton, which is anchored to sites of adhesion and can thereby be transmitted to neighboring cells and the extracellular environment. As such, focal adhesions (at the cell–matrix interface) and adherens junctions (AJs) (at the cell–cell interface) represent key force transmission and transduction complexes of the cell. In recent years, many additional components of the cell cortex, including growth factor receptors, ion channels, caveolae, and the glycocalyx, have been identified to be responsive to forces 5. In this review, we will focus on AJs, as they have emerged as central mechano-responsive components in the regulation of morphogenesis by intercellular forces.
Mechanotransduction by adherens junctions
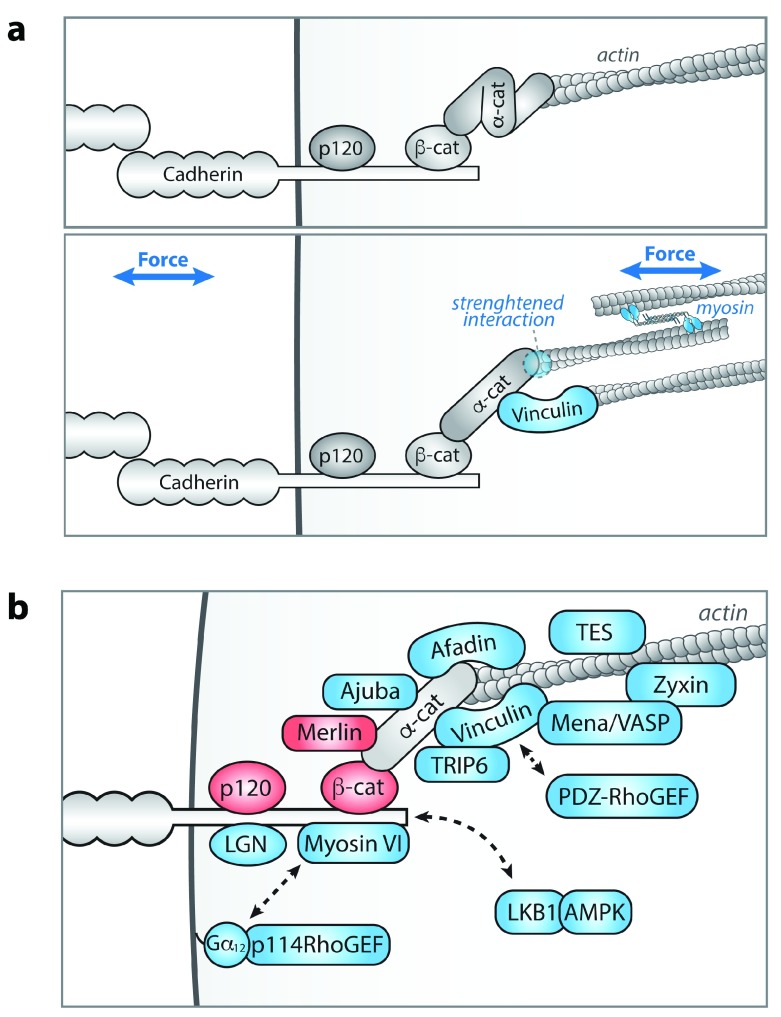
The AJ is composed of transmembrane cadherin proteins (E-cadherin being the predominant cadherin in most epithelial tissues) that form homotypic dimers between neighboring cells . The cytosolic tail of cadherins compiles a large protein complex, which dynamically connects to the actomyosin cytoskeleton 6. The core cadherin complex consists of p120-catenin, which controls cadherin membrane localization, and β-catenin and α-catenin, which provide a link with filamentous actin ( Figure 1a). Importantly, β-catenin–bound α-catenin forms a catch-bond interaction with F-actin that is strengthened when the complex experiences tensile force ( Figure 1a) 7, which is explained by the force-dependent exposure of a cryptic actin-binding site within α-catenin 8. In addition to enhanced actin binding of α-catenin, other force-induced changes occur in the cadherin complex that regulate the organization of junctional actin, thereby reinforcing cadherin junctions. Most well studied is the exposure of a binding site within α-catenin for Vinculin ( Figure 1a), which provides additional linkage of the cadherin complex with actin 9– 12 and recruits several actin-modulating proteins 13, 14. Intriguingly, Vinculin was recently shown to form a force-stabilized linkage to F-actin that is directionally asymmetric 15, suggesting that Vinculin may further contribute to junctional actin remodeling by organizing the polarity of actin filaments. In addition to Vinculin, numerous other actin assembly and remodeling proteins are recruited when the cadherin complex is under tension upon application of forces from neighboring cells or the actin cytoskeleton ( Figure 1b). These bind either directly to α-catenin (Afadin 16) or indirectly through other junctional components or the actin cytoskeleton (RhoGEF114 17, VASP 18, TES 18, and Zyxin 18). This large number of identified actin-associated proteins recruited to tensed cadherin junctions confers several layers of mechanical control of the AJ–actin link, potentially explaining the mild phenotype of selectively losing Vinculin in various tissues 19– 21.
Figure 1. Mechanotransduction by the cadherin complex.
( a) Tensile forces on the cadherin complex, exerted either by neighboring cells through its extracellular domain or by myosin-generation contraction of the actin cytoskeleton associated with its cytosolic tail, cause unfolding of α-catenin, enabling its interaction with Vinculin. The interaction between α-catenin and F-actin is regulated by force as well, as β-catenin–bound α-catenin forms a catch-bond interaction with F-actin that shifts from a weakly bound to strongly bound state with increasing tension. Mechanical force can also influence transdimer interactions formed by the extracellular domain of E-cadherin, as X-dimer configurations of the transdimer form a catch-bond interaction whose lifetime increases with force (not shown) 37. ( b) Numerous proteins that are recruited to cadherin adhesions in a tension-dependent manner have been identified and show either increased (blue) or decreased (red) association with the cadherin complex upon increased tensile force. Dashed lines indicate tension-dependent adherens junction (AJ) components of which the molecular mechanism underlying association with the cadherin complex has not yet been resolved. α-cat, α-catenin; β-cat, β-catenin; p120, p120-catenin.
By mechanically coupling the actomyosin network of neighboring cells, cadherin adhesions are essential for the transmission of forces between individual cells. As a result, cell within tissues develop collective contractility that allows them to coordinate morphogenetic cell rearrangements in tissues 2, 22, 23. Moreover, the different mechanisms to transduce force into regulation of the organization of the actin cytoskeleton and its association with the cadherin complex strengthen cell–cell adhesions, enabling them to maintain integrity of tissues upon fluctuations in intercellular stresses. However, the cellular responses to junctional force reach much further, impacting, for instance, the cell cycle and cell division 24, 25, cell migration 26, 27, and cell metabolism 28. This is mediated by force-regulated association of the cadherin complex with transcriptional activators (for example, β-catenin and YAP 24, 29), kinases (for example, LKB1/AMPK 28), and interactors of microtubules and intermediate filaments ( Figure 1b) 25, 26. E-cadherin thus should be envisioned not as a static mediator of cell–cell adhesion but rather as a dynamic sensor of tensile forces that instructs cellular behavior. In this way, E-cadherin fulfills a central role in transducing cellular forces to coordinate morphogenesis, as it instructs several processes that direct tissue shape, including collective migration, tissue growth, and cell–cell intercalation. Below, we will discuss recent advances in our understanding of the role of E-cadherin in the force-dependent regulation of these morphogenetic processes.
Cadherin mechanotransduction in collective migration
Tissue rearrangement during morphogenesis can be achieved by collective migration of groups or sheets of cells, which move while retaining adhesion between individual cells. These adhesions enable cell-to-cell communication to coordinate the direction of motion. As a result, cell clusters migrate more efficiently and persistently than isolated cells 30. Moreover, migrating as a collective enables epithelial clusters to follow specific guidance cues while single cells or monolayers lacking AJs fail to do so 27, 31– 33. It has been well established that cadherin-based adhesions enable collective migration by coupling cells to one another and by transmitting forces between cells to convey directional information (reviewed in 30, 34, 35). In migrating sheets, cells at the edge generate lamellipodia that protrude into the surrounding environment. These lamellipodia adhere to the surface and generate traction forces required for forward pulling of the cells 30, 35. Importantly, the pulling forces are exerted not only by these cells but by all cells in the cell sheet, which is coordinated via intercellular force transmission 36. Cadherin junctions are the main force transducer between cells responsible for this, as the correlation between the orientations of migration and intercellular forces is lost by blocking E-cadherin function or depleting individual AJ components 38, 39. The migration direction of individual cells in a monolayer follows the local axis of maximum intercellular stresses 40. More recently, it was shown that tension on AJs is anisotropic and highest at cell–cell contacts perpendicular to the migration direction 16. This implies that forces acting locally at cell–cell contacts may help to establish the direction of motion. Indeed, experiments with α-catenin mutants in which Vinculin binding is perturbed, showed that transduction of forces by AJs is essential for coordinated motion in epithelial monolayers 16, 41. These results are corroborated in vivo, as shown by the convergence-extension defects observed in zebrafish expressing Vinculin binding–deficient α-catenin 42.
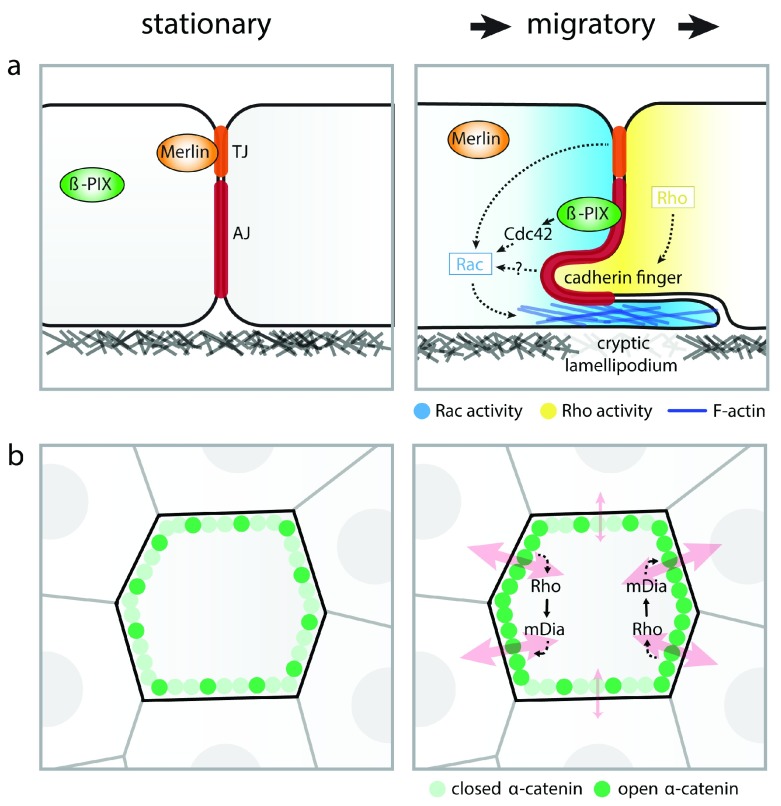
To establish directionality of collective migration, AJs not only transmit forces between cells but also transduce these forces into a cellular response to guide migration. Essentially, cell migration relies on the acquisition of a polarized cell state with Rac-induced (cryptic) lamellipodia protruding forward and Rho-mediated adhesion disassembly at the rear 43. Accumulating evidence shows that force transduction at cell–cell junctions enables cells to coordinate this polarization and concomitant migration direction to their followers ( Figure 2a) 16, 27, 44. This is mediated, at least in part, by the force-induced release of Merlin from cell contacts, which induces polarized Rac activity and formation of cryptic lamellipodia in migrating epithelial layers 44. While force-induced release of Merlin from tight junctions is implicated in this 44, the association of Merlin with the cadherin complex was recently shown to be inhibited by tensile forces as well 45. In migrating border cells in the Drosophila egg chamber, E-cadherin also transduces mechanical forces into Rac activation, resulting in protrusive activity at cell–cell contacts that are under elevated tension 27. Ectopic expression of P-cadherin in migrating myoblasts further revealed that the Cdc42-GEF β-PIX is recruited to AJs in motile cells, presumably as a result of increased junctional tension 46. This establishes the activation of Cdc42 and downstream Rac, thereby coordinating cell polarity 46. The selective function of P-cadherin, and not other cadherins, in β-PIX regulation highlights that different cadherin proteins may have distinct functions in transducing mechanical forces to establish collective cell migration. In line with this, E- and P-cadherin were shown to have distinct contributions to the development of intercellular stresses in migrating epithelia 39.
Figure 2. Mechanotransduction at cell – cell junctions during collective cell migration.
( a) Mechanotransduction at cell–cell junctions enables cells to coordinate cell polarization with their followers via several mechanisms that impinge on local activation of Rac, which establishes the formation of (cryptic) lamellipodia: (1) Tension on cell–cell junctions causes loss of Merlin from tight junctions (TJ), relieving its inhibition of Rac activation; (2) β-PIX is recruited by P-cadherin to adherens junctions (AJs) in migrating cells, presumably in a force-dependent manner, which results in activation of Cdc42 and downstream Rac1 to promote cell polarization; (3) Rho-mediated contractility at the rear of migrating cells induces the formation of cadherin fingers that extend into the trailing cells, which direct the formation of cryptic lamellipodia, presumably by contributing to the control of Rac activity. ( b) During collective migration, force-dependent conformational opening of α-catenin occurs in an anisotropic manner and is highest at cell–cell junctions perpendicular to the migratory axis. This involves a feedback mechanism through α-catenin–mediated activation of Rho and its effector mDia that induces AJ remodeling and junction strengthening. Anisotropy in the force-dependent remodeling of AJs coordinates the direction of migration between cells, possibly by establishing preferential transmission of forces between cells in the direction of migration (red arrows).
In addition to coordinating front–rear polarity, activation of Rho-family GTPases provides a positive feedback mechanism by reinforcing tension on E-cadherin junctions 16, 27. Conformational opening of α-catenin induces local activation of Rho and downstream mDia-mediated actin remodeling, which strengthens anisotropy in junctional tension in migrating epithelia ( Figure 2b) 16. Polarized Rho activity can also couple directional information between cells through the formation of specialized adhesion structures called cadherin fingers ( Figure 2a). At the rear of migrating endothelial cells, local RhoA-induced contractility triggers the formation of cadherin fingers, which become engulfed in follower cells and direct the formation of cryptic lamellipodia. The highly curved membrane in the cadherin finger is potentially instructive for Rac activation in follower cells 47, as many small GTPase regulators contain membrane curvature-recognizing domains 48. As such, an important signaling cue can be induced by tension-mediated membrane curvature at the cell–cell junction interface 49.
Although the mechanisms of force transmission and transduction by AJs discussed above apply, in principle, to all cells in a migrating group, additional mechanisms may be at play at the migratory front. Migration is typically directed by a specified leader cell that is morphologically and functionally distinct from the rest of the cell population and that initiates and organizes the migration of follower cells 50. Importantly, polarization and directed migration of the leader cell are regulated by transduction of forces at the cell–cell contact with its followers (reviewed in 30, 51). Moreover, it was recently shown that tensile forces exerted by follower cells on prospective leaders induce their leader cell phenotype 52. Another active function of the followers during collective migration was recently revealed for the very rear cells of the migrating cluster of neural crest cells 53. Reminiscent of the purse-string mechanism of wound closure, these cells contract a supracellular actin cable that causes the cell cluster to be propelled forward 53. Since both of these recently identified functions of follower and rear cells critically depend on force coordination between cells, it will be interesting to establish whether and how cadherin junctions contribute to this.
Mechanical control of tissue growth
The development of tissues into their correct size and proportions requires tight control of tissue growth and thus the rate and pattern of cell proliferation. Both of these are tightly regulated by, among other signals, mechanical forces that cells exert on each other. Seminal experiments in cultured monolayers revealed that patterns of local proliferation strongly correlate with the level of cellular traction forces 54. This was shown to be dependent on force transmission by cadherin-based adhesions 54, and specific analyses of intercellular forces demonstrated that the tendency of cells to proliferate correlates with the level of junctional tension 55, 56. Importantly, as tissues increase in cell density, junctional tension becomes reduced 57 because of a decrease in cell motility and cortical actomyosin contractility 57, 58. As such, sensing of intercellular tension provides a mechanism for cells to regulate density-dependent proliferation. At high cell density, proliferation ceases and this can be relieved by application of external stretch, as shown in both cultured cells 24, 59– 61 and the Drosophila wing disc 62. In these experiments, mechanical stretch was shown to control proliferation by inducing cell cycle entry of quiescent cells 24, 59, 61 as well as promoting progression through the subsequent cell cycle phases 24, 60, 63.
Thus far, the contribution of tension-regulated proliferation to morphogenesis has been studied mainly in the developing Drosophila wing disc, in which transduction of junctional tension is directly linked to regulation of organ size 57, 64. Moreover, mechano-sensitive control of proliferation in the wing disc explains how developing tissues can maintain homogenous patterns of proliferation despite the presence of gradients of growth factors. In the center of the wing disc, high levels of growth factor–induced proliferation and concomitant increase in cell density decrease junctional tension 57, 65. This feeds back to reduce proliferation in this region and thereby balances local proliferation rates throughout the tissue to maintain tissue organization 66. More recently, transduction of mechanical forces was also shown to regulate tissue size after development and, for instance, may adapt the size and composition of the Drosophila midgut to the feeding state of the animal 67, 68.
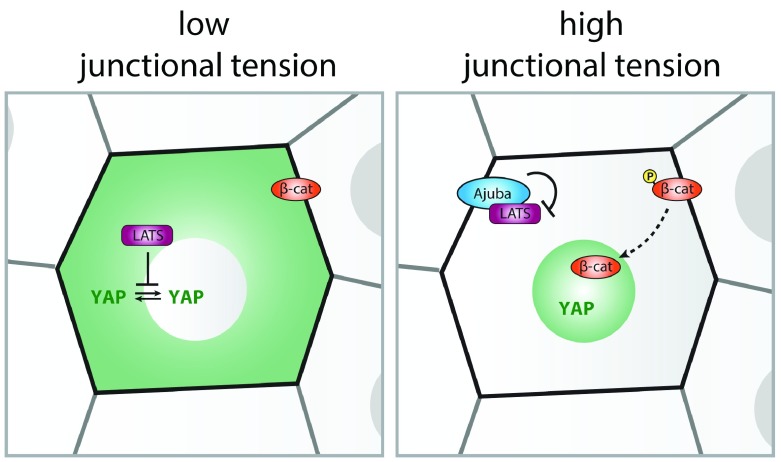
Although additional mechano-sensing complexes may be involved, E-cadherin adhesions play a key role in transducing intercellular forces to the cell cycle. It has long been established that the presence of E-cadherin adhesions can inhibit proliferation by triggering cell cycle exit, a process termed contact inhibition of proliferation 69. This involves inhibition of growth signals by E-cadherin adhesions, most notably through regulation of the Hippo signaling pathway 70. The Hippo pathway consists of a cascade of kinases that ultimately establishes the nuclear exclusion of the transcriptional activator YAP (and its homologue TAZ) through phosphorylation by LATS kinases 71. As recently demonstrated in the Drosophila wing disc and cultured epithelia, increased tension on E-cadherin junctions (for instance, by decreasing cell density or externally applied mechanical stretch) relieves this negative regulation, thereby promoting nuclear localization of YAP and cell cycle entry 24, 57, 72, 73. This is explained by force-induced opening of α-catenin that enables its interaction with proteins of the Ajuba/Zyxin family, which sequester LATS in an inactive state at AJs ( Figure 3) 57, 64, 72, 73. Several additional mechanisms may further contribute to the regulation of YAP upon fluctuations in tension on cadherin junctions 71. These may in part regulate YAP independently of the Hippo pathway and impinge on sequestration of YAP itself at cadherin junctions 74, 75, modulate nuclear import by influencing nuclear pore dynamics 34, attenuate nuclear export 45, 76, or influence YAP through modulation of the cortical cytoskeleton 59, 78. In addition to YAP signaling, force transduction by E-cadherin junctions can influence tissue growth through regulation of other proliferation pathways such as EGFR-mediated signaling 79, 80 and β-catenin/TCF-mediated transcription. The molecular mechanism of the latter is best understood as mechanical tension on the cadherin complex induces phosphorylation of β-catenin on Y654 29, 81. This site may become increasingly exposed upon elevated junctional tension, and phosphorylation results in junctional dissociation of β-catenin to promote its translocation to the nucleus and TCF-mediated transcription 82. Force-induced TCF activation has been linked to G 1/S progression in mechanically stretched epithelial cultures 24. Moreover, activation of this mechanical pathway in the mammalian intestine by ectopic application of forces to intestinal crypts was shown to trigger increased proliferation and concomitant hyperplasia 29. Importantly, mechano-sensitive β-catenin/TCF signaling downstream of E-cadherin functions beyond the regulation of cell proliferation, and fulfills an evolutionary conserved role in cellular differentiation that underlies tissue specification during embryonic development 82– 85. E-cadherin mechanotransduction was recently also linked to the control of cell metabolism through LKB1-dependent junctional recruitment and activation of AMPK and concomitant ATP production 28. Given the importance of metabolic regulation for the support of cell proliferation 86, this could provide additional mechanisms by which force transduction by AJs impacts tissue growth.
Figure 3. Regulation of YAP and β-catenin mediated transcription by junctional tension.
Phosphorylation by LATS establishes the nuclear exclusion of the transcriptional activator YAP under conditions of low junctional tension. The conformational opening of α-catenin upon elevated tension on E-cadherin adhesions (for instance, at decreased cell density) enables junctional recruitment of Ajuba-family proteins. Ajuba sequesters LATS at cell–cell contacts and keeps it inactive at this site, allowing nuclear entry of YAP. Also, the related Zyxin-family protein TRIP6 localizes to AJs, through an interaction with Vinculin, and binds and inhibits LATS (not shown). Note that additional LATS-independent mechanisms have been proposed for the intercellular tension-dependent regulation of YAP (see text). The nuclear localization of the transcriptional coactivator β-catenin is also regulated by the level of tension on cadherin adhesions. Upon increased junctional tension, a subset of the junctional β-catenin pool is phosphorylated (on Y654 in mammals), resulting in its dissociation from E-cadherin. This promotes translocation of β-catenin into the nucleus to drive TCF-dependent gene transcription. Note that loss of tension on E-cadherin has also been shown to promote junctional release and transcriptional activity of β-catenin, independent of phosphorylation of Y654 77.
Tissue growth is determined not only by the rate of proliferation but also by the loss of cells from the tissue that is similarly under control of mechanical signals. Epithelial crowding and consequent mechanical compression were demonstrated to trigger epithelial extrusion, driving loss of both apoptotic and live cells from epithelial tissues 87– 91. The stretch-sensitive calcium channel Piezo1 was identified as the main responsible mechano-transducer in this process 87. Since modulation of E-cadherin adhesions can trigger epithelial delamination 92, 93 and the distribution of tensile forces on E-cadherin junctions has been implicated in apical extrusion of apoptotic cells 94, 95, it will be interesting to test whether mechanotransduction by E-cadherin junctions plays a role in density-dependent cell extrusion as well.
Orienting cell divisions by intercellular forces
Shaping of tissues can be directed by the orientation of cell divisions and subsequent positioning of daughter cells. Division orientation is specified by the position of the mitotic spindle, which is controlled by pulling forces on astral microtubules that link the mitotic spindle to the cell cortex 96. Across metazoan species, including Xenopus, zebrafish, Drosophila, and mammals, it has been observed that epithelial divisions align with anisotropic tensile forces 97– 100. This serves to direct tissue elongation 65, 100, 101 and to dissipate high levels of anisotropic tissue tension that may arise during morphogenesis 97, 98, 101.
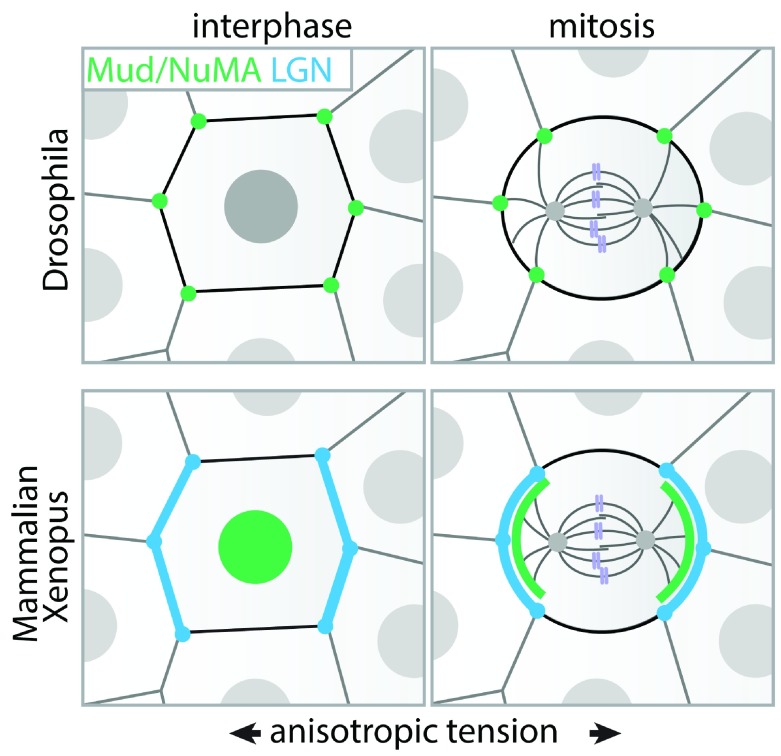
Initially, tension-oriented cell division was considered to be primarily a consequence of cell elongation along the tension axis 65, 97, 98, 101. This idea is supported by the “long axis rule”, postulated by Oscar Hertwig already in the late 19th century, stating that cells tend to divide along their longest axis. However, in epithelial monolayers, cell shape information is typically compromised by mitotic rounding. It was revealed in the Drosophila pupal notum how epithelial cells are able to preserve information of their interphase shape during mitosis 102. Tricellular junctions (TCJs), specialized adhesions at sites where three (or more) cells meet, recruit microtubule force generators via the dynein-associated protein Mud. The distribution of TCJs aligns with cell shape and mechanical stress orientation within the tissue and this spatial information is retained upon mitotic rounding ( Figure 4) 102. Future experiments may answer whether Mud recruitment to TCJs is mechanically controlled, as TCJs are sites of increased intercellular tension 103. Similar to Drosophila, the Mud homologue NuMA is required for cell divisions to align with the direction of tension in stretched mammalian epithelia 104. However, in contrast to Mud, NuMA is retained within the nucleus during interphase 105– 107. Instead, during interphase in mammalian epithelia, the NuMA-interacting protein LGN is recruited to E-cadherin junctions in a tension-dependent manner, thereby localizing in a polarized fashion under conditions of anisotropic junctional tension 25. Following mitotic entry and nuclear breakdown, the E-cadherin/LGN complex directs the recruitment of NuMA to cell–cell adhesions and enables orientation of the mitotic spindle along the axis of tissue tension ( Figure 4). LGN competes for E-cadherin binding with p120-catenin 107, 108, which may underlie its force-dependent recruitment to cell–cell junctions as p120-catenin was recently shown to relocalize from cell–cell junctions to the cytosol upon increased intercellular tension 109. Application of a low level of stretch to mammalian epithelial monolayers showed not only that force-dependent recruitment of LGN to E-cadherin is required to align divisions along the tension axis but also that this mechanism can overrule cell shape in determining division orientation 25. Shape-uncoupled orientation of division by tissue tension was recently also shown in vivo in the Drosophila mesectoderm and follicle epithelium, in which cells divide preferentially along the direction of tissue expansion that is perpendicular to the interphase long axis 110, 111. In these cells, this occurs independently of either TCJs or LGN/NuMA and instead involves anisotropic remodeling of the actomyosin cytoskeleton and cortical mechanics that may affect the ability of force generators to exert forces on the mitotic spindle 110, 111. Because of the role of E-cadherin in force-dependent remodeling of junctional actin, it will be interesting to explore whether regulation of the actomyosin cytoskeleton contributes to the ability of E-cadherin to align cell divisions with anisotropic tensile forces.
Figure 4. Regulation of cell division orientation by intercellular forces.
In the Drosophila pupal notum (top panel), the dynein-associated protein Mud is recruited to tricellular junctions (TCJs) during the G 2 phase of the cell cycle. In this way, in mitosis TCJs form cortical anchor points for astral microtubules that generate dynein-dependent pulling forces to orient the mitotic spindle. The distribution of TCJs aligns with cell shape and mechanical stress orientations within the epithelium, which is preserved upon mitotic rounding. In mammalian and Xenopus laevis epithelia (bottom panel), the Mud homologue NuMA is retained within the nucleus during interphase. Instead, the NuMA-interacting protein LGN, which directly interacts with E-cadherin, is recruited to junctions that are under elevated tension, leading to its polarization in mechanically stretched epithelial monolayers. Following mitotic entry and nuclear breakdown, the E-cadherin/LGN complex directs the recruitment of NuMA to cell–cell adhesions and thereby aligns cell divisions with the tension axis, which can overrule the interphase long axis of the cell in orienting division. Although this is not directly apparent in mammalian epithelia 25, 107, LGN is enriched at TCJs in Xenopus epithelia 113, implying that the tension-dependent recruitment of LGN by E-cadherin also provides a mechanism to align division with the position of TCJs.
Mechanical forces underlying cell–cell intercalation
In addition to being transmitted by AJs and transduced into an intracellular response, mechanical forces can regulate tissue shape by remodeling of cell–cell contacts through force-dependent regulation of AJ turnover 109. This occurs most prominently during cell intercalation, which drives convergence of the tissue along one axis and extension along the orthogonal axis 112. Cell rearrangements underlying intercalation are driven by shrinkage and disassembly of junctions perpendicular to the axis of extension, followed by formation of junctions with new neighbors. During Drosophila germband extension, shrinkage of dorsoventral junctions is controlled by polarized induction of actomyosin contractility 114– 116. The underlying increase in internalization of the cadherin complex in part involves Y667 phosphorylation of β-catenin (Y654 in mammals) 117, 118, an event that was recently shown to be directly regulated by tensile force 82. A possible explanation of the opposite effects of myosin-generated tension on cadherin junctions, either junction shrinkage or junction reinforcement (discussed earlier), may lie in the force orientation with respect to the junction. During junction shrinkage, forces parallel to the cell–cell contact orientation result in shear stress on the junction complex, which decreases E-cadherin levels at the junction 119. In contrast, force that is oriented perpendicular to anterior–posterior junctions was shown to increase junctional E-cadherin levels 119. While E-cadherin levels decrease in shrinking junctions, the tension-sensitive recruitment of the actin regulators Vinculin, Ajuba, and AIP1 is increased, enabling the junction to cope with the elevated mechanical load 119– 121. After shrinkage of dorsoventral junctions, the germband extends upon resolving the intermediate multicellular vertices and elongation of anterior–posterior junctions. For AJs to withstand the high level of tension at multicellular vertices and for subsequent vertex resolution, the force-sensitive recruitment of Ajuba is essential 121. Intriguingly, junction elongation does not happen merely as a result of tissue relaxation but instead is actively controlled by actomyosin activity as well 122, 123. Clearly, a dynamic interplay between mechanically controlled AJ turnover and strengthening is essential during intercalation, and future studies will likely provide more insights into the role of E-cadherin mechanotransduction in this.
Future perspectives
AJs have emerged as integral components in the regulation of morphogenesis by mechanical forces. In recent years, molecular details of cadherin mechanotransduction in morphogenetic processes of collective migration, cell division, and cell intercalation have started to be resolved. Future research will likely shed more light on how cadherin mechanotransduction impacts other processes that regulate tissue development and homeostasis, such as the control of cell shape, differentiation, and delamination 2, 22, 92, 124. Several important questions remain just at the start of investigation, for instance how cells discriminate between forces of different magnitudes and timescales. Different timescales add the conundrum that the short-term responses of cells to intercellular forces (for example, cell shape change or Vinculin recruitment to adhesion sites) function to dissipate force and therefore potentially prevent long-term responses 125. Moreover, it remains unanswered whether different force-dependent constituents of the AJ can be present simultaneously within the same cadherin complex, and mutual exclusivity is likely to exist (for example, between proteins competing for α-catenin binding). Understanding how the different components of cadherin mechanotransduction may be responsive to distinct magnitudes and types of force 126 will further help to explain why cells may adopt different, sometimes opposing, mechano-responses through cadherin junctions (for example, junction reinforcement or junction remodeling).
Although we have focused primarily on AJs, cell–cell contacts contain additional adhesion complexes (that is, tight junctions and desmosomes) that are exposed to external mechanical force 127, adopt tension-sensitive changes in their composition 128, and contribute to the transduction of intercellular forces 39. Moreover, intercellular forces can be sensed by mechano-sensing complexes that respond to membrane deformations, a mechanism used by mechano-sensitive ion channels that adopt open and closed conformations depending on local force-induced membrane deformations 129, 130. So far, research on mechanotransduction has predominantly approached the various mechano-sensitive complexes as isolated units. To properly understand cellular responses to forces, it will be pivotal to know how they cooperate. Interplay further exists between AJs and mechano-sensitive complexes that are not localized at cell–cell contacts, such as focal adhesions that contain numerous mechano-sensitive components that are in part shared with the AJ 6. Mechanotransduction by both adhesion complexes can converge on similar transcription factors and may cooperate in the activation of signaling pathways as has been shown for the activation of β-catenin 77. In coming years, unraveling the interplay between these different mechano-sensitive complexes will be instrumental in understanding the variety of cellular behaviors that are controlled by mechanical force.
Finally, it is becoming increasingly apparent that mechanical signals that cells receive from their environment continuously crosstalk with biochemical signals, such as growth factors and hormones. Not only can both impinge on the same intracellular signaling cascades, but several growth factor receptors are also directly (for example, Notch 131, 132) or indirectly (for example, EGFR 21 and insulin receptor 133) regulated by mechanical force. Moreover, biochemical signaling pathways may feedback to modulate the cellular mechano-response 134. A better comprehension of the dynamic interplay between mechanical and biochemical signaling will be imperative to understand the complexities of animal development.
Acknowledgments
We thank Jooske Monster (UMC Utrecht, The Netherlands) for critical reading of the manuscript.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Carien Niessen, Department of Dermatology, CECAD Cologne and Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany
Emmanuel Farge, Mechanics and Genetics of Embryonic and Tumoral Development, Inserm, UMR 168 Physico-Chimie Curie Cnrs, Institut Curie - Paris Sciences et Lettres Research University, Paris, France
René-Marc Mège, Institut Jacques Monod, Paris, France
Funding Statement
JdR is supported by the European Commission (H2020-FETPROACT-01-2016-731957), and MG is supported by the Netherlands Organisation for Scientific Research (NWO; 016.Vidi.189.166 and NWO gravitational program CancerGenomiCs.nl).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Stooke-Vaughan GA, Campàs O: Physical control of tissue morphogenesis across scales. Curr Opin Genet Dev. 2018;51:111–9. 10.1016/j.gde.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vasquez CG, Martin AC: Force transmission in epithelial tissues. Dev Dyn. 2016;245(3):361–71. 10.1002/dvdy.24384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iskratsch T, Wolfenson H, Sheetz MP: Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15(12):825–33. 10.1038/nrm3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ladoux B, Nelson WJ, Yan J, et al. : The mechanotransduction machinery at work at adherens junctions. Integr Biol (Camb). 2015;7(10):1109–19. 10.1039/c5ib00070j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gasparski AN, Beningo KA: Mechanoreception at the cell membrane: More than the integrins. Arch Biochem Biophys. 2015;586:20–6. 10.1016/j.abb.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 6. Han MKL, de Rooij J: Converging and Unique Mechanisms of Mechanotransduction at Adhesion Sites. Trends Cell Biol. 2016;26(8):612–23. 10.1016/j.tcb.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 7. Buckley CD, Tan J, Anderson KL, et al. : Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346(6209):1254211. 10.1126/science.1254211 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Ishiyama N, Sarpal R, Wood MN, et al. : Force-dependent allostery of the α-catenin actin-binding domain controls adherens junction dynamics and functions. Nat Commun. 2018;9(1):5121. 10.1038/s41467-018-07481-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Yonemura S, Wada Y, Watanabe T, et al. : alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12(6):533–42. 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Thomas WA, Boscher C, Chu YS, et al. : α-Catenin and vinculin cooperate to promote high E-cadherin-based adhesion strength. J Biol Chem. 2013;288(7):4957–69. 10.1074/jbc.M112.403774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. le Duc Q, Shi Q, Blonk I, et al. : Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189(7):1107–15. 10.1083/jcb.201001149 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Yao M, Qiu W, Liu R, et al. : Force-dependent conformational switch of α-catenin controls vinculin binding. Nat Commun. 2014;5:4525. 10.1038/ncomms5525 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Leerberg JM, Gomez GA, Verma S, et al. : Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr Biol. 2014;24(15):1689–99. 10.1016/j.cub.2014.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito S, Okuda S, Abe M, et al. : Induced cortical tension restores functional junctions in adhesion-defective carcinoma cells. Nat Commun. 2017;8(1):1834. 10.1038/s41467-017-01945-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang DL, Bax NA, Buckley CD, et al. : Vinculin forms a directionally asymmetric catch bond with F-actin. Science. 2017;357(6352):703–6. 10.1126/science.aan2556 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Matsuzawa K, Himoto T, Mochizuki Y, et al. : α-Catenin Controls the Anisotropy of Force Distribution at Cell-Cell Junctions during Collective Cell Migration. Cell Rep. 2018;23(12):3447–56. 10.1016/j.celrep.2018.05.070 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Acharya BR, Nestor-Bergmann A, Liang X, et al. : A Mechanosensitive RhoA Pathway that Protects Epithelia against Acute Tensile Stress. Dev Cell. 2018;47(4):439–452.e6. 10.1016/j.devcel.2018.09.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Oldenburg J, van der Krogt G, Twiss F, et al. : VASP, zyxin and TES are tension-dependent members of Focal Adherens Junctions independent of the α-catenin-vinculin module. Sci Rep. 2015;5:17225. 10.1038/srep17225 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Han MKL, van der Krogt GNM, de Rooij J: Zygotic vinculin is not essential for embryonic development in zebrafish. PLoS One. 2017;12(8):e0182278. 10.1371/journal.pone.0182278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alatortsev VE, Kramerova IA, Frolov MV, et al. : Vinculin gene is non-essential in Drosophila melanogaster. FEBS Lett. 1997;413(2):197–201. 10.1016/s0014-5793(97)00901-0 [DOI] [PubMed] [Google Scholar]
- 21. Rübsam M, Mertz AF, Kubo A, et al. : E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and tight junction positioning. Nat Commun. 2017;8(1):1250. 10.1038/s41467-017-01170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernandez-Sanchez ME, Brunet T, Röper JC, et al. : Mechanotransduction's impact on animal development, evolution, and tumorigenesis. Annu Rev Cell Dev Biol. 2015;31:373–97. 10.1146/annurev-cellbio-102314-112441 [DOI] [PubMed] [Google Scholar]
- 23. Mitrossilis D, Röper JC, Le Roy D, et al. : Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat Commun. 2017;8:13883. 10.1038/ncomms13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benham-Pyle BW, Pruitt BL, Nelson WJ: Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science. 2015;348(6238):1024–7. 10.1126/science.aaa4559 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Hart KC, Tan J, Siemers KA, et al. : E-cadherin and LGN align epithelial cell divisions with tissue tension independently of cell shape. Proc Natl Acad Sci U S A. 2017;114(29):E5845–E5853. 10.1073/pnas.1701703114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Weber GF, Bjerke MA, DeSimone DW: A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2012;22(1):104–15. 10.1016/j.devcel.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Cai D, Chen SC, Prasad M, et al. : Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157(5):1146–59. 10.1016/j.cell.2014.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Bays JL, Campbell HK, Heidema C, et al. : Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017;19(6):724–31. 10.1038/ncb3537 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Fernández-Sánchez ME, Barbier S, Whitehead J, et al. : Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature. 2015;523(7558):92–5. 10.1038/nature14329 [DOI] [PubMed] [Google Scholar]
- 30. Mayor R, Etienne-Manneville S: The front and rear of collective cell migration. Nat Rev Mol Cell Biol. 2016;17(2):97–109. 10.1038/nrm.2015.14 [DOI] [PubMed] [Google Scholar]
- 31. Sunyer R, Conte V, Escribano J, et al. : Collective cell durotaxis emerges from long-range intercellular force transmission. Science. 2016;353(6304):1157–61. 10.1126/science.aaf7119 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Barriga EH, Franze K, Charras G, et al. : Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature. 2018;554(7693):523–7. 10.1038/nature25742 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Vedula SR, Leong MC, Lai TL, et al. : Emerging modes of collective cell migration induced by geometrical constraints. Proc Natl Acad Sci U S A. 2012;109(32):12974–9. 10.1073/pnas.1119313109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Ladoux B, Mège RM, Trepat X: Front-Rear Polarization by Mechanical Cues: From Single Cells to Tissues. Trends Cell Biol. 2016;26(6):420–33. 10.1016/j.tcb.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ladoux B, Mège RM: Mechanobiology of collective cell behaviours. Nat Rev Mol Cell Biol. 2017;18(12):743–57. 10.1038/nrm.2017.98 [DOI] [PubMed] [Google Scholar]
- 36. Trepat X, Wasserman MR, Angelini TE, et al. : Physical forces during collective cell migration. Nat Phys. 2009;5:426–30. 10.1038/nphys1269 [DOI] [Google Scholar]; F1000 Recommendation
- 37. Rakshit S, Zhang Y, Manibog K, et al. : Ideal, catch, and slip bonds in cadherin adhesion. Proc Natl Acad Sci U S A. 2012;109(46):18815–20. 10.1073/pnas.1208349109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Tambe DT, Hardin CC, Angelini TE, et al. : Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10(6):469–75. 10.1038/nmat3025 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Bazellières E, Conte V, Elosegui-Artola A, et al. : Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17(4):409–20. 10.1038/ncb3135 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Trepat X, Fredberg JJ: Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 2011;21(11):638–46. 10.1016/j.tcb.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seddiki R, Narayana GHNS, Strale PO, et al. : Force-dependent binding of vinculin to α-catenin regulates cell-cell contact stability and collective cell behavior. Mol Biol Cell. 2018;29(4):380–8. 10.1091/mbc.E17-04-0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han MK, Hoijman E, Nöel E, et al. : αE-catenin-dependent mechanotransduction is essential for proper convergent extension in zebrafish. Biol Open. 2016;5(10):1461–72. 10.1242/bio.021378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zegers MM, Friedl P: Rho GTPases in collective cell migration. Small GTPases. 2014;5:e28997. 10.4161/sgtp.28997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Das T, Safferling K, Rausch S, et al. : A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol. 2015;17(3):276–87. 10.1038/ncb3115 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Furukawa KT, Yamashita K, Sakurai N, et al. : The Epithelial Circumferential Actin Belt Regulates YAP/TAZ through Nucleocytoplasmic Shuttling of Merlin. Cell Rep. 2017;20(6):1435–47. 10.1016/j.celrep.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 46. Plutoni C, Bazellieres E, Le Borgne-Rochet M, et al. : P-cadherin promotes collective cell migration via a Cdc42-mediated increase in mechanical forces. J Cell Biol. 2016;212(2):199–217. 10.1083/jcb.201505105 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Hayer A, Shao L, Chung M, et al. : Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells. Nat Cell Biol. 2016;18(12):1311–23. 10.1038/ncb3438 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Aspenström P: BAR Domain Proteins Regulate Rho GTPase Signaling. Adv Exp Med Biol. 2018. 10.1007/5584_2018_259 [DOI] [PubMed] [Google Scholar]
- 49. Malinova TS, Huveneers S: Sensing of Cytoskeletal Forces by Asymmetric Adherens Junctions. Trends Cell Biol. 2018;28(4):328–41. 10.1016/j.tcb.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 50. Khalil AA, Friedl P: Determinants of leader cells in collective cell migration. Integr Biol (Camb). 2010;2(11–12):568–74. 10.1039/c0ib00052c [DOI] [PubMed] [Google Scholar]
- 51. Khalil AA, de Rooij J: Cadherin mechanotransduction in leader-follower cell specification during collective migration. Exp Cell Res. 2019;376(1):86–91. 10.1016/j.yexcr.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 52. Vishwakarma M, Di Russo J, Probst D, et al. : Mechanical interactions among followers determine the emergence of leaders in migrating epithelial cell collectives. Nat Commun. 2018;9(1):3469. 10.1038/s41467-018-05927-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Shellard A, Szabó A, Trepat X, et al. : Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis. Science. 2018;362(6412):339–43. 10.1126/science.aau3301 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Nelson CM, Jean RP, Tan JL, et al. : Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102(33):11594–9. 10.1073/pnas.0502575102 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Mohan A, Schlue KT, Kniffin AF, et al. : Spatial Proliferation of Epithelial Cells Is Regulated by E-Cadherin Force. Biophys J. 2018;115(5):853–64. 10.1016/j.bpj.2018.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Uroz M, Wistorf S, Serra-Picamal X, et al. : Regulation of cell cycle progression by cell-cell and cell-matrix forces. Nat Cell Biol. 2018;20(6):646–54. 10.1038/s41556-018-0107-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Rauskolb C, Sun S, Sun G, et al. : Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell. 2014;158(1):143–56. 10.1016/j.cell.2014.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Puliafito A, Hufnagel L, Neveu P, et al. : Collective and single cell behavior in epithelial contact inhibition. Proc Natl Acad Sci U S A. 2012;109(3):739–44. 10.1073/pnas.1007809109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aragona M, Panciera T, Manfrin A, et al. : A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–59. 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Streichan SJ, Hoerner CR, Schneidt T, et al. : Spatial constraints control cell proliferation in tissues. Proc Natl Acad Sci U S A. 2014;111(15):5586–91. 10.1073/pnas.1323016111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Codelia VA, Sun G, Irvine KD: Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol. 2014;24(17):2012–7. 10.1016/j.cub.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schluck T, Nienhaus U, Aegerter-Wilmsen T, et al. : Mechanical control of organ size in the development of the Drosophila wing disc. PLoS One. 2013;8(10):e76171. 10.1371/journal.pone.0076171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gudipaty SA, Lindblom J, Loftus PD, et al. : Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543(7643):118–21. 10.1038/nature21407 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Alégot H, Markosian C, Rauskolb C, et al. : Recruitment of Jub by α-catenin promotes Yki activity and Drosophila wing growth. J Cell Sci. 2019;132(5): pii: jcs222018. 10.1242/jcs.222018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mao Y, Tournier AL, Hoppe A, et al. : Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J. 2013;32(21):2790–803. 10.1038/emboj.2013.197 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Pan Y, Heemskerk I, Ibar C, et al. : Differential growth triggers mechanical feedback that elevates Hippo signaling. Proc Natl Acad Sci U S A. 2016;113(45):E6974–E6983. 10.1073/pnas.1615012113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li Q, Nirala NK, Nie Y, et al. : Ingestion of Food Particles Regulates the Mechanosensing Misshapen-Yorkie Pathway in Drosophila Intestinal Growth. Dev Cell. 2018;45(4):433–449.e6. 10.1016/j.devcel.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. He L, Si G, Huang J, et al. : Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555(7694):103–6. 10.1038/nature25744 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. McClatchey AI, Yap AS: Contact inhibition (of proliferation) redux. Curr Opin Cell Biol. 2012;24(5):685–94. 10.1016/j.ceb.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 70. Kim NG, Koh E, Chen X, et al. : E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108(29):11930–5. 10.1073/pnas.1103345108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Mendonsa AM, Na TY, Gumbiner BM: E-cadherin in contact inhibition and cancer. Oncogene. 2018;37(35):4769–80. 10.1038/s41388-018-0304-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dutta S, Mana-Capelli S, Paramasivam M, et al. : TRIP6 inhibits Hippo signaling in response to tension at adherens junctions. EMBO Rep. 2018;19(2):337–50. 10.15252/embr.201744777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ibar C, Kirichenko E, Keepers B, et al. : Tension-dependent regulation of mammalian Hippo signaling through LIMD1. J Cell Sci. 2018;131(5): pii: jcs214700. 10.1242/jcs.214700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schlegelmilch K, Mohseni M, Kirak O, et al. : Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144(5):782–95. 10.1016/j.cell.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Giampietro C, Disanza A, Bravi L, et al. : The actin-binding protein EPS8 binds VE-cadherin and modulates YAP localization and signaling. J Cell Biol. 2015;211(6):1177–92. 10.1083/jcb.201501089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Elosegui-Artola A, Andreu I, Beedle AEM, et al. : Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017;171(6):1397–1410.e14. 10.1016/j.cell.2017.10.008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Gayrard C, Bernaudin C, Déjardin T, et al. : Src- and confinement-dependent FAK activation causes E-cadherin relaxation and β-catenin activity. J Cell Biol. 2018;217(3):1063–77. 10.1083/jcb.201706013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Mateus R, Lourenco R, Fang Y, et al. : Control of tissue growth by Yap relies on cell density and F-actin in zebrafish fin regeneration. Development. 2015;142(16):2752–63. 10.1242/dev.119701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muhamed I, Wu J, Sehgal P, et al. : E-cadherin-mediated force transduction signals regulate global cell mechanics. J Cell Sci. 2016;129(9):1843–54. 10.1242/jcs.185447 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Chiasson-MacKenzie C, Morris ZS, Baca Q, et al. : NF2/Merlin mediates contact-dependent inhibition of EGFR mobility and internalization via cortical actomyosin. J Cell Biol. 2015;211(2):391–405. 10.1083/jcb.201503081 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Benham-Pyle BW, Sim JY, Hart KC, et al. : Increasing β-catenin/Wnt3A activity levels drive mechanical strain-induced cell cycle progression through mitosis. eLife. 2016;5: pii: e19799. 10.7554/eLife.19799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Röper JC, Mitrossilis D, Stirnemann G, et al. : The major β-catenin/E-cadherin junctional binding site is a primary molecular mechano-transductor of differentiation in vivo. eLife. 2018;7: pii: e33381. 10.7554/eLife.33381 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Brunet T, Bouclet A, Ahmadi P, et al. : Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat Commun. 2013;4:2821. 10.1038/ncomms3821 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Desprat N, Supatto W, Pouille PA, et al. : Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15(3):470–7. 10.1016/j.devcel.2008.07.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Shyer AE, Rodrigues AR, Schroeder GG, et al. : Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science. 2017;357(6353):811–5. 10.1126/science.aai7868 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Zhu J, Thompson CB: Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 2019;20(7):436–450. 10.1038/s41580-019-0123-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Eisenhoffer GT, Loftus PD, Yoshigi M, et al. : Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484(7395):546–9. 10.1038/nature10999 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Marinari E, Mehonic A, Curran S, et al. : Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 2012;484(7395):542–5. 10.1038/nature10984 [DOI] [PubMed] [Google Scholar]
- 89. Saw TB, Doostmohammadi A, Nier V, et al. : Topological defects in epithelia govern cell death and extrusion. Nature. 2017;544(7649):212–6. 10.1038/nature21718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moreno E, Valon L, Levillayer F, et al. : Competition for Space Induces Cell Elimination through Compaction-Driven ERK Downregulation. Curr Biol. 2019;29(1):23–34.e8. 10.1016/j.cub.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kocgozlu L, Saw TB, Le AP, et al. : Epithelial Cell Packing Induces Distinct Modes of Cell Extrusions. Curr Biol. 2016;26(21):2942–50. 10.1016/j.cub.2016.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Miroshnikova YA, Le HQ, Schneider D, et al. : Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat Cell Biol. 2018;20(1):69–80. 10.1038/s41556-017-0005-z [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Grieve AG, Rabouille C: Extracellular cleavage of E-cadherin promotes epithelial cell extrusion. J Cell Sci. 2014;127(Pt 15):3331–46. 10.1242/jcs.147926 [DOI] [PubMed] [Google Scholar]
- 94. Michael M, Meiring JCM, Acharya BR, et al. : Coronin 1B Reorganizes the Architecture of F-Actin Networks for Contractility at Steady-State and Apoptotic Adherens Junctions. Dev Cell. 2016;37(1):58–71. 10.1016/j.devcel.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 95. Wu SK, Gomez GA, Michael M, et al. : Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat Cell Biol. 2014;16(2):167–78. 10.1038/ncb2900 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. di Pietro F, Echard A, Morin X: Regulation of mitotic spindle orientation: an integrated view. EMBO Rep. 2016;17(8):1106–30. 10.15252/embr.201642292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Campinho P, Behrndt M, Ranft J, et al. : Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat Cell Biol. 2013;15(12):1405–14. 10.1038/ncb2869 [DOI] [PubMed] [Google Scholar]
- 98. Legoff L, Rouault H, Lecuit T: A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140(19):4051–9. 10.1242/dev.090878 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Petridou NI, Skourides PA: FAK transduces extracellular forces that orient the mitotic spindle and control tissue morphogenesis. Nat Commun. 2014;5:5240. 10.1038/ncomms6240 [DOI] [PubMed] [Google Scholar]
- 100. Wang MF, Hunter MV, Wang G, et al. : Automated cell tracking identifies mechanically oriented cell divisions during Drosophila axis elongation. Development. 2017;144(7):1350–61. 10.1242/dev.141473 [DOI] [PubMed] [Google Scholar]
- 101. Wyatt TP, Harris AR, Lam M, et al. : Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc Natl Acad Sci U S A. 2015;112(18):5726–31. 10.1073/pnas.1420585112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bosveld F, Markova O, Guirao B, et al. : Epithelial tricellular junctions act as interphase cell shape sensors to orient mitosis. Nature. 2016;530(7591):495–8. 10.1038/nature16970 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 103. Higashi T, Miller AL: Tricellular junctions: how to build junctions at the TRICkiest points of epithelial cells. Mol Biol Cell. 2017;28(15):2023–34. 10.1091/mbc.E16-10-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Seldin L, Poulson ND, Foote HP, et al. : NuMA localization, stability, and function in spindle orientation involve 4.1 and Cdk1 interactions. Mol Biol Cell. 2013;24(23):3651–62. 10.1091/mbc.E13-05-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tang Z, Hu Y, Wang Z, et al. : Mechanical Forces Program the Orientation of Cell Division during Airway Tube Morphogenesis. Dev Cell. 2018;44(3):313–325.e5. 10.1016/j.devcel.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 106. Radulescu AE, Cleveland DW: NuMA after 30 years: the matrix revisited. Trends Cell Biol. 2010;20(4):214–22. 10.1016/j.tcb.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gloerich M, Bianchini JM, Siemers KA, et al. : Cell division orientation is coupled to cell-cell adhesion by the E-cadherin/LGN complex. Nat Commun. 2017;8:13996. 10.1038/ncomms13996 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 108. Conway DE, Coon BG, Budatha M, et al. : VE-Cadherin Phosphorylation Regulates Endothelial Fluid Shear Stress Responses through the Polarity Protein LGN. Curr Biol. 2017;27(14):2219–2225.e5. 10.1016/j.cub.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Iyer KV, Piscitello-Gómez R, Paijmans J, et al. : Epithelial Viscoelasticity Is Regulated by Mechanosensitive E-cadherin Turnover. Curr Biol. 2019;29(4):578–591.e5. 10.1016/j.cub.2019.01.021 [DOI] [PubMed] [Google Scholar]
- 110. Finegan TM, Na D, Cammarota C, et al. : Tissue tension and not interphase cell shape determines cell division orientation in the Drosophila follicular epithelium. EMBO J. 2019;38(3): pii: e100072. 10.15252/embj.2018100072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Scarpa E, Finet C, Blanchard GB, et al. : Actomyosin-Driven Tension at Compartmental Boundaries Orients Cell Division Independently of Cell Geometry In Vivo. Dev Cell. 2018;47(6):727–740.e6. 10.1016/j.devcel.2018.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 112. Tada M, Heisenberg CP: Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development. 2012;139(21):3897–904. 10.1242/dev.073007 [DOI] [PubMed] [Google Scholar]
- 113. Nestor-Bergmann A, Stooke-Vaughan GA, Goddard GK, et al. : Decoupling the Roles of Cell Shape and Mechanical Stress in Orienting and Cueing Epithelial Mitosis. Cell Rep. 2019;26(8):2088–2100.e4. 10.1016/j.celrep.2019.01.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bertet C, Sulak L, Lecuit T: Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429(6992):667–71. 10.1038/nature02590 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 115. Fernandez-Gonzalez R, Simoes Sde M, Röper JC, et al. : Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17(5):736–43. 10.1016/j.devcel.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 116. Rauzi M, Lenne PF, Lecuit T: Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468(7327):1110–4. 10.1038/nature09566 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 117. Levayer R, Pelissier-Monier A, Lecuit T: Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol. 2011;13(5):529–40. 10.1038/ncb2224 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 118. Tamada M, Farrell DL, Zallen JA: Abl regulates planar polarized junctional dynamics through β-catenin tyrosine phosphorylation. Dev Cell. 2012;22(2):309–19. 10.1016/j.devcel.2011.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 119. Kale GR, Yang X, Philippe JM, et al. : Distinct contributions of tensile and shear stress on E-cadherin levels during morphogenesis. Nat Commun. 2018;9(1):5021. 10.1038/s41467-018-07448-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 120. Ikawa K, Sugimura K: AIP1 and cofilin ensure a resistance to tissue tension and promote directional cell rearrangement. Nat Commun. 2018;9(1):3295. 10.1038/s41467-018-05605-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Razzell W, Bustillo ME, Zallen JA: The force-sensitive protein Ajuba regulates cell adhesion during epithelial morphogenesis. J Cell Biol. 2018;217(10):3715–30. 10.1083/jcb.201801171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Collinet C, Rauzi M, Lenne PF, et al. : Local and tissue-scale forces drive oriented junction growth during tissue extension. Nat Cell Biol. 2015;17(10):1247–58. 10.1038/ncb3226 [DOI] [PubMed] [Google Scholar]
- 123. Yu JC, Fernandez-Gonzalez R: Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila. eLife. 2016;5: pii: e10757. 10.7554/eLife.10757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wu SK, Lagendijk AK, Hogan BM, et al. : Active contractility at E-cadherin junctions and its implications for cell extrusion in cancer. Cell Cycle. 2015;14(3):315–22. 10.4161/15384101.2014.989127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wyatt T, Baum B, Charras G: A question of time: tissue adaptation to mechanical forces. Curr Opin Cell Biol. 2016;38:68–73. 10.1016/j.ceb.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 126. Charras G, Yap AS: Tensile Forces and Mechanotransduction at Cell-Cell Junctions. Curr Biol. 2018;28(8):R445–R457. 10.1016/j.cub.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 127. Price AJ, Cost AL, Ungewiß H, et al. : Mechanical loading of desmosomes depends on the magnitude and orientation of external stress. Nat Commun. 2018;9(1):5284. 10.1038/s41467-018-07523-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Spadaro D, Le S, Laroche T, et al. : Tension-Dependent Stretching Activates ZO-1 to Control the Junctional Localization of Its Interactors. Curr Biol. 2017;27(24):3783–3795.e8. 10.1016/j.cub.2017.11.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 129. Saotome K, Murthy SE, Kefauver JM, et al. : Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554(7693):481–6. 10.1038/nature25453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhao Q, Zhou H, Chi S, et al. : Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554(7693):487–92. 10.1038/nature25743 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 131. Gordon WR, Zimmerman B, He L, et al. : Mechanical Allostery: Evidence for a Force Requirement in the Proteolytic Activation of Notch. Dev Cell. 2015;33(6):729–36. 10.1016/j.devcel.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 132. Luca VC, Kim BC, Ge C, et al. : Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science. 2017;355(6331):1320–4. 10.1126/science.aaf9739 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 133. Kim J, Bilder D, Neufeld TP: Mechanical stress regulates insulin sensitivity through integrin-dependent control of insulin receptor localization. Genes Dev. 2018;32(2):156–64. 10.1101/gad.305870.117 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 134. Sehgal P, Kong X, Wu J, et al. : Epidermal growth factor receptor and integrins control force-dependent vinculin recruitment to E-cadherin junctions. J Cell Sci. 2018;131(6): pii: jcs206656. 10.1242/jcs.206656 [DOI] [PMC free article] [PubMed] [Google Scholar]