With more than 1 billion people crossing international borders each year and dramatic increases in the speed of global travel,1 there is nowhere remote in the world, and no one from whom we are disconnected. Current trends in human migration illustrate this reality. Defined as persons changing their country of usual residence,2 most migrants make a choice to move, although for many, poverty and necessity are the major reasons behind that choice. However, the world is also seeing record levels of forced migration and displacement resulting from an unparalleled number of simultaneous, complex, and protracted crises involving armed conflicts, political upheavals, natural disasters, climate change, and human rights deprivation.
Indeed, by United Nations High Commissioner for Refugees (UNHCR) estimates, there were 68.5 million forcibly displaced people worldwide by the end of 2017. Of these, 25.4 million were refugees, the highest number ever. More than half were children younger than 18 years old.3 Refugees, as defined in the 1951 Geneva Convention, are persons outside their country of nationality who are unable or unwilling to return because of a well-founded fear of persecution based on race, religion, nationality, membership in a social group, or political opinion.4 Numbers are swelling in the context of wars and political unrest in countries such as Syria, Iraq, Afghanistan, the Democratic Republic of the Congo, and Somalia; and of the persecution facing ethnic and religious minorities in Burma and other countries.
Many refugees seek asylum in nearby countries. For example, in 2016, asylum countries with the highest number of refugees included Turkey, Jordan, the Palestinian Territories, Lebanon, and Pakistan.5 Fewer than 1% of all refugees become eligible for resettlement to a third country, when limited prospects exist for either returning home or remaining permanently in the country of asylum.6 These refugees are identified by UNHCR and referred as applicants to countries participating in UNHCR’s resettlement program, including the United States, which has hosted a Refugee Admissions Program (USRAP) since 1980.7
IMMIGRANTS AND REFUGEES BOUND TO THE UNITED STATES
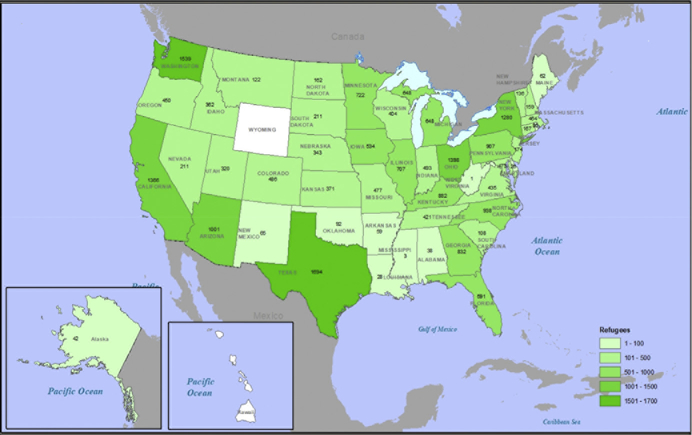
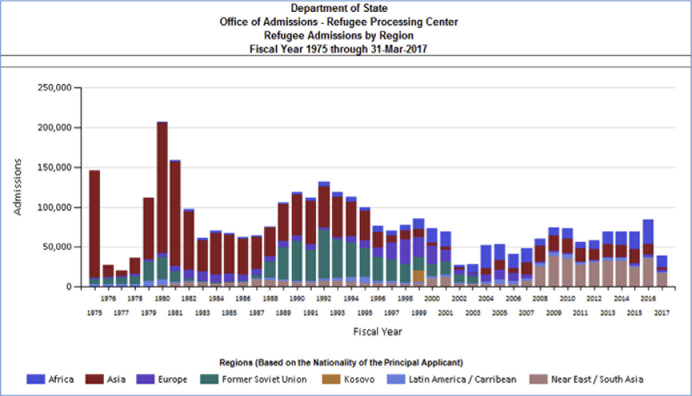
Historically, the United States has been the world’s top resettlement country.6 The peak year for refugee resettlement to the United States was 1980, when more than 200,000 refugees arrived, mainly from Cambodia and Vietnam. In federal fiscal year (FY) 2018, approximately 20,000 refugees resettled to the United States; Fig. 1 shows refugees resettled that year by receiving state, with populous states such as California, Texas, and New York among the leading receiving states. The top 5 countries of nationality for US-bound refugees in FY 2018 were the Democratic Republic of the Congo, Burma, Ukraine, Bhutan, and Eritrea,8 although resettlement numbers and origins may change annually (Fig. 2). Still, refugees constitute a tiny proportion of the international travelers arriving on our shores annually. For example, the largest annual number of refugees received in the past 2 decades was approximately 85,000, in FY 2016. This number constituted 0.05% of roughly 180 million US-bound international travelers that same year, including approximately 1 million immigrants and millions of nonimmigrant admissions, such as tourists, students, and persons on employment visas.9 The roughly 1.2 million immigrants obtaining lawful permanent residency (LPR) status during that year originated in more than 200 countries and territories; a plurality were born in Asia (approximately 39%) or North America (approximately 36%).8
Fig. 1.
Refugee arrivals by state, FY 2018. (Courtesy of DGMQ/CDC.)
Fig. 2.
Origins and numbers of US refugee admissions over time, 1975 to 2017. (Data from Refugee Processing Center. Admissions & Arrivals. Available at: http://www.wrapsnet.org/admissions-and-arrivals/. Accessed September 21, 2018.)
Health assessment represents an important component of the migration process for US-bound immigrants and refugees. These assessments support the health of migrating populations as well as US domestic health security, promote collaboration with state and international health partners, and strengthen our understanding of the health profiles of diverse arriving populations. We describe several related programs here.
MEDICAL EXAMINATION OF US-BOUND IMMIGRANTS AND REFUGEES
US-bound immigrants and refugees are required to undergo a medical examination overseas before traveling to the United States. The Centers for Disease Control and Prevention (CDC) is the federal agency with regulatory oversight of this examination, which is performed by panel physicians appointed by the US Department of State. Examinations follow technical instructions provided by CDC’s Division of Global Migration and Quarantine (DGMQ) and consist of a history and physical examination; laboratory testing for tuberculosis (TB), syphilis, and gonorrhea; assessment of vaccination status; identification of mental health conditions posing a danger to self or others; screening for substance abuse; and, for immigrants, vaccination.10
Medical examination findings and vaccination history are documented on official Department of State forms. For all refugees, and for immigrants with tuberculosis or other medical conditions of public health significance, these records are transmitted to DGMQ/CDC’s Electronic Disease Notification system, or EDN.
US-bound immigrants and refugees diagnosed with a condition of public health significance are eligible to come to the United States once the condition is treated, or, in special, rare circumstances, if they have obtained a waiver.
Medical Examination: The Tuberculosis Technical Instructions
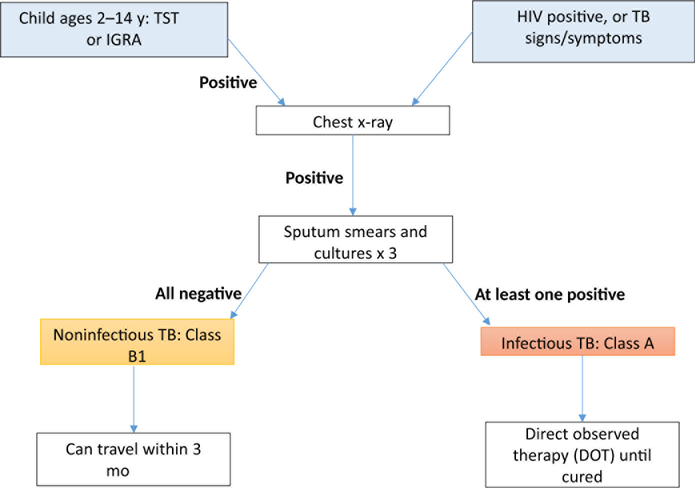
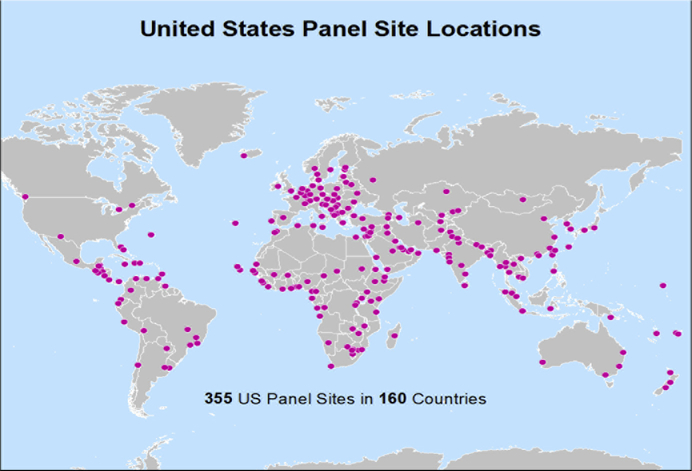
Foreign-born TB cases represent 65% of all cases of TB in the United States, a proportion that has risen over the past 2 decades.11 Therefore, TB detection and treatment are key components of the required medical evaluation of US-bound immigrants and refugees. Between 1999 and 2005, 2.7 million US-bound immigrants and 279,000 US-bound refugees were screened for TB overseas. Previous TB Technical Instructions (TBTI), developed in 1991, required only sputum smears for adult immigrants or refugees with abnormal chest radiographs. However, evidence suggested that sputum cultures could increase diagnostic yield. For example, a study conducted in 1998 to 1999 among US-bound immigrants in Vietnam demonstrated that smear-based screening was only 34% as sensitive as sputum culture, indicating that some active TB cases could be missed using sputum smear alone.12 Further, a 2009 report indicated that rates of smear-negative, radiograph-positive tuberculosis (eg, chest radiograph suggestive of active TB) were as high as 961 of 100,000.13 Hence, the TBTI were modified in 2007. Titled “Culture and Directly Observed Therapy TB Technical Instructions,” the 2007 instructions call for chest radiographs in all US-bound immigrants and refugees aged ≥15 years along with sputum smear plus culture with drug susceptibility testing in those with abnormal radiograph findings, and for anyone with human immunodeficiency virus (HIV) or symptoms suggestive of TB (Fig. 3). In addition, children aged 2 to 14 years examined in countries with TB incidence of 20 per 100,000 or higher receive a tuberculin skin test (TST, 2007–2018) or interferon gamma release assay (IGRA, 2009–present) test; if positive, a chest radiograph is required. Applicants diagnosed with active TB must complete treatment by direct observed therapy before they are cleared to travel to the United States. Implementation initially focused on high-volume countries with high TB rates, with the goal of global implementation among more than 600 Panel Physicians in 159 countries (Fig. 4).
Fig. 3.
2007 TBTI: culture and directly observed therapy. (Courtesy of DGMQ/CDC.)
Fig. 4.
Locations of US panel physicians. (Courtesy of DGMQ/CDC.)
Regional Panel Physician trainings, involving panel staff from 127 countries between 2008 and 2016 (and counting), have been key in disseminating the 2007 TBTI guidelines. In each country, DGMQ/CDC works to link Panel Physician programs with broader national TB control efforts. Data suggest that US investment in TB control programs within source countries yields greater returns on investment than improvement of screening algorithms alone,14 and TBTI implementation efforts have included laboratory capacity building, expanding many laboratories with the ability to perform TB cultures in implementing countries. Some laboratories also developed the capacity to test for resistance to second-line TB drugs, and hence to identify cases of multidrug-resistant (MDR) TB.15
A 2015 analysis found that after 2007 TBTI implementation, overseas TB detection rates in US-bound immigrants and refugees increased by >600 cases per year. In turn, the annual number of reported cases in foreign-born individuals within a year after arrival in the United States, which had previously been relatively constant, declined from 1511 cases in 2007 to 940 cases in 2012.16
Under the 2007 TBTI, children aged 2 to 14 years with positive TST or IGRA but negative chest radiograph or other TB evaluation overseas are classified as having “latent TB infection” (LTBI). These children are not treated overseas but are recommended to undergo reevaluation for TB after arrival.17 A 2010 assessment determined that only 70% of immigrant and refugee children classified with LTBI overseas (themselves constituting 12% of all children evaluated overseas) received the recommended postarrival evaluation; of those confirmed to have LTBI, only 30% completed the full course of therapy. Further, 71% of children diagnosed with LTBI overseas by TST had a negative IGRA after arrival in the United States.18 IGRA-based TB testing represents a new frontier for our program, and has replaced TST in the latest update to the TBTIs (2017).17 Use of IGRA may limit overdiagnosis of LTBI in a population screened overseas, and help improve rates of postarrival evaluation and completion of LTBI therapy.
BEYOND THE TECHNICAL INSTRUCTIONS: THE REFUGEE HEALTH INITIATIVE (1999– PRESENT)
US-bound refugees live in diverse settings, including camps, urban centers, and rural areas, in their home and asylum countries. Limitations in access to health services, imposed by war and displacement, affect many refugee populations. These factors, along with the crowded and challenging living circumstances often encountered in refugee camps or other temporary asylum settings, expose refugees to infectious disease outbreaks and other health conditions. DGMQ/CDC has partnered with camp-based and other international refugee health agencies to investigate and mitigate disease outbreaks and build laboratory capacity.
Whereas immigrants travel to the United States individually or with their families, refugees resettle in groups, on a predetermined schedule, with a 3-month to 6-month window between medical examination and departure. This window affords an opportunity to implement limited public health interventions aimed at improving refugee health and ensuring US health security. We collectively term these programs and interventions the “Refugee Health Initiative.”
The Vaccination Program for US-Bound Refugees (US Refugee Admissions Program Vaccination Program)
In the setting of displacement and upheaval, some refugees may be excluded from immunization programs in both their home countries and countries of asylum, leaving them susceptible to vaccine-preventable diseases. However, refugees are not required by statute to receive immunizations before traveling to the United States. The statute provides vaccination requirements for immigrants (LPRs); refugees do not become eligible to apply to be LPRs until 1 year after arrival to the United States and must meet vaccination requirements when they adjust their status to LPR. Vaccine-preventable disease transmission has led to serious consequences in US-bound refugees, especially children, including morbidity, disease importation, the birth of a child with severe disabilities due to congenital rubella syndrome,19 and the death of a child with measles. Further, US school entry is often delayed until receipt of Advisory Committee on Immunization Practices–recommended immunizations.
When vaccine-preventable disease outbreaks occurred near or among US-bound refugees, the strategy was initially reactive, and included specific, time-limited guidelines for immunization and delay of travel in response to each situation. However, this strategy delayed immunization until after outbreaks were detected, resulting in imported cases, did nothing to prevent future outbreaks, and was very costly. For example, international and domestic costs for just one of these outbreaks were estimated at $130,000, partly due to the high costs of travel cancellations and rebookings.20
Following these and other experiences, DGMQ/CDC developed a routine immunization schedule for US-bound refugees, in close consultation with CDC vaccine-preventable disease experts. The resulting Vaccination Program for US-bound Refugees is cofunded by DGMQ/CDC and the US Department of State, which funds the overseas medical examination of US-bound refugees; the International Organization for Migration (IOM) is the main implementing partner overseas. Starting in 2 countries, this vaccination program has expanded to nearly 60, more than three-quarters of program sites, with a goal of reaching all sites within the next year. The program is based on US standards, and includes specific guidelines for cold chain maintenance, monitoring, documentation, and adverse events reporting. The program’s immunization schedule now includes 11 vaccines, protecting against 14 diseases (Box 1).
Box 1. Components of immunization schedule for US-bound refugees, fiscal year 2018a.
Diphtheria, tetanus, and pertussis (DTP or DTaP) vaccine
Hepatitis B vaccine
H influenzae type b (Hib) vaccineb
Measles, mumps, and rubella (MMR) vaccinec
Oral polio vaccine (OPV)c or inactivated polio vaccine (IPV)
Pneumococcal vaccineb
Rotavirus vaccinec
Tetanus and diphtheria (Td) vaccine
Meningococcal ACWY vaccineb
Varicella vaccinec
Influenza vaccine (selected sites only)
a Up to 2 doses of each vaccine, depending on age and availability.
b Additional doses or separate recommendations may apply for patients with certain medical conditions.
c Vaccines contraindicated in immunocompromised or pregnant patients.
Courtesy of DGMQ/CDC.
Although immunization still is not required for resettlement, it is highly recommended to US-bound refugees, for the important health benefits and protection conferred to refugees and their receiving communities, and to prevent travel delays. Most US-bound refugees have opted in, with more than 260,000 benefiting from the Vaccination Program since 2012. In FY 2018, approximately 90% of arriving refugees received at least 1 age-appropriate dose of measles vaccine, according to DGMQ/CDC EDN data. Further, whereas in prior years many vaccine-preventable disease outbreaks (measles, mumps, polio, varicella, influenza, meningococcemia, and others) resulted in morbidity and costly travel delays, since FY 2016, the year with the largest number of resettling refugees since 1995, there have been no group travel delays associated with vaccine-preventable diseases, as of the time of writing. Net cost savings related to the USRAP Vaccination Program have been estimated at $225 per person.21
Presumptive Treatment for Parasitic Infections
Displacement combined with crowded, difficult living conditions also leaves refugees exposed to parasitic diseases and other neglected tropical diseases. For example, a 1997 assessment of US-bound Barawan Somali refugees showed that 7% had malaria and 38% had intestinal parasites22; in a 2004 assessment of resettled Lost Boys and Girls from Sudan, 44% were Schistosoma-seropositive and 46% Strongy- loides-seropositive.23 Based on this and other evidence, DGMQ/CDC developed a presumptive parasite treatment schedule for US-bound refugees, including 2 to 4 drugs targeting intestinal helminths, Strongyloides stercoralis, Schistosoma spp, and Plasmodium falciparum, at overseas departure based on country of origin and examination (Table 1).
Table 1.
Centers for Disease Control and Prevention recommendations for overseas presumptive parasite treatment in US-bound refugees
| Region | Coartem (Malaria) | Praziquantel (Schistosomiasis) | Albendazole (Soil-Transmitted Helminths) | Ivermectin (Strongyloides) |
|---|---|---|---|---|
| Africa, non-Loa Loa areas | ✓ | ✓ | ✓ | Burundi, Ethiopia, Kenya, Rwanda, Uganda, Tanzania |
| Africa, Loa Loa areas | ✓ | ✓ | ✓ | Not recommended |
| Asia | Not recommended | Not recommended | ✓ | Nepal, Malaysia, Thailand |
| Middle East | Not recommended | Not recommended | ✓ | Jordan, Iraq, Egypt |
| Latin America | Not recommended | Not recommended | ✓ | Future consideration |
Courtesy of DGMQ/CDC.
This mass drug treatment24 initiative has significantly reduced the burden of parasitic diseases in US-bound refugees. For example, a 1999 evaluation comparing refugees arriving in Minnesota before and after the presumptive treatment intervention (n = 27,000) showed a significant decline in stool helminth prevalence, from 22.5% to 7.5%, after a single dose of albendazole was instituted just before overseas departure.25 A longitudinal evaluation of US-bound refugees conducted between 2012 and 2015 showed similar declines in intestinal helminths by stool polymerase chain reaction (PCR), and, for the first time, demonstrated declines in Strongyloides prevalence both by stool PCR and serologic assays after presumptive treatment with ivermectin.26 Finally, an evaluation conducted in Minnesota showed essential elimination of malaria occurring after resettlement in arriving West African refugees after a single predeparture dose of sulfadoxine-pyrimethamine. The medication has since been changed to Coartem (artemether/lumefantrine) because of regional resistance.27
Predeparture mass drug administration programs for parasitic infections confer both health and cost benefits. A 2016 analysis estimated that although both approaches would reduce parasite burden at similar rates, it is approximately sixfold less expensive to treat presumptively before departure than to screen and treat refugees after arrival.28
Fitness to Fly: Predeparture Medical Screening
Some refugees may have other medical conditions that do not pose a public health risk, but may affect their health during travel, and must be managed before or during travel or immediately after arrival. Conditions resulting in severe illness or death in US-bound refugees during transit have included congenital cardiac disease, sickle cell disease, and malnutrition, among others.
DGMQ/CDC collaborates closely with partners such as IOM to develop and implement screening protocols to identify, manage, and stabilize patients with these and other chronic medical conditions before departure, with the goal of improving travel fitness. These protocols ensure that necessary referrals are completed and medications are refilled, that patients in need of specific interventions before travel (eg, blood transfusions for patients with hemoglobinopathies) receive them, and that the medical staff and equipment needed to facilitate a safe and comfortable flight are secured.
Site-Specific Clinical Protocols
Some clinical standard operating procedures were developed with specific populations in mind. For example, in 2014 IOM and states notified DGMQ/CDC of cases of splenomegaly, affecting approximately 15% of refugees resettling from Uganda. Contributing factors may include malaria, schistosomiasis, malnutrition, hepatitis, or hematologic conditions.29 Because appropriate pretravel management may reduce the risk of splenic rupture and other complications, evaluation and management protocols were developed, detailing important history and physical examination items, workup considerations (eg, ultrasound and testing for malaria, hepatitis, sickle cell, and anemia), and travel preparation measures.
GUIDELINES FOR THE DOMESTIC MEDICAL EXAMINATION: PROMOTING REFUGEE HEALTH AFTER ARRIVAL
In addition to the overseas activities described, DGMQ/CDC recommends a more comprehensive medical evaluation after arrival in the United States. Facilitated by state public health departments, state Refugee Health Coordinators, and other partners, this examination is not required for the resettlement process but is intended to identify health concerns and minimize morbidity. It is typically conducted 1 to 3 months after arrival. DGMQ/CDC developed evidence-based guidelines and checklists to help receiving states perform this evaluation. Items covered include the history and physical examination; screening for hepatitis and HIV infection; domestic immunization guidelines; and guidance for assessment of nutritional status and testing for elevated blood lead levels, sexually transmitted diseases (STDs), TB, malaria, and intestinal parasites.30 Immunization catch-up is typically initiated during this examination, although not all sites are able to follow patients longitudinally to completion of the full immunization series.
To further assist state health partners in identifying common health conditions in diverse US-bound populations, DGMQ/CDC has compiled health profiles, describing population background, routes of movement and asylum, and priority health conditions for the largest arriving refugee groups. These profiles are updated over time to reflect newer arriving populations.31
SUMMARY
Forced displacement and migration have increased to record levels in today’s geopolitical environment; hence, ensuring both the health of migrating populations and the health security of receiving countries is of critical importance. Based on 22 years of experience in developing and monitoring a health assessment framework for US-bound immigrants and refugees, DGMQ/CDC has identified successful strategies to improve health during planned migrations. These overseas screening, treatment, and vaccination initiatives have reduced US TB rates; decreased transmission and importation of vaccine-preventable diseases; prevented morbidity from parasitic diseases; and saved domestic health costs. Beyond their importance in the planned migration setting, these initiatives may offer a valuable template that can be adapted during unplanned migrations. For example, efforts to anticipate the flow of asylum seekers over the full course of their journey, from origin to destination, could allow for preventive and curative interventions that would improve health security. Such public health interventions can be perceived as triple-wins: good for migrants, good for sending communities, and good for receiving communities.
KEY POINTS.
With global forced displacement and migration at record levels, it is critical to ensure both the health of migrating populations and the health security of receiving countries.
Based on 22 years of experience in development and monitoring of a health assessment framework for US-bound immigrants and refugees, the Centers for Disease Control and Prevention’s Division of Global Migration and Quarantine has identified successful strategies to improve health during planned migrations, including overseas screening, treatment, and vaccination programs.
These strategies have reduced US tuberculosis rates; decreased transmission and importation of vaccine-preventable diseases; prevented morbidity from parasitic diseases; and saved domestic health costs.
This approach to screening, treatment, and prevention represents one important health model in the planned migration setting.
ACKNOWLEDGMENTS
The authors thank Zanju Wang and Courtney Chappelle of CDC’s Immigrant, Refugee, and Migrant Health Branch, Division of Global Migration and Quarantine, for their contributions to data mapping.
Footnotes
Disclosure Statement: Drs T. Mitchell, M. Weinberg, D.L. Posey, and M. Cetron have no financial relationships to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
REFERENCES
- 1.Murphy FA, Nathanson N. The emergence of new virus diseases: an overview. Sem Virol 1994;5:87–102. [Google Scholar]
- 2.Nations United. Refugees and migrants: definitions Available at: https://refugeesmigrants.un.org/definitions. Accessed September 21, 2018.
- 3.UNHCR. Figures at a glance Available at: http://www.unhcr.org/figures-at-a-glance.html. Accessed September 21, 2018.
- 4.US Department of Homeland Security. Refugees and asylees 2018. Available at: https://www.dhs.gov/immigration-statistics/refugees-asylees. Accessed September 21, 2018.
- 5.The World Bank. Refugee population by country or territory of asylum In: Data. 2018. Available at: https://data.worldbank.org/indicator/SM.POP.REFG?year_high_desc=true. Accessed September 21, 2018.
- 6.UNHCR. Resettlement 2018. Available at: http://www.unhcr.org/resettlement.html. Accessed September 21, 2018.
- 7.US Department of State US Refugee Admissions Program 2018. Available at: https://www.state.gov/j/prm/ra/admissions/index.htm. Accessed September 21, 2018.
- 8.Refugee Processing Center. Admissions & arrivals Available at: http://www.wrapsnet.org/admissions-and-arrivals/. Accessed September 21, 2018.
- 9.US Department of Homeland Security. Immigration statistics Available at: https://www.dhs.gov/immigration-statistics. Accessed September 21, 2018.
- 10.Centers for Disease Control and Prevention. Medical examination of immigrants and refugees Available at: https://www.cdc.gov/immigrantrefugeehealth/exams/medical-examination.html. Accessed September 21, 2018.
- 11.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, . Tuberculosis, data and statistics Available at: https://www.cdc.gov/tb/statistics/reports/2016/default.htm. Accessed September 21, 2018.
- 12.Maloney S, Fielding K, Laserson K, et al. Assessing the performance of overseas tuberculosis screening programs. Arch Intern Med 2006;166:234–40. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Weinberg MS, Ortega LS, et al. Overseas screening for tuberculosis in US-bound immigrants and refugees. N Engl J Med 2009;360(23):2406–15. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzman K, Oxlade O, Barr G, et al. Domestic returns from investment in the control of tuberculosis in other countries. N Engl J Med 2005;353(10):1008–20. [DOI] [PubMed] [Google Scholar]
- 15.Douglas P, Posey DL, Zenner D, et al. Capacity strengthening through premigration tuberculosis screening programmes: IRHWG experiences. Int J Tuberc Lung Dis 2017;21(7):737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Posey D, Cetron M, et al. Effect of a culture-based screening algorithm on tuberculosis incidence in immigrants and refugees bound for the United States. Ann Intern Med 2015;162(6):420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Tuberculosis technical instructions for panel physicians Available at: https://www.cdc.gov/immigrantrefugeehealth/exams/ti/panel/tuberculosis-panel-technical-instructions.html. Accessed September 21 and November 19, 2018.
- 18.Taylor EM, Painter J, Posey D, et al. Latent tuberculosis infection among immigrant and refugee children in the United States: 2010. J Immigr Minor Health 2016;18(5):966–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Brief report: Imported case of congenital rubella syndrome–New Hampshire, 2005. MMWR Morb Mortal Wkly Rep 2005;54(45):1160–1. [PubMed] [Google Scholar]
- 20.Coleman MS, Burke HM, Welstead BL, et al. Cost analysis of measles in refugees arriving at Los Angeles International Airport from Malaysia. Hum Vaccin Immun-other 2017;13(5):1084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joo H, Maskery B, Joo H, et al. A comparative cost analysis of the Vaccination Program for US-bound Refugees. Vaccine 2018;36(20):2896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Domestic intestinal parasite guidelines Available at: https://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html. Accessed September 21, 2018.
- 23.Posey DL, Blackburn BG, Weinberg M, et al. High prevalence and presumptive treatment of schistosomiasis and strongyloidiasis among African refugees. Clin Infect Dis 2007;45(10):1310–5. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Preventive therapy in human helminthiasis Geneva: (United Kingdom): World Health Organization; 2006. [Google Scholar]
- 25.Swanson S, Phares CR, Mamo B, et al. Albendazole therapy and enteric parasites in United States-bound refugees. N Engl J Med 2012;366(16):1498–507. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell T, Lee D, Weinberg M, et al. Impact of enhanced health interventions for United States-bound refugees: evaluating best practices in migration health. Am J Trop Med Hyg 2018;98(3):920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collinet-Adler S, Stauffer WM, Boulware DR, et al. Financial implications of refugee malaria: the impact of pre-departure presumptive treatment with antimalarial drugs. Am J Trop Med Hyg 2007;77(3):458–63. [PMC free article] [PubMed] [Google Scholar]
- 28.Maskery B, Coleman MS, Weinberg M, et al. Economic analysis of the impact of overseas and domestic treatment and screening options for intestinal helminth infection among US-Bound Refugees from Asia. PLoS Negl Trop Dis 2016; 10(8):e0004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goers M, Ope M, Samuels A, et al. Notes from the field: splenomegaly of unknown etiology in Congolese refugees applying for resettlement to the United States—Uganda, 2015. MMWR Morb Mortal Wkly Rep 2016;65(35):943–4. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Guidelines for the US domestic medical examination for newly arriving refugees Available at: https://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-guidelines.html. Accessed September 21, 2018..
- 31.Centers for Disease Control and Prevention. Refugee health profiles Available at: https://www.cdc.gov/immigrantrefugeehealth/profiles/index.html. Accessed September 21, 2018.