Visual Abstract
Keywords: Functional decline; geriatric nephrology; dialysis; elderly; Aged; renal dialysis; risk factors; Geriatric Assessment; Logistic Models; Prevalence; Caregivers; Activities of Daily Living; Follow-Up Studies; Frailty; Kidney Failure, Chronic; Life
Abstract
Background and objectives
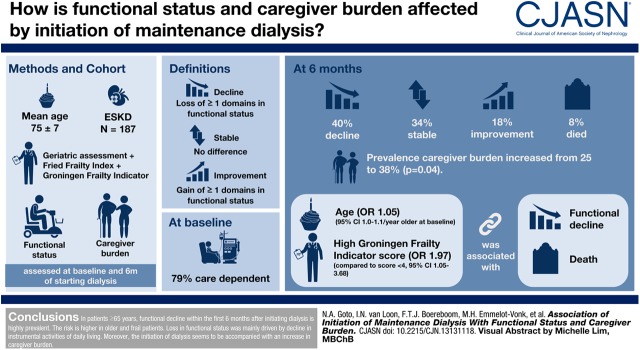
Little is known about the functional course after initiating dialysis in elderly patients with ESKD. The aim of this study was to assess the association of the initiation of dialysis in an elderly population with functional status and caregiver burden.
Design, setting, participants & measurements
This study included participants aged ≥65 years with ESKD who were enrolled in the Geriatric Assessment in Older Patients Starting Dialysis study. All underwent a geriatric assessment and a frailty screening (Fried Frailty Index and Groningen Frailty Indicator) at dialysis initiation. Functional status (activities of daily life and instrumental activities of daily life) and caregiver burden were assessed at baseline and after 6 months. Decline was defined as loss of one or more domains in functional status, stable as no difference between baseline and follow-up, and improvement as gain of one or more domains in functional status. Logistic regression was performed to assess the association between the combined outcome functional decline/death and potential risk factors.
Results
Of the 196 included participants functional data were available for 187 participants. Mean age was 75±7 years and 33% were women. At the start of dialysis, 79% were care dependent in functional status. After 6 months, 40% experienced a decline in functional status, 34% remained stable, 18% improved, and 8% died. The prevalence of high caregiver burden increased from 23%–38% (P=0.004). In the multivariable analysis age (odds ratio, 1.05; 95% confidence interval, 1.00 to 1.10 per year older at baseline) and a high Groningen Frailty Indicator compared with low score (odds ratio, 1.97; 95% confidence interval, 1.05 to 3.68) were associated with functional decline/death.
Conclusions
In patients aged ≥65 years, functional decline within the first 6 months after initiating dialysis is highly prevalent. The risk is higher in older and frail patients. Loss in functional status was mainly driven by decline in instrumental activities of daily life. Moreover, initiation of dialysis is accompanied by an increase in caregiver burden.
Introduction
The number of elderly patients on maintenance dialysis is increasing (1). This is due to aging of the population, an increase in the prevalence of CKD, and more liberal acceptance of elderly patients into dialysis programs (2). Elderly patients are a very heterogeneous group with a high prevalence of comorbidity and geriatric problems (3). One of these problems is functional dependence. This can be defined as the loss of the ability to carry out activities essential to independent living, including tasks needed for self-care (such as bathing, dressing, and continence) and more complex tasks that support independent living in a community (such as shopping, housecleaning, and telephone use) (4). The ability to perform these tasks depends on cognitive, motor (e.g., mobility), and perceptual capacities. In elderly patients on dialysis, functional dependence is highly prevalent (5,6). It is strongly associated with mortality (7,5), therapy withdrawal, and time to first hospitalization (5). Furthermore, functional dependence can negatively influence quality of life (8,9), increase caregiver burden, and increase the use of health services. Another essential point is that when it comes to prognosis, elderly patients value maintaining independence the most (10).
Therefore, it is important to understand what effect the initiation of dialysis has on the course of functional status. Moreover, it is important to try to predict in which patients functional status will improve after initiating dialysis (assumed through improvement of uremic complaints) and in which it will decline (e.g., due to burden of dialysis therapy). This can inform patients about prognosis and aid (shared) decision making regarding dialysis. Furthermore, knowledge about functional change may guide interventions to prevent functional decline or initiate improvement in high-risk patients, such as rehabilitation programs or physical training.
Unfortunately, little is known about the functional trajectory after initiating dialysis in elderly patients with ESKD. Two previous studies reported high rates of functional decline (11,12), but these studies were performed in a very specific population (nursing home patients), or were performed in a small, single-center cohort, and do not inform us about the general older dialysis population.
Therefore, the aim of this study was to assess the association of the initiation of maintenance dialysis with functional status and caregiver burden in a community-dwelling elderly population with ESKD. Furthermore, we explored which variables are associated with functional change after initiating dialysis.
Materials and Methods
Study Participants
To describe the trajectory in functional status in elderly patients initiating dialysis, data were used from the Geriatric Assessment in Older Patients Starting Dialysis study. This is a prospective, multicenter cohort study assessing the relationship of geriatric assessment with outcome in patients with ESKD. Participants were enrolled from 17 centers across the Netherlands from August 2014 to September 2017. Patients initiating dialysis (peritoneal dialysis and hemodialysis) who were aged ≥65 years were included. Participants were recruited from the predialysis outpatients clinics by their treating nephrologists. If inclusion criteria were met, participants were contacted by one of the researchers or research nurses to make an appointment for inclusion. Of the contacted participants, three were excluded because of communication problems or because they died before inclusion (n=2). Furthermore, participants refused to participate because they felt not fit enough (n=19), the family disagreed with participation (n=2), or they already participated in another study (n=1). The aim was to include patients eligible for dialysis between 3 weeks before and 2 weeks after dialysis initiation. Participants were excluded if informed consent was not provided, if they had insufficient understanding of the Dutch language, or if they suffered from a terminal nonrenal-related condition. After 6 months participants and caregivers were contacted by telephone for follow-up.
The study was conducted in accordance with the Declaration of Helsinki and approved by the medical ethics review boards of all participating hospitals. Written informed consent was obtained from all participants before enrollment.
Data Collection
Baseline data were collected by one of the two investigators or one of the two trained research nurses. Participants were either visited at home or in the dialysis center, before starting the dialysis session. Baseline data included demographic data (age, sex, living, social situation, smoking status, and alcohol use), information obtained from clinical charts (dialysis modality, cause of kidney failure), and a geriatric assessment. The geriatric assessment consisted of validated questionnaires or structured assessment of seven domains (Supplemental Table 1): activities of daily living (ADL; Katz et al. [13]), instrumental activities of daily living (IADL; Lawton and Brody [14]), comorbidity burden (Cumulative Illness Rating Scale for Geriatrics [15]), depressive symptoms (Geriatric Depression Scale [16,17]), mobility (Timed Up and Go Test [18]), nutrition (Mini Nutritional Assessment; [19]), and cognition. The latter was assessed with the Mini Mental State Examination (20), semantic fluency test (21), clock drawing test (22), and enhanced cued recall test (23). The outcome of the geriatric assessment was composed by the sum of impairment in the seven geriatric domains. Participants were considered frail if they scored two or more impairments (24,25). In addition, two frailty screening instruments were applied: the Fried frailty index (26) and Groningen Frailty Indicator (27). The Groningen Frailty Indicator is a 15-item frailty screening instrument that measures loss of function in four domains: physical (mobility, general health, malnutrition, polypharmacy, hearing, vision), social (emotional isolation), cognitive (cognitive dysfunction), and psychologic (depressed mood and feelings of anxiety). The range of the Groningen Frailty Indicator score is 0–15, with higher scores indicating more severe impairment. Participants were considered frail according to the Groningen Frailty Indicator if they scored four or more points.
All participants alive at 6 months after inclusion were interviewed by phone by a research nurse or investigator. During this interview, questionnaires about functional status (ADL and IADL) were completed.
Functional Status.
We scored overall functional status by combining ADL and the IADL; a cumulative score of 0 out of a possible 13 points indicates not care dependent, 1–5 indicates mild/moderate dependence, and >5 indicates severe care dependence (5). For baseline and follow-up, the number of dependencies in functional status was counted. The difference in number of dependencies between these moments was defined as functional outcome. Functional outcome was categorized in improvement (score ≥+1), stable (score of 0), decline (score ≤−1), and death.
Caregiver.
For all participants, a relevant caregiver was approached (if available), and when participating, informed consent was obtained. Questionnaires for caregivers were either completed during the visit to the participant or sent by mail, preferably returned within 2 weeks of enrollment of the participant. After 6 months, if the participant was still alive, caregivers were asked to fill out the questionnaires for a second time.
Caregivers received three questionnaires: the Neuropsychological Inventory (28), the Interview of Deterioration in Daily Life Dementia (29), and the Self-Perceived Pressure from Informal Care (a Dutch questionnaire assessing caregiver burden) (30). Cut-off points for the different tests are shown in Supplemental Table 1.
Statistical Analyses
Categorical variables were reported as proportions, and continuous variables were reported as means with SD or medians with interquartile range (IQR) for nonparametric data. For subgroup analysis, participants were divided into different groups according to age (65–69, 70–74, and ≥75 years) and functional dependency (independent, mildly/moderately dependent, and severely dependent). Differences between groups were assessed by chi-squared test or Fisher exact test for categorical data, one-way ANOVA for parametric continues data, and Kruskal–Wallis test for nonparametric continuous data. To assess the difference between the different age categories and functional dependencies, the chi-squared test for trend was used. Furthermore, to assess differences between baseline and follow-up data, the paired t test was used for paired parametric data, the Wilcoxon signed-rank test for nonparametric paired data, and the McNemar test for paired binary data. To assess the association between functional outcome and the results of the different caregiver questionnaires, the chi-squared test was used.
To investigate the association between functional outcome and different potential predictors, logistic regression was performed. For this analysis, functional outcome was dichotomized in a composite outcome “functional decline/death” and “stable/improvement.” A composite outcome was used because death in the chronic and frail elderly is usually preceded by slow functional decline with steadily progressive disability before dying from complications (31). Components of the geriatric assessment were assessed in a univariable analysis. Variables were considered as potential risk factors when P<0.20 in the univariable analysis, and were included in a multivariable logistic regression analysis (including age and sex). Additionally, the association between different frailty tools (geriatric assessment, Groningen Frailty Indicator, Fried Frailty Index) and the composite outcome was assessed with logistic regression.
A two-sided probability of P<0.05 was considered statistically significant. Outcomes were calculated with a 95% confidence interval (95% CI). Data were analyzed using SPSS software (IBM SPSS Statistics, version 21).
Results
During the study period, 196 participants on incident dialysis were included. Of these participants, five (3%) were lost to follow-up and four (2%) were excluded from analysis because there was too much time between inclusion and start of dialysis (>3 months), leaving 187 participants for analysis. The majority of the participants had their baseline assessments shortly after the start of dialysis (median, 8 days; IQR, 1.5–12.5). Baseline characteristics are shown in Table 1. The mean age of the population was 75±7 years and 33% of the participants were women. The main cause of ESKD was vascular disease (50%), followed by diabetes (16%). Of all participants, 77% started in-center hemodialysis and 23% started peritoneal dialysis. No differences were seen for the different age categories (Table 1).
Table 1.
Baseline characteristics of 187 patients on incident dialysis in the Geriatric Assessment in Older Patients Starting Dialysis study
| Age Category, yr | ||||
|---|---|---|---|---|
| Participant Characteristics | Total, n=187 | 65–69, n=41 | 70–74, n=49 | ≥75, n=97 |
| Age, mean (±SD) | 75 (7) | |||
| Women | 61 (33%) | 16 (39%) | 15 (31%) | 30 (31%) |
| Single/widow | 78 (42%) | 19 (46%) | 21 (43%) | 38 (39%) |
| Living at nursing home | 10 (5%) | 1 (2%) | 1 (2%) | 8 (8%) |
| Smokera | 137 (73%) | 30 (79%) | 37 (80%) | 70 (74%) |
| Current alcohol use | 73 (41%) | 13 (35%) | 19 (41%) | 41 (43%) |
| Underlying kidney disease | ||||
| Diabetes | 29 (16%) | 5 (12%) | 10 (20%) | 14 (14%) |
| Vascular | 93 (50%) | 17 (42%) | 26 (53%) | 50 (52%) |
| Other/unknown | 65 (34%) | 19 (46%) | 13 (27%) | 33 (34%) |
| Hemodialysis | 144 (77%) | 28 (68%) | 39 (80%) | 77 (79%) |
| Geriatric assessment | ||||
| Impaired ADL at baseline | 56 (30%) | 11 (27%) | 13 (27%) | 32 (33%) |
| Impaired IADL at baseline | 146 (78%) | 30 (73%) | 36 (74%) | 80 (83%) |
| Impaired cognition | 123 (67%) | 26 (65%) | 27 (57%) | 70 (73%) |
| Severe comorbidity burden | 80 (43%) | 18 (44%) | 23 (47%) | 39 (40%) |
| Severely impaired mobility | 35 (20%) | 7 (18%) | 8 (17%) | 20 (22%) |
| Symptoms of depression | 56 (30%) | 12 (29%) | 11 (22%) | 33 (34%) |
| Malnutrition | 10 (5%) | 3 (7%) | 3 (6%) | 4 (4%) |
| Frail according to GA (≥2 impairments) | 148 (77%) | 32 (78%) | 34 (69%) | 78 (80%) |
| Frail according to Fried Frailty Index (≥3) | 82 (46%) | 17 (46%) | 20 (44%) | 45 (47%) |
| Frail according to Groningen Frailty Indicator (≥4) | 115 (62%) | 24 (59%) | 29 (59%) | 62 (64%) |
The following variables had missing data: cognition (2.1%), Fried Frailty Index (4.8%), mobility (5.3%), and smoking (4.3%). All data are shown as n (%), unless otherwise indicated. ADL, activities of daily living; IADL, instrumental activities of daily living; GA, geriatric assessment.
Smoker: if the participant has smoked but stopped, or is still smoking cigarettes.
At baseline, 21% of the participants were independent in functional status, 52% were mildly/moderately dependent, and 27% were severely dependent. Prevalence of severe dependence was the highest in the age category of ≥75 years (69% versus 12% in the age category 65–69 years). Severely dependent participants experienced more symptoms of depression (54% versus 12%), were more impaired in mobility (56% versus 0%), and were more frail according to frailty tools (geriatric assessment 96% versus 24%, Fried Frailty index 84% versus 13%, Groningen Frailty Indicator 92% versus 39%) compared with the independent participants (Supplemental Table 2).
Functional Change after Initiating Dialysis
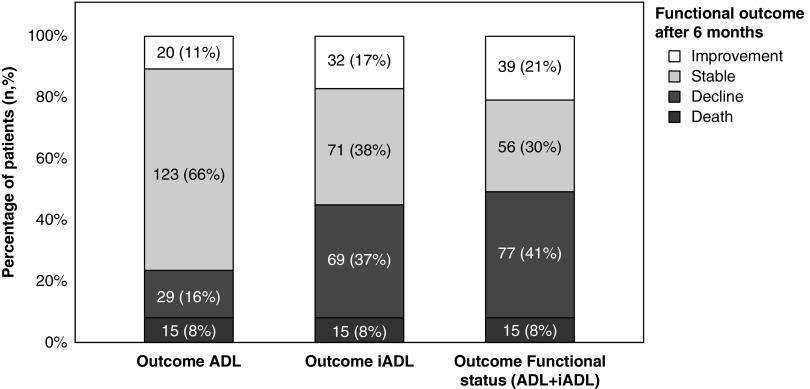
After 6 months follow-up, 8% had died and 2% received a kidney transplant. Of the participants still alive (including participants that received a kidney transplant), 40% experienced decline in functional status, 34% were stable, and 18% of the participants improved. The decline in functional status was mostly due to loss in IADL independence (37% decline versus 17% improvement). For ADL, most participants remained stable (66%). Figure 1 shows changes in ADL, IADL, and overall functional status. Specific impairments in functional status for baseline and follow-up are shown in Supplemental Table 3.
Figure 1.
Change in functional status over 6 months from dialysis initiation as defined by changes in ADL, IADL, or both (functional status). Outcome in functional status is defined as the difference in number of dependencies (ADL and IADL combined) between baseline and follow-up. All outcomes were categorized in improvement (score ≥+1), stable (score of 0), decline (score ≤−1), and death.
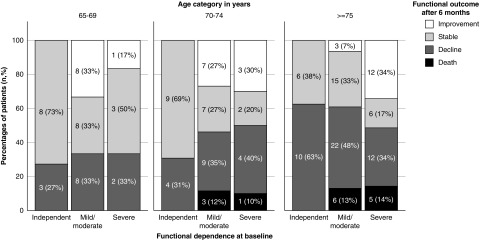
Data on functional change and mortality stratified by age and baseline functional status are shown in Table 2 and Figure 2. Elderly participants more frequently experienced poor outcome compared with younger participants (P=0.002): none of the independent participants or participants in the age category 65–69 years died. In the younger age categories, more participants improved/stabilized (65–69 years, 68%; 70–74 years, 57%; and ≥75 years 43%). In participants aged ≥75 years, more than half experienced functional decline (45%) or death (11%). Furthermore, elderly participants died more frequently compared with the younger age categories (65–69 years, 0%; 70–74 years, 8%; ≥75 years 11%). No significant differences were seen for baseline functional status and functional outcome. However, severely dependent participants tended to die more frequently compared with the participants in the more independent group (12% in the severely dependent group versus 9% in the mildly/moderately dependent group versus 0% in the independent group).
Table 2.
Change in functional status over 6 mo from dialysis initiation according to baseline age or functional dependence
| Participant Characteristics | Functional Outcome after 6 mo, n (%) | P Value | ||
|---|---|---|---|---|
| Improvement/Stable | Decline | Death | ||
| All participants | 98 (52%) | 74 (40%) | 15 (8%) | |
| Age category in years at baseline | 0.002 | |||
| 65–69 | 28 (68%) | 13 (32%) | 0 (0%) | |
| 70–74 | 28 (57%) | 17 (35%) | 4 (8%) | |
| ≥75 | 42 (43%) | 44 (45%) | 11 (11%) | |
| Functional dependence at baseline | 0.26 | |||
| Independent | 23 (58%) | 17 (43%) | 0 (0%) | |
| Mild/moderate | 48 (50%) | 39 (41%) | 9 (9%) | |
| Severe | 27 (53%) | 18 (35%) | 6 (12%) | |
Figure 2.
Change in functional status over 6 months from dialysis initiation according to baseline age and functional dependence.
Factors associated with Functional Decline after Initiating Dialysis
Table 3 shows the univariable logistic regression model for the association between demographics, components of the geriatric assessment, and the composite outcome functional decline/death within 6 months after initiating dialysis. Age, single/widow status, symptoms of depression, and an impaired mobility all had a P<0.20 and were used in a multivariable model. In the multivariable analysis, only age (odds ratio [OR], 1.05; 95% CI, 1.00 to 1.10) per year older at baseline remained independently associated with poor outcome. When the same analysis was performed with a higher cut-off score for decline (decline ≥2 or more points lost), different potential risk factors were found in univariable analysis, but also only age was independently associated with poor outcome (OR, 1.07; 95% CI, 1.02 to 1.13 per year older at baseline) (data not shown).
Table 3.
Associations between demographics, geriatric assessment, and functional decline/death
| Participant Characteristics | No. of Participants with Functional Decline/Death | Logistic Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Exposure Present | Exposure Not Present | Univariable Analysis | Multivariable Analysisa | |||||
| n (%) | n (%) | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age (continuous) | NA | NA | 1.06 | 1.01 to 1.11 | 0.02 | 1.05 | 1.00 to 1.10 | 0.04 |
| Sex (reference female) | 29/61 (48%) | 60/126 (48%) | 1.00 | 0.54 to 1.85 | 0.99 | 0.85 | 0.42 to 1.70 | 0.64 |
| Single/widow | 32/78 (41%) | 57/109 (52%) | 1.58 | 0.88 to 2.84 | 0.13 | 1.85 | 0.96 to 3.55 | 0.07 |
| Geriatric assessment | ||||||||
| Dependent in ADL at baseline | 29/56 (52%) | 60/131 (46%) | 1.27 | 0.68 to 2.38 | 0.45 | |||
| Dependent in IADL at baseline | 71/146 (49%) | 18/41 (44%) | 1.21 | 0.60 to 2.43 | 0.59 | |||
| Impaired cognition | 56/123 (46%) | 31/60 (52%) | 0.78 | 0.42 to 1.45 | 0.44 | |||
| Severe comorbidity burden | 41/80 (51%) | 48/107 (45%) | 1.29 | 0.72 to 2.31 | 0.39 | |||
| Impaired mobility | 20/35 (57%) | 63/142 (44%) | 1.67 | 0.79 to 3.53 | 0.18 | 1.53 | 0.67 to 3.47 | 0.31 |
| Symptoms of depression | 32/56 (57%) | 57/131 (44%) | 1.73 | 0.92 to 3.26 | 0.09 | 1.37 | 0.69 to 2.70 | 0.37 |
| Malnutrition | 5/10 (50%) | 84/177 (48%) | 1.11 | 0.31 to 3.96 | 0.88 | |||
OR, odds ratio; 95% CI, 95% confidence interval; NA, not applicable; ADL, activities of daily living; IADL, instrumental activities of daily living.
Adjusted for age, sex, single/widow, impaired mobility, and symptoms of depression.
When overall frailty instead of individual domains was adjusted for age and sex in the multivariable model, a high score of the Groningen Frailty Indicator was associated with the composite outcome decline/death (OR, 1.97; 95% CI, 1.05 to 3.68) when compared with a low score. Frailty according to the Fried Frailty Index or frailty according to the geriatric assessment were not significantly associated with functional decline and death when compared with the nonfrail patients (Fried Frailty Index score ≥3: OR, 1.46; 95% CI, 0.80 to 2.68; geriatric assessment ≥2 impairments: OR, 1.65; 95% CI, 0.81 to 3.35). Results are shown in Supplemental Table 4.
Caregiver
Of the 114 caregivers at baseline, 13 were excluded because the corresponding participants were excluded (n=4) or had died (n=9), and another nine caregivers were lost to follow-up. Thus, follow-up data were available for 92 caregivers. Results for the different questionnaires are shown in Table 4.
Table 4.
Caregiver questionnaires (n=92)
| Caregiver Questionnaires | Prevalence at Follow-Up, n (%) |
|---|---|
| Patient deterioration in daily activities (IDDD score ≥1) | 47 (51%) |
| Patient neuropsychiatric symptoms (NPI score ≥1) | 54 (59%) |
| Caregiver reported pressure from informal care (SPICC score ≥4) | 35 (38%) |
The following variables had missing data: NPI (1.1%) and SPICC (4.3%). IDDD, Interview for Deterioration in Daily Activities; NPI, Neuropsychiatric Inventory; SPPIC, Self-perceived Pressure from Informal Care.
More than half of the caregivers reported that the participant showed deterioration in one or more daily activities during the first 6 months after dialysis initiation. The activities most frequently affected were writing (27%), doing groceries (22%), dressing (20%), and closing zippers/buttons and tying shoelaces (20%). Overall, caregivers did not report more neuropsychiatric symptoms of the participants at follow-up (56% at baseline versus 59% at follow-up; P=0.84). However, they did see an increase in symptoms of apathy (9% versus 21%; P=0.007) and irritability (17% versus 33%; P=0.008). Additionally, caregiver burden was more prevalent during follow-up (23% moderate to high burden at baseline versus 38% at follow-up; P=0.004), and increased (median baseline, 2.0; IQR, 1.0–3.0) compared with median follow-up 3.0 (IQR, 1.0–5.0; P≤0.001).
Discussion
In this prospective, multicenter, cohort study of 187 elderly patients on incident dialysis, the prevalence of functional dependence was high; four out of five participants were dependent in functional status (30% ADL, 78% IADL) at initiation of dialysis. Furthermore, almost half of the participants experienced decline in functional status (40%) or died (8%) within the first 6 months after initiating dialysis. This decline was mostly due to loss of IADL abilities (37% decline in IADL versus 16% in ADL). Older age and a high score on the Groningen Frailty Indicator were associated with the composite outcome functional decline/death. In addition, the percentage of caregivers reporting a high burden of care, increased from 23% to 38% (P=0.004) after dialysis initiation.
To our best knowledge, this is one of the first studies that prospectively assessed functional course after initiating dialysis in a community-dwelling elderly population with ESKD. A previous study performed in nursing home patients at initiation of dialysis (n=3702) showed a very high rate of mortality and functional decline in ADL compared with our study population: within 3 months, 61% had died or had a decrease in ADL (11). Furthermore, this study showed an acceleration in decline of ADL in the 3 months before the initiation of dialysis, a stabilization between 1 and 4 months, followed by a further downward trajectory (11). However, this population of frail nursing home patients is not comparable to most elderly patients initiating dialysis. Another study (n=97) assessed the changes in living status after initiating dialysis in patients aged ≥80 years, and found that within 6 months after the start of dialysis more than 30% of patients required newly community or private-caregiver support or moved to a nursing home (12). A stabilization in functional status was seen over the next 2 years. However, this study did not report baseline data on frailty or geriatric impairments or the reason that people needed support (ADL or IADL). Therefore, it is not possible to make a comparison with our study results. Furthermore, another study that used the Karnofsky Performance Status and Short-Form 36 to evaluate functional status did not found a change in physical health after initiation of dialysis (32). However, this study is difficult to compare because of the relatively younger age of the patients that initiated dialysis (mean age was 60.6 years) and different evaluation moments. In our study, we assessed functional status only at two time points: at the start and 6 months after start of dialysis. Because functional dependence can vary even in stable dialysis patients (33), more frequent assessments, including the predialysis phase and longer follow-up, are needed to elucidate the full functional trajectory and assess the effect of hospitalization or life events.
In this study, we used a cut-off point of 1 as the definition of decline in functional status. Although this cut-off point is frequently used in prior studies (5,34,35), there is no consensus which cut-off point is clinically relevant. One of the main goals of dialysis (especially in the elderly population) is improving quality of life. Because functional status is such an important part of quality of life, a loss of one point may be clinically relevant. Furthermore, one new functional impairment could lead to another. For example, if a patient is not able to do grocery shopping anymore, this can lead to less mobilization, which can eventually lead to further loss of physical fitness, which can subsequently lead to difficulties in housekeeping, transportation, and malnutrition. This theory is also referred to as the geriatric snowball effect (36).
In our study more caregivers reported a high caregiver burden after initiating dialysis. Furthermore, caregiver burden already increased within 6 months after the initiation of dialysis. Although caregiver burden is common in the dialysis population (3,37–39) only few studied the effect of initiation of dialysis on caregivers (38). Unfortunately, most studies were performed in caregivers of relatively young patients (aged <60 years), using various outcomes (e.g., quality of life) and questionnaires, and were therefore not comparable to our study. Only one study used the Self-Perceived Pressure from Informal Care questionnaire in caregivers of elderly patients on maintenance hemodialysis (3). Although patients are comparable with our study population (same country, age, and cut-off point), this study showed a far higher burden (85%) in caregivers. One possible explanation could be that caregiver burden will even further increase 6 months after starting dialysis. As caregiver burden is associated with a decreased quality of life (39), more symptoms of depression (39), and could also lead to negative outcomes for patients (40,41), it is important to reduce and prevent caregiver burden. Therefore, physicians should periodically ask caregivers about caregiver burden to address factors that may cause distress (e.g., physical burden, psychosocial burden, behavioral problems). Additional support such as extended homecare and social work, but also education (42), can be used to decrease and prevent caregiver burden.
The major strength of this study is the large, multicenter inception cohort with extensive geriatric assessment and with few lost to follow-up. A limitation is that most patients were included just after start of dialysis. This could have led to both over- and underestimation of the functional decline at the start of study. Patients could have felt better because of an improvement of uremic complaints by dialysis and could therefore be more independent of care. It could also have led to an underestimation because the initiation period of dialysis can be challenging and could cause complaints (e.g., fatigue, cramps). Furthermore, as our 6-month mortality was lower than expected (43–45), it is likely that a relatively healthy elderly population was included into this study. A possible reason could be that patients with a more impaired health status were less likely to participate in our study. Unfortunately, we do not have any details of patients that refused to participate after being invited by their treating nephrologists. Therefore, we cannot test this hypothesis. This may imply that the findings we report may not be fully generalizable to all elderly patients initiating dialysis and, consequently, that functional decline may be even higher for this population. Another limitation is that because of the low rate of events, our multivariable models were relatively underpowered and therefore potential associations could be missed. Furthermore, no separate analysis could be performed for functional improvement because patients that were fully independent at baseline could not improve during follow-up as they already had the best score (ceiling effect). Therefore, no additional analysis for protective factors of functional decline could be performed. Because none of the patients had the worst score in functional status at baseline, this was not the case for decline.
Our results may have implications for the care of elderly patients with ESKD. In our study, the rate of functional decline 6 months after initiation of dialysis (especially in patients aged ≥75 years) is high. However, this decline was mainly through loss of IADL activities and seems therefore much less severe in our study population compared with previously mentioned studies that found severe loss of independency (11,12). Despite this, caregiver burden does increase, so the initiation of dialysis seems to have a negative effect on the situation at the start of dialysis. Moreover, the effect of functional loss is highly dependent on personal values and patient preferences. Thus, it is important to use the predialysis phase to explore an individual’s health goals (e.g., living at home, engage in social activities) and current quality of life (46). This could improve shared decision making regarding dialysis and conservative management. In addition, interventions may be initiated to prevent functional decline by engaging physical activity (47).
In conclusion, in patients aged ≥65 years, functional decline within the first 6 months after initiating dialysis is highly prevalent. Frail and older patients are especially at risk for functional decline. Moreover, the initiation of dialysis seems to be accompanied by an increase in caregiver burden. Further research should focus on improving the identification of patients at risk for functional decline and interventions that could maintain functional status. Better identification of the patient at risk for functional decline could lead to better decision making, and therefore less suffering and less health care costs. Moreover, it could lead to preventive strategies with regard to functional decline.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to all patients and medical staff who participated in this project.
This work was funded by Dianet Dialysis Stichting, the Cornelis de Visser Stichting, Stichting Medicina et Scientia, and AstraZeneca.
The funding sources had no role in the design, data collection, analysis, manuscript preparation, interpretation, or decision to submit the manuscript for publication.
Geriatric Assessment in Older Patients Starting Dialysis study investigators: Dianet Dialysis Center: Dr. Boereboom; Diakonessenhuis Utrecht: Dr. Hamaker; University Medical Center Utrecht: Dr. Abrahams, Dr. Bots, and Dr. Verhaar; St. Antonius Hospital, Nieuwegein: Dr. Vincent; Spaarne Gasthuis, Haarlem: Dr. Douma and Dr. C. Verburg; Bernhoven Hospital, Uden: Dr. Lips; Gelderse Vallei Hospital, Ede: Dr. Siezenga; Ter Gooi Hospital, Hilversum: Dr. Gamadia; Academic Medical Center, Amsterdam: I. Keur; Zaans Medical Center, Zaandam: R.J.L. Klaassen; Jeroen Bosch Hospital, Hertogenbosch: Dr. Hoogeveen; Albert Schweitzer Hospital, Dordrecht: Dr. van Bommel; St. Franciscus Hospital, Rotterdam: Dr. Schrama; Maasstad Hospital, Rotterdam: Dr. Van de Ven; and Groene Hart Hospital, Gouda: J.W. Eijgenraam.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice, “Functioning on Dialysis: An Oxymoron?,” on pages 963–964.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13131118/-/DCSupplemental.
Supplemental Table 1. Overview of testing methods.
Supplemental Table 2: Baseline characteristics for the different functional dependencies.
Supplemental Table 3. Overview of impairments in functional status at baseline and after 6 months of follow-up.
Supplemental Table 4. Association between frailty tools and functional decline/death.
References
- 1.US Renal Data System: 2017 USRDS Annual Data Report. Available at: https://www.usrds.org/2017/download/2017_Volume_1_CKD_in_the_US.pdf. Accessed August 28, 2018
- 2.Kurella M, Covinsky KE, Collins AJ, Chertow GM: Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 146: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Parlevliet JL, Buurman BM, Pannekeet MM, Boeschoten EM, ten Brinke L, Hamaker ME, van Munster BC, de Rooij SE: Systematic comprehensive geriatric assessment in elderly patients on chronic dialysis: A cross-sectional comparative and feasibility study. BMC Nephrol 13: 30, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G: Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 59: 255–263, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Jassal SV, Karaboyas A, Comment LA, Bieber BA, Morgenstern H, Sen A, Gillespie BW, De Sequera P, Marshall MR, Fukuhara S, Robinson BM, Pisoni RL, Tentori F: Functional dependence and mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 67: 283–292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook WL, Jassal SV: Functional dependencies among the elderly on hemodialysis. Kidney Int 73: 1289–1295, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Bossola M, Di Stasio E, Antocicco M, Pepe G, Tazza L, Zuccalà G, Laudisio A: Functional impairment is associated with an increased risk of mortality in patients on chronic hemodialysis. BMC Nephrol 17: 72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kutner NG, Zhang R, McClellan WM: Patient-reported quality of life early in dialysis treatment: Effects associated with usual exercise activity. Nephrol Nurs J 27: 357–367, discussion 368, 424, 2000 [PubMed] [Google Scholar]
- 9.Sclauzero P, Galli G, Barbati G, Carraro M, Panzetta GO: Role of components of frailty on quality of life in dialysis patients: A cross-sectional study. J Ren Care 39: 96–102, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Ramer SJ, McCall NN, Robinson-Cohen C, Siew ED, Salat H, Bian A, Stewart TG, El-Sourady MH, Karlekar M, Lipworth L, Ikizler TA, Abdel-Kader K: Health outcome priorities of older adults with advanced CKD and concordance with their nephrology providers’ perceptions. J Am Soc Nephrol 29: 2870–2878, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jassal SV, Chiu E, Hladunewich M: Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med 361: 1612–1613, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW: Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 185: 914–919, 1963 [DOI] [PubMed] [Google Scholar]
- 14.Lawton MP, Brody EM: Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: 179–186, 1969 [PubMed] [Google Scholar]
- 15.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF 3rd: Rating chronic medical illness burden in geropsychiatric practice and research: Application of the cumulative illness rating scale. Psychiatry Res 41: 237–248, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Yesavage JA, Sheikh JI: Geriatric Depression Scale (GDS). Clin Gerontol 5: 165–173, 1986 [Google Scholar]
- 17.Marc LG, Raue PJ, Bruce ML: Screening performance of the 15-item geriatric depression scale in a diverse elderly home care population. Am J Geriatr Psychiatry 16: 914–921, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podsiadlo D, Richardson S: The Timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39: 142–148, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Guigoz Y, Vellas B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: Presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme 1: 3–11; discussion 11-2, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975 [DOI] [PubMed] [Google Scholar]
- 21.Schmand B, Houx P, de Koning I: The Stroop Colour Word Test, the Trail Making Test, the Rivermead Behavioural Memory Test, Verbal Fluency Test. Dutch Norms, Amsterdam, Netherlands Institute of Psychologists, Section of Neuropsychology, 2012 [Google Scholar]
- 22.Royall DR, Cordes JA, Polk M: CLOX: An executive clock drawing task. J Neurol Neurosurg Psychiatry 64: 588–594, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grober E, Buschke H, Crystal H, Bang S, Dresner R: Screening for dementia by memory testing. Neurology 38: 900–903, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Mohile SG, Bylow K, Dale W, Dignam J, Martin K, Petrylak DP, Stadler WM, Rodin M: A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer 109: 802–810, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Owusu C, Koroukian SM, Schluchter M, Bakaki P, Berger NA: Screening older cancer patients for a comprehensive geriatric assessment: A comparison of three instruments. J Geriatr Oncol 2: 121–129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Peters LL, Boter H, Buskens E, Slaets JP: Measurement properties of the Groningen Frailty Indicator in home-dwelling and institutionalized elderly people. J Am Med Dir Assoc 13: 546–551, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J: The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: 2308–2314, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Teunisse S, Derix MM, van Crevel H: Assessing the severity of dementia. Patient and caregiver. Arch Neurol 48: 274–277, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Pot AM, van Dyck R, Deeg DJ: [Perceived stress caused by informal caregiving. Construction of a scale]. Tijdschr Gerontol Geriatr 26: 214–219, 1995 [PubMed] [Google Scholar]
- 31.Lunney JR, Lynn J, Hogan C: Profiles of older Medicare decedents. J Am Geriatr Soc 50: 1108–1112, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Da Silva-gane M, Wellsted D, Greenshields H, Norton S, Chandna SM, Farrington K: Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol 7: 2002–2009, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MK, Paul S, Ward SE, Gilet CA, Hladik GA: One-year linear trajectories of symptoms, physical functioning, cognitive functioning, emotional well-being, and spiritual well-being among patients receiving dialysis. Am J Kidney Dis 72: 198–204, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Govers AC, Buurman BM, Jue P, de Mol BA, Dongelmans DA, de Rooij SE: Functional decline of older patients 1 year after cardiothoracic surgery followed by intensive care admission: A prospective longitudinal cohort study. Age Ageing 43: 575–580, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Deckx L, van den Akker M, Daniels L, De Jonge ET, Bulens P, Tjan-Heijnen VC, van Abbema DL, Buntinx F: Geriatric screening tools are of limited value to predict decline in functional status and quality of life: Results of a cohort study. BMC Fam Pract 16: 30, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall RK, McAdams-DeMarco MA: Breaking the cycle of functional decline in older dialysis patients. Semin Dial 31: 462–467, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suri RS, Larive B, Garg AX, Hall YN, Pierratos A, Chertow GM, Gorodetskeya I, Kliger AS; FHN Study Group : Burden on caregivers as perceived by hemodialysis patients in the Frequent Hemodialysis Network (FHN) trials. Nephrol Dial Transplant 26: 2316–2322, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan SL, Sathick I, McKitty K, Punzalan S: Quality of life of caregivers and patients on peritoneal dialysis. Nephrol Dial Transplant 23: 1713–1719, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Belasco A, Barbosa D, Bettencourt AR, Diccini S, Sesso R: Quality of life of family caregivers of elderly patients on hemodialysis and peritoneal dialysis. Am J Kidney Dis 48: 955–963, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Thong MSY, Kaptein AA, Krediet RT, Boeschoten EW, Dekker FW: Social support predicts survival in dialysis patients. Nephrol Dial Transplant 22: 845–850, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Untas A, Thumma J, Rascle N, Rayner H, Mapes D, Lopes AA, Fukuhara S, Akizawa T, Morgenstern H, Robinson BM, Pisoni RL, Combe C: The associations of social support and other psychosocial factors with mortality and quality of life in the dialysis outcomes and practice patterns study. Clin J Am Soc Nephrol 6: 142–152, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mollaoğlu M, Kayataş M, Yürügen B: Effects on caregiver burden of education related to home care in patients undergoing hemodialysis. Hemodial Int 17: 413–420, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Couchoud C, Labeeuw M, Moranne O, Allot V, Esnault V, Frimat L, Stengel B; French Renal Epidemiology and Information Network (REIN) registry : A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant 24: 1553–1561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wick JP, Turin TC, Faris PD, MacRae JM, Weaver RG, Tonelli M, Manns BJ, Hemmelgarn BR: A clinical risk prediction tool for 6-month mortality after dialysis initiation among older adults. Am J Kidney Dis 69: 568–575, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Thamer M, Kaufman JS, Zhang Y, Zhang Q, Cotter DJ, Bang H: Predicting early death among elderly dialysis patients: Development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis 66: 1024–1032, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandel EI, Bernacki RE, Block SD: Serious illness conversations in ESRD. Clin J Am Soc Nephrol 12: 854–863, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansen KL: Exercise in the end-stage renal disease population. J Am Soc Nephrol 18: 1845–1854, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.