Abstract
Background
The Enabling Reduction of Low-grade Inflammation in Seniors (ENRGISE) Pilot Study is a multicenter randomized clinical trial examining the feasibility of testing whether omega-3 fish oil (ω-3) and the angiotensin receptor blocker losartan alone or in combination can reduce inflammation and improve walking speed in older adults with mobility impairment. We describe recruitment methods and results.
Methods
Eligible participants were 70 years and older, had elevated interleukin-6 levels (2.5–30 pg/mL) and mobility impairment.
Results
Of those who responded to recruitment, 83% responded to mailings. A total of 5,424 telephone screens were completed; of these, 2,011 (37.1%) were eligible for further screening. The most common reasons for ineligibility at the telephone screens were lack of mobility impairment or use of angiotensin receptor blockers or angiotensin-converting enzyme inhibitors (n=1.789). Of the 1,305 initial screening visits, 1,087 participants had slow gait speed (<1 m/s). Of these, 701 (64%) had elevated interleukin-6 and were eligible for second screening visits. Of the 582 second screening visits, 335 (57.6%) were eligible to be randomized. A total of 289 participants (96% of goal) were randomized: 180 in the ω-3 stratum (240% of goal); 43 in the losartan (57% of goal), and 66 in the combination (44% of goal). The telephone screen and first screening visit to randomization ratio was 19 to 1 and 4.5 to 1, respectively. The estimated cost of recruitment per randomized participant was $1,782.
Conclusion
Recruitment for ω-3 exceeded goals, but goals for the losartan and combination strata were not met due to the high proportion of participants taking angiotensin receptor blockers or angiotensin-converting enzyme inhibitors.
Keywords: Inflammation, Clinical trials, Functional performance, Physical function, Mobility impairment
In randomized clinical trials, recruitment difficulties can delay results and adversely affect budgets. Falling short of recruitment goals reduces statistical power and the trial’s capacity to fully characterize safety and subgroup response to treatment. Yet, recruitment challenges in randomized trials are common (1). A report noted that 25% of randomized trials were discontinued, 40% of these because of poor recruitment (1). Recruitment can be especially challenging in older adult populations (2,3). Older persons have more comorbidities (4), take more medications and have more frequent hospitalizations, all of which may disqualify them. Sensory deficits, cognitive impairment, caregiving responsibilities, and access to transportation may interfere. Yet, if research is to inform clinical practice, it should include the target population to the extent possible. The European Medicines Agency highlighted the need to enroll older participants, especially, those aged 75 and older into randomized clinical trials (5).
In this report, we describe the screening methods and recruitment results of the Enabling Reduction of Low-Grade Inflammation in Seniors (ENRGISE) Pilot Study. ENRGISE is a multicenter, double-blinded randomized pilot clinical trial designed to test two interventions to reduce interleukin (IL)-6 levels, an indicator of inflammation and an independent risk factor for impaired mobility and slow walking speed in older adults (6). Target enrollment was 300 men and women aged 70 or older with an average plasma IL-6 level between 2.5 and 30 pg/mL and low to moderate mobility impairment. Results from the ENRGISE Pilot Study will provide recruitment yields, feasibility, medication tolerance and adherence, and preliminary data to help justify a sample size for a more definitive randomized trial. ENRGISE is registered at clinicaltrials.gov (identifier NCT01072500).
Methods
The ENRGISE pilot is ongoing at five U.S. medical centers, Northwestern University, Tufts University, University of Florida, University of Pittsburgh, and Wake Forest School of Medicine, and was approved by the Institutional Review Boards at each center. All participants provided written informed consent. The study design for the ENRGISE pilot has been published (6). Participants were eligible if they were age 70 or older who self-reported difficulty in walking a quarter mile or climbing a flight of stairs, had a usual walking speed of less than 1 m/s on a 4-m walk, and had evidence of chronic low-grade inflammation (IL-6 level of 2.5–3.0 pg/mL averaging two measures taken 1–3 weeks apart).
Complete eligibility criteria have previously been described (6). Participants were excluded if they reported acute infection, autoimmune disease, severe arthritis, or a neurological condition that caused slow walking speed. Initially, participants were also excluded for having low vitamin D levels (serum level of 25-hydroxyvitamin D < 20 ng/mL), as required by National Institutes of Health funding announcement (RFA-AG-15-006). This requirement was dropped one third of the way through the recruitment period because of a lack of evidence that low vitamin D levels affect the ability of the interventions to reduce inflammation and mobility impairment. Those who took an angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or potassium-sparing diuretics were excluded from losartan (LO) and combination randomization. Those who consumed more than two servings per week of fish in the past year or were taking fish oil were excluded from ω-3 randomization.
Eligible participants were randomized to LO, omega-3 fish oil (ω-3), and combined LO and ω-3 in three strata each of which was placebo controlled. Randomization was stratified depending on eligibility for each group.
Study Recruitment Goals
The recruitment goal was 300 participants in three strata: n = 75, ω-3 or placebo stratum; n = 75, LO or placebo stratum; and n = 150, LO alone, ω-3 alone, combination LO plus ω-3 or placebo stratum. As described in the ENRGISE design paper (6), the target sample size (N = 300) was determined based on marginal comparisons (135 and 165 per group) between each active intervention and placebo using a one-sided hypothesis test at the 10% level. The goal was not to provide definitive evidence but to exclude small effects that would have lower clinical value. For IL-6, there was 91% power to detect a difference if the difference was at least a 15% difference. Recruitment targets were approximately 69% female, 20% racial minorities, including 5% of Hispanic ethnicity, which reflected the population distribution of these subgroups in the catchment areas.
Recruitment Strategies
A recruitment committee met bimonthly to monitor enrollment, to share recruitment strategies, and to generate innovative ideas for recruitment. The ENRGISE Administrative Coordinating Center (ACC) at University of Florida developed recruitment materials. Each clinical site used strategies based on their previous experience and used the central materials or developed their own clinic-specific materials. Each site varied slightly in their approaches but most relied on mass mailings: Northwestern, postcards; Tufts University, brochures; University of Florida, brochures; University of Pittsburgh, brochures and cover letter; and Wake Forest, site-specific brochure/postcard. Toll-free numbers were established at each center. Other recruitment strategies include electronic medical records (EMR), TV, print and radio advertisement, posters, and referral from individual practitioners. Clinical sites used specific recruitment strategies to target minorities including remailing to zip codes with a high proportion of minorities, community screenings, and outreach to minority leaders in the community.
Screening and Randomization
Participants who contacted the clinical center were screened for eligibility by telephone. Eligible and interested individuals were invited to screening visit 1 (SV1). Blood was drawn for IL-6 in those participants whose pace to complete the 4-m walk was less than 1 m/s. If the IL-6 was 2.3–30.0 pg/mL, individuals were invited to return for a second screening visit (SV2). At SV2, blood pressure and cognitive function were measured and medical history was obtained. Participants attempted the 400-m walk and those who required more than 15 minutes to complete the walk at normal pace were excluded. If participants met the eligibility criteria, a blood sample was obtained for repeat IL-6 and safety laboratory tests. If the average IL-6 at SV1 and SV2 was 2.5–30.0 pg/mL and laboratory tests were within eligibility parameters, participants were eligible for randomization.
Estimation of Recruitment Costs
Information on recruitment costs were collected by the ENRGISE ACC including information on the percent effort of the principal investigator, recruitment coordinator, and other recruitment staff. We collected the cost of mailings/postage (bulk mailings, company used to carry out the mailings, first-class mailings, returned postcard postage, preparation of mailings, and purchase of mailing lists). Printing costs (brochures, postcards, posters, cover letter) and advertising costs (print, radio, TV) were also collected. Other costs include events (mileage) and changes to advertisements. For personnel cost, because salaries vary across the sites, the percent effort per randomized participant was calculated per site and overall.
Statistical Analyses
Descriptive statistics were calculated. Categorical characteristics of participants were compared across stratum using chi-square tests, and analysis of variance was used for continuous variables. We compared the enrollment of minorities before and after the vitamin D exclusion was removed by chi-square test.
Results
Screening began in March 2016. The first participant was randomized on April 26, 2016 and the last on June, 30 2017. Direct mail using either a brochure or postcard was the most common: 83% of participants who contacted the site did so in response to mailings (Table 1). Mailing lists included purchased lists, voter registration lists, motor vehicle license lists, and research registry participant lists. The most common advertising medium was print. Several sites identified participants using EMR. Although only 253 (4.7%) participants were telephone screened in response to an EMR contact, the percent yield for randomization from this approach was 7.5% compared with 4.9% for mass mailings. The yield was slightly higher when a full brochure was mailed (5.8%) compared with a postcard (3.6%). Approximately 7.8% of participants were recruited from paid advertisements and 19 (8.9%) learned about ENRGISE because they were previously enrolled in another study at the clinical site.
Table 1.
ENRGISE Pilot Study Recruitment Sources and Strategies
| Recruitment Strategy | Percentage of Telephone Screens (%)a | Recruitment Yield: n (%) of Randomized Participantsb |
|---|---|---|
| Mass mailing | 83.3 | 222 (4.9) |
| Brochure | 48.7 | 154 (5.8) |
| Postcard | 34.7 | 68 (3.6) |
| Flyer (poster) | 1.1 | 2 (3.5) |
| Letter (EMR) | 4.7 | 19 (7.5) |
| Advertisements | 4.0 | 17 (7.8) |
| 3.2 | 13 (7.4) | |
| TV | 0.4 | 2 (10.5) |
| Radio | 0.3 | 2 (11.1) |
| Magazine | 0.1 | — |
| Referral | 2.1 | 5 (4.4) |
| Follow-up call | <0.1 | — |
| Another study | 3.9 | 19 (8.9) |
| Other | 1.4 | 7 (9.1) |
| Don’t know/refused | 0.4 | — |
Notes: aPercentage of participants who reported at the telephone screen how they heard about the study.
bOf those who heard about the study by each source, the n (%) randomized.
An overview of the steps from screening to randomization is shown in Figure 1. In total, 5,424 telephone screens were completed. Of these, 2,011 (37.1%) were eligible for SV1. Common reasons for exclusion at the telephone screen were no self-reported difficulty walking ¼ mile or climbing stairs (n = 1,183), current use of ACEIs or ARBs within 2 months (n = 623), currently taking fish oil and/or ω-3 (n = 344), use of a walker to get around (n = 246), known/active inflammatory disease (n = 230), prior or current atrial fibrillation (n = 229), reported inability to walk one block (n = 204), or reported current smoking (n = 185).
Figure 1.
ENRGISE Pilot Study: screening to randomization funnel.
Of the 2,011 eligible participants at the telephone screen, 1,305 participants attended SV1 (65% of telephone screen eligible). A total of 1,087 (83%) were eligible by the 4-m walk, and of these, 701 (64%) were eligible by IL-6 measured at SV1. A total of 582 participants attended SV2 (83% of SV1 eligible). Of these, 448 (77%) were eligible by the second IL-6 measurement and 335 (57.6%) were eligible for baseline. Of the 307 participants who attended the baseline randomization (92% of SV2 eligible), 294 were eligible to be randomized (88% of SV2 eligible), and 289 were randomized representing 98% of those eligible to be randomized at baseline.
The telephone screen to randomization ratio was approximately 19 to 1. The telephone screen to randomization ratio varied by clinical site (Table 2). For example, at Wake Forest School of Medicine, 13 participants were screened by telephone to randomize 1 participant, whereas at Northwestern University, 30 participants were screened by telephone to randomize 1 participant. Approximately, four to five participants were screened in the clinic (SV1) for every one randomization. Similar to the results of the telephone screen, there was variability across sites with Wake Forest School of Medicine randomizing one of every three participants at SV1 and Northwestern University randomizing one of every six participants.
Table 2.
ENRGISE Pilot Study Recruitment Screening Yields
| Telephone Screen | SV1 | SV2 | Baseline | Randomized | Telephone Screen to Randomizaiton Ratio | SV1 to Randomization Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Site | Number of Visits | Number of Eligible Participants | Number of Visits | Percentage of Telephone Screen Eligible Participants | Number of Eligible Participants | Number of Visits | Percentage of SV1 Eligible Participants | Number of Eligible Participants | Number of Visits | Percentage of SV2 Eligible Participants | Number of Eligible Participants | Number of Randomized Participants | ||
| All clinics | 5,424 | 2,011 | 1,305 | 65 | 701 | 582 | 83 | 335 | 307 | 92 | 294 | 289 | 19 | 4.5 |
| Wake Forest School of Medicine | 738 | 290 | 180 | 62 | 101 | 81 | 80 | 60 | 58 | 97 | 58 | 56 | 13 | 3.2 |
| Northwestern University | 1,447 | 458 | 308 | 67 | 144 | 128 | 89 | 52 | 50 | 96 | 49 | 48 | 30 | 6.4 |
| Tufts University | 800 | 356 | 243 | 68 | 139 | 106 | 76 | 67 | 66 | 99 | 63 | 63 | 13 | 3.8 |
| University of Pittsburgh | 1,334 | 447 | 250 | 56 | 153 | 123 | 80 | 73 | 62 | 85 | 59 | 58 | 23 | 4.3 |
| University of Florida | 1,105 | 450 | 324 | 80.5 | 164 | 144 | 93.5 | 83 | 71 | 83 | 65 | 64 | 17 | 5.0 |
More minority participants were eligible at SV2 after the 25-hydroxyvitamin D exclusion criterion was eliminated. Prior to the removal, there were 33 minority participants eligible at SV2 (16% of all participants eligible). After the vitamin D exclusion was removed, there were 39 minority participants eligible (30% of all participants eligible), a significant improvement, p = 0.004. Use of ACEI or ARB within 2 months was similar in minority participants and non-minority participants. The prevalence of smoking was slightly higher among minority participants than overall. A lower proportion of minority participants had too low blood pressure at SV2 compared with overall. Otherwise, the proportion excluded for various reasons did not differ by minority status.
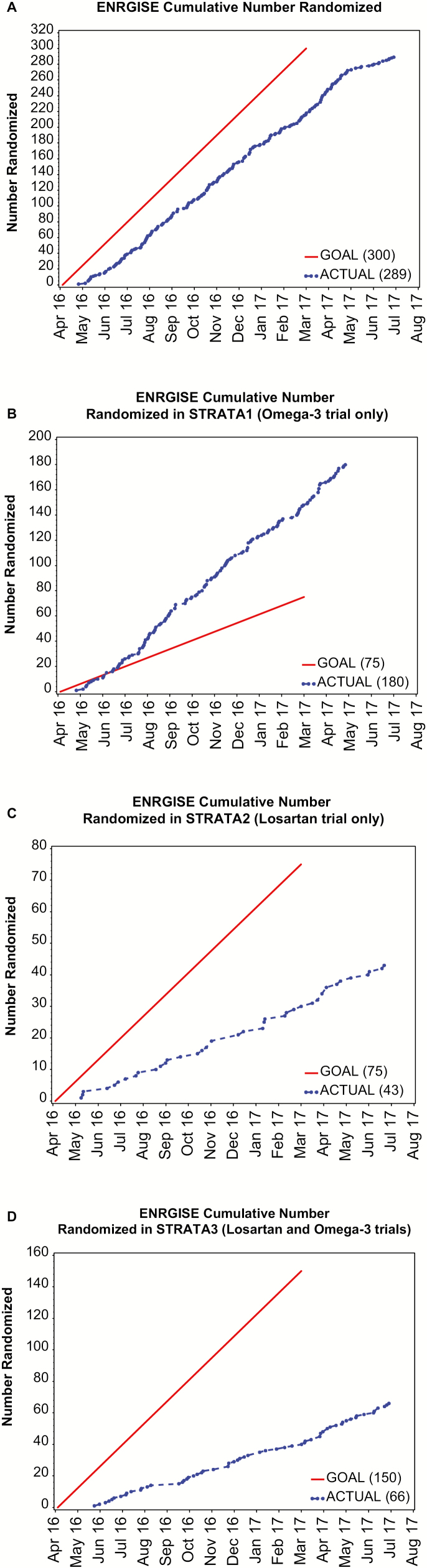
Over a recruitment period of 16 months, we randomized 289 (96% of goal) participants (Figure 2); 180 participants (240% of goal) into the ω-3 stratum; 43 participants (57% of goal) into the LO stratum; and 66 participants (44% of goal) in the combination stratum. Most randomized participants were Caucasian (n = 225, 78%). We enrolled 49 African Americans (83% of goal); 7 Hispanics (47% of goal); and 5 Asians (233% of goal).
Figure 2.
(A) Cumulative number of participants randomized in the ENRGISE Pilot Study. (B) Cumulative number of participants randomized in ω-3 stratum only. (C) Cumulative number of participants randomized in losartan (LO) stratum only. (D) Cumulative number of participants randomized in LO and ω-3 stratum trials.
Cost of Recruitment
The average cost per randomization was $1,782 but ranged from a low of $1,173 at Pittsburgh to $3,665 at Northwestern. The average personnel effort was 0.018 full-time equivalent per randomized participant but ranged from 0.012 per full-time equivalent at Wake Forest to 0.026 at the University of Florida.
Characteristics of Participants Enrolled
Characteristics of participants enrolled in the individual arms of ENRGISE are shown in Table 3. Overall, participants in the LO, ω-3, and combination strata were similar in age with an average of 78.3 years; 47% of participants were women and 78% were Caucasian. Fewer women were randomized to the LO-alone stratum compared with the combination or ω-3 stratums, but the differences were not significant. Average IL-6 levels were about 5 pg/mL. The 4-m gait speed was about 0.8 m/s indicating a mobility impaired group. The average body mass index was quite high overall at 31.5 ± 5.7 (kg/m2) (SD) but was lowest in participants randomized to the LO-alone stratum, 29.6 ± 4.9 (kg/m2). The average systolic and diastolic blood pressure varied by stratum and was lowest in participants randomized to ω-3 stratum.
Table 3.
Baseline Characteristics of Participants Enrolled Into the ENRGISE Pilot Study
| Overall (n = 289) | Omega-3 Only or Placebo (n = 180) | Losartan Only or Placebo (n = 43) | Losartan and Omega-3 or Placebo (n = 66) | p Value | |
|---|---|---|---|---|---|
| Age, y | 78.3 (5.4) | 78.3 (5.3) | 78.6 (5.1) | 78.0 (5.7) | .88 |
| Female | 137 (47.4) | 85 (47.2) | 16 (37.2) | 36 (54.5) | .21 |
| Race | .19 | ||||
| African American/Black | 49 (17.0) | 30 (16.7) | 8 (18.6) | 11 (16.7) | |
| Asian | 5 (1.7) | 2 (1.1) | 0 (0.0) | 3 (4.5) | |
| Caucasian/White | 225 (77.9) | 142 (78.9) | 31 (72.1) | 52 (78.8) | |
| Latino, Hispanic or Spanish | 7 (2.4) | 4 (2.2) | 3 (7.0) | 0 (0.0) | |
| Other/mixed | 3 (1.0) | 2 (1.1) | 1 (2.3) | 0 (0.0) | |
| Education | .25 | ||||
| Elementary school (K-08) | 3 (1.0) | 2 (1.1) | 1 (2.3) | 0 (0.0) | |
| High school/equivalent (09-12) | 87 (30.1) | 60 (33.3) | 10 (23.3) | 17 (25.8) | |
| College (13–16) | 120 (41.5) | 66 (36.7) | 20 (46.5) | 34 (51.5) | |
| Postgraduate | 69 (23.9) | 45 (25.0) | 9 (20.9) | 15 (22.7) | |
| Other | 10 (3.5) | 7 (3.9) | 3 (7.0) | 0 (0.0) | |
| Interleukin-6, pg/mL | 5.04 (5.67) | 5.36 (6.40) | 4.77 (4.08) | 4.35 (4.38) | .44 |
| 4-m gait speed, m/s | 0.82 (0.14) | 0.82 (0.12) | 0.83 (0.16) | 0.83 (0.17) | .74 |
| 400-m walk time, s | 505.4 (112.0) | 505.6 (111.5) | 499.6 (105.2) | 508.8 (118.8) | .92 |
| Body mass index, kg/m2 | 31.5 (5.7) | 31.6 (5.5) | 29.6 (4.9) | 32.3 (6.2) | .04 |
| Systolic blood pressure, mm Hg | 135.5 (18.0) | 133.3 (19.9) | 137.6 (12.9) | 140.1 (14.0) | .02 |
| Diastolic blood pressure, mm Hg | 72.2 (10.1) | 70.0 (10.0) | 74.3 (8.1) | 76.7 (9.9) | <.081 |
Note: Values are expressed as mean (SD) or n (%).
Discussion
In the ENRGISE Pilot Study, 289 participants (96% of goal) with mean age of 78.3 ± 5.3 years who were at high risk for mobility disability and had evidence of low-grade chronic inflammation were randomized. ENRGISE enrolled more than the original target of participants in the ω-3 stratum (240% of goal) as approved by the Data Safety and Monitoring Board and the sponsor. We did not meet recruitment goals for the LO stratum (57% of goal) or the combination stratum (44% of goal). Using gait speed to screen participants was effective in enriching the population for high IL-6 concentration because 64% of participants with slow gait speed had elevated IL-6 at the first screening visit. The recruitment yield was 5% of those who were telephone screened and 22% for those who were eligible at the telephone screen and participated in SV1. This low yield was due to the study eligibility criteria and not from low interest from potential participants and demonstrates the difficulty of testing the effects of commonly used medications. The high rates of ARB and ACEI interfered with reaching recruitment targets for the LO strata. Another major reason for exclusion was lack of mobility impairment.
The screening to randomization ratio varied by clinical centers with Wake Forest showing a considerable lower ratio than the other centers. Wake Forest was the only center that did not rely on central recruiting materials but developed their own materials that were targeted to their community. Wake Forest also had a large registry of participants from other studies.
The ENRGISE investigators selected interventions that are widely available, safe, tolerable, acceptable, and affordable for older adults. As described in the report by Manini and colleagues (6), we considered several interventions based on tolerability and safety, whether they reduced IL-6, their influence on physical performance, innovation for influencing mobility impairment, and biological plausibility to reduce inflammation and affordability. Based on the available evidence, LO and ω-3 were chosen because they met these criteria. However, the large number of potential participants already taking ARB and ACEI made meeting recruitment targets for LO far more difficult.
Despite targeted efforts to recruit minorities, we did not meet minority recruitment goals. This may have been largely reflected by the initial exclusion for low 25-hydroxyvitamin D levels (<20 ng/mL) because minorities, in particular African Americans, have an increased prevalence of vitamin D deficiency (7). After dropping this exclusion criterion, the proportion of minority participants eligible at SV2 doubled. We expected that more minority participants would be excluded for use of ACEIs and ARBs given the higher prevalence of hypertension among African Americans (8), but in fact, the proportion excluded for use of these medications was the same in minority participants as overall.
The cost of recruitment per randomized participant was $1,782 in ENRGISE. In comparison, the cost of recruitment per randomized participant in the LIFE Study was $840 (9). Cost of recruitment per randomized participant was $764–$868 in a frailty trial (10) and higher when recruiting participants with specific diseases ($2,000) (11). The Testosterone Trials (TTrials) did not report the cost of recruitment per randomized participant but given their high screening to randomization ratio (telephone screen to randomization, 65 to 1; SV1 to randomization, 30 to 1), it is likely that the overall cost of recruitment per randomized participant would have been much higher in the TTrials compared with ENRGISE (12).
Multiple recruitment strategies were needed to reach recruitment targets. However, most of the participants learned about the study through direct mail. This is consistent with several other major randomized clinical trials including the Systolic Hypertension in the Elderly Program (SHEP) (13), the LIFE Study (9), the TTrials (12), and the Vital D Study (14). Nevertheless, the other strategies provide complementary assistance. For example, seeing a print ad for ENRGISE in the newspaper may have encouraged participants to respond to the mailing. Response rates to mass mailings were low, less than 2% but were higher from mailings to targeted groups. For example, several sites have National Institute on Aging supported Claude D. Pepper Older Americans Independent Centers and response rates were generally higher for mailings to these “Pepper” registries, which include elders who are interested in research. An advantage of direct mail is that mailings can be targeted by age, race, sex, and zip code.
Although only 19 of the randomized participants were identified by EMR, the yield to randomization was higher (7.5%) compared with yields from mass mailings (4.9%). The yield from advertisements was also quite high (7.8%). It is quite possible that the higher yield from the EMR reflects the ability to screen out individuals who were ineligible. However, some sites were unable to identify people on ACEI or ARB through the EMR. In other cases, exclusion of all participants on ACEI or ARB medications was too restrictive and interfered with enrollment into the ω-3 trial. Wake Forest used the EMR to target participants who would be more likely to have chronic low-grade inflammation. For example, they mailed to participants from their EMR database with a body mass index greater than 30. Thus, identifying potential participants from EMR may be important for future studies.
In conclusion, we enrolled a large number of older participants with mobility limitations and evidence of low-grade chronic inflammation into the ω-3 stratum of ENRGISE. Recruitment for the LO and combination strata were lower than originally planned because many participants were taking ARBs or ACEIs. This lower enrollment rate will reduce our power for individual treatment group comparisons. Although most participants were recruited through the direct mail approach, recruitment yields were highest for EMR and advertisements. Results showed the utility of the direct mail approach and advertisements, the need to monitor recruitment carefully, the difficulty with one of our interventions (LO) given their high underlying use in the population, and the effect of a serum vitamin D minimum inclusion criterion requirement on minority enrollment. These results will inform the design of future trials of older, frail individuals.
Funding
This study was supported by a National Institutes of Health/National Institute on Aging Cooperative, Agreement No. U01AG050499. The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1P30AG028740), Wake Forest University (1 P30 AG021332), Tufts University (1P30AG031679), and University of Pittsburgh (P30 AG024827). Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1). R.A.F. is partially supported by the U.S. Department of Agriculture, under Agreement No. 58-1950-4-03. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. Abbott Laboratories provided funding for the purchase of study drug and matching placebo.
Conflict of Interest
None reported.
Supplementary Material
References
- 1. Kasenda B, von Elm E, You J, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311:1045–1051. doi: 10.1001/jama.2014.1361 [DOI] [PubMed] [Google Scholar]
- 2. Anton S, Sourdet S, Pahor M, Manini TM. Challenges in implementing large-scale clinical trials in moderately functioning older adults. In: Cherubini A, Bernabei R, Ferrucci L, Marchionni N, Studenski S, Vellas B, eds. Clinical Trials in Older Adults. Hoboken, NJ: Wiley Blackwell; 2015:125–152. [Google Scholar]
- 3. Mody L, Miller DK, McGloin JM, et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc. 2008;56:2340–2348. doi: 10.1111/j.1532-5415.2008.02015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298:1160–1162. doi: 10.1001/jama.298.10.1160-b [DOI] [PubMed] [Google Scholar]
- 5. Cerreta F, Eichler HG, Rasi G. Drug policy for an aging population – the European Medicines Agency’s geriatric medicines strategy. N Engl J Med. 2012;367:1972–1974. doi: 10.1056/NEJMp1209034 [DOI] [PubMed] [Google Scholar]
- 6. Manini TM, Anton SD, Beavers DP, et al. ; ENRGISE Pilot Study Investigators ENabling Reduction of Low-grade Inflammation in SEniors Pilot Study: concept, rationale, and design. J Am Geriatr Soc. 2017;65:1961–1968. doi: 10.1111/jgs.14965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 9. Marsh AP, Lovato LC, Glynn NW, et al. ; LIFE Study Research Group Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi: 10.1093/gerona/glt064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gill TM, McGloin JM, Gahbauer EA, Shepard DM, Bianco LM. Two recruitment strategies for a clinical trial of physically frail community-living older persons. J Am Geriatr Soc. 2001;49:1039–1045. doi: 10.1046/j.1532-5415.2001.49206.x [DOI] [PubMed] [Google Scholar]
- 11. McDermott MM, Domanchuk K, Dyer A, Ades P, Kibbe M, Criqui MH. Recruiting participants with peripheral arterial disease for clinical trials: experience from the Study to Improve Leg Circulation (SILC). J Vasc Surg. 2009;49:653–659.e4. doi: 10.1016/j.jvs.2008.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cauley JA, Fluharty L, Ellenberg SS, et al. Recruitment and screening for the testosterone trials. J Gerontol A Biol Sci Med Sci. 2015;70:1105–1111. doi: 10.1093/gerona/glv031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cosgrove N, Borhani NO, Bailey G, et al. Mass mailing and staff experience in a total recruitment program for a clinical trial: the SHEP experience. Systolic Hypertension in the Elderly Program. Cooperative Research Group. Control Clin Trials. 1999;20:133–148. doi: 10.1016/S0197-2456(98)00055-5 [DOI] [PubMed] [Google Scholar]
- 14. Sanders KM, Stuart AL, Merriman EN, et al. Trials and tribulations of recruiting 2,000 older women onto a clinical trial investigating falls and fractures: Vital D Study. BMC Med Res Methodol. 2009;9:78. doi: 10.1186/1471-2288-9-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.