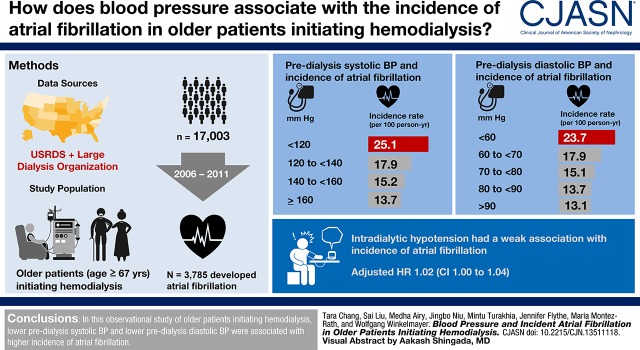
Visual Abstract
Keywords: Cardiovascular, blood pressure, hemodialysis, renal dialysis, Atrial Fibrillation, Incidence, Proportional Hazards Model, Systole, Diastole, Blood Pressure Determination, hypotension
Abstract
Background and objectives
We examined the association of predialysis systolic and diastolic BP and intradialytic hypotension with incident atrial fibrillation in older patients initiating hemodialysis.
Design, setting, participants, & measurements
We used the US Renal Data System linked to the records of a large dialysis provider to identify patients aged ≥67 years initiating hemodialysis between January 2006 and October 2011. We examined quarterly average predialysis systolic BP, diastolic BP, and proportion of sessions with intradialytic hypotension (i.e., nadir systolic BP <90 mm Hg). We applied an extended Cox model to compute adjusted hazard ratios (HRs) of each exposure with incident atrial fibrillation.
Results
Among 17,003 patients, 3785 developed atrial fibrillation. When comparing predialysis systolic BP to a fixed reference of 140 mm Hg, lower predialysis systolic BP was associated with a higher hazard of atrial fibrillation, whereas higher systolic BP was associated with a lower hazard of atrial fibrillation. When comparing across a range of systolic BP for two hypothetical patients with similar measured covariates, the association varied by mean systolic BP: at systolic BP 190 mm Hg, each 10 mm Hg lower systolic BP was associated with lower atrial fibrillation hazard (HR, 0.94; 95% confidence interval, 0.90 to 1.00), whereas at systolic BP 140 mm Hg, a 10 mm Hg lower systolic BP was associated with a higher atrial fibrillation hazard (HR, 1.12; 95% confidence interval, 1.10 to 1.14). Lower diastolic BP was associated with higher atrial fibrillation hazards. Intradialytic hypotension was weakly associated with atrial fibrillation.
Conclusions
In this observational study of older patients initiating hemodialysis, lower predialysis systolic BP and diastolic BP were associated with higher incidence of atrial fibrillation.
Introduction
Atrial fibrillation is exceptionally common in patients with ESKD, with a prevalence of >25% and a cumulative incidence during the first year of hemodialysis among older patients of nearly 15% (1). The hemodialysis procedure itself can trigger atrial fibrillation (2), due to a combination of fluctuations in BP, large fluid shifts, changes in serum potassium and calcium concentrations, and unfavorable effects on cardiac function (3–5). Given that patients with atrial fibrillation experience poorer health outcomes, including higher mortality, ischemic stroke, myocardial infarction, and heart failure (6–9), it is important to identify potentially modifiable risk factors for incident atrial fibrillation.
Risk factors for incident atrial fibrillation in patients with and without ESKD include older age, white race, a history of heart failure, and diabetes mellitus (10). In patients without ESKD, higher systolic BP and higher diastolic BP are associated with a higher risk of incident atrial fibrillation (11). Whether the same relation between BP and incident atrial fibrillation applies in patients receiving hemodialysis is unclear because lower BP is generally associated with higher risks of cardiovascular events in that population (12,13). Two single-center studies of patients on hemodialysis showed no significant association of BP with atrial fibrillation, but were limited by relatively small sample sizes (3,14). A larger study of United States patients on dialysis showed that patients in the highest (systolic BP >162 mm Hg) and lowest (systolic BP <130 mm Hg) quartiles of predialysis systolic BP had higher hazards for atrial fibrillation, but that study was on the basis of data from over two decades ago and may have included patients with preexisting atrial fibrillation (15).
We sought to examine the association of BP with incident atrial fibrillation in a large, nationally representative cohort of older patients initiating treatment for ESKD. We hypothesized that, similar to the general population, higher predialysis systolic BP and diastolic BP would be associated with higher risk of incident atrial fibrillation. We also hypothesized that patients with more frequent episodes of intradialytic hypotension would have a higher risk of incident atrial fibrillation.
Materials and Methods
Source Population
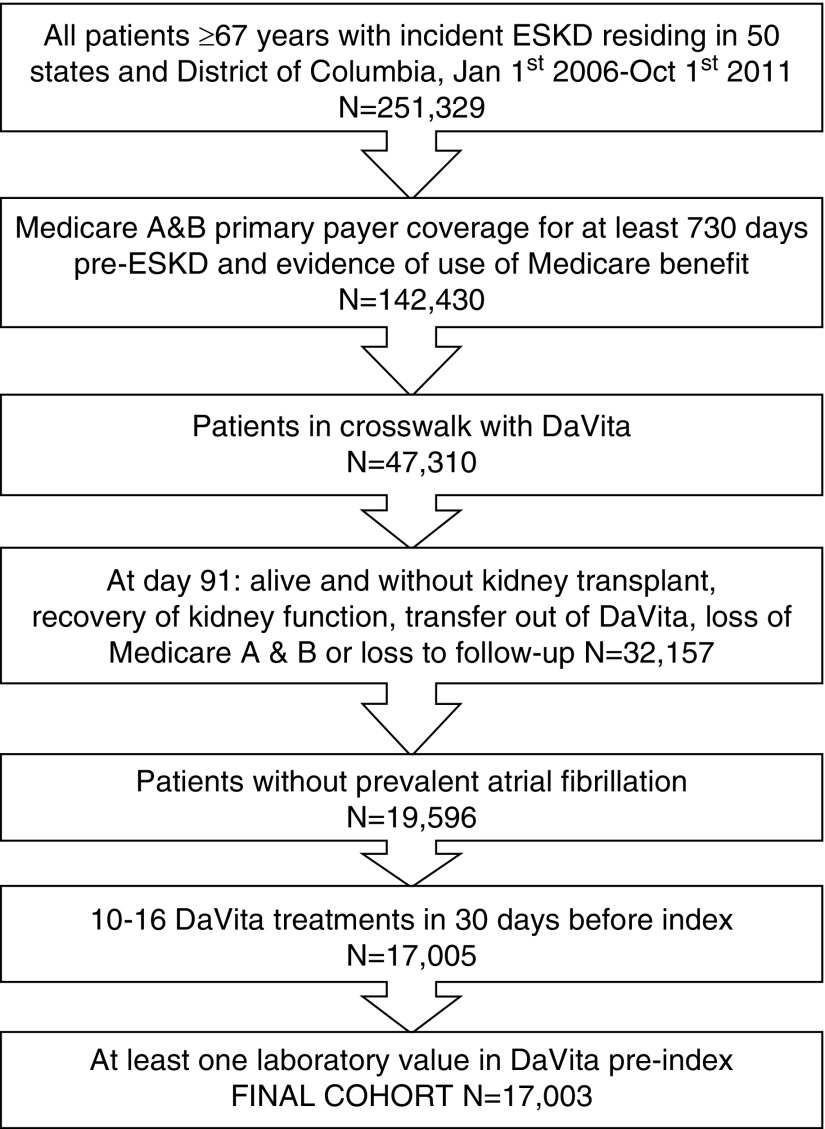
For a detailed description of our methods, please see Supplemental Appendix 1. In brief, we used data from the US Renal Data System (USRDS), the national registry for patients with ESKD (16), linked with data from the electronic health records of a large dialysis provider in the United States (DaVita, Inc., Denver, CO), using a crosswalk provided by the USRDS data coordinating center. We identified patients from the USRDS with incident ESKD ≥67 years who initiated maintenance dialysis between January 1, 2006 and October 1, 2011 in the 50 American states and the District of Columbia (Figure 1). We restricted the cohort to patients with uninterrupted coverage and proven use of Medicare Part A and B for the 2 years leading up to the date of the first dialysis to ascertain comorbid conditions, including prevalent atrial fibrillation, using Medicare claims. We selected all patients who initiated ESKD treatment with in-center hemodialysis and continued on this modality through day 91, which we defined as our index date. Patients who died, received a kidney transplant, recovered kidney function, received any peritoneal dialysis treatment, lost Medicare Part A and B coverage, or who did not have evidence of one or more dialysis session in a DaVita unit before the index date were excluded. We identified eligible subjects for incident atrial fibrillation by excluding all patients with any billing claims containing an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code of 427.3× before the index date (1).
Figure 1.
The final cohort consisted of 17,003 older patients who initiated hemodialysis in a DaVita facility between January 2006 and October 2011.
We further restricted the cohort to patients with 10–16 DaVita hemodialysis sessions in the 30 days before the index date, to exclude patients who may have been on twice weekly dialysis and patients requiring more frequent dialysis (four to five times weekly) sessions. To account for hospitalized days, we credited patients three sevenths of a hemodialysis session for each day spent in a hospital during the 30 days before the index date. For example, a patient hospitalized for 28 out of 30 days would be credited with 3/7×28=12 hemodialysis sessions and thus remain in the cohort. Finally, we excluded any patients without any laboratory results recorded in DaVita before the index date.
Outcome: Incident Atrial Fibrillation
We identified incident atrial fibrillation from any inpatient claim with ICD-9 diagnosis code of 427.3×, or from any outpatient claim with ICD-9 diagnosis code of 427.3×, provided that there was a second, subsequent inpatient or outpatient code of 427.3×, in any position, separated by at least 1 day. Of note, >85% of incident atrial fibrillation in patients on hemodialysis is first recorded in inpatient claims (17).
Exposures: BP Parameters
We defined the 90-day period from dialysis initiation to the index date as quarter 0, and continued to divide follow-up time after the index date into 90-day quarters. We averaged pre-dialysis seated systolic and diastolic BP using all available values for a given quarter. We had records of nadir intradialytic BP for each dialysis session, and defined intradialytic hypotension as any session with a nadir systolic BP <90 mm Hg (18). We calculated the proportion of dialysis sessions with intradialytic hypotension for each quarter. Using the %SPECI SAS Macro (19), we determined that modeling systolic BP and diastolic BP as a squared term and modeling intradialytic hypotension as a linear term provided the best combination of model fit and clinical applicability. However, we also conducted a companion analysis modeling the exposures as the following categorical variables: systolic BP (mm Hg) <120, 120 to <140, 140 to <160 (reference), ≥160; diastolic BP (mm Hg) <60, 60 to <70, 70 to <80, 80 to <90 (reference), ≥90; intradialytic hypotension (%) 0 (reference) >0 to <6, 6–14, >14.
Covariates
We collected baseline information at time of incident ESKD: age, sex, Medicaid dual eligibility status, incident ESKD year, reported race (white, black, Asian, Native American, and other) and Hispanic ethnicity, body mass index, and eGFR, including neighborhood-level socioeconomic data (20): median rent, median household income, percentage living below the federal poverty line, percentage unemployed, and percentage with less than a high-school education.
We ascertained the following comorbidities from claims using ICD-9 diagnosis (requiring one inpatient or two outpatient encounters separated by at least 1 day) and procedure codes (21): myocardial infarction, coronary artery disease, coronary revascularization, unstable angina, heart failure, valvular disease, ventricular fibrillation or other arrhythmia, pacemaker, hypertension, stroke or transient ischemic attack, peripheral artery disease, diabetes mellitus, hyperlipidemia, lung disease, liver disease, and depression. We ascertained baseline comorbidities using all available claims from 730 days before the index date, which were updated in each subsequent 90-day quarter. From the DaVita database, information on serum albumin and dialysis treatment length was averaged and also updated for each 90-day quarter. We ascertained whether a central venous catheter was ever used for vascular access during each quarter.
Statistical Analyses
We related the development of incident atrial fibrillation during quarter Qi with the BP parameters as well as other covariates ascertained during quarter Qi-1, where i represents the quarter indicator. We censored follow-up time at the earliest event among the following: modality change (n=2279), loss of Medicare Part A and B coverage (n=453), death (n=6065), kidney transplantation (n=233), discontinued receiving dialysis within the DaVita network (n=957), or end of study period (December 31, 2011; n=7016). The maximum follow-up time was 5.7 years (mean and median follow-up times were 1.4 and 1.0 years, respectively). We applied a Cox model as a function of a time-varying exposures and covariates (extended Cox) to compute adjusted hazard ratios (HRs) for the association with atrial fibrillation of each exposure in separate analyses. HRs were adjusted in five nested models: model 0, adjusted for year of incident ESKD; model 1, additionally adjusted for age, sex, race, and Hispanic ethnicity; model 2, additionally adjusted for census division, socioeconomic status variables, and Medicaid dual eligibility; model 3, additionally adjusted for all comorbid conditions listed above and vascular access; and model 4, additionally adjusted for albumin, eGFR, body mass index, number of dialysis sessions, and mean dialysis treatment length. We examined the correlation of the scaled Schoenfeld residuals with time and found no evidence that the log-HR changed with follow-up time for each of the three exposures and other covariates included in their respective model 4 (Schoenfeld test global P value of 0.23, 0.23, and 0.21 in model 4 for systolic BP, diastolic BP, and intradialytic hypotension, respectively).
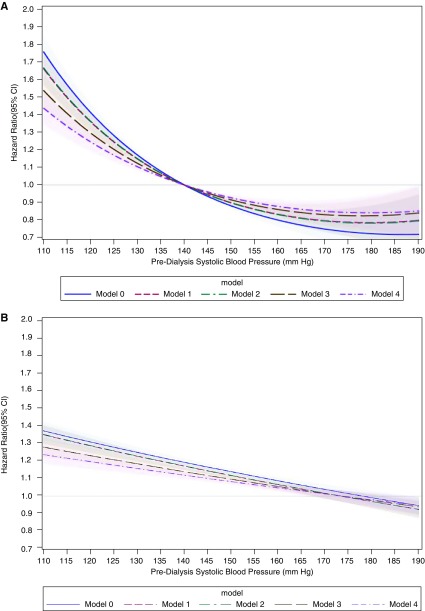
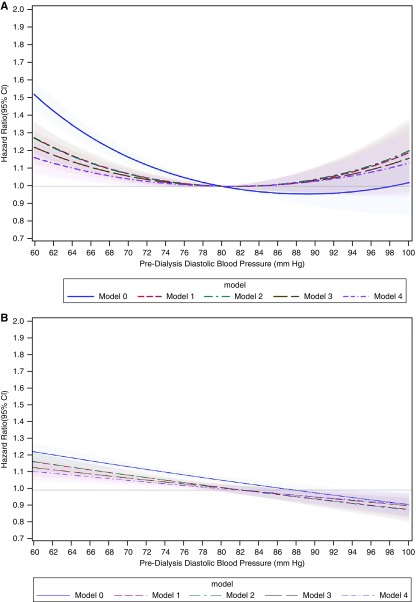
To aid the interpretation of results when modeling systolic and diastolic BP as a quadratic form, we generated two separate plots for each of these exposures. Both plots compare the hazard for atrial fibrillation between two hypothetical patients that are similar for all covariates at a time t but differ in their average BP measurements. In the first plot (A), predialysis BP anywhere in the possible range is compared with a fixed predialysis BP (140 mm Hg for systolic BP, 80 mm Hg for diastolic BP). The second plot (B) shows the HRs reflecting the atrial fibrillation hazards associated with a 10 mm Hg reduction in predialysis systolic BP or a 5 mm Hg reduction in predialysis diastolic BP across a wide range of BP values.
Missing Data
In our cohort of 17,003 patients, 2675 had at least one variable missing for every quarter the patient remained in the analytical cohort and 11,789 (11.4%) of 103,464 quarterly records had missing data. Most missing records were generated from nontime-varying variables (most common baseline eGFR, 7.7%). The most common missing record for time-varying variables was dialysis treatment length (2.4%).
Missing data were handled using multiple imputation by chained equations as implemented in R, and 12 imputed datasets were obtained for each outcome (22,23). We assumed that the data were missing at random, conditional on observed variables. In addition to the exposure and all covariates included in the analysis model, the imputation model also included the event indicator, the Nelson–Aalen estimator of the cumulative marginal hazard (24,25), and auxiliary variables (Supplemental Methods). We combined the estimates and SEM obtained from the model applied to each imputed dataset, using the Rubin rules (26).
The study was approved by Institutional Review Boards at Stanford University (protocol IRB-17904) and Baylor College of Medicine (protocol H-36408), and active Data Use Agreements with the National Institute of Diabetes and Digestive and Kidney Diseases were in place. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC), Stata version 15.1 (Stata Statistical Software: Release 15; StataCorp., College Station, TX), and R version 3.1.2.
Results
We identified 17,003 patients who met the stated inclusion and exclusion criteria (Figure 1). On average, patients with lower baseline systolic BP were older, more often male, and more were of white race than patients with higher baseline systolic BP (Table 1). They also had a higher prevalence of most comorbid conditions, with the notable exception of diabetes mellitus and, naturally, hypertension. Patients with lower baseline diastolic BP were older, of white race, and had a higher prevalence of heart failure and peripheral artery disease than patients with higher baseline diastolic BP (Supplemental Table 1). Patients with more frequent episodes of intradialytic hypotension were more often of white race, and had a higher prevalence of heart failure and diabetes mellitus (Supplemental Table 2).
Table 1.
Baseline characteristics of incident patients aged ≥65 years registered in the US Renal Data System by baseline predialysis systolic BP
| Patient Characteristics | Baseline pre-dialysis systolic BP Category (mm Hg) | ||||
|---|---|---|---|---|---|
| <120 (n=1180) | 120 to <140 (n=5207) | 140 to <160 (n=7032) | ≥160 (n=3581) | Overall (n=17,003) | |
| Demographics | |||||
| Age, yr, mean (SD) | 78 (7) | 77 (6) | 76 (6) | 75 (6) | 76 (6) |
| Male sex | 804 (68%) | 3029 (58%) | 3348 (48%) | 1254 (35%) | 8436 (45%) |
| Race | |||||
| White | 925 (78%) | 3858 (74%) | 4801 (68%) | 2220 (62%) | 11,806 (69%) |
| Black | 216 (18%) | 1120 (22%) | 1891 (27%) | 1176 (33%) | 4404 (26%) |
| Asian | 25 (2%) | 173 (3%) | 289 (4%) | 139 (4%) | 626 (4%) |
| Hispanic ethnicity | 77 (7%) | 354 (7%) | 567 (8%) | 314 (9%) | 1312 (8%) |
| Medicare/Medicaid eligible | 273 (23%) | 1290 (25%) | 1897 (27%) | 1054 (29%) | 4515 (27%) |
| CVC vascular access | 878 (74%) | 3334 (64%) | 4435 (63%) | 2385 (67%) | 11,033 (65%) |
| Census data, median (Q1–Q3) | |||||
| Unemployed | 9 (6–12) | 9 (7–12) | 9 (7–12) | 10 (7–13) | 9 (7–12) |
| Below poverty | 13 (8–10) | 13 (8–20) | 14 (8–21) | 15 (9–22) | 14 (8–21) |
| <High school education | 13 (8–21) | 13 (8–20) | 14 (9–21) | 15 (9–22) | 14 (9–21) |
| Monthly rent, $ | 886 (685–126) | 878 (701–1099) | 854 (688–1068) | 834 (678–1032) | 861 (688–1072) |
| Annual household income, $ | 49,214 (40,113–66,905) | 49,010 (39,517–65,884) | 48,125 (38,470–63,086) | 47,085 (37,132–61,418) | 48,239 (38,571–63,965) |
| Comorbid conditions | |||||
| Myocardial infarction | 108 (9%) | 384 (7%) | 432 (6%) | 203 (6%) | 1127 (7%) |
| Coronary artery disease | 531 (45%) | 1930 (37%) | 2291 (33%) | 1061 (30%) | 5814 (34%) |
| Coronary revascularization | 140 (12%) | 505 (10%) | 592 (8%) | 279 (8%) | 935 (9%) |
| Unstable angina | 64 (5%) | 279 (5%) | 356 (5%) | 203 (6%) | 902 (5%) |
| Heart failure | 625 (53%) | 2223 (43%) | 2802 (40%) | 1460 (41%) | 7110 (42%) |
| Valvular disease | 181 (15%) | 596 (11%) | 673 (10%) | 334 (9%) | 1784 (11%) |
| Ventricular fibrillation or other arrhythmia | 110 (9%) | 362 (7%) | 386 (5%) | 167 (5%) | 1028 (6%) |
| Pacemaker | 60 (5%) | 181 (4%) | 160 (2%) | 60 (2%) | 461 (3%) |
| Hypertension | 940 (80%) | 4220 (81%) | 5755 (82%) | 2985 (83%) | 13,903 (82%) |
| Stroke or transient ischemic attack | 108 (9%) | 437 (8%) | 576 (8%) | 310 (9%) | 1434 (8%) |
| Peripheral artery disease | 234 (20%) | 858 (17%) | 1041 (15%) | 492 (14%) | 2626 (15%) |
| Diabetes mellitus | 580 (49%) | 2745 (53%) | 4026 (57%) | 2301 (64%) | 9654 (57%) |
| Hyperlipidemia | 302 (26%) | 1418 (27%) | 2007 (29%) | 945 (26%) | 4673 (28%) |
| Lung disease | 369 (31%) | 1234 (24%) | 1491 (21%) | 688 (19%) | 3783 (22%) |
| Liver disease | 84 (7%) | 179 (3%) | 155 (2%) | 97 (3%) | 515 (3%) |
| Depression | 121 (10%) | 421 (8%) | 489 (7%) | 208 (6%) | 1239 (7%) |
| Biometric measurements, mean (SD) | |||||
| BMI at index date | 27 (7) | 28 (7) | 28 (7) | 28 (7) | 28 (7) |
| Missing, N(%) | 118 (10%) | 425 (8%) | 530 (8%) | 259 (7%) | 1332 (8%) |
| eGFR at dialysis initiation, ml/min per 1.73 m2 | 13.1 (5.5) | 12.5 (5.2) | 12.2 (5.0) | 11.7 (4.8) | 12.0 (5.0) |
| Missing, N (%) | 130 (11.0%) | 500 (9.6%) | 576 (8.2%) | 270 (7.5%) | 1476 (8.7%) |
| Dialysis length, hours | 3.5 (0.4) | 3.5 (0.4) | 3.4 (0.4) | 3.5 (0.4) | 3.5 (0.4) |
| Serum albumin, g/dl | 3.4 (0.5) | 3.5 (0.4) | 3.6 (0.4) | 3.5 (0.4) | 3.5 (0.4) |
| Predialysis systolic BP, mean (SD) | 113 (7) | 131 (6) | 149 (6) | 171 (9) | 146 (18) |
| Predialysis diastolic BP, mean (SD) | 63 (5) | 68 (6) | 73 (7) | 80 (8) | 72 (9) |
| Dialysis sessions with intradialytic hypotension, % mean (SD) | 35.1% (24.7%) | 13.4% (14.2%) | 7.3% (9.8%) | 5.3% (7.9%) | 10.7% (14.5%) |
All values are N (%) unless otherwise stated. Three patients were missing systolic BP at baseline and were excluded from this table. Overall, 144 patients reported Native American race and 23 patients reported other race. CVC vascular access was missing in 69 (0.4%) patients. <1% of patients had data missing for serum albumin, dialysis length, Hispanic ethnicity, and each of the census-based socioeconomic metrics. These variables cannot be tabulated owing to research regulations prohibiting the disclosure of cell sizes <10. CVC, central venous catheter; BMI, body mass index.
During 23,500 person-years of follow-up, 3785 patients developed atrial fibrillation. The unadjusted incidence rate of atrial fibrillation was highest among patients with lower predialysis systolic BP (Table 2). In sequentially adjusted nested models (Figure 2A), we also observed a curvilinear association of systolic BP with atrial fibrillation hazard. For example, when comparing predialysis systolic BP to a fixed reference of 140 mm Hg, systolic BP of 120 mm Hg was associated with a 41.3% (95% confidence interval [95% CI], 36.2% to 46.3%) higher hazard of atrial fibrillation, whereas systolic BP of 160 mm Hg was associated with a 20.0% (95% CI, 16.4% to 23.6%) lower hazard of atrial fibrillation. Results were similar when we modeled systolic BP as a categorical variable (Supplemental Table 3).
Table 2.
Observed events and unadjusted incidence rates of atrial fibrillation by category of baseline predialysis systolic BP, diastolic BP, and proportion of sessions with intradialytic hypotension (defined as nadir systolic BP <90 mm Hg)
| Exposure Variable | Total Patients (N) | Total Person-Time At Risk (Years) | Patients with Incident Atrial Fibrillation (N) | Incidence Rate (Events/100 Person-Year) |
|---|---|---|---|---|
| Predialysis systolic BP (mm Hg) | ||||
| <120 | 1180 | 1139 | 286 | 25.1 |
| 120 to <140 | 5207 | 6736 | 1207 | 17.9 |
| 140 to <160 | 7032 | 10075 | 1534 | 15.2 |
| ≥160 | 3581 | 5550 | 758 | 13.7 |
| Predialysis diastolic BP (mm Hg) | ||||
| <60 | 870 | 889 | 211 | 23.7 |
| 60 to <70 | 6377 | 8359 | 1494 | 17.9 |
| 70 to <80 | 6564 | 9460 | 1429 | 15.1 |
| 80 to <90 | 2680 | 3988 | 546 | 13.7 |
| ≥90 | 509 | 802 | 105 | 13.1 |
| Proportion of sessions with intradialytic hypotension | ||||
| 0 | 4777 | 7139 | 1074 | 15.1 |
| >0 to <6 | 4364 | 6526 | 1000 | 15.3 |
| 6–14 | 3499 | 4769 | 768 | 16.1 |
| >14 | 4360 | 5066 | 943 | 18.6 |
Total person-time at risk is censored for death, loss of Medicare Part A and B coverage, kidney transplant, dialysis modality change, leaving the DaVita network, or end of study period.
Figure 2.
Systolic BP had a curvilinear association with atrial fibrillation hazard when compared to a fixed reference (140 mm Hg), while when comparing each 10 mm Hg lower systolic BP across a range of systolic BP, the association with atrial fibrillation hazard differed depending on the average systolic BP level. (A) Predialysis systolic BP from 110 to 190 mm Hg is compared with predialysis systolic BP 140 mm Hg. (B) HRs for 10 mm Hg lower predialysis systolic BP across a range of systolic BP from 110 to 190 mm Hg. Model 0, adjusted for year of incident ESKD; model 1, additionally adjusted for age, sex, race, and Hispanic ethnicity; model 2, additionally adjusted for census division, socioeconomic status variables, and Medicaid dual eligibility; model 3, additionally adjusted for comorbid conditions and vascular access; and model 4, additionally adjusted for albumin, eGFR, body mass index, number of dialysis sessions, and mean dialysis treatment length.
When comparing the HR associated with each 10 mm Hg lower systolic BP across a range of systolic BP, we found that the association differed depending on the average systolic BP level (Figure 2B). For example, for two hypothetical patients with similar measured covariates and average systolic BP 190 mm Hg, a 10 mm Hg lower predialysis systolic BP was associated with a 5.5% (95% CI, 0.4% to 10.5%) lower risk of incident atrial fibrillation. In contrast, a 10 mm Hg lower predialysis systolic BP for two similar patients with average systolic BP of 170 mm Hg had no significant association with incident atrial fibrillation risk (HR, 1.01; 95% CI, 0.98 to 1.05), whereas at 140 mm Hg, a 10 mm Hg lower predialysis systolic BP was associated with a significantly higher incident atrial fibrillation risk (HR, 1.12; 95% CI, 1.10 to 1.14).
The unadjusted incidence rate of atrial fibrillation was highest among patients with lower predialysis diastolic BP (Table 2). When comparing predialysis diastolic BP to a fixed reference of 80 mm Hg, lower diastolic BP was associated with higher hazards of atrial fibrillation, and this association was attenuated in sequentially adjusted nested models (Figure 3A). Higher diastolic BP was also associated with higher hazard of atrial fibrillation, but the association was not statistically significant. We saw a similar curvilinear association when we modeled diastolic BP as a categorical variable (Supplemental Table 3). When comparing across a range of initial average diastolic BP, we estimated a higher atrial fibrillation incidence associated with a 5 mm Hg lower diastolic BP at levels of diastolic BP <75 mm Hg (Figure 3B).
Figure 3.
Diastolic BP had a curvilinear association with atrial fibrillation hazard when compared to a fixed reference (80 mm Hg), while when comparing each 5 mm Hg lower systolic BP across a range of systolic BP, the association with atrial fibrillation hazard differed depending on the average diastolic BP level. (A) Predialysis diastolic BP from 60 to 100 mm Hg is compared with predialysis diastolic BP 80 mm Hg. (B) HRs for 5 mm Hg lower predialysis diastolic BP across a range of diastolic BP from 60 to 100 mm Hg. Model 0, adjusted for year of incident ESKD; model 1, additionally adjusted for age, sex, race, and Hispanic ethnicity; model 2, additionally adjusted for census division, socioeconomic status variables, and Medicaid dual eligibility; model 3, additionally adjusted for comorbid conditions and vascular access; and model 4, additionally adjusted for albumin, eGFR, body mass index, number of dialysis sessions, and mean dialysis treatment length.
More frequent intradialytic hypotension was associated with higher unadjusted incidence rates of atrial fibrillation (Table 2). For a given patient with 10% higher proportion of dialysis sessions with intradialytic hypotension, they would have an associated 7% higher hazard of incident atrial fibrillation (HR, 1.07; 95% CI, 1.05 to 1.09; model 0, Table 3) in models adjusted only for incident ESKD year. However, with the inclusion of potential confounders in the models, the magnitude of association was smaller, and in fully adjusted models it was marginally significant (HR, 1.02; 95% CI, 1.00 to 1.04; model 4, Table 3).
Table 3.
Adjusted associations of intradialytic hypotension with incident atrial fibrillation
| Model | Hazard Ratio | 95% Confidence Limit |
|---|---|---|
| 0 | 1.07 | 1.05–1.09 |
| 1 | 1.06 | 1.04–1.08 |
| 2 | 1.06 | 1.04–1.08 |
| 3 | 1.03 | 1.02–1.05 |
| 4 | 1.02 | 1.00–1.04 |
Results are for each 10% higher proportion of dialysis sessions per quarter with intradialytic hypotension, defined as nadir intradialytic systolic BP <90 mm Hg. Model 0, adjusted for year of incident ESKD; model 1, additionally adjusted for age, sex, race and Hispanic ethnicity; model 2, additionally adjusted for census division, socioeconomic status variables, and Medicaid dual eligibility; model 3, additionally adjusted for comorbid conditions and vascular access; and model 4, additionally adjusted for albumin, eGFR, body mass index, and mean dialysis treatment length.
Discussion
We conducted a detailed investigation of the associations of predialysis systolic BP, diastolic BP, and intradialytic hypotension with incident atrial fibrillation a large, nationally representative cohort of older patients initiating hemodialysis in the United States. Using detailed information from each hemodialysis session, we averaged predialysis systolic BP and diastolic BP measurements across sequential 90-day time windows and showed that, compared with a fixed referent systolic BP of 140 mm Hg, patients with relatively lower systolic BP had higher hazards of incident atrial fibrillation. For predialysis diastolic BP, compared with a fixed referent diastolic BP of 80 mm Hg, atrial fibrillation rates were higher at lower levels of predialysis diastolic BP. We also presented analyses that considered the perspective of a physician seeing a patient on hemodialysis with a certain predialysis BP illustrating the associated risks of having a lower systolic BP or diastolic BP. Our analyses, although observational and therefore unable to prove causality, raise the possibility that BP lowering would reduce incident atrial fibrillation risk in patients at the upper extreme of systolic BP (approximately 180–190 mm Hg).
Our findings are consistent with two larger studies of patients on dialysis in the United States. One study examined 3621 United States patients on hemodialysis and peritoneal dialysis in 1996 and followed until 2000 (15). In that study, the lowest and highest quartiles of predialysis systolic BP, calculated from a mean of three readings, were associated with higher rates of hospitalized atrial fibrillation. The second study used data from 63,884 patients identified in the USRDS who were enrolled in a Medicaid prescription drug benefit, and found that the presence (versus absence) of coded hypertension was inversely associated with prevalent atrial fibrillation (adjusted prevalence odds ratio, 0.79); however, no actual BP measurements were available in that study (27). Similarly, a study of 1010 patients with CKD not yet on dialysis found that each 1 mm Hg higher systolic BP was associated with a 2% (95% CI, 1% to 3%) lower adjusted odds of prevalent atrial fibrillation (28).
In the non-ESKD population, orthostatic hypotension is related to incident atrial fibrillation, particularly among persons over 70 years of age (adjusted HR, 1.98; 95% CI, 1.37 to 2.86) (29). We found only a modest, marginally significant association of intradialytic hypotension with atrial fibrillation. But given that intradialytic hypotension has been associated with higher risks of death and other cardiovascular disease events such as myocardial infarction, heart failure, or stroke (18,30), avoidance of intradialytic hypotension remains clinically important.
Our findings that lower systolic BP and diastolic BP are associated with atrial fibrillation in patients with ESKD receiving hemodialysis contrast with findings regarding the association of BP and atrial fibrillation in the general population. For example, in a study of 4.3 million adults in the United Kingdom (11), each 20 mm Hg higher systolic BP was associated with a higher risk of incident atrial fibrillation (HR, 1.21; 95% CI, 1.19 to 1.22), as was each 10 mm Hg higher diastolic BP (HR, 1.21; 95% CI, 1.19 to 1.23). This generally linear association of systolic BP and diastolic BP was seen for persons aged 30–60 years, but was much flatter for patients aged >60 years. Similar findings relating a positive association among BP parameters and lifetime risk of atrial fibrillation in the general population was reported in a longitudinal study using data from the Framingham Heart Study (31).
One explanation for the discrepant findings between studies in patients with ESKD versus those without ESKD may be that patients presenting with lower predialysis BP may have difficulty with adequate ultrafiltration and achievement of postdialysis extracellular euvolemia. In turn, volume overload can lead to larger atrial size and increased pulmonary artery pressures, factors associated with atrial fibrillation in patients with ESKD (5,14). We also did not have information related to cardiac structure and function in this analysis. Moreover, there are certain risk factors for atrial fibrillation that are specific to the ESKD patient population, most notably the shifts in serum potassium and calcium concentrations that often occur during the dialysis procedure (2–4).
Another explanation for our findings may be that lower systolic BP and diastolic BP are markers of other confounding disease processes not captured in our analysis that increase atrial fibrillation risk, such as arterial stiffness or reduced ejection fraction, or poorer overall health in general. Indeed, recent secondary analyses of clinical trials in which participants were randomized to two different BP targets found that, regardless of the randomized BP treatment target assignment, lower systolic BP and diastolic BP were associated with higher risks of cardiovascular events and death (32,33). However, the beneficial association of more intensive BP lowering persisted across a range of systolic and diastolic BPs. These findings underscore the importance of randomized trials to establish the optimal BP target specifically in patients with ESKD to reduce risks of cardiovascular events, including atrial fibrillation.
Our analysis has several strengths, including the large, representative cohort of older United States patients initiating hemodialysis, adjustment for a large number of patient characteristics, and use of multiple imputation for missing data. However, there are also limitations to note in addition to the ones discussed above. First, we identified incident atrial fibrillation using billing claims rather than clinical information, although studies have demonstrated the relative validity of this approach (34,35). Second, we relied on routinely measured in-center BP measurements and did not have access to any out-of-office BP measurements, which have stronger associations with clinical outcomes (36). Finally, our findings may not generalize to younger populations, prevalent hemodialysis cohorts, patients receiving kidney replacement through other modalities, and to health care settings outside of the United States.
In conclusion, in a large, nationally representative cohort of older patients initiating hemodialysis in the United States, we show that lower predialysis systolic BP and diastolic BP were associated with higher hazards of atrial fibrillation. A 10 mm Hg lower systolic BP was associated with lower atrial fibrillation hazard only at the highest extremes of systolic BP (approximately 180–190 mm Hg). Our results provide yet more evidence that BP target trials specific to patients receiving hemodialysis are needed, given the very different associations seen compared with the general population.
Disclosures
Dr. Chang has received speaking honoraria from the American Society of Nephrology and multiple universities, consulting fees from Novo Nordisk, Janssen Research and Development LLC, and Fresenius Medical Care Renal Therapies Group LLC, and research funding from Satellite Health Care. Dr. Niu reports serving as a consultant to the University of California Davis. Dr. Turakhia reports serving as a consultant to Precision Health Economics, Medtronic, and St. Jude Medical. In the past 2 years, Dr. Flythe has received speaking honoraria from American Renal Associates, American Society of Nephrology, Dialysis Clinic, Incorporated, National Kidney Foundation, and multiple universities as well as research funding for studies unrelated to this project from the Renal Research Institute, a subsidiary of Fresenius Kidney Care North America. Dr. Flythe is on the medical advisory board to NxStage Medical and has received consulting fees from Fresenius Kidney Care North America and AstraZeneca. Dr. Winkelmayer reports having served as a scientific advisor to Akebia, Amgen, AstraZeneca, Bayer, Daichii Sankyo, Relypsa, Vifor Fresenius Medical Care Renal Pharma, and ZS Pharma; on clinical trial committees for Akebia and the Duke Clinical Research Institute; and speaking honoraria from Fibrogen. Dr. Liu, Dr. Airy, and Dr. Montez-Rath have nothing to disclose.
Supplementary Material
Acknowledgments
Dr. Chang is supported by grant R03DK113341 from the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK; Bethesda, MA). Dr. Flythe is supported by grant K23 DK109401 from the NIDDK. Dr. Winkelmayer is supported by grant R01DK095024 and from the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine.
The manuscript was reviewed and approved for publication by an officer of the NIDDK. Data reported herein were supplied by the US Renal Data System. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13511118/-/DCSupplemental.
Supplemental Appendix 1. Full methods and references.
Supplemental Table 1. Baseline characteristics of incident patients aged ≥65 years registered in the US Renal Data System by baseline predialysis diastolic BP.
Supplemental Table 2. Baseline characteristics of incident patients aged ≥65 years registered in the US Renal Data System by baseline frequency of intradialytic hypotension (defined as nadir systolic BP <90 mm Hg).
Supplemental Table 3. Association of predialysis systolic BP, diastolic BP, and intradialytic hypotension as categorical variables with incident atrial fibrillation.
References
- 1.Goldstein BA, Arce CM, Hlatky MA, Turakhia M, Setoguchi S, Winkelmayer WC: Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation 126: 2293–2301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buiten MS, de Bie MK, Rotmans JI, Gabreëls BA, van Dorp W, Wolterbeek R, Trines SA, Schalij MJ, Jukema JW, Rabelink TJ, van Erven L: The dialysis procedure as a trigger for atrial fibrillation: New insights in the development of atrial fibrillation in dialysis patients. Heart 100: 685–690, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Sacher F, Jesel L, Borni-Duval C, De Precigout V, Lavainne F, Bourdenx JP, Haddj-Elmrabet A, Seigneuric B, Keller A, Ott J, Savel H, Delmas Y, Bazin-Kara D, Klotz N, Ploux S, Buffler S, Ritter P, Rondeau V, Bordachar P, Martin C, Deplagne A, Reuter S, Haissaguerre M, Gourraud JB, Vigneau C, Mabo P, Maury P, Hannedouche T, Benard A, Combe C: Cardiac rhythm disturbances in hemodialysis patients: Early detection using an implantable loop recorder and correlation with biological and dialysis parameters. JACC Clin Electrophysiol 4: 397–408, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Vincenti A, Passini E, Fabbrini P, Luise MC, Severi S, Genovesi S: Recurrent intradialytic paroxysmal atrial fibrillation: Hypotheses on onset mechanisms based on clinical data and computational analysis. Europace 16: 396–404, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Genovesi S, Fabbrini P, Pieruzzi F, Galbiati E, Sironi E, Pogliani D, Bonforte G, Viganò MR, Stella A: Atrial fibrillation in end stage renal disease patients: Influence of hemodialysis on P wave duration and atrial dimension. J Nephrol 28: 615–621, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Freeman JV, Wang Y, Akar J, Desai N, Krumholz H: National trends in atrial fibrillation hospitalization, readmission, and mortality for Medicare beneficiaries, 1999-2013. Circulation 135: 1227–1239, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Airy M, Schold JD, Jolly SE, Arrigain S, Bansal N, Winkelmayer WC, Nally JV Jr., Navaneethan SD: Cause-specific mortality in patients with chronic kidney disease and atrial fibrillation. Am J Nephrol 48: 36–45, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee NA, Chae CU, Kim E, Moorthy MV, Conen D, Sandhu RK, Cook NR, Lee IM, Albert CM: Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail 5: 552–560, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, Piccini JP, Fonarow GC; ORBIT-AF Investigators and Patients : Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail 5: 44–52, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Lau DH, Nattel S, Kalman JM, Sanders P: Modifiable risk factors and atrial fibrillation. Circulation 136: 583–596, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Emdin CA, Anderson SG, Salimi-Khorshidi G, Woodward M, MacMahon S, Dwyer T, Rahimi K: Usual blood pressure, atrial fibrillation and vascular risk: Evidence from 4.3 million adults. Int J Epidemiol 46: 162–172, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang TI, Friedman GD, Cheung AK, Greene T, Desai M, Chertow GM: Systolic blood pressure and mortality in prevalent haemodialysis patients in the HEMO study. J Hum Hypertens 25: 98–105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Lacson E Jr., Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW: The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 48: 606–615, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Acar G, Akçay A, Doğan E, Işik IO, Sökmen A, Sökmen G, Sayarlioğlu H, Köroğlu S, Nacar AB, Tuncer C: The prevalence and predictors of atrial fibrillation in hemodialysis patients. Turk Kardiyol Dern Ars 38: 8–13, 2010 [PubMed] [Google Scholar]
- 15.Abbott KC, Trespalacios FC, Taylor AJ, Agodoa LY: Atrial fibrillation in chronic dialysis patients in the United States: Risk factors for hospitalization and mortality. BMC Nephrol 4: 1, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung J-T, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine C-E, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V: US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 73: S1–S772, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen JI, Montez-Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC: Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis 66: 677–688, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM: Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 26: 724–734, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Stedman M: A SAS macro for covariate specification in linear, logistic, and survival regression. Proceedings of the SAS Global 2017 Conference, Orlando, FL, 2017, Paper 1223–2017 [Google Scholar]
- 20.Krieger N, Waterman P, Chen JT, Soobader M-J, Subramanian SV, Carson R: Zip code caveat: Bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas--the public health disparities geocoding project. Am J Public Health 92: 1100–1102, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee JJ, Zheng Y, Montez-Rath ME, Chang TI, Winkelmayer WC: Associations of glycemic control with cardiovascular outcomes among US hemodialysis patients with diabetes mellitus. J Am Heart Assoc 6: e005581, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Buuren S, Boshuizen HC, Knook DL: Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 18: 681–694, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Van Buuren S, Brand JP, Groothuis-Oudshoorn C, Rubin DB: Fully conditional specification in multivariate imputation. J Stat Comput Simul 76: 1049–1064, 2006 [Google Scholar]
- 24.White IR, Royston P: Imputing missing covariate values for the Cox model. Stat Med 28: 1982–1998, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montez-Rath ME, Winkelmayer WC, Desai M: Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol 9: 1328–1335, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little RJ, Rubin DB: Statistical Analysis with Missing Data, New York, John Wiley & Sons, Inc., 2002 [Google Scholar]
- 27.Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, Hou Q, Shireman TI: The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 81: 469–476, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, Lerma EV: Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 173–181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko D, Preis SR, Lubitz SA, McManus DD, Vasan RS, Hamburg NM, Benjamin EJ, Mitchell GF: Relation of orthostatic hypotension with new-onset atrial fibrillation (from the Framingham Heart Study). Am J Cardiol 121: 596–601, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefánsson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, Stålhammar NO: Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 9: 2124–2132, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, McManus DD, Ko D, Weng LC, Lunetta KL, Frost L, Benjamin EJ, Trinquart L: Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: Cohort study based on longitudinal data from the Framingham Heart Study. BMJ 361: k1453, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalkman DN, Brouwer TF, Vehmeijer JT, Berger WR, Knops RE, de Winter RJ, Peters RJ, van den Born BH: J curve in patients randomly assigned to different systolic blood pressure targets: An experimental approach to an observational paradigm. Circulation 136: 2220–2229, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, Wei G, Campbell RC, Conroy M, Freedman BI, Haley W, Horwitz E, Kitzman D, Lash J, Papademetriou V, Pisoni R, Riessen E, Rosendorff C, Watnick SG, Whittle J, Whelton PK; SPRINT Research Group : Influence of baseline diastolic blood pressure on effects of intensive compared with standard blood pressure control. Circulation 137: 134–143, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S: A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 21[Suppl 1]: 141–147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR: Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 158: 111–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR: Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol 25: 1630–1646, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.