Abstract
Acute pancreatitis is a common disorder of the pancreas. It is the most frequent gastrointestinal cause for hospitalization and one of the leading causes of in-hospital deaths. Its severity ranges from mild self-limited disease to severe acute necrotizing pancreatitis characterized by systemic complications and multiorgan failure. Severe acute pancreatitis develops in about 20% of patients with acute pancreatitis and may be associated with multiorgan failure (respiratory, cardiovascular, and kidney). AKI is a frequent complication of severe acute pancreatitis and develops late in the course of the disease, usually after the failure of other organs. It carries a very poor prognosis, particularly if kidney replacement therapy is required, with mortality rates exceeding 75%. The exact pathophysiology of AKI in acute pancreatitis remains unclear but appears to result from initial volume depletion followed by complex vascular and humoral factors. Here, we provide an overview of the epidemiology, pathogenesis, causes, and management of AKI in patients with severe acute pancreatitis.
Keywords: Acute Pancreatitis; Acute Kidney Injury; Abdominal Compartment Syndrome; Kidney Replacement Therapy; Pancreatitis, Acute Necrotizing; Hypovolemia; Hospital Mortality; Renal Replacement Therapy; Multiple Organ Failure; kidney; Prognosis; hospitalization
Introduction
Acute pancreatitis is an inflammatory condition associated with a high complication rate and an increased risk of death. The diagnosis can be made by history, physical examination, and results of diagnostic tests (Table 1) (1). The revised Atlanta criteria classified acute pancreatitis according to type, severity, and phase of the disease (Table 2) (1). Imaging plays an important role in the diagnosis and staging of acute pancreatitis, establishing the cause and identifying its complications. Contrast-enhanced computed tomography is the most useful imaging technique especially when performed after 72 hours to assess the extent of the disease (1,2).
Table 1.
Diagnostic criteria of acute pancreatitis
| Diagnosis of acute pancreatitis is established by the presence of two of the following three features: |
|---|
| Abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back). |
| Serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal. |
| Characteristic findings of acute pancreatitis on contrast-enhanced computed tomography and less commonly magnetic resonance imaging or transabdominal ultrasonography |
Reprinted from reference 1, with permission.
Table 2.
The 2012 revision of the Atlanta classification of acute pancreatitis in adults
| Types |
|---|
| Interstitial edematous pancreatitis |
| Acute necrotizing pancreatitis |
| Severity |
| Mild |
| Most common form (up to 80% of cases) |
| Absence of organ failure |
| Absence of local or systemic complications |
| Usually resolves in the first week |
| Moderately severe |
| Presence of transient organ failure <48 h |
| Local complications may be present |
| Severe |
| About 20% of cases |
| Persistent organ failure for >48 h |
| Local complications include peripancreatic fluid collections, pancreatic and peripancreatic necrosis (sterile or infected), pseudocyst and walled-off necrosis (sterile or infected) |
| Poor outcome |
| Phases |
| Early: usually lasts for 1 wk |
| Late: may follow a protracted course from weeks to months |
Reprinted from reference 1, with permission.
The severe form of acute pancreatitis is associated with multiorgan failure and poor outcome. AKI has long been recognized as a common and important complication of acute pancreatitis. Unfortunately, there has been scarce information about this entity in the literature. In this review, we will provide an overview of epidemiology, pathophysiology, and management of AKI in patients with acute pancreatitis.
Epidemiology and Outcome
The prevalence of AKI in acute pancreatitis is not well documented (Table 3) (3–8). Previous studies highlighted the high prevalence of AKI in acute pancreatitis and its dismal prognosis. Most of these were limited by small samples and retrospective design. However, in a comprehensive, retrospective, observational study utilizing the National Inpatient Sample, Devani et al. reported an overall AKI prevalence of 7.9% among 3,466,493 patients hospitalized with acute pancreatitis (3). The mortality rate among AKI subgroup was significantly higher (8.8% in AKI group versus 0.7% in non-AKI; P<0.01). Prospective studies clearly needed to determine the actual incidence and prognosis of AKI in acute pancreatitis.
Table 3.
Prevalence and outcomes of AKI in acute pancreatitis in previous studies
| First Author/Country | Year | No. of Patients with Acute Pancreatitis | % of ICU Admissions | % of Patients with AKI | Definition of AKI | % of Patients with AKI who Received KRT | Mortality in Patients with Acute Pancreatitis and AKI | Scoring System |
|---|---|---|---|---|---|---|---|---|
| Tran/Netherlands (4) | 1993 | 267 | 44 | 16 | Cr> 3.2 mg/dl or a two-fold creatinine rise in patients with CKD | 26% (11/42), all died | 81% (34/42) hospital mortality | Ranson score (1±1.2 in non-AKI, 3.9±1.5 in AKI) |
| Kes/Croatia (5) | 1996 | 563 | 35 | 14 | NA | 62% (49/79), 95.9% died | 74.7% (59/79) overall mortality | Ranson score (1.1±0.7 in non-AKI, 3.8±1.4 in AKI) |
| Li/China (6) | 2010 | 228 | NA | 18.4a | Urine output <400 ml/d or Cr >2 mg/dl | NA | 66.6% (28/42) overall mortality | APACHE II score (9.65±4.40 in non-AKI, 11.71±5.60 in AKI) |
| Lin/Taiwan (7) | 2011 | 1734 | 100 | 15.0 | On the basis of ICD-9-CM codes | NA | 23.8% (62/261) ICU mortality | NA |
| Zhou/China (8) | 2015 | 414 | 100 | 69.3 | Absolute increase of serum Cr >0.3 mg/dl or 50% increase | 53.3% (153/287), 58.1% died | 44.9% (128.8/287) ICU mortality | APACHE II score 17±7.9 |
| Devani/United States (3) | 2018 | 3,466,493 | NA | 7.9 | On the basis of ICD-9-CM codes | NA | 8.8% (24,099/273,852.9) inpatient mortality | NA |
Overall mortality indicates that authors did not specify time or location of death. ICU, intensive care unit; KRT, kidney replacement therapy; Cr, serum creatinine; NA, not available; APACHE II, Acute Physiology and Chronic Health Evaluation II; ICD-9-CM, International Classification of Disease, ninth revision, Clinical Modification.
Denotes incidence. All studies are retrospective in nature.
AKI develops late in the course of acute pancreatitis, usually after failure of other organs (4,5). Remarkably, the kidney was the first organ to fail in only 8.9% of patients with AKI, and only a minority of patients develop isolated AKI (4,5,9). Retrospective studies reported risk factors for AKI in acute pancreatitis but not the need for kidney replacement therapy (KRT) (3–6). Li et al. reported history of kidney disease, hypoxemia, and abdominal compartment syndrome as risk factors (6), whereas Devani et al. found older age, male gender, sepsis, respiratory failure, intensive care unit admission, and history of CKD to increase risk for AKI (3). Of these, abdominal compartment is likely the only modifiable risk factor.
The mortality rate of patients with AKI and acute pancreatitis varied between 25% and 75% (5,7). However, Devani et al. found a three-fold fall in mortality rate among patients with AKI over the past decade (3).
Pathophysiology
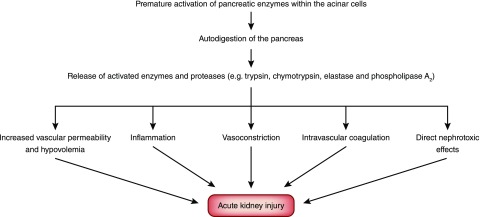
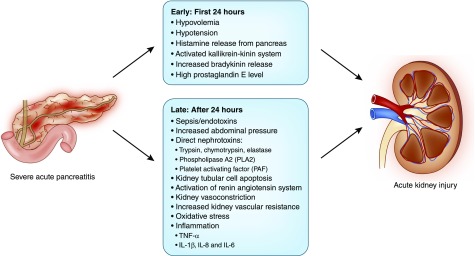
The pathophysiology of AKI in acute pancreatitis is not well studied. However, a key pathophysiologic process involves premature activation of pancreatic enzymes within the acinar cells. This leads to autodigestion of the pancreas and surrounding tissues, triggering a cascade of events that contribute to AKI (Figure 1) (10). Release into the systemic circulation of activated enzymes and proteases may cause endothelial damage leading to extravasation of fluids from the vascular space, hypovolemia, hypotension, increased abdominal pressure, intense kidney vasoconstriction, hypercoagulability, and fibrin deposition in the glomeruli. Moreover, acinar injury from autodigestion stimulates cytokine release and production of oxygen free radicals (Figure 2) (11–13).
Figure 1.
Premature activation of pancreatic enzymes within the acinar cells leads to autodigestion of the pancreas and release of enzymes and proteases which trigger a cascade of events that contribute to the pathogenesis of AKI.
Figure 2.
AKI in severe acute pancreatitis usually results from volume depletion due to extravasation of fluids from the vascular space followed by complex interactions between inflammatory, vascular and humoral factors.
Hypovolemia
Hypovolemia plays a critical role in causing AKI early during acute pancreatitis. This was first documented in dogs with experimental acute pancreatitis (11). At 4 hours after bile infusion and the development of pancreatitis, GFR fell by 40% and the plasma volume by 26% compared with the control group. There were no morphologic abnormalities in kidney biopsies, but the decline in GFR was prevented by plasma infusion. However, at 24 hours, kidney failure became unresponsive to further volume expansion. Remarkably, these results were reproduced by the infusion of trypsin, chymotrypsin, elastase, and phospholipase A2 (PLA2) into normal dogs. The authors speculated that released enzymes caused increased vascular permeability and leakage of protein-rich fluid into the interstitial compartment, leading to hypovolemia. In similar experiments in dogs, there was a rapid accumulation of ascites, increased hematocrit level, and decreased arterial pressure, suggesting hypovolemia (12). These experiments demonstrated the role for hypovolemia in AKI, at least in the initial 24 hours after the onset of acute pancreatitis (Figure 2).
Role of Toxic Substances Released from the Necrotic Pancreas
Substances released from the necrotic pancreas were implicated in the pathogenesis of AKI. These include trypsin, chymotrypsin, bradykinin, histamine, and prostaglandin E, as well as endotoxins and bacteria (11,12). Ofstad et al. (14) reported that histamine release in the pancreatic exudate of dogs with experimental acute pancreatitis caused increased vascular permeability, hypovolemia, and hypotension. In patients with acute pancreatitis and AKI, urine output improved by peritoneal lavage, suggesting that dialysis may remove substances that contributed to AKI (15). To investigate this further, Satake et al. (12) collected ascitic fluid from experimental dogs with acute pancreatitis and injected 10 ml intravenously in healthy dogs. The dogs developed transient hypotension that was not caused by hypovolemia as evidenced by lack of changes in hematocrit level. There was a significant decrease in kidney blood flow, GFR, urine output, and an increase in kidney vascular resistance, even after hypotension resolved. Kidney vasoconstriction was also documented in patients with acute pancreatitis despite adequate extracellular volume, suggesting increased sympathetic activity (16). Thus, although hypotension and hypovolemia may be the initial culprits in causing AKI early during acute pancreatitis, toxic substances in the pancreatic exudate may subsequently contribute to AKI.
Inflammation
Cytokines may contribute to the pathogenesis of AKI. TNF-α acts directly on glomeruli and tubular capillaries, leading to ischemia and tubular necrosis. It also stimulates release of other cytokines, such as IL-1β, IL-8, and IL-6, which act on endothelial cells leading to kidney ischemia, thrombosis, and release of oxygen free radicals (13). A study of 60 patients with acute pancreatitis found high levels of IL-6 and IL-8 in patients who developed AKI (17).
PLA2 rapidly deposited in proximal tubular cells of rats with experimental acute pancreatitis, causing tubular necrosis. A prospective study of 31 patients with acute pancreatitis found a positive correlation between serum PLA2 activity, as measured early during acute pancreatitis, and urinary N-acetyl-β-glucosaminidase–to–creatinine ratio, suggesting that PLA2 may hydrolyze tubular epithelial cell membrane and contribute to AKI (18). PLA2 also increases vascular permeability and generation of AA, which produces thromboxane. This potent vasoconstrictor and platelet aggregation promoter leads to microthrombi and vascular occlusion. This may be complicated by an increase in platelet-activating factor, which induces platelet activation, aggregation and generation of inflammatory mediators (13).
Inflammatory mediators may increase mucosal permeability leading to translocation of endotoxin and bacteria from the colon. Endotoxins contribute to the development of AKI by increasing endothelin level, which causes vasoconstriction, decreased kidney blood flow, and tubular necrosis (19). Also, oxygen free radicals may react with proteins and enzymes, leading to lipid peroxidation of the cell and organellar membranes, protein denaturation and increased capillary permeability, ischemia, and direct kidney cell membrane injury (13).
Finally, apoptotic cell death may also play a role in AKI. A Japanese group detected DNA fragmentation in Madin–Darby canine kidney cells exposed to pancreatitis-associated ascitic fluid. They also found nuclear and DNA fragmentation in Wister rat kidneys after intraperitoneal injection of pancreatitis-associated ascitic fluid, indicating that ascitic fluid contains factor(s) that induce apoptosis (20).
Vascular Effects of Acute Pancreatitis
Increased kidney vascular resistance was demonstrated in dogs with experimental acute pancreatitis (21). Werner et al. found an increase in kidney vascular resistance in 11 patients with acute pancreatitis. All patients became hypertensive indirectly implicating a vasopressor substance release (16). Patients with acute pancreatitis had six-fold higher plasma renin values than normal, which could be attributed to hypovolemia (22). However, plasma trypsin and kallikrein activate prorenin to renin, which in turn increases angiotensin II levels, leading to increased kidney vascular resistance, decreased effective kidney blood flow, and decline in GFR (22,23).
Abdominal Compartment Syndrome
Abdominal compartment syndrome develops when intra-abdominal pressure increases to >20 mm Hg (24,25). Patients with severe acute pancreatitis are at risk of developing abdominal compartment syndrome because of increased intra-abdominal contents by ileus, ascites, and intra-abdominal bleeding. Moreover, interstitial fluid accumulation due to increased capillary permeability and endothelial damage from volume administration, acidosis, sepsis, transfusion, coagulopathy, as well as reduced abdominal wall compliance due to edema, may play a role (24,25). The mechanism of intra-abdominal hypertension induced AKI is not clear, but intra-abdominal hypertension may compress and compromise the kidney blood flow in both the arterial and venous vasculature, leading to decreased perfusion pressure, increased venous pressure, decreased venous blood flow, and increased kidney parenchymal pressure. This results in decreased glomerular filtration pressure, and impaired microvascular function and oxygen delivery, and precipitates ischemic kidney injury.
Causes of AKI
The causes of AKI are speculative according to the above-proposed pathophysiology. Hypovolemia and sepsis can lead to prekidney AKI, acute tubular necrosis and, rarely, bilateral kidney cortical necrosis (26). As mentioned above, intra-abdominal hypertension is very common in patients with acute pancreatitis and can lead to kidney impairment. AKI also can result from associated diseases such as autoimmune disease, hemolytic-uremic syndrome, or thrombotic thrombocytopenic purpura, as well as from drugs causing acute pancreatitis and AKI, but this information is limited and on the basis of a few case reports published in the literature. Unfortunately, patients with acute pancreatitis and AKI are usually critically ill, and as a result, a kidney biopsy is rarely performed.
Management
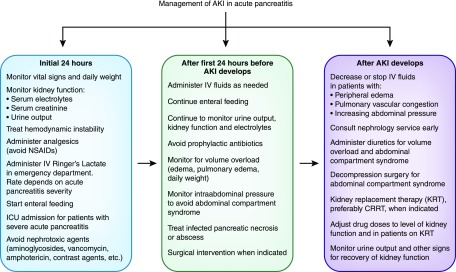
Management of AKI in severe acute pancreatitis involves intravenous fluid administration, avoidance of nephrotoxic agents, the minimizing intra-abdominal pressure, and providing KRT when indicated. The routine use of prophylactic antibiotic is not recommended unless there is evidence of active infection. If antibiotic is given, nephrotoxic ones should be avoided (Figure 3) (2,10). Similarly, not all patients with acute pancreatitis need to undergo contrast-enhanced computed tomography particularly those with mild pancreatitis and those who improve rapidly to reduce the risk of contrast nephrotoxicity. Magnetic resonance imaging can be an alternative as it has excellent soft tissue contrast, but gadolinium administration has been implicated in the development of nephrogenic systemic fibrosis. Abdominal ultrasound has little value in the diagnosis of pancreatitis or its complications, but can be useful in detecting gallstones or biliary duct stones (1,2).
Figure 3.
Management of AKI in acute pancreatitis requires a multidisciplinary approach that begins in the emergency department. This involves providing supportive care, close monitoring of kidney function and treatment of pancreatitis related complications. Escalation of therapy to a higher level of care, providing KRT and surgical treatment might be required if the initial therapy fails. CRRT, continuous RRT; ICU, intensive care unit; IV, intravenous; NSAIDs, nonsteroidal anti-inflammatory drugs.
Diagnosis of AKI is currently according to the Kidney Disease Improving Global Outcomes criteria. However, recent studies have suggested that biomarkers of kidney injury, such as urine concentrations of neutrophil gelatinase-associated lipocalin, kidney injury molecule 1, IL-18, angiopoietin 2, and procalcitonin, may help in earlier diagnosis of AKI (27,28). Procalcitonin level was superior to other inflammatory mediators in predicting AKI in patients with acute pancreatitis and in identifying patients at risk of worsening kidney function (28). Currently, there are no data to indicate that these biomarkers perform better in AKI associated with acute pancreatitis than other types of AKI. Further research will be required before biomarkers can be used to predict the onset of AKI and to guide its early management.
Fluid Management
An essential initial step in the management is the administration of ample intravenous fluids. Few human studies evaluated the optimal type, rate, duration, complications, and outcomes of fluid administration in acute pancreatitis (2). Although some clinical trials have shown a clear benefit of aggressive hydration, others reported that aggressive hydration might be associated with increased morbidity and mortality (2). The American College of Gastroenterology recommends that early aggressive intravenous hydration is most beneficial in the first 12–24 hours, but does have a little benefit beyond this time (2). The goal of fluid resuscitation is to improve the mean arterial pressure, central venous pressure, and urine output. Patients with severe hypovolemia may initially require 500–1000 ml of fluids per hour, whereas those with less severe volume depletion may require 300–500 ml per hour (10). However, infusion rate should be reduced in patients with heart failure, liver cirrhosis, and oliguric/anuric AKI. Fluid balance is commonly positive in critically ill patients with AKI and should be avoided as it can lead to increased morbidity and mortality due to pulmonary edema, tissues hypoxia, increased need for mechanical ventilation, and cardiac dysfunction (29). In these patients, loop diuretics can be considered.
In a retrospective study of 99 patients with severe acute pancreatitis in Sweden, patients who received 4000 ml or more of fluids during the first 24 hours developed significantly higher respiratory complications rates (66% versus 53%; P<0.001), and admission to intensive care units was also more common compared with patients who received <4000 ml (47% versus 20%; P<0.001), but there was no difference in patients’ outcomes (30). Aggressive volume resuscitation (≥7.5 L within 6 hours from hospital admission) was also found to be an independent predictor of developing abdominal compartment syndrome (31). Another study of 179 patients with moderately severe acute pancreatitis showed that aggressive fluid resuscitation (≥4 L) was associated with increased incidence of AKI compared the nonaggressive group (53.12% versus 25.64%; P=0.008), and longer AKI lasting time (P=0.04) (32).
By contrast, a recent four-center retrospective cohort study of 1010 patients with acute pancreatitis divided fluid administered from arrival to the emergency room to 4 hours after diagnosis of acute pancreatitis into tertiles: nonaggressive (<500 ml), moderate (500–1000 ml), and aggressive (>1000 ml). Compared with the nonaggressive fluid group, the moderate and aggressive resuscitations were associated with lower rates of complications and invasive interventions (33). Thus, although adequate intravenous fluid administration in the first 24 hours is indicated, excessive fluid administration beyond this period may be harmful and should be avoided.
It is unclear what type of fluids is most beneficial for patients with acute pancreatitis. Observational studies reported that chloride-rich solutions are associated with a higher risk of AKI and the need for KRT than balanced solutions (34). In a retrospective study of 145 patients with moderately severe and severe acute pancreatitis, hyperchloremia was associated with an increased incidence of AKI. On multivariable analysis, the increase in serum chloride was independently associated with AKI (odds ratio, 1.32; 95% confidence interval, 1.00 to 1.74; P=0.04). The authors suggested that chloride-rich solutions decreased kidney blood flow and GFR via tubuloglomerular feedback activation (35). However, randomized, controlled trials have not demonstrated the superiority of balanced solutions over chloride-rich solutions (36). The American College of Gastroenterology and International Association of Pancreatology (IAP)/American Pancreatic Association (APA) guidelines suggest that lactated Ringer solution might be the preferred fluid replacement (2,37). In a prospective, multicenter, randomized study, Wu et al.(38) reported that lactated Ringer solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Although it is unlikely that lactated Ringer solution, which contains 4 mEq/L of potassium, will cause hyperkalemia, we recommend close monitoring of serum potassium level.
Nutrition
The paradigm has shifted dramatically over the past decade, with new evidence suggesting that early enteral feeding is beneficial in acute pancreatitis. Delayed feeding for >24 hours is associated with higher rates of infected peripancreatic necrosis, multiple organ failure, and necrotizing pancreatitis (2). It was hypothesized that enteral nutrition protects the mucosal barrier of the gut and reduces bacterial translocation. Parenteral nutrition should be avoided unless the enteral route is not possible. To our knowledge, there are no studies that specifically examined the nutritional needs of patients with AKI and acute pancreatitis.
Monitoring Intra-Abdominal Pressure
Close monitoring of intra-abdominal pressure is essential in all patients with acute pancreatitis. This can be measured directly by an intraperitoneal catheter, or indirectly by gastric or urinary bladder pressure. However, transduction of bladder pressure remains the gold standard (24). Percutaneous drainage of ascites or continuous hemodiafiltration may decrease intra-abdominal pressure, but some patients require decompressive laparotomy, particularly in patients with intra-abdominal pressure >25 mm Hg (25).
The Working Group IAP/APA Acute Pancreatitis Guidelines to decrease intra-abdominal pressure in acute pancreatitis recommend: (1) nasogastric drainage, prokinetics, rectal tubes, and if necessary, endoscopic decompression; (2) volume resuscitation on demand, if volume overloaded either ultrafiltration or diuretics can be used; and (3) adequate analgesia and sedation to decrease abdominal muscle tone, and if necessary, neuromuscular blockade (37).
KRT
Indications and timing of KRT in patients with AKI after acute pancreatitis are not different from those of other critically ill patients with AKI. Urgent indications for KRT include severe hyperkalemia, severe metabolic acidosis (pH<7.1) and pulmonary edema unresponsive to diuretic therapy, irrespective of kidney function. The timing of elective initiation of KRT is debatable. A randomized, controlled trial failed to show a significant difference in the 90 days mortality between early and late initiation groups (39). The patient’s hemodynamic status usually dictates the choice of KRT modality. Intermittent hemodialysis, extended daily dialysis, or slow low-efficiency daily dialysis are reserved for hemodynamically stable patients, whereas continuous RRT (CRRT) is preferred in hemodynamically unstable patients. A few clinical trials have specifically examined the benefits of one modality of KRT over the other in these patients. A possible advantage of CRRT is its ability to decrease serum cytokine levels (Table 4). However, a low-intensity CVVH may not be adequate because of the large amount of circulating inflammatory mediators in patients with severe acute pancreatitis. In this regard, limited data suggest that high-volume CVVH, particularly when started early, may be more effective (Table 4). However, the evidence so far is not strong enough to recommend its routine use for such patients.
Table 4.
Blood purification modalities in patients with severe acute pancreatitis
| First Author/Country | Study Design (Year) | No. of Patients with Acute Pancreatitis | Blood Purification Modality | Outcomes |
|---|---|---|---|---|
| Oda/Japan (40) | Prospective observational (2005) | 17 | Continuous hemodiafiltration | Intra-abdominal pressure and IL-6 were significantly lower after 24 h of continuous hemodiafiltration initiation |
| Jiang/China (41) | Randomized controlled (2005) | 37 | Low-volume versus high-volume CVVH, early versus late CVVH | High-volume CVVH and early CVVH improved hemodynamics and survival in patients with acute pancreatitis more than low-volume CVVH and late CVVH |
| High-volume CVVH and early CVVH decreased serum concentrations of TNF-α, IL-1β, and IL-6 more efficiently than low-volume CVVH and late CVVH | ||||
| Chen/China (42) | Prospective observational (2007) | 20 | CVVH | The APACHE II score improved significantly after CVVH with improvement in endothelial dysfunction |
| Zhang/China (43) | Randomized controlled (2010) | 63 | CVVH versus standard medical therapy | CVVH improved APACHE II score and SOFA score significantly, and effectively improved gut barrier dysfunction |
| Gong/China (44) | Prospective nonrandomized (2010) | 12 | High-volume CVVH versus standard medical therapy | High-volume CVVH significantly reduced plasma inflammatory cytokines concentrations including those of IFN-γ, TNF-α, IL-1, IL-2, IL-5, and IL-13. Peripheral CD4+ and CD8+ T cells, monocyte count, and HLA-DR expression increased significantly only in the high-volume CVVH group |
| Yang/China (45) | Randomized controlled (2010) | 51 | Combined CVVH and peritoneal dialysis versus traditional therapy | Inflammatory cytokines (IL-6, IL-8, and TNF-α) decreased significantly at days 1 and 2 compared with control group |
| The APACHE II score of the combined CVVH and peritoneal dialysis significantly decreased compared with the control group | ||||
| Zhu/China (46) | Prospective nonrandomized (2011) | 75 | High-volume CVVH versus conventional treatment | The 28-d survival rate was higher in patients who accepted high-volume CVVH, especially in those without AKI. After 72 h of therapy, patients who accepted high-volume CVVH had significantly better APACHE II scores |
| Chu/China (47) | Randomized controlled (2013) | 30 | Pulse high-volume hemofiltration versus CVVH | The levels of IL-6, IL-10, and TNF-α decreased in the pulse high-volume hemofiltration group more significantly than the control group |
| Pulse high-volume hemofiltration group was superior to the control group in APACHE II score, C-reactive protein, SOFA score, and SAPS II score | ||||
| Guo/China (48) | Prospective nonrandomized (2014) | 61 | High-volume CVVH versus optimal standard therapy | High-volume CVVH was associated with a significant reduction in the incidence of kidney failure, infected pancreatic necrosis, length of hospitalization, mortality, as well as duration of kidney, respiratory, and hepatic failure |
| APACHE II score, C-reactive protein, and IL-6 levels were significantly reduced by high-volume CVVH on days 1, 3, and 7 | ||||
| Liu/China (49) | Randomized controlled (2017) | 86 | High-volume CVVH versus CVVH | The levels of IL-4, IL-6, IL-8, and IL-10, as well as procalcitonin and TNF-α decreased in the high-volume CVVH group more than in the control group |
| Xu/China (50) | Prospective nonrandomized (2017) | 25 | CVVH versus conventional treatment | CVVH more effective in decreasing intra-abdominal pressure and blood level of IL-8 |
CVVH, continuous venovenous hemofiltration; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; SAPS II, Simplified Acute Physiology Score II.
Research Needs and Conclusions
AKI is a frequent complication of acute pancreatitis and usually develops after the failure of other organs. It carries a poor prognosis, particularly if KRT is required. There is a need for prospective studies to establish the true incidence and risk factors for AKI in acute pancreatitis. The exact pathophysiology of AKI remains unclear, but appears to result from initial hypovolemia followed by complex interactions between inflammatory, vascular, and humoral factors. More studies are clearly needed particularly on the nephrotoxic potential of toxic substances released from the necrotic pancreas and inflammatory cytokines. There is also a lack of human biopsy studies in this condition because most patients are acutely ill. Prospective clinical studies that examine the impact of various therapeutic modalities on patients’ outcomes, including type and volume of intravenous fluid resuscitation, nutritional support, and KRT, are needed.
Disclosures
Dr. Nassar and Dr. Qunibi have nothing to disclose.
Acknowledgments
The authors would like to thank Dr. W. Brian Reeves and Dr. Kumar Sharma for reviewing the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group: Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut 62: 102–111, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology. American college of gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol 108: 1400–1415; 1416, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Devani K, Charilaou P, Radadiya D, Brahmbhatt B, Young M, Reddy C: Acute pancreatitis: Trends in outcomes and the role of acute kidney injury in mortality- A propensity-matched analysis. Pancreatology 18: 870–877, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Tran DD, Oe PL, de Fijter CW, van der Meulen J, Cuesta MA: Acute renal failure in patients with acute pancreatitis: Prevalence, risk factors, and outcome. Nephrol Dial Transplant 8: 1079–1084, 1993 [PubMed] [Google Scholar]
- 5.Kes P, Vucicević Z, Ratković-Gusić I, Fotivec A: Acute renal failure complicating severe acute pancreatitis. Ren Fail 18: 621–628, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Li H, Qian Z, Liu Z, Liu X, Han X, Kang H: Risk factors and outcome of acute renal failure in patients with severe acute pancreatitis. J Crit Care 25: 225–229, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Lin HY, Lai JI, Lai YC, Lin PC, Chang SC, Tang GJ: Acute renal failure in severe pancreatitis: A population-based study. Ups J Med Sci 116: 155–159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Li Y, Tang Y, Liu F, Yu S, Zhang L, Zeng X, Zhao Y, Fu P: Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology (Carlton) 20: 485–491, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Gougol A, Dugum M, Dudekula A, Greer P, Slivka A, Whitcomb DC, Yadav D, Papachristou GI: Clinical outcomes of isolated renal failure compared to other forms of organ failure in patients with severe acute pancreatitis. World J Gastroenterol 23: 5431–5437, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandol SJ, Saluja AK, Imrie CW, Banks PA: Acute pancreatitis: Bench to the bedside. Gastroenterology 132: 1127–1151, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Levy M, Geller R, Hymovitch S: Renal failure in dogs with experimental acute pancreatitis: Role of hypovolemia. Am J Physiol 251: F969–F977, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Satake K, Kanazawa G, Hiura A, Nishiwaki H, Ha SS, Chung YS, Umeyama K, Yukimura T: Renal function in experimentally induced acute pancreatitis in dogs: How it is affected by the nephrotoxic substance in pancreatic exudate from ascitic fluid. Jpn J Surg 21: 88–95, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Zhang XP, Wang L, Zhou YF: The pathogenic mechanism of severe acute pancreatitis complicated with renal injury: A review of current knowledge. Dig Dis Sci 53: 297–306, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Ofstad E, Amundsen E, Hagen PO: Experimental acute pancreatitis in dogs. II. Histamine release induced by pancreatic exudate. Scand J Gastroenterol 4: 75–79, 1969 [DOI] [PubMed] [Google Scholar]
- 15.Wall AJ: Peritoneal dialysis in the treatment of severe acute pancreatitis. Med J Aust 2: 281–283, 1965 [PubMed] [Google Scholar]
- 16.Werner MH, Hayes DF, Lucas CE, Rosenberg IK: Renal vasoconstriction in association with acute pancreatitis. Am J Surg 127: 185–190, 1974 [DOI] [PubMed] [Google Scholar]
- 17.Malmstrøm ML, Hansen MB, Andersen AM, Ersbøll AK, Nielsen OH, Jørgensen LN, Novovic S: Cytokines and organ failure in acute pancreatitis: Inflammatory response in acute pancreatitis. Pancreas 41: 271–277, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Grönroos JM, Hietaranta AJ, Nevalainen TJ: Renal tubular cell injury and serum phospholipase A2 activity in acute pancreatitis. Br J Surg 79: 800–801, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Bose SM, Verma GR, Mazumdar A, Giridhar M, Ganguly NK: Significance of serum endotoxin and antiendotoxin antibody levels in predicting the severity of acute pancreatitis. Surg Today 32: 602–607, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Takeyama Y: Significance of apoptotic cell death in systemic complications with severe acute pancreatitis. J Gastroenterol 40: 1–10, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishiwaki H, Ko I, Hiura A, Ha SS, Satake K, Sowa M: Renal microcirculation in experimental acute pancreatitis of dogs. Ren Fail 15: 27–31, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Greenstein RJ, Krakoff LR, Felton K: Activation of the renin system in acute pancreatitis. Am J Med 82: 401–404, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Uehara S, Honjyo K, Furukawa S, Hirayama A, Sakamoto W: Role of the kallikrein-kinin system in human pancreatitis. Adv Exp Med Biol 247B: 643–648, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Patel DM, Connor MJ Jr: Intra-abdominal hypertension and abdominal compartment syndrome: An underappreciated cause of acute kidney injury. Adv Chronic Kidney Dis 23: 160–166, 2016 [DOI] [PubMed] [Google Scholar]
- 25.De Waele JJ, De Laet I, Kirkpatrick AW, Hoste E: Intra-abdominal hypertension and abdominal compartment syndrome. Am J Kidney Dis 57: 159–169, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Goffin EJ, Coche EE, Lambert MJ: The case: Acute renal failure following necroticohemorrhagic pancreatitis. Kidney Int 74: 975–976, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Sporek M, Dumnicka P, Gala-Bladzinska A, Ceranowicz P, Warzecha Z, Dembinski A, Stepien E, Walocha J, Drozdz R, Kuzniewski M, Kusnierz-Cabala B: Angiopoietin-2 is an early indicator of acute pancreatic-renal syndrome in patients with acute pancreatitis. Mediators Inflamm 2016: 5780903, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang HL, Nie X, Cai B, Tang JT, He Y, Miao Q, Song HL, Luo TX, Gao BX, Wang LL, Li GX: Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: A prospective study. PLoS One 8: e82250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrier RW: Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 5: 733–739, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Eckerwall G, Olin H, Andersson B, Andersson R: Fluid resuscitation and nutritional support during severe acute pancreatitis in the past: What have we learned and how can we do better? Clin Nutr 25: 497–504, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Balogh Z, McKinley BA, Holcomb JB, Miller CC, Cocanour CS, Kozar RA, Valdivia A, Ware DN, Moore FA: Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma 54: 848–859, discussion 859–861, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Ye B, Mao W, Chen Y, Tong Z, Li G, Zhou J, Ke L, Li W: Aggressive resuscitation is associated with the development of acute kidney injury in acute pancreatitis. Dig Dis Sci 64: 544–552, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Singh VK, Gardner TB, Papachristou GI, Rey-Riveiro M, Faghih M, Koutroumpakis E, Afghani E, Acevedo-Piedra NG, Seth N, Sinha A, Quesada-Vázquez N, Moya-Hoyo N, Sánchez-Marin C, Martínez J, Lluís F, Whitcomb DC, Zapater P, de-Madaria E: An international multicenter study of early intravenous fluid administration and outcome in acute pancreatitis. United European Gastroenterol J 5: 491–498, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M: Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: An extended analysis. Intensive Care Med 41: 257–264, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Mao W, Wu J, Zhang H, Zhou J, Ye B, Li G, Gao L, Li X, Ke L, Tong Z, Li W, Li J: Increase in serum chloride and chloride exposure are associated with acute kidney injury in moderately severe and severe acute pancreatitis patients. Pancreatology 19: 136–142, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, McGuinness S, Mehrtens J, Myburgh J, Psirides A, Reddy S, Bellomo R; SPLIT Investigators; ANZICS CTG: Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: The SPLIT randomized clinical trial. JAMA 314: 1701–1710, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Working Group IAP/APA Acute Pancreatitis Guidelines: IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 13[Suppl 2]: e1–e15, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL: Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 9: 710–717.e1, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyère R, Lebert C, Bohé J, Badie J, Eraldi JP, Rigaud JP, Levy B, Siami S, Louis G, Bouadma L, Constantin JM, Mercier E, Klouche K, du Cheyron D, Piton G, Annane D, Jaber S, van der Linden T, Blasco G, Mira JP, Schwebel C, Chimot L, Guiot P, Nay MA, Meziani F, Helms J, Roger C, Louart B, Trusson R, Dargent A, Binquet C, Quenot JP; IDEAL-ICU Trial Investigators and the CRICS TRIGGERSEP Network: Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 379: 1431–1442, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Oda S, Hirasawa H, Shiga H, Matsuda K, Nakamura M, Watanabe E, Moriguchi T: Management of intra-abdominal hypertension in patients with severe acute pancreatitis with continuous hemodiafiltration using a polymethyl methacrylate membrane hemofilter. Ther Apher Dial 9: 355–361, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Jiang HL, Xue WJ, Li DQ, Yin AP, Xin X, Li CM, Gao JL: Influence of continuous veno-venous hemofiltration on the course of acute pancreatitis. World J Gastroenterol 11: 4815–4821, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen ZH, Liu ZH, Yu C, Ji DX, Li LS: Endothelial dysfunction in patients with severe acute pancreatitis: Improved by continuous blood purification therapy. Int J Artif Organs 30: 393–400, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Yuan C, Hua G, Tong R, Luo X, Ying Z: Early gut barrier dysfunction in patients with severe acute pancreatitis: Attenuated by continuous blood purification treatment. Int J Artif Organs 33: 706–715, 2010 [PubMed] [Google Scholar]
- 44.Gong D, Zhang P, Ji D, Chen Z, Li W, Li J, Li L, Liu Z: Improvement of immune dysfunction in patients with severe acute pancreatitis by high-volume hemofiltration: A preliminary report. Int J Artif Organs 33: 22–29, 2010 [PubMed] [Google Scholar]
- 45.Yang C, Guanghua F, Wei Z, Zhong J, Penghui J, Xin F, Xiping Z: Combination of hemofiltration and peritoneal dialysis in the treatment of severe acute pancreatitis. Pancreas 39: 16–19, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, Yuan J, Zhang P, Hu X, He Q, Han F, Chen J: Adjunctive continuous high-volume hemofiltration in patients with acute severe pancreatitis: A prospective nonrandomized study. Pancreas 40: 109–113, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Chu LP, Zhou JJ, Yu YF, Huang Y, Dong WX: Clinical effects of pulse high-volume hemofiltration on severe acute pancreatitis complicated with multiple organ dysfunction syndrome. Ther Apher Dial 17: 78–83, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Guo J, Huang W, Yang XN, Jin T, Altaf K, Javed MA, Lin ZQ, Huang ZW, Xue P, Johnstone M, Sutton R, Xia Q: Short-term continuous high-volume hemofiltration on clinical outcomes of severe acute pancreatitis. Pancreas 43: 250–254, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Liu C, Li M, Cao S, Wang J, Huang X, Zhong W: Effects of HV-CRRT on PCT, TNF-α, IL-4, IL-6, IL-8 and IL-10 in patients with pancreatitis complicated by acute renal failure. Exp Ther Med 14: 3093–3097, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J, Cui Y, Tian X: Early continuous veno-venous hemofiltration is effective in decreasing intra-abdominal pressure and serum interleukin-8 level in severe acute pancreatitis patients with abdominal compartment syndrome. Blood Purif 44: 276–282, 2017 [DOI] [PubMed] [Google Scholar]