Abstract
Objective:
In this pilot study we investigated the feasibility of a home-based, remotely guided exercise intervention for patients with gliomas.
Design:
Pilot randomised controlled trial with randomisation (2:1) to exercise or control group.
Subjects:
Patients with stable grade II and III gliomas.
Intervention:
The six-month intervention included 3 home-based exercise sessions per week at 60–85% of maximum heart rate. Participants wore heart rate monitors connected to an online platform to record activities, that were monitored weekly by the physiotherapist.
Main measures:
Accrual, attrition, adherence, safety, satisfaction, patient-reported physical activity, VO2peak (by maximal cardiopulmonary exercise testing) and BMI at baseline and at six months follow-up.
Results:
Thirty-four of 136 eligible patients (25%) were randomised to exercise training (N=23) or the control group (N=11), of whom 19 and 9, respectively, underwent follow-up. Mean adherence to prescribed sessions was 79%. Patients’ experiences were positive. There were no adverse events. Compared to the control group, the exercise group showed larger improvements in absolute VO2peak (+158.9 ml/min; 95%CI:−44.8 to 362.5) and BMI (−0.3 kg/m2; 95%CI: −0.9 to 0.2). The median increase in physical activity was 1,489 MET.min higher in the exercise group. The most reported reasons for non-participation were lack of motivation or time.
Conclusions:
This innovative and intensive home-based exercise intervention was feasible in a small subset of patients with stable gliomas who were interested in exercising. The observed effects suggest that the program may improve cardiorespiratory fitness. These results support the need for larger scale trials of exercise interventions in brain tumour patients.
Keywords: glioma, brain tumour, exercise, physical training, physical fitness
Introduction
After treatment with surgery, radiotherapy and/or chemotherapy, patients with gliomas with favorable prognosis may live free from severe neurological symptoms for years until the disease progresses. During this period of time, many patients may suffer from a wide range of physical, psychological/emotional and cognitive symptoms [1]. Few efforts have been undertaken to address these problems, despite the relatively young age and favorable prognosis of this clinical population.
Recently, Cormie et al. concluded, based on the evidence for beneficial effects of exercise in managing physical, psychological and cognitive symptoms in other populations, that exercise may be a promising intervention to aid in the management of the multitude of brain cancer symptoms and treatment side-effects [2]. However, there has been very limited research on the feasibility and effectiveness of exercise interventions in patients with brain tumours. Feasibility may be a particular concern in this population, as neuro-oncological symptoms may limit patients’ willingness and ability to participate in an exercise intervention (study).
To date, two small, uncontrolled (pilot) exercise intervention studies (respectively n =8, and 14 at follow-up) have been conducted in this population, the results of which suggest that an exercise program is both feasible and safe [2, 3]. In a study on exercise preferences of 106 patients with brain tumours (50% glioma), the majority of the respondents were interested in physical exercise and felt that they would be able to exercise [4]. Glioma patients also reported a strong preference for home-based exercise programs [4, 5]. Home-based interventions may be particularly important for brain cancer patients, as neurological symptoms such as epilepsy can restrict patients’ mobility (i.e., the ability to drive or use public transportation) [4].
We recently developed and pilot-tested a novel home-based aerobic exercise intervention for patients with stable, lower-grade glioma. The ultimate aim of the program was to maintain or improve cognitive function through improvement of aerobic fitness [6–8].
The primary purpose of the current paper is to present a detailed evaluation of the 6-month exercise intervention in terms of accrual, attrition, adherence, safety and patient satisfaction in a research setting. These results may help to design future studies on the benefits of exercise in brain tumour patients. In addition, we describe the direct effects of the program on cardiorespiratory fitness (VO2peak) and self-reported physical activity, as we consider these intermediate endpoints as a measure of feasibility of an exercise program designed to improve cognitive function. Detailed findings with respect to cognitive performance and patient-reported outcomes will be reported in a subsequent paper.
Methods
Study design
Ethical approval was obtained from the medical ethics committee (METC) Brabant, Tilburg, The Netherlands (N44024.008.13). The trial was registered with www.clinicaltrials.gov: NCT02303938. Enrollment started in September 2013 and ended in December 2014.
In this pilot, randomised controlled study, patients were allocated to a 6-months exercise intervention and a waiting-list control group.
Participants
We recruited patients from three Dutch hospitals: St. Elisabeth Hospital Tilburg, Haaglanden Medical Center The Hague, and Erasmus Medical Center Rotterdam. Eligibility criteria were: histologically proven or presumed (on the basis of clinical and MRI data) diffuse, low-grade (i.e., WHO grade II) glioma, or anaplastic glioma (WHO grade III); clinically stable for a minimum of 6 months prior to study entry as determined by MRI; current self-reported inactivity or only a moderate level of physical activity (i.e., <20 minutes of vigorous exercise on at least 3 days of the week) as assessed with the Physician-based Assessment and Counseling for Exercise (PACE) [9]; access to the internet; basic fluency in the Dutch language; interest in undergoing the physical exercise program under investigation; and, finally, a VO2peak, as assessed with maximal cardiopulmonary exercise test, within the range of sedentary or recreationally active reference groups [10], allowing room for further improvement of fitness.
Exclusion criteria were: anti-tumour treatment (i.e., surgery, radiotherapy, chemotherapy, corticosteroids) within 6 months prior to study entry; use of beta-blockers [11]; psychiatric or severe cognitive problems that would preclude program participation; serious orthopedic conditions, motor deficits, cardiovascular, cardiopulmonary or neurological condition; or contra-indications for exercise without face-to face supervision as assessed with the Physical Activity Readiness Questionnaire (PAR-Q) [12], or as judged by the sports physician based on the cardiopulmonary exercise test; and no room for cognitive improvement, as assessed with the neuropsychological testing.
Recruitment procedure
Potential participants underwent three screening phases. In the first screening phase, we identified potentially eligible patients via pathology databases or direct referral from the participating hospitals. Medically eligible patients received a study information letter from their physician and a reply card on which they could indicate whether they gave permission to be approached by phone. Interested patients were in this second phase called by a member of the research team who explained the study purpose and procedures, and screened for initial eligibility. In the third phase, all individual eligible patients provided informed consent, underwent neuropsychological testing, and completed self-report questionnaires on cognitive symptoms, fatigue, sleep, mood and quality life at home. Subsequently, they were invited to undergo a maximal cardiopulmonary exercise test in a sports medical center.
Eligible patients were randomly allocated in a 2:1 ratio to an exercise group or a waiting-list control group. A minimization procedure [13] was used to ensure that the two groups were balanced on age (<40, 40–50, >50), education (lower versus higher), WHO tumour grade (II versus III), disease duration (<5 versus ≥ 5 years), relative VO2 classification according to reference groups (recreational physical activity vs. sedentary [10]), and performance on the letter digit substitution task [14] (≤43 versus >43). Allocation concealment was ensured by using an online computer software program [15]. All patients were informed about their allocation by phone. Besides the sports physicians who administered the exercise tests, the other assessors, nor the patients, could be blinded to group allocation.
The decision to halt participation in case of progressive disease during the course of the study depended on further treatment and/or was left up to the individual patient and his/her treating physician.
Exercise group
The intervention comprised three home-based aerobic training sessions per week, for a duration of six months. At the start of the intervention, a physiotherapist visited participants at home. Patients received an individualized exercise prescription, based on their level of aerobic fitness, to exercise at 60–85% of their maximum heart rate (see Appendix for a more elaborate description and build-up of the activities). They could choose one or more central activities, as long as these could meet the prescribed exercise intensity. The physiotherapist instructed patients in how to use a sports watch with a heart rate monitor [16], and how to upload training data at least once a week. During the activities, this watch provided immediate feedback about heart rate and, if applicable, speed and distance. Patients kept a log of their training experiences. The physiotherapist monitored the training data on the platform on a weekly basis and provided additional personal feedback by e-mail. In case of motivational problems, fatigue, injury or technical problems, more frequent e-mail/phone contact was allowed. After the final exercise test, participants were called a last time to discuss the program and exercise test results, and to discuss continuation of physical exercise after the study period.
Waiting list control group
Patients in the waiting list control group were advised to maintain an active lifestyle, in accordance with Dutch public health guidelines [17], which were described in two motivational brochures. Patients in this group also received bimonthly phone calls from the research-assistant during which general questions about their health were asked. These calls were intended to provide some control for potential effects due to the attention that was given to the exercise group. After all assessments had been completed, participants in the control group were offered a training watch and a general exercise prescription.
Outcome measures
Baseline assessments of physical fitness, patient-reported outcomes and cognitive function were conducted before randomisation (T0) and follow-up assessments at 6 months, after patients in the exercise group had completed their training (T1).
Sociodemographic and clinical information
Patients’ age, sex and level of education were obtained via interview at baseline. Clinical information, including date of diagnosis and tumour characteristics, such as site, and histology, and anti-cancer and anti-epileptic treatment was abstracted from the medical records.
Feasibility parameters
Accrual was defined as the percentage of eligible patients who entered into the study. Attrition was defined as the percentage of randomised patients who dropped out of the study. Reasons for non-participation and discontinuation were recorded.
Individual exercise data on type of sport, duration of intervention, attended training-days, mean session duration (minutes) and mean intensity (% of maximum heart rate) were extracted from the patients’ accounts on the online platform. Adherence was then calculated as the percentage of the physical exercise sessions completed out of prescribed sessions in the period in which a patient participated in the program; ≥75% was considered sufficient. Average intensity was indicated by the average heart rate of all training sessions as a percentage of the maximum heart rate as measured during the first exercise test; a percentage of 60–85% was considered sufficient.
Finally, two physiotherapists (CK and MS) independently rated overall exercise performance for each participant as A (excellent/good), B (adequate) or C (inadequate), based on observed adherence, session duration and intensity. Disagreements were resolved by consensus. Injuries or side-effects attributable to the exercise intervention were reported in the physiotherapists’ logbook.
Patient satisfaction
Patients’ satisfaction with the program was assessed in the exercise group with a post-training, study-specific questionnaire. Qualitative data from patients’ e-mails to the physiotherapist were collected.
Self-reported physical activity
Self-reported level of physical activity was assessed by calculating MET.minutes/week acquired during walking, moderate or vigorous physical activity, as self-reported on the International Physical Activity Questionnaire (IPAQ) [18,19].
Physical outcomes
Physical fitness was assessed at baseline (T0) and after 6 months (T1) in both study groups with a maximal cardiopulmonary exercise test on a cycle ergometer with electrocardiogram and breathing gas analysis. The purpose of the test in this study was three-fold: 1) safety testing and screening, 2) baseline assessment for use in individualizing exercise prescription, and 3) (outcome) assessment of VO2peak.
The exercise test was performed in one of four participating Sports Medical Centers affiliated with the patient’s hospital. These tests were supervised by different sports physicians, who followed a protocol based on the American College of Sports Medicine (ACSM) guidelines [20], and who were blinded to allocation. Raw output data of the exercise test were used to extract absolute VO2peak data and calculate relative VO2peak (absolute VO2peak divided by body weight).
Neuropsychological assessments
Cognitive functioning was assessed at the patients’ home by a battery of neuropsychological tests of attention, memory, and executive function [21]. Patients were also given several questionnaires on self-reported cognitive symptoms, fatigue, sleep, mood and quality life to be completed and returned by mail. For the purpose of the current manuscript only the feasibility of administration of these assessments in this pilot study was evaluated.
Statistical methods
Based on historical patient census data from the three participating centres, we expected to be able to include 60 patients in the study. Due to the lack of preliminary data on the effect of exercise on cognitive function in glioma patients, there was no meaningful way to perform sample size calculations for this pilot feasibility study.
Baseline demographics, clinical and physical data were compared between the study groups, and between dropouts and patients who completed follow-up. Statistical analyses were conducted for group outcomes on absolute and relative VO2peak, BMI, weight and patient-reported physical activity. Absolute VO2peak was used as the primary outcome for analysis of changes in physical fitness. No a priori level of statistical significance was set, as the objective of the study was not to test hypotheses, but to estimate potential effect sizes. Ninety-five percent confidence intervals were calculated, along with two-sided p-values, for all comparisons.
First, we performed an intention-to-treat analysis to estimate the within-group changes in physical fitness, BMI, body weight and physical activity using paired-samples T-tests. If test assumptions were violated, a Wilcoxon signed rank test was used, and a 95% confidence interval for the median change was estimated via 1,000 bootstraps.
In addition, to estimate the change specifically attributable to the intervention, we used linear regression analyses to compare the outcomes of both groups noted above at 6 months, controlling for baseline scores. Adjusted mean between-group differences are reported with a 95% confidence interval. In case of violated assumptions for these tests, a Mann Whitney U test (inevitably without correction for baseline score) was used, and presented with bootstrap 95% confidence intervals for the difference between the group medians. Cohen’s d effect sizes were calculated for between-group effects, for which 0.2 is considered a “small” effect, 0.5 a “medium” effect and 0.8 and larger a “large” effect [22].
SPSS version 22.0 was used for all statistical analyses [23], except the calculation of 95% confidence intervals for the difference between group medians, which was done in R 3.3.1 [24].
Results
Accrual and attrition
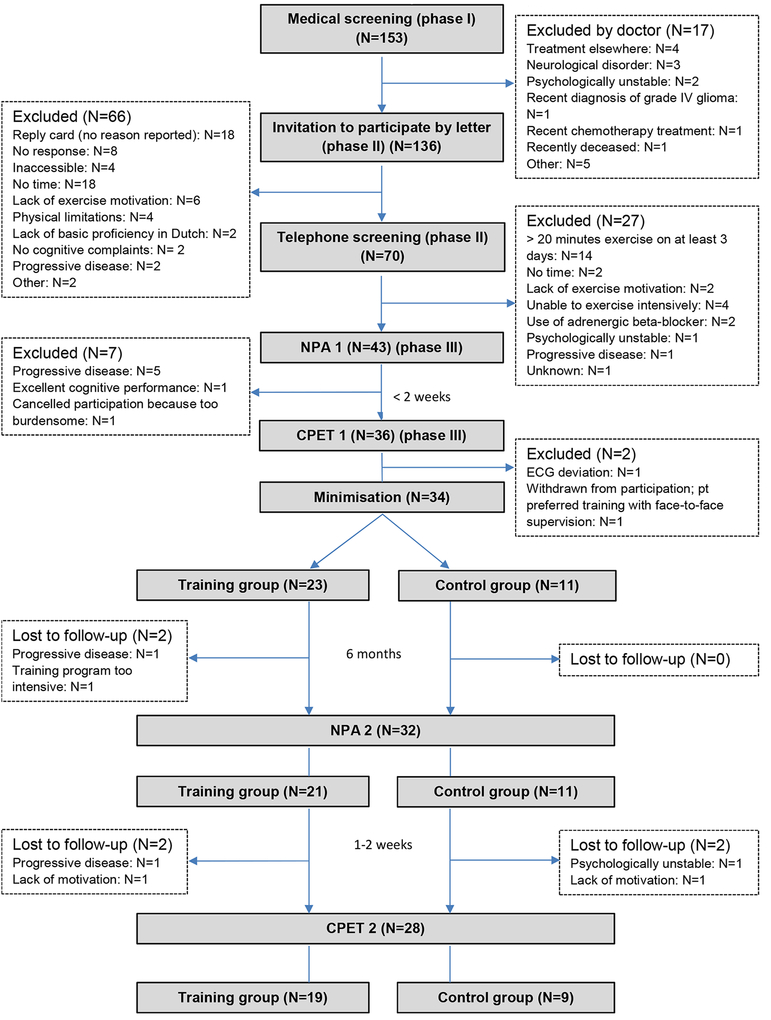
After the three phases of screening, 34 of 136 invited patients (25%) were recruited and allocated to the intervention (N=23) or the control (N=11) group. Due to personnel and time (i.e., financial) constraints, we could not prolong the recruitment phase of the study, or include an additional participating centre, to recruit the projected 60 participants. Figure 1 displays detailed information on recruitment and retention. Thirty (22%) of the patients who did not participate did not provide a reason for declining, did not respond to the invitation or could not be reached by phone. Of the 62 patients for whom a reason for non-participation was known, the most reported reasons were lack of motivation or time to exercise. Ten patients (16%) reported being physically unable to undergo intensive training or assessments. Of the 70 patients who were screened by telephone and were interested in participating, fourteen (20%) already met the criteria for a physically active lifestyle based on the PACE questionnaire. One patient withdrew after the baseline exercise test, but before randomisation, when she understood that the program did not include face-to-face training with a physiotherapist.
Figure 1. Flow of participants through the trial (accrual and attrition).
NPA= neuropsychological assessment; CPET= cardiorespiratory exercise testing; ECG: electrocardiogram.
At baseline, 29 patients (85%) had a VO2peak comparable to sedentary age-matched healthy controls, and 5 (15%) comparable to people involved in recreational sports [10]. Except for a higher proportion of grade II tumours in the exercise group, the groups were comparable at baseline with respect to sociodemographic, clinical or physical characteristics (Table 1).
Table 1.
Baseline sociodemographic, clinical and physical characteristics
| Characteristic | Intervention group (N=23) | Control group (N=11) |
|---|---|---|
| Age, years | ||
| mean (SD) | 48.0 (9.4) | 48.0 (11.9) |
| Female, N (%) | 13 (56%) | 6 (55%) |
| Education, N (%) | ||
| low | 2 (9%) | 0 (0%) |
| middle | 10 (43%) | 6 (55%) |
| high | 11 (48%) | 5 (45%) |
| WHO tumour grade, N (%) | ||
| grade II | 16 (70%) | 6 (55%) |
| grade III | 7 (30%) | 5 (45%) |
| Tumour histology, N (%) | ||
| astrocytoma | 8 (35%) | 5 (46%) |
| oligodendroglioma | 12 (52%) | 5 (46%) |
| oligoastrocytoma | 3 (13%) | 1 (8%) |
| Disease duration, years | ||
| mean (SD) | 7.6 (4.9) | 8.5 (8.6) |
| Left hemisphere, N (%) | 10 (43%) | 4 (36%) |
| Surgery, N (%) | ||
| no | 0 | 1 (9%) |
| biopsy | 4 (17%) | 1 (9%) |
| resection | 19 ( 83%) | 9 (82%) |
| Chemotherapy, N (%) | 9 (39%) | 4 (36%) |
| Radiotherapy, N (%) | 14 (61%) | 5 (45%) |
| Epilepsy | 17 (73.9%) | 8 (72.7%) |
| Anti-epileptic drugs | 14 (60.9%) | 6 (54.5%) |
| VO2 peak absolute, ml/min | ||
| mean (SD) | 2,252.0 (728.0) | 2,181.5 (741.0) |
| VO2 peak relative, ml/kg/min | ||
| mean (SD) | 26.5 (7.4) | 25.9 (4.8) |
| VO2 peak classification, N (%) [10] | ||
| recreational (VS sedentary) | 4 (17%) | 1 (9%) |
| Self-reported physical activity, MET*min/week | ||
| median (25th; 75th percentile) | 4399.5 (1468.0; 8640.0) | 4583.5 (1838.0; 13,457.0) |
| Body weight, kg | ||
| mean (SD) | 84.8(11.2) | 83.7 (22.2) |
| BMI, kg/m2 | ||
| mean (SD) | 27.3 (3.0) | 27.3 (5.5) |
| Letter digit substitution task[14], number correct | ||
| mean (SD) | 43.9 (11.5) | 43.4 (10.0) |
| SF-36 Physical Component Scale [43, 44], scale 0–100 | ||
| mean (SD) | 44.7 (8.2) | 47.0 (7.8) |
No differences were observed in baseline sociodemographic, clinical and physical characteristics between drop-outs (N=6) and patients who completed follow-up measurement (N=28), except that patients with grade III gliomas were more likely to dropout. One patient who learned during the intervention that he had progressive disease was able to undergo the follow-up exercise test.
Exercise program data: exercise preferences and adherence
A detailed overview of training variables per case in the exercise group is presented in the Online Table. Most patients (n=15 of 23) chose a combination of activities. Other patients chose a single type of exercise: indoor cycling (n=3), outdoor cycling (n=3), running/walking (n=1) or swimming (n=1). The majority (n=19) of participants completed the full 24-week exercise program (range: 23–25 weeks).
Mean adherence was 79% (SD 21%; range 39–100%; i.e. 2.4 sessions per week). Sixteen patients (70%) completed ≥75% of the prescribed training sessions and were classified as adherent, although 2 dropped out before the 6-month assessment. Reasons for non-adherence were lack of time, tiredness and fear of epileptic seizures, “lack of self-discipline for home-based exercise”, lack of motivation due to divorce, and medical advice not to swim due to ear problems (patient not interested in alternative activities). On average, patients exercised 126 minutes per (active) week (range = 26 to 322 minutes). Average exercise intensity was 76% (range = 68 to 87%) of maximum heart rate. Overall exercise performance was classified as excellent/good in 16 patients (70%), adequate in 3 (13%) and inadequate in 4 (17%).
Four participants required additional assistance with using the heart rate monitor. These problems were resolved via consultation by telephone.
Safety and injury
After the baseline exercise test, one patient was excluded from further participation for safety reasons, because of electrocardiogram (ECG) deviations. The majority of exercise participants had (had) epileptic seizures (74%) and used anti-epileptic drugs (61%), but this did not complicate exercise adherence (see Online table). However, one patient, who had already experienced frequent seizures that continued during the exercise intervention, reported serious feelings of insecurity and doubts about her capability to continue the exercise intervention. The physiotherapist and the patient together decided to decrease the frequency of training session from three to two sessions per week.
There were no exercise-induced injuries, although one patient reported aggravation of pre-existing osteoarthritis-related knee pain at the sixth month of the exercise program. The physiotherapists recommended reducing resistance during training and this decreased the pain symptoms.
Patient-reported satisfaction
Data on satisfaction were available for 20 patients. Of these, 16 patients (84%) evaluated the physical exercise program as good or excellent, and four as moderately/sufficiently satisfactory. The majority evaluated the build-up, intensity and length of the exercise sessions as good (Table 2). Seventeen patients reported that they intended to continue their training after study completion, although nine of them intended to decrease the frequency to one or two training sessions per week.
Table 2.
Post-intervention ratings of physical exercise program, training-aids and home-based guidance
| Patient rating of difficulty/quantity | (too) easy/little | just right | (too) difficult/many |
|---|---|---|---|
| Build-up of the program | 3 (15%) | 14 (70%) | 3 (15%) |
| Number of exercises per week | 0 (0%) | 11 (55%) | 9 (45%) |
| Intensity of sessions | 3 (15%) | 15 (75%) | 2 (10%) |
| Session duration | 3 (15%) | 14 (70%) | 3 (15%) |
| Difficulty Polar website | 1 (5%) | 17 (85%) | 2 (10%) |
| Difficulty Polar heart rate watch | 0 (0%) | 17 (85%) | 3 (15%) |
| Patient rating of quality | excellent/good | sufficient | insufficient/poor |
| Choice of activities | 13 (68%) | 5 (26%) | 1 (5%) |
| Experience of individualized training | 14 (74%) | 4 (21%) | 1 (5%) |
| E-mail contact with physical therapist | 16 (84%) | 2 (11%) | 1 (5%) |
| Involvement of physical therapist | 18 (95%) | 1 (5%) | 0 (0%) |
| Final program rating | 16 (84%) | 2 (11%) | 1 (5%) |
Overall, the difficulty of the training aids appeared to be acceptable. The remotely supervised character of the program, the contact with the physiotherapist by e-mail and the involvement of the physiotherapist were highly valued. However, three patients indicated that they would have preferred group training.
Some quotes of patients extracted from their e-mails to the physiotherapist are displayed in the Online Box.
Feasibility of neuropsychological and physical assessments
Administration of the neuropsychological tests at the patients’ home took about 100 minutes. Estimated time to complete the questionnaires was about 60 minutes. There were (logistical) problems with planning the baseline exercise test, explaining a large range of 6 to 75 days between these assessments in some cases, although in 91% of patients, this interval was shorter than 50 days, with a median of 20 days.
At follow-up, one exercise group patient did not return questionnaires, whereas all control group patients returned completed questionnaires. Individual patient circumstances and logistical problems with the exercise test explained intervals ranging from −35 (exercise test preceding neuropsychological assessment) to 62 days between follow-up neuropsychological and physical assessments, although in 90% of patients this interval ranged from −6 to 18 days, with a median of 4 days.
Objective physical outcomes and self-reported physical activity
Table 3 shows baseline, follow-up and change scores with respect to absolute and relative VO2peak, BMI and self-reported activity for both groups as well as between-group differences in these outcomes. In short, after 6 months, mean absolute VO2peak (the primary outcome in these analyses) in the exercise group improved 6.0% compared to baseline, and mean relative VO2peak improved 7.3%. There were substantial inter-individual differences in absolute VO2peak, with the largest improvement 26.4%, and the largest decline 18.1%. Two of the four patients with a decline had had a substantially shorter intervention duration (see Online Table). The control group (N=9) showed an average decline of 1.1% in absolute VO2peak over time and no change in mean relative VO2peak. However three patients showed an absolute VO2peak improvement (2.5, 9.0 and 17%). Between-group analyses at 6 months indicated that, after correction for baseline values, participants in the exercise group had a higher aerobic fitness compared to the control group (+158.9 ml/min; 95%CI 44.8 to 362.5, and 2.0 ml/kg/min; 95%CI: −0.4 to 4.4) , with a small effect size (d=0.24).
Table 3.
Effects of exercise program on physical outcomes
| Characteristic | Intervention group | Control group | Between group difference in outcome: mean [95%CI], p | Between group effect size (d) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline, T0 mean (SD) (n=19^) | 6 months, T1 mean (SD) (n=19) | Within group mean change: value [95%CI], p | Baseline, T0 mean (SD) (n=9^*) | 6 months, T1 mean (SD) (n=9*) | Within group mean change: value [95%CI], p | |||
| VO2 peak, absolute, ml/min | 2205.9 (666.5) | 2339.1 (750.2) | +133.2 [4.4;262.1], p=.04 | 2361.9 (696.2) | 2335.3 (620.4) | −26.6 [−144.4;91.3], p=.62 | +158.9 [−44.8;362.5], p=.12 | 0.24 |
| VO2 peak, relative, ml/kg/min | 26.0(7.4) | 27.9(8.2) | +1.9 [0.4;3.4], p=.02 | 27.6(3.4) | 27.4(3.0) | −0.1 [−1.6;1.4], p=.84 | +2.0 [−0.4; 4.4], p=.10 | 0.31 |
| BMI, kg/m2 | 27.4(3.0) | 27.0(2.9) | −0.4[−0.7;−0.1], p=.02 | 27.6(6.0) | 27.5(5.7) | 0.0 [−0.7;0.7], p=.90 | −0.3 [−0.9;0.2], p=.23 | −0.08 |
| Body weight, kg | 85.2(10.8) | 84.1(10.5) | −1.2[−2.1;0.2], p=.02 | 85.9(23.9) | 85.7 (22.8) | −0.2 [−2.4;1.9], p=.82 | −1.0 [−2.8;0.8], p=.27 | −0.08 |
| Self-reported physical activity, MET*min/wk | 4465.0 (1668.0; 8640.0) | 6876.0 (3354.0; 9966.0) | +1200.0 [213.0; 4474.5], p=.02 | 4764.0 (1759.9; 15521.3) | 4897.7 (2707.4; 4897.7) | +811 [−5918.2; 2428.5], p=.50 | +1489 [−2219.5; 5814.0], p=.40 | 0.33 |
BMI: body mass index, SD: standard deviation, 95%CI: 95% confidence interval, MET: metabolic equivalent of task;
number of participants with complete pre-post data;
n=10 for self-reported physical activity;
correction for baseline scores not possible due to use of Mann-Whitney U test.
At baseline, 19 of 23 patients (83%) were classified as overweight, of whom 6 were obese. BMI decreased in the exercise group, but not in the control group, but there were no between-group differences in mean BMI at 6 months, after correction for baseline.
Self-reported physical activity varied widely among participants. The median self-reported physical activity increased in both groups, but more strongly in the exercise group. The average increase was 126% in the exercise group compared to 23% in the control group.
Discussion
The results of this pilot RCT provide support for the feasibility of a novel, remotely-supervised, home-based aerobic exercise intervention for motivated patients with stable grades II and III gliomas However, accrual of patients to the study was limited. The results suggests a positive effect of the program on physical fitness. There were no adverse events related to the program.
Overall adherence was good; on average, participants in the exercise group adhered to 79% of the prescribed sessions (i.e., a mean of 2.4 sessions and an average training time of 126 minutes per week) and their experiences were positive, although 45% reported that the frequency of the exercise sessions was (too) high. The program included several features that are known to enhance treatment fidelity and enjoyment, and help overcome potential barriers to exercise (such as travel distance, feelings of uncertainty, motivational problems or time constraints). First, patients could exercise at home. Second, patients received regular guidance from both technology (a commercially available heart rate watch) and a physiotherapist. Remote contact is recommended in the literature as a strategy to enhance adherence to online interventions [25–27]. In previous studies of older adults, adherence to and effectiveness of blended care physical activity programs were comparable to those observed in supervised on-site programs. Third, patients were allowed, within certain limits, to choose their own activities. Current evidence suggests that exercise enjoyment is an important determinant of physical activity and exercise self-efficacy [28, 29]. These elements are aimed at implementation of regular exercise in patients’ daily routines and may aid continuing to exercise after the intervention period had ended [27, 30]. In fact, 17 of 20 of our patients reported a willingness to continue the physical exercise program after the study period, although nine intended to decrease their schedule to 1 or 2 training sessions per week. Two sessions per week may be sufficient to maintain some of the beneficial effects of exercise [31], but it is below the general exercise recommendation for cancer survivors [32].
The high level of feasibility in terms of adherence to the exercise program observed among participants contrasted with the problems experienced in recruiting patients into the study. Initially, our aim was to include 60 patients, but we were only able to recruit 34. Forty-seven percent of the 62 patients for whom a reason for declining participation was known indicated they lacked exercise motivation or time, and probably considered this long and intensive program too much for them. This number may actually be larger, as it is likely that some of the patients who did not provide a reason for declining to participate or did not respond to the invitation had similar reasons. It is difficult to determine in a randomised setting the extent to which patients decline participation because of a lack of interest in the intervention being offered, not wanting to complete questionnaires and tests, not wanting to be randomised, or a combination of these factors. We would note that previous exercise studies in cancer patients also suffered from recruitment problems. In a meta-analysis of the effectiveness of exercise interventions for fatigue in cancer patients, only 10 of the 26 studies that conducted a sample size calculation were successful in recruiting their target [33].
Although three-quarters of the participants had (had) symptoms of epilepsy, this did not complicate exercise adherence, except for one patient with very frequent seizures, for whom the program was adapted slightly. Previous studies have suggested that exercise in patients with epilepsy may result in reduction of seizure susceptibility, improvement of quality of life, reduction of anxiety and depression, and better social integration [34].
We also included outcome data on the intermediate (exercise) endpoint as a measure of feasibility of an exercise program designed to improve cognitive function through improvements in VO2peak in this specific population. As changes in VO2peak can be better understood in comparison to patients who did not exercise, we included the comparison for this outcome in our analyses. The preliminary results suggest that the program could be effective in increasing physical fitness and, perhaps, decreasing (over)weight. Absolute VO2peak, as determined with maximum exercise testing, of patients in the exercise group increased significantly, as compared to no change in the control group. Although the study was not powered for statistical analysis on group differences in outcome, in this analysis a small but clinically relevant effect [35, 36] on absolute VO2peak (a difference of 158.9 ml/min) was observed, which was comparable to the within-group analyses.
Self-reported physical activity varied widely among participants. Both groups in our study reported an increase in physical activity, although the increase was substantially larger in the exercise group. This suggests a certain level of study reactivity (i.e., that patients in the control group also changed their behaviour, simply because they were study participants), which may have led to an underestimation of the observed intervention effects. This is a common problem in exercise oncology trials [37].
Feasibility of administration of largely the same battery of neuropsychological tests and questionnaires that we used here was demonstrated in a previous large randomised controlled trial [21] in patients with stable glioma. In the current study, patients experienced the exercise tests as more burdensome than the neuropsychological tests due to their physically challenging nature and because they had to travel.
An important limitation of our study is its small sample size. By allocation of participants in a 2:1 ratio, we could make optimum use of the number of participants, as it allowed us to evaluate feasibility and potential (within-group) effects of the program in a larger group, but at the cost of limiting the precision of the between group comparison.
In addition, as discussed above, uptake was low (25%), predominantly due to patients’ lack of time or motivation to participate in the trial and/or training program. Overall, this resulted in a motivated group of participants for whom an intensive exercise program may be particularly feasible. This suggests that the intervention, in its current form, may be of interest to a smaller subset of the target population.
Furthermore, all patients were tested on a cycle ergometer, because group allocation and preferred training modality were not known until after baseline exercise testing. However, not everyone trained on a bicycle, and consequently both the baseline and follow-up tests lacked exercise specificity. This could have led to an underestimation of changes in aerobic fitness in patients who chose running or swimming as training modality.
Despite these limitations, the observed intervention effect and its specific relationship with program adherence in the current study warrant replication in a larger trial. The design of future exercise interventions and (randomised controlled) trials can hopefully benefit from the lessons we learned from this pilot study. First, we recommend to take the challenging recruitment in this patient group into account when allocating time for the recruitment phase of the study. Second, future trials could benefit from using objective measures of physical activity (i.e., accelerometers) instead of relying on self-report measures, which are prone to overestimation of physical activity levels [38, 39]. In line with the literature [40], we recommend use of cardiopulmonary exercise testing for baseline testing in order to minimise risk of exercise-related adverse events (in this study, we excluded one patient at risk). This maximum exercise test is considered the gold standard for assessment of cardiorespiratory fitness, because it provides the most accurate determination of peak oxygen uptake (VO2peak) [40]. Future trials might employ study logistics to enable adaptation of type of exercise test to training activities. The results can also be used in prescribing individual exercise programs (and their reliable evaluation in case of additional follow-up exercise testing). Finally, studies offering a program with a lower frequency of exercise activities per week might be less likely to suffer from problems with uptake, and may have a higher program adherence than our study. Although two sessions per week is below the general exercise recommendation for cancer survivors [32], patients with low physical fitness would likely still benefit from two sessions per week. During the program, the training frequency could be increased for patients who are able and willing, thus providing even better tailoring to individual needs and abilities.
The primary aim of our study was to investigate the feasibility of an aerobic exercise intervention for the improvement of cognitive function through improvement of aerobic fitness, as it has been suggested that increased cardiorespiratory fitness (i.e., VO2peak) may be associated with improvements in cognitive capacity [41, 42]. The preliminary results presented here suggest that this intensive exercise program helped to improve cardiorespiratory fitness. A forthcoming evaluation of the study results will address the question whether our physical exercise program also had an effect on cognitive functioning in this patient population. This will include detailed investigation of the effects of the exercise program on the domains of attention, memory and executive function, and on self-reported cognitive symptoms, fatigue, sleep, mood and quality life.
Given the need for management of the multitude of symptoms in this neuro-oncological patient group, and the potential applicability of exercise to aid in this management [2], the findings discussed here warrant further investigation of exercise interventions that may benefit this population.
Supplementary Material
Clinical messages.
Six-months of home-based, remotely-coached exercise is feasible in a select group of motivated glioma patients.
Recruitment of the total group of stable patients to a long and intensive exercise intervention is difficult.
Exercise may improve cardiorespiratory fitness and physical functioning in neuro-oncological patients.
Acknowledgments
We thank our research-assistants/technicians: Eva Visser, MSc; Karin Eichhorn, MSc; Fleur Franken, MSc; Sophie van der Linden, MSc; Wietske Schimmel, MSc; Eline Verhaak, MSc, as well as (the investigators of) the participating centers: Medical Center Haaglanden, The Hague; Elisabeth-TweeSteden Hospital, Tilburg; Erasmus Medical Center, Rotterdam; and the Sports Medical Centers from the: Amphia Hospital Breda, Elkerliek Hospital Helmond, Jeroen Bosch Hospital Den Bosch, Maxima Medical Center Eindhoven, Medical Center Haaglanden Den Haag, SportMedisch Advies Centrum Rotterdam; The Netherlands.
Funding support
This research was supported by a grant from the Dutch Cancer Society (UvT2010–4642).
APPENDIX
Description of the exercise program
General description
Three home-based training sessions per week, at 60–85% of maximum heart rate, for a duration of six months. Participants could choose one or more central activities (e.g., cycling, running, swimming and/or cardio-activities in the gym), based on their own preferences and capabilities, as long as they could meet their individual exercise prescription.
Introduction to the individual, home-based activities
The physiotherapist visited participants at home at the start of the intervention.
Participants received an individualized exercise prescription, based on their level of aerobic fitness as determined by cardiopulmonary exercise test at baseline and patients’ self-reported level of physical activity.
Participants also received, and were instructed in the use of, a sports watch with heart rate monitor (Polar RC3 GPS or water resistant Polar RCX5, Polar, Finland) [16].
Discussion of personal circumstances and potential barriers for exercise-adherence and how to overcome these.
Build-up of the program
Participants received individualized exercise prescriptions, adapted to individual progress, every four weeks.
During the first 11 weeks, there were one high-intensity interval session and two moderate intensity sessions per week, and a gradual increase of session duration from 20 to 45 minutes.
In week 12, patients exercised three moderate intensity sessions to allow extra recovery.
In weeks 13–23, there were two high-intensity interval training sessions and one moderate-intensity session per week of 45 minutes. High-intensity interval sessions consisted of multiple blocks (duration varied between 2 to 10 minutes, based on individual capability) at 85% of maximum heart rate (MHR), as determined during baseline cardiopulmonary exercise test, followed by recovery time at 60% of MHR. Moderate intensity sessions were at a constant intensity (also at 60% of MHR).
In week 24, three moderate-intensity sessions in order to start well-rested with the follow-up cardiopulmonary exercise test.
Technical and professional feedback
During exercise, the watch provided immediate feedback about heart rate and, if applicable, speed and distance.
Participants were requested to export training data at least once a week to an online platform, and to describe their experiences in a log.
Physiotherapist monitored the training data (number, duration, and intensity of exercise sessions, and patients’ experiences) on the platform on a weekly basis and provided personal feedback by e-mail.
In case of motivational problems, fatigue, injury or technical problems, more frequent e-mail/phone contact was allowed.
After the follow-up exercise test, participants were called to discuss the program and exercise test results, and to discuss continuation of physical exercise after the study period.
Footnotes
Competing interests
The authors declared no potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Klein M, Duffau H, De Witt Hamer PC. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J Neurooncol. 2012;108(2):309–18. doi: 10.1007/s11060-012-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cormie P, Nowak AK, Chambers SK, Galvao DA, Newton RU. The potential role of exercise in neuro-oncology. Frontiers in oncology. 2015;5:85. doi: 10.3389/fonc.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capozzi LC, Boldt KR, Easaw J, Bultz B, Culos-Reed SN. Evaluating a 12-week exercise program for brain cancer patients. Psychooncology. 2016;25(3):354–8. doi: 10.1002/pon.3842. [DOI] [PubMed] [Google Scholar]
- 4.Jones LW, Guill B, Keir ST, Carter K, Friedman HS, Bigner DD et al. Exercise interest and preferences among patients diagnosed with primary brain cancer. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2007;15(1):47–55. doi: 10.1007/s00520-006-0096-8. [DOI] [PubMed] [Google Scholar]
- 5.Nicole Culos-Reed S, Leach HJ, Capozzi LC, Easaw J, Eves N, Millet GY. Exercise preferences and associations between fitness parameters, physical activity, and quality of life in high-grade glioma patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2016. doi: 10.1007/s00520-016-3516-4 [DOI] [PubMed] [Google Scholar]
- 6.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi:nrn2298[pii] 10.1038/nrn2298[doi]. [DOI] [PubMed] [Google Scholar]
- 7.Lautenschlager NT, Cox K, Cyarto EV. The influence of exercise on brain aging and dementia. Biochimica et biophysica acta. 2012;1822(3):474–81. doi: 10.1016/j.bbadis.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Hotting K, Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neuroscience and biobehavioral reviews. 2013;37(9 Pt B):2243–57. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Long BJ, Calfas KJ, Wooten W, Sallis JF, Patrick K, Goldstein M et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med. 1996;12(2):73–81. [PubMed] [Google Scholar]
- 10.Vos JA. Ergometrie en trainingsbegeleiding [Ergometry and training supervision]. 7th ed Otterlo, the Netherlands: 2013. [Google Scholar]
- 11.West MA, Parry M, Asher R, Key A, Walker P, Loughney L et al. The Effect of beta-blockade on objectively measured physical fitness in patients with abdominal aortic aneurysms--A blinded interventional study. Br J Anaesth. 2015;114(6):878–85. doi:aev026[pii] 10.1093/bja/aev026. [DOI] [PubMed] [Google Scholar]
- 12.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Canadian journal of sport sciences = Journal canadien des sciences du sport. 1992;17(4):338–45. [PubMed] [Google Scholar]
- 13.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. A review. Control ClinTrials. 2002;23(6):662–74. [DOI] [PubMed] [Google Scholar]
- 14.Jolles J, Houx PJ, Van Boxtel MPJ, Ponds RWHM. Maastricht Aging Study: Determinants of Cognitive Aging. Maastricht, The Netherlands: Neuropsych Publishers; 1995. [Google Scholar]
- 15.ALEA. ALEA AVL. The Netherlands. https://prod.tenalea.net/avl/dm/.
- 16.Polar. Polar Personal Trainer. https://www.polarpersonaltrainer.com/.
- 17.Kemper HGC, Ooijendijk WTM, Stiggelbout M. Consensus over de Nederlandse Norm voor Gezond Bewegen [Consensus on the Dutch standard for healthy exercise]. TSG: Tijdschrift voor gezondheidswetenschappen. 2000;78(3):180–3. [Google Scholar]
- 18.IPAQ-group. IPAQ scoring protocol https://sites.google.com/site/theipaq/scoring-protocol. [Google Scholar]
- 19.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE et al. International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 20.Pescatello LS. ACSM’s guidelines for exercise testing and prescription. 9th ed Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 21.Gehring K, Sitskoorn MM, Gundy CM, Sikkes SA, Klein M, Postma TJ et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–22.doi:JC0.2008.20.5765[pii] 10.1200/JCO.2008.20.5765[doi]0.1007/s12529-016-9556-9 [DOI] [PubMed] [Google Scholar]
- 22.Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 23.Corp I IBM SPSS Statistics for Windows. 22.0 ed Armonk NY: IVBM Corp; 2013. [Google Scholar]
- 24.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for statistical computing; 2016. [Google Scholar]
- 25.Beatty L, Binnion C. A Systematic Review of Predictors of, and Reasons for, Adherence to Online Psychological Interventions. Int J Behav Med. 2016. doi: 10.1007/s12529-016-9556-9 [DOI] [PubMed] [Google Scholar]
- 26.Bluethmann SM, Vernon SW, Gabriel KP, Murphy CC, Bartholomew LK. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat. 2015;149(2):331–42. doi: 10.1007/s10549-014-3255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geraedts H, Zijlstra A, Bulstra SK, Stevens M, Zijlstra W. Effects of remote feedback in home-based physical activity interventions for older adults: a systematic review. Patient Educ Couns. 2013;91(1):14–24. doi:S0738-3991(12)00425-9[pii] 10.1016/j.pec.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Ungar N, Wiskemann J, Sieverding M. Physical Activity Enjoyment and Self-Efficacy As Predictors of Cancer Patients’ Physical Activity Level. Front Psychol. 2016;7:898. doi: 10.3389/fpsyg.2016.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valenzuela T, Okubo Y, Woodbury A, Lord SR, Delbaere K. Adherence to Technology-Based Exercise Programs in Older Adults: A Systematic Review. J Geriatr Phys Ther. 2016. doi: 10.1519/JPT.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 30.Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005(1):CD004017. doi: 10.1002/14651858.CD004017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3(5):346–56. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and science in sports and exercise. 2010;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112 [DOI] [PubMed] [Google Scholar]
- 33.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arida RM, Scorza FA, Gomes da Silva S, Schachter SC, Cavalheiro EA. The potential role of physical exercise in the treatment of epilepsy. Epilepsy Behav. 2010;17(4):432–5. doi:S1525-5050(10)00018-1[pii] 10.1016/j.yebeh.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail. 2012;5(5):579–85. doi:CIRCHEARTFAILURE.111.965186[pii] 10.1161/CIRCHEARTFAILURE.111.965186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr.,Tudor-Locke C et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Medicine and science in sports and exercise. 2011;43(8):1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 37.Steins Bisschop CN, Courneya KS, Velthuis MJ, Monninkhof EM, Jones LW, Friedenreich C et al. Control group design, contamination and drop-out in exercise oncology trials: a systematic review. PLoS One. 2015;10(3):e0120996. doi: 10.1371/journal.pone.0120996 PONE-D-14-44380[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Poppel MN, Chinapaw MJ, Mokkink LB, van Mechelen W, Terwee CB. Physical activity questionnaires for adults: a systematic review of measurement properties. Sports Med. 2010;40(7):565–600. doi:3[pii] 10.2165/11531930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Harris TJ, Owen CG, Victor CR, Adams R, Ekelund U, Cook DG. A comparison of questionnaire, accelerometer, and pedometer: measures in older people. Medicine and science in sports and exercise. 2009;41(7):1392–402. doi: 10.1249/MSS.0b013e31819b3533. [DOI] [PubMed] [Google Scholar]
- 40.Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9(8):757–65. doi:S1470-2045(08)70195-5[pii] 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 41.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological science. 2003;14(2):125–30. [DOI] [PubMed] [Google Scholar]
- 42.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic medicine. 2010;72(3):239–52. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. MedCare. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 44.Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. JClinEpidemiol. 1998;51(11):1055–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.