Abstract
Background
Kidney transplantation is reported to save costs compared to maintenance dialysis. We analyzed the current actual costs of kidney transplantation compared to dialysis, and analyzed risk factors for higher costs after transplantation.
Material/Methods
Altogether, 338 kidney transplant recipients between 2009 and 2014 were included in this study. All individual-level cost data from specialized health care and data from all reimbursed medication and travel costs were acquired from official records. Cost data were compared before and after transplantation within the same patients starting from dialysis initiation and continued until the end of follow-up at the end of 2015.
Results
Total annual costs were median 53 275 EUR per patient in dialysis, 59 583 EUR for the first post-transplantation year (P<0.001), and 12 045 EUR for the subsequent years (P<0.001 compared to dialysis). Median costs for specialized health care were 36 103 EUR/year per patient during dialysis, compared to median 51 640 EUR for the first post-transplantation year (P<0.001 compared to dialysis), whereas the median costs for the subsequent years declined to median 4895 EUR/year (P<0.001 compared to dialysis). The median annual costs for drug treatments and travel reimbursements during dialysis were higher compared to after transplantation (P<0.001). Delayed graft function and highly sensitized status were independent risk factor for higher costs during the first the post-transplantation year.
Conclusions
After the first posttransplant year the costs of a kidney transplant patient for the health care system are <1/3 of the costs seen during dialysis treatment. Delayed graft function and previous sensitization were associated with increased costs post-transplantation.
MeSH Keywords: Costs and Cost Analysis, Delayed Graft Function, Kidney Transplantation
Background
In addition to prolonged survival and increased quality of life, kidney transplantation has also been associated with significant cost savings compared to maintenance dialysis treatment in several studies [1–6]. However, comparisons with patients who stay on dialysis may be confounded by comorbidities that either prevent or delay access to transplantation and may also be associated with increased costs. In addition, local health care systems and policies greatly influence the costs of both dialysis treatment and transplantation. The health care systems may also create financial incentives that might affect the timing of access to the transplant waiting list. For example, insurance coverage has been associated with lower rate of placement on the waiting list and also longer duration of end-stage renal disease before wait listing [7,8].
Finland has a universal government health care system financed from the national social insurance, and according to recent studies the universal health coverage index in Finland is among the highest in the world with a very modest total health care spending per capita [9,10]. The costs of kidney transplantation and dialysis treatment in Finland have been analyzed in a study by Salonen et al., including patients receiving renal replacement therapy during 1991 to 1996 [5]. Several changes have occurred during the last years in the treatment of dialysis patients, with a higher prevalence of home dialysis modalities, and with more alternatives for the medical treatment of complications of end-stage renal disease, such as drugs for hyperparathyroidism, hyperphosphatemia, and anemia, which are associated with high costs. On the other hand, immunosuppressive protocols have remained quite stable after kidney transplantation with the introduction of generic immunosuppressive drugs, which may affect the costs of kidney transplantation.
The aim of this study was to analyze the current actual costs of kidney transplantation compared to dialysis in Finland with a Scandinavian setting of universal public health care, and to find potentially modifiable risk factors for high costs after transplantation.
Material and Methods
Patients
Altogether, 338 consecutive patients who received a kidney-only transplantation from the Helsinki University Hospital catchment area between 2009 and 2014 were included. One patient who received 2 transplantations during the period was excluded from the analyses. Helsinki is the only transplant center in Finland and serves 21 regional hospitals with respective nephrology departments and dialysis facilities. After the initial post-transplantation period patients return to follow-up to their regional hospital. Patients included in this study were limited to those who had complete follow-up within Helsinki University Hospital and all cost data accessible both before and after transplantation. This study has the approval of both the institutional review board of Helsinki University Hospital (326/13/03/002015) and Social Insurance Institution of Finland (KELA).
Cost data
Cost analyses were performed from the perspective of the service provider and were done using direct costs in each cost category. All individual-level cost data from the specialized health care were acquired from the hospital billing database (ECOMED). Costs were divided in the following categories: 1) operations, which includes both donor and recipient operation, travel costs from the organ procurement operation, and the costs of the transplantation office; 2) outpatient procedures; 3) laboratory testing; 4) other diagnostic testing, including radiology, pathology, and clinical physiology; 5) treatment on the ward; and 6) outpatient visits, including dialysis sessions.
In addition, individual-level data from all reimbursed medication and travel costs were acquired from the Social Insurance Institution of Finland (KELA). Social insurance covers all the costs for prescribed medications that exceed 612.62 EUR per year, and additionally 72–100% of the costs of prescription medications used for chronic illnesses. For example, diabetes medications, immunosuppressive medications, erythropoiesis stimulating agents (ESA), and phosphate binders are 100% covered by the insurance, whereas medications such as blood pressure medications and lipid lowering therapies are 72–75% covered. Only medication costs exceeding the deductible were included in the analyses.
Travel costs are covered for each patient by the social insurance when the total annual amount of travel costs exceeds the deductible, which was 157.20 EUR or 242.25 EUR during the study period. Only travel costs exceeding the deductible are included in the analyses. Data linkage was done using the unique personal identity code as a key.
Annual cost data were compared before and after transplantation from the same patients (i.e. all the patients served as their own controls), divided into 365 days periods, with the transplant date as the index date. Costs for the specialized health care could be calculated for each 365 days after and before transplantation, but the data about medication and travel costs could only be calculated as mean costs per year on the treatment for each patient before and after transplantation, due to the nature of the data received from KELA. Data collection was started from dialysis initiation and continued until the end of follow-up at the end of 2015.
Demographic data
The frequency of living donation has been very low in our country, and only 4% of the transplantations included in the study were from living donors. All the patients were on maintenance dialysis before acceptance to the kidney transplant waiting list or before a living donor transplantation. Immunosuppression after kidney transplantation was a triple-drug therapy with cyclosporine or tacrolimus, mycophenolate, and steroids. Induction with basiliximab was used only in sensitized patients. Immunosuppressive protocol has been described in more detail elsewhere [11]. All deceased donors were donors after brain death, as donation after circulatory death is not used in our country. Cytomegalovirus (CMV) seronegative recipients of an organ from a seropositive donor (D+/R−) received 6 months of prophylaxis with valganciclovir and were monitored for viremia after the end of prophylaxis; other patient groups did not receive antiviral prophylaxis but were monitored for CMV viremia after transplantation monthly for the first 3 months after transplantation, and treatment was initiated in case of symptomatic CMV infection or viral loads of 1000 IU/mL. Trimethoprim-sulfamethoxazole prophylaxis was used for all patients for 6 months after transplantation, or in case of allergy, pentamidine inhalations were used instead.
Delayed graft function was defined as the need for dialysis during the first week after transplantation. Acute rejections were diagnosed with a kidney transplant biopsy according to the Banff classification [12]. Clinical data were collected from the Finnish Transplant registry, which is a follow-up registry for all transplantation patients in Finland, obliged by law.
Statistical analyses
Differences between 2 groups in continuous variables were compared with the Mann-Whitney U test and in categorical variables with the Fisher’s exact test. When costs before and after transplantation were analyzed, each patients served as their own control (i.e., costs during dialysis and after transplantation were compared within the same patient). The nonparametric Friedman test was used for comparisons, as all distributions were not normal. Univariable and multivariable logistic regression was used to analyze risk factors. When analyzing risk factors for higher costs during the first year after transplantation, total costs during the first year after transplantation were divided into quartiles, and the highest cost quartile was the binary outcome variable in the logistic regression model. Variables that were significant or close to significant risk factors in univariable analyses (P<0.10) were selected to the multivariable model. All first-degree interactions were tested between the independent significant variables in the multivariable model, and no significant interactions were found. Calculations were performed with IBM SPSS version 21. Two-sided P-values <0.05 were considered statistically significant.
Results
Of the 337 patients who received a transplantation and were included in the analyses, delayed graft function occurred in 127 patients (38%), and 5 grafts were lost in the first 2 weeks after transplantation, 1 due to venous thrombosis, 2 due to primary nonfunction of unknown reason, and 2 due to sudden cardiac death. One-year graft survival was 97% and 3-year graft survival was 92%. The transplantation patients included in the study are characterized in Table 1. By the end of the follow-up at the end of 2015, altogether 42 patients had died, and 29 patients returned to dialysis.
Table 1.
Characterization of the patients included in the study*.
| Patients transplanted between 2009–2014 (N=338) | |
|---|---|
| Mean age | 52±14 |
| Mean duration of pretransplant dialysis (months) | 32±27 |
| Patients with diabetes (%) | 79 (23%) |
| Living donor transplantation (%) | 15 (4%) |
| Donor age | 51±15 |
| Tacrolimus-based immunosuppression (%) | 93 (28%) |
| Number of HLA mismatches | |
| AB | 1.9±0.9 |
| DR | 0.7±0.6 |
| Cold ischemia time (hrs) | 19±6 |
| Delayed graft function (%) | 127 (38%) |
| Acute rejection | |
| T cell-mediated rejection (%) | 58 (17%) |
| Antibody-mediated rejection (%) | 8 (2%) |
| Patients with positive cPRA before transplantation (%) | 177 (52%) |
| Patients with cPRA >80% before transplantation | 52 (15%) |
Mean ±1 SD, unless otherwise indicated, cPRA – calculated panel-reactive antibodies.
Mean costs before and after dialysis are depicted in Figures 1 and 2. Total annual costs were median 53 275 EUR (range 26 019 to 119 913) EUR per patient in dialysis, 59 583 EUR (range 14 966 to 232 479 EUR) for the first post-transplantation year (P<0.001), and 12 045 EUR (range 1787 to 231 112 EUR) for the subsequent years (P<0.001 compared to dialysis). Median annual costs for specialized health care were 36 103 EUR (range 4618 to 103 007 EUR) per patient during dialysis, compared to median 51 640 EUR (range 5337 to 228 128 EUR) for the first post-transplantation year (P<0.001 compared to dialysis), whereas the median costs for the subsequent years declined to median 4895 EUR (range 801 to 183 340 EUR)/year (P<0.001 compared to dialysis).
Figure 1.

Total mean costs for travel, medication, and specialized health care before and after kidney transplantation among 338 patients.
Figure 2.
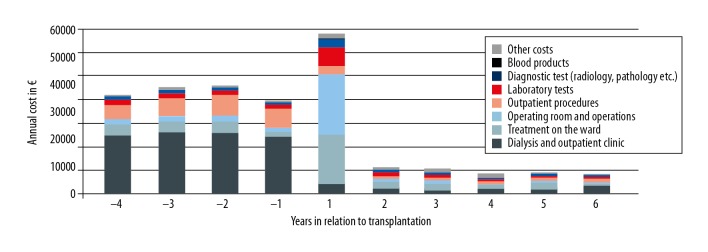
Costs for specialized health care among 338 patients before and after kidney transplantation, divided into 8 different categories: dialysis and outpatient visits, treatment on the ward, operating room and operations (including costs for organ procurement), outpatient procedures, laboratory tests, diagnostic tests, blood products, and other costs.
As expected, the most important group of cost savings after transplantation were for dialysis visits. During the first post-transplantation year, costs increased for treatment on the ward, surgical operations (mainly the transplantation operation including costs for the donor procurement), laboratory tests, and diagnostic tests, but declined substantially during the following years. The median annual costs for drug treatments during dialysis were 10 600 EUR (range 81 to 53 424 EUR), compared to 7537 EUR (range 720 to 77 105 EUR) after transplantation (P<0.001). Travel costs were significantly higher during dialysis treatment, with median annual cost of 829 EUR (range 7 to 32 629 EUR) compared to 119 EUR (0 to 14 100 EUR) after transplantation (P<0.001).
Table 2 describes the differences in median costs before and after transplantation, categorized by clinical variables. Annual costs during dialysis treatment were lower in patients on peritoneal dialysis (PD) before transplantation compared to hemodialysis (HD) (P<0.001), and similarly after transplantation patients on pretransplant PD had lower annual costs both during the first post-transplantation year and later (P=0.005 and P=0.02, respectively). During the first post-transplantation year, patients with delayed graft function had higher annual costs compared to patients with early graft function (P<0.001, Figure 3). CMV D+/R− constellation was associated with higher costs during the first post-transplantation year compared to other patients (P=0.02). No significant differences were seen in pre- or post-transplantation costs between patients with or without diabetes, highly sensitized patients (maximum pre-transplantation cPRA >80), patients receiving living or deceased donor transplantation, patients with acute rejection after transplantation, or between patients with cold ischemia time below or more than 24 hours, (data not shown).
Table 2.
Comparison of total costs (in euros) pre and posttransplantation between different patient groups, expressed as median (range).
| Patients on PD before transplantation (N=124) | Patients on HD before transplantation (N=214) | |
|---|---|---|
| Median annual costs during dialysis | 51383 (26403–107571)* | 54137 (26019–119913)* |
| Median costs for the 1st posttransplant year | 56136 (18131–165348)** | 62285 (14966–232479)** |
| Median costs for later posttransplant year | 11117 (4978–231112)*** | 12797 (1787–194078)*** |
| Patients with DGF (N=128)# | Patients with no DGF (N=205) | |
| Median annual costs during dialysis | 55423 (27278–119913) | 51578 (26019–107571) |
| Median costs for the 1st posttransplant year | 68374 (36604–232479)* | 55200 (14966–183101)* |
| Median costs for later posttransplant year | 11995 (2387–81419) | 11977 (1787–231112) |
| CMV D+/R− patients (N=72) | Other CMV combinations (N=266) | |
| Median annual costs during dialysis | 50782 (26019–98097) | 53483 (26403–119913) |
| Median costs for the 1st posttransplant year | 65105 (34887–232479)*** | 58151 (14966–216404)*** |
| Median costs for later posttransplant year | 12798 (1787–231112) | 11924 (1893–87996) |
| Patients with aRX (N=65) | Patients with no aRX (N=273) | |
| Median annual costs during dialysis | 53765 (26403–103336) | 53152 (26019–119913) |
| Median costs for the 1st posttransplant year | 64571 (36604–158356) | 58430 (14966–232479) |
| Median costs for later posttransplant year | 12492 (2605–194078) | 11995 (1787–231112) |
| Living donor transplantation (N=15) | Deceased donor transplantation (N=323) | |
| Median annual costs during dialysis | 56269 (41990–73099) | 53237 (26019–119913) |
| Median costs for the 1st posttransplant year | 48807 (39668–183101) | 59902 (14966–232479) |
| Median costs for later posttransplant year | 10655 (6925–50735) | 12132 (1787–231112) |
PD – peritoneal dialysis; HD – hemodialysis; DGF – delayed graft function; aRX – acute rejection.
P<0.001;
P=0.005;
P=0.02;
Five patients with no graft function excluded.
Figure 3.
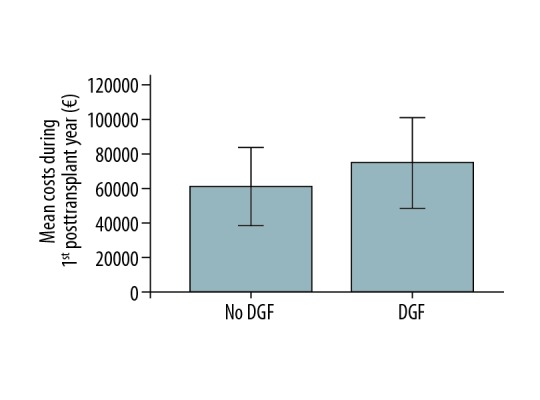
Comparison of total costs (in euros) during the first posttransplant year between patients with delayed graft function (DGF) and early graft function after kidney transplantation. Error bars represent 1 standard deviation.
Results of the univariable and multivariable logistic regression analysis of the risk factors for high costs during the first post-transplantation year (highest quartile, >71 890 EUR per year) are shown in Table 3. In univariable analyses, pre-transplantation hemodialysis, delayed graft function, increased donor age, highly sensitized status (cPRA >80%), and longer duration of pre-transplantation dialysis treatment were risk factors for high costs during the first post-transplantation year. Interestingly, CMV serostatus, diabetes, acute rejection, or recipient age were not associated with increased costs. In a multivariable model including pre-transplantation dialysis modality, pre-transplantation dialysis duration, delayed graft function, diabetes, highly sensitized status, and donor age, only delayed graft function (odds ratio, OR 2.63) and highly sensitized status (OR 2.54) were independent significant risk factors for high costs during the first post-transplantation year.
Table 3.
Univariable and multivariable logistic regression analysis of the risk factors for high costs (highest quartile) during the first year after kidney transplantation.
| Univariable OR | 95% CI | P | Multivariable OR | 95% CI | P | |
|---|---|---|---|---|---|---|
| Hemodialysis before transplantation | 2.22 | 1.23–3.98 | 0.005 | 1.48 | 0.79–2.77 | 0.22 |
| Delayed graft function | 3.49 | 2.16–5.63 | <0.001 | 2.63 | 1.55–4.48 | <0.001 |
| Diabetes | 1.70 | 0.98–2.96 | 0.06 | 1.52 | 0.84–2.75 | 0.17 |
| Cold ischemia time (hrs) | 1.01 | 0.96–1.05 | 0.73 | |||
| Acute rejection | 1.37 | 0.83–2.25 | 0.22 | |||
| CMV D+/R− status | 1.57 | 0.89–2.78 | 0.12 | |||
| Living donor transplantation | 1.11 | 0.34–3.57 | 0.87 | |||
| cPRA >80% | 2.40 | 1.29–4.46 | 0.006 | 2.54 | 1.29–4.99 | 0.007 |
| Recipient age (yrs) | 0.99 | 0.98–1.02 | 0.82 | |||
| Donor age (yrs) | 1.02 | 1.01–1.04 | 0.05 | 1.01 | 0.99–1.03 | 0.37 |
| Duration of pretransplant dialysis (months) | 1.01 | 1.00–1.02 | 0.001 | 1.01 | 0.99–1.02 | 0.08 |
Discussion
In this study we compared the current actual costs of maintenance dialysis among patients listed for kidney transplantation, and compared these costs with the post-transplantation costs within the same patients. In addition to the already previously shown fact that costs are significantly reduced after transplantation [4,5], we were able to show in a multivariable model that only delayed graft function and highly sensitized status were independent risk factors for high costs after transplantation. This has important implications for clinical treatment policies, as the frequency of delayed graft function can be reduced by actively minimizing the cold ischemia time resulting in cost savings. In a recent study from our institution, we showed that using peripheral blood lymphocytes instead of donor spleen for the prospective cytotoxic crossmatches, we were able to significantly reduce cold ischemia times and the frequency of delayed graft function [13]. This reduction in the frequency of delayed graft function was most pronounced in highly sensitized patients, underlying the potential for cost savings. In our cohort, induction therapy was used only in sensitized patients, which may also explain the relatively high frequency of delayed graft function, as the occurrence of delayed graft function may be lower among patients who receive induction therapy [14].
Several other studies have analyzed the impact of clinical variables on higher costs after transplantation. Lower renal function has been associated with increased costs after transplantation in multiple studies [15,16]. Similarly, post-transplantation complications, such as CMV and acute rejection have been associated with increased costs [17], although these findings could not be confirmed in our current analysis. In a study by Hagenmeyer et al., delayed graft function was similarly associated with higher costs after transplantation [17]. This study, however, included patients transplanted between 1998 and 2000, and excluded the costs for organ procurement, pre-transplantation care, and transplantation surgery, and did not have access to actual medication costs. In addition, only a limited number of variables was used for multivariable modelling. Our study, on the other hand, describes the current direct costs after transplantation, with access to pre- and post-transplantation data used for adjustment in the multivariable models. The definition of delayed graft function in our current study was the need for dialysis treatment during the first post-transplantation week. This definition is one of the most commonly used definitions in the literature and the only definition recorded in our transplant registry, but has several limitations based on the subjective clinical decision of the need for dialysis, and probably does not accurately describe all patients with compromised initial graft function. In addition, although multivariable model was adjusted for donor age and cold ischemia time, other unidentified characteristics related to the donor organ quality or recipient may confound our findings of the increased costs associated with delayed graft function itself.
Several previous studies have compared the costs of dialysis and transplantation [4–6], and our findings are in line with these previous studies. Comparison of actual costs is very difficult between different countries and health care systems, and major differences can be seen between countries in total spending for health care [10], which probably also has implications for the costs of pre- and post-transplantation care of patients with end-stage renal disease. Costs of dialysis and kidney transplantation were compared earlier in a Finnish study by Salonen et al. [5]. The material for their study derived from 1991 to 1996, and in addition, many costs were estimates or theoretical costs, and not all actual cost data were available for this earlier study. In a recent study from Sweden, Jarl et al. used a similar setting to our study and compared the costs of the same patients before and after transplantation [4]. According to the calculations in the Jarl et al. study, cost savings of 380 000 EUR per patient for a 10-year period could be obtained with a transplantation. Our findings of the magnitude of the cost savings are in a similar range, although our data enabled detailed analysis only from the first post-transplantation years, and not from long-term costs.
In the current study, peritoneal dialysis was associated with lower costs both during dialysis treatment and after transplantation. However, in a multivariable model dialysis modality was not a significant predictor of higher costs after transplantation, suggesting that other factors, such as younger age, fewer comorbidities, and lower frequency of delayed graft function among the patients in PD before transplantation explain the lower post-transplantation costs. Further analysis of the cost differences during dialysis treatment was outside the scope of the current study. In general dialysis treatment, especially peritoneal dialysis, was associated with lower annual costs compared to the first post-transplantation year, suggesting that the short-term economic benefit of transplantation may be questioned in patients with high risk of complications and mortality early after transplantation, such as elderly patients.
This study has some limitations of note. No cost data for primary health care were available for this study. However, most of the contacts to health care after transplantation during the first years are to specialized health care, and the effect is likely low during the first years after transplantation. Only direct costs were available for this study, and no information about disability or loss of income was available for the current study. Employment rate among end-stage renal disease patients is generally low in our population according to our recent study, and is not significantly higher after transplantation compared to peritoneal dialysis or home hemodialysis [18]. In addition, we did not have access to exact costs for each year regarding medications and travel costs, but the cost estimates are based on average costs per year per treatment modality. Presumably medication costs for the first post-transplantation year would be higher compared to later years (due to higher doses and trough level targets of immunosuppressive drugs), and as we used the mean costs during all the post-transplantation years, cost savings seen in medication costs during later years after transplantation compared to dialysis are likely even larger than we report. Medications given in the hospital are included in the costs of the treatment on the ward, reducing the possible bias. On the other hand, all these aforementioned limitations would presumable result in lower relative costs after transplantation compared to dialysis and our analyses likely underestimate the cost savings of kidney transplantation compared to dialysis. In addition, our cohort included only patients from the Helsinki metropolitan area, which is the most densely populated part of Finland, and travel costs would probably be much higher in rural areas with long distances to dialysis facilities, which similarly probably underestimates the travel cost savings achieved with a kidney transplantation. The strength of our study is a relatively large sample of kidney transplantation patients with individual-level actual cost data available from both pre- and post-transplantation period, and with multiple clinical factors available for analyzing risk factors for higher costs after transplantation.
Conclusions
In conclusion, the current costs in Finland for the first post-transplantation year exceed the costs of dialysis treatment, but in the subsequent years the costs of a kidney transplantation patient for the health care system are <1/3 of the costs seen during dialysis treatment. Cost savings are seen in all categories in the long-term, including costs for medications and travel costs. Highly sensitized patients and patients with delayed graft function are at the highest risk for increased costs during the first post-transplantation year.
Abbreviations
- CMV
cytomegalovirus
- HD
hemodialysis
- KELA
Social Insurance Institution of Finland (Kansaneläkelaitos)
- PD
peritoneal dialysis
Footnotes
Source of support: This study was funded by research grants from the Helsinki University Hospital (VTR to H.I., TYH2017)
Conflict of interest
None.
References
- 1.Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16:1859–65. doi: 10.1681/ASN.2004121092. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 4.Jarl J, Desatnik P, Peetz Hansson U, et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clin Kidney J. 2018;11:283–88. doi: 10.1093/ckj/sfx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salonen T, Reina T, Oksa H, et al. Cost analysis of renal replacement therapies in Finland. Am J Kidney Dis. 2003;42:1228–38. doi: 10.1053/j.ajkd.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Cairns JA, Fotheringham J, et al. Understanding cost of care for patients on renal replacement therapy: Looking beyond fixed tariffs. Nephrol Dial Transplant. 2015;30:1726–34. doi: 10.1093/ndt/gfv224. [DOI] [PubMed] [Google Scholar]
- 7.Schold JD, Gregg JA, Harman JS, et al. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6:1760–67. doi: 10.2215/CJN.08620910. [DOI] [PubMed] [Google Scholar]
- 8.Schold JD, Sehgal AR, Srinivas TR, et al. Marked variation of the association of ESRD duration before and after wait listing on kidney transplant outcomes. Am J Transplant. 2010;10:2008–16. doi: 10.1111/j.1600-6143.2010.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2016 Healthcare access and quality collaborators. measuring performance on the healthcare access and quality index for 195 countries and territories and selected subnational locations: A systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391:2236–71. doi: 10.1016/S0140-6736(18)30994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global Burden of Disease Health Financing Collaborator Network. Trends in future health financing and coverage. Future health spending and universal health coverage in 188 countries, 2016–40. Lancet. 2018;391:1783–98. doi: 10.1016/S0140-6736(18)30697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helanterä I, Anttila VJ, Loginov R, Lempinen M. Parainfluenza-3 infections early after kidney or simultaneous pancreas-kidney transplantation. Am J Transplant. 2017;17:809–12. doi: 10.1111/ajt.14146. [DOI] [PubMed] [Google Scholar]
- 12.Sis B, Mengel M, Haas M, et al. Banff ‘09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–71. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 13.Helanterä I, Saarinen T, Peräsaari J, et al. Shortened cold ischemia time and reduced incidence of delayed graft function for kidney transplants through enhanced tissue compatibility testing. Am J Transplant. 2018;18 Abstr D58. [Google Scholar]
- 14.Kyllönen LE, Eklund BH, Pesonen EJ, Salmela KT. Single bolus anti-thymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: Efficacy and safety. Transplantation. 2007;84:75–82. doi: 10.1097/01.tp.0000268084.64888.f3. [DOI] [PubMed] [Google Scholar]
- 15.Schnitzler MA, Johnston K, Axelrod D, et al. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation. 2011;91:1347–56. doi: 10.1097/TP.0b013e31821ab993. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain G, Baboolal K, Bennett H, et al. The economic burden of posttransplant events in renal transplant recipients in Europe. Transplantation. 2014;97:854–61. doi: 10.1097/01.TP.0000438205.04348.69. [DOI] [PubMed] [Google Scholar]
- 17.Hagenmeyer EG, Häussler B, Hempel E, et al. Resource use and treatment costs after kidney transplantation: Impact of demographic factors, comorbidities, and complications. Transplantation. 2004;77:1545–50. doi: 10.1097/01.tp.0000121763.44137.fa. [DOI] [PubMed] [Google Scholar]
- 18.Helanterä I, Haapio M, Koskinen P, et al. Employment of patients on dialysis and after kidney transplantation. Am J Kidney Dis. 2012;59:700–6. doi: 10.1053/j.ajkd.2011.08.025. [DOI] [PubMed] [Google Scholar]