Abstract
This article reviews the main toxic effect, complications and relative imaging findings of the liver that may appear during the oncologic follow up among patients affected by gastrointestinal malignancy. Awareness of the causative chemotherapeutic agent and regimens, pathophysiology and relative characteristic imaging findings of hepatic injuries is critical in order to obtain an accurate diagnosis especially when these parenchymal lesions are focal. An accurate synergic radiological diagnosis with Computed Tomography (CT) and Magnetic Resonance (MR) techniques may induce a potential termination of ineffective/toxic chemotherapy during early phases of treatment, changing the therapeutic plan in order to avoid first unnecessary liver biopsy and then invasive treatment as hepatic resection if not required. (www.actabiomedica.it)
Keywords: colorectal cancer-chemotherapy-induced focal hepatopathy, steatosis, steatohepatitis, sinusoidal obstruction syndrome, liver MR - hepatobiliary phase-Liver CT
1. Introduction
Nearly one in two men and one in three women in the United States will be affected by cancer during the lifetime (1). Colorectal cancer is the third most common type of cancer diagnosed in men and the second in women. The liver is the most frequent site of colorectal cancer metastases and up to 25% of patients present hepatic metastases at the time of diagnosis of the primary tumour (synchronous); another 25% will develop metachronous ones during the follow up (2) (3-6).
Ultrasonography (US), Magnetic Resonance Imaging (MRI) (7-17) and computed tomography (CT) (18-30) are widely used in the diagnostic setting, with or without the use of contrast agents (23, 31-33), and as guidance in many interventional radiology procedures (34-40). US is the least invasive imaging examination, well tolerated by patients (41-43).
Liver hepatectomy still represents the best curative therapeutic option for patients with colorectal metastases even if it is often preceded by chemotherapy in the preoperative setting (neoadjuvant chemotherapy) because only 15-25% of patients are fit for the curative metastasectomy at the time of presentation (44).
This medical treatment can reduce the size of colorectal liver metastases, downsize the present metastases and may provide to a presumptive treatment of micro metastases (45).
Unfortunately, micro metastases (less than 10 mm) are nearly undetectable using radiological imaging being the major cause of recurrence during follow up. Differentiation of small haemangiomas and cysts smaller than 1 cm from metastases can be difficult due to volume averaging. The sensitivity of CT for detecting lesions less than 1 cm decreases from 65%-95% to 31%-38% (46, 47).
This article reviews the toxic effect, complications and relative imaging findings of the liver that may appear during the oncologic follow up among patients affected by gastrointestinal malignancy. Radiologists should know that in addition to the desired effects on malignancy, systemic oncological therapy could determine toxic effects whose are often visible first at imaging (48, 49).
2. Background
Any type of drug is able to induce changes in biological function and so to modify cell and organs function.
This modify can be positive or negative/toxic: it depends on concentration, dose and patient’s own characteristics determining eventually drug adverse reaction that are predictable in most of cases.
Drug arrive to the target organs by steps. Pharmacokinetics studies processes that follow the administration of the drugs: absorption, metabolism and excretion. Through distribution, drugs arrive to the target organs to make its pharmacological effect (50).
Each of these steps is influenced by drug molecular structure (e.g. lipophilia), physiological characteristic such as pregnancy, age or nutritional state and patient pathologies such as hepatic or kidney’s injury, cardiovascular disease or neoplastic ones.
Hepatic metabolism represents a crucial step because in most of cases drugs have to be transformed into more hydrophilic compounds in order to be eliminated easily by kidney and/or liver.
Chemotherapy traditionally includes cytotoxic agents because their own mechanism of action consists in the capacity of induction a cell damage that can be lethal for sensible cells, through a direct damn or interference in the replicative process of the proliferating cells. These agents have low therapeutic index because they’re not specific for tumoral cells and they can cause toxicity especially to normal proliferating tissues (e.g. bone marrow).
Unfortunately, solid tumors (like colorectal malignancy) aren’t sensible to these types of drugs compared with lymphoma or testis tumor so they should be associated to others in order to improve the therapeutic effect.
Nowadays newer agents such as molecular targeted therapies and immunological agents are available in clinical practice as monotherapy or in combination with each other.
2.1 Chemotherapy for gastrointestinal malignancy
Patients with advanced stage disease could require different types of chemotherapy (preoperative, postoperative or palliative chemotherapy) (51). Preoperative therapy, so called neoadjuvant chemotherapy, offers the potential advantage of eradicating micro metastatic disease preoperatively improving progression free survival especially through innovative associations of agents with the aim to ensure a multimodal treatment for colorectal liver metastases (2). In selected patients, unresectable metastatic disease can be rendered resettable by administering “conversion chemotherapy” in order to downsize the tumor and make possible a surgical resection increasing the number of patients undergoing curative hepatectomy. The duration of both these regimens of chemotherapy should be assessed as short as possible because of the risk of hepatic injury associated (52).
2.1.1 Alchilant agents (oxaliplatin)
They have the ability to react with DNA creating irreversible damage and lethal effect to the cell. One of these drugs called oxaliplatin is frequently used in combination with 5-FU/leucovorin or capecitabine for the treatment of gastrointestinal tumors. Toxicity, generally dose dependent, is represented by peripheral neuropathy and impose dose reduction. Oxaliplatin-based chemotherapy regimens (FOLFOX, CapeOX and FLOX) are recommended by NCCN for adjuvant treatment in colorectal cancer patients (8) and as neoadjuvant therapy in combination with 5-FU in patients with colorectal liver metastases.
2.1.2 Antimetabolite agents (fluorouracil and capecitabine)
Because of their similitude with physiological metabolites, fluoropyrimidine such as fluorouracil (5-FU) can interfere with RNA synthesis and function and determine myelotoxicity as adverse reaction. 5-FU is administrated intravenously while capecitabine is a prodrug that is converted in the intestine into the active 5-FU and it’s given orally (53).
2.1.3 Topoisomerase inhibitor (irinotecan)
Irinotecan reversibly stabilizes the topoisomerase. I complex, blocking DNA synthesis with a double-strand DNA break. This event induces arrest of the cell cycle in the S-G2 phase and ultimately cause cell death (53).
2.1.4 Target therapy (bevacizumab)
Bevacizumab is a monoclonal antibody that binds to vascular endothelial growth factor (VEGF) in the circulation and inhibits its connection to the receptor VEGFR. This complex prevents new vessel formation, reduces capillary leak and normalizes tumour vasculature (54).
3. Hepatic adverse injuries
Chemotherapy induces many undesirable effects against the hepatic parenchyma that may reduce and/or make difficult the detection of the hepatic tumor burden in patients with liver metastases. As patients with metastatic tumors undergo chemotherapy with curative intent with increasing frequency, it is mandatory therefore to understand the pathophysiology of these therapy-induced liver injury in order to be familiar with their imaging features .
3.1 Sinusoidal obstruction syndrome (SOS): pathophysiology and imaging features
Rubbia et al. observed that the neoadjuvant administration of oxaliplatin in patients with colorectal liver metastases was a risk factor for the development of a specific liver injury called sinusoidal obstruction syndrome (55, 56). Bevacizumab seems to have a protective effect against oxaliplatin-related sinusoidal lesions (57). This sinusoidal injury occurred for 19–52% of patients treated by oxaliplatin-based chemotherapy (58-61). Patients could present abdominal pain, swelling, and weight gain, with or without elevation in serum enzyme levels (62).
SOS includes several pathologic conditions such as sinusoidal dilatation, peliosis, and nodular regenerative hyperplasia.
The major component initiating SOS seems to be the depolymerization of the F-actin and the increased expression of matrix metalloproteinase-9 in sinusoidal endothelial cells.
The sinusoidal wall integrity is then disrupted causing red blood cells migration into the space of Disse and deposition of collagens determining respectively peliosis and perisinusoidal fibrosis (63-66). Furthermore, the obstruction and increased pressure in the sinusoid determine presence of atrophic hepatocytes and also enlarged ones forming nodular regenerative hyperplasia (67). The discover and relative diagnosis of SOS could be important clinically for at least three reasons. First it is associated with an increased risk of morbidity after liver resection and bleeding. Particularly SOS has been associated with an increased risk for intraoperative blood transfusions, early recurrence after resection and a short overall survival after resection due to liver insufficiency (60, 68). Recently another interesting reported side effect is the development of liver nodules mimicking liver metastases (69, 70) misinterpreted as hepatic metastasis (71). Finally radiologists have to consider the development of oxaliplatin-induced SOS to avoid mistaking new-onset ascites for evidence of recurrent disease (72).
However, US findings include ascites, gallbladder wall thickening, and hepatosplenomegaly. Doppler US may show decreased flow in the portal vein (73). Common signs of a new-onset portal hypertension on CT examination could appear, including ascites, splenomegaly, periesophageal varices, and recanalization of the umbilical vein. Increased volume of the spleen has been reported to suggest sinusoidal injury (74-76); however, increased spleen size indicates portal hypertension and it is not specific for SOS (77). Han et al. reported that post-oxaliplatin “heterogeneity” of liver parenchyma, appearing as diffuse and heterogeneous hypoattenuation of the hepatic parenchyma on contrast-enhanced CT, is frequently observed in patients treated with oxaliplatin (45, 77). These findings are especially observed at the peripheral area and right hepatic lobe. At MR, diffused SOS is detectable by T2-weighted images showing a heterogeneous liver with areas of increased signal intensity corresponding to edema (47). Heterogeneous reticular pattern are also found in the hepatic parenchyma on hepatobiliary phase (HBP) MRI of the liver using gadoxetate disodium (78, 79) . However morphological imaging modalities, such as CT or US, are not enough suitable for the diagnosis of a pseudotumor caused by SOS (80). Focally lesions of SOS show an ill-defined margin (considered as the most valuable feature), non-spherical shape, isointensity on T1-weighted images, iso or hyper-signal intensity on T2-weighted images, unlike of a metastatic nodule. Gd-EOB MRI nevertheless displays a defect in the hepatocyte phase, similar to imaging findings of colorectal liver metastasis (47). Therefore, diffusion-weighted MRI, may be fundamental because the cellular density is higher in cancer than in pseudotumor (81).
Figure 1.
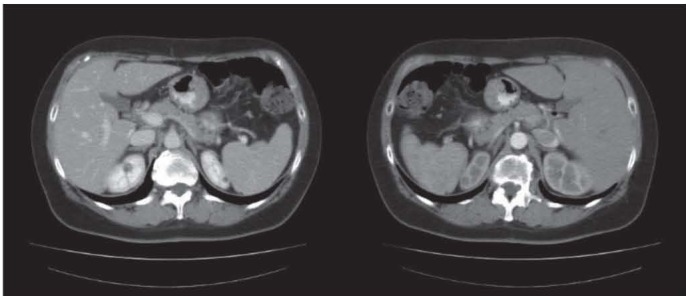
55-year old woman affected by left colon adenocarcinoma who undergoes to left hemicolectomy. We may observe in this preoperative CT diffuse low attenuation of the liver
Figure 2.
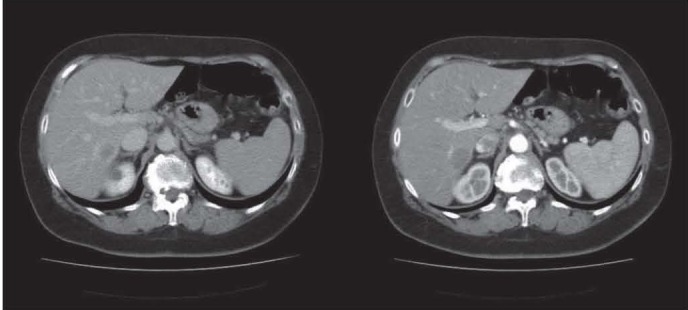
After the surgical resection of the primary tumor, the histological staging is pT3N1M1 for the presence, in the first post-operative CT, of a nodular hypodense lesion surrounded by rim enhancement with the appereance of a colorectal liver metastases
Figure 3.
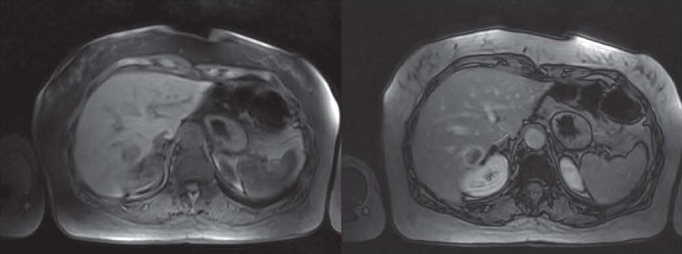
MR dynamic imaging confirms CT diagnosis of a colorectal metastase. This lesion is hypointense in T1w images before and after administration of contrast agent compared to the surrounding liver
Figure 4.
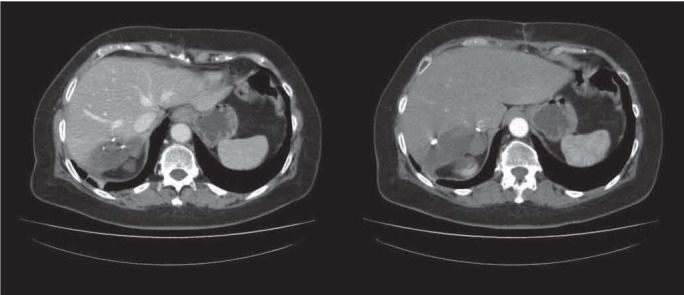
After a six-cycles-Folfox neoadjuvant regimen, these CT post-operative imaging shows common findings after surgical resection. The hepatic malignant lesion is confermed with the addiction of “blue liver” as chemotherapeutic liver adverse reaction
3.2 Focal nodular hyperplasia-like lesions
Chemotherapeutic regimens with OXP may lead to the appearance of focal nodule hyperplasia (FNH) like lesions. It is very important to differentiate this type of pseudo metastases from the real ones during follow-up. This kind of diagnosis seems to be more suitable with MR images. Commonly FNK-like lesions appear as solitary or multiple nodular and well demarcated peripherally located liver lesions exhibiting significant contrast enhancement on hepatobiliary phase (77).
Images similarly to FNH ones, representing a benign hyperplasia of the hepatic parenchyma, maybe linked to a vascular injury with increased arterial perfusion in areas with absent portal blood flow (77). In these lesions’ overexpression of OATP8 that is the uptake transporter of gadoxetic acid may be due to increased hepatocyte function to compensate diffuse liver injury (82, 83).
3.3 Pseudocirrhosis: pathophysiology and imaging features
Pseudocirrhosis describes diffuse and heterogenic hepatic parenchyma due to the contemporary presence of capsular retraction and nodular regenerative hyperplasia. This setting is however more common in patients undergoing chemotherapy for breast cancer (up to 50%) of patients (84-87). CT imaging shows first initial loss of the normal convex edge of the liver, with the presence of metastases followed by capsular retraction. It is very important to discontinue therapy in order to avoid progression in fibrosis, especially when this structural liver morphological change becomes severe with the occurrence of ascites, varices and splenomegaly, similar to true cirrhosis signs of portal hypertension. A recent case report shows the singular diagnosis of esophageal varices without liver dysfunction, after 3.5-year follow-up of the oxaliplatin-based chemotherapy (88).
3.4 Portal vein thrombosis
Portal vein branch thrombosis may appear after chemotherapy regimens with 5-FU and irinotecan (FOLFIRI) and bevacizumab (89, 90). The latter binds to the VEGF receptor and decreases the healing capacity of endothelial cells, determining bleeding and thrombosis. The mechanism by which irinotecan may determine thrombosis is not known. Patients with portal vein thrombosis are usually asymptomatic so the first diagnosis is often reached by imaging. Portal vein thrombosis is seen as a filling defect in the portal vein branch. In the arterial phase this wedge-shaped area shows increased enhancement that becomes isoattenuating compared to the liver in the further phases (90).
3.5 Steathosis and steatohepatitis: pathophysiology and imaging features
Many studies show that some chemotherapeutic agents, such as 5-FU and irinotecan, may determine chemotherapy-induced steatosis (51). The form of nonalcoholic steatohepatitis linked to chemotherapy is called chemotherapy-associated steatohepatitis (CASH) (91). The frequency of this occurrence is unknown (65, 92, 93). The combination of irinotecan and 5-fluorouracil (FOLFIRI) should be used carefully therefore in patients who are predisposed to fatty liver, mainly for those who can be eligible for liver resection. Hepatic steatosis increases morbidity after liver resection and the presence of steatohepatitis has been associated with a higher 90-day mortality rate (93, 94). It is difficult to distinguish between steatosis and steatohepatitis through imaging features. However hepatic steatosis is characterized by deposition of lipid vesicles in hepatocytes while steatohepatitis is marked by ballooning of hepatocytes, lobular inflammation, or degeneration of hepatocytes (95). At imaging, steatosis can be focal or diffuse. At ultrasonography (US), the hepatic parenchyma shows increased echogenicity while at CT low attenuation compared to the spleen (at least 10 HU at unenhanced CT) (90). At MR imaging with in-phase and out-of-phase gradient-echo sequences, the presence of signal loss (dropout) on out-of-phase images when compared with in-phase images confirms the presence of steatosis. The pattern of fatty deposition may be also focal mimicking metastases. However, in this case MRI allows to obtain a more reliable diagnosis because unlike steatosis there is no signal drop on the opposed phase in the images of metastasis (95). According to Unal et al., focal steatosis liver parenchyma may show decreased hepatocyte function and signal on MRI Gd-EOB-DTPA-enhanced liver while fat spared areas may demonstrate compensatory increased hepatocyte function on the same phase similarly to FNH-like lesions. Anyway, in the latter case diagnosis could be easily reached with T1w in- and out-of-phase (77)
4. Discussion
Follow up in oncology represents the period of time that starts after the first treatment with a curative intent. Follow up for colorectal cancer has become much longer because of the increased median overall survival of these patients due principally to the improving efficacy of modern chemotherapeutic regimens (96).
The current concept of multidisciplinary treatment and management of patient affected by colorectal malignancy has been decisive to reach optimal outcomes.
In this team, radiologists must be aware of their crucial role. Mainly during chemotherapy, imaging diagnosis is necessary to evaluate:
- treatment response;
- detection of metastases and recurrence;
- restaging of the malignancy.
CT is currently the most commonly used first-line imaging modality for oncologic monitoring because of its wide availability and reproducibility (97). CT, is also a valuable diagnostic tool for the diagnosis and the guidance of interventional procedures in a wide range of organs and in the in gastrointestinal systems (98-103).
Regarding treatment response during follow up, the effects of conventional chemotherapeutic agents are assessed generally after three to four cycles of chemotherapy (after about 1 to 2 months into the therapy) and changes in lesion sizes, as classified according to Response Evaluation Criteria in Solid Tumor (RECIST) are used to planning further decision (104, 105). However, it is already known that new imaging criteria are needed to better characterize tumor response actually. Hepatic lesions, when treated through regimens with molecularly targeted therapeutic agents, may be responding to treatment even without change in size .
Regarding the detection of metastases (hepatic tumor burden), we should remember indeed the effect of chemotherapy first on the hepatic metastases itself and then on the surrounding liver parenchymal.
Han et al demonstrated a correlation between treatment response of colorectal liver metastases and SOS in patient who have undergone oxaliplatin-based chemotherapy: the more severe is SOS, evaluated by CT parenchymal heterogeneity, the worse the tumor response is expected to be (45).
Hepatic hypoperfusion due to sinusoidal obstruction syndrome might induce hepatic hypoxia, reducing the response to chemotherapy and increasing instead the invasiveness of the tumor in the surrounding stroma (106).
Until now, in a patient with a story of gastrointestinal malignancy, radiologists have considered the appearance of each new hepatic nodule first as a new metastatic lesionn (51). This possible setting could indicate progression disease and change in therapeutic planning. It is important to recognize therefore parenchymal changes due to systemic therapy in order to make differential diagnosis especially from metastases when these structural changes are focal (pseudo metastases) (96).
During follow up with CT examination it might be possible to discover new indeterminate hepatic lesion or diffuse changes in the hepatic parenchyma that make difficult the detection of malignancy. Radiologists should be aware of the possibility that a new developing liver lesion is not always a new metastasis.
Multi-detector row CT represents the modality of choice for oncologic surveillance thanks to its availability and efficiency (23, 97, 107, 108); nowadays, for the complexity of the questions that radiologists have to answer, morphological CT study should be more often associated with other emerging functional and molecular imaging techniques.
CT perfusion parameters for example seems to predict properly the presence and extent of tumor vessels (109-112). Even if CT perfusion is a technique actually available mainly in research studies, it should be considered in future to improve earlier detection of liver malignancies and more individualized monitoring of patients during treatment, especially for molecular targeted therapies that act on on tumor perfusion.
In order to assess a better diagnosis and to quantify properly the hepatic tumor burden, liver dynamic MR examination with DWI/ADC (113) and contrast-hepatobiliary phase should be recommended. Multidetector CT has a specificity of 67% in characterizing lesions as benign or malignant, compared with 81% for MR imaging (47). The use of heavily T2-weighted images may help differentiate solid malignant lesions from hemangiomas and cysts (46).
Furthermore hepatocyte-specific contrast-enhanced MR imaging detects more metastatic lesions than does conventional MR imaging and should be used particularly for the follow-up of metastases after systemic or liver-directed therapies (114). Hepatic metastases typically appear hypointense relative to the surrounding liver parenchyma on delayed images, whereas “pseudo metastases” lesions such as focal nodular hyperplasia are visible as iso- or hyperintense. DW imaging helps the detection of small lesions and apparent diffusion coefficient (ADC) values can be useful to estimate diffusion restriction, differentiating metastatic lesions whose show high-signal-intensity with low ADC values (46, 114). Multiparametric MR examination seems to be necessary also for the pre-operative planning after neoadjuvant chemotherapy regimens with the aim to obtain the most reliable re-staging of the hepatic tumor burden. Systemic chemotherapy in the preoperative setting improves the potential benefit of surgery (115, 116) and this downsizing therapy represent the major reason for the yearly increase in the number of liver resections for colorectal liver metastases (44). Nowadays surgeons estimate that future liver remnant volume after hepatectomy can be as low as 20% if there is no evidence of injury in the remaining liver tissue (117). MR should be recommended therefore also to estimate the quality of the future remnant parenchyma.
MR pre-operative imaging features should be accurately considered because after curative resection in the context of liver surgery, chemotherapy-induced liver injury could increase the risks of intra- and postoperative complications and postoperative liver insufficiency (118). Preoperative diagnosis of these hepatic injuries seems to be important in order to choose the optimal timing for hepatic resection. Karoui et al. demonstrated that morbidity after liver resection was associated with the number of preoperative chemotherapy cycles: patients who received more than 6 cycles of chemotherapy increased morbidity (61). Another issue to consider is that the time interval between cessation of last chemotherapy predicts the possibility to have post-operative liver failure: an interval of less than four weeks was associated with more complications (59, 119).
The desirable aim would be avoiding liver needle biopsy as much as possible because of its invasive nature of carries inherent risks such as infection, requiring local anesthesia or patient sedation (104). In addition, biopsies can potentially stimulate neoangiogenesis by damaging tumor tissue and increase metastatic risk by increasing the number of circulating tumor cells (120).
5. Conclusion
It seems to be necessary to establish common standard radiological findings criteria first to recognize and assess chemotherapy liver adverse injuries (121-125) with the aim to achieve early and accurate diagnosis, especially when these parenchymal lesions are focal. An accurate synergic radiological diagnosis with CT and MR techniques may induce a potential termination of ineffective/toxic chemotherapy during early phases of treatment, changing the therapeutic plan in order to avoid first unnecessary liver biopsy and then invasive treatment as hepatic resection if not required. A more personalized approach of cancer treatment would be desirable by assessment of CT/MR imaging biomarker determining treatment response where the aim is to demonstrate that drugs may have an effect on tumor biology.
Conflict of interest:
None to declare
References
- 1.Birch JC, Khatri G, Watumull LM, Arriaga YE, Leyendecker JR. Unintended Consequences of Systemic and Ablative Oncologic Therapy in the Abdomen and Pelvis. Radiographics. 2018;38:1158–79. doi: 10.1148/rg.2018170137. [DOI] [PubMed] [Google Scholar]
- 2.Duwe G, Knitter S, Pesthy S, et al. Hepatotoxicity following systemic therapy for colorectal liver metastases and the impact of chemotherapy-associated liver injury on outcomes after curative liver resection. Eur J Surg Oncol. 2017;43:1668–81. doi: 10.1016/j.ejso.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–74. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 6.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–9. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muccio CF, Di Blasi A, Esposito G, Brunese L, D’Arco F, Caranci F. Perfusion and spectroscopy magnetic resonance imaging in a case of lymphocytic vasculitis mimicking brain tumor. Pol J Radiol. 2013;78:66–69. doi: 10.12659/PJR.884011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirillo M, Caranci F, Tortora F, et al. Structural neuroimaging in dementia. J Alzheimers Dis. 2012;29:16–19. [Google Scholar]
- 9.Di Cesare E, Cademartiri F, Carbone I, et al. Clinical indications for the use of cardiac MRI. by the SIRM Study Group on Cardiac Imaging. Radiol Med. 2013;118:752–98. doi: 10.1007/s11547-012-0899-2. [DOI] [PubMed] [Google Scholar]
- 10.Schicchi N, Valeri G, Moroncini G, et al. Myocardial perfusion defects in scleroderma detected by contrast-enhanced cardiovascular magnetic resonance. Radiol Med. 2014;119:885–94. doi: 10.1007/s11547-014-0419-7. [DOI] [PubMed] [Google Scholar]
- 11.Tarantini G, Favaretto E, Napodano M, et al. Design and methodologies of the postconditioning during coronary angioplasty in acute myocardial infarction (POST-AMI) trial. Cardiology. 2010;116:110–16. doi: 10.1159/000316967. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis F, Berritto D, Somma F, et al. Magnetic resonance imaging: A new tool for diagnosis of acute ischemic colitis? World J Gastroenterol. 2012;18:1496–501. doi: 10.3748/wjg.v18.i13.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappabianca S, Granata V, Di Grezia G, et al. The role of nasoenteric intubation in the MR study of patients with Crohn’s disease: Our experience and literature review. Radiol Med. 2011;116:389–406. doi: 10.1007/s11547-010-0605-1. [DOI] [PubMed] [Google Scholar]
- 14.Zappia M, Capasso R, Berritto D, et al. Anterior cruciate ligament reconstruction: MR imaging findings. Musculoskelet Surg. 2017;101:23–35. doi: 10.1007/s12306-017-0460-5. [DOI] [PubMed] [Google Scholar]
- 15.Barile A, Arrigoni F, Bruno F, et al. Computed Tomography and MR Imaging in Rheumatoid Arthritis. Radiol Clin North Am. 2017;55:997–1007. doi: 10.1016/j.rcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Francone M, Di Cesare E, Cademartiri F, et al. Italian registry of cardiac magnetic resonance. Eur J Radiol. 2014;83:e15–e22. doi: 10.1016/j.ejrad.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Maurizi N, Passantino S, Spaziani G, et al. Long-term Outcomes of Pediatric-Onset Hypertrophic Cardiomyopathy and Age-Specific Risk Factors for Lethal Arrhythmic Events. JAMA Cardiol. 2018;3:520–25. doi: 10.1001/jamacardio.2018.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassi R, Rambaldi PF, Di Grezia G, et al. Inflammatory bowel disease: Value in diagnosis and management of MDCT-enteroclysis and 99mTc-HMPAO labeled leukocyte scintigraphy. Abdom Imaging. 2011;36:372–81. doi: 10.1007/s00261-010-9652-2. [DOI] [PubMed] [Google Scholar]
- 19.Scialpi M, Cappabianca S, Rotondo A, et al. Pulmonary congenital cystic disease in adults. Spiral computed tomography findings with pathologic correlation and management. Radiol Med. 2010;115:539–50. doi: 10.1007/s11547-010-0467-6. [DOI] [PubMed] [Google Scholar]
- 20.Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: Comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968–78. doi: 10.1007/s11547-012-0827-5. [DOI] [PubMed] [Google Scholar]
- 21.Bertolini L, Vaglio A, Bignardi L, et al. Subclinical interstitial lung abnormalities in stable renal allograft recipients in the era of modern immunosuppression. Transplant Proc. 2011;43:2617–23. doi: 10.1016/j.transproceed.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Sverzellati N, Calabrò E, Chetta A, et al. Visual score and quantitative CT indices in pulmonary fibrosis: Relationship with physiologic impairment. Radiol Med. 2007;112:1160–72. doi: 10.1007/s11547-007-0213-x. [DOI] [PubMed] [Google Scholar]
- 23.Cappabianca S, Porto A, Petrillo M, et al. Preliminary study on the correlation between grading and histology of solitary pulmonary nodules and contrast enhancement and [18F] fluorodeoxyglucose standardised uptake value after evaluation by dynamic multiphase CT and PET/CT. J Clin Pathol. 2011;64:114–19. doi: 10.1136/jcp.2010.076562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggialetti N, Ferrari C, Minoia C, et al. Role of WB-MR/DWIBS compared to 18F-FDG PET/CT in the therapy response assessment of lymphoma. Radiol Med. 2016;121:132–43. doi: 10.1007/s11547-015-0581-6. [DOI] [PubMed] [Google Scholar]
- 25.Di Cesare E, Gennarelli A, Di Sibio A, et al. Image quality and radiation dose of single heartbeat 640-slice coronary CT angiography: A comparison between patients with chronic Atrial Fibrillation and subjects in normal sinus rhythm by propensity analysis. Eur J Radiol. 2015;84:631–36. doi: 10.1016/j.ejrad.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 26.Buffa V, Solazzo A, D’Auria V, et al. Dual-source dual-energy CT: dose reduction after endovascular abdominal aortic aneurysm repair. Radiol Med. 2014;119:934–41. doi: 10.1007/s11547-014-0420-1. [DOI] [PubMed] [Google Scholar]
- 27.Valentini V, Buquicchio GL, Galluzzo M, et al. Intussusception in Adults: The Role of MDCT in the Identification of the Site and Cause of Obstruction. Gastroenterol Res Pract. 2016;2016:5623718. doi: 10.1155/2016/5623718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Cesare E, Patriarca L, Panebianco L, et al. Coronary computed tomography angiography in the evaluation of intermediate risk asymptomatic individuals. Radiol Med. 2018;123:686–94. doi: 10.1007/s11547-018-0898-z. [DOI] [PubMed] [Google Scholar]
- 29.Di Pietto F, Chianca V, De Ritis R, et al. Postoperative imaging in arthroscopic hip surgery. Musculoskelet Surg. 2017;101:43–49. doi: 10.1007/s12306-017-0459-y. [DOI] [PubMed] [Google Scholar]
- 30.Maggialetti N, Capasso R, Pinto D, et al. Diagnostic value of computed tomography colonography (CTC) after incomplete optical colonoscopy. Int J Surg. 2016;33(1):S36–44. doi: 10.1016/j.ijsu.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 31.Splendiani A, Perri M, Marsecano C, et al. Effects of serial macrocyclic-based contrast materials gadoterate meglumine and gadobutrol administrations on gadolinium-related dentate nuclei signal increases in unenhanced T1-weighted brain: a retrospective study in 158 multiple sclerosis (MS) patients. Radiol Med. 2018;123:125–34. doi: 10.1007/s11547-017-0816-9. [DOI] [PubMed] [Google Scholar]
- 32.Tedeschi E, Caranci F, Giordano F, Angelini V, Cocozza S, Brunetti A. Gadolinium retention in the body: what we know and what we can do. Radiol Med. 2017;122:589–600. doi: 10.1007/s11547-017-0757-3. [DOI] [PubMed] [Google Scholar]
- 33.Cappabianca S, Reginelli A, Monaco L, Del Vecchio L, Di Martino N, Grassi R. Combined videofluoroscopy and manometry in the diagnosis of oropharyngeal dysphagia: Examination technique and preliminary experience. Radiol Med. 2008;113:923–40. doi: 10.1007/s11547-008-0290-5. [DOI] [PubMed] [Google Scholar]
- 34.Barile A, Bruno F, Mariani S, et al. What can be seen after rotator cuff repair: a brief review of diagnostic imaging findings. Musculoskelet Surg. 2017;101:3–14. doi: 10.1007/s12306-017-0455-2. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari F, Arrigoni F, Miccoli A, et al. Effectiveness of Magnetic Resonance-guided Focused Ultrasound Surgery (MRgFUS) in the uterine adenomyosis treatment: technical approach and MRI evaluation. Radiol Med. 2016;121:153–61. doi: 10.1007/s11547-015-0580-7. [DOI] [PubMed] [Google Scholar]
- 36.Gatta G, Parlato V, Di Grezia G, et al. Ultrasound-guided aspiration and ethanol sclerotherapy for treating endometrial cysts. Radiol Med. 2010;115:1330–39. doi: 10.1007/s11547-010-0586-0. [DOI] [PubMed] [Google Scholar]
- 37.Briganti F, Leone G, Marseglia M, Cicala D, Caranci F, Maiuri F. P64 Flow Modulation Device in the treatment of intracranial aneurysms: Initial experience and technical aspects. J Neurointerv Surg. 2016;8:173–80. doi: 10.1136/neurintsurg-2015-011743. [DOI] [PubMed] [Google Scholar]
- 38.Arrigoni F, Barile A, Zugaro L, et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol. 2017;34:55. doi: 10.1007/s12032-017-0904-7. [DOI] [PubMed] [Google Scholar]
- 39.Lagana D, Carrafiello G, Mangini M, et al. Radiofrequency ablation of primary and metastatic lung tumors: preliminary experience with a single center device. Surg Endosc. 2006;20:1262–7. doi: 10.1007/s00464-005-0607-6. [DOI] [PubMed] [Google Scholar]
- 40.Zappia M, Castagna A, Barile A, Chianca V, Brunese L, Pouliart N. Imaging of the coracoglenoid ligament: a third ligament in the rotator interval of the shoulder. Skeletal Radiol. 2017;46:1101–11. doi: 10.1007/s00256-017-2667-9. [DOI] [PubMed] [Google Scholar]
- 41.Brunese L, Romeo A, Iorio S, et al. Thyroid B-flow twinkling sign: a new feature of papillary cancer. Eur J Endocrinol. 2008;159:447–51. doi: 10.1530/EJE-07-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantisani V, Grazhdani H, Drakonaki E, et al. Strain US elastography for the characterization of thyroid nodules: Advantages and limitation. Int J Endocrinol. 2015:2015. doi: 10.1155/2015/908575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Giacomo V, Trinci M, van der Byl G, Catania VD, Calisti A, Miele V. Ultrasound in newborns and children suffering from non-traumatic acute abdominal pain: imaging with clinical and surgical correlation. J Ultrasound. 2015;18:385–93. doi: 10.1007/s40477-014-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson HL, Prats MM, Sasatomi E. Chemotherapy-induced Sinusoidal Injury (CSI) score: a novel histologic assessment of chemotherapy-related hepatic sinusoidal injury in patients with colorectal liver metastasis. BMC cancer. 2017;17:35–35. doi: 10.1186/s12885-016-2998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han NY, Park BJ, Yang KS, et al. Hepatic Parenchymal Heterogeneity as a Marker for Oxaliplatin-Induced Sinusoidal Obstruction Syndrome: Correlation With Treatment Response of Colorectal Cancer Liver Metastases. Am J Roentgenol. 2017;209:1039–45. doi: 10.2214/AJR.16.17528. [DOI] [PubMed] [Google Scholar]
- 46.Tirumani SH, Kim KW, Nishino M, et al. Update on the role of imaging in management of metastatic colorectal cancer. Radiographics. 2014;34:1908–28. doi: 10.1148/rg.347130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajpai S, Sahani DV. Recent progress in imaging of colorectal cancer liver metastases. Curr Colorectal Cancer Rep. 2009;5:99–107. [Google Scholar]
- 48.Grassi R, Cavaliere C, Cozzolino S, et al. Small animal imaging facility: New perspectives for the radiologist. Radiol Med. 2009;114:152–67. doi: 10.1007/s11547-008-0352-8. [DOI] [PubMed] [Google Scholar]
- 49.Iacobellis F, Segreto T, Berritto D, et al. A rat model of acute kidney injury through systemic hypoperfusion evaluated by micro-US, color and PW-Doppler. Radiol Med. 2018 doi: 10.1007/s11547-018-0962-8. [DOI] [PubMed] [Google Scholar]
- 50.Cortellini A, Verna L, Porzio G, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: A “hypothesis-generator” preliminary report. Thorac Cancer. 2019;10:347–51. doi: 10.1111/1759-7714.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.You S-H, Park BJ, Kim YH. Hepatic Lesions that Mimic Metastasis on Radiological Imaging during Chemotherapy for Gastrointestinal Malignancy: Recent Updates. Korean J Radiol. 2017;18:413–26. doi: 10.3348/kjr.2017.18.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–39. doi: 10.1634/theoncologist.2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engstrom PF, Arnoletti JP, Benson AB 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw. 2009;7:778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 54.Benson AB 3rd, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370–98. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 55.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–6. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 56.Rubbia-Brandt L, Lauwers GY, Wang H, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430–9. doi: 10.1111/j.1365-2559.2010.03511.x. [DOI] [PubMed] [Google Scholar]
- 57.Hubert C, Sempoux C, Humblet Y, et al. Sinusoidal obstruction syndrome (SOS) related to chemotherapy for colorectal liver metastases: factors predictive of severe SOS lesions and protective effect of bevacizumab. HPB (Oxford) 2013;15:858–64. doi: 10.1111/hpb.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cayet S, Pasco J, Dujardin F, et al. Diagnostic performance of contrast-enhanced CT-scan in sinusoidal obstruction syndrome induced by chemotherapy of colorectal liver metastases: Radio-pathological correlation. Eur J Radiol. 2017;94:180–90. doi: 10.1016/j.ejrad.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 59.Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–90. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 60.Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–24. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 61.Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richardson P, Guinan E. The pathology, diagnosis, and treatment of hepatic veno-occlusive disease: current status and novel approaches. Br J Haematol. 1999;107:485–93. doi: 10.1046/j.1365-2141.1999.01680.x. [DOI] [PubMed] [Google Scholar]
- 63.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 64.Robinson PJ. The effects of cancer chemotherapy on liver imaging. Eur Radiol. 2009;19:1752–62. doi: 10.1007/s00330-009-1333-6. [DOI] [PubMed] [Google Scholar]
- 65.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–86. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 66.Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882–90. doi: 10.1016/s0016-5085(03)01056-4. [DOI] [PubMed] [Google Scholar]
- 67.Alexandrino H, Oliveira D, Cipriano MA, Ferreira L, Tralhão JG, Castro e Sousa F. Oxaliplatin toxicity presenting as a liver nodule – case report. BMC Cancer. 2015;15:247. doi: 10.1186/s12885-015-1247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamandl D, Klinger M, Eipeldauer S, et al. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:421–30. doi: 10.1245/s10434-010-1317-4. [DOI] [PubMed] [Google Scholar]
- 69.Xiong WJ, Hu LJ, Jian YC, et al. Focal peliosis hepatis in a colon cancer patient resembling metastatic liver tumor. World J Gastroenterol. 2012;18:5999–6002. doi: 10.3748/wjg.v18.i41.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nam SJ, Cho JY, Lee HS, et al. Chemotherapy-associated hepatopathy in korean colorectal cancer liver metastasis patients: oxaliplatin-based chemotherapy and sinusoidal injury. Korean J Pathol. 2012;46:22–9. doi: 10.4132/KoreanJPathol.2012.46.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arakawa Y, Shimada M, Utsunomya T, et al. Oxaliplatin-related sinusoidal obstruction syndrome mimicking metastatic liver tumors. Hepatol Res. 2013;43:685–9. doi: 10.1111/j.1872-034X.2012.01114.x. [DOI] [PubMed] [Google Scholar]
- 72.Tisman G, MacDonald D, Shindell N, et al. Oxaliplatin toxicity masquerading as recurrent colon cancer. J Clin Oncol. 2004;22:3202–4. doi: 10.1200/JCO.2004.99.106. [DOI] [PubMed] [Google Scholar]
- 73.Lassau N, Leclere J, Auperin A, et al. Hepatic veno-occlusive disease after myeloablative treatment and bone marrow transplantation: value of gray-scale and Doppler US in 100 patients. Radiology. 1997;204:545–52. doi: 10.1148/radiology.204.2.9240551. [DOI] [PubMed] [Google Scholar]
- 74.Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28:2549–55. doi: 10.1200/JCO.2009.27.5701. [DOI] [PubMed] [Google Scholar]
- 75.Iwai T, Yamada T, Koizumi M, et al. Oxaliplatin-induced increase in splenic volume; irreversible change after adjuvant FOLFOX. J Surg Oncol. 2017;116:947–53. doi: 10.1002/jso.24756. [DOI] [PubMed] [Google Scholar]
- 76.Morine Y, Shimada M, Utsunomiya T. Evaluation and management of hepatic injury induced by oxaliplatin-based chemotherapy in patients with hepatic resection for colorectal liver metastasis. Hepatol Res. 2014;44:59–69. doi: 10.1111/hepr.12107. [DOI] [PubMed] [Google Scholar]
- 77.Unal E, Karaosmanoglu AD, Ozmen MN, Akata D, Karcaaltincaba M. Hepatobiliary phase liver MR imaging findings after Oxaliplatin-based chemotherapy in cancer patients. Abdom Radiol (NY) 2018;43:2321–28. doi: 10.1007/s00261-018-1482-7. [DOI] [PubMed] [Google Scholar]
- 78.Salvolini L, Urbinati C, Valeri G, Ferrara C, Giovagnoni A. Contrast-enhanced MR cholangiography (MRCP) with GD-EOB-DTPA in evaluating biliary complications after surgery. Radiol Med. 2012;117:354–68. doi: 10.1007/s11547-011-0731-4. [DOI] [PubMed] [Google Scholar]
- 79.Mocchegiani F, Vincenzi P, Coletta M, et al. Prevalence and clinical outcome of hepatic haemangioma with specific reference to the risk of rupture: A large retrospective cross-sectional study. Dig Liver Dis. 2016;48:309–14. doi: 10.1016/j.dld.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 80.Kawai T, Yamazaki S, Iwama A, Higaki T, Sugitani M, Takayama T. Focal Sinusoidal Obstruction Syndrome Caused by Oxaliplatin-Induced Chemotherapy: A Case Report. Hepat Mon. 2016;16:e37572. doi: 10.5812/hepatmon.37572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Namimoto T, Yamashita Y, Sumi S, Tang Y, Takahashi M. Focal liver masses: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 1997;204:739–44. doi: 10.1148/radiology.204.3.9280252. [DOI] [PubMed] [Google Scholar]
- 82.Donadon M, Di Tommaso L, Roncalli M, Torzilli G. Multiple focal nodular hyperplasias induced by oxaliplatin-based chemotherapy. World J Hepatol. 2013;5:340–4. doi: 10.4254/wjh.v5.i6.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoneda N, Matsui O, Kitao A, et al. Hepatocyte transporter expression in FNH and FNH-like nodule: correlation with signal intensity on gadoxetic acid enhanced magnetic resonance images. Jpn J Radiol. 2012;30:499–508. doi: 10.1007/s11604-012-0085-4. [DOI] [PubMed] [Google Scholar]
- 84.Blachar A, Federle MP, Brancatelli G. Hepatic capsular retraction: spectrum of benign and malignant etiologies. Abdom Imaging. 2002;27:690–9. doi: 10.1007/s00261-001-0094-8. [DOI] [PubMed] [Google Scholar]
- 85.Young ST, Paulson EK, Washington K, Gulliver DJ, Vredenburgh JJ, Baker ME. CT of the liver in patients with metastatic breast carcinoma treated by chemotherapy: findings simulating cirrhosis. AJR Am J Roentgenol. 1994;163:1385–8. doi: 10.2214/ajr.163.6.7992734. [DOI] [PubMed] [Google Scholar]
- 86.Qayyum A, Lee GK, Yeh BM, Allen JN, Venook AP, Coakley FV. Frequency of hepatic contour abnormalities and signs of portal hypertension at CT in patients receiving chemotherapy for breast cancer metastatic to the liver. Clin Imaging. 2007;31:6–10. doi: 10.1016/j.clinimag.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 87.Shirkhoda A, Baird S. Morphologic changes of the liver following chemotherapy for metastatic breast carcinoma: CT findings. Abdom Imaging. 1994;19:39–42. doi: 10.1007/BF02165859. [DOI] [PubMed] [Google Scholar]
- 88.Shigefuku R, Watanabe T, Mizukami T, et al. Esophagogastric varices were diagnosed in a non-cirrhotic liver case during long-term follow-up after oxaliplatin-based chemotherapy. Clin J Gastroenterol. 2018;11:487–92. doi: 10.1007/s12328-018-0873-1. [DOI] [PubMed] [Google Scholar]
- 89.Donadon M, Vauthey JN, Loyer EM, Charnsangavej C, Abdalla EK. Portal thrombosis and steatosis after preoperative chemotherapy with FOLFIRI-bevacizumab for colorectal liver metastases. World J Gastroenterol. 2006;12:6556–8. doi: 10.3748/wjg.v12.i40.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Viswanathan C, Truong MT, Sagebiel TL, et al. Abdominal and pelvic complications of nonoperative oncologic therapy. Radiographics. 2014;34:941–61. doi: 10.1148/rg.344140082. [DOI] [PubMed] [Google Scholar]
- 91.Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137–44. doi: 10.1007/s00534-008-0016-z. [DOI] [PubMed] [Google Scholar]
- 92.McCullough AJ. Pathophysiology of Nonalcoholic Steatohepatitis. J Clin Gastroenterol. 2006;40(1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 93.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–72. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 94.Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013;2013:815105–05. doi: 10.1155/2013/815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi JS, Kim MJ. Education and imaging: hepatobiliary and pancreatic: focal steatohepatitis mimicking a metastasis. J Gastroenterol Hepatol. 2011;26:415. doi: 10.1111/j.1440-1746.2010.06614.x. [DOI] [PubMed] [Google Scholar]
- 96.Han NY, Park BJ, Sung DJ, et al. Chemotherapy-induced focal hepatopathy in patients with gastrointestinal malignancy: gadoxetic acid--enhanced and diffusion-weighted MR imaging with clinical-pathologic correlation. Radiology. 2014;271:416–25. doi: 10.1148/radiol.13131810. [DOI] [PubMed] [Google Scholar]
- 97.Han NY, Park BJ, Kim MJ, Sung DJ, Cho SB. Hepatic Parenchymal Heterogeneity on Contrast-enhanced CT Scans Following Oxaliplatin-based Chemotherapy: Natural History and Association with Clinical Evidence of Sinusoidal Obstruction Syndrome. Radiology. 2015;276:766–74. doi: 10.1148/radiol.2015141749. [DOI] [PubMed] [Google Scholar]
- 98.Ierardi AM, Lucchina N, Petrillo M, et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med. 2014;119:483–98. doi: 10.1007/s11547-014-0417-9. [DOI] [PubMed] [Google Scholar]
- 99.Carrafiello G, Dionigi G, Ierardi AM, et al. Efficacy, safety and effectiveness of image-guided percutaneous microwave ablation in cystic renal lesions Bosniak III or IV after 24 months follow up. Int J Surg. 2013;11(1):S30–5. doi: 10.1016/S1743-9191(13)60010-2. [DOI] [PubMed] [Google Scholar]
- 100.Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34:96. doi: 10.1007/s12032-017-0946-x. [DOI] [PubMed] [Google Scholar]
- 101.Cappabianca S, Scuotto A, Iaselli F, et al. Computed tomography and magnetic resonance angiography in the evaluation of aberrant origin of the external carotid artery branches. Surg Radiol Anat. 2012;34:393–99. doi: 10.1007/s00276-011-0926-3. [DOI] [PubMed] [Google Scholar]
- 102.Reginelli A, Capasso R, Ciccone V, et al. Usefulness of triphasic CT aortic angiography in acute and surveillance: Our experience in the assessment of acute aortic dissection and endoleak. Int J Surg. 2016;33(1):S76–84. doi: 10.1016/j.ijsu.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 103.Dialetto G, Reginelli A, Cerrato M, et al. Endovascular stent-graft treatment of thoracic aortic syndromes: A 7-year experience. Eur J Radiol. 2007;64:65–72. doi: 10.1016/j.ejrad.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 104.Kim SH, Kamaya A, Willmann JK. CT perfusion of the liver: principles and applications in oncology. Radiology. 2014;272:322–44. doi: 10.1148/radiol.14130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 106.Vreuls CP, Van Den Broek MA, Winstanley A, et al. Hepatic sinusoidal obstruction syndrome (SOS) reduces the effect of oxaliplatin in colorectal liver metastases. Histopathology. 2012;61:314–8. doi: 10.1111/j.1365-2559.2012.04208.x. [DOI] [PubMed] [Google Scholar]
- 107.Pradella S, Lucarini S, Colagrande S. Liver lesion characterization: The wrong choice of contrast agent can mislead the diagnosis of hemangioma. Am J Roentgenol. 2012:199. doi: 10.2214/AJR.12.8951. [DOI] [PubMed] [Google Scholar]
- 108.Mandato Y, Reginelli A, Galasso R, Iacobellis F, Berritto D, Cappabianca S. Errors in the Radiological Evaluation of the Alimentary Tract: Part I. Semin Ultrasound CT MR. 2012;33:300–07. doi: 10.1053/j.sult.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 109.Goh V, Halligan S, Daley F, Wellsted DM, Guenther T, Bartram CI. Colorectal tumor vascularity: quantitative assessment with multidetector CT--do tumor perfusion measurements reflect angiogenesis? Radiology. 2008;249:510–7. doi: 10.1148/radiol.2492071365. [DOI] [PubMed] [Google Scholar]
- 110.Ash L, Teknos TN, Gandhi D, Patel S, Mukherji SK. Head and neck squamous cell carcinoma: CT perfusion can help noninvasively predict intratumoral microvessel density. Radiology. 2009;251:422–8. doi: 10.1148/radiol.2512080743. [DOI] [PubMed] [Google Scholar]
- 111.Kim JW, Jeong YY, Chang NK, et al. Perfusion CT in colorectal cancer: comparison of perfusion parameters with tumor grade and microvessel density. Korean J Radiol. 2012;13(1):S89–97. doi: 10.3348/kjr.2012.13.S1.S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sforza V, Martinelli E, Ciardiello F, et al. Mechanisms of resistance to anti-epidermal growth factor receptor inhibitors in metastatic colorectal cancer. World J Gastroenterol. 2016;22:6345–61. doi: 10.3748/wjg.v22.i28.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cappabianca S, Iaselli F, Reginelli A, et al. Value of diffusion-weighted magnetic resonance imaging in the characterization of complex adnexal masses. Tumori. 2013;99:210–17. doi: 10.1177/030089161309900215. [DOI] [PubMed] [Google Scholar]
- 114.Lowenthal D, Zeile M, Lim WY, et al. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol. 2011;21:832–40. doi: 10.1007/s00330-010-1977-2. [DOI] [PubMed] [Google Scholar]
- 115.Bartlett DL, Chu E. Can metastatic colorectal cancer be cured? Oncology. 2012;26:266–75. [PubMed] [Google Scholar]
- 116.Folprecht G, Gruenberger T, Bechstein W, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study) Ann Oncol. 2014;25:1018–25. doi: 10.1093/annonc/mdu088. [DOI] [PubMed] [Google Scholar]
- 117.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–80. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 118.Kneuertz PJ, Maithel SK, Staley CA, Kooby DA. Chemotherapy-associated liver injury: impact on surgical management of colorectal cancer liver metastases. Ann Surg Oncol. 2011;18:181–90. doi: 10.1245/s10434-010-1201-2. [DOI] [PubMed] [Google Scholar]
- 119.Welsh FKS, Tilney HS, Tekkis PP, John TG, Rees M. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer. 2007;96:1037–42. doi: 10.1038/sj.bjc.6603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim H, Folks KD, Guo L, et al. Early therapy evaluation of combined cetuximab and irinotecan in orthotopic pancreatic tumor xenografts by dynamic contrast-enhanced magnetic resonance imaging. Mol Imaging. 2011;10:153–67. [PMC free article] [PubMed] [Google Scholar]
- 121.Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology. 2011;258:41–56. doi: 10.1148/radiol.10092129. [DOI] [PubMed] [Google Scholar]
- 122.De Filippo M, Onniboni M, Rusca M, et al. Advantages of multidetector row CT with multiplanar reformation in guiding percutaneous lung biopsies. RAD. MED. 2008;113:945–953. doi: 10.1007/s11547-008-0325-y. ISSN: 0033-8362, doi: 10.1007/s11547-008-0325-y. [DOI] [PubMed] [Google Scholar]
- 123.Palma BD, Guasco D, Pedrazzoni M, et al. Osteolytic lesions, cytogenetic features and bone marrow levels of cytokines and chemokines in multiple myeloma patients: Role of chemokine (C-C motif) ligand20. Leukemia. 2016 Feb;30(2):409–16. doi: 10.1038/leu.2015.259. doi: 10.1038/leu.2015.259. Epub 2015 Sep 30. [DOI] [PubMed] [Google Scholar]
- 124.Bozzetti C, Nizzoli R, Tiseo M, et al. ALK and ROS1 rearrangements tested by fluorescence in situ hybridization in cytological smears from advanced non-small cell lung cancer patients. Diagnostic Cytopathology. 43:941–946. doi: 10.1002/dc.23318. ISSN: 8755-1039, doi: 10.1002/dc.23318. [DOI] [PubMed] [Google Scholar]
- 125.De Filippo M, Gira F, Corradi D, Sverzellati N, Zompatori M, Rossi C. Benefits of 3D technique in guiding percutaneous retroperitoneal biopsies. RAD. MED. 2011;116(3):407–416. doi: 10.1007/s11547-010-0604-2. ISSN: 0033-8362, doi: 10.1007/s11547-010-0604-2. [DOI] [PubMed] [Google Scholar]


