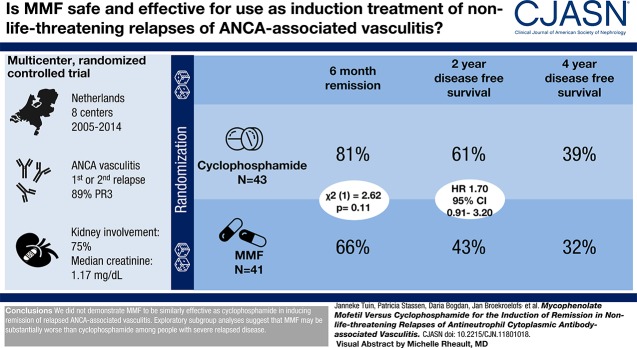
Visual Abstract
Keywords: ANCA, vasculitis, mycophenolate mofetil, cyclophosphamide, randomized controlled trials, relapse, Mycophenolic Acid, Antibodies, Antineutrophil Cytoplasmic, Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis, Recurrence, Remission Induction
Abstract
Background and objectives
Cyclophosphamide has been the mainstay of treatment of ANCA-associated vasculitis. However, cyclophosphamide has unfavorable side effects and alternatives are needed. Evidence suggests that mycophenolate mofetil can induce sustained remission in nonlife-threatening disease. The purpose of this study was to compare the efficacy and safety of mycophenolate mofetil versus cyclophosphamide for the induction treatment of nonlife-threatening relapses of proteinase 3-ANCA– and myeloperoxidase-ANCA–associated vasculitis.
Design, setting, participants, & measurements
We conducted a multicenter randomized, controlled trial. Participants with a first or second relapse of ANCA-associated vasculitis were randomized to induction treatment with cyclophosphamide or mycophenolate mofetil both in combination with glucocorticoids. Maintenance therapy consisted of azathioprine in both arms. Primary outcome was remission at 6 months, and secondary outcomes included disease-free survival at 2 and 4 years.
Results
Eighty-four participants were enrolled, of whom 41 received mycophenolate mofetil and 43 received cyclophosphamide. Eighty-nine percent of participants were proteinase 3-ANCA positive. At 6 months, 27 (66%) mycophenolate mofetil–treated participants versus 35 (81%) cyclophosphamide-treated participants were in remission (P=0.11). Disease-free survival rates at 2 and 4 years were 61% and 39% for cyclophosphamide, respectively, and 43% and 32% for mycophenolate mofetil, respectively (at 4 years, log rank test, P=0.17).
Conclusions
We did not demonstrate mycophenolate mofetil to be similarly effective as cyclophosphamide in inducing remission of relapsed ANCA-associated vasculitis. However, mycophenolate mofetil might be an alternative to cyclophosphamide for the treatment of selected patients with nonlife-threatening relapses.
Introduction
ANCA-associated vasculitides are a group of autoimmune diseases that are associated with the presence of circulating ANCA. Most patients with ANCA-associated vasculitis require significant immunosuppression during remission induction and long-term maintenance treatment. The introduction of the alkylating agent cyclophosphamide in the 1960s improved survival substantially but at the expense of serious acute and long-term side effects (1). Recently, rituximab has been proven equally effective as cyclophosphamide for induction of remission. However, in nonsevere relapsing disease, limited evidence is available regarding the effectiveness of other potentially less toxic immunosuppressive agents.
The literature suggests that mycophenolate mofetil (MMF) can safely induce sustained remission in ANCA-associated vasculitis (2–6). This prompted us to test the efficacy and safety of MMF compared with cyclophosphamide for the induction of remission in nonlife-threatening relapses, including for both proteinase 3 (PR3)-ANCA– and myeloperoxidase (MPO)-ANCA–associated vasculitis. We studied whether cyclophosphamide is superior to MMF for the induction of remission in participants with relapsing nonlife-threatening ANCA-associated vasculitis.
Materials and Methods
Study Design and Patients
This multicenter, open label, randomized trial was conducted between 2005 and 2014 at eight centers in The Netherlands. The study protocol was reviewed and approved by the local institutional review board of every participating center. The study was conducted according to the principles from the Declaration of Helsinki, and it was registered on www.clinicaltrials.gov with identifier NCT00103792.
Participants were classified as GPA or microscopic polyangiitis (MPA) according to criteria adopted from the Chapel Hill Consensus Conference Nomenclature (7). Consecutive patients with a first or second nonlife-threatening relapse of PR3- or MPO-ANCA–associated vasculitis were eligible. All patients had to provide written informed consent to participate. Patients were not eligible for study inclusion if any of the following were present: life-threatening disease defined as serious alveolar bleeding or (threatening) respiratory insufficiency, serum creatinine >5.66 mg/dl, or new dialysis dependence; ongoing high-dose maintenance therapy at the time of the relapse, more specifically cyclophosphamide ≥100 mg/d and prednisolone ≥25 mg/d; known intolerance to cyclophosphamide, MMF, or azathioprine; pregnancy; desire to have children or inadequate contraception; inability to provide informed consent; or age <18 years old.
Enrollment and Randomization
Participants were recruited from the outpatient clinics from the participating centers. Participants were enrolled by the treating physician. Patients were randomized to receive open label oral cyclophosphamide or MMF in a 1:1 ratio as induction treatment. Treatment assignment was not concealed to participants, treating physicians, or those analyzing the data. Randomization took place centrally at the University Medical Center Groningen and was performed with the use of permuted blocks of four or six. Participants were stratified according to ANCA specificity (PR3 or MPO) and whether this was a first or second relapse.
Treatment Protocol
Participants randomized to cyclophosphamide received induction treatment with oral cyclophosphamide 2 mg/kg per day (age >65 years old: 1.5 mg/kg per day) for 6 months. Participants randomized to MMF received induction treatment with MMF 1000 mg twice daily for 6 months. Both treatment groups received prednisolone in a tapering regime during induction treatment. All participants received cotrimoxazole in a dosage of 960 mg every other day for 6 months. All participants were switched to maintenance therapy with azathioprine 1.5 mg/kg per day after 6 months provided that the patient was in stable remission for at least 3 months. When remission was not attained within 6 months, therapy was considered to have failed. Azathioprine maintenance therapy was tapered after 12 months and completely withdrawn 2 years after study entry. Supplemental Material details the extended treatment protocol.
Outcomes and Definitions
Primary end point was remission at 6 months. Secondary end points were disease-free survival at 2 and 4 years, time to induction of remission, cumulative organ damage, ANCA status, and adverse events.
Remission was defined as the absence of manifestations attributable to active disease (Birmingham Vasculitis Activity Score [BVAS] =0) and C-reactive protein <10 mg/dl. Induction therapy was considered to have failed when remission was not attained within 6 months or when remission was not sustained throughout the first 6 months. A relapse was defined as the reoccurrence or new appearance of organ involvement attributable to active vasculitis and requiring increase in or reintroduction of immunosuppression. A major relapse was defined as the reoccurrence or new onset of potentially organ- or life-threatening disease activity that cannot be treated with an increase of glucocorticoids alone and requires additional escalation of therapy (i.e., the administration of cyclophosphamide). All other relapses were classified as “minor.”
A leukocyte count <4.0×109/L was defined as leukocytopenia, and a lymphocyte count <0.8×109/L was defined as lymphocytopenia. Infection was defined as disease requiring antibacterial or antiviral treatment with or without the need for hospital admission (i.e., the term does not apply to a presumably viral respiratory tract infection that did not require medical treatment).
Disease Assessments
Patient evaluation was conducted at the start of treatment, once every 4 weeks during the first 6 months, and then, once every 3 months for up to 4 years. Evaluation included a full blood count, C-reactive protein, estimated sedimentation rate, and ANCA titers. In addition, 24-hour urine analysis was performed to monitor creatinine clearance and proteinuria. Spot urine was collected for analysis of hematuria. Chest x-rays or computed tomography scans were obtained when clinically indicated. Disease activity was assessed using the BVAS (8). In addition, the Vasculitis Damage Index was used to quantify the cumulative organ damage (9). Adverse events were graded according to the National Cancer Institute’s NCI Common Terminology Criteria for Adverse Events version 4.0.
Statistical Analyses
SPSS version 23.0 (SPSS IBM, New York, NY) was used for data analysis. Data are shown as mean ± SD for normally distributed data and median and interquartile range (IQR) for nonnormally distributed data. The primary analysis was tested on the basis of an intention-to-treat method. Participants who dropped out of the study for any reason before 6 months were considered to have treatment failure. We performed an additional imputation analysis with six additional participants, assuming remission at 6 months in two additional participants on cyclophosphamide and treatment failure in additional participants on MMF.
Disease-free survival was estimated by Kaplan–Meier actuarial survival curves with log rank testing to compare groups.
As part of the exploratory analysis, treatment efficacy with consideration of disease severity was investigated by calculating tertiles of disease severity within the treatment groups. Data were presented in a bar chart figure. Time to treatment failure was estimated by Kaplan–Meier actuarial survival curves with log rank testing to compare groups.
Statistical significance was defined as a two-sided P<0.05.
On the basis of our previous study, we expected successful induction of remission in 90% of participants treated with cyclophosphamide. We considered cyclophosphamide to be superior to MMF when the relative difference in remission rate was >25%. Randomization of 90 participants was required to achieve a power of 0.8 with an α of 0.05.
Results
Participants
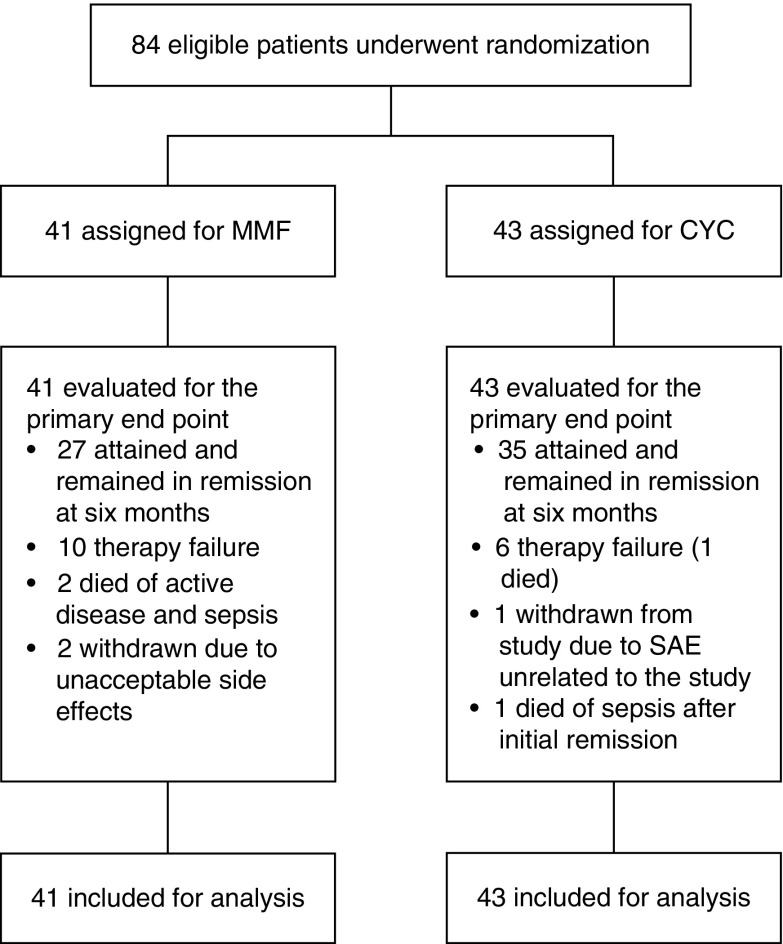
Eighty-four participants with relapsing PR3-ANCA– and MPO-ANCA–associated vasculitis were enrolled between 2005 and 2014. A list of patient inclusions by participating center is shown in Supplemental Table 1. A flowchart of all randomized and analyzed participants is shown in Figure 1. The target inclusion was 90 participants on the basis of the power calculation. Enrollment proceeded slower than anticipated, and it was decided by J.T., J.-S.S., and C.A.S. to discontinue the study prematurely in August 2014. This decision was made before performing the analysis. At that time, 84 participants were enrolled. Forty-one participants were randomized to MMF, and 43 participants received cyclophosphamide. Demographic and clinical characteristics were similar in participants randomized to cyclophosphamide and MMF at the time of randomization (Table 1). The majority of included participants were PR3-ANCA positive (89%). Sixty-two participants had experienced their first relapse (74%) at time of inclusion. The median BVAS at study entry was 15 (IQR, 13–19). Sixty-three participants had kidney involvement (75%), and the median creatinine level was 1.17 mg/dl (IQR, 0.96–1.73).
Figure 1.
Flowchart of randomized and analyzed participants. CYC, cyclophosphamide; MMF, mycophenolate mofetil; SAE, serious adverse event.
Table 1.
Baseline characteristics of all included participants with a first or second relapse of ANCA-associated vasculitis enrolled in the clinical trial of mycophenolate mofetil versus cyclophosphamide
| Patient Characteristics | All Participants, n=84 | Cyclophosphamide, n=43 | Mycophenolate Mofetil, n=41 |
|---|---|---|---|
| Age, yr, mean (SD) | 60 (±12) | 60 (±11) | 60 (±13) |
| Sex (men) | 57 (68%) | 30 (70%) | 27 (66%) |
| ANCA specificity (PR3) | 75 (89%) | 38 (88%) | 37 (90%) |
| First relapse | 62 (74%) | 31 (72%) | 31 (76%) |
| Organ involvement at study entry | |||
| ENT | 40 (48%) | 19 (44%) | 21 (51%) |
| Pulmonary | 42 (50%) | 24 (56%) | 18 (44%) |
| Kidney | 63 (75%) | 32 (74%) | 31 (76%) |
| Relapse biopsy proven | 29 (35%) | 15 (35%) | 14 (34%) |
| BVAS, median [IQR] | 15 [13–19] | 16 [13–20] | 15 [13–19] |
| CRP, mg/L, median [IQR] | 36 [12–85] | 40 [13–125] | 33 [10–57] |
| Creatinine, mg/dl, median [IQR] | 1.2 [1–1.7] | 1.1 [0.9–1.8] | 1.3 [1–1.7] |
| Creatinine >1.47 mg/dl, n | 30/83 (36%) | 16/42 (38%) | 14 (34%) |
| Vasculitis damage index | 1 [1–2] | 1 [1–3] | 1 [1–2] |
Except where indicated otherwise, values are the number (%). PR3, proteinase 3; ENT, ear, nose, and throat; BVAS, Birmingham Vasculitis Activity Score; IQR, interquartile range; CRP, C-reactive protein.
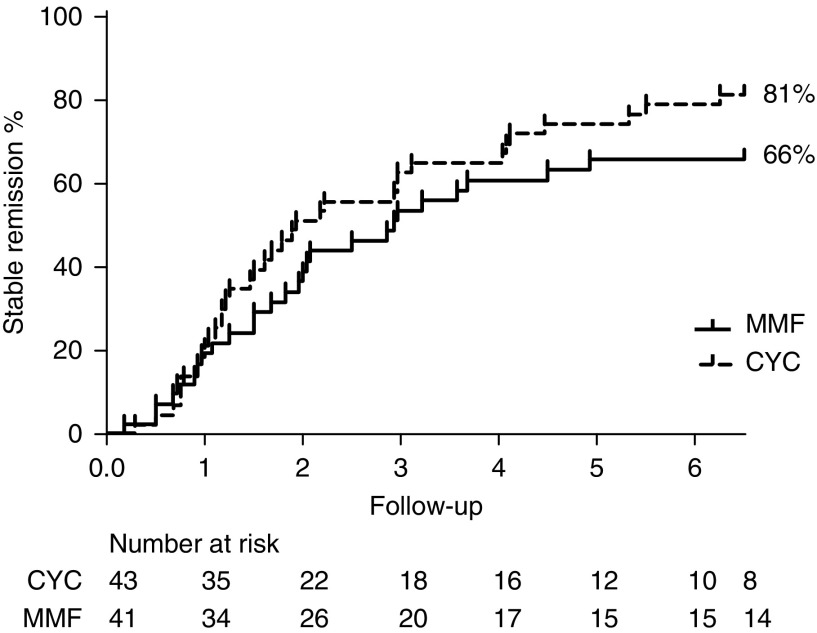
Primary Outcome: Remission Induction
Twenty-seven of 41 participants (66%) treated with MMF compared with 35 of 43 participants (81%) treated with cyclophosphamide were in stable remission at 6 months (chi square [1] =2.62, P=0.11). The relative risk for therapy failure with MMF therapy was 1.8 (95% confidence interval [95% CI], 0.9 to 3.9). Eight participants treated with cyclophosphamide were not in remission at 6 months due to progression of disease that required escalation of therapy (n=6) and resulted in death in one patient, occurrence of an adverse event unrelated to the study for which the patient was withdrawn from the study (n=1), and death due to sepsis after achieving stable remission (n=1). Therapy failed to induce stable remission within 6 months in 14 participants treated with MMF. In ten participants, disease progressed. Two participants died due to exacerbated disease combined with sepsis, and two participants discontinued therapy due to unacceptable side effects (Figure 1). All failures related to disease progression in the cyclophosphamide-treated group occurred within 5 weeks. Failure related to disease progression in the MMF group was observed in eight patients within 5 weeks, one patient after 7 weeks, and one patient after 12 weeks, and two participants showed improvement initially but had renewed disease activity after 16 weeks of therapy.
We ran an imputation analysis with six participants; when three additional cyclophosphamide-treated participants would have reached remission and three more participants on MMF would have failed, cyclophosphamide would have been superior to MMF.
Secondary Outcomes
Disease-Free Survival.
Disease-free survival rates at 2 and 4 years were 61% (n=35) and 39% (n=7), respectively, for cyclophosphamide and 43% (n=17) and 32% (n=6), respectively, for MMF (2 years log rank test, P=0.10; hazard ratio [HR], 1.70; 95% CI, 0.91 to 3.20; 4 years log rank test, P=0.17; HR, 1.49; 95% CI, 0.85 to 2.62).
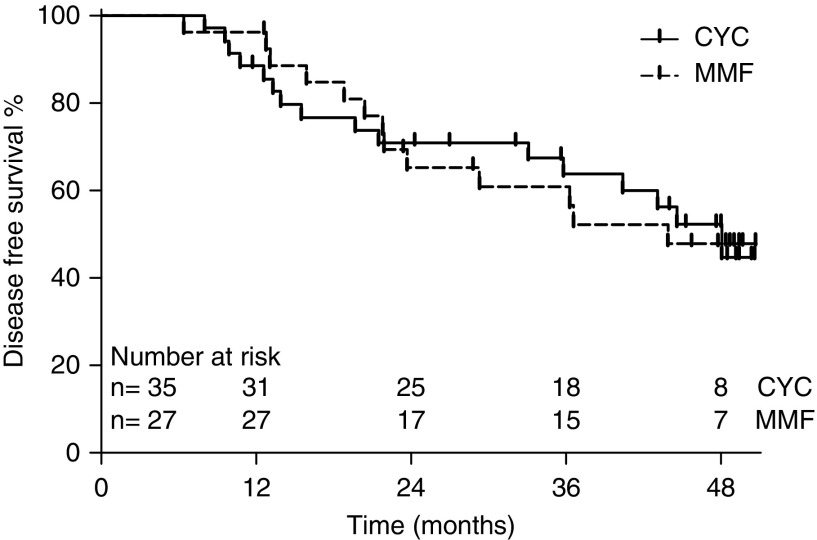
Thirteen of 27 (48%) MMF-treated participants in remission at 6 months compared with 16 of 35 (46%) cyclophosphamide-treated participants in remission at 6 months developed a relapse during follow-up (log rank test, P=0.95; HR, 1.03; 95% CI, 0.49 to 2.14) (Figure 2). The median C-reactive protein at time of relapse was 32 mg/L (IQR, 18–74) for cyclophosphamide-treated participants and 27 mg/L (IQR, 11–100) for MMF-treated participants (Mann–Whitney U, P=0.98). The median BVAS at time of relapse was 11 (IQR, 5–16) for cyclophosphamide-treated participants and 12 (IQR, 8–12) for MMF-treated participants (Mann–Whitney U, P=0.91). Ten relapses were considered minor: six relapses in cyclophosphamide-treated participants and four relapses in MMF-treated participants. Nineteen relapses were considered major: ten relapses in cyclophosphamide-treated participants and nine relapses in MMF-treated participants.
Figure 2.
Secondary outcome: disease-free survival among patients who attained remission at 6 months did not differ between CYC and MMF treated patients. CYC, cyclophosphamide; MMF, mycophenolate mofetil.
Time to Stable Remission.
Time to stable remission did not differ significantly between MMF- and cyclophosphamide-treated participants; median time to remission was 7.3 weeks (IQR, 3.7–11.7) versus 6.4 weeks (IQR, 4.0–11.9; P=0.85), respectively (Figure 3). An overview of primary and secondary outcomes is shown in Table 2.
Figure 3.
Time to stable remission according to treatment allocation did not differ between cyclophosphamide and MMF treated patients. CYC, cyclophosphamide; MMF, mycophenolate mofetil.
Table 2.
Primary and secondary outcomes
| Outcome | MMF, n=41 | Cyclophosphamide, n=43 | P Value |
|---|---|---|---|
| Preliminary Outcome | |||
| Remission at 6 mo, n (%) | 27 (66%) | 35 (81%) | 0.11 |
| Secondary Outcome | |||
| Disease-free survival at 2 yr, n (%) | 17 (43%) | 25 (61%) | 0.10 |
| Disease-free survival at 4 yr, n (%) | 6 (32%) | 7 (39%) | 0.17 |
| Time to remission, wk, median (IQR) | 7.3 [IQR, 3.7–11.7] | 6.4 [IQR, 4.0–11.9] | 0.85 |
MMF, mycophenolate mofetil; IQR, interquartile range.
Organ Damage.
At study entry, the majority of relapsing participants already had damage. The Vasculitis Damage Index rates at study entry of MMF- and cyclophosphamide-treated participants were 1.6 (SD=1.2) and 1.8 (SD=1.6), respectively. The mean Vasculitis Damage Index at 6 months for cyclophosphamide-treated participants was 2.2 (SD=1.7), and for MMF-treated participants, it was 2.1 (SD=2.0; P=0.72). At 12 months, the mean Vasculitis Damage Index for cyclophosphamide-treated participants was 2.3 (SD=1.8), and for MMF-treated participants, it was 2.3 (2.1; P=0.94).
Adverse Events.
A total of 102 adverse events occurred during this study, excluding leukopenia, thrombocytopenia, and anemia (Table 3). Four participants died during remission induction as described above. Two participants were withdrawn from the study: one MMF-treated patient because of a lung carcinoma and one cyclophosphamide-treated patient because of a pancreas carcinoma; both participants subsequently died. Three malignancies in each treatment group developed (Supplemental Table 2).
Table 3.
Adverse events
| Adverse Events | Total, n=84 | MMF, n=41 | Cyclophosphamide, n=43 |
|---|---|---|---|
| AEs, n (%) | 102 | 47 | 55 |
| AE grade 1 or 2 | 75 (74%) | 34 (72%) | 41 (75%) |
| AE grade 3 | 19 (19%) | 8 (17%) | 11 (20%) |
| AE grade 4 | 2 (2%) | 2 (4%) | 0 (0%) |
| AE grade 5 | 6 (6%) | 3 (6%) | 3 (5%) |
| Infections, n (%) | 46 | 19 | 27 |
| Episodes of infections on induction treatment | 19 (41%) | 8 (42%) | 11 (41%) |
| ≥1 infection during induction, participants | 13 | 7/34 (21%) | 6/40 (15%) |
| Grade ≥3 | 5 (11%) | 1 (5%) | 4 (15%) |
| Treatment-related AE, n participants (%) | |||
| Chemical cystitis | 1 (1%) | 0 | 1 (2%) |
| Gonadal failure | 1 (1%) | 0 | 1 (2%) |
| Diabetes mellitus | 9 (11%) | 1 (2%) | 8 (19%) |
| Malignancies | 6 | 3 | 3 |
| Intolerance MMF, n (%) | 3 (4%) | 3 (7%) | N.A. |
| Intolerance AZA, n (%) | 4 (5%) | 3 (7%) | 1 (2%) |
| Intolerance cyclophosphamide, n (%) | 0 | N.A. | 0 |
| Leukopenia, n (%) | |||
| Episodes of leukopenia on induction treatment | 15 | 3 | 12 |
| ≥1 episode of leukopenia during induction, participants | 11 | 3/35 (9%) | 8/40 (20%) |
| Episodes of leukopenia on maintenance treatment | 33 | 15 | 18 |
| ≥1 episode of leukopenia during maintenance, participants | 19 (23%) | 7 (17%) | 12 (28%) |
| Anemia, maximum AE grade, n participants (%) | |||
| Grade 1 or 2 | 73 (87%) | 35 (85%) | 38 (88%) |
| Grade 3 | 2 (2%) | 1 (2%) | 1 (2%) |
| Trombocytopenia, n participants (%) | |||
| Grade 1 | 8 (10%) | 3 (7%) | 5 (12%) |
| Grade 4a | 1 (1%) | 0 | 1 (2%) |
MMF, mycophenolate mofetil; AE, adverse event; N.A., not applicable; AZA, azathioprine.
As part of a severe pancytopenia.
Seven of 34 (21%) MMF-treated participants versus six of 40 (15%) cyclophosphamide-treated participants experienced at least one infection during induction treatment (chi square [1] =0.40, P=0.53). The relative risk for infection during induction with cyclophosphamide was 0.7 (95% CI, 0.3 to 2.0). One patient on MMF experienced a severe generalized varicella zoster infection (adverse event grade 4) during an episode of severe pancytopenia. One patient in the cyclophosphamide group experienced a severe sepsis during an episode of leukopenia on cyclophosphamide induction and subsequently died (adverse event grade 5). Two participants experienced three grade 3 infections in the cyclophosphamide group during remission maintenance on azathioprine. One patient experienced both paronychia of a finger, which was complicated by osteomyelitis and subsequent amputation of part of the finger, and a recurrent infection of the hip after total hip replacement; one patient experienced an episode of cholangitis.
Three of 35 (9%) MMF-treated participants compared with eight of 40 (20%) cyclophosphamide-treated participants experienced at least one episode of leukopenia (neutrophil count <4.0×109/L) during remission induction (chi square [1] =1.95, P=0.16). The relative risk for neutropenia during induction with cyclophosphamide was 2.3 (95% CI, 0.7 to 8.1).
One of 25 (4%) MMF-treated participants compared with eight of 38 (21%) cyclophosphamide-treated participants developed diabetes mellitus during induction treatment (Fisher exact test, P=0.08). The relative risk for diabetes mellitus during induction with cyclophosphamide was 5.3 (95% CI, 0.7 to 39.5).
ANCA.
Nine of 24 (38%) cyclophosphamide-treated participants became ANCA negative after 6 months of treatment compared with 12 of 23 (52%) MMF-treated participants (chi square [1] =0.51, P=0.47).
Exploratory Outcomes
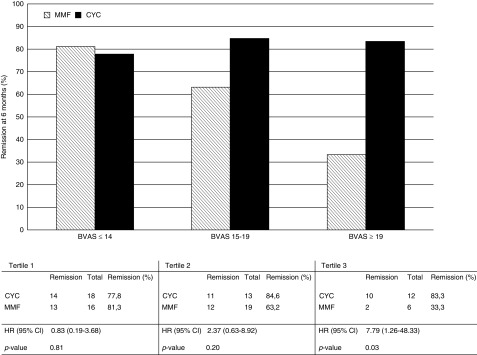
A difference in remission rate was observed between participants treated with cyclophosphamide and MMF when considering disease severity. Among participants with higher baseline disease severity, participants on MMF were less likely to attain remission at 6 months compared with participants on cyclophosphamide. The HR for failure of MMF therapy compared with cyclophosphamide in the highest tertile (BVAS>19) was 7.8 (95% CI, 1.3 to 48.3). The efficacy of cyclophosphamide seemed to be unrelated to disease severity at study entry (Figure 4).
Figure 4.
At 6 months remission rate in the highest BVAS tertile was higher in the cyclophosphamide than MMF arm. This was an exploratory subgroup analysis. Hazard ratio (HR) reflects time to treatment failure. Birmingham Vasculitis Activity Score (BVAS) tertile 1: BVAS<14; BVAS tertile 2: BVAS=14–19; and BVAS tertile 3: BVAS>19. 95% CI, 95% confidence interval; CYC, cyclophosphamide; MMF, mycophenolate mofetil.
eGFR
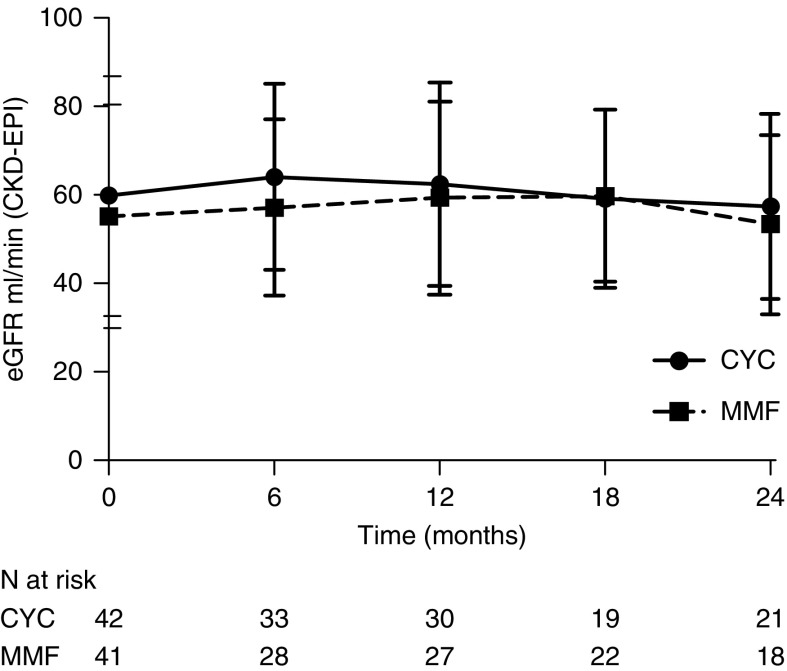
Thirty-two participants treated with cyclophosphamide (74%) compared with 31 participants treated with MMF (76%) had active kidney involvement at study entry. In this subgroup, the eGFR was similar for both treatment groups at baseline. eGFR increased to 3.8 ml/min per 1.73 m2 in the MMF-treated group versus 5.8 ml/min per 1.73 m2 in the cyclophosphamide-treated group from baseline to 6 months. eGFR during follow-up is shown in Figure 5.
Figure 5.
Course of eGFR of participants with kidney involvement during follow-up did not differ between CYC and MMF arms. The lines represent the mean eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation during follow-up. Whiskers represent one SD above and below the mean. CYC, cyclophosphamide; MMF, mycophenolate mofetil.
Protocol Violation
Not all participants received the maintenance therapy according to protocol. One patient randomized to cyclophosphamide induction therapy was azathioprine intolerant and switched back to cyclophosphamide in a tapering regimen. Three participants on MMF were azathioprine intolerant, and all three received MMF maintenance. One patient treated with cyclophosphamide induction therapy received methotrexate maintenance therapy after a prolonged period of leucopenia on cyclophosphamide. One patient treated with cyclophosphamide induction therapy was erroneously switched to MMF maintenance after induction of remission. Three participants were erroneously not switched from induction to maintenance therapy and continued on MMF. After exclusion of these participants, disease-free survival remained not significantly different (data not shown). Data on prednisolone use were incomplete. Thirteen of 21 (62%) MMF-treated participants used <10 mg of prednisolone per day at 6 months compared with 12 of 33 (36%) cyclophosphamide-treated participants.
Discussion
We investigated whether cyclophosphamide was superior to mycophenolate for remission induction in relapsing patients with ANCA vasculitis who experienced a nonlife-threatening disease manifestations. MMF induced sustained remission in a lower proportion of participants compared with cyclophosphamide, although this difference did not reach statistical significance. Among participants who attained remission, subsequent relapse rate was similar among participants who attained remission with MMF versus cyclophosphamide. No apparent differences between adverse events were observed.
In this study, 15% more participants attained remission in the cyclophosphamide arm than in the MMF arm. Nevertheless, for certain patient groups, MMF could be considered an alternative, because 66% of participants attained remission and experienced sustained remission for a similar time period compared with cyclophosphamide-treated participants.
MMF might, therefore, be an option in nonlife-threatening relapses of ANCA-associated vasculitis in patients previously treated with cyclophosphamide or patients with a contraindications for cyclophosphamide or other agents.
Many studies have focused on alternatives to cyclophosphamide with at least the same efficacy and fewer (severe) side effects over the last decades (10,11). With the exception of rituximab, many of these therapies have failed to show similar induction success as well as similar maintenance of remission (12).
The observation that MMF induced remission in a similar proportion of participants with lower disease activity suggests that MMF might be an option for patients with lower disease activity (i.e., BVAS<19 in our cohort). However, this was an exploratory outcome, and it was not the primary end point of this study. Therefore, these results should be interpreted with great caution and need additional analysis.
Our remission rate is a somewhat lower compared with that other studies. Several studies did not assess remission at 6 months, and in those studies, early relapses were not considered treatment failure (4). Remission rates reported in studies including only or primarily MPO-ANCA–positive patients yielded higher remission rates from 76% to 79% (3,5,6). The majority of our participants were PR3-ANCA positive, and this might have influenced the remission rate. PR3-ANCA– and MPO-ANCA–associated vasculitis are nowadays more and more regarded separate entities (13). Consequently, our results cannot universally be translated to MPO-ANCA–positive patients. In our study, only one half of the participants had lung or ears, nose, and throat involvement, and >75% had kidney involvement. In addition, this study only included relapsing patients, and this might represent patients with a more relapsing nature of their AAV. Furthermore, the available data showed that, in participants treated with MMF, glucocorticoids were tapered faster compared with in participants on cyclophosphamide, which could have negatively affected the overall outcome of treatment response in the MMF group.
The recently published MYCYC trial showed similar efficacy of MMF and CYC for induction of remission in patients with newly diagnosed active AAV. In this RCT, more relapses occurred in participants treated with MMF, particularly in participants with PR3-AAV. In contrast to the MYCYC trial, in this study, participants were included with relapsing AAV and had mostly been treated with CYC before; participants achieved remission earlier and were treated with MMF longer. All of these factors might have contributed to the observed differences in relapse rate between the studies (14).
Our study has severable notable strengths. It considered induction of remission as well as disease-free survival during an extensive follow-up period. In addition, this study was a randomized, controlled trial with a fairly large number of participants. However, the study had some important limitations as well. It was discontinued prematurely, it was designed as a superiority study, and the calculated power for analysis of the primary end point was not reached. Inclusion of more participants could have led to a statistically significant difference. The induction treatment with cyclophosphamide was approximately 6 months. Nowadays, patients are treated with cyclophosphamide for a shorter duration of 3–4 months, lowering cumulative cyclophosphamide exposure and possibly influencing relapse rate. Furthermore, the azathioprine maintenance dose was lower compared with that of some other studies; however, in this study, it was similar in both treatment arms.
We did not perform therapeutic drug monitoring of MMF, and the therapeutic doses are, therefore, not known. Previous studies have shown that patients with ANCA-associated vasculitis as well as healthy individuals exhibit a high interindividual variability with respect to the plasma concentrations of MPA, the active metabolite of MMF (15). It was demonstrated that MPA concentrations were associated with outcome in ANCA-associated vasculitis, and possibly, higher dosages of MMF might have resulted in better outcome (16). Additionally, therapeutic drug monitoring might be a valuable tool to improve therapy outcome.
In conclusion, we did not demonstrate MMF to be similarly effective as cyclophosphamide in inducing remission of relapsed ANCA-associated vasculitis. MMF compared with oral cyclophosphamide led to a statistically nonsignificant lower number of participants achieving remission at 6 months, and disease-free survival at 4 years of follow-up was not significantly different. MMF could be an alternative to oral cyclophosphamide for the treatment of selected patients with nonlife-threatening relapses of ANCA-associated vasculitis. Tailoring therapy in combination with therapeutic drug monitoring might further improve outcome.
Disclosures
Dr. Tuin, Dr. Stassen, Ms. Bogdan, Dr. Broekroelofs, Dr. van Paassen, Dr. Cohen Tervaert, Dr. Sanders, and Dr. Stegeman have nothing to disclose.
Supplementary Material
Acknowledgments
We thank Dr. P.L.A. van Daele, Dr. W.P. Haanstra, Dr. M.H. Hemmelder, Dr. K.W. Mui, Dr. R.R.H. Nap, and Dr. H.P.J. Willems for inclusion of participants in this trial. We thank Roche for providing study medication. Data of this trial can be shared at request. Our deidentified database will be available at request for fellow researchers from publication until June 2026 for 7 years. Data can only be shared within a secured data transfer system. We will not publish the database open access. The original study protocol is available for all who want to review it.
Roche was not involved in analyzing, writing, reviewing, or approving the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Treatment of Relapses in ANCA-Associated Vasculitis,” on pages 967–969.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11801018/-/DCSupplemental.
Supplemental Material. Treatment protocol
Supplemental Table 1. Inclusion sites.
Supplemental Table 2. Malignancies during follow-up.
References
- 1.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci AS: Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med 116: 488–498, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Joy MS, Hogan SL, Jennette JC, Falk RJ, Nachman PH: A pilot study using mycophenolate mofetil in relapsing or resistant ANCA small vessel vasculitis. Nephrol Dial Transplant 20: 2725–2732, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Hu W, Liu C, Xie H, Chen H, Liu Z, Li L: Mycophenolate mofetil versus cyclophosphamide for inducing remission of ANCA vasculitis with moderate renal involvement. Nephrol Dial Transplant 23: 1307–1312, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Stassen PM, Tervaert JW, Stegeman CA: Induction of remission in active anti-neutrophil cytoplasmic antibody-associated vasculitis with mycophenolate mofetil in patients who cannot be treated with cyclophosphamide. Ann Rheum Dis 66: 798–802, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han F, Liu G, Zhang X, Li X, He Q, He X, Li Q, Wang S, Wang H, Chen J: Effects of mycophenolate mofetil combined with corticosteroids for induction therapy of microscopic polyangiitis. Am J Nephrol 33: 185–192, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Silva F, Specks U, Kalra S, Hogan MC, Leung N, Sethi S, Fervenza FC: Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement–a prospective, open-label pilot trial. Clin J Am Soc Nephrol 5: 445–453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, Savage C, Adu D: Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 87: 671–678, 1994 [PubMed] [Google Scholar]
- 9.Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, Adu D: Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 40: 371–380, 1997 [DOI] [PubMed] [Google Scholar]
- 10.De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, Gross WL, Luqmani R, Jayne DR: Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 52: 2461–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 11.de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, Luqmani R, Pusey CD, Rasmussen N, Sinico RA, Tesar V, Vanhille P, Westman K, Savage CO; EUVAS (European Vasculitis Study Group) : Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann Intern Med 150: 670–680, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Fessler BJ, Ding L, Viviano L, Tchao NK, Phippard DJ, Asare AL, Lim N, Ikle D, Jepson B, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh K, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Mueller M, Sejismundo LP, Mieras K, Stone JH; RAVE-ITN Research Group : Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 369: 417–427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilhorst M, van Paassen P, Tervaert JW; Limburg Renal Registry : Proteinase 3-ANCA vasculitis versus myeloperoxidase-ANCA vasculitis. J Am Soc Nephrol 26: 2314–2327, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RB, Hiemstra TF, Ballarin J, Blockmans DE, Brogan P, Bruchfeld A, Cid MC, Dahlsveen K, de Zoysa J, Espigol-Frigolé G, Lanyon P, Peh CA, Tesar V, Vaglio A, Walsh M, Walsh D, Walters G, Harper L, Jayne D; European Vasculitis Study Group (EUVAS) : Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: A randomised, non-inferiority trial. Ann Rheum Dis 78: 399–405, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Chaigne B, Gatault P, Darrouzain F, Barbet C, Degenne D, François M, Szymanski P, Rabot N, Golea G, Diot E, Maillot F, Lebranchu Y, Nivet H, Paintaud G, Halimi JM, Guillevin L, Büchler M: Mycophenolate mofetil in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis: A prospective pharmacokinetics and clinical study. Clin Exp Immunol 176: 172–179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaier M, Scholl C, Scharpf D, Schmitt WH, Schwenger V, Zeier M, Sommerer C: High interpatient variability in response to mycophenolic acid maintenance therapy in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 30[Suppl 1]: i138–i145, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.