Abstract
Maturity-onset diabetes of the young (MODY) is characterized by autosomal dominant inheritance, onset before 25 years of age, absence of β-cell autoimmunity, and sustained pancreatic β-cell function. To date, mutations have been identified in at least 14 different genes, including six genes encoding proteins that, respectively, correspond to MODY subtypes 1–6: hepatocyte nuclear factor (HNF) 4α (HNF4α), glucokinase (GCK), HNF1α (HNF1α), pancreatic and duodenal homeobox 1 (PDX1), HNF1β (HNF1β), and neurogenic differentiation 1 (NEUROD1). Diagnostic tools based on currently available genetic tests can facilitate the correct diagnosis and appropriate treatment of patients with MODY. Candidates for genetic testing include nonobese subjects with hyperglycemia, no evidence of β-cell autoimmunity, sustained β-cell function, and a strong family history of similar-type diabetes among first-degree relatives. Moreover, identification of the MODY subtype is important, given the subtype-related differences in the age of onset, clinical course and progression, type of hyperglycemia, and response to treatment. This review discusses the current perspectives on the diagnosis and treatment of MODY, particularly with regard to the six major subtypes (MODY 1–6).
Keywords: MODY, molecular diagnosis, genetic testing, pharmacological treatment
Introduction
Maturity-onset diabetes of the young (MODY) was first reported in 1974 as mild familial diabetes with dominant inheritance.1 Classically, MODY was characterized by autosomal dominant inheritance, onset before 45 years of age, the absence of β-cell autoimmunity,2 absence of insulin resistance,3 and sustained β-cell function. However, the new diagnostic criteria set forth in the Practice Guideline for MODY in 20084 include onset before 25 years of age in one family member, presence of diabetes in two consecutive generations, absence of β-cell autoantibodies, and sustained endogenous insulin secretion. Preserved β-cell function is indicated by the lack of need for insulin treatment or a serum C-peptide level of >200 pmol/L even after 3 years of insulin treatment.4
Molecular methods for the diagnosis of MODY were first introduced after the 1990s. To date, mutations associated with MODY have been reported in least 14 different genes,5–14 including the following six genes encoding major factors: hepatocyte nuclear factor (HNF) 4α (HNF4α), glucokinase (GCK), HNF1α (HNF1α), pancreatic and duodenal homeobox 1 (PDX1), HNF1β (HNF1β), and neurogenic differentiation 1 (NEUROD1), which correspond to MODY subtypes 1–6, respectively. The following eight genes have been identified as possibly causative in MODY subtypes 7–14, respectively: Kruppel-like factor 11 (KLF11); carboxyl ester lipase; paired-box-containing gene 4 (PAX4); insulin (INS); B-lymphocyte kinase; adenosine triphosphate (ATP)-binding cassette, sub-family C (CFTR/MRP) member 8 (ABCC8); potassium channel, inwardly rectifying subfamily J, member 11 (KCNJ11); and adaptor protein, phosphotyrosine interaction, PH domain, and leucine zipper containing 1 (APPL1).11,12 The causative genes for MODY and their medical conditions are shown in Table 1.
Table 1.
The causative genes for maturity-onset diabetes of the young (MODY) and medical conditions associated with each MODY subtype
| MODY gene | Frequency (% in MODYs) | Pathophysiology | Other features | Possible treatment |
|---|---|---|---|---|
| HNF4α | 5 | β-Cell dysfunction | Neonatal diabetes, hyperinsulinemic hypoglycemia during infancy, low triglycerides | Sensitive to sulfonylurea |
| GCK | 15–20 | Glucose sensing defect | Stable mild fasting glucose | No medication, or Diet |
| HNF1α | 30–50 | β-Cell dysfunction | Glucosuria | Sensitive to sulfonylurea |
| PDX1 | <1 | β-Cell dysfunction | Homozygote: permanent neonatal diabetes, pancreas agenesis | Diet or OAD or insulin |
| HNF1β | 5 | β-Cell dysfunction | Renal malformations, genito-urinary tract anomalies, pancreatic hypoplasia, low birth weight | Insulin |
| NEUROD1 | <1 | β-Cell dysfunction | Neonatal diabetes, child or adult-onset diabetes neurological abnormalities. | OAD or insulin |
| KLF11 | <1 | β-Cell dysfunction | Similar to type 2 diabetes | OAD or insulin |
| CEL | <1 | Pancreas endocrine and exocrine dysfunction | Exocrine dysfunction, lipomatosis | OAD or insulin |
| PAX4 | <1 | β-Cell dysfunction | Ketoacidosis-prone | Diet or OAD or insulin |
| INS | <1 | Insulin gene mutation | Neonatal diabetes, child or adult-onset diabetes | OAD or insulin |
| BLK | <1 | Insulin secretion defect | Overweight, relative insulin secretion failure | Diet or OAD or insulin |
| ABCC8 | <1 | ATP-sensitive potassium channel dysfunction | Homozygote: permanent neonatal diabetes, Heterozygote: transient neonatal diabetes | OAD (sulfonylurea) |
| KCNJ11 | <1 | ATP-sensitive potassium channel dysfunction | Homozygote: neonatal diabetes | OAD or insulin |
| APPL1 | <1 | Insulin secretion defect | Child or adult-onset diabetes | Diet or OAD or insulin |
MODY is a rare condition, accounting for 1–5% of all cases of diabetes12,13 and 1–6% of pediatric cases of diabetes.14 Mutations in GCK, HNF1α, HNF4α, and HNF1β are the most frequently identified etiologies of MODY and, respectively, account for 32%, 52%, 10%, and 6% of all affected patients in the United Kingdom.11 However, the frequencies of these etiologies may differ among Asian and Caucasian populations. In Japan, 48.1% of pediatric cases of clinical MODY harbored already known MODY-related gene defects (GCK, 22.8%; HNF1A, 13.9%; HNF4A, 3.8%, and HNF1B, 7.6%), whereas in 51.9% cases, the causative mutations were not identified15 In Korea, only 10% of clinical MODY or childhood-onset type 2 diabetes cases harbored known MODY-related gene defects (GCK, 2.5%; HNF1A, 5%, and HNF1B, 2.5%)16 These data suggest that currently unidentified genes may cause MODY in Asian populations. The causative genes for MODY and their medical conditions are shown in Table 1.
To improve the prognosis of MODY, it is important to identify the affected subjects as early as possible. To this end, specific molecular analyses are available to predict the clinical disease course and offer the most appropriate treatment.14 However, approximately 80% of patients with MODY may be misdiagnosed with type 1 or type 2 diabetes mellitus at diagnosis,17 and current calculations indicate a delay of approximately 15 years from the diagnosis of diabetes to the genetic diagnosis of MODY.17
This review discusses the current perspectives on the diagnosis and treatment of MODY, in particular, the six major subtypes (MODY 1–6).
Diagnosis of MODY
At diagnosis, MODY cannot be distinguished easily from type 1 and type 2 diabetes mellitus based on clinical characteristics.13,14 Rather, type 1 diabetes mostly differs from MODY in terms of disease etiology, as the pathogenesis of the latter does not involve pancreatic β-cell autoimmunity. Patients with MODY usually maintain β-cell function, exhibit a stimulated serum C-peptide level exceeding 200 pmol/L, and their diabetes is well-controlled with no or low-dose insulin for at least 5 years after diagnosis.14 Although the clinical manifestations of youth-onset type 2 diabetes substantially resemble those of MODY, patients with the former condition are generally obese, whereas the latter condition is not associated with overweight. Still, approximately 10–15% of Japanese school children with type 2 diabetes are non-obese.18,19 Additionally, some patients with MODY, particularly those belonging to ethnic groups with a higher prevalence of obesity (eg, Hispanic), may become overweight or obese due to poor dietary habits and sedentary lifestyles. Furthermore, both MODY and type 2 diabetes are associated with a strong family history of diabetes. For example, studies revealed that approximately 70% of Japanese school children with type 2 diabetes had a family history of type 2 diabetes among first- and second-degree relatives. Those with nonobese type 2 diabetes had a particularly strong family background of the disease.18,19 Therefore, the correct diagnosis of MODY and determination of subtype should be based on genetic testing. Candidates for genetic testing include nonobese subjects with hyperglycemia, no evidence of β-cell autoimmunity, preserved β-cell function, and a strong family history of a similar type of diabetes among the first-degree relatives.14
Currently, genetic testing is performed worldwide to facilitate predictions of the clinical course and prognosis of MODY. Known MODY-related genes can be identified by direct sequencing with sensitivity rates as high as 100%,14 and next-generation sequencing methods (eg, gene-targeted and whole-exome sequencing) have been successfully used to identify mutations in MODY genes.16 However, genetic testing remains expensive and is necessarily limited to cases of strongly suspected MODY. These patients should be offered the most suitable treatment from among various pharmacological therapies, including oral antidiabetic drugs (OADs) and insulin.
Diagnosis of the subtypes of MODY
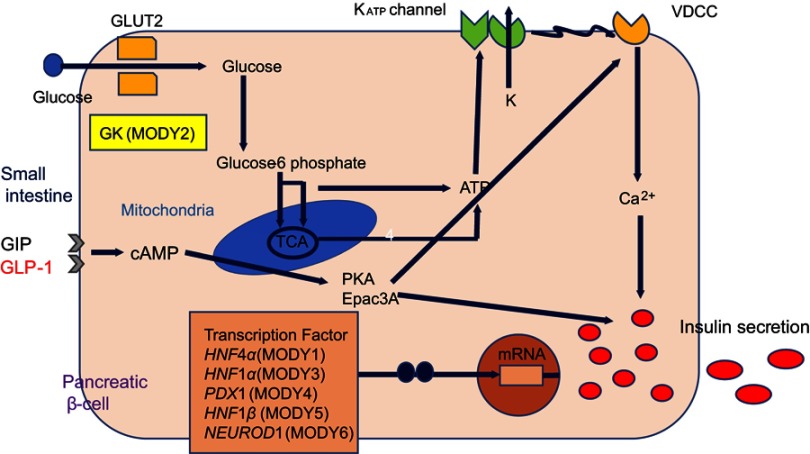
sAs shown in Table 1, at least 14 MODY subtypes have been reported, of which 1–6 are the major subtypes. As noted above, eight other subtypes have been identified, including MODY 14, which was recently associated with the causative gene APPL1 in large families.20 Most MODY-causative genes, except GCK, encode transcription factors expressed in pancreatic β-cells (Figure 1). MODY subtype determination is important, as the subtypes differ in terms of the age of onset, clinical course and progression, type of hyperglycemia, and response to treatment.14 Most patients with MODY exhibit the clinical characteristics of isolated diabetes or mild fasting hyperglycemia. However, some MODY subtypes are associated with additional manifestations, such as renal abnormalities (MODY5) or pancreatic exocrine dysfunction (MODY8). This review mainly describes the clinical characteristics of the six major subtypes of MODY.
Figure 1.
Expression of maturity-onset diabetes of the young (MODY)-causative genes in pancreatic β-cells and mechanism of insulin secretion.
Abbreviations: ATP, adenosine triphosphate; cAMP, cyclic adenosine 3ʹ,5ʹ-monophosphate; PKA, protein kinase A; Epac, exchange protein directly activated by cAMP; KATP channel, ATP-sensitive potassium channel; VDCC, voltage-dependent calcium channel; GCK, glucokinase; HNF4α, hepatocyte nuclear factor 4α; HNF1α, hepatocyte nuclear factor 1α; PDX1, pancreatic and duodenal homeobox 1; HNF1β, hepatocyte nuclear factor 1β; NEUROD1, neurogenic differentiation 1.
MODY2 (GCK-MODY)
GCK, a glucose sensor expressed in pancreatic β-cells, is a key enzyme in glucose metabolism that catalyzes the conversion of glucose to glucose-6-phosphate and thus controls glucose-mediated insulin secretion. More than 600 mutations in GCK have been identified in >1,000 families, and these alterations lead to both hyperglycemia and hypoglycemia.21 Heterozygous inactive mutations are associated with mild and often subclinical hyperglycemia from birth, and this condition gradually deteriorates with age11 Essentially, these mutations elevate the glucose threshold for insulin secretion, resulting in an elevated fasting plasma glucose level (5.5–8 mmol/L). Such patients exhibit a slight increase in the 2-hr plasma glucose level during oral glucose tolerance testing (OGTT; increase in 4.5 mmol/L = 90th percentile).4,22 These patients usually have a HbA1c level of <64 mmol/mol.23
Patients with MODY2 are usually asymptomatic. The majority are discovered through routine examinations during pregnancy or school-based urine glucose screening tests. MODY2 presents in approximately 2–6% of patients with gestational diabetes and can be distinguished based on clinical manifestations and the fasting glucose level.24,25 The birth weights of babies in MODY2 families depend on the mutation statuses of both the fetus and mother. If both harbor a GCK mutation, a maternal increase in plasma glucose will induce an appropriate insulin release in the fetus and a normal birth weight. However, if only the mother harbors the GCK mutation, maternal hyperglycemia will elicit increased fetal insulin secretion and a birth weight approximately 500 g higher than that of a baby who harbors the mutation.26 In Japan, Yorifuji et al reported that 22.8% of patients in whom MODY was detected through school urine glucose screening programs were finally diagnosed with MODY2 through genetic testing. Accordingly, MODY2 is most frequent subtype in Japanese children.15 The clinical course of this subtype may be mild and nonprogressive, and microvascular and macrovascular complications rarely occur despite long-term exposure to mild hyperglycemia.26,27
MODY 3 (HNF1α-MODY)
The transcription factor HNF1α is expressed in the liver, kidney, intestine and pancreatic β-cells. HNF1a knockout mice develop diabetes consequent to defective glucose-induced insulin secretion.28,29 Mutations in HNF1a are the most frequent causes of MODY in Europe, North America, and some Asian countries.11,14,16 In Japan, mutations in HNF1 a were detected in 13.9% of patients with clinical MODY.15 Studies of approximately 1200 families have identified >400 different HNF1a mutations,30 of which a mutation (P291fsinsC) in exon 4 is most common.31 Heterozygous mutations of HNF1a cause a progressive insulin deficiency that manifests as mild hyperglycemia in childhood and as diabetes during early adulthood.15,32 MODY3 is rarely discovered in children younger than 8 years of age.15
Hyperglycemia associated with MODY3 may be progressive and deteriorating. In these patients, the risks of microvascular and macrovascular complications are similar to those observed in patients with type 1 and type 2 diabetes.33 Interestingly, HNF1a mutation carriers develop postprandial glycosuria before the onset of diabetes due to renal tubular dysfunction and a consequently low renal threshold for glucose absorption.34 In the fetus, HNF1a mutational heterozygosity does not influence the birth weight because insulin secretion in utero remains normal.35
MODY1 (HNF4α-MODY)
The transcription factor HNF4α is expressed primarily in the liver but also in the pancreas and kidney,36 where it regulates the expression of genes required for glucose transport and metabolism. Mutations in HNF4α are relatively uncommon, accounting for approximately 5% of all MODY cases. Phenotypically, MODY1 due to heterozygous HNF4α mutation manifests as progressive insulin deficiency, similar to that observed in MODY3. Fetal HNF4α heterozygosity results in macrosomia due to hyperinsulinemia in utero and subsequent neonatal transient or persistent hypoglycemia, which is responsive to diazoxide.37 Glycosuria is not observed in MODY1, in contrast to MODY3. Hyperinsulinism associated with MODY1 generally remits during infancy, followed by a gradual decrease in endogenous insulin production and the emergence of diabetes in adolescence.14 Moreover, HNF4α has been associated with triglyceride metabolism, and mutation carriers may exhibit reduced levels of apolipoproteins (apoAII, apoCIII, and apoB).38
MODY5 (HNF1β-MODY)
The transcription factor HNF1β is involved in the organogenesis of the kidney, genito-urinary tract, liver, lungs, gut, and pancreas.39 Accordingly, renal malformations, including renal cysts, renal dysplasia, urinary tract malformation, and hypoplastic glomerulonephritic kidney disease,40 are seen in the majority of HNF1β mutation carriers and constitute the main presentation of MODY5 in children, regardless of the hyperglycemia status14 HNF1β mutation also causes renal cysts and diabetes syndrome, and these renal abnormalities are evident from the 17th week of gestation.41 Affected patients will develop renal dysfunction by 45 years of age, and half of these patients will progress to end-stage renal disease without developing diabetic kidney disease.42 Accordingly, MODY5 should be suspected in patients with diabetes and nondiabetic renal disease. Genito-urinary tract malformations (especially uterine abnormalities), liver dysfunction, and pancreatic hypoplasia have also been reported.43 Although diabetes associated with MODY5 develops in early adulthood, carriers of HNF1β mutations have significantly reduced birth weights.44 Phenotypically, MODY5 differs from MODY3. Patients with MODY5 present with dyslipidemia, including a low high-density lipoprotein level and elevated triglyceride level. Diabetes develops during adolescence or early adulthood and usually progresses to an insulin-dependent state due to pancreatic hypoplasia, with hepatic insulin resistance, relatively earlier period of the disease.11,14 Patients harboring HNF1β mutations exhibit highly variable phenotype and clinical manifestations even within affected families. Accordingly, the diagnosis of MODY5 should be made in consultation with not only diabetes specialists but also other specialists, such as nephrologists, gynecologists, and urologists.14
MODY4 (PDX1-MODY)
PDX1 is a homeodomain-containing transcription factor that acts in both the exocrine and endocrine pancreatic developmental programs and affects pancreatic development and INS expression.45 Homozygous mutations in PDX1 cause pancreas agenesis and hypoplasia and permanent neonatal diabetes,46 whereas heterozygous mutations lead to β-cell impairment and hyperglycemia, including permanent neonatal diabetes. MODY4 is a very rare subtype.
MODY6 (NEUROD1-MODY)
NEUROD1 is a basic-loop-helix transcription factor involved in pancreatic and neuronal development. It plays an important role in pancreatic β-cell maturation and maintenance. Islets lacking NEUROD1 respond poorly to glucose and show a glucose metabolic profile similar to immature β-cells. Heterozygous mutations in NEUROD1 induce childhood- or adult-onset diabetes, while homozygous mutations can cause neonatal diabetes, neurological abnormalities, and learning difficulties.47–49
The ages of onset, degrees of hyperglycemia, involvement of special tissues, and clinical features of other subtypes of MODY are shown in Table 1.
How can be MODY diagnosed correctly?
Cases of MODY are often misdiagnosed as type 1 or type 2 diabetes at presentation.13,14,19 Accordingly, an improvement in diagnostic significance will require targeted selection of subjects for genetic testing, particularly in scenarios with limited resources. Various algorithms have been proposed to select candidates for genetic testing.13,50 According to a recent diagnostic criteria,4 MODY is characterized by onset before 25 years of age, presence of diabetes in two consecutive family generations, absence of β-cell autoantibodies, and preserved endogenous insulin secretion with a serum C-peptide level of >200 pmol/L. These diagnostic criteria have been well accepted for distinguishing MODY from type 1 and type 2 diabetes. Shields et al.50 further proposed a model in which an age younger than 30 years at diagnosis was the most useful discriminator between MODY and type 2 diabetes. In that model, a family background of diabetes increased the probability of diagnosis of MODY by 23 times among patients who were first diagnosed with type 1 diabetes.51
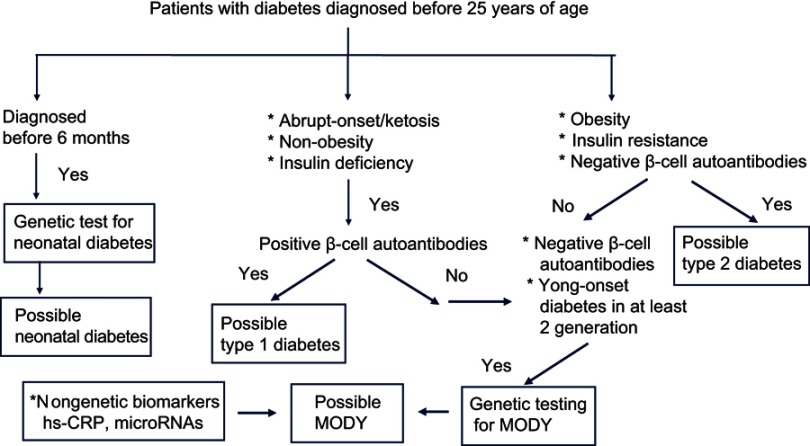
The costs and limitations of genetic testing have encouraged the discovery of nongenetic biomarkers that might be used to identify appropriate subjects for molecular diagnosis. For example, high-sensitivity C-reactive protein (hs-CRP) may be a useful biomarker in the differential diagnosis of MODY3, as affected patients have a significantly lower level of hs-CRP than those with type 1 or type 2 diabetes or MODY2.52–54 A recent study suggested that microRNAs may be useful biomarkers in carriers of MODY3-related gene mutations.55 However, some causative genes for MODY subtypes have also been associated with other forms of diabetes. KCNJ11, ABCC8, PDX1, PAX4, and NEUROD1 have been associated with permanent neonatal diabetes and type 2 diabetes, while INS is associated with both type 1 and type 2 diabetes.56,57 Moreover, variants of HNF1α, HNF4α, HNF1β, and GCK might be associated with an increased risk of type 2 diabetes.57 It is often difficult to differentiate between an incompletely penetrant MODY mutation and a rare variant of type 2 diabetes, and such distinction may be a matter of semantics.58,59 Therefore, genetic testing is not sufficient to confirm a diagnosis of MODY, which should also include clinical observation, laboratory testing (serum C-peptide, β-cell autoantibodies, and specific biomarkers). Figure 2 presents a diagnostic algorithm used to identify candidates with diabetes who should undergo genetic testing to confirm the diagnosis of MODY.
Figure 2.
Diagnostic algorithm for maturity-onset diabetes of the young (MODY).
Treatment of MODY
Correct determination of the MODY subtype is important, as this informs decisions regarding appropriate treatment and prognosis. Children and adolescents diagnosed with diabetes may initially be treated with insulin, and this regimen often continues even after the stabilization of glycemia. However, in some patients with MODY, hyperglycemia can be controlled by prescribing OADs (eg, sulfonylureas, without using insulin. Additionally, the selection of appropriate treatments for these patients is important to improve their quality of life.
MODY2 (GCK-MODY)
The majority of patients with MODY2 exhibit mildly elevated fasting plasma glucose levels but do not exhibit postprandial hyperglycemia; in other words, they secrete sufficient insulin in response to an increase in plasma glucose after consuming a meal. Therefore, dietary intervention alone is usually advised for these patients, as pharmacological intervention is not required to control hyperglycemia and prevent diabetic complications.11,14 However, some Japanese patients with MODY2 eventually require pharmacological treatment. In a study of 55 patients, Kawakita et al.60 reported that seven were treated with OADs, two with sulfonylureas, one with metformin, and two with α-glucosidase inhibitors. Asians face a risk of insulin resistance, characterized by a higher Homeostatic Model Assessment (HOMA-IR) at a relatively low body mass index (BMI),18,61 possibly due to a greater amount of visceral fat than that observed in Caucasians.62,63 Accordingly, some Japanese patients who consume carbohydrate-rich foods and have sedentary lifestyles require OADs. Asians also exhibit a relatively lower level of insulin release in response to an increase in glucose, as well as a lower homeostasis model assessment for β-cell function (HOMA-β).62,63 This pattern may also lead to an increased requirement for OADs. During pregnancy, insulin might be offered to patients with MODY2 to prevent fetal overweight.
MODY3 (HNF1α-MODY) and MODY1 (HNF4α-MODY)
Patients with MODY3 and MODY1 can initially control glycemia with diet alone, although both tend to exhibit elevated postprandial glucose levels after consuming carbohydrate-rich foods.22 Over time, however, most patients will experience deteriorating β-cell function and require pharmacological treatment. Patients with MODY3 and MODY1 are sensitive to sulfonylureas and can maintain optimal glycemic control with these drugs rather than insulin. One randomized clinical trial of gliclazide therapy demonstrated a 3.9-fold improvement in the fasting glucose levels of patients with MODY3 relative to BMI-matched patients with type 2 diabetes; this effect was particularly pronounced in children and young adults. That study also demonstrated the safety and efficacy of a switch from insulin to gliclazide despite using the former for a long duration.64 The initial sulfonylurea dose should be low to avoid hypoglycemia. Reports suggest that optimal glycemic control without problematic hypoglycemia can be maintained for decades at gliclazide doses of 20–40 mg.65,66 If hypoglycemia occurs despite dose titration, a short-acting agent (eg, meglitinide) may be considered.67 Another sulfonylurea, glimepiride, might offer a similar glucose-reducing effect with fewer episodes of hypoglycemia. It also exerts extrapancreatic effects such as decreased glucose output from the liver and enhanced sensitivity of peripheral tissues to insulin.68
Another therapeutic option involving a glucagon-like peptide (GLP-1) agonist with a similar glucose-lowering effect as sulfonylureas and a low frequency of hypoglycemia has been proposed for patients with MODY3.69,70 A double-blind, randomized crossover trial that compared a GLP-1 agonist (liraglutide) with a sulfonylurea (glimepiride) found no difference between the two drugs in terms of controlling the fasting plasma glucose and responsive postprandial plasma glucose relative to baseline. However, glimepiride was clearly associated with a higher incidence of hypoglycemia.70 We experienced similarly good glucose-reducing effects and a lack of hypoglycemia in children with MODY3 who were treated with liraglutide.71 Moreover, we previously reported that a switch from glimepiride to a dipeptidyl-peptidase-4 (DPP-4) inhibitor, alogliptin, yielded similar glycemic control without hypoglycemia in a girl with MODY1.72
During treatment, patients with MODY3 and MODY1 maintain substantial β-cell function for at least 2–4 years after diagnosis. As noted above, sulfonylureas are generally the first-line of treatment for patients with MODY3 and MODY1, despite the risk of hypoglycemia. Long-term treatment with sulfonylureas is also associated with body weight gain and the deterioration of endogenous insulin, which eventually induces insulin dependency in some patients with type 2 diabetes. In contrast, GLP-1 receptor agonists and DPP-4 inhibitors lower glucose levels and increase endogenous insulin secretion in a glucose-dependent manner. This effect occurs via a subpathway of insulin secretion in response to increased cyclic adenosine 3ʹ, 5ʹ-monophosphate (c-AMP) production, rather than by ATP production, which thus overcomes the impaired mitochondrial ATP production in response to glucose in patients with MODY3 and MODY1.28 Therefore, the mode of insulin secretion induced by GLP-1 receptor agonists and DPP-4 inhibitors may more effectively increase insulin secretion in these patients. Additionally, GLP-1 receptor agonists and DPP-4 inhibitors might prevent β-cell apoptosis and promote β-cell generation to counter the progressive impairment of β-cell function during the courses of MODY3 and MODY1.73–75 These findings suggest that GLP-1 receptor agonists and DPP-4 inhibitors may be suitable alternatives to sulfonylureas.
MODY5 (HNF1β-MODY)
Unlike patients with MODY3 and MODY1, those with MODY5 do not respond adequately to treatment with sulfonylureas, possibly due to comorbid pancreatic hypoplasia and some degree of hepatic insulin resistance.76 These patients may require intensive insulin treatment to control hyperglycemia. Additionally, microvascular complications have been described in these subjects,6 and treatment for renal disease, liver dysfunction, and dyslipidemia is necessary. Renal management is a particularly important aspect of treatment in patients harboring HNF1β mutations, as these individuals will develop renal dysfunction by 45 years of age and half will progress to end-stage renal disease.42
The current treatments of other MODY subtypes according to the molecular causes and clinical characteristics are listed in Table 1. However, standard treatments have not been established for most subtypes because of low numbers of cases and a lack of data confirming treatment efficacy. Furthermore, only metformin and insulin are approved for use in youth in the majority of countries. Sulfonylureas are approved for use in adolescents in some countries. Other OADs are not approved for use in those <18 years of age.77
Conclusion
Advanced molecular genetic analyses have led to the identification of genes associated with clinically diagnosed subtypes of MODY. Accordingly, genetic testing is useful for the correct diagnosis and appropriate treatment of patients with MODY.14 To confirm a correct diagnosis and improve prognosis, genetic testing is recommended at diagnosis or during early-stage disease, despite limitations associated with costs.
Patients with type 1 diabetes require insulin treatment for survival and metabolic control, whereas those with MODY do not usually require long-term insulin treatment. Therefore, misdiagnosis can lead to inappropriate treatment. MODY should be suspected in the presence of mild to moderate, nonketosis-prone hyperglycemia in usually nonobese patients with a strong family history of diabetes. The importance of genetic testing is emphasized by the effect of a general increase in the prevalence of obesity, which may complicate a differential diagnosis including both type 2 diabetes and MODY. Correct diagnosis of MODY is also important with respect to genetic counseling and the prevention of developing the disease.78 Rapid advances in the fields of molecular analysis and laboratory technology are expected to improve the correct diagnosis of MODY and the provision of appropriate treatment based on the individual medical condition.
Abbreviation list
MODY, maturity-onset diabetes of the young; HNF, hepatocyte nuclear factor; GCK, glucokinase; PDX1, pancreatic and duodenal homeobox 1; NEUROD1, neurogenic differentiation 1; KLF11, Kruppel-like factor 11; CEL, carboxyl ester lipase; PAX4, paired-box-containing gene 4; INS, insulin; BLK, B-lymphocyte kinase; ABCC8, adenosine triphosphate (ATP)-binding cassette, sub-family C (CFTR/MRP) member 8; KCNJ 11, potassium channel, inwardly rectifying subfamily J, member 11; APPL1, adaptor protein, phosphotyrosine interaction, PH domain, and leucine zipper containing 1; OAD, oral antidiabetic drug; OGTT, oral glucose tolerance test; HOMA-IR, homeostasis model assessment for insulin resistance; BMI, body mass index; HOMA-β, homeostasis model assessment for β-cell function; hs-CRP, high-sensitivity C-reactive protein; GLP-1, glucagon-like peptide; DPP-4, dipeptidyl-peptidase-4; c-AMP, cyclic adenosine 3ʹ, 5ʹ-monophosphate.
Disclosure
The author declares no conflicts of interest in this work.
References
- 1.Tattersall RB. Mild familial diabetes with dominant inheritance. Q J Med. 1974;43:339–357. [PubMed] [Google Scholar]
- 2.McDonald TJ, Colclough K, Brown R, et al. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from type 1 diabetes. Diabet Med. 2011;20:1028–1033. doi: 10.1111/j.1464-5491.2011.03287.x [DOI] [PubMed] [Google Scholar]
- 3.Owen KR, Roland J, Smith K, Hattersley AT. Adolescent onset type 2 diabetes in a non-obese Caucasian patient with an unbalanced translocation. Diabet Med. 2011;20:483–485. doi: 10.1046/j.1464-5491.2003.00961.x [DOI] [PubMed] [Google Scholar]
- 4.Ellard S, Ballanné-Chantelot C, Hattersley AT. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51:546–553. doi: 10.1007/s00125-008-0942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froguel PM, Vallaire F, Sun G, et al. Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin dependent diabetes mellitus. Nature. 1992;356:162–164. doi: 10.1038/356162a0 [DOI] [PubMed] [Google Scholar]
- 6.Horikawa Y, Iwasaki N, Hara M, et al. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) association with MODY. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384 [DOI] [PubMed] [Google Scholar]
- 7.Soffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY 4) linked to IPF1. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138 [DOI] [PubMed] [Google Scholar]
- 8.Vionnet N, Stoffel M, Takeda J, et al. Nonsense mutation in the glucokinase causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356:721–722. doi: 10.1038/356721a0 [DOI] [PubMed] [Google Scholar]
- 9.Yamagata K, Furuta H, Oda N, et al. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1). Nature. 1996;384:458–460. doi: 10.1038/384458a0 [DOI] [PubMed] [Google Scholar]
- 10.Yamagata K, Oda N, Kaisaki PJ, et al. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature. 1996;384:455–458. doi: 10.1038/384455a0 [DOI] [PubMed] [Google Scholar]
- 11.Kavvoura FK, Owen KR. Maturity onset diabetes of the young: clinical characteristics, diagnosis and management. Pediatr Endocrinol Rev. 2013;10:234–242. [PubMed] [Google Scholar]
- 12.Kim SH. Maturity-onset diabetes of the young: what do clinicians need to know? Diabetes Metab J. 2015;39:468–477. doi: 10.4093/dmj.2015.39.6.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanabalansingham G, Pal A, Selwood MP, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35:1206–1212. doi: 10.2337/dc11-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattersley AT, Greeley SA, Polak M, et al. ISPAD clinical practice consensus guidelines 2018: the diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2018;19(Suppl.27):47–63. doi: 10.1111/pedi.12772 [DOI] [PubMed] [Google Scholar]
- 15.Yorifuji T, Fujimaru R, Hosokawa Y, et al. Comprehensive molecular analysis of Japanese patients with pediatric-onset MODY-type diabetes mellitus. Pediatr Diabetes. 2012;13:26–32. doi: 10.1111/j.1399-5448.2011.00827.x [DOI] [PubMed] [Google Scholar]
- 16.Hwang JS, Shin CH, Yang SW, Jung SY, Huh N. Genetic and clinical characteristics of Korean maturity-onset diabetes of the young (MODY) patients. Diabetes Res Clin Pract. 2006;74:75–81. doi: 10.1016/j.diabres.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504–2508. doi: 10.1007/s00125-010-1799-4 [DOI] [PubMed] [Google Scholar]
- 18.Urakami T, Kuwabara R, Habu M, et al. Clinical characteristics of non-obese children with type 2 diabetes mellitus without involvement of β-cell autoimmunity. Diabetes Res Clin Pract. 2013;99:105–111. doi: 10.1016/j.diabres.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 19.Urakami T, Miyata M, Yoshida K, et al. Changes in annual incidence of school children with type 2 diabetes in the Tokyo metropolitan area during 1975–2015. Pediatr Diabetes. 2018;19:1385–1392. doi: 10.1111/pedi.12750 [DOI] [PubMed] [Google Scholar]
- 20.Prudente S, Jungtrakoon P, Marucci A, et al. Loss-of-function mutations in APPL1 in familial diabetes mellitus. Am J Hum Genet. 2018;97:177–185. doi: 10.1016/j.ajhg.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osbak KK, Colclough K, Saint-Martin C, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009;30:1512–1526. doi: 10.1002/humu.21110 [DOI] [PubMed] [Google Scholar]
- 22.Stride A, Vaxillaire M, Tuomi T, et al. The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia. 2002;45:427–435. doi: 10.1007/s00125-001-0770-9 [DOI] [PubMed] [Google Scholar]
- 23.Martin D, Bellarnne-Chantelot C, Deschamps I, Froguel P, Robert JJ, Velho G. Long-term follow-up of oral glucose tolerance test-derived glucose tolerance and insulin secretion and insulin sensitivity indexes in subjects with glucokinase mutations (MODY2). Diabetes Care. 2008;31:1321–1323. doi: 10.2337/dc07-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakera AJ, Spyer G, Vincent N, Ellard S, Hattersley AT, Dunne FP. The 0.1% of the population with glucokinase monogenic diabetes can be recognized by clinical characteristics in pregnancy: the Atlantic diabetes in pregnancy cohort. Diabetes Care. 2014;37:1230–1236. doi: 10.2337/dc13-2248 [DOI] [PubMed] [Google Scholar]
- 25.Rudland VL, Hinchcliffe M, Pinner J, et al. Identifying glucokinase monogenic diabetes mellitus in a multiethnic gestational diabetes mellitus cohort: new pregnancy screening criteria and utility of HbA1c. Diabetes Care. 2016;39:50–52. doi: 10.2337/dc15-1001 [DOI] [PubMed] [Google Scholar]
- 26.Steele AM, Shields BM, Shepherd M, Ellard S, Colclough K, Hattersley AT. Microvascular complication risk in patients with 50 years of moderate hyperglycemia: are target ranges for glycemia control appropriate? Abstract A77. Diabet Med. 2011;28(S1):2. doi: 10.1111/j.1464-5491.2011.03281.x [DOI] [PubMed] [Google Scholar]
- 27.Velho G, Blanche H, Vaxillaire M, et al. I dentification of 14 new glucokinase mutations and description of the clinical profile of 42 MODY-2 families. Diabetologia. 1997;40:217–224. doi: 10.1007/s001250050666 [DOI] [PubMed] [Google Scholar]
- 28.Dukes ID, Sreenan S, Roe M, et al. Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor 1alpha-deficit mouse. J Biol Chem. 1998;273:24457–24464. doi: 10.1074/jbc.273.38.24457 [DOI] [PubMed] [Google Scholar]
- 29.Pontoglio M, Sreenan S, Roe M, et al. Defective insulin secretion in hepatocyte nuclear factor 1alpha-deficit mouse. J Clin Invest. 1998;101:2215–2222. doi: 10.1172/JCI2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the hepatocyte nuclear factor 1alpha and 4alpha in maturity-onset diabetes in the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013;34:669–685. doi: 10.1002/humu.22279 [DOI] [PubMed] [Google Scholar]
- 31.Ellard S, Colclough K. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1alpha (HNF1A) and 4alpha (HNF4A) in maturity-onset diabetes in the young. Hum Mutat. 2006;27:854–869. doi: 10.1002/humu.20357 [DOI] [PubMed] [Google Scholar]
- 32.Frayling TM, Bulamn MP, Ellard S, et al. Mutations in the hepatocyte nuclear factor-1alpha gene are a common cause of maturity-onset diabetes of the young in the U.K. Diabetes. 1997;46:720–725. doi: 10.2337/diab.46.4.720 [DOI] [PubMed] [Google Scholar]
- 33.Steele AM, Shields BM, Shepherd M, Ellard S, Hattersley AT, Pearson ER. Increased all-cause and cardiovascular mortality in monogenic diabetes as a result of mutations in the HNF1A gene. Diabet Med. 2010;27:157–161. doi: 10.1111/j.1464-5491.2009.02913.x [DOI] [PubMed] [Google Scholar]
- 34.Pontoglio M, Prie D, Cheret C, et al. HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000;1:359–365. doi: 10.1093/embo-reports/kvd071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson ER, Boj SF, Steele AM, et al. Macrosomia and hyperinsulinaemic hypoglycemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007;4:e118. doi: 10.1371/journal.pmed.0040118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997;94:13209–13214. doi: 10.1073/pnas.94.24.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson ER, Pruhova S, Tack CJ, et al. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large Europea collection. Diabeteologia. 2005;48:878–885. doi: 10.1007/s00125-005-1738-y [DOI] [PubMed] [Google Scholar]
- 38.Lehto M, Bitzen PO, Isoma B, et al. Mutations in the HNF-4alpha gene affects insulin secretion and triglyceride metabolism. Diabetes. 1999;48:423–425. doi: 10.2337/diabetes.48.2.423 [DOI] [PubMed] [Google Scholar]
- 39.Barbacci E, Reber M, Ott MO, Breilat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–4805. [DOI] [PubMed] [Google Scholar]
- 40.Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet. 2006;43:84–90. doi: 10.1136/jmg.2005.032854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bingham C, Ellard S, Allen L, et al. Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor-1beta. Kidney Int. 2000;57:898–907. doi: 10.1046/j.1523-1755.2000.057003898.x [DOI] [PubMed] [Google Scholar]
- 42.Bingham C, Bulman MP, Ellard S, et al. Mutation in the transcription factor hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet. 2001;68:219–224. doi: 10.1086/316945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellanne-Chantelot C, Chauveau D, Gautier JF, et al. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004;140:510–517. [DOI] [PubMed] [Google Scholar]
- 44.Edghill EL, Bingham C, Singerland AS, et al. Hepatocyte nuclear factor-1beta mutations cause neonatal diabetes and intrauterine growth reduction: support for a critical role of HNF-1beta in human pancreatic development. Diabet Med. 2006;23:1301–1306. doi: 10.1111/j.1464-5491.2006.01999.x [DOI] [PubMed] [Google Scholar]
- 45.Stoffers DA, Thomas MK, Habener JF. Homeodomain protein IDX-1: a master regulator of pancreas development and insulin gene expression. Trends Endocrinol Metab. 1997;8:145–151. doi: 10.1016/S1043-2760(97)00008-8 [DOI] [PubMed] [Google Scholar]
- 46.Schwitzgebel VM, Mamin A, Brun T, et al. Agenesis of human pancreas due to decreased half-life of insulin promoter factor 1. J Clin Endocrinol Metab. 2003;88:4398–4406. doi: 10.1210/jc.2003-030046 [DOI] [PubMed] [Google Scholar]
- 47.Malecki MT, Jhala US, Antonellis A, et al. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet. 1999;23:323–328. doi: 10.1038/70539 [DOI] [PubMed] [Google Scholar]
- 48.Gonsorcikova L, Pruhova S, Cinek O, et al. Autosomal inheritance of diabetes in two families characterized by obesity and a novel H241Q mutation in NEUROD1. Pediatr Diabetes. 2008;9(4 Pt 2):367–372. doi: 10.1111/j.1399-5448.2008.00379.x [DOI] [PubMed] [Google Scholar]
- 49.Rubio-Cabezas O, Minton JA, Kantor I, Williams D, Ellard S, Hattersley AT. Homozygous mutations in NEUROD1 are responsible for a novel syndrome of permanent neonatal diabetes and neurological abnormalities. Diabetes. 2010;59:2326–2331. doi: 10.2337/db10-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shields BM, McDonald TJ, Ellard S, Campbell MJ, Hyde C, Hattersley AT. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia. 2012;55:1265–1272. doi: 10.1007/s00125-011-2418-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiabetesGenes. MODY Probability Calculator. Available from: http://www.diabetesgenes.org/content/mody-probability-calcualtor. Accessed June 27, 2019.
- 52.Owen KR, Thanabalasingham G, James TJ, et al. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care. 2010;33:1919–1924. doi: 10.2337/dc10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald TJ, Shields BM, Lawry J, et al. High-sensitivity CRP discriminates HNF1A-MODY from other subtypes of diabetes. Diabetes Care. 2011;34:1860–1862. doi: 10.2337/dc11-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thanabalasingham G, Shah N, Vaxillaire M, et al. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia. 2011;54:2801–2810. doi: 10.1007/s00125-010-2040-1 [DOI] [PubMed] [Google Scholar]
- 55.Bonner C, Nyhan KC, Bacon S, et al. Identification of circulating microRNAs in HNF1A-MODY carriers. Diabetologia. 2013;56:1743–1751. doi: 10.1007/s00125-013-2939-4 [DOI] [PubMed] [Google Scholar]
- 56.Bakay M, Pandey R, Hakonarson H. Genes involved in type 1 daiabets: an update. Genes. 2013;4:499–521. doi: 10.3390/genes4030499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y, Chan L. Monogenic diabetes: what it teaches us on the common forms of type 1 and type 2 diabetes. Endocr Rev. 2016;37:190–222. doi: 10.1210/er.2015-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flannick J, Ber NL, Bick AG, et al. Assessing the phenotypic effects in the general population of rare variants in genes for a dominant Mendelian form of diabetes. Nat Genet. 2013;45:1380–1385. doi: 10.1038/ng.2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomsen SK, Gloyn AL. The pancreatic β cell: recent insights from human genetics. Trends Endocrinol Metab. 2014;25:425–434. doi: 10.1016/j.tem.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawakita R, Hosokawa Y, Fujimaru R, et al. Molecular and clinical characterization of glucokinase maturity-onset diabetes of the young (GCK-MODY) in Japanese patients. Diabet Med. 2014;31:1357–1362. doi: 10.1111/dme.12487 [DOI] [PubMed] [Google Scholar]
- 61.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1 [DOI] [PubMed] [Google Scholar]
- 62.Chan JCN, Malik V, Jin WP, et al. Diabetes in Asia. Epidemiology, risk factors and pathology. Jama. 2009;301:2129–2140. doi: 10.1001/jama.2009.726 [DOI] [PubMed] [Google Scholar]
- 63.Tfayli H, Bacha F, Gungor N, Arslanian A. Phenotypic type 2 diabetes in obese youth; insulin sensitivity and secretion in islet-cell antibody negative vs. antibody positive patients. Diabetes. 2009;58:738–744. doi: 10.2337/db08-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearson RB, Starkey BJ, Poweli RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycemia and response to treatment in diabetes. Lancet. 2003;362:1275–1281. doi: 10.1016/S0140-6736(03)14571-0 [DOI] [PubMed] [Google Scholar]
- 65.Fajans SS, Brown MB. Administration of sulfonylureas can increase glucose-induced insulin secretion for decades in patients with maturity-onset diabetes of the young. Diabetes Care. 1993;16:1254–1261. doi: 10.2337/diacare.16.9.1254 [DOI] [PubMed] [Google Scholar]
- 66.Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT. A genetic diagnosis of HNF-1A diabetes alters treatment and improves glycemic control in the majority of insulin-treated patients. Diabet Med. 2009;26:437–441. doi: 10.1111/j.1464-5491.2009.02690.x [DOI] [PubMed] [Google Scholar]
- 67.Raile K, Schober K, Konrad K, et al. Treatment of young patients with HNF1A mutations (HNF1A-MODY). Diabet Med. 2015;32:526–530. doi: 10.1111/dme.12662 [DOI] [PubMed] [Google Scholar]
- 68.Campbell PK. Glimepiride: role of a new sulfonylurea in the treatment of type 2 diabetes mellitus. Ann Pharmacother. 1998;32:1044–1052. doi: 10.1345/aph.17360 [DOI] [PubMed] [Google Scholar]
- 69.Urakami T. New insights into the pharmacological treatment of pediatric patients with type 2 diabetes. Clin Pediatr Endocrinol. 2018;27:1–8. doi: 10.1297/cpe.27.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Østoft SH, Babber JI, Hausen T, et al. Glucose-lowering effect and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double blind, randomized, crossover trial. Diabetes Care. 2014;37:1797–1805. doi: 10.2337/dc13-3007 [DOI] [PubMed] [Google Scholar]
- 71.Urakami T, Habu M, Okuno M, Suzuki J, Takahashi S, Yorifuji T. Three years of liraglutide treatment offers continuously optimal glycemic control in a pediatric patient with maturity-onset diabetes of the young type 3. J Pediatr Endocrinol Metab. 2015;28:327–331. doi: 10.1515/jpem-2014-0211 [DOI] [PubMed] [Google Scholar]
- 72.Tonouchi R, Mine Y, Aoki M, Okuno M, Suzuki J, Urakami T. Efficacy and safety of alogliptin in a pediatric patient with maturity-onset diabetes of the young type 1. Clin Pediatr Endocrinol. 2017;26:183–188. doi: 10.1297/cpe.26.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31(12):2315–2317. doi: 10.2337/dc08-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide mono therapy. Diabetes Obes Metab. 2009;11(2):167–176. doi: 10.1111/j.1463-1326.2008.01016.x [DOI] [PubMed] [Google Scholar]
- 75.Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicenter, randomized, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46–55. doi: 10.1111/j.1742-1241.2008.01933.x [DOI] [PubMed] [Google Scholar]
- 76.Murphy R, Ellard S, Hattersley AT. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab. 1974;4:200–213. doi: 10.1038/ncpendmet0778 [DOI] [PubMed] [Google Scholar]
- 77.Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19(Suppl. 27):28–46. doi: 10.1111/pedi.12719 [DOI] [PubMed] [Google Scholar]
- 78.Gat-Yablonski G, Shalitin S, Phillip M. Maturity onset diabetes of the young-review. Pediatr Endocrinol Rev. 2006;3(Suppl 3):514–520. [PubMed] [Google Scholar]