Abstract
Background
Herpes zoster and its related complications are associated with significant medical burden, which negatively affects quality of life and daily functioning of the patients. The recently licensed recombinant zoster vaccine (RZV) offers high efficacy but is associated with local and systemic reactions. This study assessed the impact of RZV on the quality of life and daily functioning of participants and implications for caregivers.
Methods
Four hundred and one adults aged 50 years or older received single RZV doses at 0 and 2 months in this open-label, single-arm, multicenter study (NCT02979639). Change in mean SF-36 Physical Functioning score following first-dose administration, quality of life, reactogenicity, safety, productivity loss, and health care resource utilization was assessed. The current analysis was performed post-vaccine dose-1; safety follow-up will continue until 1 year post-dose-2.
Results
The most common solicited local symptoms were injection-site pain (77.5%), redness (23.0%), and swelling (13.3%); the most frequent solicited systemic reactions were fatigue (33.5%), headache (28.3%), and myalgia (26.8%). Grade 3 reactogenicity occurred in 9.5% of participants and was associated with a transient clinically important decrease in SF-36 Physical Functioning score (affecting activities such as walking, carrying groceries, climbing stairs) on Days 1 and 2 post-first vaccination. No clinically meaningful reductions in mean SF-36 Physical Functioning scale scores from pre- to post-RZV dose-1 were observed (mean +1.9 points, primary end point), and no overall quality-adjusted-life-year loss was recorded post-dose-1. Five participants reported lost workdays; caregiver workload was not increased.
Conclusions
Overall, the physical functioning and quality of life of older adults were not affected by a first RZV dose. The observed reactogenicity was consistent with previous studies.
Keywords: Physical function, Physical activity, Pain, RZV
Herpes zoster (HZ), resulting from the reactivation of latent varicella zoster virus (VZV), presents as a vesicular dermatomal rash usually accompanied by pain (1). The majority of patients suffering from HZ are aged more than 50 years, reflecting an age-related decline in VZV-specific T-cell immunity; incidence rates rise from 3–5 per 1,000 person-years at 50 years, to 6–8 and 8–12 per 1,000 person-years at 60 and 80 years, respectively (2).
Postherpetic neuralgia (PHN), the most common complication of HZ, may persist for months or years after infection (3). HZ and its complications cause a significant medical, social, and economic burden and have a significant impact on the quality of life (QoL) and the ability of patients to perform activities of daily living (4–7). HZ can also affect the lives of caregivers and partners (4). Inevitably, as the population ages, age-related diseases, such as HZ, will become more burdensome to society (8,9).
The incidence of HZ in older adults, and its associated complications, can be reduced through vaccination. A live-attenuated vaccine has been available since 2006 (Zostavax, a registered trademark of Merck Sharp & Dohme Corp) and an adjuvanted recombinant zoster vaccine (RZV; Shingrix, a registered trademark of GlaxoSmithKline Biologicals SA) was approved in the United States and Canada late in 2017 (10–12). RZV is at least 90% effective in preventing HZ in adults aged 50 years or older (10,11), and is cost-effective from both a payer and societal perspective (13,14).
All vaccines may produce short-lived local and systemic reactions that have the potential to affect normal activities of daily living and reduce quality-adjusted-life-year (QALY) benefits of vaccination. RZV contains the AS01B adjuvant system, and although in early trials the observed reactogenicity appeared higher than had been reported in the studies with the live vaccine, development of RZV including this dose of the adjuvant continued as greater vaccine efficacy was anticipated. In two pivotal phase III RZV efficacy trials in adults aged 50 or older (ZOE-50) and 70 years or older (ZOE-70), solicited reports of local and systemic reactions within 7 days of vaccination were 84.4% and 79.0%, with 17.0% and 11.9%, respectively, being classified as Grade 3 (redness/fever > 100 mm diameter; fever > 39.0°C; preventing normal activity) (10,11). The most frequent solicited local adverse events (AEs) recorded after RZV vaccination were injection-site pain (in up to 79.1% participants), followed by redness and swelling (10,11). These symptoms were usually of mild-to-moderate intensity and resolved within approximately 3 days or less. Grade 3 injection-site pain, defined as significant pain at rest preventing normal everyday activities, occurred in approximately 4% participants and generally lasted for less than 2 days. Solicited systemic AE, which were recorded in more than 10% participants, included myalgia, fatigue, headache, shivering, fever, and gastrointestinal (GI) symptoms. Myalgia and fatigue, which occurred in up to 45.9% and 46.3% participants, respectively, lasted an average of 2.5 days (10,11). Grade 3 solicited systemic symptoms occurred in 6.0%–11.4% participants, with an average duration less than 2 days (10,12).
It is important for vaccinators to discuss the impact of vaccination with recipients and caregivers, in terms of potential reactogenicity and QoL. In particular, having a good understanding of what to expect will inform vaccine recipients about reactogenicity and may influence the decision about returning for the second vaccine dose. As the aforementioned ZOE studies did not evaluate the impact of vaccine reactogenicity on QoL immediately after vaccination, this study was undertaken to primarily assess the effect of vaccination on the QoL of vaccine recipients, by measuring the change in SF-36 Physical Functioning (PF) scale score from pre- to post-first vaccination. Secondary objectives included changes in SF-36 PF single item scores, SF-36 role physical scores, changes in QALY, health care utilization, work loss by participants and caregivers, and the occurrence, intensity, causality, and duration of AEs.
Methods
Study Design and Participants
This phase III, single-arm, open-label study, conducted in 13 centers in the United States, commenced on January 16, 2017 (NCT02979639). This manuscript presents the prespecified first analysis of the reactogenicity data collected until 7 days after the first vaccine dose of the two-dose RZV schedule.
Men and women aged 50 years or older at enrollment, who provided written informed consent before study start, each received two 0.5-mL doses of reconstituted RZV at Months 0 and 2, administered intramuscularly into the deltoid region of the nondominant arm. Each dose contained 50 µg of glycoprotein E antigen and the GSK proprietary AS01B Adjuvant System containing MPL, QS-21, and liposome (15).
The study protocol was approved by the institutional review boards at each study center, and the study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. The exclusion criteria are described in Supplementary Material. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Reactogenicity and Safety Assessments
Systemic symptoms (fatigue, fever, GI symptoms, headache, myalgia, and shivering) were recorded before vaccination on Days −7 and 0. For 7 days post-first vaccination (Days 0–6), participants recorded solicited local (injection-site pain, redness, and swelling) and systemic symptoms (as mentioned earlier) in diary cards. Unsolicited AEs were recorded for 30 days (Days 0–29) post-first vaccination. The intensity of all AEs was graded from 1 to 3, where Grade 3 (severe) symptoms were defined as redness or swelling greater than 100 mm diameter for injection-site events; temperature greater than 39.0°C; and “preventing normal activity” for all other symptoms. The causality of all AEs was assessed by the investigator, except for solicited local symptoms, which were all considered as causally related to vaccination.
Serious AEs and potential immune-mediated diseases were recorded over the entire study period.
Quality-of-Life Assessments
Two health surveys (SF-36 (16) and EQ-5D (17)) were carried out during this trial. SF-36 is a multipurpose health survey comprising 36 questions, including scales for PF (Questions 3a–3j), Role Physical, Bodily Pain, General Health, Vitality, Social Function, Role Emotional, and Mental Health (16). Higher scores represent greater functioning/QoL, and a change from pre-vaccination of 3.3 in PF score was considered to be clinically relevant based on the threshold of minimal clinical importance defined by Angst and colleagues (18). EQ-5D is a generic measure of health status that defines health in terms of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. These five items are combined to generate health profiles, which are converted to provide a single index utility score, where a higher score represents a better QoL (17). These questionnaires have been used previously to measure the impact of HZ and PHN on QoL (19).
Participants were trained at each visit on how to complete the forms. Both questionnaires were completed at the study center during Visit 1 (7 days pre-vaccination), Visit 2 (immediately before vaccination), and Visit 3 (7 days post-first vaccination). All participants completed the PF component (Questions 3a–3j) of the SF-36 and the entire EQ-5D questionnaire at home on a daily basis for 6 days post-first vaccination.
Prevaccination values for individual participants were calculated as the mean of the Day −7 and Day 0 assessment scores. The mean post-first vaccination scores were defined as the mean of the seven assessment scores recorded from Days 1 to 7. The changes from pre-vaccination were calculated on an individual basis, as the difference between the mean prevaccination score and the mean post-first-vaccination score. The participants thereby served as their own control (ie, by using each participants’ own prevaccination value).
Health Care Resource Utilization and Missed Time From Work
Health care resource utilization associated with reactogenicity included medication associated with reactogenicity and medically attended visits and consultations. Vaccine-related lost productivity included missed time from work by employed participants and extra work expended by caregivers.
Statistical Analyses
The QoL analysis was performed on the total vaccinated cohort, which included all vaccinated participants. The results of the SF-36 and EQ-5D questionnaires were reported stratified by age (50–59, 60–69, and ≥70 years) and overall. The SF-36 PF scores were also analyzed by gender, symptom type, and maximum reactogenicity grade. The EQ-5D results (Utility scores and QALY loss) were assessed overall and in post hoc analyses by maximum reactogenicity grade. The QALY loss was estimated using the area-under-the-curve (AUC) method, where AUC was calculated using the trapezoidal rule (20). The primary analysis for safety was based on the total vaccinated cohort. All statistical analyses were performed using Statistical Analysis Software version 9.2.
Results
Participants
Of 401 participants who received the first dose of RZV, 400 (99.8%) returned their symptom sheets. Participants had a mean age of 64.6 years, and as predefined were equally distributed between the three age groups: 50–59 years (33.4%), 60–69 years (33.2%), and greater than or equal to 70 years (33.4%). The majority of participants were Caucasian (82.8%), and there were more females than males (58.6% vs 41.4%; Table 1).
Table 1.
Baseline Demographics (Total Vaccinated Cohort)
| All Participants (N = 401) | ||
|---|---|---|
| Age (y) | ||
| Mean | 64.6 | |
| SD (range) | 8.8 (50–91) | |
| N | % | |
| Gender | ||
| Female/male | 235/166 | 58.6/41.4 |
| Race | ||
| African Heritage/African American | 48 | 12.0 |
| White—Caucasian/European Heritage | 332 | 82.8 |
| Other | 21 | 5.2 |
Note: N/% = total number/percentage of participants.
Reactogenicity and Safety
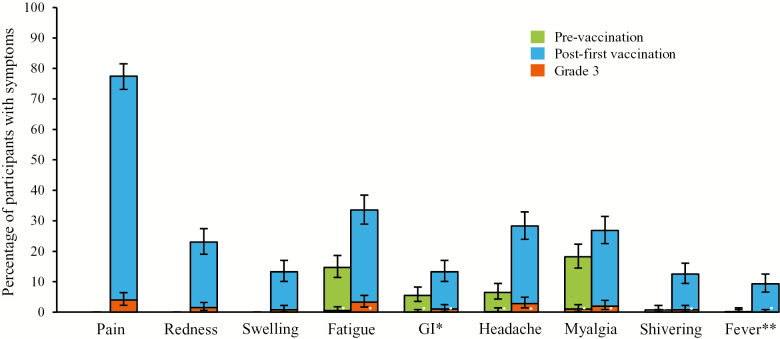
Figure 1 shows the prevaccination incidence of systemic symptoms and the incidence of solicited local and systemic symptoms up to 7 days post-first vaccination. Some participants experienced myalgia (18.2%), fatigue (14.7%), headache (6.5%), and GI symptoms (5.5%) pre-vaccination. Following the first RZV dose, the most common local injection-site reaction was injection-site pain (77.5%), followed by redness (23.0%) and swelling (13.3%); the incidence of Grade 3 solicited local AEs was less than or equal to 4%. The incidence of solicited systemic AEs all increased from pre-vaccination to 7 days post-first vaccination. The incidence of Grade 3 solicited systemic AEs was less than or equal to 3.3% in all participants, and no serious AEs or potential immune-mediated diseases were reported during the 7 days after the first dose of RZV.
Figure 1.
Percentage of participants with solicited systemic symptoms before vaccination and solicited local and systemic symptoms over Days 0–6 post-first vaccinationa (Total Vaccinated Cohort). aRecombinant zoster vaccine Dose 1 administered on Day 0. *Nausea, vomiting, diarrhea, and/or abdominal pain; **≥37.5°C. GI = gastrointestinal.
Quality-of-Life Results
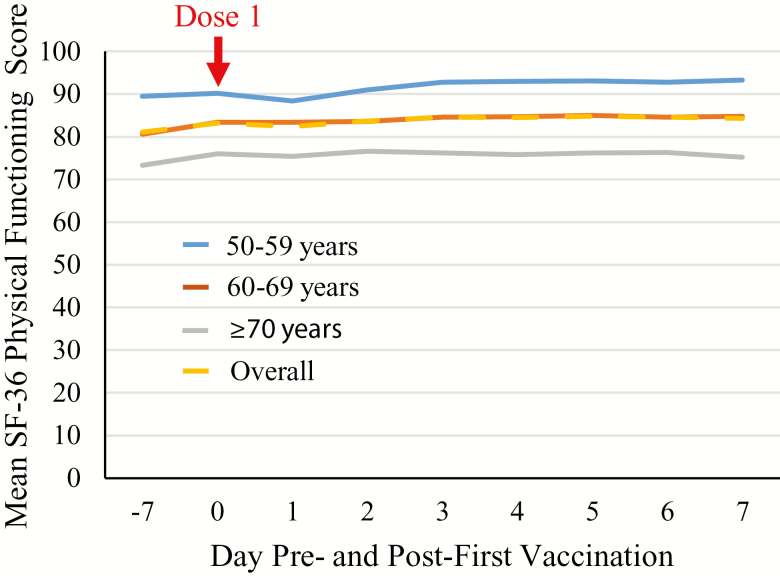
There was an overall increase in SF-36 PF score of 1.9 from pre- to post-first vaccination. Figure 2 and Table 2 present the results overall and stratified by age group. Before first vaccination, the overall SF-36 PF score was 82.2, which increased slightly post-first vaccination to 84.1 (+1.9). The prevaccination SF-36 PF scores were lower in older age groups, but the postvaccination changes were similar in the individual age groups (50–59 years: 89.9–91.9 [+2.0]; 60–69 years: 82.0–84.3 [+2.3]; ≥70 years: 74.7–76.1 [+1.5]); the changes in SF-36 PF scores were also similar in males and females. These differences were all less than 3.3, that is, below the threshold of minimal clinical importance defined by Angst and colleagues (18).
Figure 2.
Mean SF-36 Physical Functioning scale score pre- and post-first vaccinationa by age and overall (Total Vaccinated Cohort). aRecombinant zoster vaccine Dose 1 administered on Day 0.
Table 2.
Mean SF-36 PF Scale Scores Pre- and Post-first vaccinationa (Total Vaccinated Cohort)
| Pre-vaccination | Days 1–7 Post-first vaccination | Change From Pre- to Post-first vaccination | SE of the Mean Change | ||||
|---|---|---|---|---|---|---|---|
| N | Mean Score | N | Mean Score | ||||
| Overall | 401 | 82.2 | 400 | 84.1 | 1.9 | 0.52 | |
| Age category (y) | 50–59 | 134 | 89.9 | 133 | 91.9 | 2.0 | 0.78 |
| 60–69 | 133 | 82.0 | 133 | 84.3 | 2.3 | 0.75 | |
| ≥70 | 134 | 74.7 | 134 | 76.1 | 1.5 | 1.13 | |
| Reactogenicity grade | 0 | 64 | 79.6 | 63 | 84.3 | 4.7 | 1.09 |
| 1 and 2 | 299 | 83.5 | 299 | 85.5 | 1.9 | 0.61 | |
| 3 | 38 | 75.7 | 38 | 73.0 | −2.6 | 1.76 | |
| Symptom type | None | 64 | 79.6 | 63 | 84.3 | 4.7 | 1.10 |
| Local | 321 | 82.6 | 321 | 84.2 | 1.5 | 0.59 | |
| Systemic | 220 | 82.3 | 220 | 82.9 | 0.7 | 0.70 | |
| Gender | Male | 166 | 81.3 | 165 | 83.7 | 2.4 | 0.93 |
| Female | 235 | 82.8 | 235 | 84.4 | 1.6 | 0.60 | |
Notes: N/% = total number of/percentage of participants. Pre-vaccination is calculated as the mean of Day −7 and Day 0 assessments; post-first-vaccination mean score is mean of seven assessments from Day 1 to Day 7; high score represents a high level of functioning/quality of life. Change from pre-vaccination is the change from derived pre- to post-first-vaccination scores. Reactogenicity grading: 0 (none/normal); 1 (mild); 2 (moderate); 3 (severe; prevents normal activity); for swelling/redness; greatest surface diameter 0 (<20 mm); 1 (≥20 to ≤50 mm); 2 (>50 to ≤100 mm); 3 (>100 mm); for temperature: 0 (<37.5°C); 1 (37.5–38.0°C); 2 (38.1–39.0°C); 3 (>39.0°C). Participants characterized according to maximum reactogenicity grade reported within 7 days post-first vaccination.
aRecombinant zoster vaccine Dose 1 administered on Day 0.
Participants experiencing Grade 3 reactogenicity recorded a 2.6 reduction in mean PF from pre- to post-first vaccination (Table 2). On a daily basis, the PF score in participants with Grade 3 reactogenicity transiently decreased by approximately 10 points from Day 0 (75.8) to Day 1 (65.2), but had recovered by Day 3 (74.8; Table 3). Table 4 shows the changes in the other SF-36 scales from pre- to post-first vaccination ranged from −0.9 (social functioning) to +1.9 (mental health). Similar findings were seen in all age groups (data not shown).
Table 3.
Mean SF-36 PF Scale Scores Pre- and Post-first vaccinationa by Day, Maximum Reactogenicity Grade, and Symptom Type (Total Vaccinated Cohort)
| Day | Grade | Symptom Type | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 or 2 | 3 | None | Local | Systemic | |||||||
| N | Mean Score | N | Mean Score | N | Mean Score | N | Mean Score | N | Mean Score | N | Mean Score | |
| Pre-vaccination | ||||||||||||
| −7 | 64 | 76.8 | 299 | 82.8 | 38 | 75.5 | 64 | 76.8 | 321 | 81.8 | 220 | 81.8 |
| 0 | 64 | 82.3 | 299 | 84.3 | 38 | 75.8 | 64 | 82.3 | 321 | 83.5 | 220 | 82.8 |
| Post-first vaccination | ||||||||||||
| 1 | 61 | 84.8 | 299 | 84.1 | 38 | 65.2 | 64 | 84.8 | 321 | 82.0 | 220 | 79.7 |
| 2 | 62 | 84.7 | 299 | 85.5 | 38 | 68.0 | 61 | 84.7 | 321 | 83.8 | 220 | 82.3 |
| 3 | 62 | 84.9 | 298 | 85.7 | 38 | 74.8 | 62 | 84.9 | 320 | 84.5 | 220 | 83.7 |
| 4 | 62 | 84.8 | 298 | 85.6 | 38 | 75.7 | 62 | 84.8 | 320 | 84.5 | 220 | 83.6 |
| 5 | 62 | 85.0 | 298 | 85.7 | 38 | 77.2 | 62 | 85.0 | 320 | 84.8 | 220 | 84.0 |
| 6 | 62 | 85.0 | 298 | 85.7 | 38 | 74.7 | 62 | 85.0 | 320 | 84.5 | 220 | 83.7 |
| 7 | 45 | 83.1 | 220 | 85.4 | 21 | 75.5 | 45 | 83.1 | 227 | 84.7 | 156 | 82.9 |
Notes: N = total number of participants. High score represents high level of functioning/quality of life. Reactogenicity grading: 0 (none/normal); 1 (mild); 2 (moderate); 3 (severe; prevents normal activity); for swelling/redness: greatest surface diameter 0 (<20 mm); 1 (≥20 to ≤50 mm); 2 (>50 to ≤100 mm); 3 (>100 mm); for temperature: 0 (<37.5°C); 1 (37.5–38.0°C); 2 (38.1–39.0°C); 3 (>39.0°C). Participants characterized according to maximum reactogenicity grade or symptom type reported within 7 days post-first vaccination (none = participants with no solicited symptom reported; local = participants with at least one solicited local symptom reported; systemic = participants with at least one solicited general symptom reported; participants can be included in both local and systemic categories).
aRecombinant zoster vaccine Dose 1 administered on Day 0.
Table 4.
Changes in Additional SF-36 Scales From Pre- to Day 7 Post-first vaccinationa (Total Vaccinated Cohort)
| Role Physical | Bodily Pain | General Health | Vitality | Social Functioning | Role Emotional | Mental Health | |
|---|---|---|---|---|---|---|---|
| Day −7 | 84.2 | 75.1 | 77.6 | 70.2 | 91.7 | 90.8 | 83.6 |
| Day 0a | 87.8 | 78.1 | 77.8 | 73.7 | 93.3 | 92.7 | 85.8 |
| Pre-vaccination | 86.0 | 76.5 | 77.7 | 71.9 | 92.3 | 91.8 | 84.7 |
| Post-first vaccination | 87.5 | 77.2 | 77.4 | 73.8 | 92.2 | 93.3 | 87.2 |
| Change from pre- to post- first vaccination | 1.4 | 0.8 | −0.1 | 1.8 | −0.9 | 0.8 | 1.9 |
| SE of mean change | 0.89 | 1.00 | 0.52 | 0.65 | 0.82 | 0.71 | 0.49 |
Notes: Pre-vaccination is calculated as mean of Day −7 and Day 0 assessments; post-first-vaccination score measured on Day 7; high score represents high level of functioning/quality of life. Change from pre-vaccination is the change from derived pre- to post-first-vaccination scores.
aRecombinant zoster vaccine Dose 1 administered on Day 0.
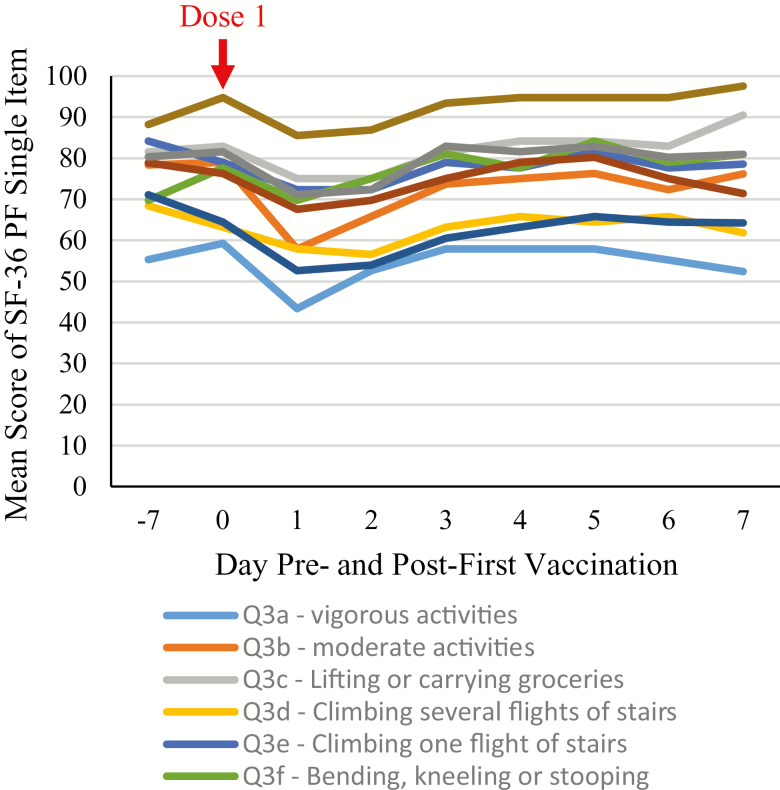
Figure 3 presents the mean PF single items from Days −7 to 7 for participants with Grade 3 reactogenicity. The items that were most affected on Day 1 were Question 3b (moderate activities, eg, moving a table, pushing a vacuum cleaner, bowling), Question 3a (vigorous activities, eg, running, lifting heavy objects, participating in strenuous sports), and Questions 3g and 3i (walking >1 mile; 100 yards, respectively). Scores had returned to prevaccination values by Day 3.
Figure 3.
Mean SF-36 Physical Functioning single items from Day −7a to Day 7 for participants with Grade 3 reactogenicity only (Total Vaccinated Cohort). aRecombinant zoster vaccine Dose 1 administered on Day 0.
EQ-5D: QALY
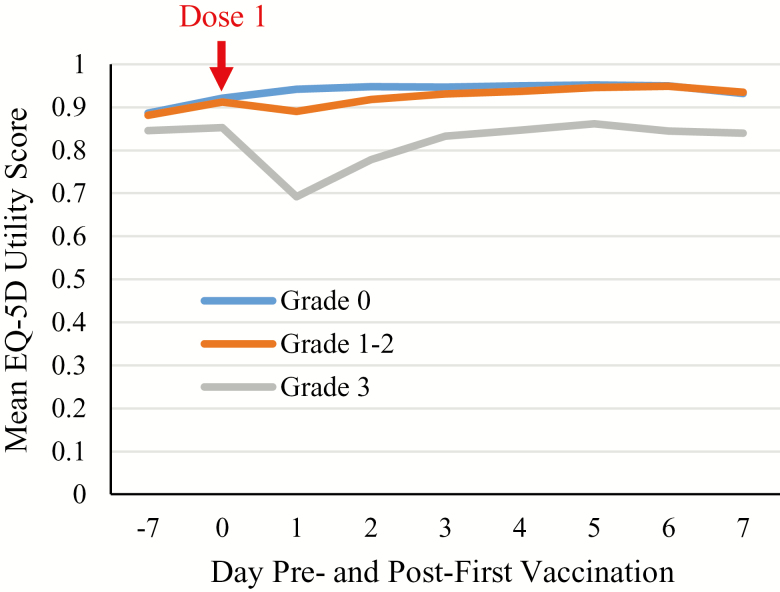
Table 5 shows the AUC of the EQ-5D utility scores pre- and post-first vaccination overall and by maximum reactogenicity grade. Overall, there was no decrease in the AUC of the EQ-5D utility scores post-first vaccination (+0.183) and thus no QALY loss. In the 38 participants with Grade 3 reactogenicity (9.5%), a relatively large decrease in mean EQ-5D utility score was observed on Day 1 (Figure 4), but these had returned to prevaccination values by Day 3. Nevertheless, the decrease on Days 1 and 2 affected the AUC score over Days 0–7, and a mean post-first-vaccination reduction of −0.247 was observed (ie, QALY loss in Grade 3 participants: [0.247]/365 = 0.000677, overall QALY loss per dose per participant: 0.000064 [ie, 0.000677 × 0.095]; Table 5).
Table 5.
Area Under the Curve of the EQ-5D Utility Scores Pre- and Post-first vaccinationa by Reactogenicity Grade and Overall (Total Vaccinated Cohort)
| Reactogenicity | |||||
|---|---|---|---|---|---|
| Grade 0 (N = 64) | Grade 1 or 2 (N = 299) | Grade 3 (N = 38) | Total (N = 401) | ||
| Pre-vaccination | Mean | 6.326 | 6.281 | 5.944 | 6.256 |
| SD | 0.7470 | 0.8039 | 1.1598 | 0.8392 | |
| Days 1–7 score (AUC) | N | 63 | 297 | 38 | 398 |
| Mean | 6.615 | 6.494 | 5.697 | 6.437 | |
| SD | 0.7503 | 0.6601 | 1.1247 | 0.7685 | |
| Change in AUC | N | 63 | 297 | 38 | 398 |
| Mean | 0.277 | 0.218 | −0.247 | 0.183 | |
| SD | 0.5498 | 0.5093 | 0.7223 | 0.5562 | |
| SE | 0.069 | 0.030 | 0.117 | 0.028 | |
Notes: AUC = area under the curve; N (column header) = total number of participants; N (rows) = number of participants with non-missing values. Pre-vaccination is calculated as AUC method applied to Day −7 and Day 0 assessments; post-first vaccination is AUC method applied to assessments from Day 1 to Day 7. Change from pre-vaccination is the change from derived pre- to post-first-vaccination AUC scores. Reactogenicity grading: 0 (none/normal); 1 (mild); 2 (moderate); 3 (severe; prevents normal activity); for swelling/redness; greatest surface diameter 0 (<20 mm); 1 (≥20 to ≤50 mm); 2 (>50 to ≤100 mm); 3 (>100 mm); for temperature: 0 (<37.5°C); 1 (37.5–38.0°C); 2 (38.1–39.0°C); 3 (>39.0°C). Participants characterized according to maximum reactogenicity grade reported within 7 d post-first vaccination.
aRecombinant zoster vaccine Dose 1 administered on Day 0.
Figure 4.
Mean EQ-5D utility score from Day −7a to Day 7 by reactogenicity grade (Total Vaccinated Cohort). aRecombinant zoster vaccine Dose 1 administered on Day 0.
Medically Attended Reactogenicity
Three participants sought medical attention for reactogenicity symptoms after the first vaccine dose. Two participants reporting Grade 1 injection-site pain/GI symptoms and Grade 2 injection-site pain/myalgia, respectively, made telephone calls on Day 0. The third patient made a visit to a general practitioner on Day 6 having experienced Grade 1 fatigue since vaccination, GI symptoms/myalgia until Day 2, headache until Day 3, and Grade 1 injection-site pain until Day 4. None of these participants reported unsolicited AEs within the 7 days post-first vaccination.
Participant Work Loss or Additional Caregiver Workload due to Vaccination
Work loss was not applicable to 215 participants, but of the 186 participants for whom this parameter was relevant, 5 (2.7%) reported missing work following vaccination due to reactogenicity symptoms. The mean work loss was 1.3 days (SD 1.0). Three of these participants had no Grade 3 reactogenicity, but one reported unsolicited AEs (dizziness [Day 1], sore throat/runny nose [Day 3]). One participant had Grade 3 myalgia and fatigue on Day 1, together with Grade 1/2 injection-site pain and headache, but did not report unsolicited AE within the 7 days post-first vaccination. The fifth participant recorded Grade 3 injection-site pain on Days 0 and 1 together with Grade 1/2 headache, myalgia, fatigue, GI symptoms, and developed bronchitis on Day 6. None of the caregivers reported an extra work burden following the first RZV dose.
Discussion
In this analysis after the first vaccine dose, the pattern of solicited AEs was consistent with previous studies (10–12). In the two ZOE-50/70 phase III trials in older adults, the overall frequencies of solicited AEs were similar after the first and second doses of RZV, although the frequencies of Grade 3 solicited systemic events were higher post-dose 2 in adults 70 years or older (10,11). In this study, notably, several of the solicited systemic AEs were already reported before vaccination (myalgia [18.2%], fatigue [14.7%], headache [6.5%], and GI symptoms [5.5%]), which can be explained by the age of the study population and the nonstringent inclusion and exclusion criteria, allowing recruitment of an overall older adult population, including people with certain comorbidities. There were no serious AEs or potential immune-mediated diseases reported during this 7-day follow-up.
The primary objective of this study was to estimate the change in the SF-36 PF scale score from pre- to post-first vaccination in relation to observed reactogenicity. Overall, there were no reductions above 3.3 (the threshold of minimal clinical importance (18)) in the mean SF-36 PF post-first vaccination. The only factor associated with a transient reduction in SF-36 PF score above this threshold was Grade 3 reactogenicity. In the 38 participants with Grade 3 reactogenicity (9.5%), the PF score decreased by approximately 10 points from Day 0 (75.8) to Day 1 (65.2), but had returned to prevaccination levels by Day 3. This reflects the transient pattern of post-first-vaccination reactogenicity, which has been previously observed to last between 1 and 3 days after RZV administration (10,11). Changes in other SF-36 scales from pre- to post-first vaccination were small (from −0.9 to +1.9), indicating that vaccination with RZV was not associated with a negative impact on these QoL parameters. It was noteworthy that for participants with Grade 1 or 2 reactogenicity, the mean PF score remained relatively stable during Days 0, 1, and 2 (ie, 84.3, 84.1, and 85.5), suggesting that PF scores were affected only in participants with Grade 3 reactogenicity. This would indicate that the classification of individuals into Grade 3 was accurate (ie, defined as preventing normal activity) and therefore provides a validation of the grading system.
Although QALY loss due to reactogenicity has been infrequently studied in cost-effectiveness models (mainly for sensitivity analysis), the overall QALY loss per vaccine dose per participant estimated here is consistent with values that can be calculated from previously presented data. For example, Ultsch and colleagues (21) assumed that all participants would have a utility loss of 0.01 lasting for 2 days (ie, QALY loss per participant: 0.00005). Van Hoek and colleagues (22) assumed 30% participants would have a utility loss of 0.05 for two days (ie, QALY loss per participant: 0.00008), and Hornberger and Robertus assumed that 0.317 participants would have a utility loss of 0.10 for 2 days (ie, QALY loss per participant: 0.00017) (23). In addition, Le and Rothberg (24) assumed that 64% participants would experience a local reaction with a QALY loss of 0.0001 and 0.1% would have a serious reaction (ie, requiring hospitalization) with a QALY loss of 0.0082 (ie, QALY loss per participant: 0.00007). Overall QALY losses due to HZ including PHN ranged from 0.021, 0.049, and 0.058 for participants aged 60–69, 70–79, and greater than or equal to 80 years, respectively (25) to 0.10 and 0.22 for those less than 70 and greater than or equal to 70 years, respectively (26). As such, the QALY loss associated with an episode of HZ (including PHN) ranges from 300 to 3,000 times higher than the potential QALY losses associated with reactogenicity, suggesting the benefits of vaccination in preventing disease clearly outweigh any potential impact of reactogenicity (26).
With respect to indirect costs associated with post-first-vaccination reactogenicity, only three participants sought medical attention, and in no case was this due to Grade 3 reactogenicity. In addition, five participants reported work loss, although only two of those experienced Grade 3 symptoms, and no extra work for caregivers was recorded. Considering that previous studies have shown that up to 64% of employed patients missed work due to HZ and PHN, or up to 76% were less effective while at work (27), any work losses as a result of vaccine reactogenicity are clearly outweighed by the benefits of vaccination.
We observed excellent compliance in participants returning their questionnaires, which is a strength of the study.
The study was limited by its open nature and lack of a control group. However, this limitation was mitigated in part by collecting prevaccination data and subsequently using the participants as their own control group, that is, analyzing changes from baseline. Increases in mean QoL scores were observed from Day −7 to Day 0 overall and from Day 0 to Day 7 predominantly in participants with Grade 0 reactogenicity. If a control group had been included in the study design, it may have been possible to adjust for this phenomenon, which is commonly referred to as a response shift (28). As participants knew that the study was being undertaken to assess the impact of reactogenicity on QoL, this might have encouraged them to report more symptoms. However, the incidence of symptoms observed in this study was comparable with previous reports. The lack of a control group may be further seen as a limitation in particular when analyzing resource use data; for example, it is not possible to determine whether all work days lost were solely due to solicited reactogenicity.
Here, we present results after one dose of RZV.
Conclusions
It is important for vaccinators to inform vaccinees about expected reactogenicity and any anticipated clinically significant impact on their QoL, both to decrease concerns about potential reactogenicity and ensure better second vaccine dose compliance. The reactogenicity observed after the first dose of RZV was comparable with results from previous studies, and the PF and QoL of individuals aged 50 years or older post-first RZV dose was not considered to be clinically affected. Grade 3 reactogenicity was, however, associated with a transient clinically important decrease in SF-36 PF score on Days 1 and 2 post-first vaccination. The work loss and resource utilization associated with reactogenicity are considered to be greatly outweighed by those avoided by preventing HZ episodes through vaccination.
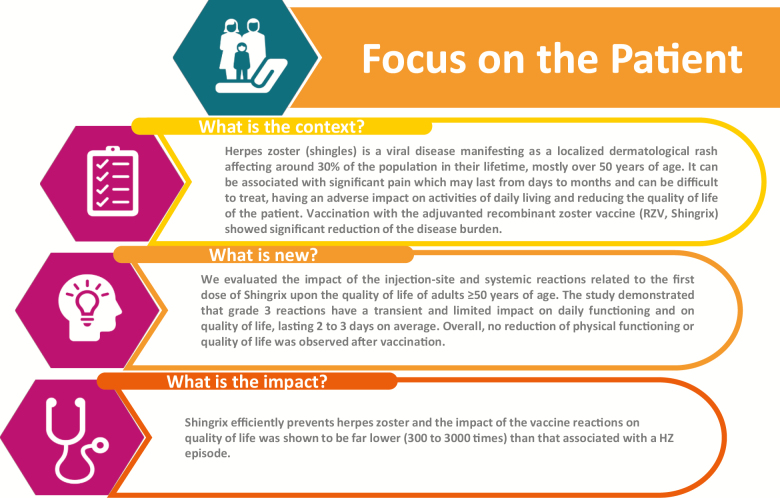
A summary contextualizing the results and their impact is displayed in the Focus on the Patient section (Figure 5) for the benefit of health care professionals.
Figure 5.
Focus on the Patient section.
Funding
This study was funded by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study and covered all costs associated with developing and publishing this manuscript. K.E.S. was supported by the National Institute on Aging, Duke Pepper Older Americans Independence Center (P30AG028716).
Conflict of Interest
K.E.S. reports grants from the GSK group of companies (GSK) during the study. M.J.L. reports grants and fees for Advisory Board from GSK during the study, as well as grants and fees for Advisory Board and speaker at international meetings from Merck outside the submitted work. M.J.L. has a patent Merck issued. D.C., K.G., L.A.F., and M.E.I. are employees and L.O. former employee of GSK. D.C. and L.O. own GSK stock options. L.O. is employee of CureVac AG and is inventor on a patent owned by GSK and relevant to RZV. S.M. is a freelance consultant for GSK. N.P.K. reports grants from GSK during the study, as well as grants from Merck, Pfizer, Sanofi Pasteur, MedImmune, Protein Science, and Dynavax outside the submitted work. K.U. reports personal fees from Janssen, Novo Nordisk, Amgen, Sanofi, and Regeneron outside the submitted work. C.F., D.B., M.C., M.N., and P.H. report no potential conflict of interest.
Supplementary Material
Acknowledgments
The authors thank the study participants, investigators, and study teams involved in this trial, as well as Alain Brecx, Marie Devèze, Kanika Dey, Vincent Dodeur, Wendy Fitzgerald, Brecht Geeraerts, Caroline Hervé, Anne Schuind, and Elisa Turriani. Medical writing services were provided by Julia Donnelly (freelancer on behalf of GSK). Editorial assistance and publication coordination were provided by Quentin Deraedt (XPE Pharma and Science c/o GSK). D.C., M.E.I., K.G., N.P.K., M.J.L., S.M., L.O., and K.E.S. conceived and designed the study. D.B., M.C., C.F., N.P.K., M.J.L., M.N., K.E.S., and K.U. collected the data. D.B., M.C., D.C., C.F., K.G., P.H., N.P.K., M.J.L., M.N., K.E.S., and K.U. performed the study. D.C., L.A.F., K.G., N.P.K., S.M., L.O., and K.E.S. analyzed the data.
References
- 1. Cohen JI. Herpes zoster. N Engl J Med. 2013;369:1766–1767. doi: 10.1056/NEJMc1310369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine. 2014;32:1645–1653. doi: 10.1016/j.vaccine.2014.01.058 [DOI] [PubMed] [Google Scholar]
- 3. Cunningham AL, Dworkin RH. The management of post-herpetic neuralgia. BMJ. 2000;321:778–779. doi: 10.1136/bmj.321.7264.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gater A, Uhart M, McCool R, Préaud E. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health. 2015;15:193. doi: 10.1186/s12889-015-1514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meyers JL, Madhwani S, Rausch D, Candrilli SD, Krishnarajah G, Yan S. Analysis of real-world health care costs among immunocompetent patients aged 50 years or older with herpes zoster in the United States. Hum Vaccin Immunother. 2017;13:1861–1872. doi: 10.1080/21645515.2017.1324373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coplan PM, Schmader K, Nikas A, et al. . Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–356. doi: 10.1016/j.jpain.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 7. Schmader KE, Sloane R, Pieper C, et al. . The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–496. doi: 10.1097/AJP.0b013e318065b6c9 [DOI] [PubMed] [Google Scholar]
- 8. Varghese L, Standaert B, Olivieri A, Curran D. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17:30. doi: 10.1186/s12877-017-0420-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics. 2013;31:125–136. doi: 10.1007/s40273-012-0020-7 [DOI] [PubMed] [Google Scholar]
- 10. Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. doi:10.1056/NEJMoa160 3800 [DOI] [PubMed] [Google Scholar]
- 11. Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184 [DOI] [PubMed] [Google Scholar]
- 12. Godeaux O, Kovac M, Shu D, et al. . Immunogenicity and safety of an adjuvanted herpes zoster subunit candidate vaccine in adults ≥50 years of age with a prior history of herpes zoster: a phase III, non-randomized, open-label clinical trial. Hum Vaccin Immunother. 2017;13:1051–1058. doi: 10.1080/21645515.2016.1265715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Oorschot DA, Anastassopoulou A, Varghese L, et al. . Cost-effectiveness assessment of herpes zoster vaccination in Germany. Value Health. 2017;20:A788. doi: 10.1016/j.jval.2017.08.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prosser LA. Economic Evaluation of Vaccination for Prevention of Herpes Zoster and Related Complications Advisory Committee on Immunization Practices; https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-03-prosser.pdf. Published October 25, 2017 Accessed February 12, 2018. [Google Scholar]
- 15. Didierlaurent AM, Laupèze B, Di Pasquale A, Hergli N, Collignon C, Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16:55–63. doi: 10.1080/14760584.2016.1213632 [DOI] [PubMed] [Google Scholar]
- 16. Ware JE Jr, Kosinski M.. SF-36 Physical and Mental Health Summary Scales: A Manual for Users of Version 1. 2nd ed. Lincoln, RI: Quality Metric Incorporated; 2001. [Google Scholar]
- 17. Kind P. The EuroQL instrument: an index of health-related quality of life. In: Spilker B, ed. Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd ed. Philadelphia, PA: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 18. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- 19. Curran C, Athan E, Diez-Domingo J, et al. . Quality of Life impact of an Investigational Subunit Adjuvanted Herpes Zoster Vaccine in Adults ≥50 Years of Age New Orleans, LA: ID Week; October 26–30, 2016. https://idsa.confex.com/idsa/2016/webprogram/Paper60532.html. Accessed February 12, 2018. [Google Scholar]
- 20. Yeh S-T. Using Trapezoidal Rule for the Area Under a Curve Calculation. SUGI 27 Proceedings 2002 http://www2.sas.com/proceedings/sugi27/p229-27.pdf. Accessed February 12, 2018.
- 21. Ultsch B, Weidemann F, Reinhold T, Siedler A, Krause G, Wichmann O. Health economic evaluation of vaccination strategies for the prevention of herpes zoster and postherpetic neuralgia in Germany. BMC Health Serv Res. 2013;13:359. doi: 10.1186/1472-6963-13-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine. 2009;27:1454–1467. doi: 10.1016/j.vaccine.2008.12.024 [DOI] [PubMed] [Google Scholar]
- 23. Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:317–325. doi: 10.7326/0003-4819-145-5-200609050-00004 [DOI] [PubMed] [Google Scholar]
- 24. Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med. 2015;163:489–497. doi: 10.7326/M15-0093 [DOI] [PubMed] [Google Scholar]
- 25. Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine. 2007;25:8326–8337. doi: 10.1016/j.vaccine.2007.09.066 [DOI] [PubMed] [Google Scholar]
- 26. Moore L, Remy V, Martin M, Beillat M, McGuire A. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc. 2010;8:7. doi: 10.1186/1478-7547-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drolet M, Levin MJ, Schmader KE, et al. . Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine. 2012;30:2047–2050. doi: 10.1016/j.vaccine.2012.01.045 [DOI] [PubMed] [Google Scholar]
- 28. Ring L, Höfer S, Heuston F, Harris D, O’Boyle CA. Response shift masks the treatment impact on patient reported outcomes (PROs): the example of individual quality of life in edentulous patients. Health Qual Life Outcomes. 2005;3:55. doi: 10.1186/1477-7525-3-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.