Abstract
Background
Treatment of musculoskeletal pain in older adults may be more effective if it incorporates integrated management of comorbid health conditions. The purpose of this study was to define empirically derived comorbidity subgroups among Medicare beneficiaries with an index condition of osteoarthritis (OA) or low back pain (LBP) as a precursor to the development of comorbidity-specific pain treatment pathways.
Methods
This study included Medicare beneficiaries participating in the Medicare Current Beneficiary Survey (MCBS) and seeking care for OA (n = 723) or LBP (n = 617) with data available for 3 years after entry into the survey. We identified 30 comorbidity diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes in claims data during beneficiaries’ first year in the survey. Latent class analysis defined comorbidity subgroups, and posterior probabilities were used to assign subgroup classification. Self-reported disability was compared over three consecutive years for each subgroup.
Results
We identified similar comorbidity subgroups for OA and LBP. The subgroups included (range of percent prevalence) low comorbidity (47.6%–54.4%), nonvascular (21.8%–28.6%), diabetes (12.2%–15.0%), renal disease with complicated hypertension (5.5%–5.8%), and complex cardiac disease/high comorbidity (3.3%–5.8%). OA and LBP subgroups with more complex comorbidity burden generally demonstrated higher disability over 3 years.
Conclusions
Five comorbidity subgroups were identified, with a large proportion of older adults classified into the subgroup defined by a low probability of most comorbidities. These findings provide direction for the development of pain treatment pathways that are tailored to address common comorbidity profiles among older adults.
Keywords: Multimorbidity, Outcomes, Physical function, Rehabilitation
Musculoskeletal pain conditions are common among older adults and have significant personal and public health consequences, including poor quality of life, high health care spending, and risk of opioid dependence (1). The age-related development of comorbid health conditions such as diabetes or heart disease often exacerbates the physical and economic burden of pain (2). Evidence suggests that comorbid conditions can directly influence causal biologic pathways for musculoskeletal pain (3). However, they may also affect response to pain-related treatment (4) or influence the appropriateness and selection of these treatments in older adults (5,6). Indeed, for many chronic health conditions such as musculoskeletal pain, there is growing concern that providing guideline-concordant care might be impractical or even harmful and could contribute to wasteful health care utilization in the presence of multiple comorbid conditions (5,6). Yet existing clinical practice guidelines for the management of musculoskeletal pain focus on treating the pain condition alone and provide inadequate direction on how to account for the moderating effects of comorbidity (5). As a result, older adults are at high risk for inadequate, costly, and potentially harmful care for their musculoskeletal pain conditions.
Comparative effectiveness research can identify more effective pain treatments for older adults with musculoskeletal pain and comorbid health conditions. However, defining comorbidity subgroups among older adults with musculoskeletal pain is an important precursor for these effectiveness studies (7). Currently, simple or weighted condition counts are the most common methods for assessing comorbidities in research and risk-adjustment applications. However, these methods may not be relevant in treatment decision-making for older adults because they do not account for the emergent health care needs that arise from interactions of various comorbid conditions (8). In this study, we derived subgroups using empirical combinations of comorbid health conditions for individuals with musculoskeletal disorders. We argue that empirical derivation of comorbidity subgroups is a more ecologically valid method of subgrouping because it identifies prevalent and naturally occurring condition combinations (9,10). We will use the results of this study to develop and compare tailored, yet scalable pain treatment pathways that integrate management of common comorbid health conditions among older adults with musculoskeletal pain. The development of such pathways will be responsive to the growing need to deliver more holistic, person-centered health care for older adults in value-based systems (1).
The primary aim of this study was to identify common comorbidity classification subgroups among older adults with musculoskeletal pain. We derived subgroups separately in cohorts of older adults with (a) osteoarthritis (OA) and (b) low back pain (LBP) because these conditions have a high prevalence in older adults, are leading causes of disability, and are common reasons to seek health care (11,12). We investigated convergence of comorbidity subgroup types between the two cohorts as an initial assessment of generalizability and to clarify whether classifications specific to an index musculoskeletal pain condition may be warranted. In a secondary aim, we examined disability for each subgroup over three consecutive years. We evaluated disability because it is the most commonly assessed outcome domain for musculoskeletal conditions (13) and an important patient-centered component of health care value. The analysis helps us to understand how common combinations of comorbid health conditions are associated with disability for older adults with OA or LBP and allows us to prioritize subgroups for future comparative effectiveness research.
Methods
Data set
This analysis utilized 5 years (2006–2010) of the 100% Cost and Use data set from the Medicare Current Beneficiary Survey (MCBS), a longitudinal, nationally representative in-person survey of randomly sampled Medicare beneficiaries, as well as matching Medicare claims data (14). Cost and Use files contain a combination of survey-reported data, Medicare Fee for Service claims data, and other health care utilization data from CMS administrative files. New participants enter the MCBS annually in rotating panels (rounds) and remain in the study for 12 rounds of data collection over 3 years.
Subjects
Individuals 65 years of age or older with a diagnosis of OA or LBP were eligible to be included in the study. Individuals who died while enrolled or otherwise unenrolled in Medicare during the 3 years of the survey were excluded.
Identification of Diagnoses
Diagnoses for OA and LBP (see Supplementary Table 1) and all comorbidities were identified from International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes in Medicare claims data (15). To ensure an analytic sample that adequately represented the clinical complexity of older adults with OA and LBP across care settings, we identified diagnoses in physician, inpatient, outpatient, skilled nursing facility, and home health claims files from each participant’s index year in the MCBS. All diagnoses were derived using claims data only to ensure a medical professional provided all diagnoses. We employed a commonly used algorithm to remove rule-out diagnoses from the analysis (16). Rule-out diagnoses are nonvalidated diagnostic codes most often found in physician and outpatient claims files. The algorithm requires that for physician and outpatient claims, a patient’s diagnoses must appear on at least two different claims that are more than 30 days apart (16).
Assignment of Comorbidities
Following identification of diagnoses, we used an established Elixhauser ICD-9-CM coding algorithm to determine the presence or absence of 30 comorbid health conditions (see Supplementary Table 2) for each individual (17). For more information on the ICD-9 codes used to define each comorbidity, we refer readers to the published Elixhauser ICD-9-CM coding algorithm by Quan and colleagues (17). We chose this coding method because it is commonly used to identify comorbidities among older adults (18), includes more ICD-9-CM codes and comorbidities compared with other indices and identifies a variety of mental health conditions (eg, alcohol and drug abuse, psychoses, depression), which were of particular interest for pain-related outcomes (19).
Sociodemographic Information
Age, sex, race, ethnicity, education level, income, smoking status, employment status, and census division were collected from the index year of entry into the MCBS for each individual.
Patient-Reported Disability
The MCBS collects self-reported measures of health-related disability annually by asking respondents to report their ability to perform various activities of daily living: stooping/crouching, walking, lifting, reaching, and writing or handling small objects (20). Item responses range from 1 (no difficulty) to 5 (unable to perform the task). Activities of daily living performance is a common measure of disability in population-based surveys (21) and among older adults (22), including those with musculoskeletal pain (23).
Statistical Analysis
Identification of comorbidity subgroups
Comorbidity subgroups were identified using latent class analysis (LCA), a probability clustering technique that identifies unobserved latent classes (24). In LCA, a class is defined by a pattern of conditional probabilities for having specific characteristics, in this case the presence of binary diagnosis indicators. Importantly, subgroups are not necessarily defined by the absolute presence or absence of specific health conditions, but rather by the relative probabilities that individuals within the subgroup have a particular condition. LCA is superior to other methods of subgrouping for the purposes of this study because it handles binary data, accommodates weighted observations, and uses a probabilistic rather than deterministic approach to subgroup allocation (24,25).
Binary indicators (ie, present or absent) for each comorbidity included in the Elixhauser index (n = 30) were entered into the LCA. To allow for proper model identification, sparsely distributed comorbidities were excluded if present in less than 5% of the analytic sample to ensure a sufficient number of individuals with each diagnosis were included (26–30). We took this approach because sparsely distributed conditions were likely to create poorly fitted models and unlikely to define the more common comorbidity subgroups of interest in this study.
We used multiple statistical and nonstatistical criteria when selecting optimal class size. Model parsimony was assessed for models of differing numbers of classes using Bayesian information criterion (BIC), and Akaike information criterion, based on the −2 log-likelihood of the model and adjusted for number of parameters and sample size (25). Bootstrapped (500 iterations) Cressie–Read statistic estimates were generated to evaluate model goodness-of-fit (30). Additional criteria used to determine optimal class solution were misclassification error rate, class size, and interpretability of classes (ie, clinical relevance of condition combinations), particularly where other criterion did not produce a clearly superior model (31). After selecting a best-fit latent class model, individuals were assigned group membership based on highest posterior probability estimates. We reported that median posterior probabilities used to classify each individual (32). These values provide an indication of ambiguity surrounding subgroup membership, where probabilities close to one are preferable. We used Latent Gold software (Statistical Innovations, Belmont, MA) to conduct LCA using survey weights, strata, and primary sampling units to account for the complex, stratified sampling design of the MCBS.
Subgroup comparisons on disability
Comorbidity subgroups were compared at each time point on disability scores using multivariate linear regression. We used case-wise deletion to handle missing data, as the incidence of missing disability or covariates was low (<1%). We included select covariates with the potential to influence disability independently in this population, including sex, race, ethnicity, education level, smoking status, and employment status. We also included number of annual medical provider events listed in the survey in an attempt to account for severity of health conditions and intensity of health care needs. Analyses were conducted using MCBS 3-year backward longitudinal weights, strata, and primary sampling units to account for nonresponse and the complex multistage sampling design of the survey (33). For these analyses, we set α value equals to .01 due to the large sample size and to account for multiple comparisons. Means and linearized standard errors accounting for the complex sampling features were computed using survey procedure methods in SAS version 9.3 (SAS Institute Inc, Cary, NC). Methodological approaches that account for the complex, stratified sampling design of the MCBS allow us to generalize our findings to the Medicare population. The University of Florida Institutional Review Board approved this study.
Results
Subgroup Analysis
The analytic data set included 12,608 individuals first enrolled in MCBS during the time from 2006 to 2008. Of those, 10,470 were 65 years or older and 7,640 were still enrolled by the end of the 3-year survey. Of the 2,830 individuals who were not enrolled at the end of the 3-year period, 1,661 died and 1,169 had unenrolled for other reasons. After employing the rule-out algorithm, 723 individuals had an OA diagnosis representing 2,663,029 individuals and 617 had an LBP diagnosis representing 2,418,650 individuals in the target population of older adult Medicare beneficiaries. Of all respondents, 158 reported both an OA and LBP diagnosis, representing 596,799 individuals in the target population. We initially considered analyzing this group with both pain diagnoses separately but concluded the sample was too small to produce valid and reliable subgroups. Therefore, these individuals were included in both analyses.
Pulmonary circulation disorders, paralysis, liver disease, peptic ulcer disease, AIDS/HIV, lymphoma, metastatic cancer, coagulopathy, obesity, weight loss, blood loss anemia, alcohol abuse, drug abuse, and psychoses had prevalence rates less than 5% for both OA and LBP diagnostic cohorts and were removed from each analysis. Supplementary Table 3 lists the distribution of comorbid conditions for OA and LBP cohorts. Additional demographic and health-related information for the sample is listed separately (Supplementary Table 4).
Model fit estimates for those with OA identified a four-class solution based on lowest sample-sized adjusted BIC (−761.52). However, the BIC value for a five-class solution was only slightly higher (−752.21, bootstrapped Cressie–Read statistic p = .24), compared with the four-class model and additional fit statistics such as classification error (.15), and class interpretability suggested a better fit compared with four-class solution. Thus, we chose a five-class model as the final solution for OA. Uncomplicated hypertension was the most common comorbidity among the OA and LBP cohorts and probability for the condition was high among all subgroups (see Supplementary Figure 1). Therefore, in developing subgroup descriptions, the probability of uncomplicated hypertension was not heavily weighted. The final subgroups for the OA cohort were identified as (percentage of cohort with subgroup classification): low comorbidity (47.6%), nonvascular (28.6%), diabetes (12.2%), renal disease with complicated hypertension (5.8%), and complex cardiac disease/high comorbidity (5.8%). Comparatively high probabilities of nonvascular conditions (besides hypertension) such as chronic pulmonary disease and hypothyroidism defined the nonvascular subgroup. Median (interquartile range) of posterior probabilities used to assign subgroup membership was 0.90 (0.75–0.96). Selected demographic information for the subgroups is provided separately in Supplementary Table 5.
Model fit estimates for those with LBP identified a five-class solution based on lowest sample-sized adjusted BIC (−858.82). Additional fit statistics such as bootstrapped Cressie–Read statistic (p = .02), classification error (0.11), and class interpretability suggested a better fitting model than four- or six-class solutions. The final subgroups were consistent with those identified in the OA diagnostic cohort (percentage of cohort with subgroup classification): low comorbidity (54.4%), nonvascular (21.8%), diabetes (15.0%), renal disease with complicated hypertension (5.5%), and complex cardiac disease/high comorbidity (3.3%). Like with the OA cohort, comparatively high probabilities of conditions such as chronic pulmonary disease and hypothyroidism defined the nonvascular subgroup (see Supplementary Figure 2). Median (interquartile range) of posterior probabilities used to assign subgroup membership was 0.95 (0.73–0.99). Selected demographic information for the subgroups is provided separately in Supplementary Table 6.
Subgroup Comparisons on Disability
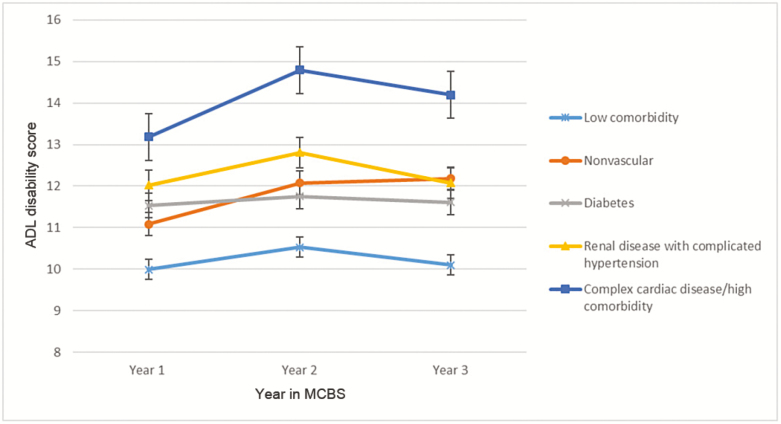
Race, ethnicity, and smoking status did not contribute significantly to the adjusted models predicting disability, and we excluded these variables from the final analysis. In the OA cohort, subgroups with higher or more complex disease burden were generally associated with higher adjusted disability scores across time (Figure 1). The low comorbidity subgroup had significantly lower disability than all other subgroups at each time point, whereas the complex cardiac disease/high comorbidity subgroup had higher disability than other subgroups. We observed a trend toward worsening disability over time for the nonvascular and complex cardiac disease/high comorbidity subgroups, but these changes did not meet statistical significance (p > .01).
Figure 1.
Osteoarthritis subgroup longitudinal profiles for activities of daily living (ADL) disability score. Error bars are standard error of measure.
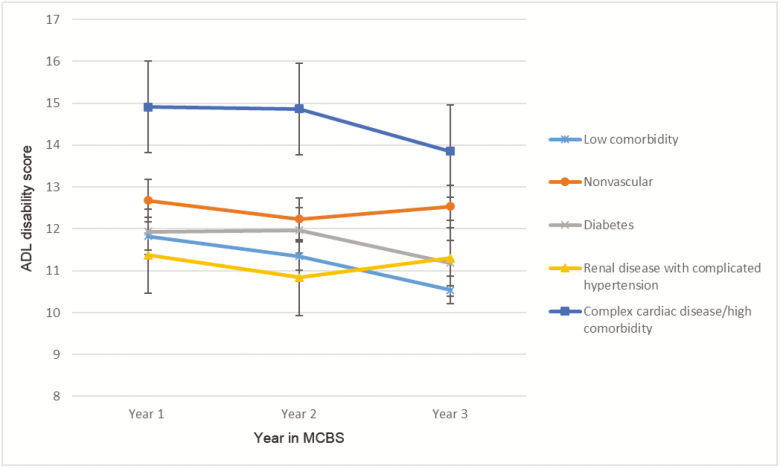
In the LBP diagnostic group, subgroups with higher disease burden also reported higher adjusted disability (Figure 2), although the low comorbidity subgroup did not demonstrate lower disability compared with other subgroups as clearly as it did in the OA cohort. Also, in contrast to the OA cohort, disability showed a trend toward improvement for the complex cardiac disease/high comorbidity subgroup, but the improvement was not statistically significant.
Figure 2.
Low back pain subgroup longitudinal profiles for activities of daily living (ADL) disability score. Error bars are standard error of measure.
Discussion
This study identified five empirically derived comorbidity subgroups in older adults with OA or LBP. The number of subgroups, their comorbidity profiles, and relative prevalence were similar among OA and LBP cohorts. These results provide initial support for subgroup generalizability across commonly experienced musculoskeletal pain conditions. However, comorbidity subgroups may be different among less common pain conditions such as fibromyalgia. Confirming these results in other pain conditions should be the target of future research. Subgroup generalizability across different pain conditions would be ideal because it enables efficient clinical implementation of comorbidity-specific pain treatment pathways.
A notable finding from this analysis is that relatively few individuals were classified into subgroups defined by high disability and comorbidity burden. This finding has important economic and policy implications for organizing treatment of musculoskeletal pain around comorbid conditions. Specifically, most older adults would be appropriate for streamlined pain treatment pathways characterized by low-intensity health care resource use. Conversely, this analysis suggests that a smaller number of older adults might require costlier, integrated pain treatment pathways that consume greater health care resources to address the complex needs of multiple health conditions. Due to their higher disability levels and potential for costlier care, the complex cardiac disease/high comorbidity subgroups might be a good initial target for comparative effectiveness research.
Our findings provide initial direction for the development of novel pain treatment pathways for older adults. Specifically, future research should develop and compare treatment pathways that address the unique biopsychosocial needs conferred by the condition combinations identified in this study. For example, individuals with disparate health conditions such as LBP and diabetes may benefit from consolidated lifestyle modification and behavioral training (8,34). For those with pain and complex cardiac disease, iterative testing of different pharmacological regimens may be indicated to identify treatment pathways that effectively address both conditions. Future studies could also evaluate the effect of holistic approaches such as mindfulness training on outcomes for both comorbid and musculoskeletal pain conditions (35,36). Alternatively, treatment pathways may simply vary the intensity, duration, or frequency of pain treatment to accommodate comorbidity burden. We propose that patients and other stakeholders representing a variety of disciplines that routinely provide health care for older adults provide input on the development and testing of these treatment pathways in future studies.
Another consideration when developing pain treatment pathways is how to manage patients with less common comorbidities, such as paralysis or metastatic cancer. We excluded these conditions in the final analysis because our goal was to identify common health condition subgroupings around which we could develop tailored yet scalable treatment pathways. The drawback of this method is that patients with less common health conditions could be misclassified, and the treatment pathways ultimately derived from the information in this study would not apply to them. These patients may benefit from individualized treatment plans aligned with the specific needs of their less common health condition(s). We contend that developing standard pain treatment options for those with common health profiles while allowing flexibility to develop more individualized treatment plans for those with unique health care profiles will deliver greater value than the current “one-size-fits-all” health care model for musculoskeletal pain.
Our results compare favorably with prior comorbidity studies in the general population of older adults that have identified a highly prevalent minimal disease subgroup and a far less prevalent subgroup characterized by the highest disease burden (28,37,38). Moreover, we found subgroups consistent with vascular and nonvascular condition profiles reported in other comorbidity studies (28,37). The specific conditions comprising each subgroup differed slightly from previous studies, probably due to differences in methodologies, data sets, and target populations. However, ecologically feasible condition combinations defined each subgroup. For instance, hypertension is a common etiology of renal disease and both share common risk factors in older adults (39). In addition, relationships between pulmonary disease and hypothyroidism have been supported (40), as well as independent links between hypothyroidism and pain conditions (41).
We did not identify distinct mental health subgroups, which was an unexpected finding considering the well-documented relationship between musculoskeletal pain and psychological distress (19). However, this data set did not include specific measures of pain-related psychological distress (eg, fear avoidance beliefs) that do not meet the diagnostic criteria for a mental health condition. Therefore, readers should not confuse the inability to identify specific mental health subgroups with the necessary absence of subgroups that exhibit high levels of pain-related psychological distress. Interestingly, probabilities for psychoses and depression were highest in subgroups defined by higher disease burden, suggesting a “high comorbidity” phenotype is also likely to have characteristics of poorer mental health.
A notable strength of our approach is use of the MCBS and matching claims, which is an ideal data set for studying health-related topics among older adults. Use of claims data ensured diagnoses were provided by a physician and less subject to recall bias. Another strength is empirical subgrouping using LCA, which is a robust modeling approach for dichotomous indicators. Classification error was approximately 15% across models, which is lower compared with prior studies using LCA in older adult cohorts (37). Nevertheless, the error rate and ambiguity around classifying approximately 10%–15% of individuals highlights inherent difficulties in classifying heterogeneous cohorts, and readers should consider this uncertainty when interpreting these results. Future research should confirm these subgroups in larger samples and different data sets.
Readers should consider some limitations when interpreting the results of this study. First, we did not identify individuals at a common point in the progression of their pain condition. Therefore, we cannot determine how chronicity of pain might influence subgroup derivation or membership. We were also unable to directly measure pain severity. As a result, we cannot evaluate its potential influence on disability for the various subgroups. Second, the use of a restrictive rule-out algorithm meant that some individuals (ie, those who rarely seek health care for pain) might have been under-represented. This may have also led to underidentification of diagnoses for which providers are less likely to rely on clinical tests to confirm (eg, LBP) or for which the duration of treatment is short and delivered in outpatient settings. In particular, the prevalence of OA was lower than previously reported estimates (42), which may be due to this conservative diagnostic approach and inclusion of Medicare fee-for-service beneficiaries only. In addition, use of the Elixhauser Comorbidity Index might have resulted in the exclusion of relevant comorbidities among older adults that are not captured by the index, such as dementia. Moreover, certain limitations are inherent when using claims data, such as the potential for missed or under-reported diagnoses (eg, obesity) and coding errors. Although we utilized standard and valid approaches to analyzing claims data, readers should consider that these findings might not be applicable to those with comorbidities not included in this analysis.
Third, we cannot distinguish the relative contributions of musculoskeletal pain and comorbid conditions to the disability measure used. However, this limitation is not unique to these analyses, and this issue highlights the challenge in addressing disability without also considering the impact of comorbidity burden. Fourth, we used data from 2006 to 2010, which were the most current data available when this study was initiated. Health care policy and coding standards change over time, which could affect the use of claims data to identify population prevalence estimates for certain health conditions. However, we believe that these study findings are still relevant for today’s population because the more common conditions identified in this study continue to be prevalent and disabling for older adults (43). Finally, we excluded respondents that died during the survey timeframe. As a result, these subgroupings and their potential treatment pathways may be most appropriate for older adults in earlier phases of their clinical conditions, where mortality is not imminent. We also caution application of these findings to individuals in long-term care facilities because they may have different health care needs compared with the independent population of older adults.
In conclusion, comorbidity-specific subgroups were similar across individuals with OA and LBP. Moreover, a highly prevalent subgroup defined by a low probability of most comorbid conditions suggests that many older adults with musculoskeletal pain may be appropriate for low cost, streamlined care pathways, whereas fewer would need more resource-intensive treatment pathways. At present, older adults with musculoskeletal conditions are entered into “one-size-fits-all” treatment pathways, while these analyses suggest that there is opportunity to tailor pain management options based on comorbidity. These results inform future research that will develop and compare comorbidity-specific pain treatment pathways for older adults. In particular, subgroups with high disability and disease burden (ie, complex cardiac disease/high comorbidity and OA) might be good initial research targets due to their potentially high risk for inadequate and costly musculoskeletal pain care.
Funding
This work was supported by the Foundation for Physical Therapy with Promotion of Doctoral Studies I and II (PODS I and II) Awards to T.A.L. and Eunice Kennedy Shriver National Institute of Child Health and Human Development by the National Institutes of Health Rehabilitation Research Career Development Program (K12-HD055929) to J.M.B.
Conflict of Interest
None reported.
Supplementary Material
References
- 1. Gatchel RJ, Reuben DB, Dagenais S, et al. . Research agenda for the prevention of pain and its impact: report of the work group on the prevention of acute and chronic pain of the federal pain research strategy. J Pain. 2018;19:837–851. doi: 10.1016/j.jpain.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 2. Ritzwoller DP, Crounse L, Shetterly S, Rublee D. The association of comorbidities, utilization and costs for patients identified with low back pain. BMC Musculoskelet Disord. 2006;7:72. doi: 10.1186/1471-2474-7-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li JX. Pain and depression comorbidity: a preclinical perspective. Behav Brain Res. 2015;276:92–98. doi: 10.1016/j.bbr.2014.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saragiotto BT, Maher CG, Hancock MJ, Koes BW. Subgrouping patients with nonspecific low back pain: hope or hype?J Orthop Sports Phys Ther. 2017;47:44–48. doi: 10.2519/jospt.2017.0602 [DOI] [PubMed] [Google Scholar]
- 5. Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PLoS One. 2011;6:e25987. doi: 10.1371/journal.pone.0025987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uhlig K, Leff B, Kent D, et al. . A framework for crafting clinical practice guidelines that are relevant to the care and management of people with multimorbidity. J Gen Intern Med. 2014;29:670–679. doi: 10.1007/s11606-013-2659-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meghani SH, Buck HG, Dickson VV, et al. . The conceptualization and measurement of comorbidity: a review of the interprofessional discourse. Nurs Res Pract. 2013;2013:192782. doi: 10.1155/2013/192782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078 [DOI] [PubMed] [Google Scholar]
- 9. Rijken M, van Kerkhof M, Dekker J, Schellevis FG. Comorbidity of chronic diseases: effects of disease pairs on physical and mental functioning. Qual Life Res. 2005;14:45–55. doi: 10.1007/s11136-004-0616-2 [DOI] [PubMed] [Google Scholar]
- 10. Ording AG, Sørensen HT. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol. 2013;5:199–203. doi: 10.2147/CLEP.S45305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Docking RE, Fleming J, Brayne C, Zhao J, Macfarlane GJ, Jones GT; Cambridge City over-75s Cohort Study Collaboration Epidemiology of back pain in older adults: prevalence and risk factors for back pain onset. Rheumatology (Oxford). 2011;50:1645–1653. doi: 10.1093/rheumatology/ker175 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Artus M, Campbell P, Mallen CD, Dunn KM, van der Windt DAW. Generic prognostic factors for musculoskeletal pain in primary care: a systematic review. BMJ Open. 2017;7:e012901. doi: 10.1136/bmjopen-2016–012901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Medicare and Medicaid Services. Medicare Current Beneficiary Survey (MCBS) https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/MCBS/. Published November 15, 2017. Accessed November 29, 2017.
- 15. United States Bone and Joint Initiative. BMUS. Rosemont, IL; 2014. http://www.boneandjointburden.org/. Accessed June 1, 2017. [Google Scholar]
- 16. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0 [DOI] [PubMed] [Google Scholar]
- 17. Quan H, Sundararajan V, Halfon P, et al. . Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 18. Yurkovich M, Avina-Zubieta JA, Thomas J, Gorenchtein M, Lacaille D. A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol. 2015;68:3–14. doi: 10.1016/j.jclinepi.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 19. Lee H, Hübscher M, Moseley GL, et al. . How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain. 2015;156:988–997. doi: 10.1097/j.pain.0000000000000146 [DOI] [PubMed] [Google Scholar]
- 20. Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467. doi: 10.2307/3349677 [PubMed] [Google Scholar]
- 21. Wiener JM, Hanley RJ, Clark R, Van Nostrand JF. Measuring the activities of daily living: comparisons across national surveys. J Gerontol. 1990;45:S229–S237. doi: 10.1093/geronj/45.6.s229 [DOI] [PubMed] [Google Scholar]
- 22. Cutler D, Landrum MB.. Investigations in the Economics of Aging. Chicago, IL: University of Chicago Press for National Bureau of Economic Research; 2012. [Google Scholar]
- 23. George LK, Ruiz D Jr, Sloan FA. The effects of total knee arthroplasty on physical functioning in the older population. Arthritis Rheum. 2008;58:3166–3171. doi: 10.1002/art.23888 [DOI] [PubMed] [Google Scholar]
- 24. Hagenaars JA, McCutcheon AL, eds. Applied Latent Class Analysis. 1st ed. Cambridge, UK: Cambridge University Press; 2009. [Google Scholar]
- 25. Vermunt JK, Magidson J. Latent class models for classification. Comput Stat Data Anal. 2003;41:531–537. doi: 10.1016/S0167-9473(02)00179-2 [Google Scholar]
- 26. Dean N, Raftery AE. Latent class analysis variable selection. Ann Inst Stat Math. 2010;62:11–35. doi: 10.1007/s10463-009-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fop M, Smart K, Murphy TB. Variable selection for latent class analysis with application to low back pain diagnosis. Ann Appl Stat. 2017;11:2080–2110. doi: 10.1214/17-aoas1061 [Google Scholar]
- 28. Islam MM, Valderas JM, Yen L, Dawda P, Jowsey T, McRae IS. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PLoS One. 2014;9:e83783. doi: 10.1371/journal.pone.0083783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miaskowski C, Dunn L, Ritchie C, et al. . Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage. 2015;50:28–37. doi: 10.1016/j.jpainsymman.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langeheine R, Pannekoek J, Van DPF. Bootstrapping goodness-of-fit measures in categorical data analysis. Sociol Methods Res. 1996;24:492–516. doi: 10.1177/0049124196024004004 [Google Scholar]
- 31. Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model Multidiscip J. 2007;14:535–569. doi: 10.1080/10705510701575396 [Google Scholar]
- 32. Nielsen AM, Vach W, Kent P, Hestbaek L, Kongsted A. Using existing questionnaires in latent class analysis: should we use summary scores or single items as input? A methodological study using a cohort of patients with low back pain. Clin Epidemiol. 2016;8:73–89. doi: 10.2147/CLEP.S103330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Briesacher BA, Tjia J, Doubeni CA, Chen Y, Rao SR. Methodological issues in using multiple years of the Medicare current beneficiary survey. Medicare Medicaid Res Rev. 2012;2:E1–E20. doi: 10.5600/mmrr.002.01.a04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lorig K, Ritter PL, Turner RM, English K, Laurent DD, Greenberg J. A diabetes self-management program: 12-month outcome sustainability from a nonreinforced pragmatic trial. J Med Internet Res. 2016;18:e322. doi: 10.2196/jmir.6484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: a randomized controlled trial. Pain Med. 2015;16:641–652. doi: 10.1111/pme.12605 [DOI] [PubMed] [Google Scholar]
- 36. Younge JO, Wery MF, Gotink RA, et al. . Web-based mindfulness intervention in heart disease: a randomized controlled trial. PLoS One. 2015;10:e0143843. doi: 10.1371/journal.pone.0143843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whitson HE, Johnson KS, Sloane R, et al. . Identifying patterns of multimorbidity in older Americans: application of latent class analysis. J Am Geriatr Soc. 2016;64:1668–1673. doi: 10.1111/jgs.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. García-Olmos L, Alberquilla A, Ayala V, et al. . Comorbidity in patients with chronic obstructive pulmonary disease in family practice: a cross sectional study. BMC Fam Pract. 2013;14:11. doi: 10.1186/1471-2296-14-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28:74–79. doi: 10.1038/jhh.2013.55 [DOI] [PubMed] [Google Scholar]
- 40. Terzano C, Romani S, Paone G, Conti V, Oriolo F. COPD and thyroid dysfunctions. Lung. 2014;192:103–109. doi: 10.1007/s00408-013-9537-6 [DOI] [PubMed] [Google Scholar]
- 41. Ørstavik K, Norheim I, Jørum E. Pain and small-fiber neuropathy in patients with hypothyroidism. Neurology. 2006;67:786–791. doi: 10.1212/01.wnl.0000234035.13779.4a [DOI] [PubMed] [Google Scholar]
- 42. Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation — United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66:246–253. doi: 10.15585/mmwr.mm6609e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.