Abstract
Studies on interventions that can delay or treat frailty in humans are limited. There is evidence of beneficial effects of angiotensin converting enzyme (ACE) inhibitors on aspects related to frailty, such as physical function, even in those without cardiovascular disease. This study aimed to longitudinally investigate the effect of an ACE inhibitor on frailty in aging male and female mice. Frailty was assessed with a clinical frailty index (FI) which quantifies health-related deficits in middle-aged (9–13 months) and older (16–25 months) mice. Chronic treatment with enalapril (30 mg/kg/day in feed) attenuated frailty in middle-aged and older female mice, and older male mice, without a long-term effect on blood pressure. Enalapril treatment resulted in a reduction in the proinflammatory cytokines interleukin (IL)-1α, monocyte chemoattractant protein-1 and macrophage inflammatory protein-1a in older female mice, and an increase in the anti-inflammatory cytokine IL-10 in older male mice compared with control animals. These sex-specific effects on inflammation may contribute to the protective effects of enalapril against frailty. This is the first study to examine the longitudinal effect of an intervention on the FI in mice, and provides preclinical evidence that enalapril may delay the onset of frailty, even when started later in life.
Keywords: Frailty index, MCP-1, MIP-1α, IL-1α, IL-10
Frailty is defined as an increased vulnerability to stressors, and can be considered a measure of variability in the health and risk status of people of the same chronological age (1). Frailty is commonly measured using a frailty index (FI), which quantifies the number of health-related deficits a person displays. Higher FI scores, which correspond to increasing frailty status, are associated with poor outcomes, including disability, institutionalization, and death (2–5). Thus, there would be clear benefits in interventions to delay or prevent frailty, not only for frail older people, but for their caregivers, families, and the health care system (6).
Clinical intervention studies for frailty have had mixed results (7–10). Randomized controlled trials of interventions are rare in the older, frail population (11,12). In addition, observational studies are limited by the heterogeneity of disease status, medication use, impaired physical function, and poor overall health status of this population, as well as a lack of agreement on definitions of frailty (10). Frailty interventions that have shown potential include certain exercise interventions (7,9), drugs that increase muscle mass (13), mesenchymal stem cell transplantation (14), and individualized multidisciplinary care interventions (15). Re-purposing medications that are currently approved for use in humans is an appealing possibility. For example, there is evidence that angiotensin converting enzyme (ACE) inhibitors, which are commonly used antihypertensive agents, have a variety of beneficial effects even in those without cardiovascular disease. These include reduced inflammation (16,17), enhanced muscle strength (18), greater muscle mass (19), and better physical function (20). Whether ACE inhibitors can attenuate frailty has not been established.
A recent advance in frailty research has been the development of frailty assessment tools for use in aging animal models (21–24). Various FI tools have been widely used to quantify frailty in rodents since the concept was first proposed by our group in 2012 (4,25–32). FI scores measured in mice are associated with deleterious age-associated changes in the atria and ventricles (28–30). Higher FI scores have also recently been shown to be associated with lower survival probability in mice (4). Importantly, cross-sectional studies have shown that FIs can be used to assess the effect of interventions on frailty in animal models (31–33). However, there have been no longitudinal studies of the impact of interventions on mouse FI scores across the life course. ACE inhibitors such as enalapril have shown potential as frailty modulators in rodent studies, with beneficial effects on grip strength and muscle quality in aging rats (34). Losartan, which is an angiotensin II receptor blocker that has effects similar to those of ACE inhibitors, also improves activity and reduces inflammation in aging mice (35).
Most prior studies of frailty in animals have used male mice and few have investigated sex differences, especially in response to interventions. This is important because there are clear sex differences in human frailty, with females almost always more frail than males (2,36–38). A recently published study from our group showed that FI scores based on clinical measures were higher in female mice than male mice, while the opposite was seen for a new FI score based on abnormalities in standard lab measurements (39). Two cross-sectional interventional frailty studies in mice that did use both sexes found that two longevity-related drugs, rapamycin (33) and resveratrol (32), were more effective in attenuating frailty in male mice compared with females. Thus, it is critically important to explore responses to therapeutic interventions in both sexes.
The aim of this article was thus to investigate the effect of chronic treatment with the ACE inhibitor enalapril, on frailty longitudinally, in middle-aged and old male and female C57BL/6 mice. We also aimed to explore mechanisms of enalapril in modulating frailty, including potential effects on blood pressure and inflammation.
Methods
Animals
C57BL/6 male and female mice were purchased from Charles River as retired breeders and aged in the Carlton Animals Care Facility. This is a conventional, clean multispecies facility that houses specific pathogen free mice and rats. Mice were housed in Individually Ventilated Caging Systems (Allentown, Inc; 21°C; 35% humidity) and regular husbandry duties were performed in Animal Transfer Stations or Biological Safety Cabinets. Mice were aged until approximately 9 months for the middle-aged group (males n = 30, females n = 32) or approximately 16 months for the older group (males n = 38, females n = 28) and then started in the current study. Animals were maintained on a 12-hour light–dark cycle, in boxes of 1–5 mice per box with ad libitum access to food and water. Animals were initially fed Prolab RMH3000 (LabDiet, MO). Once they started the experiment, mice were fed Standard Grain-Based Control Rodent Diet with bacon-flavor (#F4059; Bio-Serve, Frenchtown, NJ) containing either enalapril (280 mg/kg) or no drug. All experiments were approved by the Dalhousie University Committee on Laboratory Animals and performed in accordance with guidelines published by the Canadian Council on Animal Care. Some of the raw data for the control mice in this study (n = 30) were used for analysis that has been reported previously (39).
The food in each cage was weighed twice per month to estimate food and drug intake. Total food consumed per cage was divided by the number of mice per cage, and the number of days to calculate average daily food intake per mouse. Mice were weighed once per month. Daily drug intake was calculated from daily food intake, and mouse weight (mg/kg mouse/day).
Mouse Clinical FI Assessment
Mice were assessed for frailty using the 31-item mouse clinical FI as described previously (26,27). Briefly, mice were taken to a quiet room and allowed to acclimatize. The clinical assessment of deficits was then completed on each mouse; mice with no deficit received a score of 0, those with a mild deficit received a score of 0.5, and mice with a severe deficit received a score of 1. Values for each deficit were then summed and divided by the total number of deficits measured to yield an FI score theoretically between 0 and 1. All mice were assessed at baseline (before starting on enalapril or control food) and FI scores were balanced between control and treatment groups for each age and sex. FI assessment was then completed monthly for the duration of the experiment. Middle-aged mice of both sexes were assessed every month from 9 to 13 months of age. Older female mice were assessed each month from 16 to 21 months of age. Older male mice were assessed each month from 16 to 25 months of age, to allow them to reach the same level of frailty as the 21-month-old female mice. The experimental timeline is illustrated in Supplementary Table 1.
Blood Pressure Assessment
Blood pressure was assessed using the IITC Life Sciences tail cuff (Woodland Hills, CA) as described previously (25). For all blood pressure measurements, we used an acclimatization protocol to reduce the stress to the mice. The mice were allowed to acclimatize to the machine, without measurements, for 20 minutes on two consecutive days. On the following two consecutive days mice were allowed 15 minutes to acclimatize, then 3–5 rounds of five blood pressure measurements were made for each mouse. An average of all measurements over 2 days was used to quantify blood pressure for each mouse. Blood pressure was assessed in all mice at baseline (before the start of treatment), 6–8 weeks after treatment started and at the end of the experiment (4 months after the start of treatment for middle-aged mice and 5 or 9 months after the start of treatment for older females and males, respectively). The timeline can be found in Supplementary Table 1.
Serum Collection and Analysis
Blood samples were taken from the heart after it was removed for other studies at the completion of the experiment. For middle-aged mice, blood samples were collected from 16 males and 10 females after 4 months of treatment (eg, 13 months of age). For the older group, blood samples were collected from 14 female mice at approximately 21 months of age and 21 male mice at 25 months of age (Supplementary Table 1).
Immediately after blood collection, the sample was spun at 4°C at 9391 Gs, and the serum was collected and stored at −20°C. Serum concentrations of 23 cytokines were assessed using a mouse 23-plex cytokine assay (BioRad) which was read using a Bio-Plex MAGPIX Multiplex Reader (BioRad). All cytokines measured are listed in Supplementary Table 2. If cytokine concentrations were below the level of detection, values were replaced with the lower limit of detection/2, as we have a small sample size and less than 50% of values below the level of detection (40–42).
Statistics
Data are expressed as mean ± SEM unless otherwise indicated. Frailty scores over time were compared for each age group between sexes and treatment groups using linear mixed models (heterogenous first order autoregressive) with fixed effects of intercept, time and treatment or sex, and random effect of slope. The effect of sex, treatment, and time for each age group were also investigated with linear mixed models (fixed effects intercept, time, sex, treatment; random effect slope). Least squared differences post hoc analysis was used for all linear mixed models. Proportions of individual deficits observed in sex or treatment groups, for each age group at a particular time point, were compared with chi-squared analysis. The effect of sex and treatment group on blood pressure was compared using two-way ANOVA, with Bonferroni post hoc tests. Serum cytokine levels were compared between treatment and age groups for each sex, with two-way ANOVA and Bonferroni post hoc tests. A three-way ANOVA for age, treatment, and sex was also used to assess the effects of both sex and treatment on these outcomes. Data analysis was completed with SPSS (Version 21.0, SPSS, Inc., Chicago, IL) and SigmaPlot (Version 11.0, Systat Software, Germany). p Values less than 0.05 are considered significant.
Results
Effect of Enalapril on Frailty in Middle-Aged and Older Male and Female Mice
Doses of enalapril consumed by each mouse group in the current study were very close to the predicted dose of 30 mg/kg and overall were not significantly different between groups. For middle-aged mice, the mean enalapril dose consumed across the 4 months of treatment was 29.7 ± 1.7 mg/kg/day for females, and 29.9 ± 1.4 mg/kg/day for males. In older mice, the mean enalapril dose consumed across the 5 months of treatment for females was 31.7 ± 2.4 mg/kg/day, and across 9 months of treatment for males was 28.6 ± 1.0 mg/kg/day.
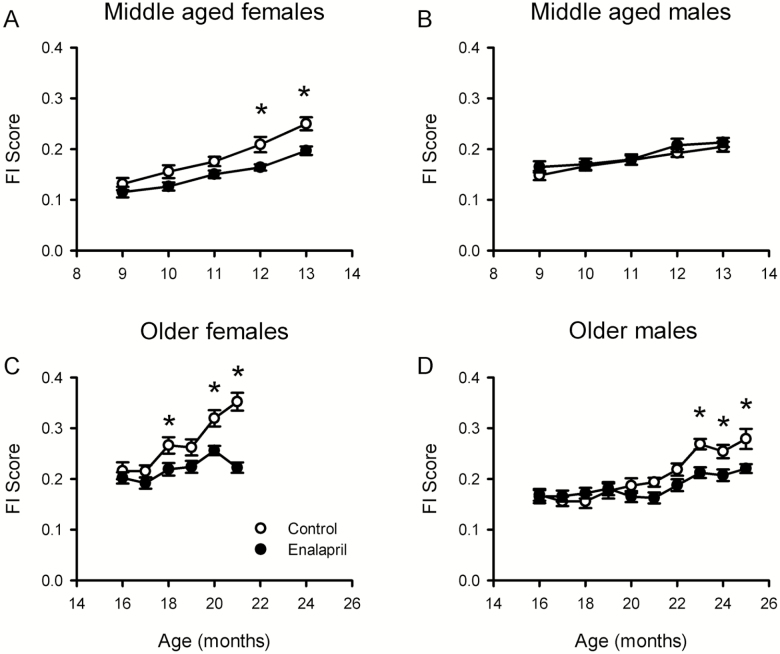
Enalapril treatment from 9 months of age attenuated the age-dependent increase in FI scores in middle-aged female mice (Figure 1A). Linear mixed model analysis demonstrated that the effects of time and treatment group on FI scores were significant. Post hoc analysis showed that female control mice had higher FI scores than those treated with enalapril and this difference was significant at 3 and 4 months of treatment. Interestingly, this effect appeared to be sex specific, as it was not seen in male mice of the same age, with linear mixed model analysis showing only an effect of time on mean FI score (Figure 1B). Thus, frailty scores increased in both sexes from 9 to 13 months of age, but enalapril treatment only attenuated frailty in females.
Figure 1.
Enalapril treatment delayed frailty in mice. (A, B) Mean ± SEM frailty index (FI) scores for enalapril-treated and control mice. Middle-aged female mice (A, n = 13–17) and middle-aged male mice (B, n = 14–15) were treated for 4 months. (C, D) Older female mice (C, n = 4–16) were treated for 5 months, and older male mice (D, n = 10–25) were treated for 9 months. Linear mixed model analysis was used to assess the effect of age and drug on FI score and enalapril dose. For FI scores, age and drug were both significant factors for middle-aged females, older females and older males. For middle-aged males only, age was significant. For enalapril dose, age was significant for middle-aged mice, and both age and sex were significant for older mice. *p < .05 compared with enalapril-treated group at the same time point.
We next determined whether enalapril attenuated FI scores when it was started in later life. When older female mice (16 months of age) were treated with enalapril for 5 months, FI scores were attenuated relative to controls (Figure 1C). Analysis showed a significant effect of time, treatment group, and their interaction on mean FI scores. Post hoc analysis showed that the treatment effect was significant after 2, 4, and 5 months. By contrast, when older males were treated with enalapril from 16 to 21 months of age, there was no significant treatment effect on frailty scores, although FI scores in control mice were higher than those in drug-treated animals at 20 and 21 months (Figure 1D). Given this, the male mice were investigated for a longer period to allow them to reach the same level of frailty as the older females. By 23 months of age, the control males had a mean FI score of 0.27 ± 0.01, which was similar to the females at age 18 months. Although FI scores increased with age in the control older male mice, this was markedly attenuated by enalapril treatment (Figure 1D). Linear mixed model analysis demonstrated that the effects of time, treatment, and their interaction on FI scores were significant (Figure 1D). Post hoc analysis showed that FI scores were significantly higher in control male mice than drug-treated mice at 23, 24, and 25 months of age. These findings indicate that, even though there were sex-specific differences in the development of frailty, chronic treatment with enalapril reduced FI scores in both sexes even when it was started in later life, and the effect of enalapril appeared to be greater in females than males.
Effect of Enalapril on Individual Items in the FI
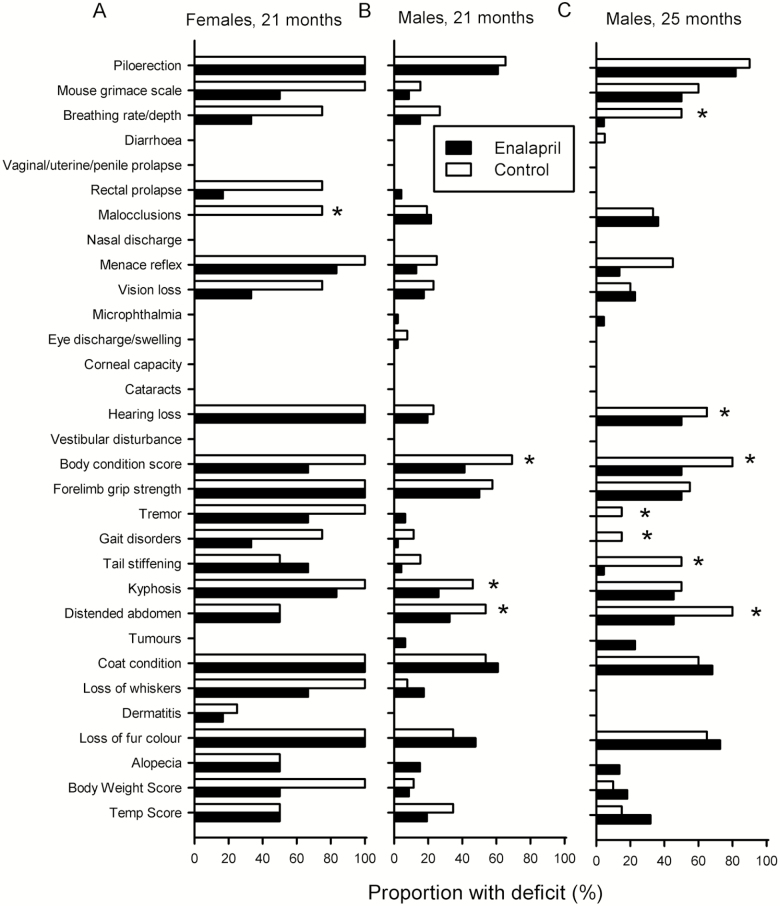
To determine whether enalapril improved certain deficits or groups of deficits rather than overall frailty, individual items in the FI were compared between control and enalapril-treated mice (Figure 2). There were no significant differences in the proportions of middle-aged mice with specific deficits in control and treated mice, except that more vision loss was seen in control females compared with drug-treated females (data not shown). In older females (21 months), only malocclusions occurred more often in controls than in drug-treated mice, although most deficits were reduced by enalapril treatment (Figure 2A). In 21-month-old males, there were significantly more distended abdomen, kyphosis, and body condition score deficits in control than in than drug-treated mice (Figure 2B). For 25-month-old males (with FI scores comparable to 21-month-old females), controls had a greater proportion of deficits in breathing rate, hearing loss, body condition score, tremor, gait disorders, tail stiffening, and distended abdomen than the control mice (Figure 2C). These data indicate that the beneficial effects of enalapril on overall frailty reflect the sum of many small treatment effects on a variety of individual deficits, rather than effects on one or two items. These effects also differ between the sexes.
Figure 2.
Control and enalapril-treated mice display a range of individual FI deficits. The proportion of mice scored for a specific deficit in enalapril-treated and control mouse groups. (A) Older females at 21 months of age, (B) older males at 21 months of age, and (C) older males at 25 months of age. (A) 6 drug, 4 control; (B) 23 drug, 12 control; (C) 11 drug, 10 control. *p < .05 with chi-squared analysis, compared with enalapril-treated group.
Effect of Enalapril on Blood Pressure in Middle-Aged and Older Male and Female Mice
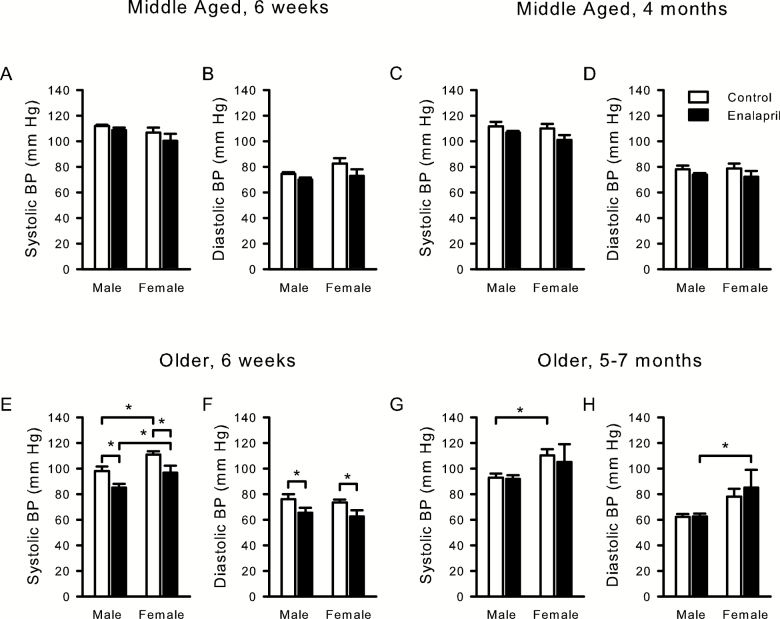
To determine whether antihypertensive effects of enalapril could contribute to its effect on frailty, blood pressure was measured in all groups after 6 weeks of treatment and at the end of treatment (Figure 3). Two-way ANOVA analysis of sex and treatment effects in middle-aged mice showed a significant effect of sex on systolic blood pressure (SBP), and of treatment on diastolic blood pressure (DBP) at 6 weeks, although post hoc analysis showed no differences (Figure 3A and B). Critically, by the end of the experiment when effects on frailty in females were detectable, there was no effect of treatment or sex on blood pressure in middle-aged mice (Figure 3C and D).
Figure 3.
Blood pressure in middle-aged and older mice treated with enalapril. Systolic and diastolic blood pressure (BP) measured in middle-aged (A–D) and older (E–H) mice either 6 weeks after treatment started (A, B, E, F) or at the end of treatment (4 months for middle-aged, C, D; 5/7 months for older mice, G, H). Two-way ANOVA, with post hoc analysis, was used to analyze the effects of treatment and sex on systolic and diastolic blood pressure at each time point. Middle-aged 6 weeks male 6 drug, 6 control and female 3 drug, 5 control; middle-aged end of treatment male 5 drug, 5 control and female 6 drug, 6 control; older 6 weeks male 13 control, 11 drug and female 9 control, 8 drug; older end of treatment male 13 control, 19 drug and female 4 control, 5 drug. *p < .05 compared with corresponding control group.
In older mice, at 6 weeks after the start of treatment, there was a significant effect of enalapril on both SBP and DBP. Post hoc analysis showed that enalapril reduced SBP and DBP in both males and females when compared with controls (Figure 3E and F). This suggests that the drug had mild blood pressure-lowering effects early in the course of treatment. However, by the end of treatment, there were no significant effects of enalapril on either SBP or DBP in older males or females (Figure 3G and H). This indicates that blood pressure normalized after five or more months of treatment and suggests that the effects of enalapril on frailty were unlikely to be due to effects on blood pressure.
There were also some sex differences in blood pressure, at least in the older mice. After 6 weeks of treatment, there was a significant effect of sex on SBP and, at the end of treatment, there was a significant effect of sex on both SBP and DBP. Post hoc analysis showed that older females had significantly higher SBP than older males at 6 weeks (Figure 3E and G), higher SBP at end of treatment in control mice only and higher DBP at the end of treatment in enalapril-treated mice only (Figure 3H).
Effect of Enalapril on Pro- and Anti-inflammatory Cytokine Levels in Aging Male and Female Mice
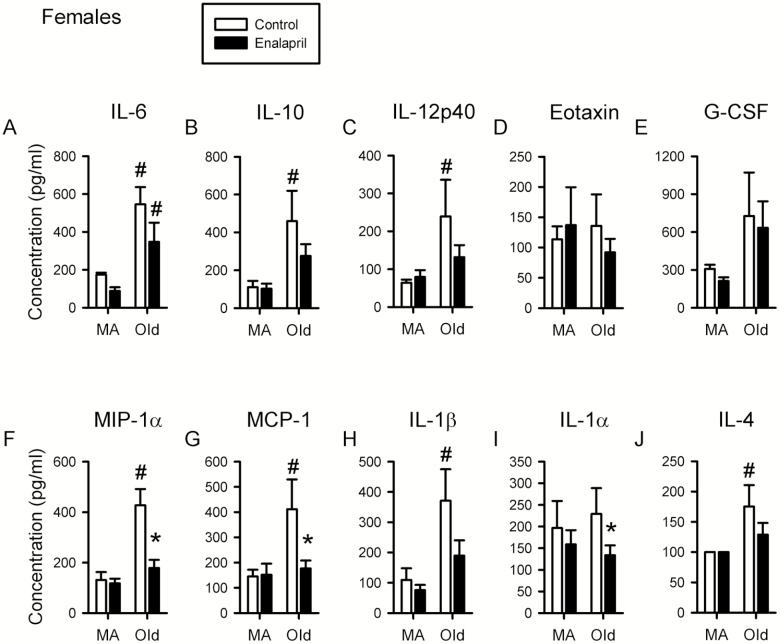
To determine whether the effects of enalapril on frailty may be partly due to effects on inflammation, serum levels of a variety of cytokines were measured (Supplemental Table 2). A two-way ANOVA was used to assess the effects of age and enalapril treatment on inflammatory cytokine levels in both sexes. In females, there was a significant effect of age on interleukin (IL)-6, IL-10, IL-12p40, IL-1β, IL-4, monocyte chemoattractant protein (MCP)-1, and macrophage inflammatory protein (MIP)-1α levels (Figure 4A–C, G, H, and J). Post hoc analysis revealed that each of these proinflammatory cytokines increased with age in control mice and that IL-6 also increased with age in drug-treated mice (Figure 4A). Interestingly, enalapril reduced the levels of most cytokines in the older female group and this effect was statistically significant for the cytokines MIP-1α and MCP-1 (Figure 4F and G). IL-1α was not significantly increased with age, but was reduced in enalapril-treated mice compared with control older female mice (Figure 4H).
Figure 4.
Proinflammatory cytokine levels are increased with age and attenuated by enalapril treatment in females. Levels of cytokines interleukin (IL)-6, IL-10, IL-12p40, eotaxin, granulocyte-colony stimulating factor (G-CSF), macrophage inflammatory protein (MIP)-1α, monocyte chemoattractant protein (MCP)-1, IL-1β, IL-1α, and IL-4 were assessed in serum from female mice (A–J). Levels for female mice were assessed at 13 months of age after 4 months of treatment (middle-aged) and at 21 months of age after 5 months of treatment (older). Two-way ANOVA, with post hoc analysis, was used to examine the effect of age and treatment on serum levels of each cytokine. Middle-aged female 6 drug, 5 control; older female 9 drug, 5 control. *p < .05 compared with corresponding control group. #p < .05 compared with corresponding middle-aged group.
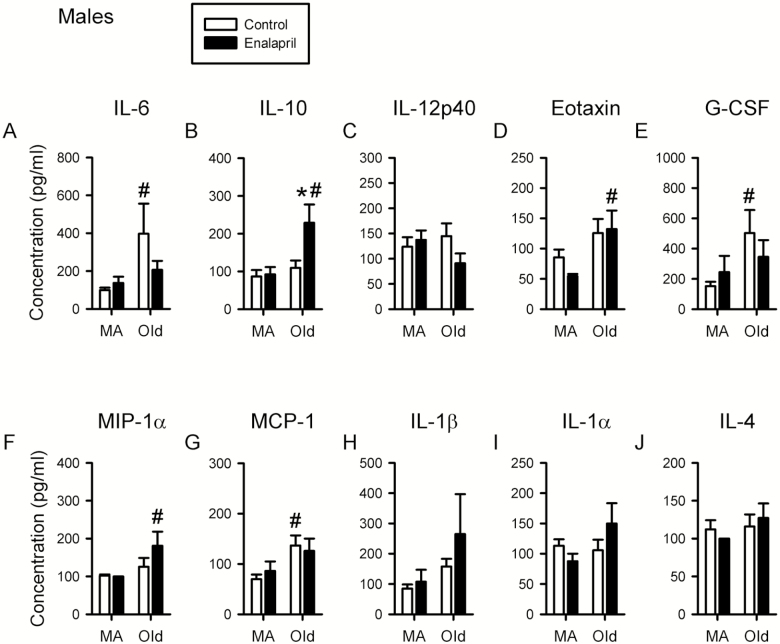
In males, there was a significant effect of age on IL-6, IL-10, IL-13, eotaxin, granulocyte-colony stimulating factor (G-CSF), MCP-1, and MIP-1α (Figure 5A, B, and D–G, Supplementary Table 2). Post hoc analysis showed that IL-6, IL-13, MCP-1 and G-CSF increased with age in control mice (Figure 5A, E, and G, Supplementary Table 2), while IL-10, eotaxin and MIP-1α increased with age in drug-treated mice (Figure 5B, D, and F). In terms of the effects of enalapril, there were clear sex-specific differences. In contrast to what was observed in females, enalapril treatment had little effect on the levels of proinflammatory cytokines in older males (Figure 5). However, enalapril treatment significantly increased levels of the anti-inflammatory cytokine IL-10 in older males (Figure 5B).
Figure 5.
Proinflammatory cytokine levels are increased with age in males but enalapril treatment increases anti-inflammatory cytokine levels. Levels of cytokines interleukin (IL)-6, IL-10, IL-12p40, eotaxin, granulocyte-colony stimulating factor (G-CSF), macrophage inflammatory protein (MIP)-1α, monocyte chemoattractant protein (MCP)-1, IL-1β, IL-1α, and IL-4 were assessed in serum from male mice (A–J). Cytokine levels for male mice were assessed in serum at 13 months of age after 4 months of treatment (middle-aged) and at 25 months of age after 7 months of treatment (older). Two-way ANOVA, with post hoc analysis, was used to examine the effect of age and treatment on serum levels of each cytokine. Middle-aged male 8 drug, 9 control; older male 12 drug, 9 control. *p < .05 versus corresponding control group. #p < .05 versus corresponding middle-aged group.
Mean data for all cytokines measured in males and females at both ages are shown in Supplementary Table 2. As a secondary analysis, a three-way ANOVA of age, drug, and sex was completed to investigate sex differences in cytokine levels. This showed a significant effect of sex on serum levels of IL-12p40, IL-10, IL-1α, MCP-1, and MIP-1α. Post hoc analysis showed that for each of these cytokines, levels were higher in older females than older males. Additionally, IL-1α levels were also higher in middle-aged females compared with middle-aged males (Supplementary Table 2). Taken together with the reduction in proinflammatory cytokine levels in older females, and increased anti-inflammatory cytokine levels in older males with enalapril treatment, these observations suggest that patterns of inflammation, and their response to treatment, differ in a sex-specific manner in aging mice.
Discussion
This study was the first to examine the longitudinal effect of an intervention on the FI in aging mice. Chronic treatment with the ACE inhibitor enalapril attenuated frailty in middle-aged and older females, and in older males. The mechanism of this protective effect did not appear to relate to effects on blood pressure. Modulation of inflammation by ACE inhibitor treatment may contribute to the protective effects of enalapril against frailty, and this occurred in a sex-specific manner.
Chronic treatment of female C57BL/6 mice with enalapril, whether in middle-age or advanced age, caused a marked reduction in frailty when compared with age-matched females who did not receive drug. Although enalapril had no effect on frailty in middle-aged male mice, drug treatment did attenuate FI scores in older male animals. This exciting result provides the first direct evidence that ACE inhibitors may delay the onset of frailty. Previous clinical and preclinical studies have suggested beneficial effects of ACE inhibitors on aspects related to frailty, such as muscle strength and physical function (18,20). This study shows that ACE inhibitors may also reduce the overall accumulation of health-related deficits with aging in mice. It is important to note that the FI tool used here is a measure of overall health and explores deficits across a range of different domains, but it does not contain measures of cardiovascular function per se. Investigation of changes in the specific items that make up the FI tool showed that enalapril treatment resulted in changes across a wide range of deficits in males and females. Therefore, the beneficial effect of enalapril is the sum of many small effects across multiple systems, which can be assessed with the mouse FI tool.
In the current study, there were clear male–female differences in the effects of enalapril on frailty. Enalapril-reduced FI scores in middle-aged female mice, but had no effect in age-matched males. In older males, beneficial effects on FI scores were only seen once mice had reached 23 months of age, after 7 months of treatment. One explanation for these sex-specific differences may be that the FI scores did not increase with age at the same rate in both sexes. For example, control females had a mean FI score of 0.25 ± 0.01 at 13 months of age, whereas males had a mean FI score of 0.20 ± 0.01 at the same age. In the older group, 18-month-old females had similar FI scores to 23-month-old males. This implies that male mice accumulated health deficits at a slower rate than females, and thus had lower FI scores at all ages assessed. It may be that significant attenuation of frailty with enalapril was not seen in males because the control male mice had not become frail enough yet. This male–female difference is consistent with what is seen in humans, where females are more frail than males at all ages (2,36–38). Although information is limited, three studies suggest similar findings in mice, with females having higher FI scores than males (26,33,39). These sex-specific findings highlight the importance of investigating interventions in both sexes.
Results of this study indicate that the mechanism of protection against frailty was unlikely to be related to the antihypertensive effects of enalapril. There was no effect of the drug on blood pressure in middle-aged mice, even though enalapril attenuated FI scores in females at this age. Six weeks of enalapril treatment did lower both SBP and DBP in older mice, which is consistent with drug exposure in the older mice, but changes in FI scores were not seen at this age. Blood pressure normalized after five plus months on treatment when effects on FI scores were evident, which strongly suggests that effects on frailty were not linked to antihypertensive effects of the drug. Interestingly, a clinical study of enalapril treatment in normotensive patients also reported that enalapril had no sustained effect on blood pressure, despite seeing improved physical function in drug-treated individuals (20).
A contributing mechanism to the attenuation of frailty with enalapril may be its effects on inflammation. In this study, chronic treatment with enalapril modified cytokines differentially in older male and female mice. In male mice, enalapril treatment resulted in an increase in the anti-inflammatory cytokine IL-10 compared with control mice. This implies that in older male mice, the upregulation of anti-inflammatory pathways may contribute to the reduction in frailty by enalapril. This is supported by human studies which have shown associations between increasing frailty and lower IL-10 levels (43,44). Additionally, Walston and colleagues (45) developed a mouse knock-out for IL-10, which they propose is a mouse model of frailty. Knock-out mice display higher levels of proinflammatory cytokines and reduced muscle strength, along with extremely low levels of IL-10 (45–47). Interestingly, we did not see a decrease in proinflammatory cytokines in older enalapril-treated male mice. There were trends toward this however with IL-6, IL-13, MCP-1, and G-CSF increasing with age in control but not drug-treated male animals. A larger sample size for serum collection may allow this effect to be seen more clearly. In contrast to the older males, enalapril reduced levels of the proinflammatory cytokines IL-1α, MCP-1, and MIP-1α in older females. Interestingly, there is evidence for links between higher levels of MIP-1α and frailty in humans, although no sex effect was observed (48). Increasing MCP-1 was also shown in a recent study to be associated with age in male and female rodents and with both age and frailty in humans of both sexes (49). IL-1α is a key senescence-associated cytokine so would be expected to be associated with aging and frailty (50,51). Higher levels of IL-1α in females compared with males have been seen in young healthy humans, and in in vitro studies (52,53), although the relationship between IL-1α levels, frailty, and sex has not yet been explored. Interestingly, previous studies have shown that there are clear sex differences in response to life span-extending compounds in mice, with compounds that target inflammation such as aspirin and nordihydroguaiaretic acid increasing life span in males but not females (54). It is interesting that we see an effect of enalapril, which targets inflammation, on frailty in both females and males. It would be interesting to investigate further the effects of enalapril on life span, and conversely the effects of other life span-increasing anti-inflammatory drugs on frailty to understand the effect of sex on the potential interaction, or disconnect, between health span and life span extension. Overall, our results support the view that inflammation plays an important role in frailty, and that enalapril may act to attenuate frailty by increasing anti-inflammatory cytokines in male mice, and decreasing proinflammatory cytokines in female mice.
On the other hand, enalapril treatment had no significant effect on inflammatory cytokines in middle-aged female mice, despite the reduction in FI scores in females with treatment. This suggests that other mechanisms may contribute to the attenuation of frailty by enalapril. As would be expected from results of clinical studies (55–58), levels of many inflammatory cytokines in the current study were higher in older mice, than in middle-aged mice. It is possible, therefore that enalapril may only have an effect on inflammation once it is dysregulated in aging. Given the multidimensional nature of frailty it is likely that interventions that target multiple systems would have the greatest beneficial effects. Other mechanisms that may contribute to protective effects of enalapril on frailty could include effects on muscle, which have been seen in human studies with ACE inhibitors (59,60), or beneficial effects on the heart and vasculature that are not related to blood pressure (61). Future studies should explore these mechanisms further.
Other animal studies exploring interventions for frailty have also seen potentially beneficial effects. Kane and colleagues (32) investigated the effect of either calorie restriction or resveratrol on mouse clinical FI scores in a cross-sectional study and found beneficial effects of both interventions in male mice. Antoch and colleagues (33) used a different rodent FI tool, and saw that a high fat diet increased FI scores in male mice, while rapamycin prevented this increase in frailty. Two studies using mouse assessments based on frailty phenotype tools, which assess a person or mouse as frail based on their performance in five functional tests, found that exercise interventions could also delay the onset of frailty (62,63). Along with the current study, these studies demonstrate the value of animal frailty assessment tools in screening potential clinical interventions for the treatment of frailty.
In addition to the sex differences in frailty observed in this study, we also saw sex differences in blood pressure and inflammation with aging. Older females had higher SBP than older males when measured at both timepoints. This supports observations in humans that show females are more likely to have higher SBP than males as they age (64). We also observed clear sex differences in inflammation with age. Levels of the proinflammatory cytokines IL-12p40, MIP-1α, MCP-1, and IL-1α, and the anti-inflammatory cytokine IL-10 were all higher in older female mice than older male mice. This implies that, with aging, there may be more inflammation in female mice than in male mice. Again, this is consistent with observations in people, where inflammation is higher in females than males at all ages (37,65). A recent study from our group found sex differences in the association between frailty and inflammatory cytokines in aged male and female mice (39). In older female mice, increasing frailty scores were associated with increased levels of IL-6, IFN-γ and IL-9, while increasing frailty in older males was associated with increasing IL-12p40 levels (39). Interestingly, IL-6 was also increased with age, and appeared to be attenuated by enalapril treatment in female mice in the current study, suggested a particular role of IL-6 in female aging and frailty. Gordon and Hubbard (66) propose that increased inflammation in aging females may contribute to their higher levels of frailty when compared with aging males. It is also possible that if enalapril acts, at least in part, by reducing inflammation, that this sex difference in age-related inflammation may explain why the drug works at an earlier time point in females in than males.
Although this study provides preclinical evidence for the potential use of ACE inhibitors to attenuate frailty in humans, there are still several questions that remain to be answered. The optimal time to start treatment, and the optimum treatment time-frame are still not clear. The observation that frailty was attenuated in female mice started at both 9 and 16 months of age suggests that treatment is still beneficial even if started later in life. It would be interesting in future studies to determine whether enalapril could reverse higher levels of frailty, by starting treatment once mice have higher FI scores. Additionally, it would be interesting to stop treatment and observe whether the beneficial effects persist beyond the treatment period.
There are some limitations to this study that should be acknowledged. The limited sample sizes for some of the blood pressure measurements may reduce the power to conclude whether or not there were blood pressure changes with treatment and across sexes. Although our results are statistically significant, they should be confirmed with larger sample numbers. Furthermore, the distribution of the cytokine data were not always completely normal when assessed with Shapiro–Wilk tests. Even so, we have used two- and three-way ANOVA analysis for this data given the robustness of the ANOVA for not completely normal data distribution (67). Our study design did not allow us to determine whether enalapril treatment extended life span, because in most cases the mice were censored as tissue was collected to use in other experiments. It would be interesting to determine whether enalapril treatment affected life span as well as health span in future studies.
In conclusion, this study found that chronic enalapril treatment attenuated frailty in middle-aged and older females, and in older males. Sex-specific effects on inflammation may contribute, at least in part, to the mechanisms underlying this protection. This article highlights the importance of using both males and females in aging and intervention studies, and provides preclinical evidence that enalapril may delay the onset of frailty, even when started later in life.
Funding
S.E.H. was supported by Canadian Institutes of Health Research grants (MOP 126018 and MOP 97973). A.E.K. was supported by a Reynolds Post-Doctoral Fellowship from the Department of Pharmacology.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgments
The authors are grateful to Peter Nicholl for expert technical assistance.
References
- 1. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 2. Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123:1457–1460. doi: 10.1016/S0047-6374(02)00082-9 [DOI] [PubMed] [Google Scholar]
- 3. Rockwood K, Song X, Mitnitski AB. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Can Med Assoc J. 2011;138:487–494. doi: 10.1503/cmaj.110626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rockwood K, Blodgett JM, Theou O, et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. 2017;7:43068. doi: 10.1038/srep43068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci. 2004;59:1310–1317. doi: 10.1093/gerona/59.12.1310 [DOI] [PubMed] [Google Scholar]
- 6. Cesari M, Prince M, Thiyagarajan JA, et al. Frailty: an emerging public health priority. J Am Med Dir Assoc. 2016;17:188–192. doi: 10.1016/j.jamda.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 7. Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46:383–392. doi: 10.1093/ageing/afw247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeffery CA, Shum DW, Hubbard RE. Emerging drug therapies for frailty. Maturitas. 2013;74:21–25. doi: 10.1016/j.maturitas.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 9. Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143. doi: 10.1016/j.pcad.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 10. Cesari M, Fielding R, Benichou O, et al. Pharmacological interventions in frailty and sarcopenia: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2015;4:114–120. doi: 10.14283/jfa.2015.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med. 2011;26:783–790. doi: 10.1007/s11606-010-1629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Konrat C, Boutron I, Trinquart L, Auleley GR, Ricordeau P, Ravaud P. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. PLoS One. 2012;7:e33559. doi: 10.1371/journal.pone.0033559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bendayan M, Bibas L, Levi M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part II. Ongoing and unpublished randomized trials. Prog Cardiovasc Dis. 2014;57:144–151. doi: 10.1016/j.pcad.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 14. Tompkins BA, DiFede DL, Khan A, et al. Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase II randomized, double-blind, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2017;72:1513–1522. doi: 10.1093/gerona/glx137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cameron ID, Fairhall N, Langron C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang Y, Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol. 2015;179:137–145. doi: 10.1111/cei.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/S0140-6736(02)08024-8 [DOI] [PubMed] [Google Scholar]
- 19. Di Bari M, van de Poll-Franse LV, Onder G, et al. ; Health, Aging and Body Composition Study. Antihypertensive medications and differences in muscle mass in older persons: the health, aging and body composition study. J Am Geriatr Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x [DOI] [PubMed] [Google Scholar]
- 20. Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177:867–874. doi: 10.1503/cmaj.061339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kane AE, Hilmer SN, Mach J, Mitchell SJ, de Cabo R, Howlett SE. Animal models of frailty: current applications in clinical research. Clin Interv Aging. 2016;11:1519–1529. doi: 10.2147/CIA.S105714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howlett SE. Assessment of frailty in animal models. Interdiscip Top Gerontol Geriatr. 2015;41:15–25. doi: 10.1159/000381131 [DOI] [PubMed] [Google Scholar]
- 23. Seldeen KL, Pang M, Troen BR. Mouse models of frailty: an emerging field. Curr Osteoporos Rep. 2015;13:280–286. doi: 10.1007/s11914-015-0283-y [DOI] [PubMed] [Google Scholar]
- 24. Mohler MJ, Fain MJ, Wertheimer AM, Najafi B, Nikolich-Žugich J. The frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol. 2014;54:6–13. doi: 10.1016/j.exger.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 25. Parks RJ, Fares E, Macdonald JK, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67:217–227. doi: 10.1093/gerona/glr193 [DOI] [PubMed] [Google Scholar]
- 26. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi: 10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feridooni HA, Sun MH, Rockwood K, Howlett SE. Reliability of a frailty index based on the clinical assessment of health deficits in male C57BL/6J mice. J Gerontol A Biol Sci Med Sci. 2015;70:686–693. doi: 10.1093/gerona/glu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feridooni HA, Kane AE, Ayaz O, et al. The impact of age and frailty on ventricular structure and function in C57BL/6J mice. J Physiol. 2017;594(23):7105–7126. doi: 10.1113/JP274134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moghtadaei M, Jansen HJ, Mackasey M, et al. The impacts of age and frailty on heart rate and sinoatrial node function. J Physiol. 2016;00:1–22. doi: 10.1113/JP272979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jansen HJ, Moghtadaei M, Mackasey, et al. Atrial structure, function and arrhythmogenesis in aged and frail mice. Sci Rep. 2017;7:44336. doi: 10.1038/srep44336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huizer-Pajkos A, Kane AE, Howlett SE, et al. Adverse geriatric outcomes secondary to polypharmacy in a mouse model: the influence of aging. J Gerontol A Biol Sci Med Sci. 2016;71:571–577. doi: 10.1093/gerona/glv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kane AE, Hilmer SN, Boyer D, et al. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol Ser A Biol Sci Med Sci. 2016;71:333–339. doi: 10.1093/gerona/glu315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Antoch MP, Wrobel M, Kuropatwinski KK, et al. Physiological frailty index (PFI): quantitative in-life estimate of individual biological age in mice. Aging (Albany NY). 2017;9:615–626. doi: 10.18632/aging.101206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carter CS, Giovannini S, Giovaninni S, et al. Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 × Brown Norway rats. Age (Dordr). 2011;33:167–183. doi: 10.1007/s11357-010-9196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin CH, Yang H, Xue QL, et al. Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp Gerontol. 2014;58:174–178. doi: 10.1016/j.exger.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65:246–255. doi: 10.1093/geronb/gbp102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. doi: 10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 38. Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53:40–47. doi: 10.1111/j.1532-5415.2005.53008.x [DOI] [PubMed] [Google Scholar]
- 39. Kane AE, Keller K, Heinze-Milne S, Grandy S, Howlett SE. A murine FI based on clinical and laboratory measurements: links between frailty and pro-inflammatory cytokines differ in a sex-specific manner. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly117. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hewett P, Ganser GH. A comparison of several methods for analyzing censored data. Ann Occup Hyg. 2007;51:611–632. doi: 10.1093/annhyg/mem045 [DOI] [PubMed] [Google Scholar]
- 41. Ogden TL. Handling results below the level of detection. Ann Occup Hyg. 2010;54:255–256. doi: 10.1093/annhyg/mep099 [DOI] [PubMed] [Google Scholar]
- 42. Ganser GH, Hewett P. An accurate substitution method for analyzing censored data. J Occup Environ Hyg. 2010;7:233–244. doi: 10.1080/15459621003609713 [DOI] [PubMed] [Google Scholar]
- 43. Van Den Biggelaar AHJ, Huizinga TWJ, De Craen AJM, et al. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp Gerontol. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 44. Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16:249–252.doi: 10.1007/BF03327392 [DOI] [PubMed] [Google Scholar]
- 45. Walston J, Fedarko N, Yang H, et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ko F, Yu Q, Xue QL, et al. Inflammation and mortality in a frail mouse model. Age (Dordr). 2012;34:705–715. doi: 10.1007/s11357-011-9269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akki A, Yang H, Gupta A, et al. Skeletal muscle ATP kinetics are impaired in frail mice. Age (Dordr). 2014;36:21–30. doi: 10.1007/s11357-013-9540-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Su L, Hao Q-KK, Liu S, Dong B-RR. Monocytes related inflammatory biomarkers are associated with frailty syndrome. Int J Gerontol. 2017;11:225–229. doi: 10.1016/j.ijge.2017.08.004 [DOI] [Google Scholar]
- 49. Yousefzadeh MJ, Schafer MJ, Noren Hooten N, et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell. 2018;(October):1–7. doi: 10.1111/acel.12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCarthy DA, Clark RR, Bartling TR, Trebak M, Melendez JA. Redox control of the senescence regulator interleukin-1α and the secretory phenotype. J Biol Chem. 2013;288:32149–32159. doi: 10.1074/jbc.M113.493841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mariotti M, Castiglioni S, Bernardini D, Maier JA. Interleukin 1 alpha is a marker of endothelial cellular senescent. Immun Ageing. 2006;3:4. doi: 10.1186/1742-4933-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lynch EA, Dinarello CA, Cannon JG. Gender differences in IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist secretion from mononuclear cells and urinary excretion. J Immunol. 1994;153:300–306. [PubMed] [Google Scholar]
- 53. Sadeghi M, Daniel V, Naujokat C, Weimer R, Opelz G. Strikingly higher interleukin (IL)-1alpha, IL-1beta and soluble interleukin-1 receptor antagonist (sIL-1RA) but similar IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, tumour necrosis factor (TNF)-alpha, transforming growth factor (TGF)-beta and interferon IFN-gamma urine levels in healthy females compared to healthy males: protection against urinary tract injury?Clin Exp Immunol. 2005;142:312–317. doi: 10.1111/j.1365-2249.2005.02924.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Austad SN, Bartke A. Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology. 2016;62:40–46 doi: 10.1159/000381472 [DOI] [PubMed] [Google Scholar]
- 55. Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Epps P, Oswald D, Higgins PA, et al. Frailty has a stronger association with inflammation than age in older veterans. Immun Ageing. 2016;13:27. doi: 10.1186/s12979-016-0082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 58. Li H, Manwani B, Leng SX. Frailty, inflammation, and immunity. Aging Dis. 2011;2:466–473. [PMC free article] [PubMed] [Google Scholar]
- 59. Cozzoli A, Nico B, Sblendorio VT, et al. Enalapril treatment discloses an early role of angiotensin II in inflammation- and oxidative stress-related muscle damage in dystrophic mdx mice. Pharmacol Res. 2011;64:482–492. doi: 10.1016/j.phrs.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sartiani L, Spinelli V, Laurino A, et al. Pharmacological perspectives in sarcopenia: a potential role for renin-angiotensin system blockers?Clin Cases Miner Bone Metab. 2015;12:135–138. doi: 10.11138/ccmbm/2015.12.2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nissen SE, Tuzcu EM, Libby P, Persell SD. Treatment of normotensive coronary patients with amlodipine or enalapril: effects on cardiovascular outcomes. J Clin Outcomes Manag. 2005;12:78–79. [Google Scholar]
- 62. Graber TG, Ferguson-Stegall L, Liu H, Thompson LV. Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci. 2015;70:1045–1058. doi: 10.1093/gerona/glu163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, et al. A new frailty score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J Gerontol A Biol Sci Med Sci. 2017;72:885–891. doi: 10.1093/gerona/glw337 [DOI] [PubMed] [Google Scholar]
- 64. Pinto E. Blood pressure and ageing. Postgrad Med J. 2007;83:109–114. doi: 10.1136/pgmj.2006.048371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci. 2011;66:493–500. doi: 10.1093/gerona/glr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gordon EH, Hubbard RE. The pathophysiology of frailty: why sex is so important. J Am Med Dir Assoc. 2018;19:4–5. doi: 10.1016/j.jamda.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 67. Lix LM, Keselman JC, Keselman HJ. Consequences of assumption violations revisited : a quantitative review of alternatives to the one-way analysis of variance “F” test. Rev Educ Res. 1996;66:579–619 doi: 10.3102/00346543066004579 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.