Visual Abstract
Keywords: chronic kidney disease; tuberculosis; Epidemiology and outcomes; Incidence; Prevalence; risk factors; albuminuria; Mycobacterium tuberculosis; Control Groups; renal dialysis; Body Mass Index; Retrospective Studies; Renal Insufficiency, Chronic; Comorbidity; Renal Replacement Therapy; hypertension; Pulmonary Disease, Chronic Obstructive; diabetes mellitus; Smoking; National Health Programs
Abstract
Background and objectives
The incidence and risk of Mycobacterium tuberculosis in people with predialysis CKD has rarely been studied, although CKD prevalence is increasing in certain countries where Mycobacterium tuberculosis is endemic. We aimed to investigate the association between predialysis CKD and active Mycobacterium tuberculosis risks in a nation with moderate Mycobacterium tuberculosis risk.
Design, setting, participants, & measurements
In this nationwide retrospective cohort study, we reviewed the National Health Insurance Database of Korea, screening 17,020,339 people who received a national health screening two or more times from 2012 to 2016. Predialysis CKD was identified with consecutive laboratory results indicative of CKD (e.g., persistent eGFR <60 ml/min per 1.73 m2 or dipstick albuminuria). People with preexisting active Mycobacterium tuberculosis or kidney replacement therapy were excluded. A 1:1 matched control group without CKD was included with matching for age, sex, low-income status, and smoking history. The risk of incident active Mycobacterium tuberculosis, identified in the claims database, was assessed by the multivariable Cox regression model, which included both matched and unmatched variables (e.g., body mass index, diabetes, hypertension, places of residence, and other comorbidities).
Results
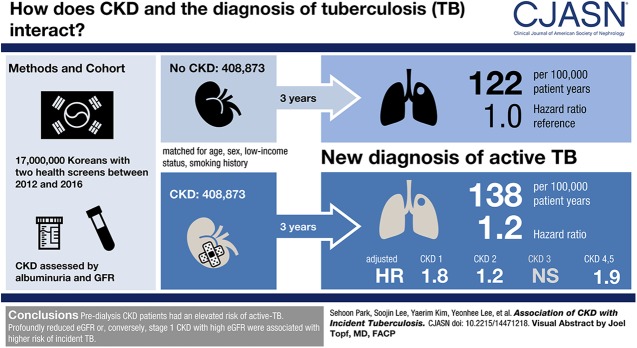
We included 408,873 people with predialysis CKD and the same number of controls. We identified 1704 patients with active Mycobacterium tuberculosis (incidence rate =137.5/100,000 person-years) in the predialysis CKD group and 1518 patients with active Mycobacterium tuberculosis (incidence rate =121.9/100,000 person-years) in the matched controls. The active Mycobacterium tuberculosis risk was significantly higher in the predialysis CKD group (adjusted hazard ratio, 1.21; 95% confidence interval, 1.13 to 1.30). The risk factors for active Mycobacterium tuberculosis among the predialysis CKD group were old age, men, current smoking, low income, underlying diabetes, chronic obstructive pulmonary disease, and Kidney Disease Improving Global Outcomes CKD stage 1 (eGFR≥90 ml/min per 1.73 m2 with persistent albuminuria) or stage 4/5 without dialysis (eGFR<30 ml/min per 1.73 m2).
Conclusions
In the Korean population, the incidence of active Mycobacterium tuberculosis was higher in people with versus without predialysis CKD.
Introduction
CKD is a rapidly growing global medical problem (1–3). In addition to the previously highlighted risk of adverse cardiovascular events in people with CKD, noncardiovascular comorbidities, including infections, significantly affect the prognosis of people with CKD (4). Among the infectious conditions, Mycobacterium tuberculosis (TB), which is one of the top ten causes of global deaths (5), is endemic in certain nations where CKD prevalence is increasing (6).
It is well established that dialysis-dependent patients or kidney transplant recipients have an elevated risk for TB (7–9), and the linkage has been explained by their immunosuppressed states and shared risk factors (10). Considering that one’s immune function starts to become impaired from the early stages of CKD (11,12), it was suggested that TB risk may also be higher in those people with mild to moderate CKD. However, large-scale evidence regarding TB risk in people with predialysis CKD has rarely been reported due to the lack of a cohort that included a sufficient number of people with predialysis CKD in a nation with a certain level of TB prevalence (6). Because people with CKD may require additional clinical attention for their vulnerability to TB (13), such evidence is essential to guide surveillance for active TB and help health care providers manage TB in a globally increasing population with predialysis CKD (1,2).
In this study, we aimed to study the linkage between CKD and active TB by investigating a large-scale predialysis CKD cohort including nearly a half a million people with CKD confirmed with sequential laboratory examinations and comparing their TB risks with a matched control group. We hypothesized that analyzing the large-scale database in a country with a moderately active TB incidence (Korea) would reveal the association between CKD and risk of active TB.
Materials and Methods
Ethical Considerations
The institutional review board of Seoul National University Hospital (institutional review board no. E-1801–027–913) approved the study. The attending government organization approved the usage of the National Health Insurance Service (NHIS, no.2018-1-148). This study was conducted in accordance with the Declaration of Helsinki.
Study Setting
The study was a nationwide population-based cohort study reviewing the National Health Insurance Database (NHID), which was provided by the NHIS of the Republic of Korea (14). From the year 2010, Korea’s NHIS has provided additional insurance coverage for all patients diagnosed with active TB to decrease the burden of TB in the nation, and specific insurance codes were obligatorily applied to patients with active TB after confirmation of their diagnosis. Because the NHID includes complete information on insured medical services throughout the country, the claims database enabled us to review all patients with active TB in the nation by means of their unique insurance codes (15).
In Korea, similar to in other nations, dialysis dependency and transplantation are considered as the risk factors for active TB, but no additional recommendation for active TB surveillance was present for the predialysis CKD population. Patients with latent TB did not receive the specific insurance codes and were not considered in this study.
The general health screening program is run by the NHIS, which is provided for office workers/nonworkplace subscribers every 2 years and nonoffice workers every year. The proportion of people receiving this charge-free health screening was >70% in the year 2011 among a target population of approximately 15 million people. Because general health screening measures serum creatinine values and urine dipstick albuminuria every time, CKD stages were identifiable by reviewing the information. The registered centers that provide the general health screening are quality controlled and regulated by a government organization; however, their laboratory equipment manufacturers are not unified.
Study Population
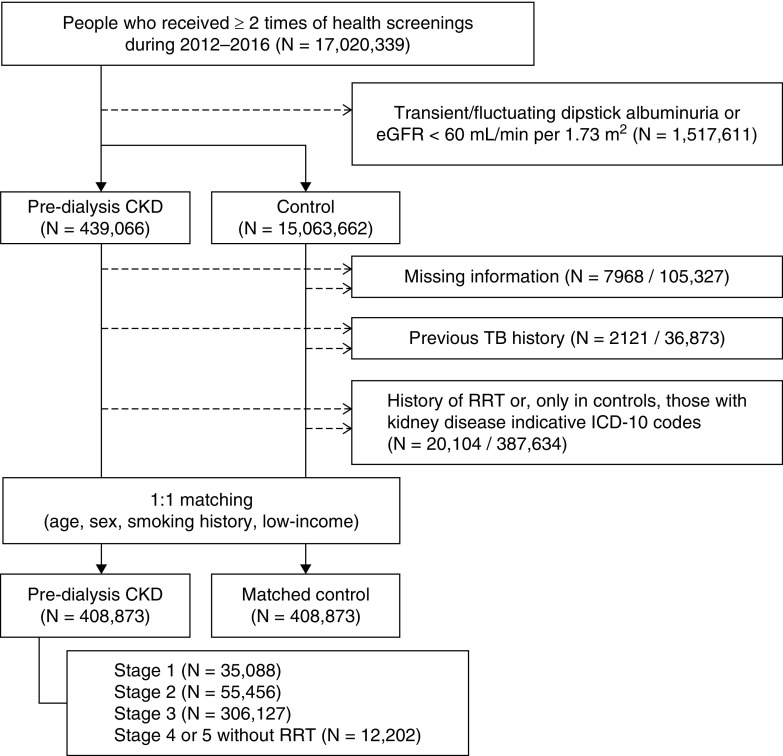
We screened the adult (age ≥19 years old) health examinees who received two or more examinations from 2012 to 2016 including non-isotope dilution mass spectrometry serum creatinine measurements by the Jaffe kinetic method, because this method was used for the majority of serum creatinine measurements in the health screening dataset. Patients excluded on the basis of the criteria were (1) those with a previous TB history identified by the specific insurance or International Classifications of Diseases, tenth revision (ICD-10) diagnostic codes; (2) those with a history of kidney replacement therapy or kidney transplantation at baseline or before diagnosis of TB; and (3) those who had their serum creatinine measured by a method other than the Jaffe kinetic method. To include those who had stable CKD or CKD-free state, (4) those who had transient or fluctuating laboratory results indicative of CKD or laboratory findings indicative of newly developed CKD during the study period were excluded. Because we intended to collect a control group free from CKD, (5) those who had CKD-related ICD-10 diagnostic codes were also excluded from the control group (Supplemental Material) (16). The examples of the study population, the index inclusion date, the follow-up duration, and the excluded patients are shown in Supplemental Figure 1.
Study Groups
The study group included people with predialysis CKD. The presence of predialysis CKD was determined when evidence of CKD (i.e., the presence of dipstick albuminuria [1+ or more] or eGFR<60 ml/min per 1.73 m2 calculated from the Modification of Diet in Renal Disease equation) was observed for two or more consecutive health examinations and until the last examination. Among those sequential health examinations indicative of CKD, the first examination date was the inclusion date for the predialysis CKD group when baseline information was collected and follow-up was initiated. To stratify the predialysis CKD stages determined by the Kidney Disease Improving Global Outcomes guidelines (17), we defined four subgroups with the eGFR and albuminuria values from the first included health examination. CKD stage 1 (eGFR≥90 ml/min per 1.73 m2) and stage 2 (eGFR≥60 and <90 ml/min per 1.73 m2) included individuals who had successive albuminuria but had no reduced eGFR at baseline. Health examinees with CKD stage 3 (eGFR≥30 and <60 ml/min per 1.73 m2) and stage 4 or 5 without dialysis (eGFR<30 ml/min per 1.73 m2) were also identified.
The control group was 1:1 matched from a population of health examinees who did not have any evidence of CKD (reduced eGFR or albuminuria) during all included health examinations, and the first examination date was the inclusion date. The matching variables included age, sex, low-income status, and smoking history. Because we intended to construct a matched control group that included an age-, sex-, and social factors–matched general population, comorbidities (e.g., hypertension and diabetes) and body mass index (BMI) variables were not matched, because that would have included people with substantial comorbidities in the control group. However, because hypertension, diabetes, BMI, and other comorbidity variables are still important potential confounders, the variables were adjusted in additional multivariable analyses.
Study Outcomes
The main study outcome was incident active TB during the follow-up, and the follow-up was performed until the outcome, death, or censoring on December 31, 2016. Respiratory (A15 and A16), nonrespiratory (A17 and A18), and miliary TB (A19) events were separately identified with repetitive identification of the corresponding ICD-10 diagnostic codes.
Next, we investigated prognosis after active TB diagnosis. Because direct causes of death were unavailable in the NHID, we implemented an operational definition for TB-associated mortality, which was defined as 1-year all-cause mortality from a TB diagnosis.
Data Collection
Demographic and clinical characteristics at the inclusion dates were collected as baseline variables. The details are shown in Supplemental Material.
Statistical Analyses
We presented continuous variables as medians (interquartile ranges) and categorical variables as numbers (percentages). We used the chi-squared test and the Mann–Whitney U test or Kruskal–Wallis test to investigate the differences between the study groups. The Kaplan–Meier survival curve was plotted, with the P value calculated from the log–rank method. The Cox regression analyses were performed to investigate the risk of active TB within the study groups. The main multivariable model included matched variables (age, sex, low-income status, and smoking history) and other unmatched variables (place of residence, history of diabetes mellitus, hypertension, cancer, chronic obstructive pulmonary disease, immunosuppressants usage, and baseline BMI). After an interaction term analysis to confirm whether a significant difference of active TB risk was present according to CKD stages, risk of active TB of each CKD stage subgroup was compared with that of the matched controls. The risk of 1-year mortality was investigated with the same method as the multivariable Cox regression analysis above for those who were diagnosed with active TB. The risk factors for active TB among the predialysis CKD group were analyzed with a multivariable Cox regression analysis by using the backward elimination method. No missing values were present in the final group of studied people, because we excluded the patients with missing variables. The SAS 9.4 program (SAS Institute) was used to perform the statistical analysis. Two-sided P values of <0.05 indicated a statistically significant difference.
Results
Study Population
After exclusion (Figure 1), we identified 408,873 health examinees with predialysis CKD. According to baseline laboratory findings, health examinees with predialysis CKD were divided into CKD stage 1 (n=35,088), stage 2 (n=55,456), stage 3 (n=306,127), and stage 4/5 without dialysis (n=12,202).
Figure 1.
Study flow diagram. ICD-10, International Classifications of Diseases, tenth revision; TB, tuberculosis.
Baseline Characteristics
The matched variables were identically distributed between the predialysis CKD and control groups (Table 1). The median age was 65 years old, and 51% of the participants were men. The predialysis CKD group had higher BMI and higher proportions of hypertension and diabetes. None of the matched controls had a dipstick albuminuria or reduced eGFR <60 ml/min per 1.73 m2. The baseline characteristics were also significantly different according to the stages of CKD (Supplemental Table 1), because younger people were more likely to have CKD stage 1, and individuals with advanced stages of CKD more commonly had hypertension and diabetes comorbidities.
Table 1.
Baseline characteristics of the study participants in health examinations of the National Health Insurance Service of the Republic of Korea from 2012 to 2016
| Variables | Matched Controls, n=408,873 | Predialysis CKD, n=408,873 |
|---|---|---|
| Matched and adjusted variables | ||
| Age, yr | 65 [56–72] | 65 [56–72] |
| Men | 208,181 (51) | 208,181 (51) |
| Low incomea | 89,541 (22) | 89,541 (22) |
| Smoking history | ||
| Never | 266,213 (65) | 266,213 (65) |
| Previous | 78,438 (19) | 78,438 (19) |
| Current | 64,222 (16) | 64,222 (16) |
| Unmatched but adjusted variables | ||
| Body mass index, kg/m2 | 23.8 [21.8–25.8] | 24.6 [22.5–27.0] |
| Place of residence | ||
| Urban | 175,445 (43) | 182,642 (45) |
| Rural | 233,428 (57) | 226,231 (55) |
| Previous immunosuppressant usage | 77,316 (19) | 78,575 (19) |
| Comorbidities | ||
| Hypertension | 177,336 (43) | 277,032 (68) |
| Diabetes mellitus | 61,186 (15) | 135,305 (33) |
| Congestive heart failure | 7780 (2) | 19,759 (5) |
| Dementia | 11,764 (3) | 15,346 (4) |
| Peripheral vascular disease | 43,406 (11) | 59,963 (15) |
| Cancer | 19,562 (5) | 24,795 (6) |
| Liver disease | 5227 (1) | 8431 (2) |
| Chronic obstructive pulmonary disease | 78,327 (19) | 87,427 (21) |
| Unadjusted variables | ||
| Creatinine, mg/dl | 0.8 [0.7–1.0] | 1.2 [1.0–1.4] |
| eGFR, ml/min per 1.73 m2 | 86 [74–98] | 55 [48–59] |
| Dipstick albuminuria | ||
| None or trace | 408,873 (100) | 277,544 (68) |
| 1+ | 0 (0) | 69,817 (17) |
| ≥2+ | 0 (0) | 61,512 (15) |
Low income was defined as a total income <20th percentile for the nation.
Active TB Risks for Predialysis CKD
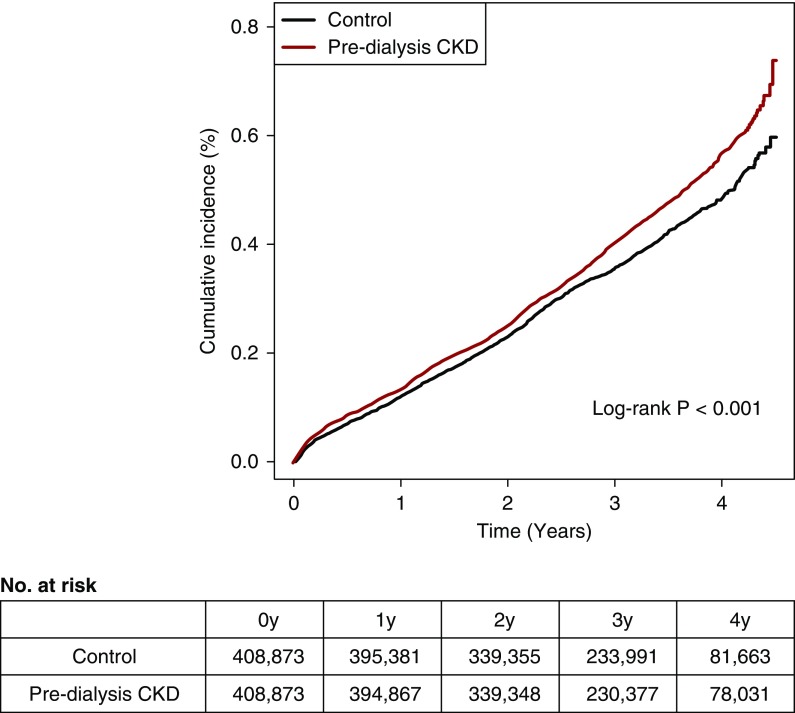
The median follow-up duration was 3.03 years in the CKD group and 3.04 years in the matched control group. The incidence rate was 137.5 per 100,000 person-years in the predialysis group, with 1704 patients with active TB, and this was higher than the 121.9 per 100,000 person-years of the matched control group, with 1518 patients with active TB (Figure 2); this result remained significant in the multivariable analysis (Table 2). When specific CKD stages were considered, the association of CKD with active TB risk significantly varied by stage of CKD (interaction P value <0.001). The incidence rate of TB reached 241.6 per 100,000 person-years in the people with stage 4/5 CKD not on dialysis, which was approximately double of that in the matched control group. When multiple characteristics were adjusted, we found that the risk of active TB was prominently elevated in individuals with CKD stage 1 and individuals with CKD stage 4/5 without dialysis compared with the matched controls. The result was similarly shown in both the rural and urban areas, and the incident rate was higher in those who lived in rural regions, particularly in those who had CKD stage 3 (Supplemental Table 2).
Figure 2.
Higher active tuberculosis cumulative incidence was shown in the predialysis CKD group when compared to the matched control group. The x axis indicates the time (years), and the y axis indicates the cumulative incidence (percentage) of active tuberculosis during follow-up. The red graph indicates the cumulative incidence of the predialysis CKD group, and the black graph indicates the cumulative incidence of the matched control group. The P value calculated from the log rank method is shown. The survival table showing the number at risk is below the survival graph.
Table 2.
Incidence and risk of tuberculosis in people with predialysis CKD compared with the matched control group
| Study groups | N of TB/Total | Incidence Rate (/100,000 PY) | Univariable | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |||
| Matched control | 1518/408,873 | 121.9 | Reference | Reference | Reference | |||
| Predialysis CKDc | 1704/408.873 | 137.5 | 1.13 (1.06 to 1.21) | <0.001 | 1.13 (1.06 to 1.21) | <0.001 | 1.21 (1.13 to 1.30) | <0.001 |
| Stage 1 | 117/35,088 | 122.7 | 1.02 (0.84 to 1.23) | 0.88 | 1.63 (1.34 to 1.97) | <0.001 | 1.82 (1.49 to 2.21) | <0.001 |
| Stage 2 | 157/55,456 | 98.7 | 0.81 (0.69 to 0.96) | 0.01 | 1.05 (0.89 to 1.24) | 0.56 | 1.19 (1.00 to 1.41) | 0.04 |
| Stage 3 | 1336/306,127 | 141.3 | 1.16 (1.08 to 2.25) | <0.001 | 1.08 (1.00 to 1.16) | 0.05 | 1.16 (1.07 to 1.25) | <0.001 |
| Stage 4/5 without dialysis | 94/12,202 | 241.6 | 1.98 (1.61 to 2.44) | <0.001 | 1.97 (1.60 to 2.42) | <0.001 | 1.85 (1.50 to 2.28) | <0.001 |
TB, tuberculosis; PY, person-years; HR, hazard ratio; 95% CI, 95% confidence interval.
Model 1 was adjusted for age, sex, low-income states, and smoking histories.
Model 2 was adjusted for age, sex, low-income status, smoking histories, places of residence (rural or urban), history of diabetes mellitus, hypertension, cancer, chronic obstructive pulmonary disease, immunosuppressants usage history, and baseline body mass index.
Stages of CKD were determined as stage 1 (eGFR≥90 ml/min per 1.73 m2 with consecutive albuminuria), stage 2 (eGFR≥60 and <90 ml/min per 1.73 m2 with consecutive albuminuria), stage 3 (eGFR≥30 and <60 ml/min per 1.73 m2), and stage 4/5 without dialysis (eGFR<30 ml/min per 1.73 m2 but not on kidney replacement therapy).
Risk Factors for Active TB Among People with Predialysis CKD
Within the predialysis CKD group, older individuals and men had a higher risk of active TB (Table 3). Current smokers, but not individuals with a previous smoking history, had a significantly higher risk of TB, and low-income status was another risk factor for active TB. A higher BMI was associated with a lower risk for active TB. Regarding other medical histories, usage of immunosuppressants, diabetes, and chronic obstructive pulmonary disease were independent risk factors for TB in people with predialysis CKD. Regarding CKD stages, compared with the CKD stage 2 subgroup, both the subgroup with CKD stage 1 and the subgroup with CKD stage 4/5 not on dialysis were associated with a significantly higher risk for active TB.
Table 3.
Risk factors for tuberculosis in people with predialysis CKD
| Variables | Univariable Cox Regression | Backward Elimination | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| Age (continuous), per 10-yr increment | 1.37 (1.31 to 1.44) | <0.001 | 1.37 (1.31 to 1.42) | <0.001 |
| Men (versus women) | 1.35 (1.19 to 1.53) | <0.001 | 1.33 (1.18 to 1.51) | <0.001 |
| Smoking history | ||||
| Ex-smoker (versus never smoker) | 0.96 (0.83 to 1.11) | 0.57 | 0.95 (0.82 to 1.10) | 0.52 |
| Current smoker (versus never smoker) | 1.18 (1.01 to 1.37) | 0.03 | 1.18 (1.01 to 1.37) | 0.04 |
| Low income (versus not) | 1.15 (1.03 to 1.28) | 0.02 | 1.15 (1.03 to 1.29) | 0.01 |
| Stages of CKD (versus stage 2)a | ||||
| Stage 1 | 1.48 (1.16 to 1.88) | 0.001 | 1.49 (1.17 to 1.89) | 0.001 |
| Stage 2 | Reference | Reference | ||
| Stage 3 | 1.05 (0.88 to 1.25) | 0.58 | 1.03 (0.87 to 1.22) | 0.75 |
| Stage 4/5 without dialysis | 1.67 (1.29 to 2.17) | <0.001 | 1.63 (1.26 to 2.11) | <0.001 |
| Body mass index (continuous), per 5-kg/m2 increment | 0.45 (0.42 to 0.49) | <0.001 | 0.45 (0.42 to 0.49) | <0.001 |
| Previous usage of immunosuppressants | 1.26 (1.13 to 1.41) | <0.001 | 1.26 (1.13 to 1.41) | <0.001 |
| Comorbidities | ||||
| Hypertension (versus none) | 0.95 (0.85 to 1.06) | 0.36 | ||
| Diabetes mellitus (versus none) | 1.46 (1.32 to 1.62) | <0.001 | 1.48 (1.34 to 1.63) | <0.001 |
| Congestive heart failure (versus none) | 1.23 (1.02 to 1.48) | 0.03 | ||
| Dementia (versus none) | 0.91 (0.73 to 1.14) | 0.42 | ||
| Liver disease (versus none) | 0.95 (0.68 to 1.33) | 0.77 | ||
| Peripheral vascular disease (versus none) | 1.12 (0.99 to 1.27) | 0.06 | ||
| Cancer (versus none) | 0.97 (0.81 to 1.17) | 0.76 | ||
| Chronic obstructive pulmonary disease (versus none) | 1.42 (1.19 to 1.68) | <0.001 | 1.44 (1.30 to 1.59) | <0.001 |
| Clinical diagnosis for glomerular or tubulointerstitial disease (versus none) | 0.99 (0.80 to 1.22) | 0.89 | ||
HR, hazard ratio; 95% CI, 95% confidence interval.
Stages of CKD were determined as stage 1 (eGFR≥90 ml/min per 1.73 m2 with consecutive albuminuria), stage 2 (eGFR≥60 and <90 ml/min per 1.73 m2 with consecutive albuminuria), stage 3 (eGFR≥30 and <60 ml/min per 1.73 m2), and stage 4/5 without dialysis (eGFR<30 ml/min per 1.73 m2 but not on kidney replacement therapy).
Pulmonary, Nonpulmonary, and Miliary TB in Predialysis CKD
When we separated the active TB sites, pulmonary, nonpulmonary, and miliary TB risks were higher in the predialysis CKD group compared with the control group (Supplemental Table 3). The finding that CKD stage 1 and CKD stage 4/5 without dialysis were associated with significantly higher risks of active TB was similar when we limited to pulmonary TB. Regarding miliary TB, the CKD stage 1 group also showed certain high adjusted hazard ratios, but no statistical significance was observed, and the number of patients was too small (n=34) to perform a robust analysis.
TB-Associated Mortality in Predialysis CKD
Of those who were diagnosed with TB, we identified 149 patients who were deceased within 1 year after their active TB diagnosis in the predialysis CKD group and 87 in the matched control groups (Table 4). The risk of TB-associated death was approximately 1.5-fold in those with predialysis CKD compared with the controls. When tolerating the limited number of death events in each CKD stage, the risk of TB-associated death after active TB diagnosis was higher in CKD stage 1, CKD stage 3, and CKD stage 4/5 without dialysis compared with the matched controls, but this was not observed in CKD stage 2.
Table 4.
One-year all-cause mortality for participants diagnosed with active tuberculosis
| Study groups | N of deaths/TB | Univariable Analysis | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | ||
| Matched control | 87/1518 | Reference | Reference | Reference | |||
| Predialysis CKDc | 149/1704 | 1.54 (1.18 to 2.01) | <0.001 | 1.77 (1.36 to 2.31) | <0.001 | 1.68 (1.28 to 2.22) | <0.001 |
| Stage 1 | 7/117 | 0.99 (0.46 to 2.15) | 0.99 | 2.60 (1.17 to 5.81) | 0.02 | 2.16 (0.96 to 4.87) | 0.06 |
| Stage 2 | 6/157 | 0.68 (0.30 to 1.55) | 0.36 | 1.20 (0.52 to 2.77) | 0.67 | 1.09 (0.47 to 2.53) | 0.84 |
| Stage 3 | 121/1336 | 1.59 (1.21 to 2.10) | <0.001 | 1.67 (1.13 to 2.21) | <0.001 | 1.60 (1.20 to 2.13) | 0.001 |
| Stage 4/5 without dialysis | 15/94 | 3.00 (1.73 to 5.19) | <0.001 | 3.78 (2.19 to 6.55) | <0.001 | 3.49 (2.01 to 6.09) | <0.001 |
TB, tuberculosis; HR, hazard ratio; 95% CI, 95% confidence interval.
Model 1 was adjusted for age, sex, low-income states, and smoking histories.
Model 2 was adjusted for age, sex, low-income status, smoking histories, places of residence (rural or urban), history of diabetes mellitus, hypertension, cancer, chronic obstructive pulmonary disease, immunosuppressants usage history, and baseline body mass index.
Stages of CKD were determined as stage 1 (eGFR≥90 ml/min per 1.73 m2 with consecutive albuminuria), stage 2 (eGFR≥60 and <90 ml/min per 1.73 m2 with consecutive albuminuria), stage 3 (eGFR≥30 and <60 ml/min per 1.73 m2), and stage 4/5 without dialysis (eGFR<30 ml/min per 1.73 m2 but not on kidney replacement therapy).
Discussion
In this nationwide population-based study in a nation with moderate TB prevalence, we identified a higher risk of active TB in people with predialysis CKD than in those without CKD. Both the examinees with CKD stage 1 and those with advanced stages of CKD showed prominently elevated active TB risks. Among those who were diagnosed with active TB, the people with predialysis CKD had a worse 1-year mortality outcome than the patients with TB without underlying CKD.
The main strength of our study is that we showed that the incidences and significantly higher risks of active TB in people with predialysis CKD, which was rarely studied in a large-scale predialysis CKD cohort, and we identified the risk factors for active TB in people with predialysis CKD. Infection along with malignancy are the most important noncardiovascular complications related to the mortality of people with CKD (4). Patients who were on kidney replacement therapy were known to have higher risks for TB or other infectious diseases and this outcome was explained by their immunosuppressed status and shared risk factors (7–9). However, TB risks in globally increasing number of people with mild to moderate CKD were hard to assess in a large population-based study (6). Previous studies made efforts to reveal the association between TB and CKD, but their limitations were lack of laboratory confirmation for CKD and inclusion of insufficient number of people with predialysis CKD. (18,19). We overcame this hurdle by analyzing a unique cohort of nationwide health screening examinees and examined the TB risks in each CKD stage with tolerable statistical powers. Considering the recent incidence of active TB in Korea (approximately 90–100 per 100,000 person-years) and that we included a CKD population with older age, the number of identified patients with TB in the study was acceptable (20).
Previously, the TB risk was assumed to be higher for more severe degrees of CKD (6). This was true for those who had CKD stage 2 or higher, because a linear association between eGFR and risk of active TB was demonstrated within these people; poor immune function in individuals with more profound kidney function impairment may explain this outcome (10,21). However, CKD stage 1 was also associated with prominently elevated active TB risks, but CKD stage 2, which also had persistent albuminuria, showed insignificant association with the risk of TB. CKD stage 1 may indicate kidney hyperfiltration, which has been reported to be related to several adverse clinical outcomes (22–24), or simply persistent albuminuria with low serum creatinine. A possible mechanism underlying this result may be that extremely high eGFR is related to undernutrition, because creatinine-based eGFR is innately affected by such factors; also, the critical linkage between malnutrition and TB has been widely recognized (25). Alternately, hyperfiltration, if considering CKD stage 1 as a hyperfiltrative condition, commonly occurs in people with certain conditions, such as diabetes, obesity, or secondary focal segmental glomerulosclerosis, which may partially explain this result. However, persistent albuminuria, which was present in all participants with early-stage CKD, may have different meaning from eGFR regarding risk of TB, although both are used as important markers of CKD. Because the cause of this association could not be directly uncovered by this study, a future study with a hypothesis that the nature of CKD stage 1 might not be in the linear spectrum of CKD stages may be considered.
Traditional active TB risk factors were confirmed to also be important in people with predialysis CKD (e.g., smoking, lower BMI, older age, diabetes, immunosuppressants exposure, chronic obstructive pulmonary disease, and men) (25–27). Those with predialysis CKD and multiple conventional risk factors, particularly CKD stage 1 or 4/5 without dialysis, may be the group of interest for additional active TB screening. The importance of such an approach should be emphasized, because people with predialysis CKD also had a higher risk of 1-year mortality after diagnosis of active TB than the matched controls.
Several limitations of this study warrant additional research. First, although South Korea is one of the countries with moderate TB and CKD prevalence (20), the strength of association between TB and CKD may possibly be different in other nations, particularly in low- or middle-income regions (6). Because the countries may be the primary sites with poorly controlled TB risks in an emerging CKD population, efforts to confirm the linkage between TB and CKD should also be considered in those nations. Second, as stated above, the mechanisms for different TB risks according to each CKD stage could not be confirmed. Third, the TB-associated mortality was not conclusive: direct identification of causes of death was unavailable, limited numbers of death events were identified, and a potential immortal time bias was present, because study participants received at least multiple health examinations with several-year intervals. Fourth, unincluded confounders might have been present in our matching process. However, it turned out that we matched most of the factors significantly related to TB risk in our risk factor analysis, and the overall results remained after adjustment that included other significant risk variables. Because the incidence of TB was prominently higher in CKD stage 1 or CKD stage 4/5 without dialysis than that of general population in Korea, the effect of unmatched variables would not crucially alter the main findings of our study. Fifth, not all people were eligible for or received the general health screening, and therefore, potential selection bias remains, although we studied one of the largest cohorts of people with predialysis CKD.
In conclusion, people with predialysis CKD had an elevated risk of active TB. Profoundly reduced eGFR or conversely, CKD stage 1 was associated with the higher TB risk. Health care providers should be aware of the risk of TB and consequent worse prognosis in people with predialysis CKD.
Disclosures
Dr. Park, Dr. Soojin Lee, Dr. Yaerim Kim, Dr. Yeonhee Lee, Dr. Kang, Dr. Semin Cho, Dr. Kyungdo Han, Dr. Seoung Seok Han, Dr. Hajeong Lee, Dr. Jung Pyo Lee, Dr. Joo, Dr. Lim, Dr. Yon Su Kim, and Dr. Dong Ki Kim have nothing to disclose.
Supplementary Material
Acknowledgments
This study was supported by grant HI18C1604 from the Ministry of Health and Welfare, Republic of Korea.
Data are available through the Korean National Health Insurance Sharing Service. Researchers who wish to access the data can apply at (https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do) and request access to NHIS-2018-1-148.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.14471218/-/DCSupplemental.
Supplemental Figure 1. Examples of the study groups and excluded cases.
Supplemental Material. Collected data and variable definition.
Supplemental Table 1. Baseline characteristics of people with predialysis CKD according to their stages of CKD.
Supplemental Table 2. Incidence and risk of tuberculosis in subgroups divided according to place of residence.
Supplemental Table 3. Incidences and risks of pulmonary, nonpulmonary, and miliary tuberculosis.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Meguid El Nahas A, Bello AK: Chronic kidney disease: The global challenge. Lancet 365: 331–340, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J, Kim YK: Current characteristics of dialysis therapy in Korea: 2016 Registry data focusing on diabetic patients. Kidney Res Clin Pract 37: 20–29, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jager DJ, Vervloet MG, Dekker FW: Noncardiovascular mortality in CKD: An epidemiological perspective. Nat Rev Nephrol 10: 208–214, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA: Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 380: 2095–2128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanowski K, Clark EG, Levin A, Cook VJ, Johnston JC: Tuberculosis and chronic kidney disease: An emerging global syndemic. Kidney Int 90: 34–40, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Al-Efraij K, Mota L, Lunny C, Schachter M, Cook V, Johnston J: Risk of active tuberculosis in chronic kidney disease: A systematic review and meta-analysis. Int J Tuberc Lung Dis 19: 1493–1499, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Pradhan RP, Katz LA, Nidus BD, Matalon R, Eisinger RP: Tuberculosis in dialyzed patients. JAMA 229: 798–800, 1974 [PubMed] [Google Scholar]
- 9.Singh N, Paterson DL: Mycobacterium tuberculosis infection in solid-organ transplant recipients: Impact and implications for management. Clin Infect Dis 27: 1266–1277, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B: Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3: 1526–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J: Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int 65: 1031–1040, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB: Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 16: 3728–3735, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Richardson RM: The diagnosis of tuberculosis in dialysis patients. Semin Dial 25: 419–422, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Park S, Lee S, Kim Y, Lee Y, Kang MW, Han K, Han SS, Lee H, Lee JP, Joo KW, Lim CS, Kim YS, Kim DK: Risk of cancer in pre-dialysis chronic kidney disease: A nationwide population-based study with a matched control group. Kidney Res Clin Pract 38: 60–70, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, Lee EJ, Ae Shin S: Data resource profile: The national health information database of the national health insurance service in South Korea. Int J Epidemiol 46: 799–800, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA: New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 57: 1288–1294, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 18.Cheng KC, Liao KF, Lin CL, Liu CS, Lai SW: Chronic kidney disease correlates with increased risk of pulmonary tuberculosis before initiating renal replacement therapy: A cohort study in Taiwan. Medicine (Baltimore) 97: e12550, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran E, Baharani J, Dedicoat M, Robinson E, Smith G, Bhomra P, Thien OS, Ryan R: Risk factors associated with the development of active tuberculosis among patients with advanced chronic kidney disease. J Infect 77: 291–295, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Yim JJ: Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis 21: 1913–1920, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ: Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol 26: 1426–1433, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reboldi G, Verdecchia P, Fiorucci G, Beilin LJ, Eguchi K, Imai Y, Kario K, Ohkubo T, Pierdomenico SD, Schwartz JE, Wing L, Saladini F, Palatini P: Glomerular hyperfiltration is a predictor of adverse cardiovascular outcomes. Kidney Int 93: 195–203, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Melsom T, Stefansson V, Schei J, Solbu M, Jenssen T, Wilsgaard T, Eriksen BO: Association of increasing GFR with change in albuminuria in the general population. Clin J Am Soc Nephrol 11: 2186–2194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hood ML: A narrative review of recent progress in understanding the relationship between tuberculosis and protein energy malnutrition. Eur J Clin Nutr 67: 1122–1128, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Lönnroth K, Roglic G, Harries AD: Improving tuberculosis prevention and care through addressing the global diabetes epidemic: From evidence to policy and practice. Lancet Diabetes Endocrinol 2: 730–739, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Kirenga BJ, Ssengooba W, Muwonge C, Nakiyingi L, Kyaligonza S, Kasozi S, Mugabe F, Boeree M, Joloba M, Okwera A: Tuberculosis risk factors among tuberculosis patients in Kampala, Uganda: Implications for tuberculosis control. BMC Public Health 15: 13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.