Abstract
Background
Frailty and multimorbidity are independent prognostic factors for mortality, but their interaction has not been fully explored. We investigated the importance of multimorbidity patterns in older adults with the same level of frailty phenotype.
Methods
In a cohort of 7,197 community-dwelling adults aged 65 years and older, physical frailty status (robust, pre-frail, frail) was defined using shrinking, exhaustion, inactivity, slowness, and weakness. Latent class analysis was used to identify individuals with multimorbidity patterns based on 10 self-reported chronic conditions. We estimated hazard ratios (HR) and incidence rate differences (IRDs) for mortality comparing multimorbidity patterns within each frailty state.
Results
Five multimorbidity classes were identified: minimal disease (24.7%), cardiovascular disease (29.0%), osteoarticular disease (27.3%), neuropsychiatric disease (8.9%), and high multisystem morbidity (10.0%). Within each frailty state, the mortality rate per 1,000 person-years over 4 years was greatest in the neuropsychiatric class and lowest in the minimal disease class: robust (56.3 vs 15.7; HR, 2.11 [95% CI: 1.05, 4.21]; IRD, 24.1 [95% CI: −11.2, 59.3]), pre-frail (85.3 vs 40.4; HR, 1.74 [95% CI: 1.28, 2.37]; IRD, 27.1 [95% CI: 7.6, 46.7]), and frail (218.1 vs 96.4; HR, 2.05 [95% CI: 1.36, 3.10]; IRD, 108.4 [95% CI: 65.0, 151.9]). Although HRs did not vary widely by frailty, the excess number of deaths, as reflected by IRDs, increased with greater frailty level.
Conclusions
Considering both multimorbidity patterns and frailty is important for identifying older adults at greater risk of mortality. Of the five patterns identified, the neuropsychiatric class was associated with lower survival across all frailty levels.
Keywords: Frailty, Multimorbidity, Latent class analysis, Heterogeneity, Risk assessment
Frailty, an age-related clinical syndrome of decreased resilience in stressors, affects 15% of the community-dwelling older adults in the United States (1). Increasing degree of frailty (from robust, pre-frail to frail) is associated with greater morbidity, functional decline, and mortality (2,3) in wide-ranging contexts, including cancer (4,5), cardiovascular (6), operative (7), and intensive care (8). Based on this evidence, frailty assessment has become a crucial part of the clinical decision-making to better tailor the care of older adults (9,10). Importantly, evidence suggests that frail individuals are not a clinically homogeneous group and that further risk stratification may enable identification of subgroups at greater risk (11). Moreover, accumulation of chronic diseases, along with age-associated physiologic changes, results in multisystem dysregulation, leading to frailty (12). Distinct chronic disease profiles, or patterns (13) of multimorbidity (defined as the co-occurrence of two or more chronic conditions) (14), may represent clinically different paths to frailty and provide additional prognostic information within individuals with the same level of frailty (15).
Multimorbidity has been quantified in several ways, such as simple count of conditions, weighted count, and specific comorbidity indices (16). Recently, latent class analysis (LCA) was used to identify groups of individuals who have similar multimorbidity patterns. These patterns were associated with quality of life, functioning, hospitalizations, and emergency department visits (17,18). Rather than simple counts or weighted comorbidity indices, multimorbidity patterns may be more suitable to examine clinically relevant subgroups within older individuals with the same multimorbidity burden and frailty status.
In this study, we investigated the associations of multimorbidity patterns and frailty (19) with mortality using nationally representative data from the National Health and Aging Trends Study(NHATS). We hypothesized that clinically meaningful multimorbidity patterns could be identified in individuals with the same frailty level, some of which may portend poorer prognosis.
Materials and Methods
Data Source and Sample
Data are from the first five yearly rounds of NHATS, starting in 2011. The NHATS is a survey of a nationally representative sample of Medicare beneficiaries aged 65 years and older. Participants (or proxies if participants were unable to self-report) were interviewed in person and assessed for physical performance at baseline. Our sample was restricted to the 7,197 community-dwelling participants interviewed in 2011 without any exclusion criterion. Further details about the recruitment strategy and design of the NHATS have been described elsewhere (20). This study was approved by the institutional review board of the Brigham and Women’s Hospital, Boston, Massachusetts.
Measurement of Frailty
Frailty was operationalized using the physical frailty phenotype based on five criteria: shrinking, exhaustion, low physical activity, slow gait speed, and weak grip strength (6). Exhaustion was present when participants reported having low energy or being easily exhausted in the last month. Low physical activity was defined as not having taken part in vigorous activities or never walked for exercise in the last month. Weakness was defined as the best of two dominant handgrip strength measurements being at or below the 20th percentile within eight sex–body mass index categories (Supplementary Table A1) using a Jamar Plus+ Digital Hand Dynamometer. Slowness was defined as gait speed from the best of two timed 3 m walk tests being at or below the 20th percentile within four sex–height categories. Shrinking was present when participants reported involuntarily losing 10 pounds or more over the last year. If participants were unable to complete the handgrip strength measurement or timed walk, they were considered impaired in line with recommended practice and other published work (1,21). Participants meeting three or more criteria were considered “frail”; those with one or two criteria, “pre-frail”; and those without any criterion, “robust.”
Chronic conditions, multimorbidity, and covariates
Ten chronic conditions were assessed by asking participants whether a doctor had told them that they had hypertension, diabetes mellitus, heart disease, stroke, lung disease, arthritis, osteoporosis, cancer, depressive or anxiety symptoms, and dementia. These were used to perform LCA (see Analysis section) to identify multimorbidity patterns. Demographic variables included age (65–69, 70–74, 75–79, 80–84, 85–89, and ≥90 years) and sex. For imputation of missing baseline data (see Analysis section), we additionally used information on 15 questions assessing activities of daily living and instrumental activities of daily living, 12 physical function questions (eg, ability to climb stairs, carry heavy objects), and 9 questions pertaining to geriatric syndromes (eg, pain, falls).
Outcome
All-cause mortality was assessed yearly for 4 years until 2015 and a proxy respondent interview was administered for participants who died between rounds. Loss to follow-up for mortality ascertainment was 32.0% at year 5.
Analysis
Analyses were performed using R 3.4.3 (R Foundation for Statistical Computing, Vienna) and Stata 14.2 (College Station, TX). Single multivariable imputation using chained equations (MICE package) was used to impute baseline missing data for each component of frailty phenotype (8.0% for grip strength, 6.8% for gait speed, and less than 2.5% for all other variables), using available information on demographic, frailty, chronic conditions, disability, and physical function variables as predictors. LCA (poLCA package) was performed using 10 chronic conditions in the entire cohort. We examined two to seven multimorbidity classes, and selected the final model based on Bayesian information criterion and clinical meaning of the resulting multimorbidity classes. Each participant was assigned to a multimorbidity class based on the highest probability of class membership. For each frailty state (robust, pre-frail, and frail), Cox proportional hazards and additive Poisson models (22) with robust variance estimators were used to estimate hazard ratios (HRs) and incidence rate differences (IRDs) for mortality comparing different multimorbidity patterns. In addition, we estimated HRs and IRDs for mortality comparing different frailty states within each multimorbidity pattern. Models were adjusted for age (categories of 5 years modeled as a linear term) and sex. Due to nonconvergence of some age- and sex-adjusted additive Poisson models, we had to combine adjacent age categories for ages 65–69, 70–74, and 75–79 for the analysis of the frailty group and we did not adjust for race. A two-sided p value less than .05 was considered statistically significant.
Results
Among the 7,197 community-dwelling participants, 2,213 (30.7%) were robust, 3,647 (50.7%) were pre-frail, and 1,337 were (18.6%) frail. The full demographic characteristics and prevalence of chronic conditions are shown in Table 1. At baseline, the median age at was between 75 and 79 years. Age of participants was evenly distributed with approximately a fifth of all participants in each age group of 5 years when considering those older than 85 years as a single category. There were 4,147 (57.6%) women, and 4,861 (67.5%) individuals were white. Hypertension (67.3%) was the most common chronic condition, followed by arthritis (55.7%). A quarter (25.6%) of individuals reported cancer and 5.5% dementia. Over 4 years, 1,212 (16.8%) participants died. Mortality rate was highest in the frail group (136.0 deaths/1,000 person-years [PYs]), followed by the pre-frail group (48.7 deaths/1,000 PYs) and the robust group (20.5 deaths/1,000 PYs).
Table 1.
Characteristics of Community-Dwelling Adults Aged 65 Years or Older According to Multimorbidity Patterns in the National Health and Aging Trends Study 2011
| Characteristics | Total | Minimal Disease | CVD | Osteoarticular | Neuropsychiatric | High Multisystem Morbidity |
|---|---|---|---|---|---|---|
| Sample size (%) | 7,197 (100) | 1,780 (24.7) | 2,087 (29.0) | 1,968 (27.3) | 641 (8.9) | 721 (10.0) |
| Age, years (%) | ||||||
| 65–69 | 1,392 (19.3) | 483 (27.1) | 375 (18.0) | 365 (18.5) | 44 (6.9) | 125 (17.3) |
| 70–74 | 1,541 (21.4) | 419 (23.5) | 490 (23.5) | 403 (20.5) | 78 (12.2) | 151 (20.9) |
| 75–79 | 1,461 (20.3) | 319 (17.9) | 453 (21.7) | 420 (21.3) | 115 (17.9) | 154 (21.4) |
| 80–84 | 1,422 (19.8) | 286 (16.1) | 435 (20.8) | 394 (20.0) | 159 (24.8) | 148 (20.5) |
| 85–89 | 859 (11.9) | 160 (9.0) | 224 (10.7) | 236 (12.0) | 154 (24.0) | 85 (11.8) |
| 90+ | 522 (7.3) | 113 (6.3) | 110 (5.3) | 150 (7.6) | 91 (14.2) | 58 (8.0) |
| Women (%) | 4,147 (57.6) | 863 (48.5) | 974 (46.7) | 1,413 (71.8) | 372 (58.0) | 525 (72.8) |
| Race (%) | ||||||
| White | 4,861 (67.5) | 1,282 (72.0) | 1,309 (62.7) | 1,467 (74.5) | 381 (59.4) | 422 (58.5) |
| Non-white | 2,336 (32.5) | 498 (28.0) | 778 (37.3) | 501 (25.5) | 260 (40.6) | 299 (41.5) |
| Chronic conditions (%) | ||||||
| Arthritis | 4,006 (55.7) | 291 (16.3) | 840 (40.2) | 1,753 (89.1) | 401 (62.6) | 721 (100.0) |
| Osteoporosis | 1,472 (20.5) | 63 (3.5) | 47 (2.3) | 783 (39.8) | 143 (22.3) | 436 (60.5) |
| Lung disease | 1,099 (15.3) | 64 (3.6) | 262 (12.6) | 325 (16.5) | 78 (12.2) | 370 (51.3) |
| Cancer | 1,843 (25.6) | 181 (10.2) | 666 (31.9) | 651 (33.1) | 144 (22.5) | 201 (27.9) |
| Diabetes | 1,819 (25.3) | 139 (7.8) | 1,040 (49.8) | 19 (1.0) | 138 (21.5) | 483 (67.0) |
| Heart disease | 2,184 (30.3) | 138 (7.8) | 935 (44.8) | 279 (14.2) | 385 (60.1) | 447 (62.0) |
| Hypertension | 4,845 (67.3) | 417 (23.4) | 1,998 (95.7) | 1,327 (67.4) | 431 (67.2) | 672 (93.2) |
| Stroke | 826 (11.5) | 15 (0.8) | 274 (13.1) | 28 (1.4) | 297 (46.3) | 212 (29.4) |
| Psychiatric disease | 1,907 (26.5) | 142 (8.0) | 414 (19.8) | 406 (20.6) | 463 (72.2) | 482 (66.9) |
| Dementia | 397 (5.5) | 7 (0.4) | 0 (0.0) | 29 (1.5) | 331 (51.6) | 30 (4.2) |
| No. of chronic conditions (SD) | 2.6 (1.6) | 0.7 (0.6) | 2.9 (1.0) | 2.6 (0.9) | 3.8 (1.4) | 5.1 (1.2) |
| Frailty status (%) | ||||||
| Robust | 2,213 (30.7) | 917 (51.5) | 610 (29.2) | 576 (29.3) | 52 (8.1) | 58 (8.1) |
| Pre-frail | 3,647 (50.7) | 769 (43.2) | 1,147 (55.0) | 1,099 (55.8) | 285 (44.5) | 347 (48.1) |
| Frail | 1,337 (18.6) | 94 (5.3) | 330 (15.8) | 293 (14.9) | 304 (47.4) | 316 (43.8) |
Note: CVD = cardiovascular disease.
Multimorbidity Patterns
Out of the models that allowed two-to-seven classes, the four- and five-class models yielded similarly optimal fit based on Bayesian information criterion (Supplementary Table A2). The five-class model, which offered the most reasonable clinical interpretability, was selected. The detailed class selection process and the comparison between distribution of chronic conditions within the four- and five-class models are reported in Supplementary Methods.
The five classes were named according to chronic conditions having excess prevalence in each class compared to the population prevalence. Overall, the model classified 35.5% of individuals with the probability ≥.70 and 93.2% with the probability ≥.40 into one of the following classes: minimal disease (n = 1,780, 24.7 %), cardiovascular disease (CVD; n = 2,087, 29.0%), osteoarticular disease (n = 1,968, 27.3%), neuropsychiatric disease (n = 641, 8.9%), and high multisystem morbidity (n = 721, 10.0%). Posterior probabilities by classes are reported in Supplementary Table A4. Compared to the population averages, the minimal disease class was composed of individuals with lower prevalence of all conditions (mean number of conditions: 0.7), whereas the high multisystem morbidity class had higher prevalence for all conditions except dementia (mean number of conditions: 5.1). The neuropsychiatric class (mean number of conditions: 3.8) was driven mainly by stroke, psychiatric disorders, and dementia. The CVD class (mean number of conditions: 2.9) had a higher prevalence of diabetes, hypertension, and heart disease, along with a slightly higher prevalence of cancer and stroke. The osteoarticular class (mean number of conditions: 2.6) had a higher prevalence of arthritis, osteoporosis, and cancer.
Multimorbidity Class, Frailty Status, and Mortality
The prevalence of multimorbidity patterns differed by frailty state. The most common multimorbidity pattern in robust individuals was minimal disease (41.4%), followed by CVD (27.6%) and osteoarticular (26.0%). In comparison, all multimorbidity patterns except minimal disease were similarly prevalent in frail individuals: CVD (24.7%), osteoarticular (21.9%), neuropsychiatric disease (22.7%), and high multisystem morbidity patterns (23.6%).
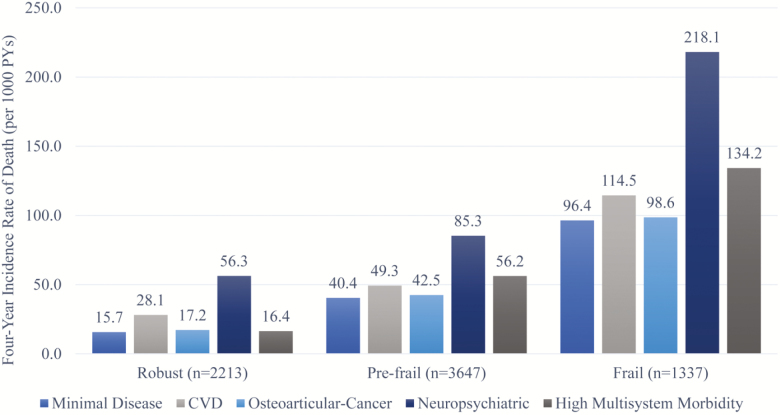
Mortality rate was consistently lowest in the minimal disease class and highest in neuropsychiatric class within each frailty state (15.7 vs 56.3/1,000 PYs for the robust, 40.4 vs 85.3/1,000 PYs for the pre-frail HR, and 96.4 vs 218.1/1,000 PYs for the frail; Figure 1). Notably, individuals with neuropsychiatric pattern in a given frailty state seemed to have a mortality rate that was comparable to that of individuals with the next higher frailty level. The greatest rate of mortality (218.1/1,000 PYs) was observed in those with frailty and neuropsychiatric pattern. Supplementary Table A5 shows yearly loss to follow-up overall and by multimorbidity classes.
Figure 1.
Unadjusted death rates according to multimorbidity patterns and frailty showing progressively greater mortality rates with increasing frailty state. The neuropsychiatric patterns showed consistently greater mortality rates within each frailty level, and comparable to that of the other multimorbidity patterns with an adjacent higher frailty level. CVD = cardiovascular disease.
Multivariable analysis showed widely varying age- and sex-adjusted HRs and IRDs for mortality across multimorbidity classes within each frailty state (Table 2). In robust participants, CVD class (HR, 1.59; 95% CI: 1.08, 2.36) and neuropsychiatric class (HR, 2.11; 95% CI: 1.05, 4.21) were associated with a greater hazard of death compared with the minimal disease class. In pre-frail participants, the neuropsychiatric class (HR, 1.74; 95% CI: 1.28, 2.37) and high multisystem morbidity class (HR, 1.52; 95% CI: 1.10, 2.10) were associated with a greater risk of death. In frail participants, the neuropsychiatric class was associated with a greater risk of death (HR, 2.05; 95% CI: 1.36, 3.10). On the IRD scale, the results were qualitatively comparable to those for the HRs. The largest IRD was observed for the neuropsychiatric class among the frail participants (IRD, 108.4; 95% CI: 65.0, 151.9). With increasing levels of frailty, the HRs comparing multimorbidity patterns did not vary widely. However, the number of excess deaths driven by multimorbidity patterns, as reflected by IRDs, was greater in frail populations.
Table 2.
Associations of Multimorbidity Patterns With Mortality of 4 Years Among Each Frailty State (Robust, Pre-frail, and Frail)
| Total, n | Death, n | IR, per 1,000 PYs | HR (95% CI)† | IRD (95% CI)†, per 1,000 PYs | |
|---|---|---|---|---|---|
| Robust | 2,213 | 147 | 20.5 | ||
| Minimal disease | 917 | 46 | 15.7 | Reference | Reference |
| CVD | 610 | 55 | 28.1 | 1.59 (1.08, 2.36) | 8.8 (1.1, 16.5) |
| Osteoarticular | 576 | 33 | 17.2 | 1.00 (0.63, 1.57) | 1.7 (−3.5, 6.8) |
| Neuropsychiatric | 52 | 10 | 56.3 | 2.11 (1.05, 4.21) | 24.1 (−11.2, 59.3) |
| High multisystem morbidity | 58 | 3 | 16.4 | 1.04 (0.32, 3.36) | 2.6 (−15.9, 21.1) |
| Pre-frail | 3,647 | 561 | 48.7 | ||
| Minimal disease | 769 | 96 | 40.4 | Reference | Reference |
| CVD | 1,147 | 180 | 49.3 | 1.27 (0.99, 1.62) | 8.9 (1.6, 16.2) |
| Osteoarticular | 1,099 | 150 | 42.5 | 1.09 (0.84, 1.41) | 2.1 (−4.3, 8.5) |
| Neuropsychiatric | 285 | 72 | 85.3 | 1.74 (1.28, 2.37) | 27.1 (7.6, 46.7) |
| High multisystem morbidity | 347 | 63 | 56.2 | 1.52 (1.10, 2.10) | 17.0 (4.2, 29.7) |
| Frail | 1,337 | 504 | 136.0 | ||
| Minimal disease | 94 | 26 | 96.4 | Reference | Reference |
| CVD | 330 | 106 | 114.5 | 1.12 (0.73, 1.72) | 32.0 (−2.4, 66.3) |
| Osteoarticular | 293 | 83 | 98.6 | 0.93 (0.60, 1.45) | 10.9 (−22.2, 44.1) |
| Neuropsychiatric | 304 | 169 | 218.1 | 2.05 (1.36, 3.10) | 108.4 (65.0, 151.9) |
| High multisystem morbidity | 316 | 120 | 134.2 | 1.47 (0.96, 2.25) | 51.7 (16.0, 87.3) |
Note: CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio; IR = incidence rate; IRD = incidence rate difference; PYs = person-years.
†Adjusted for age and sex.
Increasing frailty level was consistently associated with a greater gradient of mortality within each multimorbidity pattern (Supplementary Table A6).
Discussion
Using LCA derived in a nationally representative sample, our study shows that the mortality risk differs widely according to the patterns of multimorbidity among individuals with the same frailty state. In particular, those with the neuropsychiatric pattern (ie, those with stroke, psychiatric disease, and dementia) have a higher mortality risk than those with other patterns within each frailty state. A large volume of the literature has demonstrated that frailty is associated with adverse clinical outcomes in individuals with a single disease or in specific clinical contexts (eg, perioperative) (2,5,23). These analyses are typically performed by examining the association between frailty and outcomes within a single disease or adjusting for comorbidities using regression models. Such analyses show that frailty can capture heterogeneity, which is unaccounted for by the comorbidity-based models (24). However, they ignore prognostic importance of multimorbidity patterns within each frailty state. Importantly, our results show that these differences convey meaningful prognostic information and should be additionally considered. Frailty is recognized as a heterogeneous syndrome (25,26), but its translation into clinical care, as proposed in consensus reports and clinical guidelines (9,10,25,27), requires categorizing older adults with sufficient homogeneity for decision-making. Determination of frailty phenotype status may not be sufficient to identify subgroups whose clinical management should be differentiated. Looking beyond frailty status to consider multimorbidity will lead to better understanding of heterogeneity in survival and improve risk stratification.
A few researchers have attempted to examine risk stratification beyond frailty status. Aarts and coworkers (15) investigated the combined associations of multimorbidity, disability, and frailty, showing that individuals with frailty phenotype and multimorbidity (defined as the co-occurrence of two or more diseases) had a higher risk of mortality during a follow-up of 8 years than individuals with frailty alone (44.8% vs 28.1%) and the non-frail (16.6%). However, no comparison was performed by multimorbidity levels (15). Another study found that distinct mobility limitation profiles were associated with institutionalization and mortality within very frail older adults (11). Lutomski and coworkers (28) demonstrated the importance of considering frailty, multimorbidity, disability, and their interactions to understand the quality of life and health care costs of older populations. Although the outcomes of this cross-sectional study were different from mortality outcome of our analysis, their findings support our results by showing the importance of both frailty and multimorbidity.
The interplay between frailty status and multimorbidity classes shows distinct patterns of the mortality association. In analyses adjusted for age and sex, these multimorbidity patterns reveal that mortality in the neuropsychiatric group was significantly higher than that in the minimal disease group within all frailty states. Dementia, depression, and stroke are independently associated with mortality (29–31). These conditions seem to preserve their prognostic value beyond the frailty phenotype, which does not include cognitive or affective dimensions. An alternative definition of frailty, which includes cognitive and affective dimensions, might capture some of this heterogeneity within the frailty levels (32,33). We also observed that the high multisystem morbidity group was associated with increased mortality compared with the minimal disease group in the pre-frail and frail status, but not in the robust. This finding may be due to the different severity of disease within the high multimorbidity group across frailty state (ie, frail individuals may have more advanced diseases than robust individuals). Another possible explanation is that older adults with high multimorbidity who have not yet developed pre-frail or frail state may be resilient to the negative effect of multiple comorbidities. Further research on this group could identify factors that enhance resilience, or the ability to resist frailty and mortality, despite a high burden of multimorbidity, such as lower inflammatory markers, higher education, or social capital (34).
The CVD pattern was associated with increased mortality in the robust but not in frail individuals on the multiplicative scale, which suggests that the mortality risk in those with CVD class may become similar to the risk in those with minimal class as the frailty level increases. Others have also found that CVD does not modulate the baseline association between frailty and mortality in the frail group (35). Interestingly, although individuals in the osteoarticular group had a greater prevalence of chronic conditions (eg, arthritis, osteoporosis) than the minimal disease group, they did not have greater mortality risk once stratified by their frailty status. A simple count of chronic conditions would have inappropriately distinguished these two groups, potentially losing meaningful information in comparison to an approach preserving a qualitative account of chronic conditions (16). The limitation of simple count is also manifested when comparing the high multisystem morbidity group with a higher count of chronic conditions yet lower mortality than the neuropsychiatric group (5.1 vs 3.8 conditions on average).
The five multimorbidity patterns we identified are concordant with previous studies. In a systematic review of 14 articles using various methods to establish multimorbidity patterns, Prados-Torres and coworkers (36) reported three stable patterns: cardiovascular and metabolic, musculoskeletal, and mental health conditions. These are consistent with the CVD, osteoarticular, and neuropsychiatric classes we described. Whitson and coworkers (18) used LCA in a sample of the Medicare population and identified 6 multimorbidity patterns based on 13 diagnoses. Although we labelled some patterns differently, the patterns in our study and their distribution in the population correspond to the first five in the analysis by Whitson and coworkers; their sixth pattern (cardio-stroke-cancer) drew members from the “very sick” (labelled “high multisystem morbidity” in our study) and “vascular” (“CVD” in our study). Agreement between the multimorbidity patterns we obtained and those previously identified using different methods and populations reinforces the robustness of our patterns.
The main strengths of our study included a large, nationally representative sample that allowed a stratified analysis by frailty status and five clinically meaningful multimorbidity groups rather than sole presence or absence of multimorbidity. In addition, we examined differences in mortality on the relative and absolute rate scale. By comparing differences on the IRD scale, it is clear that the differences between multimorbidity patterns are greatest among the frail participants, illustrating the relevance of the scale used to assess heterogeneity. Our study has a few important limitations that deserve mention. First, we used the frailty phenotype to assess frailty (6). Our findings might have been different if the deficit accumulation index, which includes chronic conditions as well as cognitive and affective domains (37), had been used. Therefore, the specific definition of frailty that is used may prove important (38). Second, we considered that the inactivity criterion for frailty phenotype was present based on not having taken part in vigorous activities or never walked for exercise in the last month instead of a validated questionnaire of physical activity, which may lead to underestimation of this criterion. Third, multimorbidity class membership was determined based on the predictive probability calculated from LCA. Although each class seemed to show clinically distinct patterns of chronic conditions, an individual’s multimorbidity class may not be evident in some cases. Moderate posterior probabilities of classification may limit clinical translation of our findings. Fourth, we used 10 chronic conditions commonly available in epidemiologic studies for LCA, but these were not determined a priori (36). Our results might have been different if other chronic conditions had been included, as specific multimorbidity patterns may be sensitive to the number of available chronic conditions (39). Moreover, the comparison of simple counts of chronic conditions within multimorbidity patterns may depend on the number of chronic conditions. Fifth, the self-reported physician-diagnosed chronic conditions were subject to informational bias (40). Finally, loss to follow-up over the 4 years of the study was 32% and our results may be biased if censoring was informative.
Conclusion
Looking beyond frailty phenotype state and considering multimorbidity patterns can identify older adults at greater risk of mortality. The neuropsychiatric pattern was consistently associated with lowest survival in all frailty states. Assessing frailty, multimorbidity, and their interplay can potentially improve risk prediction and facilitate individualized care of aging populations.
Funding
This work was supported by the Fondation du Centre hospitalier de lʹUniversité de Montréal to Q.D.N.; the National Institute on Aging (R01AG046206 to M.C.O. and K08AG051187 to D.H.K.); the Boston Claude D. Pepper Older Americans Independence Center/Pilot and Exploratory Studies Core (P30AG031679 to D.H.K.); and the Boston Royal Center Pilot Award (P30AG048785 to D.H.K.).
Supplementary Material
Acknowledgements
The sponsor had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
Dr Kim provides paid consultative services to Alosa Health, a nonprofit educational organization with no relationship to any drug or device manufacturers.
References
- 1. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70:1427–1434. doi: 10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. ; Gerontopole Brussels Study Group. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17:1163.e1–1163.e17. doi: 10.1016/j.jamda.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 3. Wu C, Smit E, Xue QL, Odden MC. Prevalence and correlates of frailty among community-dwelling Chinese older adults: the China Health and Retirement Longitudinal Study. J Gerontol A Biol Sci Med Sci. 2017;73:102–108. doi: 10.1093/gerona/glx098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012;13:e437–e444. doi: 10.1016/S1470-2045(12)70259-0 [DOI] [PubMed] [Google Scholar]
- 5. Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26:1091–1101. doi: 10.1093/annonc/mdu540 [DOI] [PubMed] [Google Scholar]
- 6. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 7. Robinson TN, Walston JD, Brummel NE, et al. Frailty for surgeons: review of a National Institute on Aging conference on frailty for specialists. J Am Coll Surg. 2015;221:1083–1092. doi: 10.1016/j.jamcollsurg.2015.08.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Maguet P, Roquilly A, Lasocki S, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4 [DOI] [PubMed] [Google Scholar]
- 9. Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF; American College of Surgeons National Surgical Quality Improvement Program; American Geriatrics Society Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–466. doi: 10.1016/j.jamcollsurg.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 10. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. doi: 10.1016/j.jacc.2013.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montero-Odasso M, Bergman H, Béland F, Sourial N, Fletcher JD, Dallaire L. Identifying mobility heterogeneity in very frail older adults. Are frail people all the same?Arch Gerontol Geriatr. 2009;49:272–277. doi: 10.1016/j.archger.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 12. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garin N, Koyanagi A, Chatterji S, et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J Gerontol A Biol Sci Med Sci. 2016;71:205–214. doi: 10.1093/gerona/glv128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what’s in a name? A review of literature. Eur J Gen Pract. 1996;2(2):65–70. doi: 10.3109/13814789609162146 [Google Scholar]
- 15. Aarts S, Patel KV, Garcia ME, et al. Co-presence of multimorbidity and disability with frailty: an examination of heterogeneity in the frail older population. J Frailty Aging. 2015;4:131–138. doi: 10.14283/jfa.2015.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56:221–229.doi: 10.1016/S0895-4356(02)00585-1 [DOI] [PubMed] [Google Scholar]
- 17. Olaya B, Moneta MV, Caballero FF, et al. Latent class analysis of multimorbidity patterns and associated outcomes in Spanish older adults: a prospective cohort study. BMC Geriatr. 2017;17:186. doi: 10.1186/s12877-017-0586-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitson HE, Johnson KS, Sloane R, et al. Identifying patterns of multimorbidity in older Americans: application of latent class analysis. J Am Geriatr Soc. 2016;64:1668–1673. doi: 10.1111/jgs.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vetrano DL, Palmer K, Marengoni A, et al. Frailty and multimorbidity: a systematic review and meta-analysis [published online ahead of print May 3, 2018]. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly110 [DOI] [PubMed] [Google Scholar]
- 20. Kasper JD, Freedman VA. Findings from the 1st round of the National Health and Aging Trends Study (NHATS): introduction to a special issue. J Gerontol B Psychol Sci Soc Sci. 2014;69 (suppl 1):S1–S7. doi: 10.1093/geronb/gbu125 [DOI] [PubMed] [Google Scholar]
- 21. Kasper J, Freedman V, Niefeld M. Construction of performance-based summary measures of physical capacity in the National Health and Aging Trends Study NHATS Technical Paper #4. 2013. http://nhats.org/scripts/documents/NHATS_SPPB_Technical_Paper_Jul2013.pdf. Accessed October 10, 2017.
- 22. Boshuizen HC, Feskens EJ. Fitting additive Poisson models. Epidemiol Perspect Innov. 2010;7:4. doi: 10.1186/1742-5573-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg. 2016;151:538–545. doi: 10.1001/jamasurg.2015.5085 [DOI] [PubMed] [Google Scholar]
- 24. Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116:179–185.doi: 10.1016/j.amjmed.2003.09.031 [DOI] [PubMed] [Google Scholar]
- 25. Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement. The Frailty Operative Definition-Consensus Conference Project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62–67. doi: 10.1093/gerona/gls119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muscedere J, Andrew MK, Bagshaw SM, et al. Screening for frailty in Canada’s health care system: a time for action. Can J Aging. 2016;35:281–297. doi: 10.1017/S0714980816000301 [DOI] [PubMed] [Google Scholar]
- 28. Lutomski JE, Baars MA, Boter H, et al. [Frailty, disability and multi-morbidity: the relationship with quality of life and healthcare costs in elderly people]. Ned Tijdschr Geneeskd. 2014;158:A7297. [PubMed] [Google Scholar]
- 29. James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82:1045–1050. doi: 10.1212/WNL.0000000000000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761 [DOI] [PubMed] [Google Scholar]
- 31. Koton S, Schneider ALC, Rosamond WD, et al. Stroke incidence and mortality trends in US Communities, 1987 to 2011. JAMA. 2014;312(3):259. doi: 10.1001/jama.2014.7692 [DOI] [PubMed] [Google Scholar]
- 32. Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2216. doi: 10.1111/j.1532-5415.2008.02008.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57:453–461. doi: 10.1111/j.1532-5415.2008.02136.x [DOI] [PubMed] [Google Scholar]
- 34. Sanders JL, Arnold AM, Hirsch CH, et al. Effects of disease burden and functional adaptation on morbidity and mortality on older adults. J Am Geriatr Soc. 2016;64:1242–1249. doi: 10.1111/jgs.14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaes B, Depoortere D, Van Pottelbergh G, Matheï C, Neto J, Degryse J. Association between traditional cardiovascular risk factors and mortality in the oldest old: untangling the role of frailty. BMC Geriatr. 2017;17:234. doi: 10.1186/s12877-017-0626-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67:254–266. doi: 10.1016/j.jclinepi.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 37. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839. doi: 10.1111/j.1532-5415.2009.02225.x [DOI] [PubMed] [Google Scholar]
- 39. Calderón-Larrañaga A, Vetrano DL, Onder G, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. 2017;72:1417–1423. doi: 10.1093/gerona/glw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.