Abstract
Percutaneous Needle Biopsy (PNB) is the insertion of a needle into a suspected lesion or an organ with the aim to obtain cells or tissue for diagnosis. It’s a relatively non-invasive procedure and is performed by radiologist under guidance of imaging techniques such as ultrasound (US), computed tomography (CT), fluoroscopy, magnetic resonance imaging (MRI), and positron emission tomography CT (PET-CT). The choice of imaging technique depends on the evaluation of the target lesion and patient compliance. PNB includes two categories: fine-needle aspiration biopsy (FNAB) that is the use of a thin needle (18-25 gauge) to extract cells for cytological evaluation; and core needle biopsy (CNB) that is the use of a larger needle (9-20 gauge) to extract a piece of tissue for histological evaluation. The indications for biopsy are the characterization of nature (benign or malignant) of a lesion, diagnosis and staging of tumor, and biological or immunohistochemical/genetic analisys on tissue. Success of PNB is the procurement of sufficient material to characterize lesions and to guide the patient outcome. Major complications are rare. PNB became a useful technique in diagnosis and study of retroperitoneal lesions, because of a more suitable access to specific intra-abdominal structures, lowering the risk of injury of interposed structures (such as bowel, great vessels). (www.actabiomedica.it)
Keywords: biopsy, retroperitoneum, tumor, computed tomography
Introduction
Image-guided Percutaneous Needle Biopsy (PNB) is an interventional procedure performed by radiologists (1-5) with the aim to obtain cells or tissue for diagnosis by the insertion of a needle into a suspected lesion.
It’s a relatively non-invasive procedure, and it has absolute advantages compared to open or excisional biopsy.
Success of PNB is related to proper patient selection, preparation and adequate procedural planning (6-8).
Planning and procedural phases
PNB implicates the involvement of interventional radiologists in multidisciplinary boards (9-21). The radiologyst has a key role in the pre-procedural phase: to evaluate potential contraindications and risks of PNB, to confirm the indications for PNB and to identify the optimal target and the selection of the proper imaging guidance.
Indications to PNB are the characterization of nature (benign or malignant) of a lesion (22), the diagnosis and staging of a tumor, and biological or immunohistochemical/genetic analisys on tissue (7, 23).
Although PNB is a relatively non-invasive procedure, there are some contraindications, such as the alteration of coagulation status (specially if it can’t be correctable) and bleeding risk, the patient’s clinical status (to tolerate bleeding or anesthesia) and cooperation.
The main imaging-guide modalities are ultrasound (US) (24-26) and computed tomography (CT) (27-35); other uncommon imaging techniques are fluoroscopy, magnetic resonance imaging (MRI) (36-47), and positron emission tomography CT (PET-CT) (6, 7, 48).
US guidance has a wide use because of portability, lack of ionizing radiation, and low operating cost. Real time imaging allows to visualize and track the needle throughout its entire pathway and is useful even in lesions moving on respiratory motion; Color-Doppler (24) aid in vascular structures visualization. Furthermore, in selected patients, US contrast-injection increases lesion characterization on the surrounding tissue. Freehand or needle-guided technique are both suitable. Compared to the freehand technique, the guided technique is limited by a fixed angle. Limits of the US-guidance technique are the operator experience and the appropriate acoustic window view, such as the difficulty to penetrate air-filled structures and bone (49).
Compared to US, CT has a better preprocedural planning of PNB, because of its high spatial resolution and large field of view. It permits multiplanar reformations (MPR) to obtain a more adequate path of needle. An intravenous contrast injection may be required to increase accuracy on lesion visualization.
Other imaging guidance modalities are: CT-fluoroscopy, that allows a real-time visualization of the needle, advancement reducing procedural time, but it exposes operators and patients to radiation doses; MR-guidance, despite excellent soft tissue contrast and lack of ionizing radiation, isn’t currently feasible because of increased costs and procedure time, the lack in appropriate open-scanner and MRI-compatible instruments;
PNB includes two basic techniques for sample acquisition: fine needle aspiration biopsy (FNAB) and core needle biopsy (CNB) (Figg. 1-6) (50, 51).
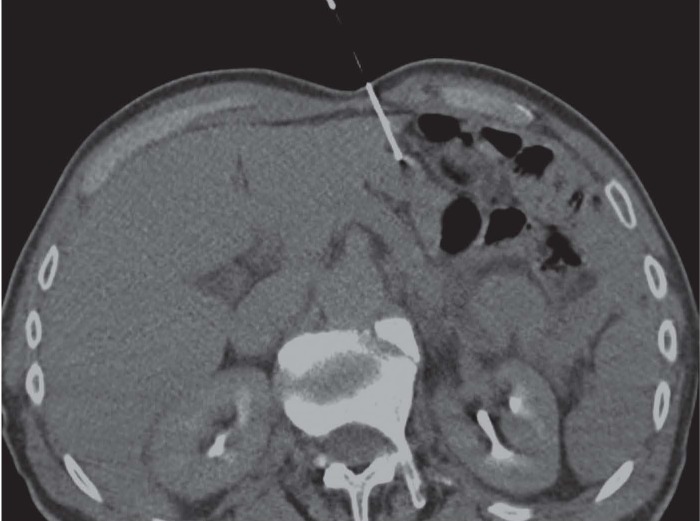
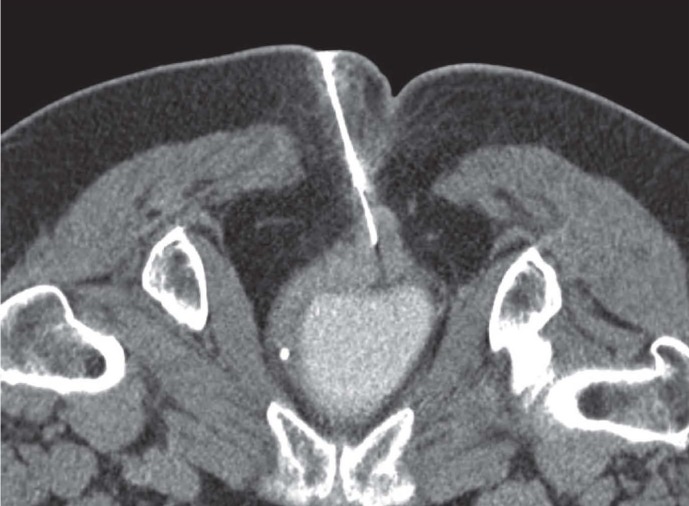
Figure 1.
A 72 years old man with history of total gastrectomy for ADK. CT-guided CNB on supine patient for histological evaluation of epigastric solid lesion
Figure 6.
A 75 years old woman with solid exophytic lesion of left kidney. CT-guided CNB on prone position showed a renal cell carcinoma
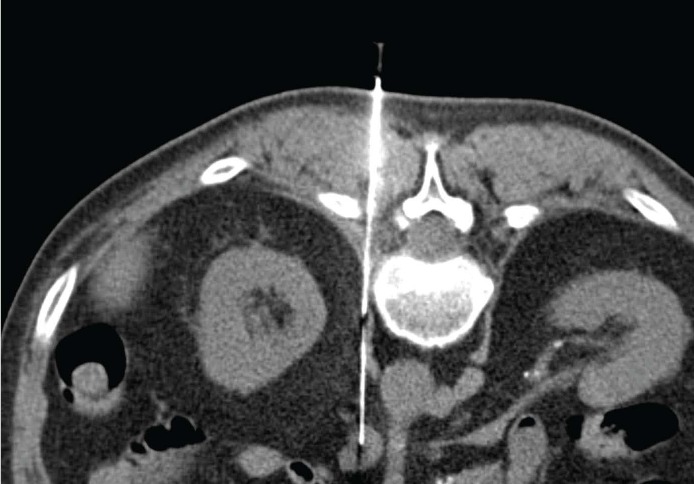
Figure 2.
A 64 years old man with outcome of pulmonary lobectomy for primitive lung cancer. CT-guided CNB on prone position of retroperitoneal node: the sample permitted to confirm the metastatic nature
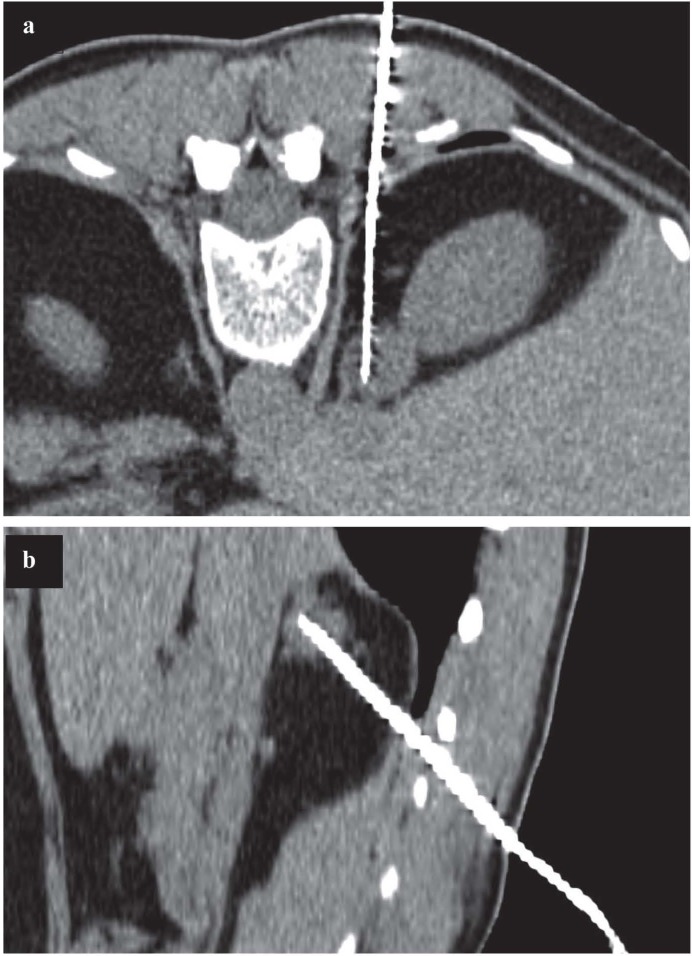
Figure 3.
54 years old woman with previous cervical and endometrial squamous cells carcinoma, with indeterminate right adrenal solid lesion having elevated metabolic activity at PET examination. CT guided CNB on prone position in axial view (a) and parasagittal reconstruction (b), permitted the histological diagnosis of adenoma
Figure 4.
An 81 years old woman with outcome of anterior resection of the rectum for ADK with focal thickening of posterior wall. CT-guided FNAB on prone position of the lesion confirmed recurrence of the tumor
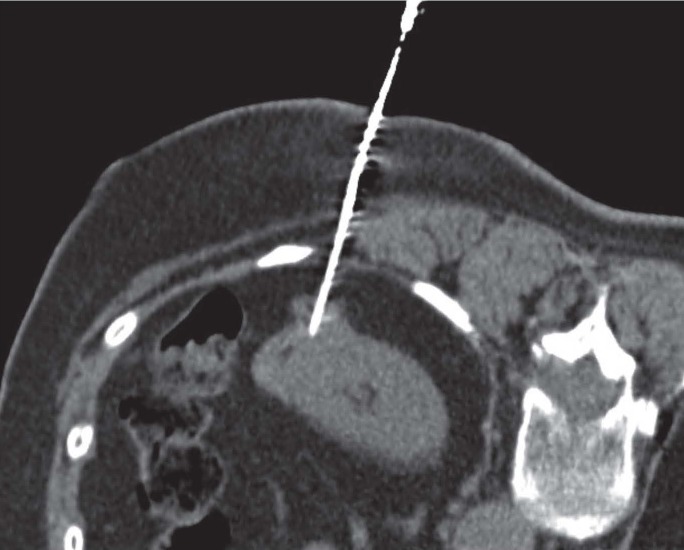
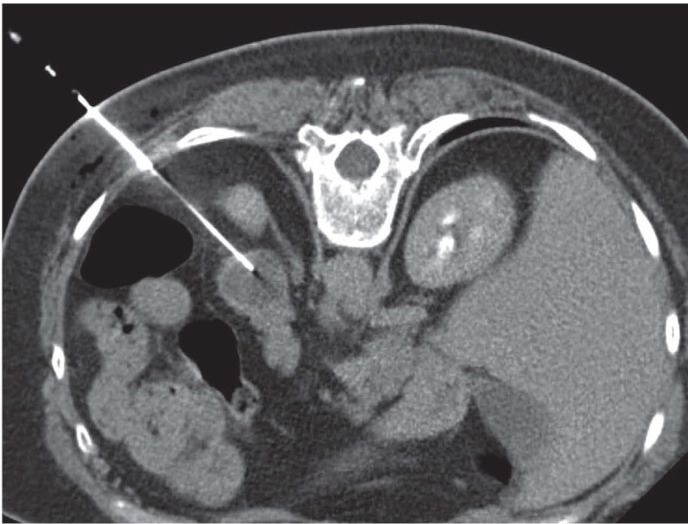
Figure 5.
A 65 years old woman with abdominal pain; the abdominal CT demonstrate a pancreatic tail lesion. CT-guided PNB on prone position of pancreatic tail lesion showed a neuroendocrine tumor
FNAB device extracts individual cells for cytological evaluation using a small needle (18-25G) with inner stylet. Once in place, the stylet is removed, a syringe is attached to the needle, and cells are aspirated. Small lesions, necrotic tumors or lesions close to critical structures are its main targets. The most commonly devices used in retroperitoneal biopsies are the spinal needles and the Chiba needles (52).
CNB devices use larger needles (9-20G) with different mechanisms (manually or automatically cutting systems) to extract a piece of tissue for complete histologic evaluation (53).
A safe and proven technique is the use of coaxial needle: the biopsy needle is introduced coaxially into a guide needle (9-19G), previously advanced nearby the target. It doesn’t increase the recurrence of complications and allow multiple specimen samples in a single puncture and decrease the tumor cells seeding risk along the needle tract (48, 54-58).
The extracted samples are then smeared on glass slides and fixed (FNAB) or placed in formalin (CNB); for bacteriological analysis the sample is sent in saline for culture (59, 60).
Post-procedural phase
Retroperitoneal PNB is considered a minimally invasive and safe procedure.
There are major and minor complications, related to the technique (bleeding, infection, perforation, tract seeding) or to organ specific injury (such as haematuria, pneumothorax, haemoptysis, air embolism).
After the procedure and before discharge, imaging control is generally obtained and documented to detect immediate possible complications; equally, vital signs monitoring and clinical observation are required for a few hours following the procedure. In case of major complications, hospitalization in appropriate environment should be guaranteed.
Technical success of PNB varies greatly depending upon the size and location of the target, benign or malignant nature of the lesion, number of samples obtained, availability of an onsite cytopathologist, IRs’ and pathologists’ experience, equipment availability (61).
Clinical success of PNB is the usefulness of the procedure in terms of improvement of patient care.
In case of non-diagnostic biopsy a repeated biopsy should be considered, such as different techniques or approaches modalities (surgical biopsy or open access) (62-67).
Conclusions
Retroperitoneal Percutaneous Needle Biopsy is a minimally invasive, well established and safe procedure, with a low rate of complications and high diagnostic yield.
Radiologist plays a critical role in the entire management of the patient, since the procedure planning until the patient discharge.
PNB is gaining an even more crucial role, specially with the development of molecular personalized treatment, so avoiding in several patients more invasive diagnostic procedure.
Ethical approval:
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest:
None to declare
References
- 1.Giordano AV, Arrigoni F, Bruno F, et al. Interventional Radiology Management of a Ruptured Lumbar Artery Pseudoaneurysm after Cryoablation and Vertebroplasty of a Lumbar Metastasis. Cardiovasc Intervent Radiol. 2017;40:776–79. doi: 10.1007/s00270-016-1551-7. [DOI] [PubMed] [Google Scholar]
- 2.Barile A, Arrigoni F, Bruno F, et al. Present role and future perspectives of interventional radiology in the treatment of painful bone lesions. Future Oncol. 2018;14:2945–55. doi: 10.2217/fon-2017-0657. [DOI] [PubMed] [Google Scholar]
- 3.Arrigoni F, Bruno F, Zugaro L, et al. Developments in the management of bone metastases with interventional radiology. Acta Biomed. 2018;89:166–74. doi: 10.23750/abm.v89i1-S.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barile A, Arrigoni F, Zugaro L, et al. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol. 2017;34:53. doi: 10.1007/s12032-017-0909-2. [DOI] [PubMed] [Google Scholar]
- 5.Barile A, La Marra A, Arrigoni F, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89:20150355. doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veltri A, Bargellini I, Giorgi L, Almeida P, Akhan O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB) Cardiovasc Intervent Radiol. 2017;40:1501–13. doi: 10.1007/s00270-017-1658-5. [DOI] [PubMed] [Google Scholar]
- 7.Carberry GA, Lubner MG, Wells SA, Hinshaw JL. Percutaneous biopsy in the abdomen and pelvis: a step-by-step approach. Abdom Radiol (NY) 2016;41:720–42. doi: 10.1007/s00261-016-0667-1. [DOI] [PubMed] [Google Scholar]
- 8.Shao H, McCarthy C, Wehrenberg-Klee E, et al. CT-Guided Percutaneous Needle Biopsy of Retroperitoneal and Pelvic Lymphadenopathy: Assessment of Technique, Diagnostic Yield, and Clinical Value. J Vasc Interv Radiol. 2018;29:1429–36. doi: 10.1016/j.jvir.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Basile A, Carrafiello G, Ierardi AM, Tsetis D, Brountzos E. Quality-improvement guidelines for hepatic transarterial chemoembolization. Cardiovasc Intervent Radiol. 2012;35:765–74. doi: 10.1007/s00270-012-0423-z. [DOI] [PubMed] [Google Scholar]
- 10.Carrafiello G, Mangini M, Fontana F, et al. Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: Preliminary experience. Cardiovasc Intervent Radiol. 2010;33:367–74. doi: 10.1007/s00270-009-9745-x. [DOI] [PubMed] [Google Scholar]
- 11.Laganà D, Carrafiello G, Mangini M, et al. Indications for the use of the Amplatzer vascular plug in interventional radiology. Radiol Med. 2008;113:707–18. doi: 10.1007/s11547-008-0306-1. [DOI] [PubMed] [Google Scholar]
- 12.Carrafiello G, Laganà D, Nosari AM, et al. Utility of computed tomography (CT) and of fine needle aspiration biopsy (FNAB) in early diagnosis of fungal pulmonary infections. Study of infections from filamentous fungi in haematologically immunodeficient patients. Radiol Med. 2006;111:33–41. doi: 10.1007/s11547-006-0004-9. [DOI] [PubMed] [Google Scholar]
- 13.Mangini M, Laganà D, Fontana F, et al. Use of Amplatzer Vascular Plug (AVP) in emergency embolisation: Preliminary experience and review of literature. Emergency Radiology. 2008;15:153–60. doi: 10.1007/s10140-007-0696-8. [DOI] [PubMed] [Google Scholar]
- 14.Laganà D, Carrafiello G, Mangini M, et al. Radiofrequency ablation of primary and metastatic lung tumors: Preliminary experience with a single center device. Surgical Endoscopy and Other Interventional Techniques. 2006;20:1262–67. doi: 10.1007/s00464-005-0607-6. [DOI] [PubMed] [Google Scholar]
- 15.Dionigi G, Dionigi R, Rovera F, et al. Treatment of high output entero-cutaneous fistulae associated with large abdominal wall defects: single center experience. Int J Surg. 2008;6:51–6. doi: 10.1016/j.ijsu.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 16.De Filippo M, Onniboni M, Rusca M, et al. Advantages of multidetector-row CT with multiplanar reformation in guiding percutaneous lung biopsies. Radiol Med. 2008;113:945–53. doi: 10.1007/s11547-008-0325-y. [DOI] [PubMed] [Google Scholar]
- 17.Ierardi AM, Lucchina N, Petrillo M, et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med. 2014;119:483–98. doi: 10.1007/s11547-014-0417-9. [DOI] [PubMed] [Google Scholar]
- 18.Carrafiello G, Dionigi G, Ierardi AM, et al. Efficacy, safety and effectiveness of image-guided percutaneous microwave ablation in cystic renal lesions Bosniak III or IV after 24 months follow up. Int J Surg. 2013;11(1):S30–5. doi: 10.1016/S1743-9191(13)60010-2. [DOI] [PubMed] [Google Scholar]
- 19.Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34:96. doi: 10.1007/s12032-017-0946-x. [DOI] [PubMed] [Google Scholar]
- 20.Vivarelli M, Vincenzi P, Montalti R, et al. ALPPS Procedure for Extended Liver Resections: A Single Centre Experience and a Systematic Review. PLoS One. 2015;10:e0144019. doi: 10.1371/journal.pone.0144019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dialetto G, Reginelli A, Cerrato M, et al. Endovascular stent-graft treatment of thoracic aortic syndromes: A 7-year experience. Eur J Radiol. 2007;64:65–72. doi: 10.1016/j.ejrad.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Pradella S, Lucarini S, Colagrande S. Liver lesion characterization: the wrong choice of contrast agent can mislead the diagnosis of hemangioma. AJR Am J Roentgenol. 2012;199:W662. doi: 10.2214/AJR.12.8951. [DOI] [PubMed] [Google Scholar]
- 23.Cortellini A, Verna L, Porzio G, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: A “hypothesis-generator” preliminary report. Thorac Cancer. 2019;10:347–51. doi: 10.1111/1759-7714.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacobellis F, Segreto T, Berritto D, et al. A rat model of acute kidney injury through systemic hypoperfusion evaluated by micro-US, color and PW-Doppler. Radiol Med. 2018 doi: 10.1007/s11547-018-0962-8. [DOI] [PubMed] [Google Scholar]
- 25.Gatta G, Parlato V, Di Grezia G, et al. Ultrasound-guided aspiration and ethanol sclerotherapy for treating endometrial cysts. Radiol Med. 2010;115:1330–39. doi: 10.1007/s11547-010-0586-0. [DOI] [PubMed] [Google Scholar]
- 26.Grassi R, Cavaliere C, Cozzolino S, et al. Small animal imaging facility: New perspectives for the radiologist. Radiol Med. 2009;114:152–67. doi: 10.1007/s11547-008-0352-8. [DOI] [PubMed] [Google Scholar]
- 27.Barile A, Bruno F, Arrigoni F, et al. Emergency and Trauma of the Ankle. Semi Musc Rad. 2017;21:282–89. doi: 10.1055/s-0037-1602408. [DOI] [PubMed] [Google Scholar]
- 28.Scialpi M, Cappabianca S, Rotondo A, et al. Pulmonary congenital cystic disease in adults. Spiral computed tomography findings with pathologic correlation and management. Radiol Med. 2010;115:539–50. doi: 10.1007/s11547-010-0467-6. [DOI] [PubMed] [Google Scholar]
- 29.Maggialetti N, Capasso R, Pinto D, et al. Diagnostic value of computed tomography colonography (CTC) after incomplete optical colonoscopy. Int J Surg. 2016;33(1):S36–44. doi: 10.1016/j.ijsu.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 30.Di Cesare E, Gennarelli A, Di Sibio A, et al. Image quality and radiation dose of single heartbeat 640-slice coronary CT angiography: A comparison between patients with chronic Atrial Fibrillation and subjects in normal sinus rhythm by propensity analysis. Eur J Radiol. 2015;84:631–36. doi: 10.1016/j.ejrad.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Regine G, Stasolla A, Miele V. Multidetector computed tomography of the renal arteries in vascular emergencies. Eur J Radiol. 2007;64:83–91. doi: 10.1016/j.ejrad.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 32.De Cecco CN, Buffa V, Fedeli S, et al. Preliminary experience with abdominal dual-energy CT (DECT): True versus virtual nonenhanced images of the liver. Radiol Med. 2010;115:1258–66. doi: 10.1007/s11547-010-0583-3. [DOI] [PubMed] [Google Scholar]
- 33.Buffa V, Solazzo A, D’Auria V, et al. Dual-source dual-energy CT: dose reduction after endovascular abdominal aortic aneurysm repair. Radiol Med. 2014;119:934–41. doi: 10.1007/s11547-014-0420-1. [DOI] [PubMed] [Google Scholar]
- 34.Valentini V, Buquicchio GL, Galluzzo M, et al. Intussusception in Adults: The Role of MDCT in the Identification of the Site and Cause of Obstruction. Gastroenterol Res Pract. 2016;2016:5623718. doi: 10.1155/2016/5623718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Cesare E, Patriarca L, Panebianco L, et al. Coronary computed tomography angiography in the evaluation of intermediate risk asymptomatic individuals. Radiol Med. 2018;123:686–94. doi: 10.1007/s11547-018-0898-z. [DOI] [PubMed] [Google Scholar]
- 36.Masciocchi C, Arrigoni F, Ferrari F, et al. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med Oncol. 2017;34:52. doi: 10.1007/s12032-017-0906-5. [DOI] [PubMed] [Google Scholar]
- 37.Splendiani A, D’Orazio F, Patriarca L, et al. Imaging of post-operative spine in intervertebral disc pathology. Musculoskelet Surg. 2017;101:75–84. doi: 10.1007/s12306-017-0453-4. [DOI] [PubMed] [Google Scholar]
- 38.Arrigoni F, Gregori LM, Zugaro L, Barile A, Masciocchi C. MRgFUS in the treatment of MSK lesions: A review based on the experience of the university of L’aquila, Italy. Transl Cancer Res. 2014;3:442–48. [Google Scholar]
- 39.Battipaglia G, Avilia S, Morelli E, Caranci F, Perna F, Camera A. Posterior reversible encephalopathy syndrome (PRES) during induction chemotherapy for acute myeloblastic leukemia (AML) Ann Hematol. 2012;91:1327–28. doi: 10.1007/s00277-011-1398-6. [DOI] [PubMed] [Google Scholar]
- 40.Tedeschi E, Caranci F, Giordano F, Angelini V, Cocozza S, Brunetti A. Gadolinium retention in the body: what we know and what we can do. Radiol Med. 2017;122:589–600. doi: 10.1007/s11547-017-0757-3. [DOI] [PubMed] [Google Scholar]
- 41.Briganti F, Leone G, Marseglia M, Cicala D, Caranci F, Maiuri F. P64 Flow Modulation Device in the treatment of intracranial aneurysms: Initial experience and technical aspects. J Neurointerv Surg. 2016;8:173–80. doi: 10.1136/neurintsurg-2015-011743. [DOI] [PubMed] [Google Scholar]
- 42.Arrigoni F, Barile A, Zugaro L, et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol. 2017;34:55. doi: 10.1007/s12032-017-0904-7. [DOI] [PubMed] [Google Scholar]
- 43.Cirillo M, Caranci F, Tortora F, et al. Structural neuroimaging in dementia. J Alzheimers Dis. 2012;29:16–19. [Google Scholar]
- 44.Mocchegiani F, Vincenzi P, Coletta M, et al. Prevalence and clinical outcome of hepatic haemangioma with specific reference to the risk of rupture: A large retrospective cross-sectional study. Dig Liver Dis. 2016;48:309–14. doi: 10.1016/j.dld.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Schicchi N, Valeri G, Moroncini G, et al. Myocardial perfusion defects in scleroderma detected by contrast-enhanced cardiovascular magnetic resonance. Radiol Med. 2014;119:885–94. doi: 10.1007/s11547-014-0419-7. [DOI] [PubMed] [Google Scholar]
- 46.Tarantini G, Favaretto E, Napodano M, et al. Design and methodologies of the postconditioning during coronary angioplasty in acute myocardial infarction (POST-AMI) trial. Cardiology. 2010;116:110–16. doi: 10.1159/000316967. [DOI] [PubMed] [Google Scholar]
- 47.Salvolini L, Urbinati C, Valeri G, Ferrara C, Giovagnoni A. Contrast-enhanced MR cholangiography (MRCP) with GD-EOB-DTPA in evaluating biliary complications after surgery. Radiol Med. 2012;117:354–68. doi: 10.1007/s11547-011-0731-4. [DOI] [PubMed] [Google Scholar]
- 48.De Filippo M, Saba L, Rossi E, et al. Curved Needles in CT-Guided Fine Needle Biopsies of Abdominal and Retroperitoneal Small Lesions. Cardiovasc Intervent Radiol. 2015;38:1611–6. doi: 10.1007/s00270-015-1107-2. [DOI] [PubMed] [Google Scholar]
- 49.Lorentzen T, Nolsoe CP, Ewertsen C, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part I. General Aspects (long Version) Ultraschall Med. 2015;36:E1–14. doi: 10.1055/s-0035-1553593. [DOI] [PubMed] [Google Scholar]
- 50.Gupta P, Rajwanshi A, Nijhawan R, et al. Fine needle aspiration in retroperitoneal lesions. APMIS. 2017;125:16–23. doi: 10.1111/apm.12627. [DOI] [PubMed] [Google Scholar]
- 51.Tomozawa Y, Inaba Y, Yamaura H, et al. Clinical value of CT-guided needle biopsy for retroperitoneal lesions. Korean J Radiol. 2011;12:351–7. doi: 10.3348/kjr.2011.12.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandt KR, Charboneau JW, Stephens DH, Welch TJ, Goellner JR. CT- and US-guided biopsy of the pancreas. Radiology. 1993;187:99–104. doi: 10.1148/radiology.187.1.8451443. [DOI] [PubMed] [Google Scholar]
- 53.Misra RK, Mitra S, Jain RK, Vahikar S, Bundela A, Misra P. Image-guided fine needle cytology with aspiration versus non-aspiration in retroperitoneal masses: is aspiration necessary? J Pathol Transl Med. 2015;49:129–35. doi: 10.4132/jptm.2015.01.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta S, Wallace MJ, Cardella JF, et al. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol. 2010;21:969–75. doi: 10.1016/j.jvir.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Shyn PB, Tatli S, Sahni VA, et al. PET/CT-guided percutaneous liver mass biopsies and ablations: targeting accuracy of a single 20 s breath-hold PET acquisition. Clin Radiol. 2014;69:410–5. doi: 10.1016/j.crad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Winter TC, Lee FT Jr, Hinshaw JL. Ultrasound-guided biopsies in the abdomen and pelvis. Ultrasound Q. 2008;24:45–68. doi: 10.1097/RUQ.0b013e318168c869. [DOI] [PubMed] [Google Scholar]
- 57.Sainani NI, Arellano RS, Shyn PB, Gervais DA, Mueller PR, Silverman SG. The challenging image-guided abdominal mass biopsy: established and emerging techniques ‘if you can see it, you can biopsy it’. Abdom Imaging. 2013;38:672–96. doi: 10.1007/s00261-013-9980-0. [DOI] [PubMed] [Google Scholar]
- 58.Akan H, Ozen N, Incesu L, Gumus S, Gunes M. Are percutaneous transgastric biopsies using 14-, 16- and 18-G Tru-Cut needles safe? An experimental study in the rabbit. Australas Radiol. 1998;42:99–101. doi: 10.1111/j.1440-1673.1998.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 59.Stewart CJ, Coldewey J, Stewart IS. Comparison of fine needle aspiration cytology and needle core biopsy in the diagnosis of radiologically detected abdominal lesions. J Clin Pathol. 2002;55:93–7. doi: 10.1136/jcp.55.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol. 2011;66:1007–14. doi: 10.1016/j.crad.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 61.Harisinghani MG, Gervais DA, Hahn PF, et al. CT-guided transgluteal drainage of deep pelvic abscesses: indications, technique, procedure-related complications, and clinical outcome. Radiographics. 2002;22:1353–67. doi: 10.1148/rg.226025039. [DOI] [PubMed] [Google Scholar]
- 62.Wittmann TA, Abel EJ. Percutaneous biopsy in large, locally advanced or metastatic renal tumors. Urol Oncol. 2017;35:87–91. doi: 10.1016/j.urolonc.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Bertolini L, Vaglio A, Bignardi L, et al. Subclinical interstitial lung abnormalities in stable renal allograft recipients in the era of modern immunosuppression. Transplantation Proceedings. 2011;43:2617–2623. doi: 10.1016/j.transproceed.2011.06.033. ISSN: 0041-1345, doi: 10.1016/j.transproceed.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 64.Palma BD, Guasco D, Pedrazzoni M, et al. Osteolytic lesions, cytogenetic features and bone marrow levels of cytokines and chemokines in multiple myeloma patients: Role of chemokine (C-C motif) ligand20. Leukemia. 2016 Feb;30(2):409–16. doi: 10.1038/leu.2015.259. doi: 10.1038/leu.2015.259. Epub 2015 Sep 30. [DOI] [PubMed] [Google Scholar]
- 65.Bozzetti C, Nizzoli R, Tiseo M, et al. ALK and ROS1 rearrangements tested by fluorescence in situ hybridization in cytological smears from advanced non-small cell lung cancer patients. Diagnostic Cytopathology. 43:941–946. doi: 10.1002/dc.23318. ISSN: 8755-1039, doi: 10.1002/dc.23318. [DOI] [PubMed] [Google Scholar]
- 66.De Filippo M, Gira F, Corradi D, Sverzellati N, Zompatori M, Rossi C. Benefits of 3D technique in guiding percutaneous retroperitoneal biopsies. RAD. MED. 2011;116(3):407–416. doi: 10.1007/s11547-010-0604-2. ISSN: 0033-8362, doi: 10.1007/s11547-010-0604-2. [DOI] [PubMed] [Google Scholar]
- 67.Barile A, Bruno F, Mariani S, et al. What can be seen after rotator cuff repair: a brief review of diagnostic imaging findings. Musculoskelet Surg. 2017 Mar;101(1):3–14. doi: 10.1007/s12306-017-0455-2. doi: 10.1007/s12306-017-0455-2. Epub 2017 Feb 13. Review. [DOI] [PubMed] [Google Scholar]