Abstract
Cyanobacteria harmful algal blooms are increasing in frequency and cyanotoxins have become an environmental and public concern in the U.S. and worldwide. In this Review, the majority of reported studies and developments of electrochemical affinity biosensors for cyanotoxins are critically reviewed and discussed. Essential background information about cyanobacterial toxins and electrochemical biosensors is combined with the rapidly moving development of electrochemical biosensors for these toxins. Current issues and future challenges for the development of useful electrochemical biosensors for cyanotoxin detection that meet the demands for applications in field freshwater samples are discussed. The major aspects of the entire review article in a prescribed sequence include (i) the state-of-the-art knowledge of the toxicity of cyanotoxins, (ii) important harmful algal bloom events, (iii) advisories, guidelines, and regulations, (iv) conventional analytical methods for determination of cyanotoxins, (v) electrochemical transduction, (vi) recognition receptors, (vii) reported electrochemical biosensors for cyanotoxins, (viii) summary of analytical performance, and (ix) recent advances and future trends. Discussion includes electrochemical techniques and devices, biomolecules with high affinity, numerous array designs, various detection approaches, and research strategies in tailoring the properties of the transducer–biomolecule interface. Scientific and engineering aspects are presented in depth. This review aims to serve as a valuable source to scientists and engineers entering the interdisciplinary field of electrochemical biosensors for detection of cyanotoxins in freshwaters.
Keywords: Electrochemical biosensors, immunosensors, aptasensors, detection methods, sensing, monitoring, toxicity, toxins, cyanotoxins, microcystins, cylindrospermopsin, anatoxin-a, saxitoxin, water
Graphical Abstract
Harmful algal blooms of cyanobacteria (Cyano-HABs) produce color, odor, and taste problems and generate highly toxic compounds, known as cyanotoxins. Cyano-HABs are increasing in frequency and cyanotoxins have become an environmental and public concern in the U.S. and worldwide. The most commonly found and studied group of cyanotoxins is cyclic heptapeptides, the microcystins. However, other cyanotoxins are of growing concern as well, due to their apparent increasing prevalence. From a toxicological and legislative point of view, the most relevant cyanotoxins are variants of microcystin, cylindrospermopsin, anatoxin-a, and saxitoxin. Conventional analytical methods for the determination of cyanotoxins are usually conducted in certified laboratories using advanced instrumentation. However, most of these techniques are cumbersome, expensive, time-consuming, and not suitable for point-of-use water monitoring. In addition, some of these methods lack sensitivity and specificity. There is a need for development of an advanced, small, and portable device that can overcome the drawbacks of current methods and can be used in situ and online or real-time. For the past decade, many approaches have emerged toward the development of new online/real-time biosensors with high affinity to cyanotoxins. Researchers worldwide have focused their efforts particularly on electrochemical biosensing development, which is the main subject of this Review.
Excellent reviews are accessible in the literature regarding the current state of knowledge and drinking water treatment options,(1–3) the toxicological impacts on aquatic ecosystems(4,5)and mammalian systems,(6,7) and drinking water management processes.(8) Furthermore, there are other excellent reviews that present the state-of-art of biosensors for microcystin detection(9) and electrochemical biosensors for a variety of toxins found in food and water.(10–12) These reviews are all restricted to include only a few targeted applications of each review’s time frame. The Singh et al.(9) literature review focused on biosensors for microcystin with different types of transducers (such as optical, electrochemical, thermal, etc.). That review presents a brief summary for each transducer, which is a good introduction to the general field of biosensors. The Campas et al.(10) literature review refers to electrochemical biosensors for a variety of toxins in food safety and environmental applications. This important review introduces the incorporation of nanomaterials into the field. However, it provides only a small overview on microcystin detection using electrochemical biosensors. Likewise, Reverté et al.(11) published their article in 2016, an updated version of Campàs et al.(10) The field evolved tremendously just in the next few years leading to the Zhang et al.(12) excellent summary of 25 papers describing recent progress of successful algal toxin detection in water using electrochemical biosensing techniques. This rapidly moving field is undergoing a transition from development of sensor concepts that are demonstrated primarily on relatively simple samples of cyanotoxins in purified water or buffer to cyanotoxins in real samples of surface waters. Our review article fills a void by combining essential background information about cyanobacterial toxins and electrochemical biosensors with the rapidly moving development of electrochemical biosensors for these toxins. Scientific and engineering aspects are presented in sufficient depth for the article to serve as a valuable source to scientists and engineers entering the field. Current issues and future challenges for the development of useful electrochemical biosensors for cyanotoxin detection that meet the demands for applications in field freshwater samples are discussed.
In this Review, the majority of reported studies and developments of electrochemical affinity biosensors for cyanotoxins are reviewed and scrutinized. Introductory parts include the current state-of-the-art in toxicity of cyanotoxins and conventional analytical methods and they are briefly covered. Discussion includes electrochemical techniques and devices, biomolecules with high affinity for cyanotoxins, and advances in electrochemical affinity biosensing of cyanotoxins. The majority of reported applications are presented, focusing on LOD achieved, dynamic ranges, and specificity accomplished. Potential improvements in analytical performance are presented in depth: first, by understanding the fundamental concepts and, second, through tailoring the properties of the transducer–biomolecule interface. Finally, the latest advances and future trends on devices showing potential for electrochemical biosensing application are summarized. This Review incorporates a complete guide into the interdisciplinary research of electrochemical biosensors for all cyanotoxins of current interest.
Cyanotoxins
For the past three decades, cyanobacteria are known to be notorious for their production and release of potent toxins during harmful bloom events which are now common worldwide.(13)These toxic secondary metabolites can be grouped as hepatotoxic, neurotoxic, and dermatoxic. The most common cyanobacterial toxins (cyanotoxins) are described briefly below and their toxicity is summarized in Table1.
Table 1.
Summary of cyanotoxins toxicity mechanisms and health effects
| Cyanotoxin | Mechanism of Toxicity | Health Effects |
|---|---|---|
| Phosphatase 1 & 2A inhibition | Gastroenteritis, liver damage, tumor promotion | |
| Microcystins | Apoptosis induction | |
| Cylindrospermopsins | Protein synthesis inhibitionOverall, unknown | Gastroenteritis, liver and kidney damage, headache, fatigue |
| Anatoxin-a | Nicotinic cholinergic agonist | Muscle spasm, fatigue, paralysis, respiratory arrest and death |
| Homoanatoxin-a | Resistant to cholinesterase degradation | |
| Anatoxin-a(s) | Acetylcholinesterase inhibitor | Tremors, convulsion, salivation, respiratory failure, death |
| Saxitoxin (paralytic shellfish poisons) | Block voltage-gated sodium channel | Nausea, vomiting, diarrhea, paralysis, death |
Hepatotoxins
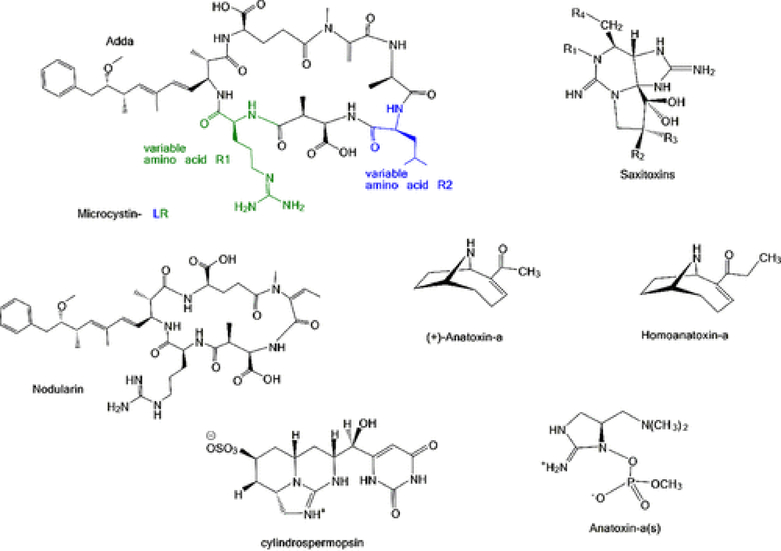
Microcystins (MC) are a group of cyclic heptapeptide hepatotoxins with over 200 variants differing by methylation, epimerization, hydroxylation, amino acid sequence, and toxicity.(14)They are the most common cyanotoxins in freshwater during harmful bloom events. MCs are potent inhibitors of serine/threonine phosphatases which control the cell cycle, metabolic regulation, protein synthesis, growth factor signaling pathways, transcriptional regulation, and neurotransmission in animals and plants. The inhibition of such important enzymes has major effects on important cellular processes. The toxicity of MCs is primarily attributed to the inhibition of ser/thr protein phosphatases resulting in disruption of signal transduction leading to their toxic effects. The microcystin congener with variable amino acid R1—Leucine and R2—Arginine (MC-LR; Figure 1) is the most common variant frequently found in harmful blooms of cyanobacteria. Nodularin (NOD; Figure 1), a cyclic pentapeptide, is another potent inhibitor of eukaryotic phosphatase similar to MC toxins. Like MCs, NOD is primarily considered a potent hepatotoxin since NOD targets the liver mainly, but can also affect other organs and tissues. NOD has 10 variants, and NOD-R is the most common. Cylindrospermopsin (CYN; Figure 1) is a sulfated-guanidine alkaloid with a tricyclic structure where the substituted uracil moiety is important in the toxicity of the toxin. It has been shown to be hepatotoxic in vivo, cytotoxic, dermatoxic, genotoxic, nephrotoxic, developmentally toxic, and potentially carcinogenic. CYN was originally discovered in a tropical cyanobacterium, Cylindrospermopsis raciborskii, an organism that caused major human poisoning in the Palm Island mystery disease.(15) However, in recent years, CYN has been increasingly detected in temperate freshwater.(16) CYN exerts greatest damage to the liver, but other organs such as heart, kidney, lung, and intestine can be affected. Unlike MCs and NODs, CYN does not inhibit protein phosphatase, but is a strong inhibitor of protein synthesis in vitro.
Figure 1.
Chemical structures of hepatotoxins and neurotoxins.
Neurotoxins
Anatoxins are neurotoxins that can be divided into anatoxin-a, homoanatoxin-a, and anatoxin-a(s). Anatoxin-a (ATX-a, Figure 1) was first implicated in the deaths of cows after ingesting contaminated lake water with algal bloom in Saskatchewan, Canada, in 1961.(17,18)Intraperitoneal injection of the cells or cell culture filtrates in mice resulted in convulsion, tremor, paralysis, and then death in minutes. Accordingly, it was initially named Very Fast Death Factor. Homoanatoxin-a is almost structurally identical with ATX-a, including an adjusted methylene group. Both anatoxins share toxicological properties and are analogs of the tropane alkaloid, cocaine. ATX-a has similar action to acetylcholine, a neurotransmitter released at the nerve junction to transmit signal to other cells. ATX-a binds to the acetylcholine receptor on muscle cells triggering muscle contraction. However, ATX-a is more potent and is resistant to degradation by acetylcholinesterase, leading to overstimulation of muscles.(19) Persistent stimulation causes muscular fasciculation, seizure, fatigue, and paralysis and can result in respiratory arrest and death. Anatoxin a(s) (ATX-a(s), Figure 1) is a phosphate ester of cyclic N-hydroxyguanidine and is mainly known as a natural organophosphate nerve agent similar to sarin, soman, and VX. Like ATX-a, ATX-a(s) is also a potent acetylcholinesterase inhibitor but ATX-a(s) has 10-fold greater toxicity. ATX-a(s) irreversibly binds to the active site of acetylcholinesterase preventing the degradation of acetylcholine in the peripheral and parasympathetic nervous system. Continuous stimulation of muscles can lead to tremors, convulsion, respiratory failure, brain damage, and death. The letter s refers to salivation, a common symptom in mammals.(20) Saxitoxin (STX) and its congeners are a group of neurotoxins commonly known as paralytic shellfish poisons (PSPs, “red tides” toxins; Figure 1). They are the most significant harmful algal bloom toxins in terms of public health (especially in the food industry). The eukaryotic dinoflagellates are the main source of PSPs in marine waters, whereas cyanobacteria are the major producers in freshwater. PSPs comprise 57 structurally related tricyclic guanidine alkaloids with varying toxicity. Depending upon the functional groups R1, R2, R3, and R4 (Figure 1), they are broadly classified into saxitoxins, neosaxitoxins, gonyautoxins, and decarbamoyl saxitoxins. PSTs are potent, naturally occurring water-soluble neurotoxins that block the signal transmission of the voltage-gated sodium channel of excitable cell membranes. Specifically, STX binds to site 1 to block the opening of sodium channels and prevents the conductance of signals along the neuron resulting in muscle paralysis and possibly death due to respiratory failure. STX is the most studied representative toxin and the most toxic (LD50 = 5 μg/kg) and it exhibits 1000 times higher toxicity than the nerve gas sarin. Serious human outbreaks of PSP in the US and worldwide are attributed to consumption of contaminated fish and shellfish. Humans have been reported to show characteristic neurological symptoms of nausea, vomiting, diarrhea, and sometimes death within 2–12 h.(21)
Important Cyanobacterial Harmful Bloom Events
Human exposure to cyanotoxins through drinking water consumption has been documented in the past decades worldwide.(22) In 1979, over 100 children on Palm Island Queensland, Australia, exhibited gastroenteritis and were admitted to hospitals. Local officials associated the outbreak with the local water supply source at Solomon Dam and cyanotoxins such as CYN. CYN was not known at the time; it was characterized years later by Ohtani et al.(23) The first and only documented outbreak of illness involving at least 52 deaths of dialysis patients attributed to cyanobacterial toxins was in Caruaru, Brazil, 1996.(24) A harmful bloom of Microcystis aeruginosa in Lake Taihu, Wuxi, China in May 2007, resulted in color, taste, and odor problems for approximately two million people who depend on the lake as their drinking water source. The harmful bloom was attributed to extensive eutrophication and industrial and domestic wastewater discharges. The Cyano-HAB pollution resulted in public panic, inadequancy of bottled water, and deleterious economic impacts in the area. Similarly, the frequent occurrence of Cyano-HABs in Lake Erie in the US is especially problematic. Outbreaks have worsened in the past few years, affecting the drinking water quality in the surrounding states. The most affected residents were from northern Ohio, where two drinking water utilities were forced to shut down. In 2013, six cases of acute gastrointestinal illness related to cyanotoxins were reported in Carroll Township, and 110 cases in Toledo in 2014.(25) Recurring blooms can be found in some of the world’s largest inland freshwater ecosystems, including Lake Erie and Lake Michigan (USA–Canada), Lake Okeechobee (Florida, USA), Lake Pontchartrain (Louisiana, USA), Lake Victoria (Africa), Lake Taihu (China), and estuarine and coastal waters, e.g., the Baltic Sea, Caspian Sea, tributaries of Chesapeake Bay, North Carolina’s Albemarle-Pamlico Sound, Florida Bay, the Swan River Estuary in Australia, and the Patos and other coastal lagoon estuaries in Brazil, to mention a few.(22)
Alerts, Advisories, Guidelines, and Regulations
In 1998, the World Health Organization (WHO, 2003) established a provisional drinking water guideline of 1 ng/mL (or 1 μg/L) for total MC-LR (free and bound), the most common variant of the MC family of cyanotoxins. Since then other countries have used this value to set their health alert or advisories, guidelines, and/or regulations for drinking water. For recreational water bodies, guidance or regulations are based on cell density (which can correspond to toxin level), biovolume, and pigment levels. In most cases, countries established a tier alert level based on adverse health effects with possible site recreation closure or warning to the public. No guidelines have been published by the WHO for other cyanotoxins, primarily due to insufficient toxicological data needed to establish concentration limits on cyanotoxins such as CYNs, anatoxins, and STXs. Also, Australia established a drinking water guideline for total MCs (MC-LR toxicity equivalent) at 1.3 ng/mL. Health advisories were also set for CYN at 1 ng/mL and STX at 3 ng/mL. Canada reaffirmed its maximum acceptable concentration of 1.5 ng/mL for total MCs in drinking water. France, Spain, Singapore, and Brazil established 1 ng/mL for total MCs in drinking water.(26) The European Union national drinking water legislation is based on the Drinking Water Directive which does not specifically address cyanotoxins.(27)
The US Environmental Protection Agency (USEPA), however, has not established regulations for cyanotoxins. The USEPA’s Office of Water listed cyanobacteria, and their associated toxins on the drinking water Candidate Contaminant List (CCL) 1 (1998) and CCL 2 (2005).(28)Cyanotoxins were included on CCL 3 (2009) and the CCL 4 (2016). CCL is a list of chemical and microbial contaminants in drinking water that require research for possible guidance or regulation. MCs, CYN, STX, and ATX-a are the cyanotoxins included in the latest CCL 4. In 2015, the USEPA published a ten-day health advisory in drinking water for MCs of 0.3 ng/mL for bottle-fed infants and preschool children, and 1.6 ng/mL for school-age or older adults; for CYN, 0.7 ng/mL for bottle-fed infants and preschool children, and 3 ng/mL for school-age and adults. Analytical methods for MC, CYN, and anatoxin have been developed (USEPA 2015a, 2015b) to be used for the Unregulated Contaminant Monitoring Rule to gather nationwide occurrence data in drinking water utilities.(29) Several states in the US have established health advisories or action levels in managing source and drinking water. For further information on regulations and guidelines for cyanotoxins in different countries Chorus, D. I. (2012) is an excellent source.(30)
Analytical Methods for Determination of Cyanotoxins
Early methods to detect cyanobacterial toxins were performed on animals injected with contaminated materials, cyanobacteria cells, cell cultures, and extracts. Seventeen different biotests using crustaceans, protozoans, insects, rotifers, cnidarians, nematodes, oligochaetes, and plants have been developed.(31) Alternative chemical and functional analyses are now replacing animal tests due to ethical consideration, prolonged procedure, high cost, and issues of low sensitivity and nonspecificity. Animal bioassay replacements for cyanotoxin toxicity determination include cell cultures using hepatocytes, intestine, fibroblasts, and neuroblastoma.
Biochemical assays which include immunoassays, enzymatic, and receptor assays are sensitive, rapid, and suitable for large-scale screening. Numerous immunoassays have been conducted and used to detect MCs/NOD, CYN, anatoxins, and STXs. Enzyme-linked immunosorbent assays (ELISA) are routinely used to analyze cyanotoxins in water. They can be configured as qualitative (simple positive or negative test) or semiquantitative tests. Also, they are easy to perform, do not require highly skilled personnel, and are relatively inexpensive. Like any test, immunoassays have limitations. Commercially available kits are routinely used in water quality laboratories to screen ground and surface water samples. They can detect both toxins produced by inactive and active microcystin genotypes of cyanobacteria,(32) both toxic and nontoxic variants, and are predisposed to matrix interference. Detection of various MC variants can be variable even using monoclonal antibodies specific for the highly conserved 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid (Adda) component of all MCs and NODs.(33,34) Despite their limitations, commercial ELISA methods allow rapid on-site detection of toxins without pretreatment and are used as a screening method to minimize the number of samples for further analyses with more accurate identification and quantification methods. Biochemical methods for the detection of cyanotoxins have been developed as well. Ser/thr phosphatase inhibition assays for MCs and NODs, cholinesterase inhibition test for anatoxin-(a)s, and receptor binding assays for PSPs and ATX-a are available. Protein phosphatase inhibition assays are based on MCs inhibition of ser/thr phosphatase 1 and 2A. Like immunoassays, the method does not differentiate toxic and nontoxic variants, cross-reacts with other toxins or compounds in water, and can be affected by matrices (other endogenous phosphatases, metals, and organic materials). Receptor bioassays (RBA) are gaining acceptance as tools for detection and quantitation of PSTs and their naturally occurring analogues, referred to here as STXs, in shellfish and water.(35) Assays developed are based on the ability of STXs to bind to mammalian sodium channels and saxiphilin, a protein belonging to the transferrin family in the xanthid crab, Liomera tristis.(36,37) An interlaboratory study comparing a precolumn oxidation HPLC method with a mouse bioassay showed correlation with RBA using rat brain membrane.(35) The ability of ATX-a and homoana-a to bind to nicotinic acetylcholine receptor also led to the development of receptor binding assays.(35) However, nicotinic acetylcholine receptor can also bind with spirolides, gymnodimines, and other marine toxins.(38) Binding of ATX-a(s) with the catalytic site of acetylcholinesterase enzyme is also the main mechanism of action of organophosphates and carbamates, commonly used as insecticides. ATX-a(s) inhibits acetylcholinesterase allowing acetylcholine, a neurotransmitter at the nerve synapse in the peripheral and central nervous systems, to continuously contract skeletal muscles or relax the heart. This problem was circumvented by Devic et al.(39) who engineered cholinesterases specifically sensitive to cyanobacterial toxin, ATX-a(s).
Several analytical techniques that involve state-of-the-art equipment have been used for the detection and subsequent quantification of cyanobacterial toxins. Liquid chromatography (LC) with photodiode array detection, mass spectrometry (MS), matrix-assisted laser desorption ionization/time-of-flight (MALDI-TOF), fluorescence, and electrochemistry are chemical methods to detect and quantify cyanotoxins. Identification and quantification of multiple classes of cyanotoxins (MCs, NOD, CYN, anatoxin) in a single analysis by LC/MS/MS is also available.(40,41) Simultaneous detection of multiple classes is ideal since harmful blooms of cyanobacteria can contain multiple cyanotoxin-producing species and many toxin-producing species produce more than one type of toxin and/or variants.(42) The US EPA developed LC with MS/MS detection for the determination of MCs, NOD, CYN, and ATX-a (USEPA Methods 544 and 545) in drinking water which will be used for the Agency’s Unregulated Contaminant Monitoring Regulation List 4. An alternative screening method to detect total microcystin is based on the detection of 2-methyl-3-methoxy-4-phenylbutyric acid (MMPB) as an oxidation product of microcystins.(43) Gas chromatography with MS and flame ionization detection and capillary electrophoresis with UV or MS detection have been used as chemical methods to detect and quantify cyanotoxins. These methods however require more complex procedures compared with LC/MS or LC/MS/MS methods.(44) MS-based methods are the only methods that unambiguously identify and quantify the different variants of microcystins, while methods based on assays using antibodies or protein phosphatases do not discriminate various variants. Chromatographic methods are very sensitive and precise; however, these methods can be hampered by interfering sample components (salts, metals, organic, and inorganic compounds) even with laborious and time-consuming cleanup procedures such as solid-phase extraction techniques. Chromatographic methods are also limited by the availability of standards, long processing time, cumbersome procedure, highly skilled analyst requirement, expensive instrument, and lack of portability.
Small molecules, like cyanotoxins, can be more difficult to “capture” with conventional analytical techniques. Limited efforts have been made so far to develop methods and instrumentation for the in situ or real-time detection of these toxins in natural environments. Fast-acting neurotoxic cyanotoxins require immediate detection to prevent exposure. Anatoxins are also chemically unstable and decompose rapidly. However, their half-lives are long enough to cause problems in the ecosystems (i.e., animal and fish deaths). Additionally, increasingly harmful bloom incidents and the likelihood of cyanotoxins in drinking water sources are propelling the need for the development of a highly selective, sensitive, fast-responding (seconds to minutes), and fouling-resistant method that can detect and monitor common potent cyanotoxins. The development of microsensing devices to detect cyanotoxins in water could rectify some problems associated with the current methods to detect cyanobacterial toxin contamination in water. Electrochemical affinity biosensors appear to be a very promising alternative technique for detection of cyanotoxins. Electrochemical affinity biosensors have low detection limits and higher target selectivity and specificity. Advanced portable biosensors would allow immediate assessment of water bodies and water treatment deficiencies so remedial measures could be put in place rapidly.
Electrochemical Biosensors
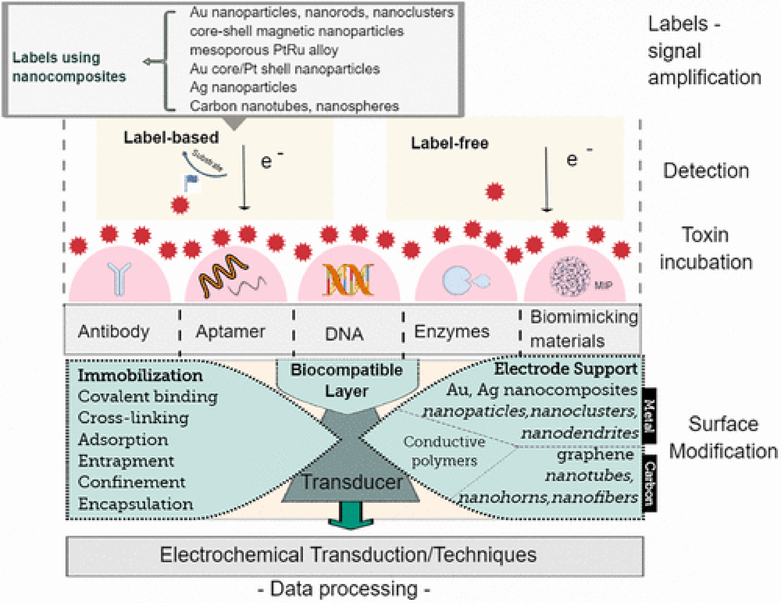
A biosensor contains a biological recognition element that specifically reacts with the target of interest (Figure 2). The biological event takes place at the interface between the bulk solution of the sample and the surface of a transducer which converts the event into a measurable signal. An electrochemical biosensor uses an electrode as the transducer to convert the biological change into an electrical signal, voltage, or current. The transduction (electrochemical technique) and signal processor collect, amplify, and display the signal. Electrochemical biosensors are efficient for small target compound detection, as they combine the sensitivity of electrochemistry with the high specificity of a biological reaction. Electrochemical biosensors have important characteristics, such as a dynamic concentration range, rapid response within seconds to minutes, amenability to miniaturization, and compensation for any drifts caused by temperature, pH, or other environmental factors. Electrochemical biosensors may be reliable, precise, and practical. An ideal biosensor incorporates features of minimal training and power requirements, portability (i.e., hand-held and lightweight), and most importantly, presents meaningful results using less sample volume and reagents. When integrated with novel transducer and interface designs, electrochemical microsystems have recently provided excellent analytical methodologies for the detection of toxins in water compared with other detection systems. For instance, novel transducers include metal or carbon-based composites to enhance the response of the electrode (usually referred to as “electrode support”).(10) Indeed, biosensors can achieve low detection limits, due to the selective binding or reaction of the biological recognition element to the target analyte. Incorporation of biochemistry and nanotechnology in electrochemical biosensors has been reported to enhance the signal transduction to reach femtomolar concentrations.(45,46)
Figure 2.
Schematic description of components and operation principle for electrochemical biosensors used in detection of cyanotoxins.
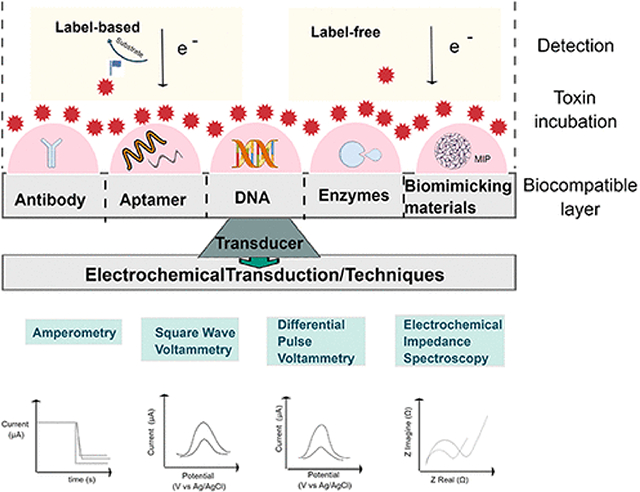
Electrochemical biosensors can be classified into biocatalytic devices and affinity sensors, depending on the nature and detection mechanism of the biomolecular element used.(47)Biocatalytic devices use the enzyme-target reaction to produce electroactive species, whereas affinity sensors monitor the interaction between bioreceptor and target to produce measurable signal. Immunosensors, aptasensors, and DNA sensors are examples of subclasses of electrochemical affinity biosensors. Additionally, a combination of the mode of signal transduction and biological receptor could describe an electrochemical affinity sensor. For example, when an electrochemical biosensor involves impedance spectroscopy and antibodies, these biosensors are often often termed impedimetric immunosensors. Finally, affinity sensors may use labels to improve detection, often described as “labeled” versus “label-free” based detection. Electrochemical biosensors specifically for cyanotoxins will be discussed for the remainder of this Review.
Electrochemical Transduction
Electrochemical Techniques and Methodologies
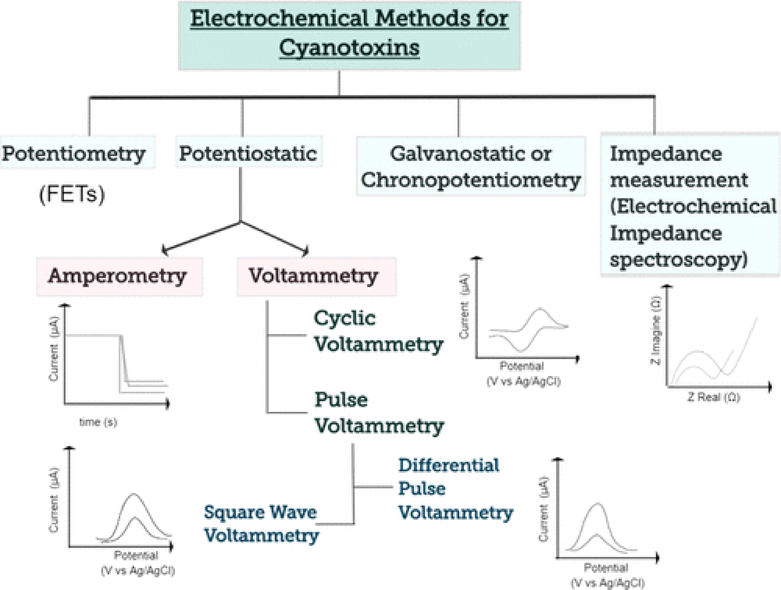
A variety of electrochemical techniques have been applied to the detection of toxins by biosensors (Figure 3). These techniques fall into the following general categories depending on the electrical signal that is applied to the electrochemical cell, which consists of the sensor, a reference electrode and an optional auxiliary (counter) electrode to provide current: potentiostatic—a controlled potential is applied to the electrochemical cell and current is measured; galvanostatic—current is applied and potential is measured; potentiometric—the cell potential is measured under the condition of near zero current; and impedance—potential is applied to the cell and the current response is measured and analyzed so that impedance (complex resistance) is obtained.(48,49) These general categories are further classified into specific methods. First, amperometry and voltammetry, are the most common potentiostatic electrochemical techniques used in affinity biosensors. Both techniques apply a (constant, scanning, or pulsing) potential to a working electrode (WE) which is the sensor versus a reference electrode and measure the current. Amperometry uses a fixed potential and measures the changes in current over time. In contrast, voltammetry scans a set potential range. Both techniques have a wide dynamic range with low limits of quantification. Several voltammetric methods have been used in electrochemical biosensors for cyanotoxins, including cyclic voltammetry (CV),(50) normal pulse voltammetry (NPV), differential pulse voltammetry (DPV), and square-wave voltammetry (SWV).(51) CV is the most versatile electrochemical technique for the general study of electroactive species. CV is usually the first experiment performed in an electrochemical study of a compound or an electrode surface. A characteristic CV experiment consists of scanning from an initial potential at a fixed rate to a switching potential at which the scan direction is reversed toward a final potential and measuring the resulting current. The voltammogram is obtained by plotting current as a function of applied potential. This technique may consist of a single scan or multiple scans (i.e., cycling the potential).(52) Several techniques use the application of potential pulses to the WE to improve the LOD by minimizing charging current associated with changing the electrode potential. NPV consists of a series of potential pulses of increasing amplitude for which the current response is measured near the end of each pulse after interfering charging current has decayed away. DPV is the application of a constant amplitude small potential pulse that is scanned through a fixed potential range. Here the current is sampled immediately before the pulse is applied and at the end of the pulse and the difference in the two currents is displayed for the voltammogram. An increasing popular pulse-voltammetric technique with excellent analytical sensitivity is SWV. A symmetrical square wave is superimposed on a staircase waveform where the forward pulse of the square wave is coincident with the staircase step and the difference in current between a forward and a reverse pulse is measured. This technique is popular because it employs faster scan rates than NPV and DPV and can have a lower LOD.(51) Enhanced sensitivity can be attained by stripping square wave voltammetry (SWSV) which combines SWV with an electrolytic or adsorptive accumulation of the target analyte at the WE as a preconcentration step that greatly improves the LOD.(53) Furthermore, amperometric detection is a superior technique in a flow electrode system or when using a rotating or vibrating WE.(52) In such mass transfer conditions, amperometry is considered the most suitable method, because it minimizes background signal from the charging current that would interfere with the electrochemical quantification. Amperometric detection can also monitor enzyme reactions in biocatalytic biosensors. Galvanostatic techniques are used much less than potentiostatic techniques. The primary technique is chronopotentiometry in which the current between the WE and counter electrode is controlled, and the resulting potential is measured across the WE and reference electrode. Chronopotentiometry allows the exploration of ion depletion at a membrane–sample interface, which can be observed as an inflection of the potential–time trace. Potentiometric techniques to detect pollutants have also been applied.(54)Potentiometric biosensors operate under conditions of near-zero current and measure the change in electrical potential at the WE when the target analyte binds to the immobilized biorecognition agent on the surface.(55) Finally, electrochemical impedance spectroscopy (EIS) is a powerful technique in electrochemical affinity biosensors whose usage has grown dramatically in the past ten or so years because it can be used to measure analytes without using labels to create a detectable signal, leading to simpler procedures for measurement. An alternating potential signal is applied to the electrochemical cell whose frequency is varied over a wide range to obtain the impedance spectrum.(51) The resistive and capacitive components of impedance contribute important information to study and interpret interface properties and surface reactions. Changes in electron-transfer resistance at the WE yield direct monitoring of the analyte binding to a recognition element (e.g., antibody, aptamer). Label-free impedimetric immunosensors such as aptasensors to detect small molecules in environmental applications have gained popularity among the biosensor scientific community due to their avoidance of environmental interferences. Electrochemical biosensors for cyanotoxins using the described techniques are presented in detail below.
Figure 3.
Categorization of electrochemical techniques used for detection of cyanotoxins.
Electrodes and Customized Devices
The choice of materials for the transducer or detector device is critical in fabricating any biosensor. The majority of the chemical or biochemical reactions take place at the WE surface of the transducer. In general, there are two important components/compartments of a WE platform. The first part is the material that is used as the base electrode or main substrate for connection with the signal processing instrument. This material should be highly conductive, and have even morphology and long-term stability. Some of the materials that have been commonly used are metals like gold, platinum, palladium, and carbon-based material (e.g., glassy carbon).(56) Overall, gold has performed best in many applications due to its inertness, corrosion resistance, high conductivity, stability, and bioactivity retention (ease of biocomponents attachment through gold-self-assembled monolayers).(57) A second component such as carbon nanotubes (CNTs),(58) graphene, nanoparticles (NPs),(59) or quantum dots (QDs)(60) of the WE can be added on top of the base electrode to increase the surface area, improve conductivity, and impart specific electrocatalytic properties. Due to rapid advancement in nanotechnology and reproducible synthesis of these materials, the LOD of biosensors using nanostructured electrodes has significantly improved down to the femtomolar (fM) range.(61–63) For MC-LR biosensors, different types of materials like gold,(64) glassy carbon,(65) and indium tin oxide (ITO)(66) have been used as WEs. The carbon-based solid contact electrodes appear to perform better than the metal ones.(67) Metal NPs,(68) graphene,(69) carbon nanofibers (CNF),(70) and molybdenum disulfide (MoS2)(71) are some of the materials that have been used to enhance the sensitivity of these electrodes. Other materials like nanoporous Pt–Ru alloy(72) and molecularly imprinted polymers (MIP)(73) have also been used successfully. Finally, ITO electrodes have also been used in electrochemical biosensors. The applicability of these electrodes in photoelectrochemical analysis is their main benefit.
The geometry and size of the WE play an important role in sensor performance. Early sensor development used traditional macro electrodes, which were then shrunk to micro electrodes and finally nanoelectrodes with advanced materials and instrumentation. This has further led to miniaturization, optimization, computerization, and simplification of the detection procedures. The use of screen printing for the development of “lab-on-a-chip” attracted a lot of attention. Screen-Printed Electrodes (SPEs), besides being disposable, offer solutions to some problems caused by using the conventional solid electrodes, like memory effect and large solution volume requirements. Screen-printing technologies gave prominence to electrochemical biosensors toward point-of-use application. SPEs can be printed on paper,(74) plastic,(75) and ceramic substrates(76) for convenient handling and ease of use. Furthermore, SPE can be modified with nanomaterials,(77) polymers,(78) and immobilized with biological components(79) with ease, thereby improving the sensitivity and selectivity of the biosensors. Some extensive reviews on SPEs have been published.(80–84) To further enhance the SPEs, the use of interdigitated electrodes (IDEs) has been shown to be beneficial for sensor performance. These electrodes can be used to measure large current operating at low voltage, which is usually achieved by reducing the distance between the electrodes. The use of interdigitated WE also increases the surface area of the electrode. Sensor geometry can be modified by increasing the area of the counter electrode while keeping the areas of the other two electrodes the same. This reduces the current density needed for electrochemical reactions at the counter electrode.(85) Lately, ion-selective electrodes(86) and ion-sensitive field-effect transistors (FET)(87) emerged in electrochemical biosensors using MIP and biological elements (e.g., antibody), respectively.
Electrical Interface Modification
The affinity biosensor interface is the sensing medium between the bulk solution (or sample) and the transducer surface of the biosensor where a biological event is taking place. Initial modification of the WE surface is very important, since this establishes the link between analyte and WE. Surface alteration also aids in stabilization of the biological system, improving the biosensor operational and storage stability. This surface modification (Figure 2) can be done by altering the electrode surface and introducing sites of known functional groups (functionalization) for subsequent binding with nanoparticles, the biorecognition element, or the analyte of interest (immobilization).(88,89) Functionalization of the surface can be done by covalent or noncovalent bonding. Immobilization of nanocomposites or molecules falls into four general categories: covalent binding, adsorption, cross-linking, and entrapment.(90) The modification of the surface electrode depends on the material of the electrode, the biological entity, and the special electrode architecture to connect those two.
On metal electrode surfaces, such as gold, chemisorption of reactive headgroup molecules usually results in self-assembling into molecular monolayers.(91) The thiol-end of these molecules reacts spontaneously with a gold surface to form monolayers. Several applications use this adsorption technique to immobilize silver or gold nanoparticles to increase the surface area of the electrode.(11) The assembled molecules or nanoparticles can further react with the target or other molecules to create a specific microarray for the detection of target analyte. The ease of surface modification and attachment of biomolecules through self-assembled monolayers (SAM) and their high stability are acknowledged advantages of gold electrode surfaces.(92–94)
For carbon-based supports, covalent binding, adsorption, and entrapment of carbon nanocomposites into polymers are common immobilization techniques.(11,95) Covalent functionalization leads to changes in material structure. For instance, covalent modification of CNTs or graphene leads to the attachment of a functional group like −NH2, −COOH, and −OH to sp2 carbon via a chemical bond which alters its chemical properties and introduces defect sites in the carbon structure.(96) Noncovalent functionalization involves surface interaction via weak forces like hydrogen bonding or van der Waals forces.(97) This may involve physical adsorption of functional group molecules or weak ionic interactions. To achieve these types of surface alterations, either wet or dry techniques can be adopted. Electrochemical and acid treatments are most commonly used methods for wet functionalization. An account of wet functionalization of graphene, including covalent and noncovalent immobilization of functional groups, has been reported. For example, covalent immobilization of aryl radical molecules on graphene through electrografting is a common functionalization process.(98) Wet surface alteration is efficient; however, its major disadvantage is waste generation, impurity incorporation, and longer processing times. Employing dry functionalization may eliminate the drawbacks of wet processes. Plasma treatment(99) and irradiation with e-beam(100) and γ rays(101) are some of the dry functionalization techniques. Ammonia plasma treatment of graphene(102) and CNTs(103)has successfully incorporated N-based species in the carbon structure, the species of which have been confirmed by X-ray photoelectron spectroscopy. NH3-based plasma treatment is beneficial especially for detection of biological species (e.g., nitrogen-fixing bacteria, cyanotoxin, protein, and DNA). These species possess NH-type linkage which can easily be attracted or coupled to the N-type activated surface via weak interactions.(104)
Recognition Receptors
Several high-affinity biomolecules have been used for the selective binding of cyanotoxin molecules. Most electrochemical biosensors for cyanotoxins are based on antigen–antibody affinity interactions. However, recognition receptors with high affinity to the analyte, such as aptamers,(105) appear to be a promising alternative. Engineered natural receptors, such as antibody fragments,(106) affimer proteins,(107) and artificial receptors,(108) have been developed and reported in the literature. Of these engineered recognition receptors for cyanotoxins, affimer proteins are the only example not to have been reported in the literature, to the best of our knowledge.
Antibodies
In the 1950s, antibodies were introduced as highly selective analytical reagents for immuno-based detection assays.(109) Polyclonal antibodies (pAb) were used. Later in 1975, the development of monoclonal antibodies (mAb) improved the specificity of existing assays.(110) Antibodies provided remarkable selectivity for a wide range of target analytes from small molecules to large biological organisms such as bacteria. Antibodies could easily be incorporated into assays using labels such as radioactive isotopes for radioimmunoassay. Immunoassay was combined with electrochemical detection in 1979 and was developed in the 1980s.(111) In early developments of electrochemical immunosensors, antibodies were immobilized directly on the electrode.(47,112) Immunoglobulin G (IgG), a class of antibody, is preferentially used in immunosensors. Each mAb antibody recognizes a single epitope whereas pAb are antibodies that recognize different epitopes on the toxin antigen.
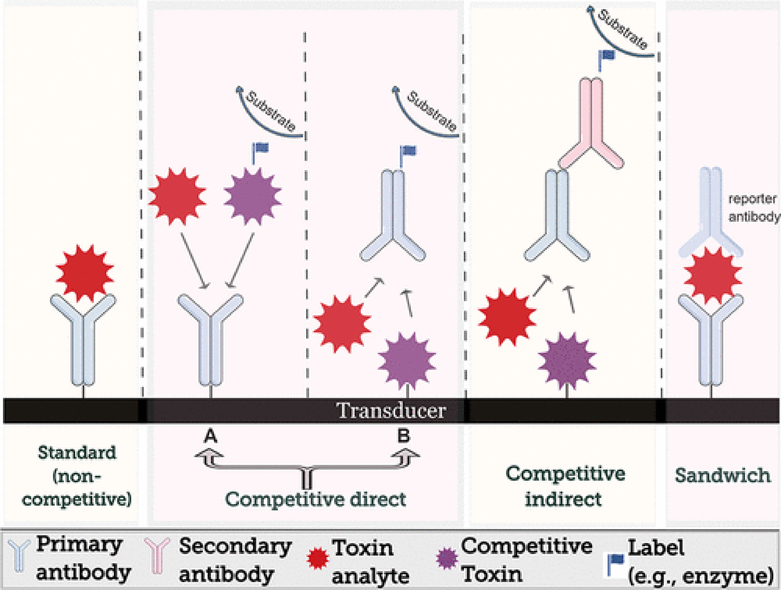
In many ways, general antibody array formats of a biosensor probe are mimicking ELISA and there are multiple designs in use (represented in Figure 4). Those include standard (noncompetitive) direct configuration, competitive direct, or indirect formats. A sandwich configuration is less commonly used, due to the small size and their few binding sites of toxins. When transducer probes consist of a microarray of immobilized antibodies on the transducer surface, standard (noncompetitive) direct assays, competitive direct microassays, or sandwich assays are used. Standard assay configuration includes direct binding of the target toxin to the immobilized antibody. The accumulation of the target on the electrode surface cause a change in the observed electrochemical signal. In the case of competitive direct assays, free target molecules in a sample competes with labeled target molecule to bind an antibody on the surface of the electrode. The labeled, unbound analyte is separated or washed away, and the remaining labeled, bound analyte on the biosensor surface is measured (Figure 4A). Alternatively, when the toxin is adsorbed on the surface of the electrode, antibodies play the role of the reporters. The latter can be used in label-free competitive direct format (Figure 4B).(113) When an enzyme-labeled secondary antibody is used, a competitive indirect format is formed. Label-free designs tend to minimize the analysis steps and create a rapid and possible online detection of toxins. Recently, label free formats are becoming more popular in the field of electrochemical biosensors.
Figure 4.
Schematic representation of assay configurations commonly used for detection of cyanotoxins.
Both pAb and mAb antibodies against MCs have been developed and used in several immunoassays over the years. The first pAb antibodies specific to MCs were reported in 1988(114) and several others followed.(115–118) The majority of antibodies specific to MCs were developed from mice,(119) and in some cases from the eggs of immunized chickens.(120)Newer antibody production methodologies used MC conjugates to carrier protein (e.g., keyhole limpet hemocyanine, bovine serum albumin). This novel method was introduced by Metcalf et al.(116) and used by several researchers. A MC-LR-bovine serum albumin (BSA) conjugate used by Sheng et al.(117) showed quite similar cross-reactivities for several MC variants and NOD. PAbs against two different variants, the MC-LR and MC-Arginine-Arginine (RR) with comparable cross-reactivities, have been developed as well.(118) Additionally, Baier et al.(121) used conjugates of MC-LR, MC-RR, and NODs with polylysine to raise pAbs; however, the cross-reactivities were similar. This repeated pattern of similarities in cross-reactivity between MC variants and NOD might be attributed to the Adda group of these toxins. In general, highly selective with high affinity mAbs are preferred. The first mAbs against MC-LR were developed by Nagata et al.(122) Due to the popularity of mAbs for smaller molecules with limited binding sites, researchers produced antibodies specifically against the common Adda amino acid side chain of all MCs and NODs which also showed cross-reactivities with the different congeners.(33,121,123,124) The Adda and mAbs are suitable for application in indirect competitive ELISA targeting the Adda group for “total” MCs and NODs.(125)
Despite the availability of commercial immunoassays (ELISA) for CYN, STX, and ATX-a reliable supply of antibodies is limited. A research group from UK(126) developed and characterized both mAbs and pAbs specific to cylindrospermopsin. Also, pAbs and mAbs against STXs have been isolated,(127–131) but ATX-a and ATX-a(s) antibodies do not appear to be reported in literature. The limited commercialization of antibodies against CYN, STX, and ATX-a hampered the development of immunosensors for these toxins. Recently, research groups have directed their attention to the selection of aptamers with high affinity toward these toxins, which are presented later in this Review.
Antibody fragments (e.g., Fab, Fv, scFv) are a good biomolecule alternative for electrochemical affinity biosensor applications. Due to their small size and high specificity to the targets, antibody fragments may increase the selectivity and sensitivity. They can minimize nonspecific adsorption or binding, lower steric hindrance, and fill properly orientated antigen binding fragments onto a limited surface area. In fact, antibody fragments are especially powerful when conjugated to nanoparticles.(132) Antibody fragments, known as side-chain antibody fragments, can be specifically engineered. Also, chemical fragmentation can be generated by treating antibodies with proteases or reducing agents to digest or break antibodies into small fragments that contain the antibody binding region for a specific antigen.(133–135) Genetically engineered single-chain antibody fragments for MC-LR were attempted twice by the Porter research group.(136,137) Both used a näve human phage display library to isolate the fragments. The first attempt resulted in relatively low affinity to MC-LR.(136) The second attempt resulted in antibody fragments with higher affinity to MC-LR; however, strong cross-reactivity to MC-RR was observed.(137) Recently, development of a single chain fragment variable molecule with highly specific traits to MC-LR and several MC congeners displayed almost even cross-reactivities to a range of MC-congeners.(138) We found no reports for the use of antibody fragments in electrochemical biosensor applications for cyanotoxins.
Aptamers
The evolution of nucleic acid ligands that could specifically bind to target where RNA oligonucleotides fold in such a way to bind with high affinity to proteins and small ligands was discovered by Tuerk et al.(139) and Ellington et al.(140) Later, single-strand DNA aptamers were introduced.(141) Aptamers are short, engineered single-strand DNA or RNA that fold and bind to a ligand by complementary shape interactions, mimicking antibody–ligand binding affinity. Aptamers with high affinity and specificity can be selected in vitro for a wide range of molecules, such as small chemicals, lipids, or proteins. Aptamers have gained increasing popularity over other natural receptors in detection tools, such as biosensors.(142)For example, compared to antibodies or enzymes, aptamers have the advantages of easy modification, high reproducibility, high stability, and low cost. Additionally, some aptamers yield important conformational changes upon target binding, enabling high versatility in the design of electrochemical biosensors. Depending on the conformational changes and the size of the targets, adaptation to electrochemical techniques and microassay formats can enhance the detection. Furthermore, the small size and flexibility of aptamers enable aptamer immobilization in higher densities than immunoassays, which is of critical importance in building microarrays, especially in complex systems. Finally, the aptamer modified surfaces have the advantage of reusability after regeneration of the aptamer to the initial standing or folding position.
Aptamers, as recognition elements, are an excellent alternative for the detection of toxic MCs. The first study on a DNA aptamer specific to MC-LR was presented by Nakamura et al.(143)This group used a typical in vitro selection of aptamers with the assistance of polymerase chain reaction amplification. Unfortunately, SPR analysis showed the aptamers to have very low affinity to MC-LR.(143) In vitro selection of ssDNA aptamers for MC-LR, LA, and YR with low dissociation constants have been developed by Ng et al.(144) However, three sequences had high affinity and selectivity to LR, LA, and YR. Aptamers with relatively low affinity may exhibit high electrochemical signals depending on the different molecular conformations during binding of the analyte target.(144) An innovative aptamer systematic evolution of ligand by exponential enrichment (SELEX) approach for MC-RR involves graphene oxide (GO). Unbound sequences were adsorbed on the GO through π–π stacking interactions for aptamer SELEX selection.(145) RNA-based aptamers for MC-LR have been reported as well. In vitro selection of RNA aptamers specific to MC-LR has been reported by Gu et al.(146)Enriched RNAs were cloned into E. coli cells. One clone had high binding of 0.5 μM to MC-LR.(146) Adsorption studies with GO showed a promising application of these RNAs for MC-LR detection.(147)
Aptamers for CYN, ATX-a, and STX have also been developed. Elshafey et al.(148)successfully employed SELEX selection of aptamers with high affinity and selectivity to CYN. A first attempt on selection of aptamers specific to ATX-a was in 2009. However, relevant DNA sequences and affinity constants were not reported in the literature.(149) In 2015, Elshafey et al.(150) developed an efficient fluorescent and electrochemical detection method with high binding affinities to ATX-a. One aptamer exhibited the lowest dissociation constant of roughly 27.17 nM, which was calculated using a Langmuir isotherm with an electrochemical-based method.(150) In 2013, Handy et al.,(151) generated DNA STX aptamers using a hapten–carrier conjugate as the SELEX target. Zheng et al.(152) optimized the aptamers developed by Handy et al.(151) by site-directed mutagenesis and truncation to showcase the formation of a G-quadruplex upon toxin binding. In 2013, Hu et al.(153)incorporated a SELEX method with an alternative aptamer highly specific to STX that imitates antibody–antigen interaction.
DNA, Enzymes, and Artificial Receptors
Interactions of double-stranded DNA (dsDNA) and cyanotoxins may lead to either DNA damage or agglomeration.(154) There are two well-known dsDNA and small molecule interactions: noncovalent (outer electrostatic binding, groove binding, and intercalation) and covalent (chemical modification of various DNA constituents) interactions. Intercalation and groove binding are the two most common modes by which small molecules bind directly and selectively to dsDNA. Small analytes can fit between dsDNA base pairs by intercalation, resulting in simultaneous displacement of a planar aromatic ring and unwinding of the DNA helix. In contrast, configurational entropy changes can cause groove binding, which involves covalent and noncovalent (electrostatic) interactions that do not perturb the DNA duplex structure to any great extent.(155) For example, plasmid DNA interactions with MC-LR showed high affinities between the molecules through the groove binding mode in a recently published report of Shi et al.(156) On the other hand, the covalent interaction of hepatotoxins with dsDNA may induce conformational changes in DNA, cleavage of hydrogen bonds, and/or oxidative damage to DNA bases. The attack of a hydroxyl radical to deoxyguanosine leads to DNA polymerase misreading and results in the production of the common biomarker 8-oxo-deoxyguanosine (8-oxoGua). After exposure to hepatotoxins, 8-oxoGua has been detected in vitro in cultured rat hepatocytes and in vivo in rat liver.(157) Interactions of calf thymus ssDNA and dsDNA with hepatotoxins was first introduced by Santos et al.; MC-LR and NOD cause dsDNA aggregation and damage, resulting in the formation of DNA abasic sites.(158)Additionally, the nucleotide structure of CYN may not lead to oxidative DNA damage, but could employ significant increase in DNA strand breaks.(159) Overall, cyanotoxin interactions with DNA may potentially lead to new biosensing designs.
Enzyme inhibition in the presence of a substrate can generate biocatalytic biosensors when enzymes are used as the biorecognition element. However, enzyme activation may develop assisted affinity biosensors when enzymes serve as labels. Cyanotoxins are toxins known to inhibit specific enzymes, such as phosphatases and acetylcholinesterase. Hepatotoxins, like MCs, inhibit protein phosphatase activity,(160) whereas anatoxin-a is an irreversible active site-directed inhibitor of acetylcholinesterase.(161) Furthermore, activation of the enzymes horseradish peroxidase (HRP) or alkaline phosphatase is commonly used for enzyme labels in electrochemical affinity biosensors. Artificial molecules mimicking enzymes, deoxyribozymes (DNAzymes), are used as labels to enhance sensitivity in biosensing strategies. DNAzymes show high catalytic activities toward specific substrates with improved stability over natural enzymes. DNAzymes can be denatured and renatured several times without substantial loss of activity. One popular kind of DNAzyme is the G-quadruplex-DNAzyme. In this DNAzyme, a guanine (G)-rich nucleic acid sequence folds into a parallel or an antiparallel G-quadruplex and it can easily bind to other target-probe DNA sequences. Due to its ability to catalyze the reduction of H2O2, the complex of hemin/G-quadruplex DNAzyme attracted a lot of attention. Hemin/G-quadruplex DNAzyme is a G-rich sequence coupled with hemin and this complex can easily replace natural enzymes in certain applications. Recently, this HRP-mimicking DNAzyme has been extensively used as a biocatalytic label to replace enzymes in electrochemical affinity biosensors.(162)
Electrochemical Biosensors for Cyanotoxins
An array of comparative studies in electrochemical biosensors for cyanotoxins was obtained from scientific reports and they are organized in Tables2 (label-based immunosensors), 3(label-free immunosensors), 4 (DNA and aptasensors), and presented in this Review.
Table 2.
Label-Based Immunosensors for MC-LR
| recognition element | assay format | electrode | novelty/biosensor improvement | electroch. technique | working range (ng/mL) | LOD (pg/mL) | water samples | ref |
|---|---|---|---|---|---|---|---|---|
| Mab | indirect competitive | ITO | Multiple amplification, Enzyme-free, Support; Au nanodendrites, Label; SiO2@MSN, DNAzymes | DPV | 0.0005–25 | 0.3 | DI | (170) |
| mAb | competitive sandwich | Gold | Support; MoS2–Au NCs, Label; Au@Pt NPs | DPV | 0.001–103 | 0.3 | TW, LW, RW | (168) |
| mAb | indirect competitive | GCE | Support; PEG/CNFs, Label; Au NPs | DPV | 0.0025–5 | 1.68 | Polluted Water | (95) |
| mAb | competitive | SWCNTs | Enzyme-free, Label; DNAzyme/CNTs | DPV | 0.01–7.0 | 2.31 | Reservoir | (169) |
| mAb | competitive | GCE | Support; Au NPs | EIS | 0.01–100 | 4 | LW | (163) |
| mAb | indirect competitive | ITO | Support; CNT@Co silicate, Label; multi-HRP–(Fe3O4 nanoclusters/PDA–Au NPs)–Ab2 | CV | 0.005–50 | 4 | TW, LW (Boyang Lake) | (164) |
| mAb | competitive sandwich | Gold | Support; MoS2/ AuNRs | DPV | 0.01–20 | 5 | TW, LW | (71) |
| mAb | indirect competitive | ITO | Support; magnetic graphene composite, Label; Ab2–(AuNR– circular DNA)- HRP | CV | 0.01–50 | 7 | LW | (66) |
| mAb | sandwich | GCE | Support; graphene sheet, Label; mesoporous PtRu-Ab2 | Amperometry | 0.01–28 | 9.63 | Polluted Water | (72) |
| mAb | indirect competitive | SPE | Support; nitrogen-doped graphene hydrogel, Label; multi-HRP-(mesoporous CNS/TH/Au NPs)-Ab2 | CV | 0.01–10 | 9.7 | spiked LW (Poyang Lake) | (167) |
| mAb | indirect competitive | GCE | Support; graphene sheet-chitosan, Label; HRP-CNS-Ab | DPV | 0.05–15 | 16 | Reservoir,TW, RW | (165) |
| PAb | indirect competitive | GCE | Support; SWNHs | DPV | 0.05–20 | 30 | LW | (166) |
| mAb | direct | Gold | QDs/Ab probe | SWSV | 0.227–50 | 99 | Microcystis aerug. Cultures | (171) |
Label-Based Immunosensors
Label-based immunosensors are a common method in electrochemical biosensing for cyanotoxins (Table2). A general format for detection of cyanotoxins is competitive (direct or indirect) transduction and rarely sandwich type of transduction. The advantage of enzyme labels is amplification of signal, through the production of enzyme product in the presence of a substrate. HRP is the most common enzyme used in electrochemical MC detection. The use of nanotechnology on either the electrode support or label can further amplify the electrical signal and result in ultrasensitive detection.(11) A successful direct competitive detection using biocatalytic precipitate reaction of a substrate by H2O2 and HRP was developed by Hou et al.(163) A glassy carbon electrode modified with gold nanoparticles (Au NPs) and stock MC-LR was immobilized on the electrode. After the competition between the immobilized MC-LR and the MC-LR in the analyte, mAb-HRP conjugate was used to accelerate the oxidation of 4-chloro-1-naphthol (substrate) to its precipitate form. The impedimetric detection of MC-LR was based on the mass loading of the precipitate on the electrode. Increasing enzyme precipitate served as the label, which caused increasing impedance signal with decreasing MC-LR concentration. The biosensor exhibited high sensitivity, a LOD of 4 pg/mL and high specificity against MC-LR; however, specificity studies were limited only between MC-LR and MC-RR variants.(163) Similar LOD (4 pg/mL) was achieved by Gan et al.(164) in an indirect competitive assay. Dual amplification was accomplished using CNT onto Co-silicate as a support, and HRP-secondary antibody conjugate based on magnetic core–shell nanocomposites as a label. The latter consisted of iron (II,III) oxide (Fe3O4) nanoclusters/polydopamine/gold nanoparticles. Another HRP-based detection using DPV detection and MoS2 with Au NRs as electrode support accomplished a LOD of 5 pg/mL.(71) He et al.(66) reported a successful HRP-labeled competitive immunosensor. An ITO modified electrode using nanomaterials, antibodies, and DNA molecules after its circularization was developed for MC-LR detection with LOD of 7 pg/mL. Graphene with magnetic properties increased electrode surface area, whereas gold nanorods with circular DNA (by rolling circle replication) conjugation helped to increase the impedance signal. A calibration curve was established based on CV measurements after the addition of 2 mM H2O2 in 1 mM hydroquinone solution.(66) Furthermore, a DPV biosensor for MC-LR was developed using immobilized graphene sheets with chitosan on glassy carbon electrode and conjugated carbon nanospheres with mAbs for signal amplification. Optimization steps (incubation time, H2O2 concentration, and ratio of H2O2:Ab) were important to reach a LOD of 16 pg/mL using a highly sensitive indirect competitive immunosensor.(165) Single-walled carbon nanohorns (SWNHs) were introduced on a glassy carbon electrode by Zhang et al.(166) Indirect competitive detection was based on HRP-H2O2 reaction and product precipitate concentration was monitored by DPV. The cone-shaped tips of SWNHs enhanced the immobilization capability of MC-LR and the minimum detection was 30 pg/mL. Fabrication reproducibility was verified by interassay; however, the relative standard deviation showed variability of 5.9–7.7%.(166) Nitrogen-doped graphene hydrogel SPE support was prepared by self-polymerization of dopamine (PDA) on GO, followed by a hydrothermal reaction. HRP and secondary antibodies were conjugated with captured Au NPs on mesoporous carbon nanospheres (CNS), using thionine (TH) as an electron mediator. This dual amplified biosensor reached a LOD of 9.7 pg/mL.(167)
Signal amplification through Au nanocomposites that enable the electron transfer has been developed. Two successful strategies to amplify a biosensor for MC-LR have been reported. MoS2 nanosheets with gold nanoclusters (MoS2/Au NCs) and Au core/Pt shell nanoparticles (Au@Pt NPs) were employed in a labeled competitive assay using DPV and 0.3 pg/mL LOD for MC-LR was achieved.(168) A labeled competitive immunosensor using a GC electrode modified with CNFs and Au NPs as a signal label resulted in a LOD of 1.68 pg/mL and a wide linear range of MC-LR concentrations (0.0025–5 ng/mL).(95) Both studies reached very low LODs, as calculated with a signal-to-noise ratio (S/N) of 3.
DNAzyme and its conjugates can be employed as a replacement for HRP in enzyme-free electrochemical immune-based detection of MC-LR. Tian et al.(169) developed a similar enzyme-based detection using horseradish peroxidase-mimicking DNAzyme on CNTs. The detection label was MC-LR conjugated with DNAzyme on CNTs. The sensor is based on the catalytic reduction of H2O2 and the LOD was 2.31 pg/mL. Another enzyme-free approach was introduced by Gan et al.(170) Monodisperse core–shell mesoporous silica nanoparticles (SiO2@MSN)-functionalized DNAzyme concatemers were synthesized to load hemin, which mimics an enzyme. DNAzyme concatemer consisted of the intercalation of methylene blue (MB) and DNA strands into the silica. G-quadruplex-hemin mimics the HRP activity, where MB is the electron mediator. π–π stacking interactions between the hemin-G-quadruplex and MB-DNA enhance the catalytic activity of DNAzyme. The biosensor platform was fabricated with gold nanodendrites due to their potential for multiple detection and binding sites in dendritic structures. Nanodendrites have a special morphology of repetitively long, dense, and sharp branched nanocomposites. DPV electrochemical measurements showed a promising application for MC-LR with high specificity in clean water. A low LOD of 0.3 pg/mL was achieved for clean water, however biosensor performance in real water samples was not tested.(170) Additionally, Wei et al.(72) developed an enzyme-free biosensor using nanomaterials–antibodies conjugation in a sandwich assay. They used graphene supported electrodes with immobilized Ab and detection was acquired through a label of conjugated secondary Abs with PtRu nanoparticles that were responsible for electrooxidation of H2O2. Characterization of the biosensor was carried out with impedance. Detection by amperometric measurements over time gave a detection limit of 9.63 pg/mL.(72)
Alternative to enzyme-type amplification, quantum dots (QD) have been tested in an electrochemical immunosensor for an indirect MC-LR detection. After the antibody-QD binding to different concentrations of MC-LR in prepared microtiter plate wells, the cadmium ions from the QDs are released. The semiconductor nanocrystal has a CdSe core encapsulated in a shell of ZnS and polymer. Gold electrode and SWSV were used for measuring the acidic dissolution of Cd2+. After optimization steps, a LOD of 99 pg/mL was achieved.(171)
Label-Free Immunosensors
In label-free immunosensors the electrode supports play a significant role for ultrasensitive detection of MC-LR (Table3). Graphene–gold nanocomposite conducting polymer holding carboxyl groups (polyDPB), gold nanoparticles layer (Au NP), and finally ionic liquid (IL) layer was used by Ruiyi et al.(45) as a GCE electrode support. This multilayered GCE modified electrode was able to detect MC-LR down to a remarkably low LOD of 0.000 037 pg/mL. However, the working range was limited between 1 × 10–7 – 8 × 10–8 ng/mL. The Yang research group employed CV, EIS, and DPV measurements in exploring biosensor properties in two different approaches. First, a label-free antibody-based electrochemical biosensor was developed using Au NPs/silicon template/MB/chitosan nanocomposites. The achieved LOD was at the level of 100 pg/mL.(172) However, in 2016, a G4-polyamidoamine (PAMAM) dendrimer and Ag nanocubes fabrication remarkably decreased the LOD down to 17 pg/mL.(173) Furthermore, immuno-field effect transistor based biosensors with single wall carbon nanotubes (SWCNT) incorporating IDEs appear promising for MC-LR detection.(87) Due to a displacement of the immobilized antibodies, these FET immunosensors were able to detect electrochemically the MC-LR with a LOD of 0.6 pg/mL and high specificity.(87) Sun et al.(174)had two successful attempts in developing electrochemical impedimetric biosensors for MC-LR. In 2010, a gold electrode modified with Au NPs was used to increase surface area. An antibody-based direct assay approach was performed. They explored different possible effects for sensor optimization. Interestingly, the influence on the Au NPs particle size was evaluated and 15 nm was used during fabrication to reach an ultralow detection of 18.2 pg/mL MC-LR.(174) In 2013, they followed a similar strategy; however alternatively to Au NPs, they used multiwalled carbon nanotubes (MWCNTs) functionalized with room temperature IL. They tested three room temperature ILs, but 1-amyl-2,3-dimethyl-imidazolium hexafluorophosphate, a more hydrophobic room temperature IL, was used for development of the MC-LR biosensor. A sensor with long-term stability and high specificity was developed, reaching a LOD of 1.7 pg/mL.(175) Dionysiou and colleagues synthesized carbon nanomaterials, such as graphene and CNTs, to develop carbon-based WEs for biosensor application. They effectively employed an impedimetric competitive label-free electrochemical bioassay for the detection of MC-LR. First, MWCNTs probes underwent alkaline functionalization to introduce necessary sites for MC-LR immobilization. Later competitive detection was employed using mAbs in MC-LR samples. The detection limit was 40 pg/mL.(176) In 2017, the same group used 3D foam graphene as WE to achieve a similar detection limit of 50 pg/mL.(177)
Table 3.
Label-Free Immunosensors
| cyanotoxin | recognition element | assay format | electrode | novelty/biosensor improvement | electroch. technique | working range (ng/mL) | LOD (pg/mL) | water samples | ref |
|---|---|---|---|---|---|---|---|---|---|
| MC-LR | mAb | direct | chitosan modified | Support; PAMAM, Ag NCs | DPV | (0.05–25) × 103 | 17 | LW | (173) |
| MC-LR | PAb | direct | gold | Support; Au NPs | EIS | 0.05–300 | 18.2 | DI | (174) |
| MC-LR | PAb | direct | GCE | Support; graphene-Au NP nanocomposite and polyDPB, and IL | DPV | (1 × 10−7)–(8 × 10−8) | 0.000037 | TW, LW, RW, GW | (45) |
| MC-LR | mAb | direct | IDE | Support; SWCNTs | chemiresistance | 0.001–1 | 0.6 | TW LW, RW | (87) |
| MC-LR | PAb | direct | GCE | Support; MWCNT/IL | EIS | 0.005–1 | 1.7 | DI, BW, TW | (175) |
| MC-LR | Mab | direct | Au | Support; Au NPs | DPV | 0.05–15 | 20 | Crude algae | (68) |
| MC-LR | mAb | indirect competitive | MWCNTs | MWCNTs probe | EIS | 0.05–20 | 40 | DI | (176) |
| MC-LR | mAb | indirect competitive | 3D foam G | Graphene probe | EIS | 0.05–20 | 50 | TW | (177) |
| MC-LR | mAb | direct | Au | Flow-through, support; thiourea SAM/Ag NPs | potentiometric step (capacitance) | 10−5–1 | 0.007 | BW, TW, Raw W | (46) |
| SXT | mAb | noncompetitive | Cu-graphene | Flow-through, support; polymeric lipid membrane/graphene | potentiometric | 1–1000 | 1000 | LW, shellfish | (55) |
Label-free flow-through biosensors for cyanotoxins are only a few. Capacitive immunosensors for the detection of MC-LR have reached an extremely LOD (0.007 pg/mL). A gold electrode was modified with thiourea SAM and silver nanoparticles (Ag NP). Antibodies were immobilized on the Ag NPs and direct label-free detection was tested.(46) In 2011, Dawan et al.(178) compared the flow-through capacitive immunosensor with and without Ag NPs. Also, the immunosensor performance using Au NPs was tested, and resulted in similar linearity to the Ag NPs-based immunosensor.(178) Finally, a miniaturized potentiometric STX biosensor using anti-STX incorporating lipid films on graphene nanosheets with 1 ng/mL detection limit has been recently reported. An adequate selectivity for detection over a wide range (299 pg/mL – 299 ng/mL) of toxin concentrations and a fast response time of ca. 5–20 min has been achieved.(55)
Aptasensors
Aptasensors for the detection of cyanotoxins have been recently developed (Table4). In 2013, the Chen research group reported the first aptamer-based impedimetric biosensor(64) in an attempt to decrease the detection limits of their previously reported sensor.(68) Optimization of aptamer immobilization and MC-LR incubation time with gold electrode provided a lower detection limit of 18 pg/mL. Specificity studies were also limited between the MC-LR and MC-RR variants.(64) Application of SWV and aptamer-based biosensors for MC-LR was approached by the Zourob research group.(69,144) In 2012 the selected aptamers were tested for their ability to specifically select different variants of MC, including MC-LR, MC-Leucine-Alanine (LA), and MC-Tryptophan-Arginine (YR). Three ssDNA sequences were distinguished between the selected aptamers with low dissociation constants and showed high affinity toward (i) MC-LR, (ii) MC-LR and MC-LA, (iii) MC-LR, MC-LA, and MC-YR for biosensing application. The LODs for a gold-based direct MCs detection were in the low concentration of 10 pg/mL.(144) Later in 2014, they developed a biosensor, where aptamer adsorbed on graphene-based SPEs utilizing π–π stacking interactions and successfully lowered the detection limit down to 1.9 pg/mL. Interestingly, they used spiked fish extracts for samples as part of the biosensor performance evaluation.(69) Also, an aptasensor for direct detection of MC-LR using a GCE-modified electrode with cobalt(II) metallodendrimer (SDD-Co(II)) and Ag NPs has been developed. Detection was monitored with CV and the LOD was 40 pg/mL.(179) Furthermore, two different groups reported label-free impedimetric aptasensors for CYN, both detecting lower concentrations than the suggested EPA guideline of 0.7 ng/mL. Both publications investigated optimization conditions and tested the performance in spiked water samples.(148,180) The impedimetric signal changes were attributed to conformational changes upon binding of CYN that enable electron transfer with the electrode. One group used a gold modified electrode with thiol SAMs for aptamer immobilization and accomplished a 39 pg/mL LOD.(148) The other group used a modified GCE with TH–graphene nanocomposites before immobilizing the aptamer and achieved an LOD of 117 pg/mL.(180) In a similar fashion, a label-free impedimetric aptasensor for ATX-a was established from the Zourob research group.(150) In another case, the selectively binding aptamer to STX reported by Handy et al.(151) was later utilized by Hou et al.(181) to develop an amperometric sensor. Gold electrode modified with carbon nanotubes on a SAM, and methylene blue as mediator was introduced. DPV oxidation peak current decreased with increasing STX concentration and gave a LOD 0.11 mg/mL. Using a SAM with advanced nanomaterials increased the sensor sensitivity.(181)
Table 4.
Aptasensors and dsDNA-Based Biosensors
| cyanotoxin | recognition element | electrode | novelty/biosensor improvement | electroch. technique | working range (ng/mL) | LOD (pg/mL) | water samples | ref |
|---|---|---|---|---|---|---|---|---|
| MC-LR | aptamer | Gold | choice recognition element | EIS | 0.05–100 | 18 | LW, RW, TW | (64) |
| MC-LR | aptamer | Graphene - SPE | choice recognition element, Graphene-SPE | SWV | 0.0001–1 | 1.9 | Fish Samples | (69) |
| MC-LR | aptamer | GCE modified | SDD-Co(II) Ag NPs | CV | 0.1–1.1 | 40 | DI, TW, WW | (179) |
| MC-LR | Calf thymus dsDNA | Gold | choice recognition element | DPV, EIS | 0.004–0.512 | 1.4 | TW | (186) |
| MCs | aptamers | Gold | different MC variants | SWV | 0.01–10 | 10 | DI | (144) |
| CYN | ssDNA Aptamer | GCE | TH–Graphene | EIS | 0.39 to 78 | 117 | LW | (180) |
| CYN | ssDNA aptamer | Gold | choice recognition element | EIS | 0.042–124.6 | 39 | TW | (148) |
| SXT | aptamer | Gold | MB-COOH-MWCNTs-SAM | DPV | 0.27–9.0 | 113.7 | Spiked Mussel | (181) |
| ATX | aptamer | Gold | choice recognition element | EIS | 0.17–17 | 82.6 | Spiked Drinking Water | (150) |
DNA Biosensors
Several genosensors and DNA hybridization-based sensors have been developed for cyanobacteria detection (such as Microcystis species).(157,182–185) Recently, a calf thymus DNA-based electrochemical biosensor for MC-LR has been developed. Conformational changes of calf thymus showed a good alternative electrochemical method for label-free MC-LR detection; however, in the presence of high concentrations of MC-RR selectivity toward MC-LR was decreased.(186) In the literature, there is an extensive development with numerous array designs and detection approaches for DNA-based electrochemical biosensing.(187)
Electrochemical Biocatalytic Biosensors
Enzyme activity inhibition is widely used as a detection tool for cyanotoxins in colorimetric,(188) fluorescene,(189) and electrochemical biocatalytic assays.(39,190–195) Campas et al.(190) introduced the incorporation of protein phosphatase inhibition for electrochemical biosensor application. Later, the same group translated the enzymatic inhibition of protein phosphatase 2 (PP2A) in a current intensity signal using chronoamperometry to reach a LOD or 37 ng/mL (35% inhibition of PP2A).(191) In 2008, a successful attempt to improve the sensitivity was based on dephosphorylation and recycling of nonelectroactive p-aminophenyl phosphate by PP2A and NADH oxidase, respectively.(192) A remarkable 775-fold decrease in detection limit was achieved. Catanante et al.(195) utilized the PP1 activity in an electrochemical DPV biosensor application for MC-LR detection with a LOD of 0.93 ng/mL. Also, a chronopotentiometric assay for MC-LR based on enzymatic inhibition achieved a LOD of 0.5 ng/mL.(196) With less success in terms of LOD (1 ng/mL), acetylcholinesterase activity inhibition was used for an electrochemical biosensor for ATX-a (S) detection.(39)
Artificial Receptors-Based “Biosensors”
Finally, artificial recognition elements or biomimicking materials, such as MIPs, are of growing interest for several applications. They are considered lower-cost alternatives to antibodies and some researchers name them “artificial antibodies”.(197) Artificial biosensors have been developed with the sensing layer consisting of imprinted sol–gel materials capable of establishing surface interactions with MC-LR.(67) A special tailoring of molecularly imprinted materials highly specific to MC-LR using CNTs was performed by Queiros et al.(86)They used IES electrodes and the sensing membrane of the “biosensor” consisted of ionophore, PVC and plasticizer, where ionophore was varying between molecularly imprinted CNTs and nonimprinted CNTs. Artificial antibodies (imprinted polymers) displayed good LOD in a potentiometric sensor application for MC-LR, below the guideline value established by WHO.(198)
Summary of Analytical Performance
The analytical performance of an electrochemical biosensor includes the evaluation of cross reactivity studies between different cyanotoxins, selectivity studies toward other interfering substances in surface freshwater, and stability studies of the sensor. Overall, electrochemical affinity biosensors have accomplished excellent LODs of toxins in water compared with the electrochemical biocatalytic biosensors. There are reported LODs for MC-LR at the low level of femtomolar scale.(45) In the field of electrochemical biosensors for cyanotoxins, most studies commonly use the method of S/N > 3 to determine an initial LOD. Admittedly, it is a question in the scientific community, at what extend these low LODs can be reproduced in real water samples, which are far more complex than laboratory samples. Most studies in affinity biosensors compared the analytical performance of the sensor when each substance was tested alone in a sample. Only a few studies tested for possible interference in the presence of two or more substances, including the target toxin.(149,163) MC-LR specific biosensors were tested over a variety of MC variants, NOD or other commonly found substances in freshwater.(64,168,175,179) CYN biosensors were challenged with MC-LR and ATX-a, whereas ATX-a biosensor performance was tested for cross-reactivity over ATX-a, MC-LR, or mixtures of cyanotoxins.(150) For STX electrochemical biosensors, the sensors were tested in waters contaminated with okadaic acid, neosaxitoxin, and gonyautoxins.(181)Despite the extensive past and present research, researchers are focusing more on the novelty of a sensor and reaching low detection limits, rather than increasing both sensitivity and specificity for an applicable sensor with environmental samples. Comprehensive reports on analytical performance for a feasible application are summarized. Among the label-based biosensors, Hou et al.(163) and Gan et al.(164) did excellent work considering the importance of stability and specificity of their sensor. Also, the Zhao et al.(165) sensor was extensively challenged with real water samples in the presence of ions and substances commonly found in freshwater. In label-free biosensors, Ruiyi et al.(45) reported a remarkable stability of 60 days and both ELISA and HPLC were employed in water sample recovery evaluation. In the aptasensors category, Eissa et al.(69) evaluated their sensor stability, nonspecific adsorption, and specificity and tested fish samples. Elshafey et al.(148) reported similar assessment of analytical performance to the latter aptasensor; however, they added testing of the reproducibility of their sensor. Finally, Loyprasert et al.(46) published a reusable label-free and flow-through biosensor that combines extremely high sensitivity (due to nanoparticles incorporation) and excellent sample recovery along with long sensor stability. The unique part of their sensor performance evaluation was the establishment of a new calibration curve, specific for real water samples. Nonetheless, the main drawback of the latter sensor is that a flow-through system may not be applicable for field measurements.
Recent Advances and Future Trends
Nanoparticles and their composites with polymers or carbon are widely used in designing microassays and electrodes in electrochemical biosensors for toxins.(11,10)Nanocomposites enhance the analytical properties of the electrochemical transduction. An approach is to modify the supporting WE for better immobilization or interaction within the transduction microassay and biological system. A full replacement of existing WEs with nanocomposites, such as MWCNT or graphites, can lead to conductivity enhancement and introduce higher density biomolecule layers, both of vital importance for an electrochemical biosensor. Besides, they can be used as labels in competition assays or overall signal enhancers, such as gold nanoparticles. Depending on their assigned role, different properties of NP and nanocomposites with multiple designs, structures, or conjugations with other molecules have been used in recent advances of electrochemical biosensors for detection of cyanotoxins.
Recently, metallic nanoparticles have proven to be of considerable importance for electrochemical biosensor applications because of their excellent properties. Au NPs provide high chemical stability, good biocompatibility, and the ability to facilitate excellent electron transfer between the analyte and electrode surface. Electrode surface modification with functional groups (−SH, −NH2, −CN) can coordinate metallic nanoparticles immobilization to create a multilayered nanocomposite modified electrode. Amine or thiol linkers, such as cysteamine,(68) polytyramine,(199) and thiourea SAM(46) create the foundation for Au NPs or Ag NPs immobilization. It is worth highlighting that Loyprasert et al.(46) and Dawan et al.(178) developed nanomaterial-modified electrodes flow-through capacitive immunosensors with extremely low detection limits for the detection of MC-LR.(46) Due to their large surface area, Au NPs can increase the immobilization of biomolecules and, hence, the bioreceptor density on the surface of the WE. Stable higher densities of bioreceptor improves the capabilities and performance of a biosensor. Au NPs with functional groups (−OH, −NH2, and −COOH) have been combined with antibodies, target analyte, or enzymatic compounds. Au NPs are widely used as label supports or signal enhancers in electrochemical biosensors.(11)Depending on the design of the microarray layers of an electrochemical transducer, different advanced Au NPs conjugations have been reported. Coupling of key biomolecules or the target molecule on the surface of Au NPs through covalent and noncovalent interactions is commonly applied. Recent publications on biosensors for small molecules detection use Au NPs as signal labels.(95) Advances with a dual amplification approach used MoS2/AuNCs and Au core with Pt shell nanoparticles.(168) Involvement of Au nanorods composites can result in an amplified biosensor as well.(66) Also, modified biosensing approaches using Ag nanocomposites, such as nanocubes(173) and nanoparticles,(179) exhibit signal amplification as well. Also, metallodendrimer encapsulated nanoparticles(179) and metallonanodendrimer structures(170) have become attractive candidates in electrochemical biosensor applications, due to the symmetry of branching, internal cavities, and additional binding sites. A unique case of metallic nanoparticles is QDs, which are semiconductor nanocrystals that are used most in optical applications. However, their electrocatalytic characteristics have been used as electrochemical labels in immunosensors, like the one developed by Yu et al.(171)
Carbon-based nanomaterials have been extensively used in the fabrication of biosensors. SWCNTs,(169,200) single-walled carbon nanohorns (SWCNHs),(166) and CNS(165) have been incorporated for electrode modifications or playing a label role for signal amplification. Besides, MWCNTs(176) and 3D graphene(177) have been used as the actual WE. The first successful MWCNT-based electrochemical biosensor has been developed by Han et al.(176)Recent developments in the internal structure of graphene by incorporating a new form of graphene—3D graphene—has led to efficient low LOD electrochemical sensors.(201) This new form of graphene offers important advantages such as high surface area, conductivity, and reproducibility.(202) Recently, there has been a greater thrust on hybrid materials which have better electrical, chemical, and physical characteristics than the parent materials. A 3D graphene and carbon nanofiber hybrid material was reported recently which provides a greater surface area as it utilizes the nanostructure of both carbon nanomaterials.(203)Owing to its high conductivity and large surface area, this material is a potential candidate for sensing electrodes. Also, there has been some progress on alternates to zero band gap single-layer graphene electrodes. The use of semiconducting metal chalcogenide based atomic thin electrodes for biosensing has been reported some time back.(204) The Lv et al. group(205) recently reported a novel MC-LR sensor using a nanoparticle grafted MoS2nanosheets platform, while another group(206) used WS2 nanosheet as a transducer for fabrication of a MC-LR sensor. Another relatively new approach for artificial biosensing electrode material is MIP.(207) A highly efficient photoelectrochemical sensor with MIP polypyrrole/Cu2O film having the LOD around 0.23 pg/mL was reported recently.(208) Another MIP-based sensor with TiO2/CNT nanostructure was reported having a LOD in the pg/mL range.(209) Interestingly, some recent developments combine carbon and metallic nanomaterials to serve similar or different purposes.(11) One example is the modifications of glassy carbon electrodes that include electrodeposited well-dispersed graphene oxide-Au NPs nanocomposites.(45) This combination served as a very good electrode support that has a synergistic effect on the overall performance of the sensor: higher sensitivity, stability, and reproducibility. Another combination of nanomaterials has been used for different purposes. A biosensor using nanomaterials–antibodies conjugations and a sandwich assay was developed by Wei et al.(72) Conductivity and surface area was increased with graphene modified electrodes and detection was acquired with a label of antibodies-PtRu alloy. The sensor had a remarkable stability of up to 2 months.(72) The application of variations on nanomaterials will influence the production of the next generation of reliable sensors in terms of electron transfer, bioactive agent immobilization, and overall stability.
Advances with nonmetallic magnetic monodisperse spherical particles (beads) have been used as well. The well-known magnetic beads have been recently used as an electrode-coating support(194,210) or as a label.(211) The convenience of the easy magnetic bead handling and the wide variation of magnetic bead surface modifications for immobilization of biorecognition molecules makes them attractive in electrochemical biosensors. Magnetic beads with hydrophilic surfaces are particularly convenient for nucleic acids, providing reproducible magnetic separation. For these reasons, magnetic beads have been extensively used in aptamers selection and biosensing.(194,210,211) In our opinion, magnetic beads will be employed more in aptamer-based biosensors for small toxins.
Recent trends for biosensor development have focused on miniaturization of the sensor designs. Improvements in SPEs for biosensors have led to further miniaturization attempts which include fabrication of microelectrodes,(212) ultramicroelectrodes,(213) and nanoelectrodes.(214) Various advanced top-down techniques have been employed to construct nanoelectrodes (one dimension under 100 nm) like nanoimprint, e-beam lithography,(215) ion beam lithography,(216) and scanning probe lithography.(217) Apart from lithographic techniques, another interesting way of fabricating nanoelectrodes was reported in which the fabrication of a circular silver nanoelectrode around 10 nm in diameter was done by interfacial reactions.(218) This miniaturization, besides having the obvious benefit of size, offers some other important advantages that include waste reduction, in vivo analysis, greater current density, and higher mass transfer characteristics. For example, a low LOD (0.6 pg/mL) paper-based MC-LR sensor has been reported in the past by Wang et al.(219) Future prospects will certainly include use of the above-mentioned techniques for fabrication of more highly efficient MC-LR sensing portable devices which have long run stability, repeatability, flexibility, and lower LODs. As mentioned in the earlier section of this Review, interdigitated devices offer significant advantage over conventional electrodes like enhanced current output, high signal-to-noise ratio, high sensitivity, and shorter response times. Another intriguing development in the future could be successful combination of MIP, atomic thin semiconducting interdigitated nanoelectrodes(220) for development of highly efficient and flexible sensors. Electrochemical impedance spectroscopy using interdigitated array electrodes (IDA) is an attractive combination as well as it offers the possibility of label-free detection using batch fabricated microfabricated devices. EIS detection with IDAs could be a powerful approach for detection of small molecules. These devices can easily be coupled with microfluidic sample preparation platforms to implement low cost, point-of-use analysis.(221–224) The development of “lab-on-a-chip” can be made with many channels, which allows multiple analysis of targets and reduces incubation times, volume of reagents, and cost.
The development of aptamers specific to different cyanotoxins and advanced aptamer-based assays in electrochemical biosensors has shown great potential and possible replacement for immunosensors. The small size of cyanotoxins and MCs cross-reactivities limit the detection of different MC variants. Aptamers can differentiate between MC variants, but signal production may be small, due to minimal conformational changes with a small size target. To enhance the signal, a sandwich assay is routinely used for detection of higher molecular weight molecules. However, this might not be applicable for detection of small toxins, since primary binding with antibodies or aptamers may leave little or no space for interaction with other biomolecular receptors. Nonetheless, advances in the design of the transducer interface for cyanotoxin detection needs improvement. Displacement of a complementary DNA upon target binding has been proposed to enhance signal amplification and sensitivity in terms of detection limits for small toxins. Another option is a competitive approach, where displacement of RNA aptamer from its complex with the target on the surface when free target molecules are in the bulk solution. The involvement of nanomaterials may also enhance the signal production. In our opinion, aptamers and label-free aptasensors incorporating nanomaterials, microfluidics on IDAs, or other miniaturized platforms are future trends toward the building of more reliable electrochemical biosensors for small toxins like cyanotoxins.
Although environmental biosensors can be constructed using the advanced characteristics of novel nanocomposites, high affinity biomolecules, and high-performance electrodes, the in situ and real-time monitoring of cyanotoxins is still a challenge. Most of electrochemical biosensors for cyanotoxins are successfully tested in buffered solutions or distilled water samples spiked with toxins. However, matrix effects invariably influence the analytical performance of a sensor. Also, the stability of sensors after multiple uses needs extensive evaluation. Biofouling prevention is an important precaution to be established before commercialization of environmental biosensors. Another challenge is sample pretreatment requirement before analysis. Certain applications need sophisticated sample pretreatment (e.g., solid-phase extraction) which make real-time monitoring difficult or unfeasible. Furthermore, simultaneous detection of MC-variants using a lab-on-a-chip biosensor is of relevant importance, but cross-reactivity between MC-variants may give overestimated results. Although MC-LR biosensors were extensively studied, there is an urgent need for the development of biosensors for other MC variants, CYN, SXT, and ATX-a.
Overall, the implementation of biosensors in real samples and detection of cyanotoxins in picomolar or femtomolar concentrations requires more effort. Validated low femtomolar LODs (if achieved) can certainly help, because using diluted samples can minimize the matrix effect during detection or avoid complex pretreatment procedures. Certainly, low LODs need reevaluation in real water samples to accomplish accurate and precise quantification of cyanotoxins. Reaching low detection limits in real water samples is still a big challenge in electrochemical biosensors. Undoubtedly, the field of electrochemical biosensors for cyanotoxins is moving rapidly. Currently, researchers are testing complex samples and that will probably add complications, such as causing sensor fouling and lowering sensitivity and selectivity. Estimation of LODs in real water samples needs extensive research. This has yet to be determined. Uniformity in the methodology used for LOD determination would be tremendously beneficial for the sensor community. Excellent sources on procedures and methods for determination of LOD in clean water or a specific matrix are the suggested procedures by EPA(225) and the book by Harris and Lucy (2016).(226) Application of these methods in determination of LOD would improve tremendously the quality assurance of a sensor.
Certainly, electrochemical biosensors show similar capabilities as ELISA. After optimization in water samples, they may be used as a routine quantitative screening and point-of-use water monitoring, mainly because of their high portability. Yet, MS-based methodologies are the only recognized analytical techniques that can distinguish between variants or transformation products. Quantification of MC variants in low concentrations using electrochemical biosensors is the biggest challenge to overcome. Ultimately, selection of aptamers or other high affinity biomolecules for specific MC variants is expected to be developed. When combined with innovative signal transduction technology, micro and nanosystems with multimicrofluidic channels, electrochemical biosensors may finally achieve specific variant detection at low concentrations. The interdisciplinary field of electrochemical biosensors is developing rapidly and new sensor applications/technologies on multicyanotoxin analysis are expected to emerge to properly assist the assessment of toxicity in real time. In the near future, breakthroughs on electrochemical biosensors will certainly contribute to fast cyanotoxins detection and efficient drinking water quality control, providing people with clean and safe drinking water.
Vocabulary
Harmful algal blooms of cyanobacteria, massive proliferation of cyanobacteria due to increasing nutrient levels (eutrophication) from human activities, impact significantly drinking and recreational water quality, prevalent in surface waters, including lakes, rivers, estuaries and drinking water reservoirs; Enzyme-linked immunosorbent assay, a plate-based method that detects and quantifies substances such as peptides and proteins, this method is based on the estimation of conjugated enzyme activity via incubation with a substrate; Lab-on-a-chip, a microfabricated fluidics device designed to perform several high-resolution biochemical analyses on a single integrated circuit; Point-of-use water monitoring, testing conducted at or near the site to provide real-time monitoring; Self-assembled monolayers, crystalline-like monolayers formed spontaneously on a substrate surface through adsorption and the adsorbed molecules possess a headgroup that has a strong affinity to the substrate; Systematic evolution of ligand by exponential enrichment, in vitro selection of preferred binding sequences from a pool of random sequences; Molecularly imprinted polymer, the product of polymerization of monomers in the presence of a template molecule that is extracted afterward, leaving behind complementary cavities in the polymer matrix.
Acknowledgments
This Review was prepared with the financial support of the National Science Foundation, Division of Chemical, Bioengineering, Environmental & Transport Systems, with award No. 1706489.
Abbreviations
- Cyano-HABs
harmful algal blooms of cyanobacteria
- LOD
limit of detection
- MC
microcystin
- MC-LR
Microcystin-Leucine-Arginine
- NOD
Nodularin
- CYN
Cylindrospermopsin
- ATX-a
anatoxin-a
- ATX-a(s)
anatoxin-a(s)
- STX
saxitoxin
- PSP
paralytic shellfish poisons
- WHO
World Health Organization
- USEPA
US Environmental Protection Agency
- CCL
Candidate Contaminant List
- ELISA
enzyme-linked immunosorbent assay
- LC
liquid chromatography
- MS
mass spectrometry
- RBA
receptor bioassays
- WE
working electrode
- CV
cyclic voltammetry
- SWV
square wave voltammetry
- SWSV
stripping wave square voltammetry
- EIS
electrochemical impedance spectroscopy
- fM
femtomolar
- CNF
carbon nanofiber
- MIP
molecularly imprinted polymers
- SPE
screen-printed electrode
- IDE
interdigitated electrodes
- FET
field–effect transistor
- CNT
carbon nanotube
- NP
nanoparticles
- mAb
monoclonal antibody
- pAb
polyclonal antibody
- IgG
immunoglobulin G, MC-RR
- MC
Arginine-Arginine
- Adda
3-amino-9- methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid
- SELEX
systematic evolution of ligand by exponential enrichment
- GO
graphene oxide
- dsDNA
double strand DNA
- 8-oxoGua
8-oxo-deoxyguanosine
- HRP
horseradish peroxidase
- DNAzymes
deozyribozymes
- Au NPs
gold nanoparticles
- SWNH
single-walled carbon nanohorn
- CNS
carbon nanospheres
- MoS2
Molybdenum disulfide
- SAM
self-assembled monolayer
- Au NC
gold nanoclusters
- Pt NPs
platinum nanoparticles
- GCE
glassy carbon electrode
- CNF
carbon nanofiber
- SiO2@MSN
monodisperse core–shell mesoporous silica nanoparticles
- MB
methylene blue
- QD
quantum dots
- PAMAM
G4-polyamidoamine dendrimer
- SWCNT
single-walled carbon nanotube
- MWCNT
multiwalled carbon nanotubes
- IL
ionic liquid
- SDD-Co(II)
cobalt(II) salicylaldiimine metallodendrimer
- IDA
interdigitated array electrodes
- ITO
indium tin oxide
- Au@Pt NPs
gold core/platinum shell nanoparticles
- PEG
polyethylene glycol
- CNT@Cosilicate
carbon nanotube/cobalt silicate core–shell nanocomposite
- Fe3O4
iron (II,III) oxide
- PDA
polydopamine
- TH
thionine
- Ab2
secondary antibody
- CNS
carbon nanosphere
- TW
tap water
- LW
lake water
- RW
river water
- DI
distilled water
- Ag NCs
Ag nanocubes
- polyDPB
functional conducting polymer
- GW
groundwater
- BW
bottled water
- Ag NP
Ag nanoparticles
- MB
methylene blue
- COOH
carboxyl group
- WW
wastewater
Footnotes
The authors declare no competing financial interest.
References
- 1.Antoniou MG; de la Cruz AA; Dionysiou DD Cyanotoxins: New Generation of Water Contaminants. J. Environ. Eng 2005, 131 (9), 1239–1243, DOI: 10.1061/(ASCE)0733-9372(2005)131:9(1239) [DOI] [Google Scholar]
- 2.Westrick JA; Szlag DC; Southwell BJ; Sinclair J A Review of Cyanobacteria and Cyanotoxins Removal/Inactivation in Drinking Water Treatment. Anal. Bioanal. Chem 2010, 397 (5), 1705–1714, DOI: 10.1007/s00216-010-3709-5 [DOI] [PubMed] [Google Scholar]
- 3.He X; Liu YL; Conklin A; Westrick J; Weavers LK; Dionysiou DD; Lenhart JJ; Mouser PJ; Szlag D;Walker HW Toxic Cyanobacteria and Drinking Water: Impacts, Detection, and Treatment. Harmful Algae 2016,54, 174–193, DOI: 10.1016/j.hal.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 4.Zanchett G; Oliveira-Filho EC Cyanobacteria and Cyanotoxins: From Impacts on Aquatic Ecosystems and Human Health to Anticarcinogenic Effects. Toxins 2013, 5 (10), 1896–1917, DOI: 10.3390/toxins5101896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catherine Q; Susanna W; Isidora ES; Mark H; Aurélie V; Jean-François H A Review of Current Knowledge on Toxic Benthic Freshwater Cyanobacteria - Ecology, Toxin Production and Risk Management. Water Res. 2013,47 (15), 5464–5479, DOI: 10.1016/j.watres.2013.06.042 [DOI] [PubMed] [Google Scholar]
- 6.Stewart I; Seawright AA; Shaw GR Cyanobacterial Poisoning in Livestock, Wild Mammals and Birds – an Overview In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer New York:New York, NY, 2008; Vol. 619, pp 613–637. [DOI] [PubMed] [Google Scholar]
- 7.McLellan NL; Manderville RA Toxic Mechanisms of Microcystins in Mammals. Toxicol. Res. (Cambridge, U. K.) 2017, 6 (4), 391–405, DOI: 10.1039/C7TX00043J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westrick JA; Szlag D A Cyanotoxin Primer for Drinking Water Professionals. J. - Am. Water Works Assoc 2018, 110 (8), E1–E16, DOI: 10.1002/awwa.108830319139 [DOI] [Google Scholar]
- 9.Singh S; Srivastava A; Oh HM; Ahn CY; Choi GG; Asthana RK Recent Trends in Development of Biosensors for Detection of Microcystin. Toxicon 2012, 60 (5), 878–894, DOI: 10.1016/j.toxicon.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 10.Campàs M; Garibo D; Prieto-Simón B Novel Nanobiotechnological Concepts in Electrochemical Biosensors for the Analysis of Toxins. Analyst 2012, 137 (5), 1055–1067, DOI: 10.1039/c2an15736e [DOI] [PubMed] [Google Scholar]
- 11.Reverté L; Prieto-Simón B; Campàs M New Advances in Electrochemical Biosensors for the Detection of Toxins: Nanomaterials, Magnetic Beads and Microfluidics Systems. A Review. Anal. Chim. Acta 2016, 908, 8–21, DOI: 10.1016/j.aca.2015.11.050 [DOI] [PubMed] [Google Scholar]
- 12.Zhang W; Dixon MB; Saint C; Teng KS; Furumai H Electrochemical Biosensing of Algal Toxins in Water: The Current State-of-the-Art. ACS. Sensors 2018, 3 (7), 1233–1245, DOI: 10.1021/acssensors.8b00359 [DOI] [PubMed] [Google Scholar]
- 13.Dionysiou D Overview: Harmful Algal Blooms and Natural Toxins in Fresh and Marine Waters – Exposure, Occurrence, Detection, Toxicity, Control, Management and Policy. Toxicon 2010, 55 (5), 907–908, DOI: 10.1016/j.toxicon.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 14.Catherine A; Bernard C; Spoof L; Bruno M Microcystins and Nodularins. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis 2017, 107–126, DOI: 10.1002/9781119068761.ch11 [DOI] [Google Scholar]
- 15.De La Cruz AA; Hiskia A; Kaloudis T; Chernoff N; Hill D; Antoniou MG; He X; Loftin K; O’Shea K;Zhao C A Review on Cylindrospermopsin: The Global Occurrence, Detection, Toxicity and Degradation of a Potent Cyanotoxin. Environ. Sci. Process. Impacts 2013, 15 (11), 1979–2003, DOI: 10.1039/c3em00353a [DOI] [PubMed] [Google Scholar]
- 16.Sinha R; Pearson LA; Davis TW; Burford MA; Orr PT; Neilan BA Increased Incidence of Cylindrospermopsis Raciborskii in Temperate Zones – Is Climate Change Responsible?. Water Res. 2012, 46 (5),1408–1419, DOI: 10.1016/j.watres.2011.12.019 [DOI] [PubMed] [Google Scholar]
- 17.Méjean A; Paci G; Gautier V; Ploux O Biosynthesis of Anatoxin-a and Analogues (Anatoxins) in Cyanobacteria. Toxicon 2014, 91, 15–22, DOI: 10.1016/j.toxicon.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 18.Sivonen K; Jones G Cyanobacterial Toxins In Toxic Cyanobacteria in Water; CRC Press, 1999; pp 41–111. [Google Scholar]
- 19.Hiripi L; Kovacs A; Kiss T Membrane Effects of Toxins Isolated from a Cyanobacterium. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol 2002, 131, 167–176, DOI: 10.1016/S1532-0456(01)00290-3 [DOI] [PubMed] [Google Scholar]
- 20.Aráoz R; Molgó J; Tandeau de Marsac N Neurotoxic Cyanobacterial Toxins. Toxicon 2010, 56 (5), 813–828, DOI: 10.1016/j.toxicon.2009.07.036 [DOI] [PubMed] [Google Scholar]
- 21.Wiese M; D’Agostino PM; Mihali TK; Moffitt MC; Neilan BA Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8 (7), 2185–2211, DOI: 10.3390/md8072185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paerl HW; Otten TG Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol 2013, 65 (4), 995–1010, DOI: 10.1007/s00248-012-0159-y [DOI] [PubMed] [Google Scholar]
- 23.Ohtani I; Moore RE; Runnegar MTC Cylindrospermopsin: A Potent Hepatotoxin from the Blue-Green Alga Cylindrospermopsis Raciborskii. J. Am. Chem. Soc 1992, 114 (20), 7941–7942, DOI: 10.1021/ja00046a067 [DOI] [Google Scholar]
- 24.Azevedo SMFO; Carmichael WW; Jochimsen EM; Rinehart KL; Lau S; Shaw GR; Eaglesham GK Human Intoxication by Microcystins during Renal Dialysis Treatment in Caruaru - Brazil. Toxicology 2002, 181–182, 441– 446, DOI: 10.1016/S0300-483X(02)00491-2 [DOI] [PubMed] [Google Scholar]
- 25.Benedict KM; Reses H; Vigar M; Roth DM; Roberts VA; Mattioli M; Cooley LA; Hilborn ED; Wade TJ; Fullerton KE Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water — United States, 2013–2014. MMWR. Morb. Mortal. Wkly. Rep 2017, 66 (44), 1216–1221, DOI: 10.15585/mmwr.mm6644a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chorus I Cyanotoxins : Occurrence, Causes, Consequences; Springer: New York, 2001. [Google Scholar]
- 27.European Commission. Drinking water legislation - Environment - European Commission; http://ec.europa.eu/environment/water/water-drink/legislation_en.html (accessed Feb 22, 2019.
- 28.EPA. Drinking Water Contaminant Human Health Effects Information; https://www.epa.gov/dwstandardsregulations/drinking-water-contaminant-human-health-effects-information (accessed Feb 22, 2019).
- 29.D’Anglada L Strong V, J. Drinking Water Health Advisory Documents for Cyanobacterial Toxins; Epa 820R15100; U.S EPA (United States Enviromental Protection Agency), 2015; p 5. [Google Scholar]
- 30.Chorus DI Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Federal Environmental Agency (Umweltbundesamt): Dessau, Germany, 2012; p 151. [Google Scholar]
- 31.Maršálek B; Bláha L Comparison of 17 Biotests for Detection of Cyanobacterial Toxicity. Environ. Toxicol.2004, 19 (4), 310–317, DOI: 10.1002/tox.20020 [DOI] [PubMed] [Google Scholar]
- 32.Kurmayer R; Christiansen G; Fastner J; Börner T Abundance of Active and Inactive Microcystin Genotypes in Populations of the Toxic Cyanobacterium Planktothrix Spp. Environ. Microbiol. 2004, 6 (8), 831–841, DOI: 10.1111/j.1462-2920.2004.00626.x [DOI] [PubMed] [Google Scholar]
- 33.Fischer WJ; Garthwaite I; Miles CO; Ross KM; Aggen JB; Richard Chamberlin A; Towers NR;Dietrich DR Congener-Independent Immunoassay for Microcystins and Nodularins. Environ. Sci. Technol 2001,35 (24), 4849–4856, DOI: 10.1021/es011182f [DOI] [PubMed] [Google Scholar]
- 34.Akter S; Vehniäinen M; Meriluoto J; Spoof L; Lamminmäki U Non-Competitive ELISA with Broad Specificity for Microcystins and Nodularins. Adv. Oceanogr. Limnol 2017, 8 (1), 121–130, DOI: 10.4081/aiol.2017.6349 [DOI] [Google Scholar]
- 35.Van Dolah FM; Fire SE; Leighfield TA; Mikulski CM; Doucette GJ Determination of Paralytic Shellfish Toxins in Shellfish by Receptor Binding Assay: Collaborative Study. J. AOAC Int 2012, 95 (3), 795–812, DOI: 10.5740/jaoacint.CS2011_27 [DOI] [PubMed] [Google Scholar]
- 36.Llewellyn LE; Negri AP; Doyle J; Baker PD; Beltran EC; Neilan BA Radioreceptor Assays for Sensitive Detection and Quantitation of Saxitoxin and Its Analogues from Strains of the Freshwater Cyanobacterium, Anabaena Circinalis. Environ. Sci. Technol 2001, 35 (7), 1445–1451, DOI: 10.1021/es001575z [DOI] [PubMed] [Google Scholar]
- 37.Ruberu SR; Langlois GW; Masuda M; Kittredge C; Perera SK; Kudela RM Receptor Binding Assay for the Detection of Paralytic Shellfish Poisoning Toxins: Comparison to the Mouse Bioassay and Applicability under Regulatory Use. Food Addit. Contam., Part A 2018, 35 (1), 144–158, DOI: 10.1080/19440049.2017.1369584 [DOI] [PubMed] [Google Scholar]
- 38.Rubio F; Kamp L; Carpino J; Faltin E; Loftin K; Molgó J; Aráoz R Colorimetric Microtiter Plate Receptor-Binding Assay for the Detection of Freshwater and Marine Neurotoxins Targeting the Nicotinic Acetylcholine Receptors. Toxicon 2014, 91, 45–56, DOI: 10.1016/j.toxicon.2014.08.073 [DOI] [PubMed] [Google Scholar]
- 39.Devic E, Li D; Dauta A; Henriksen P; Codd GA; Marty JL; Fournier D, Detection of Anatoxin-a(s) in Environmental Samples of Cyanobacteria by Using a Biosensor with Engineered Acetylcholinesterases. Appl. Environ. Microbiol 2002, 68 (8), 4102–4106, DOI: 10.1128/AEM.68.8.4102-4106.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham JL; Loftin KA; Meyer MT; Ziegler AC Cyanotoxin Mixtures and Taste-and-Odor Compounds in Cyanobacterial Blooms from the Midwestern United States. Environ. Sci. Technol 2010, 44 (19), 7361–7368, DOI: 10.1021/es1008938 [DOI] [PubMed] [Google Scholar]
- 41.Oehrle SA; Southwell B; Westrick J Detection of Various Freshwater Cyanobacterial Toxins Using Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry. Toxicon 2010, 55 (5), 965–972, DOI: 10.1016/j.toxicon.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 42.Zervou SK; Christophoridis C; Kaloudis T; Triantis TM; Hiskia A New SPE-LC-MS/MS Method for Simultaneous Determination of Multi-Class Cyanobacterial and Algal Toxins. J. Hazard. Mater 2017, 323, 56–66, DOI: 10.1016/j.jhazmat.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 43.Sano T; Nohara K; Shiraishi F; Kaya K A Method for Micro-Determination of Total Microcystin Content in Waterblooms of Cyanobacteria (Blue-Green Algae). Int. J. Environ. Anal. Chem 1992, 49 (3), 163–170, DOI: 10.1080/03067319208027567 [DOI] [Google Scholar]
- 44.Kaushik R; Balasubramanian R Methods and Approaches Used for Detection of Cyanotoxins in Environmental Samples: A Review. Crit. Rev. Environ. Sci. Technol 2013, 43 (13), 1349–1383, DOI: 10.1080/10643389.2011.644224 [DOI] [Google Scholar]
- 45.Ruiyi L; Qianfang X; Zaijun L; Xiulan S; Junkang L Electrochemical Immunosensor for Ultrasensitive Detection of Microcystin-LR Based on Graphene-Gold Nanocomposite/Functional Conducting Polymer/Gold Nanoparticle/Ionic Liquid Composite Film with Electrodeposition. Biosens. Bioelectron. 2013, 44 (1), 235–240, DOI: 10.1016/j.bios.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 46.Loyprasert S; Thavarungkul P; Asawatreratanakul P; Wongkittisuksa B; Limsakul C; Kanatharana P Label-Free Capacitive Immunosensor for Microcystin-LR Using Self-Assembled Thiourea Monolayer Incorporated with Ag Nanoparticles on Gold Electrode. Biosens. Bioelectron. 2008, 24 (1), 78–86, DOI: 10.1016/j.bios.2008.03.016 [DOI] [PubMed] [Google Scholar]
- 47.Ronkainen NJ; Halsall HB; Heineman WR Electrochemical Biosensors. Chem. Soc. Rev 2010, 39 (5),1747–1763, DOI: 10.1039/b714449k [DOI] [PubMed] [Google Scholar]
- 48.Bard AJ; Faulkner LR Electrochemical Methods : Fundamentals and Applications; Wiley, 2001. [Google Scholar]
- 49.Prentice GA Electrochemical Engineering Principles; Prentice Hall, 1997. [Google Scholar]
- 50.Kissinger PT; Heineman WR Cyclic Voltammetry. J. Chem. Educ 1983, 60 (9), 702, DOI: 10.1021/ed060p702 [DOI] [Google Scholar]
- 51.Kissinger PT; Ridgway TH Small-Amplitude Controlled-Potential Techniques In Laboratory Techniques in Electroanalytical Chemistry, Revised and Expanded; CRC Press, 2019; pp 141–164. [Google Scholar]
- 52.Heineman WR; Kissinger PT; Kissinger PT Large-Amplitude Controlled-Potential Techniques InLaboratory Techniques in Electroanalytical Chemistry, Revised and Expanded; CRC Press, 2018; pp 51–126. [Google Scholar]
- 53.Wang J Electrochemical Preconcentration In Laboratory Techniques in Electroanalytical Chemistry, Revised and Expanded; CRC Press, 2018; pp 719–738. [Google Scholar]
- 54.Heineman WR; Kissinger PT; Kissinger PT Large-Amplitude Controlled-Current Techniques InLaboratory Techniques in Electroanalytical Chemistry, Revised and Expanded; CRC Press, 2018; pp 127–140. [Google Scholar]
- 55.Bratakou S; Nikoleli GP; Siontorou CG; Nikolelis DP; Karapetis S; Tzamtzis N Development of an Electrochemical Biosensor for the Rapid Detection of Saxitoxin Based on Air Stable Lipid Films with Incorporated Anti-STX Using Graphene Electrodes. Electroanalysis 2017, 29 (4), 990–997, DOI: 10.1002/elan.201600652 [DOI] [Google Scholar]
- 56.O’Neill RD; Chang SC; Lowry JP; McNeil CJ Comparisons of Platinum, Gold, Palladium and Glassy Carbon as Electrode Materials in the Design of Biosensors for Glutamate. Biosens. Bioelectron. 2004, 19 (11),1521–1528, DOI: 10.1016/j.bios.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 57.Collinson MM Nanoporous Gold Electrodes and Their Applications in Analytical Chemistry. ISRN Anal. Chem. 2013, 2013, 1–21, DOI: 10.1155/2013/692484 [DOI] [Google Scholar]
- 58.Jain R; Sharma S Glassy Carbon Electrode Modified with Multi-Walled Carbon Nanotubes Sensor for the Quantification of Antihistamine Drug Pheniramine in Solubilized Systems. J. Pharm. Anal 2012, 2 (1), 56–61, DOI: 10.1016/j.jpha.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma X; Chen M Electrochemical Sensor Based on Graphene Doped Gold Nanoparticles Modified Electrode for Detection of Diethylstilboestrol. Sens. Actuators, B 2015, 215, 445–450, DOI: 10.1016/j.snb.2015.04.016 [DOI] [Google Scholar]
- 60.Martynenko IV; Litvin AP; Purcell-Milton F; Baranov AV; Fedorov AV; Gun’ko YK Application of Semiconductor Quantum Dots in Bioimaging and Biosensing. J. Mater. Chem. B 2017, 5 (33), 6701–6727, DOI: 10.1039/C7TB01425B [DOI] [PubMed] [Google Scholar]
- 61.Wei D; Bailey MJA; Andrew P; Ryhänen T Electrochemical Biosensors at the Nanoscale. Lab Chip 2009,9 (15), 2123–2131, DOI: 10.1039/b903118a [DOI] [PubMed] [Google Scholar]
- 62.Lord H; Kelley SO Nanomaterials for Ultrasensitive Electrochemical Nucleic Acids Biosensing. J. Mater. Chem 2009, 19 (20), 3127–3134, DOI: 10.1039/b814569e [DOI] [Google Scholar]
- 63.Rasheed PA; Sandhyarani N Electrochemical DNA Sensors Based on the Use of Gold Nanoparticles: A Review on Recent Developments. Microchim. Acta 2017, 184 (4), 981–1000, DOI: 10.1007/s00604-017-2143-1 [DOI] [Google Scholar]
- 64.Lin Z; Huang H; Xu Y; Gao X; Qiu B; Chen X; Chen G Determination of Microcystin-LR in Water by a Label-Free Aptamer Based Electrochemical Impedance Biosensor. Talanta 2013, 103, 371–374, DOI: 10.1016/j.talanta.2012.10.081 [DOI] [PubMed] [Google Scholar]
- 65.Sun X; Guan L; Shi H; Ji J; Zhang Y; Li Z Determination of Microcystin-LR with a Glassy Carbon Impedimetric Immunoelectrode Modified with an Ionic Liquid and Multiwalled Carbon Nanotubes. Microchim. Acta 2013, 180 (1–2), 75–83, DOI: 10.1007/s00604-012-0912-4 [DOI] [Google Scholar]
- 66.He Z; Wei J; Gan C; Liu W; Liu Y A Rolling Circle Amplification Signal-Enhanced Immunosensor for Ultrasensitive Microcystin-LR Detection Based on a Magnetic Graphene-Functionalized Electrode. RSC Adv. 2017,7 (63), 39906–39913, DOI: 10.1039/C7RA07696G [DOI] [Google Scholar]
- 67.Queirós RB; Noronha JP; Marques PVS; Fernandes JS; Sales MGF Determination of Microcystin-LR in Waters in the Subnanomolar Range by Sol–gel Imprinted Polymers on Solid Contact Electrodes. Analyst 2012, 137 (10), 2437, DOI: 10.1039/c2an35141b [DOI] [PubMed] [Google Scholar]
- 68.Tong P; Tang S; He Y; Shao Y; Zhang L; Chen G Label-Free Immunosensing of Microcystin-LR Using a Gold Electrode Modified with Gold Nanoparticles. Microchim. Acta 2011, 173 (3–4), 299–305, DOI: 10.1007/s00604-011-0557-8 [DOI] [Google Scholar]
- 69.Eissa S; Ng A; Siaj M; Zourob M Label-Free Voltammetric Aptasensor for the Sensitive Detection of Microcystin-LR Using Graphene-Modified Electrodes. Anal. Chem 2014, 86 (15), 7551–7557, DOI: 10.1021/ac501335k [DOI] [PubMed] [Google Scholar]
- 70.Zhang J; Sun Y; Dong H; Zhang X; Wang W; Chen Z An Electrochemical Non-Enzymatic Immunosensor for Ultrasensitive Detection of Microcystin-LR Using Carbon Nanofibers as the Matrix. Sens. Actuators, B 2016,233, 624–632, DOI: 10.1016/j.snb.2016.04.145 [DOI] [Google Scholar]
- 71.Zhang Y; Chen M; Li H; Yan F; Pang P; Wang H; Wu Z; Yang W A Molybdenum Disulfide/Gold Nanorod Composite-Based Electrochemical Immunosensor for Sensitive and Quantitative Detection of Microcystin-LR in Environmental Samples. Sens. Actuators, B 2017, 244, 606–615, DOI: 10.1016/j.snb.2017.01.030 [DOI] [Google Scholar]
- 72.Wei Q; Zhao Y; Du B; Wu D; Cai Y; Mao K; Li H; Xu C Nanoporous PtRu Alloy Enhanced Nonenzymatic Immunosensor for Ultrasensitive Detection of Microcystin-LR. Adv. Funct. Mater 2011, 21 (21), 4193–4198, DOI: 10.1002/adfm.201100773 [DOI] [Google Scholar]
- 73.Chen K; Liu M; Zhao G; Shi H; Fan L; Zhao S Fabrication of a Novel and Simple Microcystin-LR Photoelectrochemical Sensor with High Sensitivity and Selectivity. Environ. Sci. Technol 2012, 46 (21), 11955–11961, DOI: 10.1021/es302327w [DOI] [PubMed] [Google Scholar]
- 74.Cinti S; Mazzaracchio V; Cacciotti I; Moscone D; Arduini F Carbon Black-Modified Electrodes Screen-Printed onto Paper Towel, Waxed Paper and Parafilm M®. Sensors 2017, 17 (10), 2267, DOI: 10.3390/s17102267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasudivan S; Zhiping W Fine Line Screen Printed Electrodes for Polymer Microfluidics In 2010 12th Electronics Packaging Technology Conference, EPTC 2010; IEEE, 2010; pp 89–93. [Google Scholar]
- 76.Miserere S; Ledru S; Ruillé N; Griveau S; Boujtita M; Bedioui F Biocompatible Carbon-Based Screen-Printed Electrodes for the Electrochemical Detection of Nitric Oxide. Electrochem. Commun. 2006, 8 (2), 238–244, DOI: 10.1016/j.elecom.2005.11.016 [DOI] [Google Scholar]
- 77.Zhang JG; Kang TF; Xue R; Sun X An Immunosensor for Microcystins Based on Fe3O4@Au Magnetic Nanoparticle Modified Screen-Printed Electrode. Fenxi Huaxue/ Chin. J. Anal. Chem 2013, 41 (9), 1353–1358, DOI: 10.1016/S1872-2040(13)60679-9 [DOI] [Google Scholar]
- 78.Filik H; Aslıhan Avan A Conducting Polymer Modified Screen-Printed Carbon Electrode Coupled with Magnetic Solid Phase Microextraction for Determination of Caffeine. Food Chem. 2018, 242, 301–307, DOI: 10.1016/j.foodchem.2017.09.068 [DOI] [PubMed] [Google Scholar]
- 79.Silva Nunes G; Jeanty G; Marty J-L Enzyme Immobilization Procedures on Screen-Printed Electrodes Used for the Detection of Anticholinesterase Pesticides: Comparative Study. Anal. Chim. Acta 2004, 523 (1), 107–115, DOI: 10.1016/j.aca.2004.03.100 [DOI] [Google Scholar]
- 80.Alonso-Lomillo MA; Domínguez-Renedo O; Arcos-Martínez MJ Screen-Printed Biosensors in Microbiology; a Review. Talanta 2010, 82 (5), 1629–1636, DOI: 10.1016/j.talanta.2010.08.033 [DOI] [PubMed] [Google Scholar]
- 81.Taleat Z; Khoshroo A; Mazloum-Ardakani M Screen-Printed Electrodes for Biosensing: A Review (2008–2013). Microchim. Acta 2014, 181 (9–10), 865–891, DOI: 10.1007/s00604-014-1181-1 [DOI] [Google Scholar]
- 82.Albareda-Sirvent M; Merkoçi A; Alegret S Configurations Used in the Design of Screen-Printed Enzymatic Biosensors. A Review. Sens. Actuators, B 2000, 69 (1–2), 153–163, DOI: 10.1016/S0925-4005(00)00536-0 [DOI] [Google Scholar]
- 83.Renedo OD; Alonso-Lomillo MA; Martínez MJA Recent Developments in the Field of Screen-Printed Electrodes and Their Related Applications. Talanta 2007, 73 (2), 202–219, DOI: 10.1016/j.talanta.2007.03.050 [DOI] [PubMed] [Google Scholar]
- 84.Mistry KK; Layek K; Mahapatra A; RoyChaudhuri C; Saha H A Review on Amperometric-Type Immunosensors Based on Screen-Printed Electrodes. Analyst 2014, 139 (10), 2289, DOI: 10.1039/c3an02050a [DOI] [PubMed] [Google Scholar]
- 85.Jothimuthu P; Wilson RA; Herren J; Haynes EN; Heineman WR; Papautsky I Lab-on-a-Chip Sensor for Detection of Highly Electronegative Heavy Metals by Anodic Stripping Voltammetry. Biomed. Microdevices 2011,13 (4), 695–703, DOI: 10.1007/s10544-011-9539-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Queirós RB; Guedes A; Marques PVS; Noronha JP; Sales MGF Recycling Old Screen-Printed Electrodes with Newly Designed Plastic Antibodies on the Wall of Carbon Nanotubes as Sensory Element for in Situ Detection of Bacterial Toxins in Water. Sens. Actuators, B 2013, 189, 21–29, DOI: 10.1016/j.snb.2012.11.112 [DOI] [Google Scholar]
- 87.Tan F; Saucedo NM; Ramnani P; Mulchandani A Label-Free Electrical Immunosensor for Highly Sensitive and Specific Detection of Microcystin-LR in Water Samples. Environ. Sci. Technol 2015, 49 (15), 9256–9263, DOI: 10.1021/acs.est.5b01674 [DOI] [PubMed] [Google Scholar]
- 88.Sharma S; Byrne H; O’Kennedy RJ Antibodies and Antibody-Derived Analytical Biosensors. Essays Biochem. 2016, 60 (1), 9–18, DOI: 10.1042/EBC20150002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu Z; Zhang YHP Use of Nonimmobilized Enzymes and Mediators Achieved High Power Densities in Closed Biobatteries. Energy Sci. Eng 2015, 3 (5), 490–497, DOI: 10.1002/ese3.91 [DOI] [Google Scholar]
- 90.Sassolas A; Blum LJ; Leca-Bouvier BD Immobilization Strategies to Develop Enzymatic Biosensors.Biotechnol. Adv 2012, 30 (3), 489–511, DOI: 10.1016/j.biotechadv.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 91.Samanta D; Sarkar A Immobilization of Bio-Macromolecules on Self-Assembled Monolayers: Methods and Sensor Applications. Chem. Soc. Rev 2011, 40 (5), 2567–2592, DOI: 10.1039/c0cs00056f [DOI] [PubMed] [Google Scholar]
- 92.Shen H; Mark JE; Seliskar CJ; Mark HB; Heineman WR Blocking Behavior of Self-Assembled Monolayers on Gold Electrodes. J. Solid State Electrochem 1997, 1 (2), 148–154, DOI: 10.1007/s100080050039 [DOI] [Google Scholar]
- 93.Newton L; Slater T; Clark N; Vijayaraghavan A Self Assembled Monolayers (SAMs) on Metallic Surfaces (Gold and Graphene) for Electronic Applications. J. Mater. Chem. C 2013, 1 (3), 376–393, DOI: 10.1039/C2TC00146B [DOI] [Google Scholar]
- 94.Vericat C; Vela ME; Benitez G; Carro P; Salvarezza RC Self-Assembled Monolayers of Thiols and Dithiols on Gold: New Challenges for a Well-Known System. Chem. Soc. Rev 2010, 39 (5), 1805–1834, DOI: 10.1039/b907301a [DOI] [PubMed] [Google Scholar]
- 95.Zhang J; Sun Y; Dong H; Zhang X; Wang W; Chen Z An Electrochemical Non-Enzymatic Immunosensor for Ultrasensitive Detection of Microcystin-LR Using Carbon Nanofibers as the Matrix. Sens. Actuators, B 2016,233, 624–632, DOI: 10.1016/j.snb.2016.04.145 [DOI] [Google Scholar]
- 96.Balasubramanian K; Burghard M Chemically Functionalized Carbon Nanotubes. Small 2005, 1 (2), 180–192, DOI: 10.1002/smll.200400118 [DOI] [PubMed] [Google Scholar]
- 97.Bai H; Xu Y; Zhao L; Li C; Shi G Non-Covalent Functionalization of Graphene Sheets by Sulfonated Polyaniline. Chem. Commun 2009, 0 (13), 1667, DOI: 10.1039/b821805f [DOI] [PubMed] [Google Scholar]
- 98.Hirsch A; Englert JM; Hauke F Wet Chemical Functionalization of Graphene. Acc. Chem. Res 2013, 46(1), 87–96, DOI: 10.1021/ar300116q [DOI] [PubMed] [Google Scholar]
- 99.Dey A; Chroneos A; Braithwaite NSJ; Gandhiraman RP; Krishnamurthy S Plasma Engineering of Graphene. Appl. Phys. Rev 2016, 3 (2), 021301, DOI: 10.1063/1.4947188 [DOI] [Google Scholar]
- 100.Li B; Feng Y; Ding K; Qian G; Zhang X; Liu Y Effect of Electron Beam Irradiation on Multi-Walled Carbon Nanotubes. Trans. Nonferrous Met. Soc. China 2014, 24 (3), 764–769, DOI: 10.1016/S1003-6326(14)63123-X [DOI] [Google Scholar]
- 101.Ansón-Casaos A; Puértolas JA; Pascual FJ; Hernández-Ferrer J; Castell P; Benito AM; Maser WK;Martínez MT The Effect of Gamma-Irradiation on Few-Layered Graphene Materials. Appl. Surf. Sci 2014, 301,264–272, DOI: 10.1016/j.apsusc.2014.02.057 [DOI] [Google Scholar]
- 102.Lin Y-C; Lin C-Y; Chiu P-W Controllable Graphene N-Doping with Ammonia Plasma. Appl. Phys. Lett 2010, 96 (13), 133110, DOI: 10.1063/1.3368697 [DOI] [Google Scholar]
- 103.Khare BN; Wilhite P; Quinn RC; Chen B; Schingler RH; Tran B; Imanaka H; So CR; Bauschlicher CW; Meyyappan M Functionalization of Carbon Nanotubes by Ammonia Glow-Discharge: Experiments and Modeling. J. Phys. Chem. B 2004, 108 (24), 8166–8172, DOI: 10.1021/jp049359q [DOI] [Google Scholar]
- 104.Mahmoudifard M; Soleimani M; Vossoughi M Ammonia Plasma-Treated Electrospun Polyacrylonitryle Nanofibrous Membrane: The Robust Substrate for Protein Immobilization through Glutaraldhyde Coupling Chemistry for Biosensor Application. Sci. Rep 2017, 7 (1), 1, DOI: 10.1038/s41598-017-10040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song S; Wang L; Li J; Fan C; Zhao J Aptamer-Based Biosensors. TrAC, Trends Anal. Chem 2008, 27(2), 108–117, DOI: 10.1016/j.trac.2007.12.004 [DOI] [Google Scholar]
- 106.Sharma S; Byrne H; O’Kennedy RJ Antibodies and Antibody-Derived Analytical Biosensors. Essays Biochem. 2016, 60 (1), 9–18, DOI: 10.1042/EBC20150002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrigno PK Non-Antibody Protein-Based Biosensors. Essays Biochem. 2016, 60 (1), 19–25, DOI: 10.1042/EBC20150003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cieplak M; Kutner W Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition?. Trends Biotechnol. 2016, 34 (11), 922–941, DOI: 10.1016/j.tibtech.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 109.Yalow RS; Berson SA Assay of Plasma Insulin in Human Subjects by Immunological Methods. Nature 1959, 184 (4699), 1648–1649, DOI: 10.1038/1841648b0 [DOI] [PubMed] [Google Scholar]
- 110.Köhler G; Milstein C Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity.Nature 1975, 256 (5517), 495–497, DOI: 10.1038/256495a0 [DOI] [PubMed] [Google Scholar]
- 111.Heineman W; Anderson C; Halsall H Immunoassay by Differential Pulse Polarography. Science 1979, 204(4395), 865–866, DOI: 10.1126/science.441741 [DOI] [PubMed] [Google Scholar]
- 112.Heineman WR; Halsall HB Strategies for Electrochemical Immunoassay. Anal. Chem 1985, 57 (12),1321A–1331A, DOI: 10.1021/ac00289a804 [DOI] [PubMed] [Google Scholar]
- 113.Rogers KR Principles of Affinity-Based Biosensors. Mol. Biotechnol. 2000, 14 (2), 109–130, DOI: 10.1385/MB:14:2:109 [DOI] [PubMed] [Google Scholar]
- 114.Brooks WP Codd GA Immunoassay of Hepatotoxic Cultures and Water Blooms of Cyanobacteria Using Microcystis Aeruginosa Peptide Toxin Polyclonal Antibodies. Environ. Technol. Lett 1988, 9 (12), 1343–1348, DOI: 10.1080/09593338809384699 [DOI] [Google Scholar]
- 115.Chu FS; Huang X; Wei RD; Carmichael WW Production and Characterization of Antibodies against Microcystins. Appl. Environ. Microbiol 1989, 55 (8), 1928–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Metcalf JS; Bell SG; Codd GA Production of Novel Polyclonal Antibodies against the Cyanobacterial Toxin Microcystin-LR and Their Application for the Detection and Quantification of Microcystins and Nodularin.Water Res. 2000, 34 (10), 2761–2769, DOI: 10.1016/S0043-1354(99)00429-7 [DOI] [Google Scholar]
- 117.Sheng JW; He M; Shi HC; Qian Y A Comprehensive Immunoassay for the Detection of Microcystins in Waters Based on Polyclonal Antibodies. Anal. Chim. Acta 2006, 572 (2), 309–315, DOI: 10.1016/j.aca.2006.05.040 [DOI] [PubMed] [Google Scholar]
- 118.Young FM; Metcalf JS; Meriluoto JAO; Spoof L; Morrison LF; Codd GA Production of Antibodies against Microcystin-RR for the Assessment of Purified Microcystins and Cyanobacterial Environmental Samples.Toxicon 2006, 48 (3), 295–306, DOI: 10.1016/j.toxicon.2006.05.015 [DOI] [PubMed] [Google Scholar]
- 119.Baier W; Loleit M; Fischer B; Jung G; Neumann U; Weiß M; Weckesser J; Hoffmann P; Bessler W;Mittenbühler K Generation of Antibodies Directed against the Low-Immunogenic Peptide-Toxins Microcystin-LR/RR and Nodularin. Int. J. Immunopharmacol 2000, 22 (5), 339–353, DOI: 10.1016/S0192-0561(99)00086-7 [DOI] [PubMed] [Google Scholar]
- 120.McDermott CM; Feola R; Plude J Detection of Cyanobacterial Toxins (Microcystins) in Waters of Northeastern Wisconsin by a New Immunoassay Technique. Toxicon 1995, 33 (11), 1433–1442, DOI: 10.1016/0041-0101(95)00095-4 [DOI] [PubMed] [Google Scholar]
- 121.Baier W; Loleit M; Fischer B; Jung G; Neumann U; Weiß M; Weckesser J; Hoffmann P; Bessler WG;Mittenbühler K Generation of Antibodies Directed against the Low-Immunogenic Peptide-Toxins Microcystin-LR/RR and Nodularin. Int. J. Immunopharmacol 2000, 22 (5), 339–353, DOI: 10.1016/S0192-0561(99)00086-7 [DOI] [PubMed] [Google Scholar]
- 122.Nagata S; Soutome H; Tsutsumi T; Hasegawa A; Sekijima M; Sugamata M; Harada K-I; Suganuma M; Ueno Y. Novel Monoclonal Antibodies against Microcystin and Their Protective Activity for Hepatotoxicity.Nat. Toxins 1995, 3 (2), 78–86, DOI: 10.1002/nt.2620030204 [DOI] [PubMed] [Google Scholar]
- 123.Zeck A; Weller MG; Bursill D; Niessner R Generic Microcystin Immunoassay Based on Monoclonal Antibodies against Adda. Analyst 2001, 126 (11), 2002–2007, DOI: 10.1039/b105064h [DOI] [PubMed] [Google Scholar]
- 124.Khreich N; Lamourette P; Renard PY; Clavé G; Fenaille F; Créminon C; Volland H A Highly Sensitive Competitive Enzyme Immunoassay of Broad Specificity Quantifying Microcystins and Nodularins in Water Samples. Toxicon 2009, 53 (5), 551–559, DOI: 10.1016/j.toxicon.2008.12.021 [DOI] [PubMed] [Google Scholar]
- 125.United States Environmental Protection Agency. Method 546: Determination of Total Microcystins and Nodularins in Drinking Water and Ambient Water by Adda Enzyme-Linked Immunosorbent Assay; No. 815-B-16–011, 21; United States Environmental Protection Agency: Washington, DC, 2016. [Google Scholar]
- 126.Elliott CT; Redshaw CH; George SE; Campbell K First Development and Characterisation of Polyclonal and Monoclonal Antibodies to the Emerging Fresh Water Toxin Cylindrospermopsin. Harmful Algae 2013, 24, 10–19, DOI: 10.1016/j.hal.2012.12.005 [DOI] [Google Scholar]
- 127.Micheli L; Di Stefano S; Moscone D; Palleschi G; Marini S; Coletta M; Draisci R; Delli Quadri F Production of Antibodies and Development of Highly Sensitive Formats of Enzyme Immunoassay for Saxitoxin Analysis. Anal. Bioanal. Chem 2002, 373 (8), 678–684, DOI: 10.1007/s00216-002-1399-3 [DOI] [PubMed] [Google Scholar]
- 128.Hack R; Renz V; Märtlbauer E; Terplan G A Monoclonal Antibody to Saxitoxin. Food Agric. Immunol 1990, 2 (1), 47–48, DOI: 10.1080/09540109009354701 [DOI] [Google Scholar]
- 129.Chanh TC; Hewetson JF Polyclonal Anti-Idiotypes Induce Specific Anti-Saxitoxin Antibody Responses.Immunopharmacology 1993, 26 (3), 225–233, DOI: 10.1016/0162-3109(93)90038-R [DOI] [PubMed] [Google Scholar]
- 130.Bürk C; Usleber E; Dietrich R; Märtlbauer E Production and Characterization of Antibodies against Neosaxitoxin Utilizing a Novel Immunogen Synthesis Procedure. Food Agric. Immunol 1995, 7 (4), 315–322, DOI: 10.1080/09540109509354891 [DOI] [Google Scholar]
- 131.Bark C; Usleber E; Dietrich R; Martlbauer E Production and Characterization of Antibodies against Neosaxitoxin Utilizing a Novel Immunogen Synthesis Procedure. Food Agric. Immunol 1995, 7 (4), 315–322, DOI: 10.1080/09540109509354891 [DOI] [Google Scholar]
- 132.Wang Y; Tang LJ; Jiang JH Surface-Enhanced Raman Spectroscopy-Based, Homogeneous, Multiplexed Immunoassay with Antibody-Fragments-Decorated Gold Nanoparticles. Anal. Chem 2013, 85 (19),9213–9220, DOI: 10.1021/ac4019439 [DOI] [PubMed] [Google Scholar]
- 133.Holliger P; Hudson PJ Engineered Antibody Fragments and the Rise of Single Domains. Nat. Biotechnol 2005, 23 (9), 1126–1136, DOI: 10.1038/nbt1142 [DOI] [PubMed] [Google Scholar]
- 134.Sharma H; Mutharasan R Half Antibody Fragments Improve Biosensor Sensitivity without Loss of Selectivity. Anal. Chem 2013, 85 (4), 2472–2477, DOI: 10.1021/ac3035426 [DOI] [PubMed] [Google Scholar]
- 135.Akiyoshi DE; Sheoran AS; Rich CM; Richard L; Chapman-Bonofiglio S; Tzipori S Evaluation of Fab and F(Ab’)2 Fragments and Isotype Variants of a Recombinant Human Monoclonal Antibody against Shiga Toxin 2. Infect. Immun 2010, 78 (3), 1376–1382, DOI: 10.1128/IAI.00867-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mcelhiney J; Lawton LA; Porter AJR Detection and Quantification of Microcystins (Cyanobacterial Hepatotoxins) with Recombinant Antibody Fragments Isolated from a Näve Human Phage Display Library. FEMS Microbiol. Lett 2000, 193 (1), 83–88, DOI: 10.1111/j.1574-6968.2000.tb09406.x [DOI] [PubMed] [Google Scholar]
- 137.Mcelhiney J; Drever M; Lawton LA; Porter AJ Rapid Isolation of a Single-Chain Antibody against the Cyanobacterial Toxin Microcystin-LR by Phage Display and Its Use in the Immunoaffinity Concentration of Microcystins from Water. Appl. Environ. Microbiol 2002, 68 (11), 5288–5295, DOI: 10.1128/AEM.68.11.5288-5295.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murphy C; Stack E; Krivelo S; McPartlin DA; Byrne B; Greef C; Lochhead MJ; Husar G; Devlin S;Elliott CT Detection of the Cyanobacterial Toxin, Microcystin-LR, Using a Novel Recombinant Antibody-Based Optical-Planar Waveguide Platform. Biosens. Bioelectron 2015, 67, 708–714, DOI: 10.1016/j.bios.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 139.Tuerk C; Gold L Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249 (4968), 505–510, DOI: 10.1126/science.2200121 [DOI] [PubMed] [Google Scholar]
- 140.Ellington AD; Szostak JW In Vitro Selection of RNA Molecules That Bind Specific Ligands. Nature 1990,346 (6287), 818–822, DOI: 10.1038/346818a0 [DOI] [PubMed] [Google Scholar]
- 141.Ellington AD; Szostak JW Selection in Vitro of Single-Stranded DNA Molecules That Fold into Specific Ligand-Binding Structures. Nature 1992, 355 (6363), 850–852, DOI: 10.1038/355850a0 [DOI] [PubMed] [Google Scholar]
- 142.Cunha I; Biltes R; Sales M; Vasconcelos V Aptamer-Based Biosensors to Detect Aquatic Phycotoxins and Cyanotoxins. Sensors 2018, 18 (7), 2367, DOI: 10.3390/s18072367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakamura C; Kobayashi T; Miyake M; Shirai M; Miyakea J Usage of a DNA Aptamer as a Ligand Targeting Microcystin. Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A 2001, 371 (1), 369–374, DOI: 10.1080/10587250108024762 [DOI] [Google Scholar]
- 144.Ng A; Chinnappan R; Eissa S; Liu H; Tlili C; Zourob M Selection, Characterization, and Biosensing Application of High Affinity Congener-Specific Microcystin-Targeting Aptamers. Environ. Sci. Technol 2012, 46(19), 10697–10703, DOI: 10.1021/es301686k [DOI] [PubMed] [Google Scholar]
- 145.Wu S; Li Q; Duan N; Ma H; Wang Z DNA Aptamer Selection and Aptamer-Based Fluorometric Displacement Assay for the Hepatotoxin Microcystin-RR. Microchim. Acta 2016, 183 (9), 2555–2562, DOI: 10.1007/s00604-016-1904-6 [DOI] [Google Scholar]
- 146.Gu K; Famulok M In Vitro Selection of Specific Aptamers against Microcystin-LR. Zhonghua Yu Fang Yi Xue Za Zhi 2004, 38 (6), 369–373 [PubMed] [Google Scholar]
- 147.Hu X; Mu L; Wen J; Zhou Q Immobilized Smart RNA on Graphene Oxide Nanosheets to Specifically Recognize and Adsorb Trace Peptide Toxins in Drinking Water. J. Hazard. Mater 2012, 213–214 (214), 387– 392, DOI: 10.1016/j.jhazmat.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 148.Elshafey R; Siaj M; Zourob M In Vitro Selection, Characterization, and Biosensing Application of High-Affinity Cylindrospermopsin-Targeting Aptamers. Anal. Chem 2014, 86 (18), 9196–9203, DOI: 10.1021/ac502157g [DOI] [PubMed] [Google Scholar]
- 149.Jackson GW; Strych U; Frank E; Willson RC; Ballerstadt R; McNichols RJ Portable FRET Sensing of Proteins, Hormones, and Toxins Using DNA Aptamers and Quantum Dots In Nanotech Conference & Expo 2009, Vol 2, Technical Proceedings; 2009; pp 205–208. [Google Scholar]
- 150.Elshafey R; Siaj M; Zourob M DNA Aptamers Selection and Characterization for Development of Label-Free Impedimetric Aptasensor for Neurotoxin Anatoxin-A. Biosens. Bioelectron. 2015, 68, 295–302, DOI: 10.1016/j.bios.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 151.Handy SM; Yakes BJ; DeGrasse JA; Campbell K; Elliott CT; Kanyuck KM; DeGrasse SL First Report of the Use of a Saxitoxin-Protein Conjugate to Develop a DNA Aptamer to a Small Molecule Toxin. Toxicon 2013, 61 (1), 30–37, DOI: 10.1016/j.toxicon.2012.10.015 [DOI] [PubMed] [Google Scholar]
- 152.Zheng X; Hu B; Gao SX; Liu DJ; Sun MJ; Jiao BH; Wang LH A Saxitoxin-Binding Aptamer with Higher Affinity and Inhibitory Activity Optimized by Rational Site-Directed Mutagenesis and Truncation. Toxicon 2015, 101, 41–47, DOI: 10.1016/j.toxicon.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 153.Hu P; Liu Z; Tian R; Ren H; Wang X; Lin C; Gong S; Meng X; Wang G; Zhou Y Selection and Identification of a DNA Aptamer That Mimics Saxitoxin in Antibody Binding. J. Agric. Food Chem 2013, 61 (14),3533–3541, DOI: 10.1021/jf400880r [DOI] [PubMed] [Google Scholar]
- 154.Oliveira Brett AM; Diculescu VC; Chiorcea-Paquim AM; Serrano SH Chapter 20 DNA-Electrochemical Biosensors for Investigating DNA Damage. Compr. Anal. Chem. 2007, 49, 413–437, DOI: 10.1016/S0166-526X(06)49020-6 [DOI] [Google Scholar]
- 155.Oliveira SCB; Oliveira-Brett AM DNA-Electrochemical Biosensors: AFM Surface Characterisation and Application to Detection of in Situ Oxidative Damage to DNA. Comb. Chem. High Throughput Screening 2010, 13(7), 628–640, DOI: 10.2174/1386207311004070628 [DOI] [PubMed] [Google Scholar]
- 156.Shi Y; Guo C; Sun Y; Liu Z; Xu F; Zhang Y; Wen Z; Li Z Interaction between DNA and Microcystin-LR Studied by Spectra Analysis and Atomic Force Microscopy. Biomacromolecules 2011, 12 (3), 797–803, DOI: 10.1021/bm101414w [DOI] [PubMed] [Google Scholar]
- 157.Valério E; Abrantes LM; Viana AS 4-Aminothiophenol Self-Assembled Monolayer for the Development of a DNA Biosensor Aiming the Detection of Cylindrospermopsin Producing Cyanobacteria. Electroanalysis 2008,20 (22), 2467–2474, DOI: 10.1002/elan.200804350 [DOI] [Google Scholar]
- 158.Santos PVF; Lopes IC; Diculescu VC; Oliveira-Brett AM DNA - Cyanobacterial Hepatotoxins Microcystin-LR and Nodularin Interaction: Electrochemical Evaluation. Electroanalysis 2012, 24 (3), 547–553, DOI: 10.1002/elan.201100516 [DOI] [Google Scholar]
- 159.Štraser A; Filipič M; Gorenc I; Žegura B The Influence of Cylindrospermopsin on Oxidative DNA Damage and Apoptosis Induction in HepG2 Cells. Chemosphere 2013, 92 (1), 24–30, DOI: 10.1016/j.chemosphere.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 160.MacKintosh C; Beattie KA; Klumpp S; Cohen P; Codd GA Cyanobacterial Microcystin-LR Is a Potent and Specific Inhibitor of Protein Phosphatases 1 and 2A from Both Mammals and Higher Plants. FEBS Lett. 1990,264 (2), 187–192, DOI: 10.1016/0014-5793(90)80245-E [DOI] [PubMed] [Google Scholar]
- 161.Beasley VR; Coppock RW; Simon J; Ely R; Buck WB; Corley RA; Carlson DM; Gorham PR Apparent Blue-Green Algae Poisoning in Swine Subsequent to Ingestion of a Bloom Dominated by Anabaena Spiroides. J. Am. Vet. Med. Assoc 1983, 182 (4), 413–414 [PubMed] [Google Scholar]
- 162.Alizadeh N; Salimi A; Hallaj R Hemin/G-Quadruplex Horseradish Peroxidase-Mimicking DNAzyme: Principle and Biosensing Application In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin, Heidelberg, 2017; pp 1–22. [DOI] [PubMed] [Google Scholar]
- 163.Hou L; Ding Y; Zhang L; Guo Y; Li M; Chen Z; Wu X An Ultrasensitive Competitive Immunosensor for Impedimetric Detection of Microcystin-LR via Antibody-Conjugated Enzymatic Biocatalytic Precipitation. Sens. Actuators, B 2016, 233, 63–70, DOI: 10.1016/j.snb.2016.04.034 [DOI] [Google Scholar]
- 164.Gan C; Ling L; He Z; Lei H; Liu Y In-Situ Assembly of Biocompatible Core-Shell Hierarchical Nanostructures Sensitized Immunosensor for Microcystin-LR Detection. Biosens. Bioelectron 2016, 78, 381–389, DOI: 10.1016/j.bios.2015.11.072 [DOI] [PubMed] [Google Scholar]
- 165.Zhao H; Tian J; Quan X A Graphene and Multienzyme Functionalized Carbon Nanosphere-Based Electrochemical Immunosensor for Microcystin-LR Detection. Colloids Surf., B 2013, 103, 38–44, DOI: 10.1016/j.colsurfb.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 166.Zhang J; Lei J; Xu C; Ding L; Ju H Carbon Nanohorn Sensitized Electrochemical Immunosensor for Rapid Detection of Microcystin-LR. Anal. Chem 2010, 82 (3), 1117–1122, DOI: 10.1021/ac902914r [DOI] [PubMed] [Google Scholar]
- 167.Gan C; Sun Z; Ling L; He Z; Lei H; Liu Y Construction of Portable Electrochemical Immunosensors Based on Graphene Hydrogel@polydopamine for Microcystin-LR Detection Using Multi-Mesoporous Carbon Sphere-Enzyme Labels. RSC Adv. 2016, 6 (57), 51662–51669, DOI: 10.1039/C6RA07881H [DOI] [Google Scholar]
- 168.Pang P; Teng X; Chen M; Zhang Y; Wang H; Yang C; Yang W; Barrow CJ Ultrasensitive Enzyme-Free Electrochemical Immunosensor for Microcystin-LR Using Molybdenum Disulfide/Gold Nanoclusters Nanocomposites as Platform and Au@Pt Core-Shell Nanoparticles as Signal Enhancer. Sens. Actuators, B 2018,266, 400–407, DOI: 10.1016/j.snb.2018.03.154 [DOI] [Google Scholar]
- 169.Tian J; Zhao H; Yuan F; Quan X; Chen S Ultrasensitive Immunoassay of Microcystins-LR Using G-Quadruplex DNAzyme as an Electrocatalyst. Int. J. Environ. Anal. Chem 2014, 94 (10), 988–1000, DOI: 10.1080/03067319.2014.891109 [DOI] [Google Scholar]
- 170.Gan C; Wang B; Huang J; Qileng A; He Z; Lei H; Liu W; Liu Y Multiple Amplified Enzyme-Free Electrochemical Immunosensor Based on G-Quadruplex/Hemin Functionalized Mesoporous Silica with Redox-Active Intercalators for Microcystin-LR Detection. Biosens. Bioelectron 2017, 98, 126–133, DOI: 10.1016/j.bios.2017.06.038 [DOI] [PubMed] [Google Scholar]
- 171.Yu HW; Lee J; Kim S; Nguyen GH; Kim IS Electrochemical Immunoassay Using Quantum Dot/Antibody Probe for Identification of Cyanobacterial Hepatotoxin Microcystin-LR. Anal. Bioanal. Chem 2009,394 (8), 2173–2181, DOI: 10.1007/s00216-009-2910-x [DOI] [PubMed] [Google Scholar]
- 172.Fu X; Feng Y; Niu S; Zhao C; Yang M; Yang Y Sensitive Detection of Microcystin-LR by Using a Label-Free Electrochemical Immunosensor Based on Au Nanoparticles/Silicon Template/Methylene Blue Nanocomposite. J. Nanosci. Nanotechnol 2013, 13 (12), 8245–8252, DOI: 10.1166/jnn.2013.7936 [DOI] [PubMed] [Google Scholar]
- 173.Fu X; Zhao C; Liu X; Zhao J; Niu X; Zheng L; Yang Y A Layer-by-Layer Assembly Label-Free Electrochemical Immunosensor for the Detection of Microcystin-LR Based on CHIT/PAMAM Dendrimer/Silver Nanocubes. Int. J. Environ. Anal. Chem 2016, 96 (3), 284–297, DOI: 10.1080/03067319.2016.1150460 [DOI] [Google Scholar]
- 174.Sun X; Shi H; Wang H; Xiao L; Li L A Simple, Highly Sensitive, and Label-Free Impedimetric Immunosensor for Detection of Microcystin-Lr in Water. Anal. Lett 2010, 43 (4), 533–544, DOI: 10.1080/00032710903406912 [DOI] [Google Scholar]
- 175.Sun X; Guan L; Shi H; Ji J; Zhang Y; Li Z Determination of Microcystin-LR with a Glassy Carbon Impedimetric Immunoelectrode Modified with an Ionic Liquid and Multiwalled Carbon Nanotubes. Microchim. Acta 2013, 180 (1–2), 75–83, DOI: 10.1007/s00604-012-0912-4 [DOI] [Google Scholar]
- 176.Han C; Doepke A; Cho W; Likodimos V; De La Cruz AA; Back T; Heineman WR; Halsall HB; Shanov VN; Schulz MJ A Multiwalled-Carbon-Nanotube-Based Biosensor for Monitoring Microcystin-LR in Sources of Drinking Water Supplies. Adv. Funct. Mater 2013, 23 (14), 1807–1816, DOI: 10.1002/adfm.201201920 [DOI] [Google Scholar]
- 177.Zhang W; Han C; Jia B; Saint C; Nadagouda M; Falaras P; Sygellou L; Vogiazi V; Dionysiou DDA 3D Graphene-Based Biosensor as an Early Microcystin-LR Screening Tool in Sources of Drinking Water Supply.Electrochim. Acta 2017, 236, 319–327, DOI: 10.1016/j.electacta.2017.03.161 [DOI] [Google Scholar]
- 178.Dawan S; Kanatharana P; Wongkittisuksa B; Limbut W; Numnuam A; Limsakul C; Thavarungkul P Label-Free Capacitive Immunosensors for Ultra-Trace Detection Based on the Increase of Immobilized Antibodies on Silver Nanoparticles. Anal. Chim. Acta 2011, 699, 232–241, DOI: 10.1016/j.aca.2011.05.038 [DOI] [PubMed] [Google Scholar]
- 179.Bilibana MP; Williams AR; Rassie C; Sunday CE; Makelane H; Wilson L; Ntshongontshi N; Jijana AN; Masikini M; Baker PGL Electrochemical Aptatoxisensor Responses on Nanocomposites Containing Electro-Deposited Silver Nanoparticles on Poly(Propyleneimine) Dendrimer for the Detection of Microcystin-LR in Freshwater. Sensors 2016, 16 (11), 1901, DOI: 10.3390/s16111901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhao Z; Chen H; Ma L; Liu D; Wang Z A Label-Free Electrochemical Impedance Aptasensor for Cylindrospermopsin Detection Based on Thionine–graphene Nanocomposites. Analyst 2015, 140 (16), 5570–5577, DOI: 10.1039/C5AN00704F [DOI] [PubMed] [Google Scholar]
- 181.Hou L; Jiang L; Song Y; Ding Y; Zhang J; Wu X; Tang D Amperometric Aptasensor for Saxitoxin Using a Gold Electrode Modified with Carbon Nanotubes on a Self-Assembled Monolayer, and Methylene Blue as an Electrochemical Indicator Probe. Microchim. Acta 2016, 183 (6), 1971–1980, DOI: 10.1007/s00604-016-1836-1 [DOI] [Google Scholar]
- 182.Erdem A; Kerman K; Mer B; Ozkan D; Kara P; Oz O DNA Biosensor for Microcystis Spp. Sequence Detection by Using Methylene Blue and Ruthenium Complex as Electrochemical Hybridization Labels. Turk J. Chem 2002, 26, 851–862 [Google Scholar]
- 183.Yan F; Erdem A; Meric B; Kerman K; Ozsoz M; Sadik OA Electrochemical DNA Biosensor for the Detection of Specific Gene Related to Microcystis Species. Electrochem. Commun 2001, 3 (5), 224–228, DOI: 10.1016/S1388-2481(01)00149-7 [DOI] [Google Scholar]
- 184.Lan M; Chen C; Zhou Q; Teng Y; Zhao H; Niu X Voltammetric Detection of Microcystis Genus Specific-Sequence with Disposable Screen-Printed Electrode Modified with Gold Nanoparticles. Adv. Mater. Lett 2010, 1(3), 217–224, DOI: 10.5185/amlett.2010.7144 [DOI] [Google Scholar]
- 185.K’Owino IO; Mwilu SK; Sadik OA Metal-Enhanced Biosensor for Genetic Mismatch Detection. Anal. Biochem 2007, 369 (1), 8–17, DOI: 10.1016/j.ab.2007.06.046 [DOI] [PubMed] [Google Scholar]
- 186.Zhang K; Ma H; Yan P; Tong W; Huang X; Chen DDY. Electrochemical Detection of Microcystin-LR Based on Its Deleterious Effect on DNA. Talanta 2018, 185, 405–410, DOI: 10.1016/j.talanta.2018.03.051 [DOI] [PubMed] [Google Scholar]
- 187.Drummond TG; Hill MG; Barton JK Electrochemical DNA Sensors. Nat. Biotechnol 2003, 21 (10),1192–1199, DOI: 10.1038/nbt873 [DOI] [PubMed] [Google Scholar]
- 188.Sassolas A; Catanante G; Hayat A; Marty J-L Development of an Efficient Protein Phosphatase-Based Colorimetric Test for Okadaic Acid Detection. Anal. Chim. Acta 2011, 702 (2), 262–268, DOI: 10.1016/j.aca.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 189.Fontal OI; Vieytes MR; Baptista de Sousa JMV; Louzao MC; Botana LM A Fluorescent Microplate Assay for Microcystin-LR. Anal. Biochem 1999, 269 (2), 289–296, DOI: 10.1006/abio.1999.3099 [DOI] [PubMed] [Google Scholar]
- 190.Campàs M; Szydlowska D; Trojanowicz M; Marty J-L Towards the Protein Phosphatase-Based Biosensor for Microcystin Detection. Biosens. Bioelectron 2005, 20 (8), 1520–1530, DOI: 10.1016/j.bios.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 191.Campàs M; Szydłowska D; Trojanowicz M; Marty JL Enzyme Inhibition-Based Biosensor for the Electrochemical Detection of Microcystins in Natural Blooms of Cyanobacteria. Talanta 2007, 72 (1), 179–186, DOI: 10.1016/j.talanta.2006.10.012 [DOI] [PubMed] [Google Scholar]
- 192.Campàs M; Olteanu MG; Marty JL Enzymatic Recycling for Signal Amplification: Improving Microcystin Detection with Biosensors. Sens. Actuators, B 2008, 129 (1), 263–267, DOI: 10.1016/j.snb.2007.08.009 [DOI] [Google Scholar]
- 193.Lotierzo M; Abuknesha R; Davis F; Tothill IE A Membrane-Based ELISA Assay and Electrochemical Immunosensor for Microcystin-LR in Water Samples. Environ. Sci. Technol 2012, 46 (10), 5504–5510, DOI: 10.1021/es2041042 [DOI] [PubMed] [Google Scholar]
- 194.Reverté L; Garibo D; Flores C; Diogène J; Caixach J; Campàs M Magnetic Particle-Based Enzyme Assays and Immunoassays for Microcystins: From Colorimetric to Electrochemical Detection. Environ. Sci. Technol 2013, 47 (1), 471–478, DOI: 10.1021/es304234n [DOI] [PubMed] [Google Scholar]
- 195.Catanante G; Espin L; Marty JL Sensitive Biosensor Based on Recombinant PP1α for Microcystin Detection. Biosens. Bioelectron 2015, 67, 700–707, DOI: 10.1016/j.bios.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 196.Yu N; Ding J; Wang W; Wang X; Qin W Pulsed Galvanostatic Control of a Solid-Contact Ion-Selective Electrode for Potentiometric Biosensing of Microcystin-LR. Sens. Actuators, B 2016, 230, 785–790, DOI: 10.1016/j.snb.2016.02.121 [DOI] [Google Scholar]
- 197.Idil N; Mattiasson B Imprinting of Microorganisms for Biosensor Applications. Sensors 2017, 17 (4), 708, DOI: 10.3390/s17040708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chorus I; Bartram J, Eds.; Toxic Cyanobacteria in Water: A guide to their public health consequences, monitoring and management; World Health Organization; https://www.who.int/water_sanitation_health/resourcesquality/toxcyanbegin.pdf (accessed Apr 18, 2019). [Google Scholar]
- 199.Lebogang L; Mattiasson B; Hedström M Capacitive Sensing of Microcystin Variants of Microcystis Aeruginosa Using a Gold Immunoelectrode Modified with Antibodies, Gold Nanoparticles and Polytyramine.Microchim. Acta 2014, 181 (9–10), 1009–1017, DOI: 10.1007/s00604-014-1199-4 [DOI] [Google Scholar]
- 200.Wang L; Chen W; Xu D; Shim BS; Zhu Y; Sun F; Liu L; Peng C; Jin Z; Xu C Simple, Rapid, Sensitive, and Versatile SWNT-Paper Sensor for Environmental Toxin Detection Competitive with ELISA. Nano Lett. 2009, 9 (12), 4147–4152, DOI: 10.1021/nl902368r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang W; Han C; Jia B; Saint C; Nadagouda M; Falaras P; Sygellou L; Vogiazi V; Dionysiou DDA 3D Graphene-Based Biosensor as an Early Microcystin-LR Screening Tool in Sources of Drinking Water Supply.Electrochim. Acta 2017, 236, 319–327, DOI: 10.1016/j.electacta.2017.03.161 [DOI] [Google Scholar]
- 202.Mensing JP; Poochai C; Kerdpocha S; Sriprachuabwong C; Wisitsoraat A; Tuantranont A Advances in Research on 2D and 3D graphene-based supercapacitors. Adv. Nat. Sci.: Nanosci. Nanotechnol 2017, 8, 2–9, DOI: 10.1088/2043-6254/aa7214 [DOI] [Google Scholar]
- 203.Mishra S; Nguyen H; Adusei PK; Hsieh YY; Shanov V Plasma Enhanced Synthesis of N Doped Vertically Aligned Carbon Nanofibers on 3D Graphene. Surf. Interface Anal 2019, 51 (2), 290–297, DOI: 10.1002/sia.6604 [DOI] [Google Scholar]
- 204.Zhu C; Zeng Z; Li H; Li F; Fan C; Zhang H Single-Layer MoS2-Based Nanoprobes for Homogeneous Detection of Biomolecules. J. Am. Chem. Soc 2013, 135 (16), 5998–6001, DOI: 10.1021/ja4019572 [DOI] [PubMed] [Google Scholar]
- 205.Lv J; Zhao S; Wu S; Wang Z Upconversion Nanoparticles Grafted Molybdenum Disulfide Nanosheets Platform for Microcystin-LR Sensing. Biosens. Bioelectron 2017, 90, 203, DOI: 10.1016/j.bios.2016.09.110 [DOI] [PubMed] [Google Scholar]
- 206.Qin X; Wang Y; Song B; Wang X; Ma H; Yuan J Homogeneous Time-Resolved Fluoroimmunoassay of Microcystin-LR Using Layered WS2 Nanosheets as a Transducer. Methods Appl. Fluoresc 2017, 5 (2), 024007, DOI: 10.1088/2050-6120/aa6c00 [DOI] [PubMed] [Google Scholar]
- 207.Ertürk G; Mattiasson B Capacitive Biosensors and Molecularly Imprinted Electrodes. Sensors 2017, 17(2), 390, DOI: 10.3390/s17020390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen J; Gao P; Wang H; Han L; Zhang Y; Wang P; Jia N PPy/Cu2O Molecular Imprinting Composite Film Based Visible Light-Responsive Photoelectrochemical Sensor for Microcystin-LR. J. Mater. Chem. C 2018, 6(15), 3937–3944, DOI: 10.1039/C7TC05743A [DOI] [Google Scholar]
- 209.Liu M; Ding X; Yang Q; Wang Y; Zhao G; Yang N A PM Leveled Photoelectrochemical Sensor for Microcystin-LR Based on Surface Molecularly Imprinted TiO2@CNTs Nanostructure. J. Hazard. Mater 2017, 331,309–320, DOI: 10.1016/j.jhazmat.2017.02.031 [DOI] [PubMed] [Google Scholar]
- 210.Wang L; Kang TF; Lu LP; Zhang JG; Xue R; Cheng SY Microcystin-(Leucine-Arginine) Immunosensor Based on Iron(II, III) Magnetic Nanoparticles. Anal. Lett 2014, 47 (18), 2939–2949, DOI: 10.1080/00032719.2014.919506 [DOI] [Google Scholar]
- 211.Ge S; Liu W; Ge L; Yan M; Yan J; Huang J; Yu J In Situ Assembly of Porous Au-Paper Electrode and Functionalization of Magnetic Silica Nanoparticles with HRP via Click Chemistry for Microcystin-LR Immunoassay. Biosens. Bioelectron 2013, 49, 111–117, DOI: 10.1016/j.bios.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 212.Yang H; Rahman MT; Du D; Panat R; Lin Y 3-D Printed Adjustable Microelectrode Arrays for Electrochemical Sensing and Biosensing. Sens. Actuators, B 2016, 230, 600–606, DOI: 10.1016/j.snb.2016.02.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gillis WF; Lissandrello CA; Shen J; Pearre BW; Mertiri A; Deku F; Cogan S; Holinski BJ; Chew DJ; White AE Carbon Fiber on Polyimide Ultra-Microelectrodes. J. Neural Eng 2018, 15 (1), 016010, DOI: 10.1088/1741-2552/aa8c88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Puczkarski P; Swett JL; Mol JA Graphene Nanoelectrodes for Biomolecular Sensing. J. Mater. Res.2017, 32 (15), 3002–3010, DOI: 10.1557/jmr.2017.256 [DOI] [Google Scholar]
- 215.Sandison ME; Cooper JM Nanofabrication of Electrode Arrays by Electron-Beam and Nanoimprint Lithographies. Lab Chip 2006, 6 (8), 1020, DOI: 10.1039/b516598a [DOI] [PubMed] [Google Scholar]
- 216.Winston D; Manfrinato VR; Nicaise SM; Cheong LL; Duan H; Ferranti D; Marshman J; McVey S;Stern L; Notte J Neon Ion Beam Lithography (NIBL). Nano Lett. 2011, 11 (10), 4343–4347, DOI: 10.1021/nl202447n [DOI] [PubMed] [Google Scholar]
- 217.Akai T; Abe T; Shimomura T; Kato M; Ishibashi M; Heike S; Choi B-K; Hashizume T; Ito K Fabrication of Four-Probe Fine Electrodes Using Scanning-Probe Nanofabrication. Jpn. J. Appl. Phys 2003, 42, 4764–4766, DOI: 10.1143/JJAP.42.4764 [DOI] [Google Scholar]
- 218.Zhu X; Qiao Y; Zhang X; Zhang S; Yin X; Gu J; Chen Y; Zhu Z; Li M; Shao Y Fabrication of Metal Nanoelectrodes by Interfacial Reactions. Anal. Chem 2014, 86 (14), 7001–7008, DOI: 10.1021/ac501119z [DOI] [PubMed] [Google Scholar]
- 219.Wang L; Chen W; Xu D; Shim BS; Zhu Y; Sun F; Liu L; Peng C; Jin Z; Xu C Simple, Rapid, Sensitive, and Versatile SWNT–Paper Sensor for Environmental Toxin Detection Competitive with ELISA. Nano Lett. 2009, 9 (12), 4147–4152, DOI: 10.1021/nl902368r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sharma D; Lee J; Shin H An Electrochemical Immunosensor Based on a 3D Carbon System Consisting of a Suspended Mesh and Substrate-Bound Interdigitated Array Nanoelectrodes for Sensitive Cardiac Biomarker Detection. Biosens. Bioelectron 2018, 107, 10–16, DOI: 10.1016/j.bios.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 221.Narakathu BB; Atashbar MZ; Bejcek BE Improved Detection Limits of Toxic Biochemical Species Based on Impedance Measurements in Electrochemical Biosensors. Biosens. Bioelectron. 2010, 26, 923–928, DOI: 10.1016/j.bios.2010.06.051 [DOI] [PubMed] [Google Scholar]
- 222.Rana S; Page RH; Mcneil CJ Comparison of Planar and 3-D Interdigitated Electrodes as Electrochemical Impedance Biosensors. Electroanalysis 2011, 23 (10), 2485–2490, DOI: 10.1002/elan.201100353 [DOI] [Google Scholar]
- 223.Arya SK; Zhurauski P; Jolly P; Batistuti MR; Mulato M; Estrela P Capacitive Aptasensor Based on Interdigitated Electrode for Breast Cancer Detection in Undiluted Human Serum. Biosens. Bioelectron 2018, 102,106–112, DOI: 10.1016/j.bios.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 224.Ding S; Mosher C; Lee XY; Das SR; Cargill AA; Tang X; Chen B; McLamore ES; Gomes C;Hostetter JM Rapid and Label-Free Detection of Interferon Gamma via an Electrochemical Aptasensor Comprising a Ternary Surface Monolayer on a Gold Interdigitated Electrode Array. ACS Sensors 2017, 2 (2), 210–217, DOI: 10.1021/acssensors.6b00581 [DOI] [PubMed] [Google Scholar]
- 225.United States Environmental Protection Agency. Definition and Procedure for the Determination of the Method Detection Limit, Revision 2; https://www.epa.gov/sites/production/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf (accessed Apr 16, 2019).
- 226.Harris DC; Lucy CA Quality Assurance and Calibration Methods In Quantitative chemical analysis; W.H. Freeman & Company, 2016; pp 102–105. [Google Scholar]