Abstract
The function of the pharynx, an organ in the model system Caenorhabditis elegans, has been correlated with life span and motility (another measure of health) since 1980. In this study, in order to further understand the relationship between organ function and life span, we measured the age-related decline of the pharynx using an electrophysiological approach. We measured and analyzed electropharyngeograms (EPG) of wild type animals, short-lived hsf-1 mutants, and long-lived animals with genetically decreased insulin signaling or increased heat shock pathway signaling; we recorded a total of 2,478 EPGs from 1,374 individuals. As expected, the long-lived daf-2(e1370) and hsf-1OE(uthIs235) animals maintained pharynx function relatively closer to the youthful state during aging, whereas the hsf-1(sy441) and wild type animals’ pharynx function deviated significantly further from the youthful state at advanced age. Measures of the amount of variation in organ function can act as biomarkers of youthful physiology as well. Intriguingly, the long-lived animals had greater variation in the duration of pharynx contraction at older ages.
Keywords: Variation, Health span, C. elegans, Physiology, Organ Function
Entropy dictates that all ordered structures in the universe decay over time. Thus, aging is a cosmic process that occurs to both animate and inanimate material; even atoms decay—just on a longer timescale. However, energy can be expended (and the amount of entropy in the universe increased) in order to generate or repair ordered, animate, or inanimate structures. Yet, despite living organisms’ ability to generate and repair ordered structures, in virtually all sexually reproducing animals that reach a fixed size, physiological capacities decline after the onset of reproductive maturity.
Aging is the term used to describe a series of stochastic (1–4), but also probabilistic (3,5), molecular changes that degrade the composition, and thus any functionality of, both animate and inanimate materials. Brick and steel decay just as flesh and bone do. However, biological systems are unique in that they can deploy molecular repair processes encoded in their genome that can prevent, slow or repair molecular damage accrued over time. Thus, modern gerontologists have been engaged in identifying molecular repair pathways utilized by long lived animals, be they a relatively long-lived species (6,7) or a long-lived mutant within a species (8,9). In identifying these molecular repair pathways that may slow or reverse aging in biological systems, it is useful to devise means to measure effective rates of loss of functional capacities, from the level of the organelle all the way to the level of the organism. Hence, any successes of efforts to slow the aging process are measured in terms of effects on measured physiological capacities.
Indeed, many measures of physiological functionality have been used to assess the biological age of humans and animals. For example, grip strength has been shown to decline with age (10); so has maximum heart rate (11). Thus, an individual having a grip strength or a maximum heart rate above the level of the average age value of that parameter could indicate an individual biologically younger than her/his chronological age. Being below that average maximum heart rate for a given age could mean that one is biologically older than their chronological age, meaning one might expect to have a shorter life span. Thus, the study of these functional measures of health can help assess individual health—they can act as biomarkers (12). Measures of functional health with age, or biomarkers of aging, also help determine if drugs or treatments designed to elicit the activation of pro-longevity molecular repair pathways have actual effects on functional physiology before the terminal endpoint of life span can be measured.
Here, we used Caenorhabditis elegans as a model to understand how organ function declines, and how organ functionalities are maintained, during the course of aging in long-lived mutants. C. elegans is a 1-mm soil nematode utilized for basic biology research (13), including the basic biology of aging (8,9,14–17). They exist as males and hermaphrodites, with the latter gender encompassing the vast majority of scientific inquiries. C. elegans hermaphrodites live for about three weeks when raised at 20° (3 days of development and 17–20 days of adult life on average). Their genomes are editable (18), and they are transparent with a fixed somatic cell lineage (19,20), making them ideal for studies into the molecular, genetic, and cellular basis of the aging process. In this study, we focus on electrical measures of organ function, here, the C. elegans the pharynx, to observe the declining function of an organ with age in wild type, short-lived, and long-lived genotypes.
The C. elegans pharynx is a violin-shaped organ composed of 60 cells; 20 are neurons, 20 are muscles, and 20 are glandular or structural (21). The organ contracts autonomously, but also alters its contraction behavior in response to a known network of only 12 neurons (22–24). Thus, the organ is similar to a heart insofar as it is a fluid-pumping organ composed of muscle cells, with an intrinsic rhythmic contraction rate that can be modulated by neuronal input.
The functionality of the C. elegans pharynx has been correlated with differences in life span and health (motility) since 1980 (25). Many prior reports (not all of which are referenced here) examining aging and pharynx function measured pumping rates (pumps per minute) or calcium signals to determine functionality of the pharynx (25–31), or even the ability to ingest beads as a metric of pharynx function (32) during the course of aging.
Here we are reporting an electrophysiological analysis of the function of the pharynx in relation to life span and health span in different genetic backgrounds (Figure 1). In this report, we used a microfluidic device (Nemametrix, Inc., Eugene) to immobilize animals and record electrical signals from their pharynges. As expected, we found that a decline in function, relative to the youthful state, was associated with increased chronological age in wild-type animals. Compared to wild type animals, we found that long-lived mutants retained youthful organ function, and the short-lived mutant lost organ function before wild type animals, though at a similar rate. Interestingly, we found that some measures of variation in organ function were different in long-lived mutants. In humans, increased variation in beat-to-beat interval of the heart is correlated with better, more youthful cardiac function (33). Thus, this report further demonstrates that measures of variation are also useful ways of understanding and identifying consequential differences in physiology. Future studies screening for pro-longevity drugs, treatments or mutations may be aided by considering measures of organ function including mean-independent measures of variation.
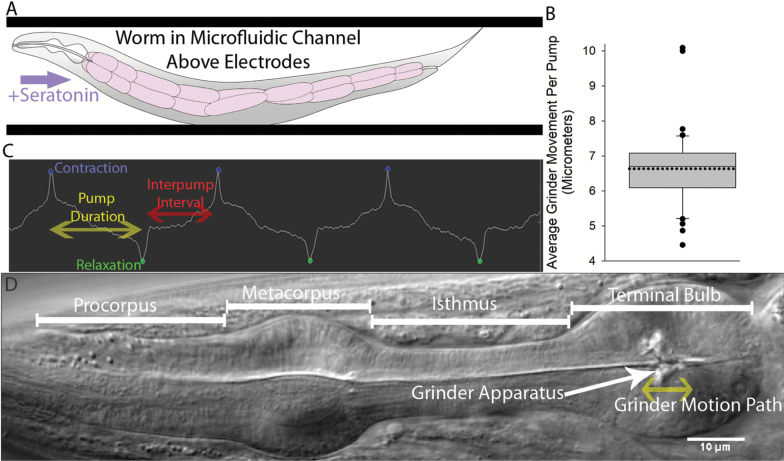
Figure 1.
Overview of electropharyngeogram measurement scheme. (A) Shows a cartoon schematic of the worm loaded into the fluidic chamber above the electrode. (B) Shows the average movement of the grinder apparatus in the pharynx for a contraction. (C) Shows the electrical waveform generated during the measurement of three distinct pumps, detailing the peaks for contraction and relaxation, as well as the pump duration and interpump interval (IPI). (D) Shows a Nomarski DIC micrograph of a C. elegans pharynx, detailing the regions of the organ, including the grinder apparatus (white arrow), which visibly moves during pump contractions; the anterior–posterior motion path of the grinder is indicated by the yellow bidirectional arrow below the grinder.
Materials and Methods
See Supporting Material for materials and methods.
Results
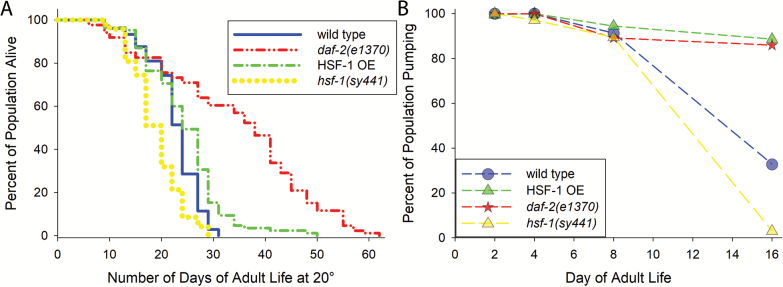
Figure 1 shows an overview of the electropharyngeogram (EPG) experiments. Figure 1A shows a cartoon diagram of the worm loaded into the microfluidic chamber above an electrode. Figure 1B shows the distance the grinder apparatus of the pharynx is observed to move in response to serotonin-induced pumping in the absence of food in the fluidic chamber. The waveform generated by measurement of the electrical signal from the C. elegans pharynx is shown in Figure 1C. Figure 1D shows the wild type pharynx and the motion path of the grinder apparatus on the cuticle of pm6 (pharyngeal muscle cell group 6; consists of three cells). Figure 2A shows the life spans of the animals we measured were similar to prior reports (17,34–36). Figure 2B shows the percent of the population of each genotype pumping at each time point during the aging process.
Figure 2.
Life spans and percentages of each population actively pumping at different ages. (A) Shows the life spans of the genotypes we measured electropharyngeograms for in this study. All genotypes were relatively long or short lived as previously reported. (B) Shows the percent of the population of each genotype pumping at each measurement point.
Wild Type Animals
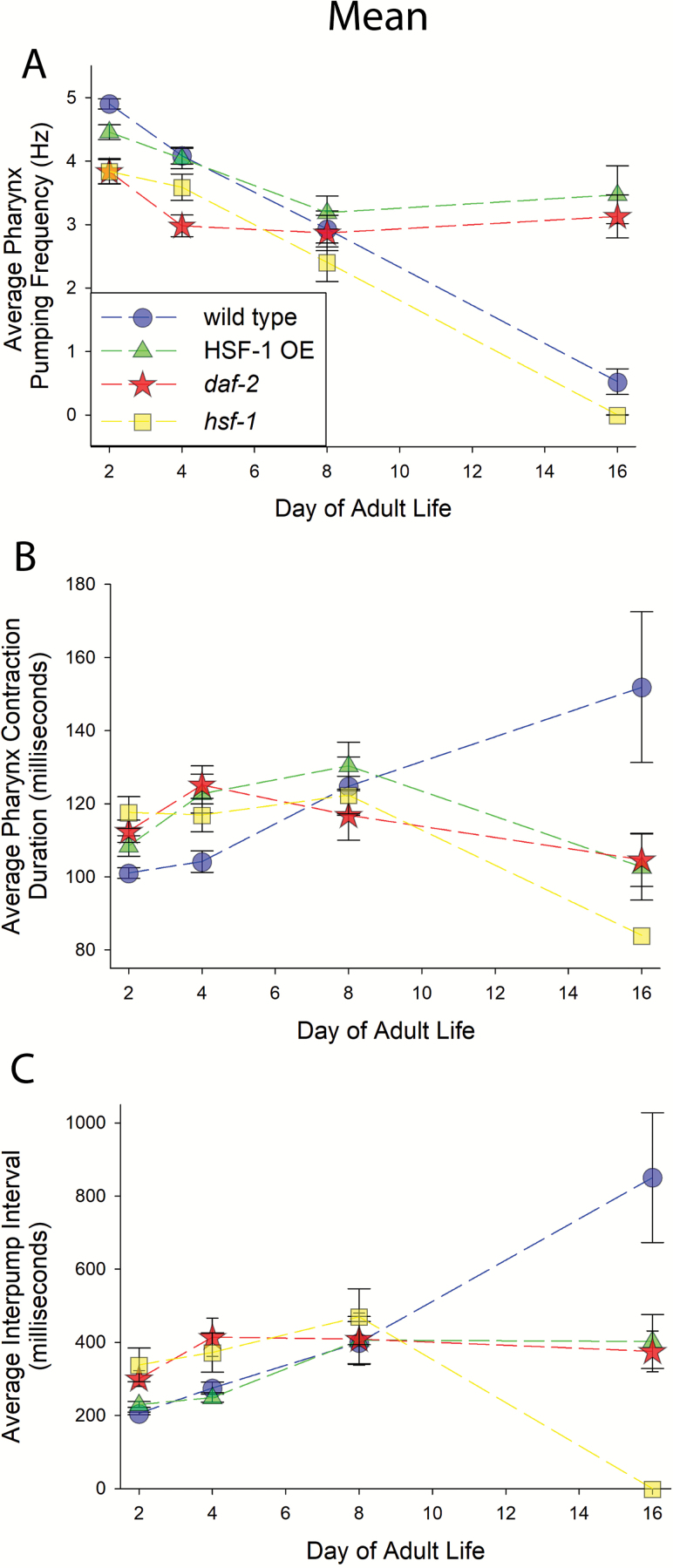
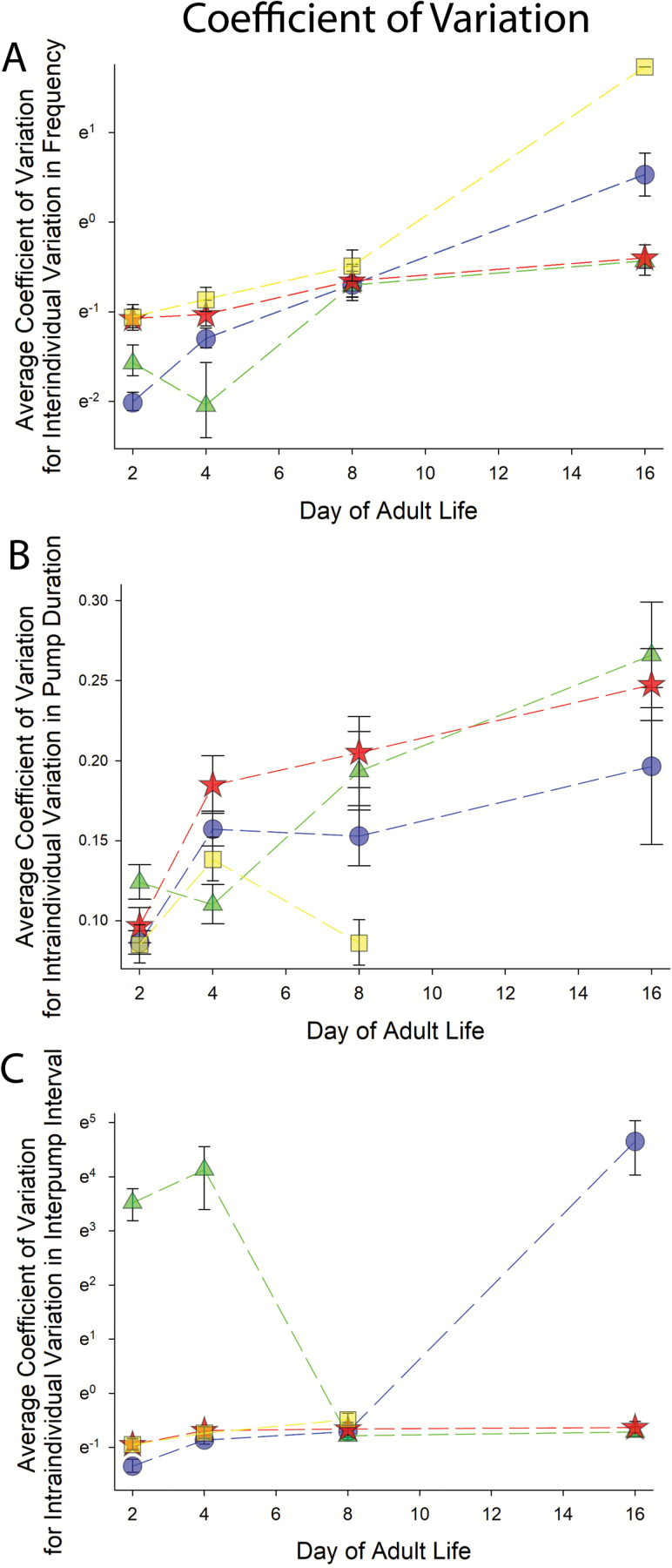
Figures 3 and 4 show the results of recording over 700 EPGs from over 350 individual wild type animals during the course of aging, on days 2, 4, 8, and 16 of adulthood (1–4 days short of typical average wild type life spans at 20°). In wild type animals, the frequency of pharyngeal contractions declined steadily with age; there was a significant difference in pump frequency on all days measured (Figure 3A). The duration of pharyngeal contractions increased with age, starting at day 8 (Figure 3B). The interpump interval (IPI) also increased, starting at day 4, increasing on day 8 and then remaining stable until day 16 (statistically, see Supplemental File F1 and Figure 3C). The amount of interindividual variation in the frequency of pumping in the population increased with age (Figure 4A). The coefficient of variation (CV) for individual animals’ pump duration increased with age relative to the youthful state (Figure 4B); the CV for IPI remained stable until day 8, and then shot up at day 16 (Figure 4C). Additional details of statistical analyses are shown in Materials and Methods in Supporting Materials, and in Supplemental File F1.
Figure 3.
Average values for different properties of pharynx function measured via electropharyngeograms. Relationship of genotypes to symbols in legend in (A) is maintained throughout figure. Axes are labeled. Error bars are 95% confidence intervals. Statistical details are provided in Supplemental File 1. (A) Shows average frequency. (B) Shows average pump duration. There is no data shown for hsf-1 on day 16 because there was only one animal that pumped one time in that group, so there was no variation in something measured only once. (C) Shows average interpump interval (IPI). Again, there is no data shown for hsf-1 animals on day 16 because there was only one individual that pumped one time, so there was no interpump interval (IPI).
Figure 4.
Average amount of variation in properties of pharynx function measured via electropharyngeograms. Symbols represent the same genotypes as in Figure 3. Natural log scales are used on the y axes of (A) and (C) due to the relatively high range measured parameters. (A) Shows average coefficient of variation for interindividual variation in frequency. The high value for hsf-1 at day 16 was driven by a single individual that pumped once, with every other 16 day old hsf-1 adult we measured failing to pump. (B) shows average intraindividual variation in pump duration. (C) shows average intraindividual variation in interpump interval (IPI).
Short-lived hsf-1 Mutants
Results of the analyses of EPGs of over 250 hsf-1(sy441) mutant animals are shown in Figures 3 and 4. Short-lived hsf-1 mutants had a nearly identical rate of pharyngeal decline compared to wild type, but started at a lower pumping frequency than wild type and pumped at a significantly lower frequency at each time point (Figure 3A). The pump duration did not significantly change with age, but was significantly different than wild type on days 2, 4, and 16 (Figure 3B). IPI increased slightly on day 8 relative to the youthful state; IPIs were significantly different than wild type on days 2 and 4 of adulthood (Figure 3C).
The amount of interindividual variation in frequency increased with age and was greater than wild type (significantly on days 2 and 4), though the day 16 measurements were almost all zero pumps with a single individual responding (Figure 4A). The amount of variation in pump duration went up on day 4 of adulthood; it was significantly different than wild type on days 4 and 8 (Figure 4B). The amount of variation in IPI did not detectably change with age (Figure 4C).
Long-lived HSF-1 Overexpression Animals
Figures 3 and 4 show the results from recording over 300 animals with extra copies of hsf-1 integrated into their genome (HSF-1 OE animals). HSF-1 OE animals’ pumping frequency declined until day 8 of adulthood and remained stable until at least day 16 (Figure 3A). These animals also had a higher frequency of pumping, relative to wild type at day 16 of adulthood (Figure 3A). The engineered HSF-1 OE animals had the same pump duration on days 2 and 16 of adulthood, but significantly different pump durations for all other day comparisons; pump duration was significantly different than wild type on days 2, 4, and 16 of adulthood (Figure 3B). IPI was significantly different than the youthful 2-day old state on days 8 and 16 of adulthood (Figure 3C); statistical comparison to wild type shown in Supplemental File 1.
The interindividual variation in frequency increased on days 8 and remained stable until at least day 16, relative to the youthful state; it was different than wild type on every day except day 8 of adulthood (Figure 4A). Intraindividual variation in pump duration was different than the youthful state on days 8 and 16 of adulthood; it was significantly different than wild type at each time point (Figure 4B). Intraindividual variation in IPI was significantly different than the youthful state (day 2 adults are considered the youthful state) on days 4, 8, and 16; it was significantly different than wild type on days 2, 4, and 16 of adulthood (Figure 4C).
Long-lived daf-2 Mutants
We analyzed approximately 300 long-lived daf-2(e1370) animals to generate the data shown in Figure 1. Pumping frequency of long-lived daf-2 mutants declined relative to the youthful state on day 4 of adulthood and remained stable until day 16 of adulthood; this parameter was significantly different than wild type on every single day (Figure 3A). Pump duration peaked on day 4 of adulthood, which was the only day that was significantly different than the youthful 2-day old adult state (Figure 3B). Pump duration was significantly different than wild type on days 2, 4, and 16 (Figure 3B). The IPI of daf-2 mutants was significantly different from the youthful state on days 4 and 8, but not on day 16 (Figure 3C). The mutant IPI was different from wild type on days 2 and 4 (Figure 3C). We did not detect a difference between daf-2 and wild type on day 16, presumably because of relatively low N and multiple comparisons in the Two-way ANOVA followed by a Holm–Sidak test we performed (Supplemental File 1), but there is a statistically significant difference from wild type if one compares just wild type and daf-2 on day 16 via an ANOVA on ranks.
The interindividual variation in pump frequency increased slightly on day 8 and remained stable through day 16; the CV for frequency was lower in wild type on day 2 of adulthood and lower in daf-2 animals on day 16 of adulthood (Figure 4A). There was significant increase in the amount of variation in the pump duration relative to the youthful state; there was elevated variation relative to wild type on days 4, 8, and 16 (Figure 4B). There was no change detected in the CV for the IPI during the course of aging to day 16 of adulthood. The IPI CV was significantly different than wild type on day 16 of adulthood (Figure 4C).
Discussion
Aging and the Decline of Physiological Capacities
Organs begin to fail with advanced chronological age. In order to preserve or restore youthful capacities, organ function must be preserved or repaired. Consistent with the idea that youthful organ function is essential for extended existence, longer-lived mutants maintained relatively youthful pharynx function at advanced ages where wild type animals’ pharynx function had deteriorated. The shorter lived hsf-1 mutant lost function before all other genotypes, presumably because of problems attributable to proteostasis failures caused by lack of wild type Heat Shock Factor 1 transcription factor activity (37). Thus, we would expect any long-lived mutant or pro-longevity treatment (drug/environment) to maintain vital organ function better than wild type animals at advanced ages, unless there is some other compensatory mechanism that allows a long-lived animal to exist with a lesser functioning organ. To date, enhanced maintenance of functionality of the C. elegans pharynx at advanced ages seems to be associated with longevity (e.g., (27)). However, it will be interesting to see if any of the metrics afforded by the EPG allow scientists to predict life span or health span (measured by motility) as can be done in both C. elegans (38,39) and Drosophila (40) by expression level of reporter genes.
Health span of Long-lived Animals: Is the Pharynx a Good Measure of Health? Does Lasting Longer Mean Decaying Longer?
The function of the pharynx at advanced age seems to be a good metric of health. The key is measurement at advanced age. Many longevity mutants, such as the ones we examined here may have lower frequencies of pumping that are better maintained throughout time. It may be that the candle burns a bit slower, but a bit longer. Another example, mutations in eat-2 decrease youthful pumping rate and extend life span (41); the eat-2 mutants may even maintain their low pumping frequency late into life, but our instrument was unable to detect signal from eat-2(ad465) mutants, and we were unable to locate any prior reports wherein eat-2 pumping had been measured at advanced age. Thus, to date, in C. elegans, pharynx function at advanced age appears to indicate longevity and health. The functional pharynx seems to be a fair measure of wild type health as consumption of nutrients is essential for organismic health. The pharynx measure should be carefully considered when measuring mutants that innately disable pharynx function to achieve dietary restriction; we think the main consideration is to not focus on the starting level of pharynx functionality, and instead focus on functionality at advanced ages and/or the rate at which function declines during aging for a given genotype. Indeed, it seems that many longevity mutants may have less robust pumping in young adulthood, as suggested by the lower frequency of pumping for long-lived animals on day-2 of adulthood (Figure 1A). Long-lived mutants are also often less fit in terms of adaptation to environmental changes (temperature shifts), rate of development to sexual maturity and self-progeny production early in adulthood (42,43), suggesting that decreased evolutionary fitness may be a general feature of physiological states associated with mutations that increase life span.
Intuitively, it seems that increased life span requires increased duration of functional physiology, perhaps, minimally, the preservation of functional cellular capacities. It is not clear how much increased functional physiology solely at the cellular level translates to macroscopic functional physiology, in terms of systems being able to perform youthful tasks at the organismic level—tasks like moving one’s body. A few functioning autonomous cells in an otherwise decrepit tissue cannot perform the functions of the whole tissue, though an animal may still be alive. Thus, it would seem that cellular abundance/physiological capacity and cell–cell communication must also be preserved. The concern is that extending life span may not always extend health span, which is a reflection of functional capacities of tissues and organs.
Indeed, some recent reports have created some controversy regarding whether or not treatments that extend life span extend health span. Longer-lived mutants may (44) or may not (45) spend a greater portion of their lives in a functionally impaired state, depending on how one defines functionally impaired and designs the assays and equation to measure total health span. Thus, the decreased health span of daf-2(e1370) mutants in [44] seemed to be due to the decision to measure motility on food, and goes away if animals are measured on plates without food (45). The decision by both groups to normalize to maximum life span might cause results to be different than if they normalized to average life span.
Our data clearly demonstrate that, although the long-lived mutants have reduced pharynx functionality relative to wild type early in life, they maintain this functionality beyond a point where wild type animals have declined precipitously. Thus, we would argue that for this single measure of health span, it has been extended in both the daf-2 mutant and the HSF-1 overexpressing animals. Clearly, however, this is only one measure of health span in worms. A challenge for the field moving forward will be to develop consensus regarding how to integrate multiple measures of organ function into a quantitative index of health, perhaps similar to the frailty indices that have been validated for both humans (46,47) and mice (48,49).
Intraindividual Variation and Interindividual Variation in Complex Quantitative Traits
Measures of the amount of variation in various quantitative traits can be informative, and even diagnostic. Beat-to-beat variation in heart rate has been shown to be a sign of youth; that is, increased beat-to-beat variation in heart function is considered a youthful trait. Aging decreases beat-to-beat variation, and lowered beat-to-beat variation is considered a risk of future functional decline (33). Indeed, people with mutations in a mitochondrial, pro-longevity gene NDUF11A have reduced measures of heart rate variation (Root mean square of beat to beat variation), putting them at risk for future heart disease (50). The most direct comparison with the worm pharynges would seem to be the CV of the IPI, which was not elevated in long-lived mutants. However, at older ages, pump duration CV was elevated in long-lived mutants, indicating that this could also be another mean-independent biomarker of longevity (Figure 4B). Note that, unlike beat-to-beat variation in humans, CV for pump duration does not naturally decline with age; here elevated values at older ages are simply associated with the two long-lived genotypes.
Our previous work found that daf-2(e1370) mutants showed reduced interindividual variation in expression of a life span biomarker. Here we found that long-lived daf-2 mutants and HSF-1 OE animals both had reduced interindividual variation in frequency at advanced ages. Their population profile, in terms of interindividual variability, was more youthful at advanced age than wild type animals (day 16, Figure 4A). It is not yet clear if reduced interindividual variation in some parameters may be indicative of longevity, but the little evidence we have to date suggests that the amounts of variation in different complex quantitative traits can be studied genetically (51), and may be informative about how the aging process manifests in populations (52). These aforementioned studies, and this study, identified signaling systems and environmental conditions that can influence the amount of spread in the following complex quantitative traits: gene expression (51,52), life span (52), and pharynx pumping (this study). Thus, it is our hope that these reports will both inform other scientists as to the way the aging process manifests in individuals and populations, and persuade other scientists to consider measures of variation in addition to measures of mean when trying to classify and understand different aspects of aging biological systems.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (F32AG054098 to J.C.R., T32AG000057 in support of M.C. and N.B., P30AG013280 to M.K. and R00AG045341 to A.M.) and an Alzheimer’s Disease Research Foundation Seed Grant to A.M. and M.K. which was provided by the University of Washington Alzheimer’s Disease Research Center which is also supported by the National Institute on Aging at the National Institutes of Health (5P50AG005136-34). Some strains were provided by the C.G.C., which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40OD010440).
Conflicts of Interest
None.
Supplementary Material
Acknowledgements
We would like to thank Dr. George Martin for thoughtful discussion of the results. A.M., M.K., M.C., N.B., and J.C.R. designed the study. J.C.R., N.B., M.M. and B.M. maintained stocks and recorded EPGs. F.F. and M.C. measured grinder movement. A.M. and M.C. analyzed the data. A.M. wrote the original draft. A.M., M.K., M.C., J.C.R., and N.B. revised the manuscript.
References
- 1. Hayflick L. Biological aging is no longer an unsolved problem. Ann N Y Acad Sci. 2007;1100:1–13. doi:10.1196/annals.1395.001 [DOI] [PubMed] [Google Scholar]
- 2. Hayflick L. Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genet. 2007;3:e220 doi:10.1371/journal.pgen.0030220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirkwood TB, Finch CE.. Chance, Development, and Aging. New York: Oxford University Press; 2000. [Google Scholar]
- 4. Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. doi:10.1038/270301a0 [DOI] [PubMed] [Google Scholar]
- 5. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi:10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011; 66:741–750. doi:10.1093/gerona/glr044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ungvari Z, Csiszar A, Sosnowska D, et al. Testing predictions of the oxidative stress hypothesis of aging using a novel invertebrate model of longevity: the giant clam (Tridacna derasa). J Gerontol A Biol Sci Med Sci. 2013. 68:359–67. doi:10.1093/gerona/gls159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson TE. Caenorhabditis elegans 2007: the premier model for the study of aging. Exp Gerontol. 2008;43:1–4. doi:10.1016/j.exger.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGue M, Christensen K. Growing old but not growing apart: twin similarity in the latter half of the lifespan. Behav Genet. 2013;43:1–12. doi:10.1007/s10519-012-9559-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleg JL, O’Connor F, Gerstenblith G, et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol (1985). 1995;78:890–900. doi:10.1152/jappl.1995.78.3.890 [DOI] [PubMed] [Google Scholar]
- 12. Baker GT 3rd, Sprott RL. Biomarkers of aging. Exp Gerontol. 1988;23:223–239. doi:10.1016/0531-5565(88)90025-3 [DOI] [PubMed] [Google Scholar]
- 13. Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson TE, Wood WB. Genetic analysis of life-span in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1982;79:6603–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klass MR. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev. 1983;22:279–286. doi:10.1016/0047-6374(83)90082-9 [DOI] [PubMed] [Google Scholar]
- 16. Klass M, Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976;260:523–525. doi:10.1038/260523a0 [DOI] [PubMed] [Google Scholar]
- 17. Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi:10.1016/0047-6374(77)90043-4 [DOI] [PubMed] [Google Scholar]
- 18. Frokjaer-Jensen C. Exciting prospects for precise engineering of Caenorhabditis elegans genomes with CRISPR/Cas9. Genetics. 2013;195:635–642. doi:10.1534/genetics.113.156521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi:10.1016/0012-1606(77)90158-0 [DOI] [PubMed] [Google Scholar]
- 20. Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. [DOI] [PubMed] [Google Scholar]
- 21. Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:299–325. doi:10.1098/rstb.1976.0085 [DOI] [PubMed] [Google Scholar]
- 22. Avery L. Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J Exp Biol. 1993;175:283–297. [DOI] [PubMed] [Google Scholar]
- 23. Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–485. doi:10.1016/0896-6273(89)90206-7 [DOI] [PubMed] [Google Scholar]
- 24. Avery L, Horvitz HR. A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant. Cell. 1987;51:1071–1078. doi:10.1016/0092-8674(87)90593-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hosono R, Sato Y, Aizawa SI, Mitsui Y. Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegans. Exp Gerontol. 1980;15:285–289. doi:10.1016/0531-5565(80)90032-7 [DOI] [PubMed] [Google Scholar]
- 26. Bolanowski MA, Russell RL, Jacobson LA. Quantitative measures of aging in the nematode Caenorhabditis elegans. I. Population and longitudinal studies of two behavioral parameters. Mech Ageing Dev. 1981;15:279–295. doi:10.1016/0047-6374(81)90136-6 [DOI] [PubMed] [Google Scholar]
- 27. Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi:10.1111/j.1474-9726.2006.00192.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2004;101:8084–8089. doi:10.1073/pnas.0400848101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dancy BM, Brockway N, Ramadasan-Nair R, Yang Y, Sedensky MM, Morgan PG. Glutathione S-transferase mediates an ageing response to mitochondrial dysfunction. Mech Ageing Dev. 2016;153:14–21. doi:10.1016/j.mad.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alvarez-Illera P, Sanchez-Blanco A, Lopez-Burillo S, Fonteriz RI, Alvarez J, Montero M. Long-term monitoring of Ca2+ dynamics in C. elegans pharynx: an in vivo energy balance sensor. Oncotarget. 2016;7:67732–67747. doi:10.18632/oncotarget.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alvarez-Illera P, García-Casas P, Arias-Del-Val J, Fonteriz RI, Alvarez J, Montero M. Pharynx mitochondrial [Ca(2+)] dynamics in live C. elegans worms during aging. Oncotarget. 2017;8:55889–55900. doi:10.18632/oncotarget.18600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith ED, Kaeberlein TL, Lydum BT, et al. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev Biol. 2008;8:49 doi:10.1186/1471-213X-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogliari G, Mahinrad S, Stott DJ, et al. Resting heart rate, heart rate variability and functional decline in old age. CMAJ. 2015;187:E442–E449. doi:10.1503/cmaj.150462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi:10.1038/366461a0 [DOI] [PubMed] [Google Scholar]
- 35. Steinkraus KA, Smith ED, Davis C, et al. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi:10.1111/j.1474-9726.2008.00385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baird NA, Douglas PM, Simic MS, et al. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science. 2014;346:360–363. doi:10.1126/science.1253168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hajdu-Cronin YM, Chen WJ, Sternberg PW. The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics. 2004;168:1937–1949. doi:10.1534/genetics.104.028423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi:10.1038/ng1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mendenhall AR, Tedesco PM, Taylor LD, Lowe A, Cypser JR, Johnson TE. Expression of a single-copy hsp-16.2 reporter predicts life span. J Gerontol A Biol Sci Med Sci. 2012;67:726–733. doi:10.1093/gerona/glr225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang J, Tower J. Expression of hsp22 and hsp70 transgenes is partially predictive of drosophila survival under normal and stress conditions. J Gerontol A Biol Sci Med Sci. 2009;64:828–838. doi:10.1093/gerona/glp054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1998;95:13091–13096. doi:10.1073/pnas.95.22.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walker DW, et al. Evolution of lifespan in C. elegans. Nature. 2000;405:296–297. doi:10.1038/35012693 [DOI] [PubMed] [Google Scholar]
- 43. Mendenhall AR, Wu D, Park SK, et al. Genetic dissection of late-life fertility in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2011;66:842–854. doi:10.1093/gerona/glr089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc Natl Acad Sci USA. 2015;112:E277–E286. doi:10.1073/pnas.1412192112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hahm JH, Kim S, DiLoreto R, et al. C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat Commun. 2015;6:8919 doi:10.1038/ncomms9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Howlett SE, Rockwood K. New horizons in frailty: ageing and the deficit-scaling problem. Age Ageing. 2013;42:416–423. doi:10.1093/ageing/aft059. [DOI] [PubMed] [Google Scholar]
- 47. Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ. 1994;150:489–495. [PMC free article] [PubMed] [Google Scholar]
- 48. Rockwood K, Blodgett JM, Theou O, et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. 2017;7:43068 doi:10.1038/srep43068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi:10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kerr KF, Avery CL, Lin HJ, et al. Genome-wide association study of heart rate and its variability in Hispanic/Latino cohorts. Heart Rhythm. 2017;14:1675–1684. doi:10.1016/j.hrthm.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mendenhall A, Crane MM, Tedesco PM, Johnson TE, Brent R. Caenorhabditis elegans genes affecting interindividual variation in life-span biomarker gene expression. J Gerontol A Biol Sci Med Sci. 2017. doi:10.1093/gerona/glw349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mendenhall A, Crane MM, Leiser S, et al. Environmental canalization of life span and gene expression in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2017;72:1033–1037. doi:10.1093/gerona/glx017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.