Abstract
Background
Rotator cuff injury is the most common cause of shoulder disability, and although the repair technique has improved, the rate of rotator cuff reduction after repair is still high. The fibrocartilage region, which appears to be histologically inserted, cannot be regenerated. In recent years, studies have reported that mesenchymal stem cells (MSCs) have enhanced cartilage regeneration in the tendon and bone interface after rotator cuff repair, which has become a hot topic of research.
Material/Methods
Two mesenchymal stem cell types, SMSC (synovial-derived mesenchymal stem cells) and BMSC (bone marrow-derived mesenchymal stem cells) were intervened using kartogenin (KGN). The cytotoxicity was evaluated and the proliferation of the 2 cells was observed. Four commonly used cartilage phenotype genes were detected by quantitative real-time polymerase chain reaction, and the cartilage differentiation of MSCs induced by KGN was explored. The bidirectional regulation of the expression of BMP-7 and the downstream gene Smad5 was observed by constructing a lentiviral overexpression vector containing the target gene BMP-7. To explore whether BMP-7/Smad5 pathway activation promotes differentiation of SMSCs into chondrocytes.
Results
KGN can induce the selective differentiation of endogenous MSCs into chondrocytes by activating the BMP-7/Smad5 pathway, which promotes the regeneration of interfacial cartilage, and improves the quality of tendon healing of the tendon after rotator cuff repair.
Conclusions
This study found a new biological intervention method to promote the effect of tendon on bone healing after rotator cuff repair.
MeSH Keywords: Abnormal Karyotype, Cartilage, Mesenchymal Stromal Cells, Rotator Cuff
Background
Rotor cuff injury is the most common cause of clinically scapular dysfunction in patients, and it usually leads to long-term pain and limited mobility in the shoulder joint [1]. Despite the continuous advancement of rotator cuff repair surgery, non-operative physiotherapy to keep range of movement, rotator cuff and deltoid strength, as well as scapula-stabilizing which utilizes surgical methods to repair the tendon [2], there continues to be increased risk of re-tearing of the tendons after surgical reconstruction [3]. Pathological studies have shown that highly specialized fibro-cartilaginous transition layers between the rotator cuff and the bone cannot regenerate after repair, which leads to patients who cannot be restored to normal function after surgical reconstruction [4,5].
A novel biological treatment method is required to regenerate the torn tendon and lessen scar formation [6,7]. Mesenchymal stem cells (MSCs) have been shown to have great potential to differentiate into a large variety of cell types, such as chondrocytes, neural cells, and endothelial cells, thus stimulating cartilage regeneration without exogenous seed cells and scaffolds, which could be used to alleviate the risk of tendons re-tearing [8]. Gomes et al. found that injecting autologous bone marrow (BM) mononuclear cells after surgery could increase patient’s functional status [9].
Kartogenin (KGN) is a recently characterized drug with small molecule that promotes the selective differentiation of endogenous MSCs into chondrocytes, thus stimulating cartilage regeneration [10]. In addition, limb skeletal growth and tendon development both can be enhanced by KGN [11,12]. Therefore, KGN has positive effects on cartilage and tendon regeneration, which makes it possible that KGN could be used to promote tendon to bone healing in our study. Otherwise, it has been reported that MSCs from adjacent synovium, tendons and other tissues can promote tendon to bone healing after rotator cuff injury [13–15].
Therefore, we had the hypothesis that KGN could induce the differentiation of endogenous MSCs into chondrocytes, thus stimulating cartilage regeneration at the interface of tendon to bone healing, which would reproduce the normal morphology of the interface, and the improved quality of tendon to bone healing would reduce the re-tear rate of repaired rotator cuff.
Material and Methods
Synovial-derived MSCs
We isolated synovial-derived MSCs (SMSCs) from a total of 12 healthy human knee synovial tissues as described by Pei et al. [16]. The primary culture of the cells was for 2 weeks in SMSCs culture medium (Dakco Biotechnology Co., Ltd.), subculture was for 2 generations until the cell density reached 80% of the bottom of the culture dish. The third generation (P3) SMSCs were used in the study; culture conditions were gas phase: air, 95%; carbon dioxide, 5%; temperature: 37°C, incubator humidity of 70–80%. The present study was approved by the Ethical Review Committee in the Changzheng Hospital of Second Military Medical University. Written informed consent was obtained from all patients.
Human bone marrow-derived mesenchymal stem cells (BMSCs)
We collected human bone marrow-derived mesenchymal stem cells (BMSCs) from 8 healthy human bone marrow samples according to the method of Hoffmann et al. [17]. Culture of the primary cell was for 2 weeks in BMSCs culture medium (Dakco Biotechnology Co., Ltd.), subculture was for 2 generations until the cell density reached 80% of the bottom of the culture dish. The third generation (P3) BMSCs cells were used in the study; culture conditions were gas phase: air, 95%; carbon dioxide, 5%; temperature: 37°C, incubator humidity of 70–80%).
Cells treated with KGN
The KGN primary stock was diluted with phosphate-buffered saline (PBS) solution into 2 working fluid concentrations, 100 μmol/L and 10 mmol/L. We treated the SMSCs and BMSCs at a final concentration of 10 nM, 100 nM, 1 μM, 10 μM, and 100 μM KGN in culture medium for 72 hours; the control group was treated with the same volume of DMSO.
Cell viability was determined with Cell Counting Kit-8
We collected P3 SMSCs and BMSCs by digesting cells with 0.25% trypsin and 0.02% EDTA, seeded 2×104 cells per well in 96-wells plates and incubated with growth media containing KGN as described. We measured cell viability by Cell Counting Kit-8 (CCK-8) method on consecutive 6 days. The values represent the average of 3 replicates and a representative experiment from at least 2 independent experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
We extracted RNA from cells using TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa, Cat.# 9767). RNA samples were reverse transcribed applying PrimeScript™ RT Master Mix (Perfect Real Time) (TaKaRa, Cat.# RR036Q). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed applying TB Green™ Fast qPCR Mix (TaKaRa, Cat.# RR430S). Primers were synthesized by Shanghai R&S Biotechnology Co., Ltd.
The sequences of the primers applied for qRT-PCR are recorded in Table 1. The values represent the average of 3 replicates from at least 2 independent experiments.
Table 1.
Primer sequence used in this study.
| Name of primer | Primer sequence (5′-3′) |
|---|---|
| Aggrecan-F | GCACCCAGCACAATGAAGA |
| Aggrecan-R | AATAAAGCCATGCCAATCTCA |
| Type II collagen-F | TTCAGGGTTACCAGGGTTCA |
| Type II collagen-R | TCCACCATCCCAGATTTGC |
| hCOL2A1-F | TTCATCCCACCCTCTCACA |
| hCOL2A1-R | GGCATTTGACTCACACCAGTTA |
| hSOX9-F | ACTGGGAACAACCCGTCTA |
| hSOX9-R | TGGTCCTCTCTTTCTTCGG |
| hTIMP1-F | GAAGTCAACCAGACCACCTTA |
| hTIMP1-R | TATCCGCAGACACTCTCCA |
| hBMP7-F1 | TACGCCGCCTACTACTGTGA |
| hBMP7-R1 | GTTGATGAAGTGGACCAGCG |
| hSMAD5-F1 | TGTTGGTGGAGAGGTGTATGC |
| hSMAD5-R1 | TGGTTGACAGATTGAGCCAGA |
Generation of lentivirus
HEK-293T cells (cells number: 3×106) were seeded in 100 mm dish with DMEM supplemented with 10% fetal bovine serum (FBS).Then pLenti-BMP7-EGFP-IRES 20 μg, Packaging Mix plasmids (Invitrogen), were transfected using POLO Deliverer™ 3000 Transfection Reagent (Shanghai R&S Biotechnology Co., Ltd). After 48 hours incubation, the DMEM media containing lentivirus particles were collected, filtered through a 0.45 μm filter, and concentrated using ultra-centrifuge at 4°C, 2 2000 rpm for 2 hours. Finally, 1.04×108 Tu/mL viruses were concentrated in 1 mL DMEM medium. Also, control lentiviral (Lenti-EGFP NC) was performed by this method. To select virus infected cells, 8 ug/mL of puromycin was used 24 hours post infection. The Cy3-siRNA and control IRES-EGFP was purchased from Shanghai R&S. Infection of siRNA was performed as described.
Fluorescence immunocytochemistry
SMSCs and BMSCs were infected with pLenti-BMP7-EGFP-IRES (Lenti-EGFP NC) and Cy3-siRNA (IRES-EGFP NC) post 6 hours, then the culture medium was replaced with fresh complete medium containing 2 ug/mL KGN, with continued culture for 48 hours on cell slides. Then the following procedure was followed: 1) carefully remove the cell slides from the 24-well plate with small forceps, place them on a glass slide, and mark them. 2) Cells were fixed with 4% paraformaldehyde for 15 minutes, then washed twice with PBS. 3) Cell membranes were ruptured using 0.5% Triton-100 (prepared in PBS) for 5 to 10 minutes, increasing cell membrane permeability. 4) specimens were blocked with blocking solution (4% BSA, PBST) for 1 hour at room temperature; then incubated with primary antibody. The primary antibody was diluted with freshly prepared blocking solution, BMP7 protein antibody (Sanying, Cat.# 12221-1-AP) at 1: 25; P-Smad5 protein antibody (Abcam, Cat.# ab76296) at 1: 50. The primary antibody was incubated overnight at 4°C. 5) The cells were washed 5 times with PBS. 6) Cells were incubation with the selected secondary antibody corresponding to the primary antibody with different fluorescein label according to the needs of the experiment; the secondary antibody was diluted with freshly prepared blocking solution. The secondary antibody was labeled with FITC, and the dilution ratio is 1: 1000. 7) The secondary antibody was incubated for 1 hour at room temperature. 8) The cells were washed 5 times with PBS. 9) The cells were stained with DAPI for 10 minutes, and then the cells were washed 5 times with PBS. 10) We absorbed excess water from the cell slides and covered slides by seal slides with anti-quenching sealing liquid. 11) We then observed the slides under a confocal microscope (LSM710, ZEISS) and photographed the slides.
Results
Morphology of SMSCs and SMSCs
We observed the SMSCs under a vertical microscope, and observed that the cell morphology of BMSCs was similar to that of SMSCs. The early cells were small and spindle shaped. As the cell growth density increased, cells gradually became fusiform shape (Figure 1A). When BMSCs were cultured for 2 weeks, the cell confluent was 80%, after subculture, BMSCs showed a long spindle shape, and when the cell colonies were full, they showed sunflower-like or fingerprint-like distribution. The third generation BMSCs grew well and the cell morphology was stable, showing a long fusiform shape (Figure 1C). Therefore, based on the observations of the primary cell culture experiments, the overall growth cycle and cell morphology of the 2 cells types SMSCs and BMSCs were very similar, showing good quality in cell proliferation and cell viability and long fusiform shape with uniform morphology. Both SMSCs and BMSCs did not show significant differences in morphology after 72 hours of treatment with KGN, and KGN has not toxic to SMSCs and BMSCs (Figure 1B, 1D).
Figure 1.
(A–D) Morphology of SMSCs and BMSCs. NC, without KGN treatment, 100×; KNG treatment for 72 hours, 100×. SMSCs – synovial-derived mesenchymal stem cells; BMSCs – bone marrow-derived mesenchymal stem cells; KGN – kartogenin.
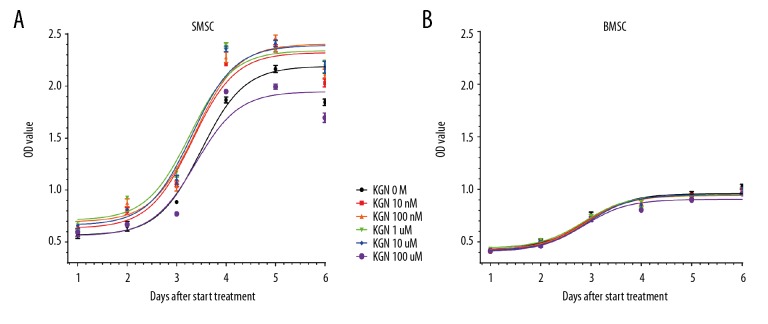
Cell growth curve
The cell growth curve results showed that the proliferation of SMSCs cells did not increase significantly on Day 1, 2, and 3, peaked on Day 4, maintained on Day 5, and decreased slightly on Day 6. With KGN at concentrations of 10 nmol/L~10 μmol/L, the proliferation of SMSCs cells was significantly promoted when compared with the control DMSO treatment group, and 10 μmol/L KNG exhibited the best promoted effected (Figure 2A). However, in contrast, KGN at the concentration of 10 μmol/L promoted the proliferation of BMSCs, showed a significant proliferation promoted effect only during the second to fourth day after intervention. BMSCs cell proliferation was not obvious in the previous 2 days, and it began to rise on the third day, peaked on the fifth day, and slightly decreased on the sixth day (Figure 2B).
Figure 2.
(A, B) Growth curve. SMSCs and BMSCs with the intervention of KGN, applied the CCK-8 method to determine cell viability. SMSCs – synovial-derived mesenchymal stem cells; BMSCs – bone marrow-derived mesenchymal stem cells; KGN – kartogenin. CCK-8 – Cell Counting Kit-8.
Interestingly, the proliferative potential of both cell types decreased slightly on the sixth day, which may be related to contact inhibition. We observed that as the culture time was prolonged, the proliferation levels of both cell types was increasing, and the trends of the proliferation levels of the 2 cell types were substantially uniform. Most importantly, we found that no matter which concentration of KGN was used to intervene in the cells, compared to the control group, the overall trend of the proliferation of the 2 mesenchymal stem cells in the KGN drug group was not significantly affected, and it remained stable and cells continued to proliferate. This indicated that KGN had low cytotoxicity and ideal safety.
KGN induced MSCs and BMSCs differentiation into cartilage
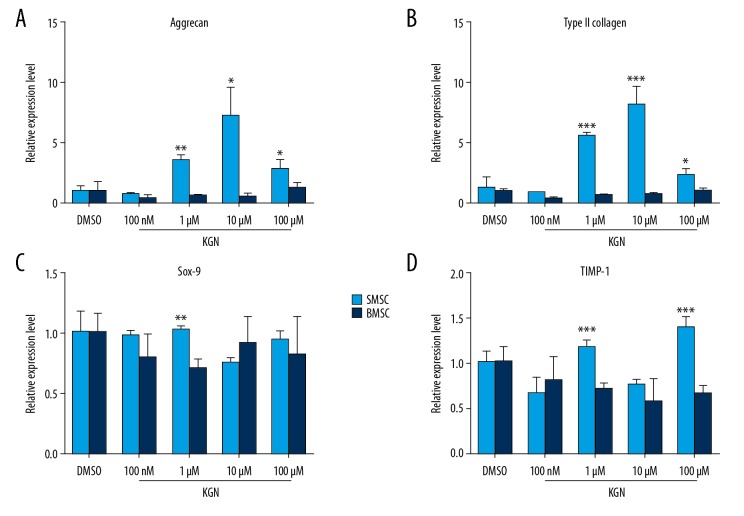
In order to explore the intervention of KGN to induce chondrogenic differentiation of various tissue-derived mesenchymal stem cells, we performed a preliminary study using qRT-PCR to detect 4 commonly used cartilage phenotype genes, including glycosaminoglycan (aggrecan), type II collagen, Sox-9, and tissue inhibitor of metalloproteinases-1 (TIMP-1) in the concentration of 10 nmol/L to 100 μmol/L. Result showed that the expression of aggrecan and type II collagen genes in SMSCs significantly increased under the intervention of KGN at 1 μmol/L and 10 μmol/L, and the expression of the TIMP-1 gene in SMSCs also increased under the intervention of KGN at 100 μmol/L. While in BMSCs, only with the expression of aggrecan and type II collagen genes increased under the intervention of KGN at 100 μmol/L, but without statistically significant. This indicated that KGN could induce MSCs differentiate into cartilage, and the response of SMSCs to KGN was stronger than that of BMSCs, indicating the ability of SMSCs to differentiate into cartilage was better than BMSCs (Figure 3).
Figure 3.
(A–D) KGN induced MSCs and BMSCs differentiate into cartilage. SMSCs and BMSCs experienced 72-hour treatment with KGN, applied qRT-PCR to determinate gene expression level. KGN – kartogenin; SMSCs – synovial-derived mesenchymal stem cells; BMSCs – bone marrow-derived mesenchymal stem cells; qRT-PCR – quantitative real-time polymerase chain reaction.
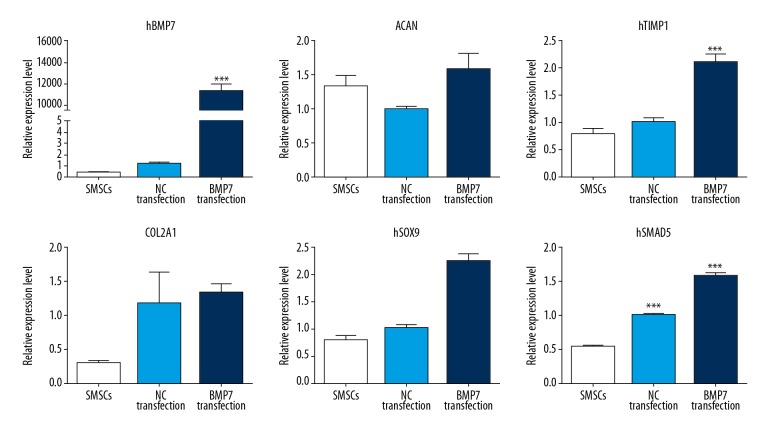
BMP-7 activated Smad5
We successfully constructed the lentiviral overexpression vector pLenti6.3-BMP-7-IRES-EGFP containing the target gene BMP-7, and verified that the optimal lentivirus titer for virus infection was 1.04×108 Tu/ml. After the SMSCs were infected with BMP-7 lentivirus, the expression of the BMP-7 gene increased significantly, far exceeding the normal group and the NC group (P<0.05), indicating that the lentivirus successfully infected the cells, and the target gene BMP-7 was over expression. Moreover, when BMP-7 expression was upregulated, the expression of Smad5 and 4 cartilage phenotype genes was also upregulated (P<0.05), indicating that BMP-7 can further regulate the expression of downstream gene Smad5. BMP-7/Smad5 pathway activation promotes SMSCs differentiate into chondrocytes (Figure 4).
Figure 4.
BMP-7 activates Smad5. Infected SMSCs with BMP-7 lentivirus, BMP-7 over expression could activate BMP-7. SMSCs – synovial-derived mesenchymal stem cells.
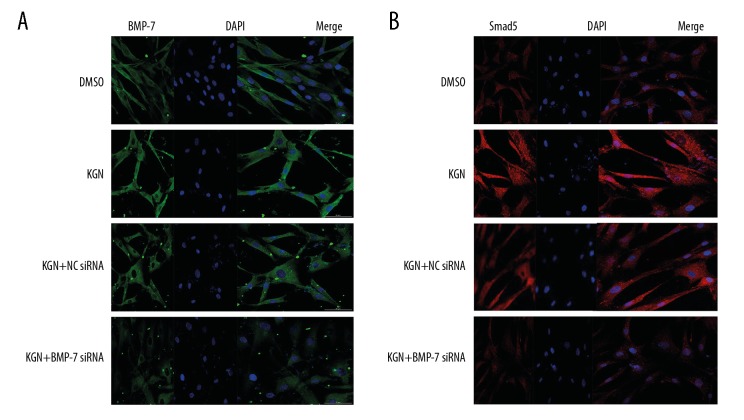
Immunofluorescence detection of BMP-7 and Smad5 protein
To explore the mutual regulation relationship between BMP-7 and Smad5 in the process of KGN-activated SMSCs differentiate into chondrogenic signaling pathway, we performed a cell immunofluorescence confocal assay. We found that the expression of BMP-7 and Smad5 proteins in KGN-treated cells was significantly increased compared with the DMSO group, indicating that KGN promotes upregulation of BMP-7 and Smad5 genes in SMSCs. Compared with the NC siRNA+KGN group and the KGN group, the expression of BMP-7 and Smad5 protein in the BMP-7 siRNA+KGN group decreased significantly, which was close to results for the DMSO group, indicating that BMP-7 gene was downregulated. After that, the Smad5 gene expression was also downregulated, indicating that the 2 genes are in the same cellular signaling pathway, and the BMP-7 gene could regulate Smad5, and these 2 genes could synergistically promote the SMSCs differentiate into chondrocytes (Figure 5).
Figure 5.
(A, B) Immunofluorescence detection of BMP-7 and Smad5 protein.
Discussion
Both human SMSCs and BMSCs have multi-differentiation potential. In our study we found that KGN had no toxicity for SMSCs and BMSCs. KGN at 10 nmol/L~10 μmol/L promoted SMSCs proliferation at every timepoint of detection, especially KGN at 10 μmol/L. KGN at 10 μmol/L promoted BMSCs proliferation during the second and the fourth days after the intervention of KGN.
The expression of aggrecan and type II collagen genes in SMSCs significantly increased under the intervention of KGN at 1 μmol/L and 10 μmol/L, and the expression of TIMP-1 gene in SMSCs also increased under the intervention of KGN at 100 μmol/L. Only the expression of aggrecan and type II collagen genes in BMSCs increased under the intervention of KGN at 100 μmol/L, without statistical difference. It was proven that KGN could induce the differentiation of MSCs into cartilage, and the response of SMSCs to KGN was stronger than that of BMSCs, indicating the ability of SMSCs to differentiate into cartilage was better than BMSCs.
A KGN drug delivery system with fibrin glue as the carrier was successfully prepared. Fluorescence spectroscopy suggested that the loss of KGN was negligible during the preparation of the KGN drug delivery system, and KGN could be released slowly from the system over time to impact on the surrounding microenvironment.
Target genes BMP-7 and Smad5 were selected by qRT-PCR screening; these genes have been associated with KGN promoting the selective differentiation of endogenous MSCs into chondrocytes to improve tendon to bone healing [18]. Gene intervention technology, immunofluorescence staining, qRT-PCR, and western blot proved that KGN induced the differentiation of endogenous MSCs into cartilage by activating the BMP-7/Smad5 pathway.
In vivo, the expression of BMP-7 and Smad5 at the interface of tendon to bone healing under KGN intervention at 6 and 12 weeks after rotator cuff repair increased significantly, indicating that KGN improved tendon to bone healing by activating the BMP-7/Smad5 pathway.
Conclusions
Based on these results, we found that KGN could induce the selective differentiation of endogenous MSCs into chondrocytes to promote cartilage regeneration at the interface by activating the BMP-7/Smad5 pathway; the normal morphology of tendon-bone junction seemed to be reproduced. Finally, KGN successfully improved the quality of tendon to bone healing after rotator cuff repair.
Therefore, this study found a new kind of biological intervention method for promoting tendon to bone healing after rotator cuff repair. The results of this study will help to further acknowledge the unique application value of KGN in the research field of tendon to bone healing, providing important evidence for more in-depth application research and related mechanism research in the future. However, this study did not conduct research on living animals or at clinical levels. The results of cell-level studies are not necessarily applicable to in vivo studies. Therefore, the experimental conclusions need further study.
Footnotes
Source of support: This work was supported by Youth Foundation of Shanghai Municipal Commission of Health and Family Planning (20164Y0117)
Conflicts of interest
None.
References
- 1.Didesch JT, Tang P. Anatomy, etiology, and management of scapular winging. J Hand Surg Am. 2019;44(4):321–30. doi: 10.1016/j.jhsa.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Deprés-Tremblay G, Chevrier A, Snow M, et al. Rotator cuff repair: A review of surgical techniques, animal models, and new technologies under development. J Shoulder Elbow Surg. 2016;25(12):2078–85. doi: 10.1016/j.jse.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Brodell JD, Jr, MacDonald A, Perkins JA, et al. Deltoid-spring ligament reconstruction in adult acquired flatfoot deformity with medial peritalar instability. Foot Ankle Int. :2019. doi: 10.1177/1071100719839176. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Rothrauff BB, Pauyo T, Debski RE, et al. The rotator cuff organ: Integrating developmental biology, tissue engineering, and surgical considerations to treat chronic massive rotator cuff tears. Tissue Eng Part B Rev. 2017;23(4):318–35. doi: 10.1089/ten.teb.2016.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yonemitsu R, Tokunaga T, Shukunami C, et al. Fibroblast growth factor 2 enhances tendon-to-bone healing in a rat rotator cuff repair of chronic tears. Am J Sports Med. 2019;47(7):1701–12. doi: 10.1177/0363546519836959. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Li R, Rong W, et al. Therapeutic effect of hepatocyte growth factor-overexpressing bone marrow-derived mesenchymal stem cells on CCl4-induced hepatocirrhosis. Cell Death Dis. 2018;9(12):1186. doi: 10.1038/s41419-018-1239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q, Yu Y, Reisdorf RL, et al. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. 2019;192:189–98. doi: 10.1016/j.biomaterials.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Kwon DR, Park GY, Moon YS, Lee SC. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cells combined with polydeoxyribonucleotides on full-thickness rotator cuff tendon tear in a rabbit model. Cell Transplant. :2018. doi: 10.1177/0963689718799040. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellera Gomes JL, da Silva RC, Silla LM, et al. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373–77. doi: 10.1007/s00167-011-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Im GI. Application of kartogenin for musculoskeletal regeneration. J Biomed Mater Res A. 2018;106(4):1141–48. doi: 10.1002/jbm.a.36300. [DOI] [PubMed] [Google Scholar]
- 11.Decker RS, Koyama E, Enomoto-Iwamoto M. Mouse limb skeletal growth and synovial joint development are coordinately enhanced by kartogenin. Dev Biol. 2014;395(2):255–67. doi: 10.1016/j.ydbio.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annamalai RT, Hong X, Schott NG, et al. Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects. Biomaterials. 2019;208:32–44. doi: 10.1016/j.biomaterials.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhang J, Chang F, et al. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater Sci Eng C Mater Biol Appl. 2018;88:79–87. doi: 10.1016/j.msec.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Cheng Y, Chen J, et al. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103–11. doi: 10.1016/j.actbio.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZZ, Wang SJ, Zhang JY, et al. 3D-printed poly (ɛ-caprolactone) scaffold augmented with mesenchymal stem cells for total meniscal substitution: A 12- and 24-week animal study in a rabbit model. Am J Sports Med. 2017;45(7):1497–511. doi: 10.1177/0363546517691513. [DOI] [PubMed] [Google Scholar]
- 16.Pei M, Zhang Y, Li J, Chen D. Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev. 2013;22(6):889–900. doi: 10.1089/scd.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann A, Floerkemeier T, Melzer C, Hass R. Comparison of in vitro cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. J Tissue Eng Regen Med. 2017;11(9):2565–81. doi: 10.1002/term.2153. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Ma J, Mu R, et al. Bone morphogenetic protein 7 promotes odontogenic differentiation of dental pulp stem cells in vitro. Life Sci. 2018;202:175–81. doi: 10.1016/j.lfs.2018.03.026. [DOI] [PubMed] [Google Scholar]