Abstract
Background
Frailty phenotype and deficit-accumulation frailty index (FI) are widely used measures of frailty. Their performance in predicting recovery after surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR) has not been compared.
Methods
Patients undergoing SAVR (n = 91) or TAVR (n = 137) at an academic medical center were prospectively assessed for frailty phenotype and FI. Outcomes were death or poor recovery, defined as a decline in ability to perform 22 daily activities and New York Heart Association class 3 or 4 at 6 months after surgery. The predictive ability of frailty phenotype versus FI and their additive value to a traditional surgical risk model were evaluated using C-statistics, net reclassification improvement (NRI), and integrated discrimination improvement.
Results
TAVR patients had higher prevalence of phenotypic frailty (85% vs 38%, p < .001) and greater mean FI (0.37 vs 0.24, p < .001) than SAVR patients. In the overall cohort, FI had a higher C-statistic than frailty phenotype (0.74 vs 0.63, p = .01) for predicting death or poor recovery. Adding FI to the traditional model improved prediction (NRI, 26.4%, p = .02; integrated discrimination improvement, 7.7%, p < .001), while adding phenotypic frailty did not (NRI, 4.0%, p = .70; integrated discrimination improvement, 1.6%, p = .08). The additive value of FI was evident in TAVR patients (NRI, 42.8%, p < .01) but not in SAVR patients (NRI, 25.0%, p = .29). Phenotypic frailty did not add significantly in either TAVR (NRI, 6.8%, p = .26) or SAVR patients (NRI, 25.0%, p = .29).
Conclusions
Deficit-accumulation FI provides better prediction of death or poor recovery than frailty phenotype in older patients undergoing SAVR and TAVR.
Keywords: Frailty, Aortic valve replacement, Functional status
Aortic stenosis (AS) is a disease of significant morbidity and mortality that affects approximately 290,000 older adults in the United States (1). While half of the patients with severe AS are referred for aortic valve replacement, only about 40% of patients go on to have surgery due to high operative risk (2). Transcatheter aortic valve replacement (TAVR) has recently been established as an alternative option with survival benefit for high-risk patients (3), but some patients die or live with poor quality of life (4). Traditional risk prediction models, such as the Society of Thoracic Surgeons predicted risk of mortality (STS-PROM), are suboptimal in predicting poor outcomes (5), failing to incorporate geriatric syndromes, such as frailty, which have been shown to be independently associated with poor outcomes after cardiac surgery (6).
Frailty is a syndrome that is characterized by “homeostenosis,” reduced physiologic reserve to tolerate stressors, which leads to increased vulnerability to adverse health outcomes (6). It is an independent risk factor of poor outcomes following cardiac surgery, including prolonged hospitalization (7), delirium (8), functional decline (9), or discharge to institution (10). However, studies in cardiac surgery have used various definitions of frailty, many of which have not been previously validated (6). Others adopted simple assessments (eg, gait speed (11)). Furthermore, outcomes have typically focused on mortality and major cardiovascular events rather than functional outcomes (6).
There are two widely accepted approaches to measuring frailty in geriatrics: the frailty phenotype (12) and deficit-accumulation frailty index (FI) (13–15). Frailty phenotype is defined based on weight loss, exhaustion, physical inactivity, slowness, and weakness (1). An FI quantifies a total burden of age-related health deficit across multiple domains (16). In community-dwelling older populations, an FI provides better risk prediction than phenotypic frailty (17), but the prognostic value of these approaches has not been compared in the setting of cardiac surgery.
We aimed to evaluate the performance of frailty phenotype and FI for prediction of mortality and poor functional recovery in older adults undergoing TAVR and surgical aortic valve replacement (SAVR). We hypothesized that, when added to a traditional surgical risk model based on age, sex, and STS-PROM, FI would improve prognostic accuracy more than frailty phenotype.
Methods
Study Design and Participants
The FRAILTY-AVR Study was a prospective cohort study of older adults undergoing TAVR and SAVR at 14 academic centers in Canada, the United States, and France (NCT01845207). The design, conduct, and main results were published (18). Briefly, patients were eligible if they were 70 years or older and underwent TAVR or SAVR for severe AS. Exclusion criteria were (a) emergent surgery or surgery involving the aorta or another heart valve; (b) clinical instability precluding assessment; (c) severe neuropsychiatric impairment (eg, a Mini-Mental State Examination score <15 points or active psychosis); or (d) non-English speaking. The investigators at the Beth Israel Deaconess Medical Center, Boston, MA, conducted the FRAILTY-AVR Functional Outcomes Study to assess the longitudinal changes in functional status. Between 2014 and 2016, we screened 446 patients and enrolled 246 patients (103 SAVR and 143 TAVR patients) who met the selection criteria (Supplementary Figure 1). This analysis included 91 SAVR and 137 TAVR patients who had available functional status data at 6 months. This study was approved by the Institutional Review Board and a written consent was obtained.
Preoperative Evaluation
A trained research assistant or nurse interviewed patients to obtain medical history, including the New York Heart Association (NYHA) classification, geriatric conditions and functional limitations in 22 daily activities and physical tasks: seven activities of daily living, seven instrumental activities of daily living, five tasks in the Nagi scale (19), and three tasks in the Rosow-Breslau scale (Supplementary Table 1) (20). Minnesota Leisure Time Activity Questionnaire (21), Mini-Mental State Examination, 5-item Geriatric Depression Scale (22), and physical performance tests were also administered. The average gait speed (m/s) was calculated from five trials of 5-meter walk at usual pace and the average grip strength (kg) from three measurements using a Jamar hydraulic dynamometer in the dominant hand. A study geriatrician reviewed medical records to extract body mass index, comorbidities, medications, and test results. The STS-PROM (23) and Charlson comorbidity index were calculated (24).
Frailty Assessment
We determined frailty status according to the frailty phenotype (12) and comprehensive geriatric assessment-based frailty index (CGA-FI) (13–15). Because modifications of the original criteria impact classification of frailty status and predictive ability (25), frailty phenotype was defined by cut-points reported in the original publication, based on weight loss, exhaustion, physical inactivity, slowness, and weakness (Supplementary Table 2) (12). Patients were classified as robust if they had no components, pre-frail if they had 1–2 components, and frail if they had ≥3 components. A CGA-FI was calculated by the proportion of deficits among 48 health-related items that were assessed on CGA (eg, symptoms, diagnoses, functional limitations, performance tests, and test results) (Supplementary Table 1). Patients were classified into three levels of frailty based on the CGA-FI distribution: mild (<0.23 [25th percentile]), moderate (0.23 [25th percentile] to <0.41 [75th percentile]), and severe (≥0.41 [75th percentile]).
Recovery Status
Trained research assistants interviewed patients or their proxies via telephone to assess vital status, NYHA class and functional limitations in 22 activities and physical tasks at 3 and 6 months after the surgery (Supplementary Table 1). A functional status score was calculated as the total number of activities that one could perform independently (range: 0–22). A hierarchical recovery profile was defined based on vital status, functional status score, and NYHA class at 6 months: (a) alive with a functional status score unchanged or improved from baseline, regardless of NYHA class (good recovery); (b) alive with functional decline, yet NYHA class 1 or 2 (partial recovery); (c) alive with functional decline and NYHA class 3 or 4 (poor recovery); and (d) death. Functional decline was defined by comparing baseline score with the average scores at 3 and 6 months. It was assumed that global functional status would be more important than disease-specific NYHA class, and that given the progressive nature of severe AS, stable (ie, no decline) functional status over 6 months would be acceptable.
Statistical Analysis
Preoperative characteristics were compared between SAVR and TAVR cohorts using Wilcoxon Rank Sum test or chi-square test. Because TAVR patients were clinically different from SAVR patients, we analyzed the total cohort as well as each cohort separately. Missing grip strength (n = 8) and gait speed (n = 35) were imputed using a conditional mean based on self-reported functional limitations in relevant physical tasks. We examined the distribution of the two frailty measures and their agreement using the Spearman correlation coefficient.
We first compared prediction of recovery status at 6 months between categorical frailty phenotype (robust, pre-frail, frail) versus categorical FI (mild, moderate, severe frailty), using chi-square test. As there were no robust patients, frailty phenotype only had two categories in this analysis (pre-frail and frail). Association of frailty measures as continuous variables with poor recovery or death was then compared using logistic regression to adjust for age, sex, and STS-PROM (as well as procedure type for the overall cohort analysis). The area under the receiver operating characteristic curves was compared between the two frailty measures treated as continuous variables using 2,000 bootstrap resampling. We also estimated sensitivity, specificity, positive predictive value, and negative predictive value at different cut-points and determined an optimal cut-point that maximized the sum of sensitivity and specificity for each frailty measure (26).
Lastly, we evaluated the performance of a continuous frailty phenotype and three-level CGA-FI, when added to a traditional risk model based on age, sex, and STS-PROM. Models were assessed in terms of (a) calibration using Hosmer-Lemeshow chi-square statistic, (b) C-statistics, (c) net reclassification improvement (NRI), and (d) integrated discrimination improvement (27). The NRI assesses the total proportion of subjects correctly moved between risk strata (ie, cases increases in risk strata and non-cases decreasing). The integrated discrimination improvement measures the separation of average predicted risk between cases and non-cases. The risk categories for NRI were defined as low (<13% [25th percentile]), moderate (13 [25th percentile] to <21% [75th percentile]), and high (≥21% [75th percentile]), based on the predicted risk from the traditional model.
Analyses were performed in Stata Release 14 (StataCorp., College Station, TX) and R software version 3.4.2. A 2-sided p-value < .05 was considered statistically significant.
Results
Cohort Characteristics
Compared with TAVR patients (Table 1), SAVR patients were younger (mean age: 78.1 vs 84.2 years) and had less symptoms (NYHA class 3 or 4: 62.1% vs 88.1%), lower STS-PROM (2.9% vs 5.8%), lower Charlson comorbidity index (2.1 vs 3.6), and higher MMSE score (26.9 vs 25.0). They also had faster gait speed (0.9 m/s vs 0.6 m/s), stronger grip strength (25.0 kg vs 16.7 kg), and less activity of daily living (5.8% vs 16.1%) and instrumental activity of daily living disability (48.5% vs 79.7%).
Table 1.
Characteristics of Patients Undergoing Aortic Valve Replacement
| Characteristics | SAVR | TAVR | p-Value |
|---|---|---|---|
| N (%) or Mean ± SD | N (%) or Mean ± SD | ||
| Sample size | 91 | 137 | |
| Age, years | 77.8 ± 5.3 | 84.5 ± 5.8 | <.001 |
| Female | 44.0% | 51.8% | .24 |
| White race | 95.6% | 98.5% | .18 |
| NYHA class 3/4 | 63.7% | 88.3% | <.001 |
| Aortic valve area, cm2 | 0.7 ± 0.2 | 0.7 ± 0.2 | .091 |
| STS predicted risk of mortality, % | 2.8 ± 1.4 | 5.9 ± 3.0 | <.001 |
| Charlson comorbidity index | 2.2 ± 1.7 | 3.6 ± 2.3 | <.001 |
| Atrial fibrillation | 23.1% | 48.2% | <.001 |
| Chronic kidney disease | 29.7% | 37.2% | .24 |
| Chronic lung disease | 24.2% | 34.3% | .10 |
| Congestive heart failure | 31.9% | 57.7% | <.001 |
| Diabetes | 28.6% | 27.7% | .89 |
| Myocardial infarction | 15.4% | 25.6% | .07 |
| Stroke or transient ischemic attack | 6.6% | 21.9% | .002 |
| MMSE score,a points | 27.1 ± 2.5 | 25.1 ± 3.2 | <.001 |
| Gait speed,a m/s | 0.9 ± 0.3 | 0.6 ± 0.2 | <.001 |
| Grip strength,a kg | 25.0 ± 1.0 | 16.8 ± 0.7 | <.001 |
| Frailty phenotypea | 37.9% | 84.6% | <.001 |
| CGA-FI | 0.24 ± 0.11 | 0.37 ± 0.11 | <.001 |
| ADL disability | 5.5% | 16.8% | .01 |
| IADL disability | 48.4% | 79.6% | <.001 |
Notes: ADL = activity of daily living; CGA-FI = comprehensive geriatric assessment-based frailty index; IADL = instrumental activity of daily living; MMSE = Mini-Mental Status Examination; NA = not applicable; NYHA = New York Heart Association; SAVR = surgical aortic valve replacement; SD = standard deviation; STS = Society of Thoracic Surgeons; TAVR = transcatheter aortic valve replacement.
aMissing information was imputed for MMSE (5 patients), gait speed (35 patients), grip strength (8 patients), and frailty phenotype (36 patients).
Preoperative Frailty Assessment: Frailty Phenotype Versus CGA-FI
The overall prevalence of phenotypic frailty was 65.0% (the remaining 35.0% were pre-frail, and no patients were robust) and the mean CGA-FI was 0.32 (range: 0.02–0.66). The degree of frailty was greater among TAVR patients than SAVR patients according to the frailty phenotype (SAVR vs TAVR: 34.4% vs 82.5%) and CGA-FI (mean: 0.24 vs 0.37) (Table 1). Frailty phenotype was highly correlated with CGA-FI in SAVR patients (Spearman correlation coefficient: 0.73), and moderately correlated in TAVR patients (Spearman correlation coefficient: 0.48) (Supplementary Figure 2).
Frailty and Recovery Status at 6 Months
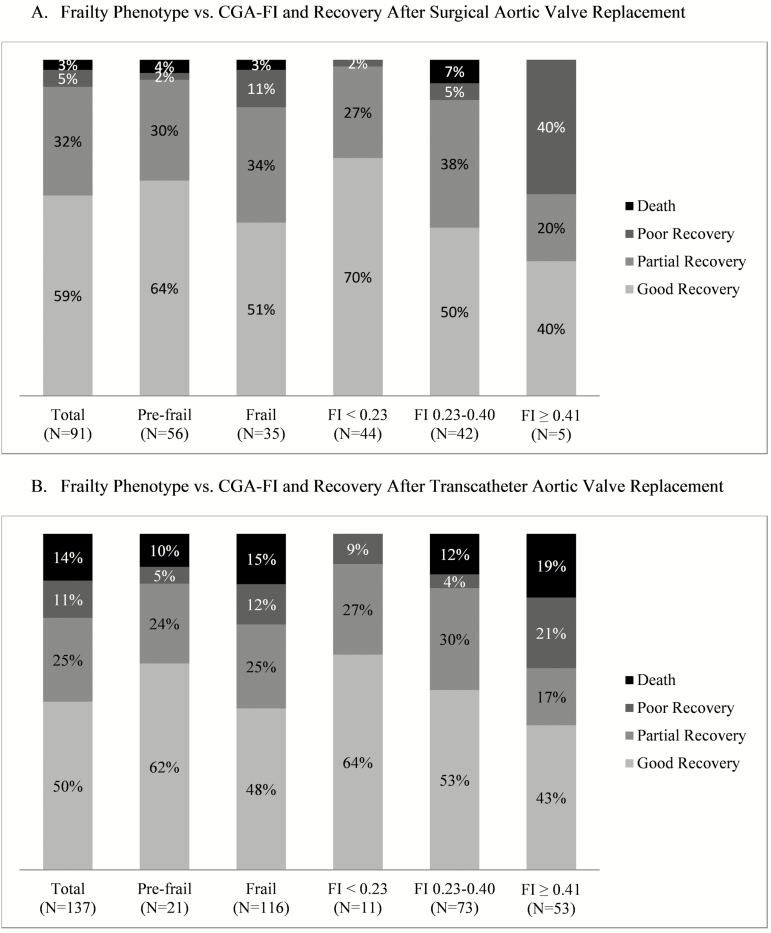
Of the 91 SAVR patients assessed at 6 months, 54 (59.3%) returned to functional baseline or improved (good recovery); 29 (31.9%) had functional decline with minimal symptoms (partial recovery); 5 (5.5%) had functional decline with severe symptoms (poor recovery); and 3 (3.3%) died (Figure 1). Of the 137 TAVR patients assessed at 6 months, the corresponding numbers were 69 (50.4%), 34 (24.8%), 15 (11.0%), and 19 (13.9%), respectively. As previously noted, no patients were robust, thus frailty phenotype was categorized into pre-frail and frail. Recovery status did not differ significantly by frailty phenotype in the SAVR cohort (p = .22) and TAVR cohort (p = .60) (Figure 1). In comparison, increasing CGA-FI was associated with lower risk of good recovery and greater risk of poor recovery or death in the SAVR cohort (p = .006) and TAVR cohort (p = .03).
Figure 1.
Frailty phenotype versus frailty index in predicting poor recovery or death after aortic valve replacement. (A) Frailty phenotype versus CGA-FI and recovery after surgical aortic valve replacement. (B) Frailty phenotype versus CGA-FI and recovery after transcatheter aortic valve replacement. CGA-FI, Comprehensive Geriatric Assessment – Frailty Index. Good recovery was defined as alive with functional status same as or improved from baseline; partial recovery was defined as alive with functional status lower than baseline and the New York Heart Association (NYHA) class 1 or 2; poor recovery was defined as being alive with functional status lower than baseline and the NYHA class 3 or 4. There were no robust patients according to the frailty phenotype in the study population.
The CGA-FI was more consistently associated with poor recovery or death than frailty phenotype (Table 2). In the combined cohort, patients with frailty phenotype had a greater risk of poor recovery or death than those without; however, associations were attenuated after adjusting for age, sex, and STS-PROM (frail vs pre-frail: 23.8% vs 7.8%; p = .08). In contrast, even after adjustment, increasing CGA-FI categories was associated with poor recovery or death in the combined cohort (mild, moderate, and severe frailty: 3.6%, 14.8%, and 39.7%; p = .002). The results in each procedural cohort showed similar trends but had limited statistical power.
Table 2.
Frailty and Risk of Poor Recovery After Aortic Valve Replacement
| Poor Recovery n/N (%) | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | |
|---|---|---|---|
| Combined | |||
| Frailty phenotypeb | |||
| Pre-frail | 6/77 (7.8%) | Reference | Reference |
| Frail | 36/151 (23.8%) | 3.7 (1.5–9.2) | 2.5 (0.9–6.7) |
| p-value (df = 1) | 0.005 | 0.08 | |
| CGA-FI | |||
| Mild (<0.23) | 2/55 (3.6%) | Reference | Reference |
| Moderate (0.23–0.40) | 17/115 (14.8%) | 4.6 (1.0–20.7) | 3.8 (0.8–18.0) |
| Severe (≥0.41) | 23/58 (39.7%) | 17.4 (3.9–78.6) | 12.2 (2.3–63.7) |
| p-value (df = 2) | <0.001 | 0.002 | |
| SAVR | |||
| Frailty phenotypeb | |||
| Pre-frail | 3/56 (5.4%) | Reference | Reference |
| Frail | 5/35 (14.3%) | 2.9 (0.7–13.2) | 2.7 (0.6–13.6) |
| p-value (df = 1) | 0.16 | 0.22 | |
| CGA-FI | |||
| Mild (<0.23) | 1/44 (2.3%) | Reference | Reference |
| Moderate (0.23–0.40) | 5/42 (11.9%) | 5.8 (0.7–52.0) | 4.3 (0.4–42.1) |
| Severe (≥0.41) | 2/5 (40.0%) | 28.7 (2.0–414.2) | 33.9 (1.8–657.8) |
| p-value (df = 2) | 0.03 | 0.06 | |
| TAVR | |||
| Frailty phenotypeb | |||
| Pre-frail | 3/21 (14.3%) | Reference | Reference |
| Frail | 31/116 (26.7%) | 2.2 (0.6–7.9) | 2.2 (0.6–8.0) |
| p-value (df=1) | 0.23 | 0.24 | |
| CGA-FI | |||
| Mild (<0.23) | 1/11 (9.1%) | Reference | Reference |
| Moderate (0.23–0.40) | 12/73 (16.4%) | 2.0 (0.2–16.8) | 2.0 (0.2–17.0) |
| Severe (≥0.41) | 21/53 (39.6%) | 6.6 (0.8–55.1) | 5.5 (0.6–47.9) |
| p-value (df = 2) | 0.006 | 0.03 | |
Notes: CGA-FI = comprehensive geriatric assessment-based frailty index; CI = confidence interval; df = degree of freedom; OR = odds ratio; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement.
aAdjusted for age, sex, and Society of Thoracic Surgeons predicted risk of mortality (and procedure type for analysis of the combined cohort).
bNo patients were robust according to frailty phenotype.
Predicting Poor Recovery or Death at 6 Months Using Frailty Assessment
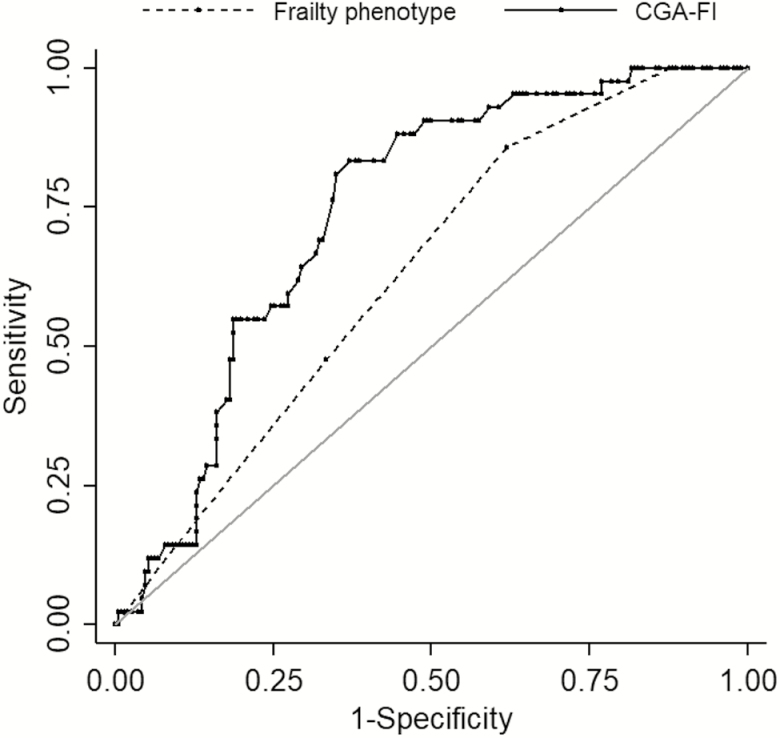
In predicting poor recovery or death, CGA-FI performed better than frailty phenotype (C-statistics for frailty phenotype vs CGA-FI: 0.63 vs 0.74; p = .01) (Figure 2). The optimal cut-point was ≥2 for the frailty phenotype scale (sensitivity: 0.86, specificity: 0.38) and ≥0.33 for CGA-FI (sensitivity: 0.83, specificity: 0.63). The sensitivity, specificity, positive predictive value, and negative predictive value of the two frailty measures at alternative cut-points are presented in Supplementary Table 3. When each cohort was analyzed separately, CGA-FI had higher C-statistics than frailty phenotype in SAVR patients (frailty phenotype vs CGA-FI: 0.69 vs. 0.81; p = .06) and TAVR patients (0.54 vs 0.65; p = .07), although these differences were not statistically significant.
Figure 2.
Receiver operating characteristic curves of frailty phenotype and frailty index to predict poor recovery or death at 6 months after aortic valve replacement. The C-statistic for CGA-FI was significantly higher than that for frailty phenotype (0.74 vs 0.63; p = .01). The optimal cut-point was ≥2 for the frailty phenotype scale (sensitivity: 0.86, specificity: 0.38) and ≥0.33 for CGA-FI (sensitivity: 0.83, specificity: 0.63). CGA-FI, comprehensive geriatric assessment-based frailty index.
Adding CGA-FI to a traditional model based on age, sex, and STS-PROM improved prediction more than adding frailty phenotype (Table 3). In the combined cohort, adding frailty phenotype correctly reclassified 4.0% of patients (p = .70) with an integrated discrimination improvement of 1.6% (p = .08). By comparison, incorporation of CGA-FI categories resulted an NRI of 26.4% (p = .02) and integrated discrimination improvement of 7.7% (p < .001). In the TAVR cohort, only 6.8% of patients with frailty phenotype (p = .26) versus 42.8% of patients were correctly reclassified with CGA-FI (p = .001). In the SAVR cohort, 21.4% of patients were correctly reclassified with frailty phenotype, in comparison to 25.0% correct reclassification with CFA-FI, although neither of these differences were statistically significant. Reclassification tables for frailty phenotype and CGA-FI in the total combined cohort are presented in Supplementary Table 4.
Table 3.
Contribution of Frailty Phenotype and Comprehensive Geriatric Assessment-Frailty Index to a Traditional Surgical Risk Assessment Model in Predicting Poor Recovery or Death at 6 mo After Aortic Valve Replacement
| Population | Model Performance | Traditional Modela | Traditional Model + FP | Traditional Model + CGA-FI |
|---|---|---|---|---|
| Combined | Hosmer-Lemeshow chi-square | 7.83 | 12.35 | 9.98 |
| Total N = 228 | C-Statistic | 0.66 | 0.67; p = .48b | 0.75; p = .02c |
| Poor recovery or | NRI, % | NA | 4.0; p = .70b | 26.4; p = .02c |
| Death N = 42 | IDI, % | NA | 1.6; p = .08b | 7.7; p < .001c |
| SAVR | Hosmer-Lemeshow chi-square | 6.06 | 10.40 | 5.12 |
| Total N = 91 | C-Statistic | 0.79 | 0.82; p = .41b | 0.87; p = .24c |
| Poor recovery or | NRI, % | NA | 25.0; p = .29b | 25.0; p = .29c |
| Death N = 8 | IDI, % | NA | 4.5; p = .28b | 9.1; p = .23c |
| TAVR | Hosmer-Lemeshow chi-square | 7.41 | 6.21 | 8.59 |
| Total N = 137 | C-Statistic | 0.63 | 0.64; p = .64b | 0.70; p = .18c |
| Poor recovery or | NRI, % | NA | 6.8; p = .26b | 42.8; p = .001c |
| Death N = 34 | IDI, % | NA | 0.2; p = .63b | 4.7; p = .03c |
Notes: CGA-FI = comprehensive geriatric assessment-based frailty index; FP = frailty phenotype; IDI = integrated discrimination improvement; NA = not applicable; NRI = net reclassification index; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement.
aThe traditional surgical risk assessment model is based on age, sex, and Society of Thoracic Surgeons predicted risk of mortality.
b p-value compares traditional model plus frailty phenotype vs traditional model alone.
c p-value compares traditional model plus comprehensive geriatric assessment-based frailty index vs traditional model alone.
Discussion
While measures of frailty improve preoperative risk assessment beyond traditional risk models in older patients undergoing SAVR and TAVR (28–31), the frailty phenotype and deficit accumulation FI have not been directly compared in this setting. In our cohort in which frailty phenotype was highly prevalent, the frailty phenotype provided limited risk discrimination for poor recovery or death. By contrast, CGA-FI, which quantifies the severity of frailty based on a larger number of variables in multiple domains, improved prediction over a traditional cardiac surgery-specific assessment. These results suggest that selection of frailty measures should carefully consider characteristics of patient populations.
Research on frailty assessment in cardiac surgical procedures has thus far focused on devising disease-specific frailty instruments, rather than validating well-established frailty instruments in current use. In a recent systematic review (6), we found that numerous disease-specific frailty assessments were developed, but very few have been validated in an independent sample (9,30–33). It remains uncertain whether the predicted risk calculated using weights from the original study population is generalizable to a different population. While several validated frailty measures exist in geriatrics (12,14,15,34), little attention has been paid to assess the comparative performance of existing measures in aortic valve replacement. Moreover, previous studies have emphasized prediction of mortality or cardiovascular events rather than functional recovery, which may be more meaningful to patients (35).
Our results are consistent with previous studies in general populations which demonstrated better prediction of mortality with a more comprehensive deficit-accumulation FI than frailty phenotype, which largely focuses on physical frailty (36,37). Incorporating CGA-FI to a traditional risk model resulted in improved prediction, while adding frailty phenotype did not. In particular, additional improvement over the traditional risk model with CGA-FI was evident in TAVR cohort. This suggests that frailty phenotype may not effectively capture multisystem impairments in TAVR patients. The frailty phenotype classified 82.5% of TAVR patients as frail, limiting further risk stratification among the frailest patients.
As in previous studies (2,3), we observed that the maximum value of CGA-FI was 0.66. This limit is typically around 0.6–0.7, suggesting that survival may not be possible beyond this limit. Such feature offers a useful framework of preoperative risk assessment and decision-making. For instance, a patient with a CGA-FI of 0.5 has little reserve to tolerate additional stress; thus potential benefits should be carefully weighed against the risk of poor recovery and death from new deficits. While such interpretation is intuitive, our ability to quantify frailty from a one-time preoperative evaluation might have been limited. Prediction was more difficult in medically complex TAVR patients than SAVR patients, reflecting the inherent variability and heterogeneity in TAVR patients. The risk of poor recovery or death was 40% among severely frail patients by CGA-FI, suggesting that a high value of CGA-FI alone is not deterministic of futility. Functional impairment mainly driven by severe AS may recover post-procedure. One recent study found that phenotypically frail patients had greater improvement in patient-centered outcomes following TAVR and SAVR (38). Thus, while frailty assessment may serve to inform expected clinical outcomes, multidisciplinary efforts and shared decision-making remain a critical part of the evaluation process.
Logistical considerations in choosing a frailty measure include time, resources, and expertise to conduct the assessment. CGA is longer to administer and requires expertise in geriatrics; in exchange, it provides a multi-faceted quantitative assessment to deliver more individualized perioperative and post-acute care. Frailty phenotype, which does not include assessments of cognitive function and functional limitations, may be less useful for this purpose. Nonetheless, how to practically implement CGA-FI in the busy workflow of preoperative clinics remains a challenge. A more feasible approach may be to apply a simple screening, such as gait speed, to identify high-risk patients for CGA and perioperative co-management (11). Calculation of CGA-FI can be also be automated in electronic medical records or web-based calculators.
Strengths of our study include prospective assessment of frailty using the original frailty phenotype criteria and the deficit-accumulation FI; adoption of a patient-centered outcome that combines mortality, functional status, and NYHA class; and a high retention rate (>90%). Our study has limitations that deserve mention. We aimed to compare the performance of the two frailty measures, not the outcomes of SAVR versus TAVR. Although the comparison of outcomes between SAVR and TAVR by different levels of frailty is crucial for decision-making, such interpretation is subject to bias in the absence of randomization. Our study was underpowered to evaluate the performance of frailty measures within each procedural cohort. For instance, a non-significant NRI of 25.0% in SAVR cohort may be clinically important. In addition, the 18 patients whose recovery status was missing tended to be less frail at baseline (frailty phenotype: 50% vs 67%; mean CGA-FI: 0.28 vs 0.32). Thus, the risk of poor recovery or death might have been overestimated. Finally, the outcome risk in our study reflects our institution’s earlier experience with high-risk patients. Absolute risks may not generalize to patients treated at other institutions or to a more contemporary cohort.
In conclusion, our study shows that preoperative frailty status is associated with death or poor functional recovery at 6 months after SAVR and TAVR. In this population with high prevalence of frailty, a CGA-FI, which incorporates multiple dimensions of health, has an advantage over the frailty phenotype in preoperative risk prediction. Further research is needed to determine how CGA-FI can be practically incorporated in routine care.
Funding
The FRAILTY-AVR Functional Outcomes Study was conducted with the support of a KL2/Catalyst Medical Research Investigator Training award (an appointed KL2 award) and an additional support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award KL2 TR001100-01 and UL1 TR001102) and the Training Program in Cardiovascular Research award (T32-HL007374) from the National Heart, Lung, and Blood Institute, National Institutes of Health. L.A.L. was supported by grant R01AG025037, R01AG041785, and P30AG031679 from the National Institute on Aging. D.H.K. is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, American Federation for Aging Research, John A. Hartford Foundation, and Atlantic Philanthropies. He is also supported by the Boston Claude D. Pepper Older Americans Independence Center (P30AG031679) and Boston Roybal Center Pilot Award (P30AG048785). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic healthcare centers, or the National Institutes of Health. The funding sources had no role in the design, collection, analysis, or interpretation of the data, or the decision to submit the manuscript for publication.
Supplementary Material
Acknowledgments
S.S. and D.H.K. have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy for the data analysis. Study concept and design: J.A., L.A.L., D.H.K.; Acquisition, analysis, and interpretation of data: All authors; Drafting of the manuscript: S.S. and D.H.K.; Critical revision of the manuscript for important intellectual content: All authors; Statistical analysis: S.S. and D.H.K.; Administrative, technical, or material support: Lipsitz, D.H.K.; Study supervision: L.A.L., D.H.K.
Conflict of Interest
L.A.L. holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. J.J.P. reports receiving institutional grants from Medtronic, Boston Scientific, and Edwards Lifesciences, and serves on advisory boards for Boston Scientific and Edwards Lifesciences. K.G. is a consultant to Medtronic, Inc. D.H.K. provides paid consultative services to Alosa Health, a nonprofit educational organization with no relationship to any drug or device manufacturers.
References
- 1. Osnabrugge RL, Mylotte D, Head SJ, et al. . Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update a Report from the American Heart Association. Circulation. 2016;33(4). doi:10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3. Osnabrugge RL, Arnold SV, Reynolds MR, et al. ; CoreValve U.S. Trial Investigators Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv. 2015;8:315–323. doi: 10.1016/j.jcin.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anand A, Harley C, Visvanathan A, et al. . The relationship between preoperative frailty and outcomes following transcatheter aortic valve implantation: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2017;3:123–132. doi: 10.1093/ehjqcco/qcw030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sintek M, Zajarias A. Patient evaluation and selection for transcatheter aortic valve replacement: the heart team approach. Prog Cardiovasc Dis. 2014;56:572–582. doi: 10.1016/j.pcad.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 6. Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: a systematic review. Ann Intern Med. 2016;165:650–660. doi: 10.7326/M16-0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green P, Woglom AE, Genereux P, et al. . The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abawi M, Nijhoff F, Agostoni P, et al. . Incidence, predictive factors, and effect of delirium after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2016;9:160–168. doi: 10.1016/j.jcin.2015.09.037 [DOI] [PubMed] [Google Scholar]
- 9. Schoenenberger AW, Stortecky S, Neumann S, et al. . Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684–692. doi: 10.1093/eurheartj/ehs304 [DOI] [PubMed] [Google Scholar]
- 10. Huded CP, Huded JM, Friedman JL, et al. . Frailty status and outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2016;117:1966–1971. doi: 10.1016/j.amjcard.2016.03.044 [DOI] [PubMed] [Google Scholar]
- 11. Lilamand M, Dumonteil N, Nourhashémi F, et al. . Gait speed and comprehensive geriatric assessment: two keys to improve the management of older persons with aortic stenosis. Int J Cardiol. 2014;173:580–582. doi: 10.1016/j.ijcard.2014.03.112 [DOI] [PubMed] [Google Scholar]
- 12. Fried LPP, Tangen CMM, Walston J, et al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 13. Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x [DOI] [PubMed] [Google Scholar]
- 14. Jones D, Song X, Mitnitski A, Rockwood K. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population-based study of elderly Canadians. Aging Clin Exp Res. 2005;17:465–471. [DOI] [PubMed] [Google Scholar]
- 15. Evans SJ, Sayers M, Mitnitski A, Rockwood K. The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing. 2014;43:127–132. doi: 10.1093/ageing/aft156 [DOI] [PubMed] [Google Scholar]
- 16. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 62/7/722 [pii]. [DOI] [PubMed] [Google Scholar]
- 17. Rockwood K, Blodgett JM, Theou O, et al. . A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. 2017;7:43068. doi: 10.1038/srep43068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afilalo J, Lauck S, Kim DH, et al. . Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 19. Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467. [PubMed] [Google Scholar]
- 20. Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. [DOI] [PubMed] [Google Scholar]
- 21. Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. [DOI] [PubMed] [Google Scholar]
- 22. Hoyl MT, Alessi CA, Harker JO, et al. . Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc. 1999;47:873–878. [DOI] [PubMed] [Google Scholar]
- 23. Online STS Adult Cardiac Surgery Risk Calculator http://riskcalc.sts.org/stswebriskcalc/#/. Accessed April 23, 2018.
- 24. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 25. Theou O, Cann L, Blodgett J, Wallace LM, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res Rev. 2015;21:78–94. doi: 10.1016/j.arr.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 26. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 27. Steyerberg EW, Vickers AJ, Cook NR, et al. . Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chauhan D, Haik N, Merlo A, et al. . Quantitative increase in frailty is associated with diminished survival after transcatheter aortic valve replacement. Am Heart J. 2016;182:146–154. doi: 10.1016/j.ahj.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 29. Afilalo J, Lauck S, Kim DH, et al. . Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR Study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 30. Stortecky S, Schoenenberger AW, Moser A, et al. . Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489–496. doi: 10.1016/j.jcin.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 31. Sündermann S, Dademasch A, Rastan A, et al. . One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact Cardiovasc Thorac Surg. 2011;13:119–123; discussion 123. doi: 10.1510/icvts.2010.251884 [DOI] [PubMed] [Google Scholar]
- 32. Arnold S, Kirtane A, Kodali S, et al. . Predictors of poor outcome after transcatheter aortic valve replacement in the partner trial. J Am Coll Cardiol. 2013;61(10 suppl 1):E1978. doi: 10.1016/S0735-1097%2813%2961978-6 [Google Scholar]
- 33. Seiffert M, Sinning JM, Meyer A, et al. . Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol. 2014;103:631–640. doi: 10.1007/s00392-014-0692-4 [DOI] [PubMed] [Google Scholar]
- 34. Puthoff ML. Outcome measures in cardiopulmonary physical therapy: short physical performance battery. Cardiopulm Phys Ther J. 2008;19:17–22. [PMC free article] [PubMed] [Google Scholar]
- 35. Coylewright M, Palmer R, O’Neill ES, Robb JF, Fried TR. Patient-defined goals for the treatment of severe aortic stenosis: a qualitative analysis. Health Expect. 2016;19:1036–1043. doi: 10.1111/hex.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. doi: 10.1093/ageing/aft160 [DOI] [PubMed] [Google Scholar]
- 37. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 38. Kotajarvi BR, Schafer MJ, Atkinson EJ, et al. . The impact of frailty on patient-centered outcomes following aortic valve replacement. J Gerontol A Biol Sci Med Sci. 2017;72:917–921. doi: 10.1093/gerona/glx038 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.