Summary
This study systematically assessed the effectiveness of lifestyle interventions on glycemic indicators among adults (≥18years) without IGT or diabetes. Randomized controlled trials using physical activity (PA), diet (D), or their combined strategies (PA+D) with follow-up ≥12 months were systematically searched from multiple electronic-databases between inception and April 17, 2015. Outcome measures included fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), fasting insulin (FI), homeostasis model assessment-estimated insulin resistance (HOMA-IR), and bodyweight. Included studies were divided into low-range (FPG <5.5mmol/L or HbA1c <5.5%) and high-range (FPG ≥5.5mmol/L or HbA1c ≥5.5%) groups according to baseline glycemic levels. Seventy-one studies met inclusion criteria. Random-effect models demonstrated that compared with usual care, lifestyle interventions achieved significant reductions in FPG (−0.14mmol/L[95%CI, −0.18, −0.09]), HbA1c (−0.05%[−0.08, −0.03]), FI (%change: −15.18%[− 20.01–10.35]), HOMA-IR (%change: −22.66%[−29.19, −16.14]), and bodyweight (%change: −4.00%[−4.73, −3.26]). The same effect sizes in FPG reduction (0.08) appeared among both low-range and high-range groups. Similar effects were observed among all groups regardless of lengths of follow-up. D and PA+D interventions had larger effects on glucose reduction than PA alone. Lifestyle interventions significantly improved FPG, HbA1c, FI, HOMA-IR, and bodyweight among adults without IGT or diabetes, and might reduce progression of hyperglycemia to type 2 diabetes mellitus.
Keywords: Lifestyle intervention, glucose regulation, systematic review, meta-analysis
1. Introduction
Diabetes imposes a large burden on human health, society, and the economy due to its wide-ranging complications and extensive treatment costs [1]. Physical inactivity, unhealthy diet, and obesity are well-established risk factors for type 2 diabetes mellitus, and structured lifestyle interventions incorporating behavior change, dietary modifications, and regular moderate-intensity physical activity resulting in modest weight reduction have been shown to reduce type 2 diabetes mellitus incidence [2–4]. However, for practical reasons of statistical power and study cost, the major diabetes prevention trials have focused on the subset of individuals with impaired glucose tolerance (IGT) rather than these other risk factors [2–4]. This has raised considerable debate about whether structured lifestyle interventions should be limited to people with IGT or could be applied more broadly to the population that includes individuals without IGT.
In studies among persons with normal glucose levels, researchers need large sample sizes and long follow-up periods for exploring the effect of lifestyle interventions on reducing the incidence of diabetes, making these studies costly and difficult to conduct [5]. However, a systematic review and meta-analysis of aggregate data from studies of lifestyle interventions among people without IGT may provide evidence of the impact of such interventions on risk factors for diabetes or on the potential to prevent type 2 diabetes mellitus.
We conducted a systematic review to assess the aggregated impact of lifestyle interventions on glycemic indicators among adults (≥18 years) without IGT or diabetes.
2. Methods
2.1. Data source and searches
We developed a study protocol following Cochrane Collaboration standards [6]. We systemically searched MEDLINE, EMBASE, CINAHL, Web of Science, Cochrane Library, and Psychlnfo databases, from inception to April 17, 2015. Medical Subject Headings, text words, and search strategies are available from the authors. We examined reference lists of all studies and relevant reviews for potential additional studies. We directly contacted authors to clarify unclear data.
2.2. Study selection
We selected randomized controlled trials (RCTs) published in any language that examined lifestyle strategies involving physical activity (PA), diet (D), or their combination (PA+D) among adults (≥18 years), and with at least 1 glycemic indicator reported as the intervention outcome (e.g., fasting plasma glucose [FPG], glycated hemoglobin A1c [HbAlc], fasting insulin [FI], and homeostasis model assessment-estimated insulin resistance [HOMA-IR]) with a follow-up interval ≥12 months. Included studies investigated individuals without IGT or diabetes. We excluded all studies among individuals with IGT confirmed by 2-hour oral glucose tolerance test (OGTT) (75g). We included studies regardless of baseline glucose levels. However, we classified studies with mean baseline FPG <5.5mmol/L or HbA1c <5.5% as the low-range group, and with mean baseline FPG ≥5.5mmol/L or HbA1c ≥5.5% as the high-range group, and analyzed data as a whole and by glycemic level groups.
2.3. Data extraction and quality assessment
One primary reviewer and a secondary reviewer independently assessed the manuscript titles and abstracts for inclusion. If any disagreement occurred between the 2 reviewers, a third reviewer reviewed the item, and consensus was reached by a discussion. The study team extracted data regarding demographic and intervention characteristics. Primary outcomes in this review included FPG, A1C, FI, and HOMA-IR. In our synthesis models, we used percent changes in FI and HOMA-IR, rather than raw values, to account for non-standardized insulin measurements. We also examined percent body weight change from baseline.
In our review, all interventions were classified into 3 categories: PA, D, and their combination. PA interventions included any strategy to increase physical activity levels using counseling, exercise prescription, or a supervised or unsupervised exercise program. The D interventions included any strategy used to reduce or control calorie intake, such as very low-calorie diet (<800 kcal/d) or low-calorie diet (800 to 1500 kcal/d). PA+D interventions usually also employed a behavioral modification component, such as counseling, education, cognitive-behavioral therapy, or social support.
We assessed study quality by examining potential selection, attrition, and detection bias [6]. We excluded from our main analyses studies of poor quality (e.g., studies with attrition ≥30%). However, we conducted a sensitivity analysis to compare pooled effects between studies with potentially significant bias and those without. For example, for those studies with attrition ≥30%, data were not used in our primary meta-analyses, but were used in our sensitivity analyses.
2.4. Data Synthesis and Analysis
If 2 or more studies with similar intervention and comparison groups reported a similar outcome of interest, we conducted a meta-analysis to determine pooled effects. We calculated the mean difference between baseline and follow-up measures for the intervention and comparison groups (delta I and delta C) and the standard error of each difference. We used 3 data synthesis strategies to estimate pooled effects: stratification by glycemic levels, stratification by follow-up duration, and stratification by type of intervention.
We used the DerSimonian and Laird random-effects model [7] to determine pooled effects. We used meta-regression to determine whether various study-level characteristics (mean age, follow-up duration, duration of the intervention, number of intervention contacts, attrition, and year of publication) affected the between-group change in FPG, and we examined interaction terms for all models. We also used meta-regression to test if there is an association between the magnitude of body weight change and the magnitude of FPG change. The meta-regression was conducted using SPSS (version 20.0, Armonk, NY: IBM Corp.). We used the chi-squared test to examine heterogeneity, and Cochrane Review Manager software (version 5.1; Copenhagen, Denmark) to calculate pooled effects. Effect size was defined by the mean difference between delta I and delta C divided by the standard deviation of the mean.
If a comparison group in a study used a similar approach as the intervention group, but only differed in dose, intensity, or frequency (e.g., diet plan A vs diet plan B; or swimming vs walking), we analyzed the effects of treatment in a single-arm model to determine within-group changes (between post-intervention and pre-intervention in 1 arm) for both intervention and comparison group. These effects were also estimated by using the DerSimonian and Laird random-effects model. We did not conduct sensitivity analysis for these studies.
3. Results
Seventy-one studies [8–78] (plus 30 additional publications based on those studies [79–108]) encompassing 13943 participants (Table 1: range, 20 to 1089) fulfilled the inclusion criteria (Figure 1). Follow-up duration ranged from 12 to 54 months. The weighted mean age of the participants was 50.9 years (range, 30.2 to 70.4 year), and mean body mass index (BMI) was 30.1 kg/m2 (range, 23.3 to 38.7 kg/m2). Mean baseline FPG, HbAlc, FI, and HOMA-IR were 5.3 mmol/L, 5.4%, 13.7 μU/ml, and 3.9, respectively. More studies took place in a community setting vs a clinic setting (52 vs 19). Sampling methods varied, but most participants were recruited through screening programs. Attrition ranged from 0% to 60%, and was 30% or more in 15 studies [8,20–22,31,44,46,49,51,57,60,62,64,70,78]; higher attrition rates were associated with longer follow-up duration. Thirty-eight studies with FPG <5.5mmol/L or HbA1c <5.5 % were classified as low-range glycemic level studies, and 33 studies with FPG ≥5.5mmol/L or HbA1c ≥5.5% were classified as high-range glycemic level studies (Table 1).
Table 1:
Characteristics of Study Participants
| Citation | Sample size |
Length of follow- up (month) |
Age at baseline (years) mean (SD) |
Sex (% female) |
Setting; Race/ethnicity |
BMI at baseline (kg/m2) mean (SD) |
FPG at baseline (mmol/L) mean (SD) |
HbAlc at baseline (%) mean (SD) |
Insulin at baseline (μU/ml) mean (SD) |
Inclusion criteria | Sampling method |
Attrition (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ackermann et al. 2008 | 92 | 12 | 58.3 (10.1) | 55.4 | Community (YMCA) Indianapolis Indiana 81.5% white 12.0% black | 31.4 (4.9) | NR | 5.6 (0.5) | NR | People with ADA risk score>10 and casual capillary blood glucose (CCBG) of 6.1–11.0 mmol/L | Recruited from YMCA by a community- based screening | 32.6% |
| Almeida et al. 2011 | 53 | 12 | range: 20–29: 12 30–39: 26 >40:15 | 18.9 | Clinic Sao Paulo Brazil | 23.3 (2.7) | 4.7 (0.6) | NR | NR | Aged 20–59yrs; without hyperlipidemia, hypertriglyceridemia, hyperglycemia, obesity, cancer, anabolic, or corticosteroid drugs use, or pregnancy | Recruited from a reference HIV clinic | 20.8% |
| Anderson et al. 2014 Craigie etal. 2011 |
329 | 12 | 63.6 (6.8) | 26.0 | Community Scotland UK 99.0% white 1.0% others | 30.7 (4.2) | 6.1(2.0) | 6.0(1.1) | 10.6(8.6) HOMA-IR: 3.0 (2.9) | Aged 50–74yrs; BMI>25kg/m2; with polypectomy for adenoma, without pregnancy, DM | Recruited from a bowel screening program | 7.3% |
| Anderssen et al.& 1998 Jacobs et al. 2009 The ODES Investigators 1993 Torjesen et al.1997 | 219 | 12 | 44.9 (2.5) | 9.6 | Community Oslo Norway | 28.8(3.4) | 5.6 (0.7) | NR | 23.2(13.3) | BMI>24 kg/m2DBP: 86–99 mmHg TC: 5.20–7.74 mmol/L HDL-C<1.2 mmol/L Fasting TG>1.4 mmol/L | Recruited from a continuously ongoing screening program in Oslo | 4.6% |
| Arguin et al. 2012 | 25 | 12 | 60.5 (6.0) | 100.0 | Community Sherbrooke Quebec Canada | NR (body weight mean (SD) 79.6 (10.7) | 5.0 (0.4) | NR | NR | Sedentary obese postmenopausal women without: (1) abnormal fasting lipid profile; (2) CVD; and (3) DM | Using a computergenerated randomization list | 12.0% |
| Bazzano et al. 2014 | 148 | 12 | 46.8(10.1) | 88.5 | Community New Orleans LA 45.3% white 51.4% black 2.0% Hispanic | 35.4 (4.2) | 5.2 (0.6) | NR | 17.3 (10.0) | Obese people (BMI: 30–45 kg/m2) without DM and CVD | Recruited from community screening and TV ads | 17.8% |
| Bo et al. 2007&2009 | 375 | 48.0 | 55.7(5.7) | 58.2 | Community Asti Italy | 29.7 (4.4) | 5.8 (0.8) | NR | median (interquartile range) 20.4 (24.0) for IG; 21.3 (31.2) for CG | People with MetS defined by FPG>6.1 mmol/L, without DM and CVD | Recruited from a metabolic screening program | 10.7% |
| Bouchonville et al. 2014 Villareal et al. 2011 | 107 | 12.0 | 69.7 (4.0) | 62.6 | Community St. Louis MO | 37.2 (5.0) | 5.5 (0.6) | NR | 16.6(10.6) HOMA-IR: 4.2 (3.0) | Old (>65yrs) and obese (>30 kg/m2) people without DM | Recruited from ads | 13.0% |
| Brinkworth et al. 2004 | 58 | 12 | 50.2 (NR) | 77.6 | Community Adelaide Australia | 34.0 (NR) | 5.4 (0.2) | NR | 16.0(1.3) HOMA-IR: 3.8 (0.4) | Obese, hyperinsulinemic persons aged 20–65yrs, insulin >12 mu/1 without DM, | NR | 25.9% |
| Broekhuizen et al. 2012 | 340 | 12 | 45.3 (12.9) | 56.7 | Community Amsterdam The Netherland | 26.5 (5.0) | 4.9 (0.9) | NR | NR | Aged 18–70 yrs, with familial hypercholesterolemia, a LDL-C level>75th percentile | Recruited from the national cascade screening program | 7.4% |
| Burke et al. 2007 & 2008 | 241 | 36 | 56.2(7.3) | 55.6 | Community Perth Australia | 30.1 (2.7) | 5.0 (0.9) | NR | 1.8 (0.9) HOMA-IR: 2.4 (1.7) | Overweight, age>40yrs using 1 or 2 drugs to treat HT >3 months without DM, chronic renal failure, CVD | Recruited bymediaadvertising | 16.2% |
| Burtscher et al. 2009&2012 | 36 | 12 | 57.5 (6.9) | 55.6 | Clinic Innsbruck Austria | 29.0 (3.9) | 6.0 (0.4) | NR | NR | Patients with IFG (FPG: 5.6– 6.9 mmol/L), aged 40–65yrs; BMI>25 kg/m2, and without DM | Recruited byphysiciansthroughmemberscreening | 0.0% |
| Choo et al. 2014 | 110 | 12 | 43.1 (9.0) | 100.0 | Community Seoul South Korea | 28.5 (3.8) | 5.0 (0.8) | NR | NR | Aged 18–65yrs; elevated waist circumference (>85cm), abdominal obesity without DM and CVD | Recruited via poster, leaflet, telephone, and ads | 55.5% |
| Clifton et al. 2008 | 119 | 12 | 49.0 (9.0) | 100.0 | Community Adelaide Australia | 32.8(3.5) | 6.1 (0.6) | NR | 9.9 (4.7) | Women, aged 20–65yrs, BMI:27–40kg/m2, without DM, or renal or liver disease | Recruited from public ads and screened by questionnaires | 33.6% |
| Cole et al. 2013 | 94 | 12 | 58.3 (9.6 | 46.0 | Community San Antonio Texas 64.0% white 17.0% black 19.0% Flispanic | 30.8 (4.9) | 6.1 (0.5) | 5.9 (0.5) | NR | Aged 18yrs+; without DM, but with pre-DM, by ADA IFG (5.6–6.9 mmol/L) | Recruited from a pre-DM education class | 31.0% |
| Coon et al. 1989 | 20 | 12 | 59.5 (7.5) | 0.0 | Community Baltimore MD | 29.0 (3.0) | 5.4 (0.5) | NR | 13.0(6.0) | Aged 45yrs+, healthy persons without DM | Recruited by ads | 0.0% |
| Cox et al 2006 & 2008 & 2010 | 116 | 12 | 55.5 (4.7) | 100.0 | Community Berth Australia | 26.4 (3.3) | 5.1 (0.4) | NR | 6.2 (3.9) | Aged 50–70 yrs; BMI<34 kg/m2; non-smoker, with sedentary lifestyle, without DM | Recruited by ads. | 25.9% |
| Ditschuneit et al. 1999 & 2001 | 100 | 24 | 45.7(10.6) | 79.0 | Clinic sUlm Germany | 33.4 (3.6) | 5.0 (0.6) | NR | 21.5 (8.1) | Age>18yrs, BMI between 25 and 40 kg/m2 without endocrine disorders | Recruited by referring to the obesity clinics | 27.0% |
| Donnelly et al. 2000 | 22 | 18 | 51.5 (8.5) | 100.0 | Community Kearney NE | 31.2 (4.0) | 5.5 (0.8) | NR | 14.0 (9.8) | BMI>25 kg/m2, low aerobic capacity, at risk for continued weight gain | NR | 0.0% |
| Esposito et al. 2003 | 120 | 24 | 34.6 (5.0) | 100.0 | Clinic Naples Italy | 34.9 (2.4) | 5.9 (0.8) | NR | 14.0 (4.0) HOMA-IR: 3.7 (0.5) | Obese premenopausal women, aged 20–46yrs; without DM, IGT (7.8–11.0 mmol/L), CAD, pregnancy. OGTT confirmed | Recruited from a outpatient department | 6.7% |
| Esposito et al. 2004a | 110 | 24 | 43.3 (5.0) | 0.0 | Clinic Naples Italy | 36.7 (2.4) | 5.8 (0.6) | NR | 20.0 (7.5) | Obese men with erectile dysfunction, aged 35–55yrs; without DM and IGT, OGTT confirmed | Recruited from a outpatient department list | 5.5% |
| Esposito et al. 2004b (JAMA v.292) & 2009 | 180 | 24 | 43.9 (6.2) | 45.0 | Clinic Naples Italy | 28.0 (3.3) | 6.3 (0.6) | NR | 15.5 (6.5) HOMA: 3.8 (0.7) | Sedentary people with MetS defined by FPG>6.1 mmol/L, | Recruited from a screening program | 8.9% |
| Fatouros et al. 2005 | 50 | 12 | 70.4(3.8) | 0.0 | Community Alexandroupolis Greece | 29.5 (3.3) | 5.9 (0.7) | NR | 14.2(3.1) HOMA-IR: 3.7 (2.8) | Inactive old men, nonsmoker, without DM, FPG<7 mmol/L | Recruited from a volunteer database in localcommunity | 0.0% |
| Fernandez et al. 2012 | 40 | 12 | 40.9(13.5) | 67.5 | Community Leon Spain | 31.8(2.4) | 4.6 (0.9) | NR | 21.2(27.4) | Aged 18–70yrs; BMI: 28–35 kg/m2; without DM and pregnancy | Recruited from a clinic trial | 60.0% |
| Ferrara et al. 2012 | 188 | 24 | 56.4(9.5) | 47.9 | Clinic Naples Italy | 29.2 (4.5) | 5.6 (1.5) | NR | NR | People with HT | Recruited from a outpatient clinic | 0.0% |
| Fisher et al. 2012 | 97 | 12 | age range: 21–46 y | 100.0 | Community Birmingham AL 46.4% white 53.6% black | 28.0(1.0) | 4.8 (0.4) | NR | 11.4(3.6) | Aged 21–46yrs; BMI: 27–30 kg/m2; non-smoker, with sedentary lifestyle premenopausal women | Recruited from a previous parent study | 0.0% |
| Fogelholm et al. 2000 | 82 | 24 | age range: 30–45y | 100.0 | Community Tampere Finland | 34.0 (3.6) | 5.1 (0.5) | NR | 12.7(5.2) | Aged 30–45yrs, BMI: 30–45 kg/m2, physically inactive | Recruited by ads | 9.8% |
| Fonolla et al. 2009 | 297 | 12 | 46.0 (8.4) | 15.5 | Community Granada Spain | 28.8(5.0) | 5.6 (1.9) | NR | NR | People with moderate risk of CVD, without DM, or pregnancy | Recruited from a screening program | 14.8% |
| Frank et al. 2005 | 173 | 12 | 60.7 (6.7) | 100.0 | Community Seattle Washington | 30.4 (3.9) | 5.4 (0.5) | NR | 17.9(8.3) HOMA: 4.4 (2.2) | Postmenopausal women, aged 50–75yrs, sedentary at baseline BMI>25 kg/m2 without DM, nonsmoker | Recruited through mailings and media ads | 1.7% |
| Groeneveld et al. 2008 & 2010 | 816 | 12 | 46.6 (9.0) | 0.0 | Community Amsterdam The Netherlands | 28.5 (3.5) | NR | 5.7 (0.4) | NR | Male construction workers with elevated risk of CVD | Recruited from periodical health screening | 27.6% |
| Fleshka et al. 2003 | 423 | 24 | 44.5 (10.0) | 84.6 | Clinics NY, Medison, Baton Rouge, Boulder, Davis, Durham, Woodbury | 33.7 (3.6) | 5.0 (0.7) | NR | 18.0 (9.5) (IU/L) | Aged 18–65yrs; BMI: 27–40 kg/m2; with FPG<7.8 mmol/L | Recruited from existing clinic records, or by ads | 27.0% |
| Imayama et al. 2013Foster-Schubert et al. 2012 Mason et al. 2011&2013 | 439 | 12 | 58.0(5.0) | 100.0 | Community Seattle WA 85.0% white | 30.9 (4.1) | 5.4 (0.5) | NR | 12.9(8.1) HOMA-IR: 3.1 (2.1) | Aged 50–75yrs; BMI: >25 kg/m2; <100 min/w PA; postmenopausal; without DM; FPG<7.0 mmol/I, | Recruited from mass mailing ads | 9.1% |
| Kanaya et al. 2012 Delgadillo et al. 2010 | 238 | 12 | 56.5 (16.5) | 73.5 | Community Berkeley, Oakland, etc CA22.5% white 23.0% black 37.0% Hispanic | 30.0 (5.7) | 5.2 (0.7) | NR | NR | Aged 25yrs+; a capillary blood glucose:5.9–8.9 mmol/L, without DM | Recruited from a community- based education outreach | 12.2% |
| Kanaya et al. 2014 | 180 | 12 | 55.0 (7.0) | 72.0 | Clinics San Francisco, San Diego CA65.0% white | 34.3 (6.7) | 5.8 (0.7) | 5.9 (0.4) | 27.5 (17.6) HOMA-IR: 7.2 (5.1) | Aged 21–65yrs; with MetS (FPG:5.6–6.9 mmol/L), HT, and underactive lifestyle (<150min/w of moderate intensity activity), without DM | Recruited by ads and flyers in community and clinical settings | 21.1% |
| Katula et al. 2010&2011&2013 | 301 | 24 | 57.9(9.5) | 57.5 | Community Winston-Salem NC 73.8% white 24.6% black | 32.7 (4.0) | 5.9 (0.6) | NR | 16.7(9.8) HOMA-IR: 4.4 (2.9) | Patients with pre-DM defined by FPG of 5.3–6.9 mmol/L and BMI of 25–39 kg/m2 and without DM and CVD | Recruited from mass mailing, community health fair or referrals | 12.6% |
| Kawano et al. 2009 | 217 | 17 | 60.9(13.8) | 66.5 | Community Saga City Japan | 23.7 (4.4) | 5.1 (0.5) | 5.1 (0.3) | NR | People with FPG: 5.6–7.8 mmol/L, or HbAlc: 5.5–6.0% | Recruited from health checkup | 27.2% |
| Keogh et al. 2007 | 36 | 12 | 48.6 (5.2) | 68.0 | Community Adelaide Australia | 32.9 (4.5) | 5.9 (0.5) | NR | 14.8(11.0) | Overweight or obese people, aged 20–65yrs; BMI: 27–40 kg/m2; without DM, with FPG<7.0 mmol/L | Recruited from newspaper ads | 30.6% |
| Lawton et al. 2009 | 1089 | 24 | 58.9(6.9) | 100.0 | Clinics Wellington New Zealand | 29.2 (6.0) | 5.0 (0.6) | 5.5 (0.6) | 6.9 (4.7) | Physically inactive women, aged 40–74yrs without medical condition | Recruited by invitation letters or practice register | 7.4% |
| Lim et al. 2010 | 113 | 12 | 47.0 (10.0) | 82.3 | Community Adelaide Australia | 32.0(6.0) | 5.4 (0.6) | NR | 9.1 (4.6) | Aged 20–65yrs, BMI: 28–40 kg/m2, with at least one CVD risk factor, without DM | Recruited by ads | 38.9% |
| Lombard et al. 2010 | 250 | 12 | 40.4 (4.8) | 100.0 | Community Melbourne Australia | 27.8(5.4) | 4.6 (0.4) | NR | NR | Women with a child in schools without pregnancy and serious medical conditions | Recruited by invitation in school newsletter | 14.0% |
| Ma et al. 2009&2013 | 241 | 15 | 52.9(10.6) | 47.0 | Clinic San Francisco CA78.0% white 17.0% Asian | 32.0 (5.4) | 5.6 (0.5) | NR | NR | Patients with age>18 yrs, BMI>25 kg/m2, with pre-DM defined by FPG of 5.6–6.9 mmol/L, or MetS | Recruited from a single primary care clinic | 8.3% |
| Marsh etal. 2010 | 96 | 12 | 30.2(5.2) | 100.0 | Clinic Sydney Australia | 34.5 (4.2) | 4.8 (0.7) | NR | 15.6(10.8) | Women, aged 18–40yrs; BMI<25 kg/m2, with polycystic ovary syndrome, without pregnancy and DM | Recruited from a screening program | 49.0% |
| McAuley et al. 2005&2006 | 93 | 12 | Range: 30– 70y | 100.0 | Community Dunedin New Zealand | 35.7 (5.0) | 5.1 (0.6) | NR | 13.9(6.9) | Overweight women, aged 30– 70yrs; BMI>27 kg/m2; without pregnancy | Recruited by local ads | 18.3% |
| Mellberg et al. 2014 | 70 | 24 | 59.9(5.7) | 100.0 | Community Umea Sweden | 32.7 (3.5) | 5.2 (1.1) | NR | 8.7 (4.4) | Postmenopausal non-smoking women, BMI>27 kg/m2, without DM, FPG<7.0 mmol/L | Recruited by newspapers ads | 30.0% |
| Muto et al. 2001 | 326 | 18 | 42.5 (3.7) | 0.0 | Community Tokyo Japan | 24.7 (3.0) | 5.6 (1.3) | NR | NR | Male workers with at least one abnormality, including FPG>5.6 mmol/L | Recruited from a building maintenance company | 7.4% |
| Narayan et al. 1998 | 95 | 12 | Range: 25– 50y | 75.8 | Community Pima AZ | Range:20.2–59.9 | Range: 4.2–6.5 | Range: 4.5–6.3 | Range: 24–137 (pM) | Overweight or obese people, aged 25–54yrs; BMI>25kg/m2, without DM, OGTT<7.8 mmol/L | Recruited from an ongoing epidemiological study | 2.0% |
| Nilsson et al. 1992 | 94 | 12 | 55.0 (7.2) | NR | Community Dalby Sweden | Weight(kg):81.4(11.6) | 5.0 (0.5) | NR | 17.6 (8.9) | Patients with or without FIT, but no DM | Recruited from a cross- sectional study | 8.5% |
| Nilsson et al 2001 | 113 | 18 | 49.7 (6.2) | 60.9 | Community Flelsingborg Sweden | 27.8(5.6) | 4.9 (1.2) | NR | 8.7 (5.7) | Aged 40–50yrs; with a cardiovascular risk score sum of >9 | Recruited from a screening program | 18.6% |
| Ockene et al. 2012 Merriam et al. 2009 | 312 | 12 | 52.0(11.2) | 74.4 | Community Lawrence MA | 33.9 (5.6) | 5.8 (0.7) | NR | 20.0(13.6) HOMA-IR: 5.2 (3.8) | Age>25yrs+, BMI>24kg/m2, with risk for DM, but without DM | Recruited from the Greater Lawrence Family Flealth Center patient panel | 7.4% |
| Poston et al. 2006 | 250 | 12 | 41.0(8.5) | 92.4 | Community Huston TX | 36.1 (3.1) | 4.5 (0.7) | NR | NR | Overweight or obese people, aged 25–55yrs; BMI: 27–40 kg/m2; without DM or pregnancy for women, FPG<7.0 mmol/L, confirmed byOGTT | Recruited from a screening program | 45.6% |
| Potteiger et al. 2003 & 2002 | 66 | 16 | NR | 57.6 | Community Denver CO | Range: 25– 34.9 | 5.5 (0.4) | NR | 11.4(4.4) | Sedentary people without DM and heart disease | Recruited from part of the Midwest Exercise Trial | 10.1% |
| Reid etal. 2014 | 426 | 12 | 51.5 (11.6) | 61.3 | Clinic Ottawa Canada 95.3% white | 29.4 (5.7) | 5.1 (0.6) | NR | NR | Obese people with coronary risk, without DM, pregnancy, FPG<7.0 mmol/L | Recruited from a care cardiac center by ads and flyers | 25.8% |
| Rossner et al. 1997 | 93 | 12 | 41.0 (NR) | 67.7 | Clinics Stockhlom Sweden | 38.7 (4.5) | 5.2 (1.3) | NR | NR | Obese people with BMI> 30 kg/m2, without DM | Recruited from hospital waiting list | 38.7% |
| Ryttig et al. 1997 | 81 | 28 | 42.5 (10) | 54.3 | Clinics Stockhlom Sweden | 37.7 (4.6) | 5.5 (1.2) | NR | NR | Obese people, aged 21–64yrs; BMI:>30 kg/m2; without DM or pregnancy | Recruited from hospital waiting list | 4.9% |
| Sartorelli et al. 2005 | 104 | 12 | 45.5 (9.1) | 79.8 | Community Sao Paulo Brazil | 28.7 (2.5) | 5.2 (0.5) | NR | NR | Overweight or obese people, aged 30–65yrs; BMI: 24–35 kg/m2; without DM, or pregnancy | Recruited from a screening program of high-risk group for DM, or from ads | 31.7% |
| Simkin-Silverman et al. 1995 & 1998 & 2003Kuller et al. 2001 &2006&2012 | 535 | 54 | 47.0(1.0) | 100.0 | Community Allegheny PN 92.0% white | 25.1 (3.3) | 5.4 (0.8) | NR | NR | Premenopausal women, aged 44–50yrs; BMI: 20–34 kg/m2; FPG<7.8 mmol/L | Recruited from the Women’s Healthy Lifestyle Project | 2.8% |
| Staten et al. 2004 | 361 | 12 | 57.2 (4.8) | 100.0 | Community Tucson AZ 100% Hispanics | 29.5 (5.3) | 5.9 (2.5) | NR | NR | Uninsured Hispanic women, age>50yrs, | Recruited from clinicregistration | 33.4% |
| Stefanick et al. 1998 | 377 | 12 | 52.1 (7.3) | 47.7 | Community Palo Alto CA | 26.7 (3.0) | 5.3 (0.5) | NR | NR | Postmenopausal women, aged 45–64yrs; men aged 30–64yrs; without DM, FPG<7.8 mmol/L, OGTT confirmed | Recruited from the Diet and Exercise for Elevated Risk Trial | 27.0% |
| Tapsell et al. 2014 | 120 | 12 | 48.9 (9.3) | 75.0 | Community Wollongong Australia | 30.0(2.7) | 5.3 (0.5) | NR | Median (IQR): 10.7 (7.5–13.7) for IG 11.4(8.3– 15.1) for CG | Healthy adults aged 18–65yrs, BMI: 25–35 kg/m2, without DM | Recruited by ads in the local media | 22.5% |
| ter Bogt et al. 2009 | 457 | 12 | 56.1 (7.8) | 57.9 | Community Bilthoven The Netherlands | 29.6 (3.4) | 5.2 (0.6) | NR | NR | Overweight or obese people, aged 40–70yrs; BMI: 25–40 kg/m2; with HT or dyslipidemia, without DM | Recruited from a screening program | 9.0% |
| Thompson et al. 2005 | 90 | 12 | 41.4(8.9) | 85.6 | Clinic Knoxville TN | 34.8(3.1) | 5.2 (0.5) | NR | 11.0(6.5) | Obese people, aged 25–70yrs; BMI: 30–40 kg/m2; without DM or pregnancy | Recruited from ad posters | 13.3% |
| Tsai et al. 2010 | 50 | 12 | 49.4(11.9) | 88.0 | Clinic Philadelphia PN 19.0% white 81.0% black | 36.5 (6.0) | 5.5 (1.5) | NR | NR | Overweight or obese people with BMI: 27–50 kg/m2, without serious psychiatric illness | Recruited from flyers, and referrals from PCPs | 6.0% |
| Vainionpaa et al. 2007 | 120 | 12 | Range: 35– 40y | 100.0 | Community Oulu Finland | 25.3 (4.6) | 4.8 (0.5) | NR | 4.9 (3.3) | Women aged 35–40yrs, without chronic disease | Recruited from the National Population Register of Finland | 33.3% |
| Vetter et al. 2013 Wadden et al. 2011 | 390 | 24 | 51.5 (11.5) | 79.7 | Clinic Philadelphia PA 59.0% white 38.5%black | 38.5 (4.7) | 5.8 (1.8) | NR | 13.5 (10.0) HOMA-IR: 3.4 (2.9) | Aged 21yrs+; BMI: 30–50 kg/m2; with MetS (FPG> 6.1 mmol/L); without cardiovascular events | Recruited from primary care practices | 13.8% |
| von Thiele Schwarz et al. 2008 | 195 | 12 | 46.6(10.8) | 100.0 | Community Stockholm Sweden | NR | 5.0 (0.5) | 4.4 (0.3) | NR | Working age women without DM and pregnancy | Recruited from a public dental health care organization | 9.2% |
| Watanabe et al. 2003 | 173 | 12 | 55.1 (7.1) | 0.0 | Community Tokyo Japan | 24.4 (2.9) | 5.8 (0.6) | NR | NR | Male workers with risk for DM, aged 35–70yrs; OGTT confirmed | Recruited from annual checkup list | 9.8% |
| Weinstock et al. 1998 | 45 | 23 | 43.3 (7.4) | 100.0 | Community Syracuse NY | 35.9 (6.0) | 5.1 (0.6) | NR | 15.4 (6.9) | Women without DM, CAD, and pregnancy | Recruited from a cohort study | 0.0% |
| Weiss et al. 2006 | 48 | 12 | 56.8 (3.0) | 63.2 | Community St. Louis MO | 27 .3 (2 . 1) | 5.3 (0.4) | NR | 7.8 (5.1) | Sedentary people, aged 50– 60yrs; BMI:23.5–29.9kg/m2; non-smoker without DM. FPG<7.0 mmol/L, OGTT confirmed | Recruited from a screening program | 4.2% |
| Wing et al. 1995 | 202 | 18 | 37.4 (5.3) | 48.1 | Community Pittsburgh PA | 30 .9 (2 . 1 ) | 5.5 (0.7) | NR | 27.1 (15.6) | Aged 25–45yrs; 13.6–31.8 kg above ideal body weight, without serious disease | Recruited from newspaper or radio ads | 21.3% |
| Wing et al. 1998 | 154 | 24 | 45.7 (4.4) | 79.0 | Community Pittsburgh PA | 3 5 . 9 ( 4.3) | 5.9 (0.6) | 7.2 (0.5) | 15.9 (13.4) | Overweight people, aged 40– 55yrs; with diabetic parents | Recruited from newspaper ads | 22.0% |
| Wycherley et al. 2012 | 123 | 12 | 50.8 (9.3) | 0.0 | Clinic Adelaide Australia | 33 . 0 (3.9) | 5.8 (0.7) | NR | 10.0 (6.7) | Overweight or obese males, aged 20–65yrs; BMI: 27–40 kg/m2, without DM | Recruited by a screening program | 44.7% |
| Mean (SD) Total Range |
1394320-1089 | 12–54 | 50.9 (8.4) | 0–100 | 30.1 (4.4) 23.3–38.7 | 5.3 (1.0) | 5.4 (0.6) | 13.7 (9.0) HOMA-IR: 3.9 (2.8) | 0–60.0 | |||
Abbreviations: BG: blood glucose; BMI: body mass index; CAD: coronary Artery Disease; CVD: cardiovascular disease; DBP: diastolic blood pressure; DM: diabetes mellitus; FBG: fasting blood glucose; FPG: fasting plasma glucose; HbAlc: glycated hemoglobin A1c; HDL: high density cholesterol; HT: hypertension; IGT: impaired glucose tolerance; LDL: low density cholesterol MetS: metabolic syndrome; NR: not reported; OGTT: oral glucose tolerance test; PCP: primary care physician; PG: plasma glucose; SD: standard deviation
Fig. 1 --.
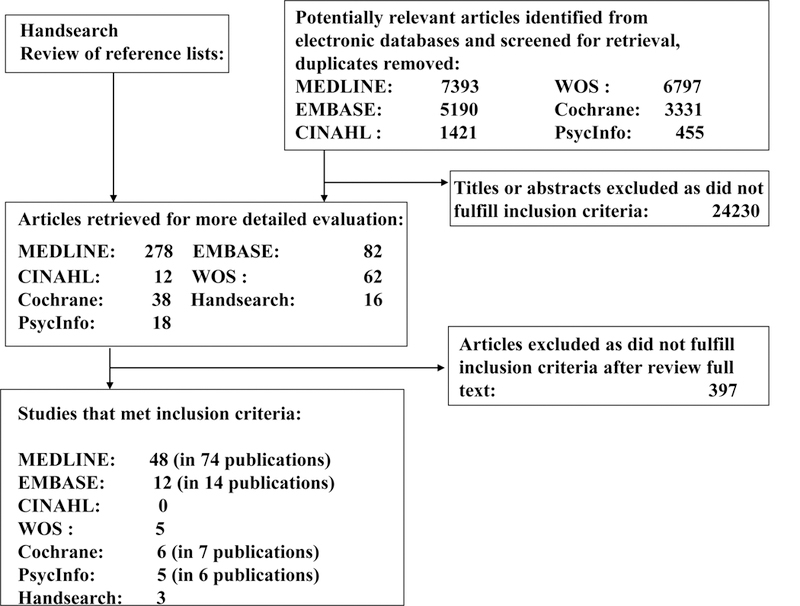
Study flow diagram. CINAHL, Cumulative Index to Nursing and Allied Health Literature. EMBASE, Excerpta Medica Database. MEDLINE, Medical Literature Analysis and Retrieval System Online. PsycInfo, Psychological Infomration Database. WOS, Web of Science.
We observed considerable heterogeneity in the treatments provided to both intervention and comparison groups (Table S1 and Table S2, presented online as supplementary materials). In 27 studies, a similar approach was used in both intervention and control groups (data from these studies were synthesized by a single-arm model). In the other 44 studies, the control group received usual care (UC). In the 44 studies that compared an intervention to UC, 32 had 2 arms [8–10,14,17,18,22,27–29,32,38,40,42,43,45,47,52–59,62,67,69,70,72,76,104], 5 studies [34,48,71,72,75] had 3 arms, and 7 studies [11,15,30,39,46,65,77] had 4 arms (e.g., PA, D, PA+D and control arm). The randomization procedure was described in 43 studies (Table S2). Allocation concealment (i.e., blinding) was adequately reported in 26 studies. Meta-regression analyses indicated that there was no significant interaction between the between-group change in FPG and any study-level characteristic. An Egger’s plot demonstrated a symmetrical shape distribution (except for 2 outliers) which supports a hypothesis of no publication bias.
3.1. Changes in Glycemic Indicators
In 52 studies or study arms comparing interventions to UC with attrition <30%, the pooled effect estimate from all studies demonstrated that compared to UC, lifestyle interventions—including PA, D, or PA+D—achieved significant reductions in FPG (−0.14mmol/L [95% CI, −0.18, −0.09]), HbA1c (−0.05% [−0.08, −0.03]), FI (percent change: −15.18% [−20.01, −10.35]), HOMA-IR (percent change: −22.66% [−29.19, −16.14]), and body weight (percent change: −4.00%[−4.73, - 3.26]) (Table 2). Sensitivity analyses including studies with attrition ≥30% in the model produced similar results.
Table 2,
Lifestyle Interventional Effect: Meta-Analyses Results
| FPG (mmol/L) | HbA1c (%) | % Change in Fasting Insulin | % Change in HOMA-IR | Weight Loss (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies (sample size) |
pooled effect mean (Effect size) (95%CI) |
Hetero- geneity p value |
Studies (sample size) |
pooled effect mean (Effect size) (95%CI) |
Hetero- geneity p value |
Studies (sample size) |
pooled effect mean (Effect size) (95%CI) |
Hetero- geneity p value |
Studies (sample size) |
pooled effect mean (Effect size) (95%CI) |
Hetero- geneity p value |
Studies (sample size) |
pooled effect mean (Effect size) (95%CI) |
Hetero- geneity p value |
|
| LI vs UC (all studiesa) | 52 (8919) | −0.14 (0.07) (−0.18, −0.09) | <0.01 | 9 (2617) | −0.05 (0.06) (−0.08, −0.03) | 0.54 | 33 (5308) | −15.18 (0.08) (−20.01, −10.35) | <0.01 | 19 (2846) | −22.66 (0.13) (−29.19, −16.14) | <0.01 | 45 (8167) | −4.00 (0.12) (−4.73, −3.26) | <0.01 |
| LI vs UC (all studiesb) | 59 (9446) | −0.12 (0.05) (−0.17, −0.08) | <0.01 | 10 (2709) | −0.05 (0.06) (−0.08, −0.02) | 0.57 | 37 (5501) | −14.38 (0.08) (−19.04, −9.72) | <0.01 | NA | 53 (8786) | −3.80 (0.12) (−4.47, −3.13) | <0.01 | ||
| LI vs UC (Group 1c) | 23 (4263) | −0.08 (0.08) (−0.11, −0.04) | 0.05 | 2 (246) | −0.07 (0.12) (−0.14, −0.01) | 0.29 | 12 (1551) | −11.69 (0.11) (−16.99, −6.38) | 0.04 | 5 (837) | −13.11 (0.08) (−24.60, −1.61) | <0.01 | 18 (3165) | −3.71 (0.11) (−4.86, −2.56) | <0.01 |
| LI vs UC (Group 2d) | 29 (4656) | −0.19 (0.08) (−0.26, −0.12) | <0.01 | 7 (2371) | −0.05 (0.04) (−0.08, −0.02) | 0.52 | 21 (3747) | −16.56 (0.08) (−23.14, −9.98) | <0.01 | 14 (2009) | −28.05 (0.17) (−35.43, −20.67) | <0.01 | 27 (5002) | −4.19 (0.12) (−5.19, −3.18) | <0.01 |
| LI vs UC (F/U=12 mo) | 43 (7221) | −0.10 (0.06) (−0.14, −0.07) | <0.01 | 5 (2306) | −0.05 (0.07) (−0.08, −0.02) | 0.41 | 25 (4521) | −15.45 (0.08) (−21.22, −9.69) | <0.01 | 15 (2147) | −24.39 (0.09) (−36.10, −12.69) | <0.01 | 37 (6627) | −3.66 (0.10) (−4.53, −2.80) | <0.01 |
| LI vs UC (13–23 mo) | 8 (1560) | −0.15 (0.12) (−0.21, −0.09) | 0.91 | 1 (158) | −0.10 (0.19) (−0.18, −0.02) | NA | 4 (496) | −11.04 (0.09) (−22.33, 0.25) | 0.24 | 1 (158) | −14.63 (0.13) (−32.44, 3.18) | NA | 6 (1289) | −3.28 (0.16) (−4.39, −2.17) | 0.09 |
| LI vs UC (≥24 mo) | 15 (3423) | −0.12 (0.04) (−0.23, −0.001) | <0.01 | 4 (1242) | −0.03 (0.03) (−0.08, 0.01) | 0.78 | 15 (3426) | −11.30 (0.05) (−18.68, −3.91) | <0.01 | 7 (1567) | −20.07 (0.13) (−27.73, −12.40) | <0.01 | 15 (3424) | −3.58 (0.09) (−4.98, −2.19) | <0.01 |
| PA vs UC | 14 (1813) | −0.07 (0.08) (−0.11, −0.03) | 0.74 | 3 (1227) | −0.04 (0.06) (−0.08, 0.01) | 0.94 | 9 (1555) | −7.61 (0,05) (−15.52, 0.30) | 0.06 | 5 (233) | −7.25 (0.08) (−19.02, 4.51) | 1.00 | 12 (1663) | −1.55 (0.08) (−2.53, −0.57) | 0.38 |
| D vs UC | 7 (499) | −0.17 (0.15) (−0.27, −0.08) | 0.46 | 1 (50) | 0.00 (0.00) (−0.23, 0.23) | NA | 5 (321) | −13.73 (0.10) (−28.64, 1.18) | 0.06 | 2 (282) | −24.24 (0.22) (−37.21, −11.27) | 0.94 | 6 (433) | −6.21 (0.24) (−8.63, −3.19) | <0.01 |
| PA+D vs UC | 31 (6607) | −0.15 (0.06) (−0.21, −0.09) | <0.01 | 5 (1340) | −0.07 (0.09) (−0.11, −0.02) | 0.21 | 19 (3432) | −18.25 (0.10) (−24.18, −12.32) | <0.01 | 12 (2431) | −24.69 (0.13) (−32.15, −17.23) | <0.01 | 27 (6071) | −4.15 (0.12) (−5.02, −3.27) | <0.01 |
Abbreviations: CI: confidence interval; D: diet; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin A1c; HOMA-IR: homeostasis model assessment of insulin resistance; LI: lifestyle intervention; mo: month; PA: physical activity; UC: usual care; vs: versus
All studies with attrition <30%
All studies with attrition <30% plus studies with attrition ≥30%
All studies with attrition <30% and participants with FPG<5.5 mmol/L or HbA1c <5.5%
All studies with attrition <30% and participants with FPG≥5.5 mmol/L or HbA1c≥5.5%
3.1.1. Comparison According to Participant Baseline Glycemic Status (Limited to studies with attrition <30% thereafter)
In studies among persons with low-range glycemic status, lifestyle interventions were associated with significantly reduced FPG (−0.08mmol/L [−0.11, −0.04]), HbA1c (−0.07% [−0.14, −0.01]), FI (percent change: −11.69% [−16.99, −6.38]), HOMA-IR (percent change: −13.11%[−24.60, −1.61]), and body weight (percent change: −3.71% [−4.86, −2.56]). In studies among persons with high-range glycemic status, lifestyle interventions decreased FPG (−0.19mmol/L [−0.26, −0.12]), HbA1c (−0.05% [−0.08, −0.02]), FI (percent change: −16.56% [−23.14, −9.98]), HOMA-IR (percent change: −28.05% [−35.43, −20.67]), and body weight (percent change: −4.19% [−5.19, − 3.18]). Notably, intervention effects on FPG differed in absolute values (−0.08mmol/L in low- range vs −0.19mmol/L in high-range groups), but the effect sizes were the same across groups (0.08).
3.1.2. Comparison According to Length of Follow-Up
Similar reductions in FPG and percent body weight were achieved in 12 months of follow-up (− 0.10mmol/L [−0.14, −0.07], and −3.66% [−4.53, −2.80]), 13–23 months (−0.15mmol/L[−0.21, - 0.09], and −3.28% [−4.39, −2.17]), and ≥24 months (−0.12mmol/L [−0.23, −0.001], and −3.58%[- 4.98, −2.19]). Meta-regression analyses demonstrated that the association between the magnitude of percent body weight change and the magnitude of FPG change was not significant (P = 0.183, and the R2 for correlation between percent body weight change and FPG change was very low [0.037]).
3.1.3. Comparison According to Intervention Modality
Analyses stratified by intervention types showed that each type was effective, with D vs UC achieving the highest reduction in FPG (−0.17mmol/L [−0.27, −0.08]), followed by PA+D vs UC (−0.15mmol/L [−0.21, −0.09]), and then PA vs UC (−0.07mmol/L [−0.11, −0.03]). Reduction in body weight followed a similar pattern, with greater weight loss among comparisons of D vs UC (−6.21% [−8.63, −3.19]) and PA+D vs UC (−4.15% [−5.02, −3.27]) than for PA vs UC (−1.55% [2.53, −0.57]). Similar patterns were also observed for percent changes in FI and HOMA-IR with PA+D vs UC (FI: −18.25% [−24.18, −12.32], HOMA-IR: −24.69% [−32.15, −17.23]), followed by D vs UC (FI: −13.73% [−28.64, 1.18], HOMA-IR: −24.24% [−37.21, −11.27]), and PA vs UC (FI: - 7.61% [−15.52, 0.30], HOMA-IR: −7.25% [−19.02, 4.51]). Pooled effects of interventions on FPG are shown in Figure 2 and meta-analyses results in a single arm model are presented online as Table S3.
Fig. 2 --.
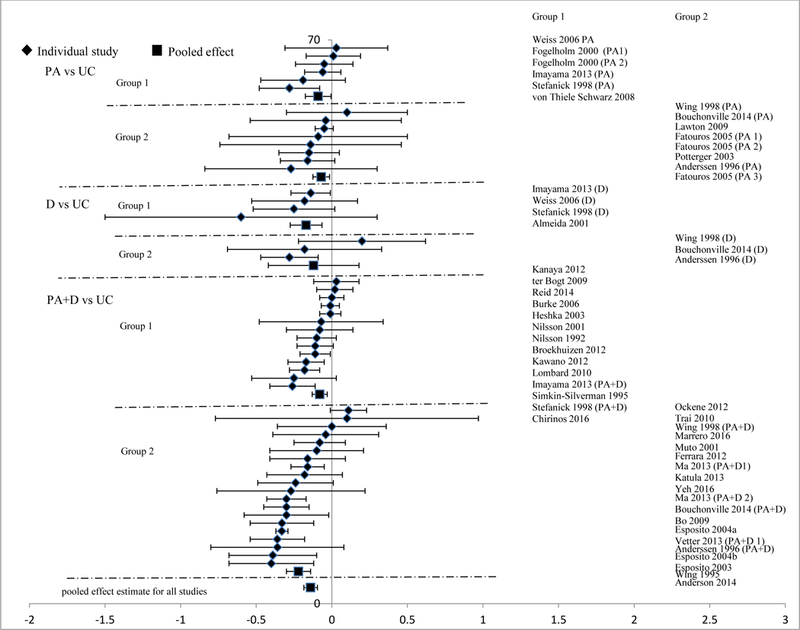
Changes in fasting plasma glucose in the intervention versus usual care groups (mmol/L). Group 1: low-range glycemic group (FPG <5.5mmol/L or HbA1c <5.5%). Group 2: high-range glycemic group (FPG ≥5.5 mmol/L or HbA1c ≥5.5%). D, diet. PA, physical activity. UC, usual care. vs versus.
4. Discussion
The target population for type 2 diabetes mellitus prevention has been a topic of debate since the completion of major diabetes prevention trials [109]. The difficulty stems from observations that diabetes prevalence has increased across all segments of society [110], yet the evidence for preventive interventions is mainly limited to persons with IGT [2–4,111,112]. This comprehensive review of the effects of structured lifestyle interventions yielded 3 main findings.
First, lifestyle interventions among individuals with lower risk than those with IGT and diabetes led to significant improvements in FPG, HbA1c, and FI among the full range of baseline risk, with no apparent differences measured by effect sizes between studies of persons with low-range vs high-range glycemic levels. The average magnitude of effect of 1-year change in FPG of about −0.3mmol/L was about 40% of the magnitude of effect seen among persons with IGT in the U.S. Diabetes Prevention Program and the Finnish Diabetes Prevention Study, wherein lifestyle interventions resulted in a −0.2mmol/L glucose change and a 58% reduced incidence of diabetes [2]. The findings from our meta-analyses imply that the reduction in glucose may bring about similar diabetes risk reductions among population without IGT.
Second, interventions that focused only on PA without a concomitant calorie restriction had weaker effects on glycemic indicators than studies that used PA and calorie restriction, or calorie restriction alone. Third, interventions were effective across a wide variation of follow-up durations, from 1 year in 43 studies, to more than 2 years in 15 studies. Taken as a whole, our findings suggest that multi-faceted interventions combining PA and D are likely to be effective in improving glucose regulation and reducing risk for diabetes in populations with average low- range and high-range glucose levels.
Several components of lifestyle interventions have been associated with improved insulinmediated glucose transport and therein reduce insulin resistance and progression to glucose intolerance. Our findings were generally supportive of the diabetes prevention trials, which suggest that multi-component interventions, including elements of calorie restriction, PA, and behavioral support are most effective in improving glucose tolerance. However, our study did not find a significant correlation between the magnitude of weight loss and magnitude of glucose benefit. Our study found slightly weaker effects for exercise-only interventions (e.g., PA alone resulted in only 1.55% weight loss, far lower than the 5% recommended by American Diabetes Association (ADA)) [113]. In addition to non-insulin mediated glucose transport in skeletal muscle, exercise programs have been shown to have important independent effects on insulinmediated glucose regulation, markers of inflammation, insulin resistance, blood pressure, lipid profile, fitness, and improved lean-to-fat mass ratio [114]. Given the fact that PA provides more benefits than just weight loss does, we need to take those extra benefits into account when we interpret our findings.
Our finding of no difference in effect by follow-up duration has potentially encouraging implications for the implementation of preventive interventions. Weight loss programs typically lead to a nadir of weight loss around 6 months followed by a gradual weight regain. Even programs with intensive attention to weight maintenance typically result in a 50% average weight regain over 3 to 4 years. Our findings that changes in glucose were as great in studies with longer (≥ 2 years) as shorter (12 months) follow-up duration suggest that the between-group improvement in FPG could persist [27–29,42,76]. These findings echo the extended benefits found in the Da Qing legacy study [115]. It is worth noting that most of these studies applied a PA+D strategy and included some behavioral modification components.
Our findings have important implications for the definitions of diabetes risk groups as well as clinical and public health strategies to reduce diabetes incidence. Despite strong evidence that lifestyle interventions can reduce diabetes incidence, the RCT evidence is limited to individuals with IGT. However, roughly 60% of individuals with ADA-defined pre-diabetes (measured by IFG: 5.6–6.9mmol/L) [116] and 70% of those with World Health Organization-defined intermediate hyperglycemia (measured by IFG: 6.1–7.7mmol/L) [117] do not have IGT. Individuals with isolated IFG are thought to be more affected by beta cell dysfunction than by insulin resistance and thus may be less likely to benefit from lifestyle interventions. This has fueled continued debate over who should be targeted for diabetes prevention programs. Our findings suggest that lifestyle interventions are likely to have important benefits across the full distribution of HbA1c and fasting glucose and insulin levels. However, the types of interventions that should be applied to individuals with low to moderate levels of glycemic risk are ultimately influenced by economic factors as well as the effectiveness of interventions. Economic analyses have shown that structured lifestyle interventions are considerably more cost-effective when applied to persons at the high end of the FPG and HbA1c distribution [118,119]. Comprehensive strategies to reduce incidence likely require graduated tiers of interventions. Population-wide approaches to improve nutrition and PA will likely provide benefit to the entire population, but the magnitude of that benefit is unknown. A comprehensive approach that includes both effective population-wide approaches along with structured lifestyle interventions proven to be effective should be the goal.
There are several limitations in our study. First, only 2 studies reported the number of cases of diabetes, thus precluding even pooled estimates of the effect of the interventions on diabetes incidence rates. This reflects the fact that an intervention trial of diabetes incidence conducted among persons in the low- to high-range glucose status (from normal glucose [<5.5mmol/L] - below the IGT threshold) would require large sample sizes (i.e. several thousand participants) over several years.
Second, the large number of studies evaluated lends itself to many sources of heterogeneity, including intervention type, dose, intensity, and frequency, as well as individual risk status and levels of adherence. Our analyses of heterogeneity were conducted at the study level rather than at the individual level, and thus may have lacked sensitivity to detect the impact of such factors on glycemic indicators. Although the study population was diverse in terms of age, race/ethnicity, and sex, we were unable to test whether intervention effectiveness varied across these factors. An advantage of such diversity in our review, however, is that the effect sizes may be more reflective of real-world variation than those observed in a single large RCT.
Third, although we attempted to quantify and stratify by level of glycemic risk, there was considerable heterogeneity within studies that prevented a clean classification. As a result, there was likely considerable overlap in participant characteristics between the low-range group and high-range group in our study. A precise determination of how intervention effectiveness varies by baseline levels of glycemia may require individual level data.
Despite these limitations, this is the first comprehensive compilation of the impact of lifestyle interventions on risk for progression of dysglycemia among individuals below the IGT threshold. This comprehensive systematic review suggests that lifestyle change is important for diabetes prevention across the full spectrum of risk, complementing the major trials of diabetes incidence that focused on individuals with IGT. Decisions about how to implement prevention in practice ultimately depend on a wider set of factors, including the cost of delivering different types of interventions and the disease incidence level in the target population. For example, structured lifestyle interventions have been found to be considerably more cost-effective among persons with higher levels of HbA1c or FPG because applying interventions to persons with a higher incidence of diabetes lead to greater reduction in costs of complications and health care utilization. Thus, multiple intervention tiers may be warranted for diabetes prevention, with intense structured programs delivered to persons at higher risk, and population-targeted policies aimed at making healthier food and physical activity choices easier for the lower end of the diabetes risk distribution.
Supplementary Material
Acknowledgement:
We thank Drs. Ann Albright, Elizabeth Luman, and Sharon Saydah for their very helpful comments on revising our manuscript.
No specific funding was received for this study. This study was supported by the Centers for Disease Control and Prevention. No funding bodies had any role in the study design, data collection, analysis, decision to publish or preparation of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC
Footnotes
Conflicts of interest disclosures:
No actual or potential conflicts of interest exist.
References
- [1].Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Available at http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Last accessed 19 April 2015.
- [2].Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997; 20: 537–44. [DOI] [PubMed] [Google Scholar]
- [4].Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–50. [DOI] [PubMed] [Google Scholar]
- [5].Johnson M, Jones R, Freeman C, Woods HB, Gillett M, Goyder E et al. Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence. Diabet Med 2013; 30: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. [Version 5.1.0 [updated March 2011]]. 2011. The Cochrane Collaboration; Available at www.cochrane-hand-book.org. Last accessed 12 March 2015. [Google Scholar]
- [7].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1954; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- [8].Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 2008; 35: 357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Almeida LB, Segurado AC, Duran ACF, Jaime PC. Impact of a nutritional counseling program on prevention of HAART-related metabolic and morphologic abnormalities. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV 2011; 23: 755–63. [DOI] [PubMed] [Google Scholar]
- [10].Anderson AS, Craigie AM, Caswell S, Treweek S, Stead M, Macleod M et al. The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ 2014; 348: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Anderssen SA, Hjermann I, Urdal P, Torjesen PA, Holme I. Improved carbohydrate metabolism after physical training and dietary intervention in individuals with the “atherothrombogenic syndrome’. Oslo Diet and Exercise Study (ODES). A randomized trial. J Intern Med 1996; 240: 203–9. [DOI] [PubMed] [Google Scholar]
- [12].Arguin H Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: a pilot study. Menopause 2012; 19: 870–6. [DOI] [PubMed] [Google Scholar]
- [13].Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y et al. Effects of low- carbohydrate and low-fat diets: a randomized trial. Ann Intern Med 2014; 161: 309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bo S, Ciccone G, Baldi C, Benini L, Dusio F, Forastiere G et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J Gen Intern Med 2007; 22: 1695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bouchonville M, Armamento-Villareal R, Shah K, Napoli N, Sinacore DR, Qualls C et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond) 2014; 38: 423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brinkworth GD, Noakes M, Keogh JB, Luscombe ND, Wittert GA, Clifton PM. Long¬term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord 2004; 28: 661–70. [DOI] [PubMed] [Google Scholar]
- [17].Broekhuizen K, van Poppel MN, Koppes LL, Kindt I, Brug J, van Mechelen W. No significant improvement of cardiovascular disease risk indicators by a lifestyle intervention in people with familial hypercholesterolemia compared to usual care: results of a randomised controlled trial. BMC Res Notes 2012; 5: 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Burke V, Beilin LJ, Cutt HE, Mansour J, Williams A, Mori TA. A lifestyle program for treated hypertensives improved health-related behaviors and cardiovascular risk factors, a randomized controlled trial. J Clin Epidemiol 2007; 60: 133–41. [DOI] [PubMed] [Google Scholar]
- [19].Burtscher M, Gatterer H, Kunczicky H, Brandstatter E, Ulmer H. Supervised exercise in patients with impaired fasting glucose: impact on exercise capacity. Clin J Sport Med 2009; 19: 394–8. [DOI] [PubMed] [Google Scholar]
- [20].Choo J, Lee J, Cho JH, Burke LE, Sekikawa A, Jae SY. Effects of weight management by exercise modes on markers of subclinical atherosclerosis and cardiometabolic profile among women with abdominal obesity: a randomized controlled trial. BMC Cardiovasc Disord 2014; 14: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Clifton PM, Keogh JB, Noakes M. Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr 2008; 87: 23–9. [DOI] [PubMed] [Google Scholar]
- [22].Cole RE, Boyer KM, Spanbauer SM, Sprague D, Bingham M. Effectiveness of prediabetes nutrition shared medical appointments: prevention of diabetes. Diabetes Educ 2013; 39: 344–53. [DOI] [PubMed] [Google Scholar]
- [23].Coon PJ, Bleecker ER, Drinkwater DT, Meyers DA, Goldberg AP. Effects of body composition and exercise capacity on glucose tolerance, insulin, and lipoprotein lipids in healthy older men: a cross-sectional and longitudinal intervention study. Metabolism 1989; 38: 1201–9. [DOI] [PubMed] [Google Scholar]
- [24].Cox KL, Burke V, Beilin LJ, Puddey IB. A comparison of the effects of swimming and walking on body weight, fat distribution, lipids, glucose, and insulin in older women-the Sedentary Women Exercise Adherence Trial 2. Metabolism: Clinical and Experimental 2010; 59:1562–73. [DOI] [PubMed] [Google Scholar]
- [25].Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr 1999; 69: 198–204. [DOI] [PubMed] [Google Scholar]
- [26].Donnelly JE, Jacobsen DJ, Heelan KS, Seip R, Smith S. The effects of 18 months of intermittent vs. continuous exercise on aerobic capacity, body weight and composition, and metabolic fitness in previously sedentary, moderately obese females. Int J Obes Relat Metab Disord 2000; 24: 566–72. [DOI] [PubMed] [Google Scholar]
- [27].Esposito K, Marfella R, Ciotola M, Palo C, Giugliano F, Giugliano G et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004; 292: 1440–6. [DOI] [PubMed] [Google Scholar]
- [28].Esposito K, Giugliano F, Di PC, Giugliano G, Marfella R, D’Andrea F et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004; 291: 2978–84. [DOI] [PubMed] [Google Scholar]
- [29].Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA: Journal of the American Medical Association 2003; 289: 1799–804. [DOI] [PubMed] [Google Scholar]
- [30].Fatouros IG, Tournis S, Leontsini D, Jamurtas AZ, Sxina M, Thomakos P et al. Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity related. J Clin Endocrinol Metab 2005; 90: 5970–7. [DOI] [PubMed] [Google Scholar]
- [31].Fernandez AC, Casariego AV, Rodriguez IC, Pomar MDB. One-year effectiveness of two hypocaloric diets with different protein/carbohydrate ratios in weight loss and insulin resistance. Nutr Hosp 2012; 27: 2093–101. [DOI] [PubMed] [Google Scholar]
- [32].Ferrara AL, Pacioni D, Di Fronzo V, Russo BF, Staiano L, Speranza E et al. Lifestyle Educational Program Strongly Increases Compliance to Nonpharmacologic Intervention in Hypertensive Patients: A 2-Year Follow-Up Study. J Clin Hypertens (Greenwich) 2012; 14: 767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fisher G, Hunter GR, Gower BA. Aerobic exercise training conserves insulin sensitivity for 1 yr following weight loss in overweight women. J Appl Physiol 2012; 112: 688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fogelholm M, Kukkonen-Harjula K, Nenonen A, Pasanen M. Effects of walking training on weight maintenance after a very-low-energy diet in premenopausal obese women: a randomized controlled trial. Arch Intern Med 2000; 160: 2177–84. [DOI] [PubMed] [Google Scholar]
- [35].Fonollá J, López-Huertas E, Machado FJ, Molina D, Alvarez I, Mármol E et al. Milk enriched with “healthy fatty acids” improves cardiovascular risk markers and nutritional status in human volunteers. Nutrition (Burbank) 2009; 25: 408–14. [DOI] [PubMed] [Google Scholar]
- [36].Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res 2005; 13: 615–25. [DOI] [PubMed] [Google Scholar]
- [37].Groeneveld IF, Proper KI, van der Beek AJ, van Mechelen W. Sustained body weight reduction by an individual-based lifestyle intervention for workers in the construction industry at risk for cardiovascular disease: results of a randomized controlled trial. Prev Med 2010; 51: 240–6. [DOI] [PubMed] [Google Scholar]
- [38].Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD et al. Weight Loss with Self-help Compared with a Structured Commercial Program: A Randomized Trial. Journal of the American Medical Association 2003; 289: 1792–8. [DOI] [PubMed] [Google Scholar]
- [39].Imayama I, Alfano CM, Mason C, Wang C, Duggan C, Campbell KL et al. Weight and metabolic effects of dietary weight loss and exercise interventions in postmenopausal antidepressant medication users and non-users: a randomized controlled trial. Prev Med 2013; 57: 525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kanaya AM, Santoyo-Olsson J, Gregorich S, Grossman M, Moore T, Stewart AL. The Live Well, Be Well study: a community-based, translational lifestyle program to lower diabetes risk factors in ethnic minority and lower-socioeconomic status adults. Am J Public Health 2012; 102: 1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kanaya AM, Araneta MR, Pawlowsky SB, Barrett-Connor E, Grady D, Vittinghoff E et al. Restorative yoga and metabolic risk factors: the Practicing Restorative Yoga vs. Stretching for the Metabolic Syndrome (PRYSMS) randomized trial. J Diabetes Complications 2014; 28: 406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Katula JA, Vitolins MZ, Morgan TM, Lawlor MS, Blackwell CS, Isom SP et al. The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med 2013; 44: S324–S332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kawano M, Shono N, Yoshimura T, Yamaguchi M, Hirano T, Hisatomi A. Improved cardio-respiratory fitness correlates with changes in the number and size of small dense LDL: Randomized controlled trial with exercise training and dietary instruction. Intern Med 2009; 48: 25–32. [DOI] [PubMed] [Google Scholar]
- [44].Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. Br J Nutr 2007; 98: 852–9. [DOI] [PubMed] [Google Scholar]
- [45].Lawton BA, Rose SB, Raina Elley C, Dowell AC, Fenton A, Moyes SA. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. BJSM online 2009; 43: 120–6. [PubMed] [Google Scholar]
- [46].Lim SS, Noakes M, Keogh JB, Clifton PM. Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control. Nutr Metab Cardiovasc Dis 2010; 20: 599–607. [DOI] [PubMed] [Google Scholar]
- [47].Lombard C, Deeks A, Jolley D, Ball K, Teede H. A low intensity, community based lifestyle programme to prevent weight gain in women with young children: cluster randomised controlled trial. BMJ 2010; 341: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE): a randomized controlled trial. BMC Fam Pract 2009; 10: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr 2010; 92: 83–92. [DOI] [PubMed] [Google Scholar]
- [50].McAuley KA, Smith KJ, Taylor RW, McLay RT, Williams SM, Mann JI. Long-term effects of popular dietary approaches on weight loss and features of insulin resistance. Int J Obesity 2006; 30: 342–9. [DOI] [PubMed] [Google Scholar]
- [51].Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C et al. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr 2014; 68: 350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Muto T, Yamauchi K. Evaluation of a multicomponent workplace health promotion program conducted in Japan for improving employees’ cardiovascular disease risk factors. Prev Med 2001; 33: 571–7. [DOI] [PubMed] [Google Scholar]
- [53].Narayan KM, Hoskin M, Kozak D, Kriska AM, Hanson RL, Pettitt DJ et al. Randomized clinical trial of lifestyle interventions in Pima Indians: a pilot study. Diabet Med 1998; 15: 66–72. [DOI] [PubMed] [Google Scholar]
- [54].Nilsson PM, Lindholm LH, Schersten BF. Life style changes improve insulin resistance in hyperinsulinaemic subjects: a one-year intervention study of hypertensives and normotensives in Dalby. J Hypertens 1992; 10: 1071–8. [PubMed] [Google Scholar]
- [55].Nilsson PM, Klasson EB, Nyberg P. Life-style intervention at the worksite - Reduction of cardiovascular risk factors in a randomized study. Scandinavian Journal of Work, Environment and Health 2001; 27: 57–62. [DOI] [PubMed] [Google Scholar]
- [56].Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health 2012; 102: 336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Poston WSC, Haddock CK, Pinkston MM, Pace P, Reeves RS, Karkoc N et al. Evaluation of a primary care-oriented brief counselling intervention for obesity with and without orlistat. Journal of Intern Med 2006; 260: 388–98. [DOI] [PubMed] [Google Scholar]
- [58].Potteiger JA, Jacobsen DJ, Donnelly JE, Hill JO, Midwest Exercise T. Glucose and insulin responses following 16 months of exercise training in overweight adults: the Midwest Exercise Trial. Metabolism 2003; 52: 1175–81. [DOI] [PubMed] [Google Scholar]
- [59].Reid RD, McDonnell LA, Riley DL, Mark AE, Mosca L, Beaton L et al. Effect of an intervention to improve the cardiovascular health of family members of patients with coronary artery disease: a randomized trial. CMAJ 2014; 186: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rossner S, Flaten H. VLCD versus LCD in long-term treatment of obesity. Int J Obes Relat Metab Disord 1997; 21: 22–6. [DOI] [PubMed] [Google Scholar]
- [61].Ryttig KR, Flaten H, Rossner S. Long-term effects of a very low calorie diet (Nutrilett) in obesity treatment. A prospective, randomized, comparison between VLCD and a hypocaloric diet+behavior modification and their combination. Int J Obes Relat Metab Disord 1997; 21: 574–9. [DOI] [PubMed] [Google Scholar]
- [62].Sartorelli DS, Sciarra EC, Franco LJ, Cardoso MA. Beneficial effects of short-term nutritional counselling at the primary health-care level among Brazilian adults. Public Health Nutr 2005; 8: 820–5. [DOI] [PubMed] [Google Scholar]
- [63].Simkin-Silverman LR, Wing RR, Boraz MA, Kuller LH. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med 2003; 26: 212–20. [DOI] [PubMed] [Google Scholar]
- [64].Staten LK, Gregory-Mercado KY, Ranger-Moore J, Will JC, Giuliano AR, Ford ES et al. Provider counseling, health education, and community health workers: The Arizona WISEWOMAN project. Journal of Womens Health 2004; 13: 547–56. [DOI] [PubMed] [Google Scholar]
- [65].Stefanick ML, Mackey S, Sheehan M, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med 1998; 339: 12–20. [DOI] [PubMed] [Google Scholar]
- [66].Tapsell LC, Batterham MJ, Thorne RL, O’Shea JE, Grafenauer SJ, Probst YC. Weight loss effects from vegetable intake: a 12-month randomised controlled trial. Eur J Clin Nutr 2014; 68: 778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].ter Bogt NC, Bemelmans WJ, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain: One-year results of a randomized lifestyle intervention. Am J Prev Med 2009; 37: 270–7. [DOI] [PubMed] [Google Scholar]
- [68].Thompson WG, Rostad Holdman N, Janzow DJ, Slezak JM, Morris KL, Zemel MB. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes Res 2005; 13: 1344–53. [DOI] [PubMed] [Google Scholar]
- [69].Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: Results of a randomized controlled pilot study. Obesity (Silver Spring) 2010; 18: 1614–18. [DOI] [PubMed] [Google Scholar]
- [70].Vainionpaa A, Korpelainen R, Kaikkonen H, Knip M, Leppaluoto J, Jamsa T. Effect of impact exercise on physical performance and cardiovascular risk factors. Med Sci Sports Exerc 2007; 39: 756–63. [DOI] [PubMed] [Google Scholar]
- [71].Vetter ML, Wadden TA, Chittams J, Diewald LK, Panigrahi E, Volger S et al. Effect of lifestyle intervention on cardiometabolic risk factors: results of the POWER-UP trial. Int J Obes (Lond) 2013; 37: S19–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].von Thiele Schwarz U, Lindfors P, Lundberg U. Health-related effects of worksite interventions involving physical exercise and reduced workhours. Scandinavian Journal of Work, Environment and Health 2008; 34: 179–88. [DOI] [PubMed] [Google Scholar]
- [73].Watanabe M, Yamaoka K, Yokotsuka M, Tango T. Randomized controlled trial of a new dietary education program to prevent type 2 diabetes in a high-risk group of Japanese male workers. Diabetes Care 2003; 26: 3209–14. [DOI] [PubMed] [Google Scholar]
- [74].Weinstock RS, Dai H, Wadden TA. Diet and exercise in the treatment of obesity: effects of 3 interventions on insulin resistance. Arch Intern Med 1998; 158: 2477–83. [DOI] [PubMed] [Google Scholar]
- [75].Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 2006; 84:1033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wing RR, Jeffery RW. Effect of modest weight loss on changes in cardiovascular risk factors: Are there differences between men and women or between weight loss and maintenance? Int J Obes (Lond) 1995; 19: 67–73. [PubMed] [Google Scholar]
- [77].Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998; 21: 350–9. [DOI] [PubMed] [Google Scholar]
- [78].Wycherley TP, Brinkworth GD, Clifton PM, Noakes M. Comparison of the effects of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutr Diabetes 2012; 2: e40–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Anderssen SA, Holme I, Urdal P, Hjermann I. Associations between central obesity and indexes of hemostatic, carbohydrate and lipid metabolism. Results of a 1-year intervention from the Oslo Diet and Exercise Study. Scand J Med Sci Sports 1998; 8: 109–15. [DOI] [PubMed] [Google Scholar]
- [80].Bo S, Gambino R, Ciccone G, Rosato R, Milanesio N, Villois P et al. Effects of TCF7L2 polymorphisms on glucose values after a lifestyle intervention. Am J Clin Nutr 2009; 90: 1502–8. [DOI] [PubMed] [Google Scholar]
- [81].Burke V, Mansour J, Beilin LJ, Mori TA. Long-term follow-up of participants in a health promotion program for treated hypertensives (ADAPT). Nutr Metab Cardiovasc Dis 2008; 18: 198–206. [DOI] [PubMed] [Google Scholar]
- [82].Burtscher M, Gatterer H, Dunnwald T, Pesta D, Faulhaber M, Netzer N et al. Effects of supervised exercise on gamma-glutamyl transferase levels in patients with isolated impaired fasting glucose and those with impaired fasting glucose plus impaired glucose tolerance. Exp Clin Endocrinol Diabetes 2012; 120: 445–50. [DOI] [PubMed] [Google Scholar]
- [83].Cox KL, Burke V, Beilin LJ, Grove JR, Blanksby BA, Puddey IB. Blood pressure rise with swimming versus walking in older women: the Sedentary Women Exercise Adherence Trial 2 (SWEAT 2). J Hypertens 2006; 24: 307–14. [DOI] [PubMed] [Google Scholar]
- [84].Cox KL, Burke V, Beilin LJ, Derbyshire AJ, Grove JR, Blanksby BA et al. Short and long-term adherence to swimming and walking programs in older women--the Sedentary Women Exercise Adherence Trial (SWEAT 2). Prev Med 2008; 46: 511–7. [DOI] [PubMed] [Google Scholar]
- [85].Craigie AM, Caswell S, Paterson C, Treweek S, Belch JJ, Daly F et al. Study protocol for BeWEL: the impact of a BodyWEight and physicaL activity intervention on adults at risk of developing colorectal adenomas. BMC Public Health 2011; 11: 184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Delgadillo AT, Grossman M, Santoyo-Olsson J, Gallegos-Jackson E, Kanaya AM, Stewart AL. Description of an academic community partnership lifestyle program for lower income minority adults at risk for diabetes. Diabetes Educ 2010; 36: 640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ditschuneit HH, Flechtner-Mors M. Value of structured meals for weight management: risk factors and long-term weight maintenance. Obes Res 2001; 9: 284S–289S. [DOI] [PubMed] [Google Scholar]
- [88].Esposito K, Ciotola M, Giugliano F, Maiorino MI, Autorino R, De SM et al. Effects of intensive lifestyle changes on erectile dysfunction in men. J Sex Med 2009; 6: 243–50. [DOI] [PubMed] [Google Scholar]
- [89].Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012; 20: 1628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Groeneveld IF, Proper KI, van der Beek AJ, van Duivenbooden C, van Mechelen W. Design of a RCT evaluating the (cost-) effectiveness of a lifestyle intervention for male construction workers at risk for cardiovascular disease: the health under construction study. BMC Public Health 2008; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jacobs DR Jr., Sluik D, Rokling-Andersen MH, Anderssen SA Drevon CA. Association of 1-y changes in diet pattern with cardiovascular disease risk factors and adipokines: results from the 1-y randomized Oslo Diet and Exercise Study. Am J Clin Nutr 2009; 89: 509–17. [DOI] [PubMed] [Google Scholar]
- [92].Katula JA, Vitolins MZ, Rosenberger EL, Blackwell C, Espeland MA, Lawlor MS et al. Healthy Living Partnerships to Prevent Diabetes (HELP PD): design and methods. Contemp Clin Trials 2010; 31: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project.[Erratum appears in Diabetes Care. 2012 Feb;35(2):455]. Diabetes Care 2011; 34: 1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women’s Healthy Lifestyle Project: A randomized clinical trial: results at 54 months. Circulation 2001; 103: 32–7. [DOI] [PubMed] [Google Scholar]
- [95].Kuller LH, Kinzel LS, Pettee KK, Kriska AM, Simkin-Silverman LR, Conroy MB et al. Lifestyle Intervention and Coronary Heart Disease Risk Factor Changes over 18 Months in Postmenopausal Women: The Women On the Move through Activity and Nutrition (WOMAN Study) Clinical Trial. J Womens Health (Larchmt) 2006; 15: 962–74. [DOI] [PubMed] [Google Scholar]
- [96].Kuller LH, Gabriel KK, Kinzel LS, Underwood DA, Conroy MB, Chang Y et al. The Women on the Move Through Activity and Nutrition (WOMAN) Study: Final 48-month results. Obesity (Silver Spring) 2012; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med 2013; 173: 113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med 2011; 41: 366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mason C, Risques RA, Xiao L, Duggan CR, Imayama I, Campbell KL et al. Independent and combined effects of dietary weight loss and exercise on leukocyte telomere length in postmenopausal women. Obesity (Silver Spring) 2013; 21: E549–E554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin- resistant obese women. Diabetologia 2005; 48: 8–16. [DOI] [PubMed] [Google Scholar]
- [101].Merriam PA, Tellez TL, Rosal MC, Olendzki BC, Ma Y, Pagoto SL et al. Methodology of a diabetes prevention translational research project utilizing a community-academic partnership for implementation in an underserved Latino community. BMC Med Res Methodol 2009; 9: 20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Potteiger JA, Jacobsen DJ, Donnelly JE. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int J Obes Relat Metab Disord 2002; 26: 87–9. [DOI] [PubMed] [Google Scholar]
- [103].Simkin-Silverman LR WRBMMEKL. Maintenance of cardiovascular risk factor changes among middle-aged women in a lifestyle intervention trial. Women’s Health 1998; 4: 255–71. [PubMed] [Google Scholar]
- [104].Simkin-Silverman L, Wing RR, Hansen DH, Klem ML, Pasagian-Macaulay AP, Meilahn EN et al. Prevention of cardiovascular risk factor elevations in healthy premenopausal women. Prev Med 1995; 24: 519–17. [DOI] [PubMed] [Google Scholar]
- [105].The ODES investigators. The Oslo Diet and Exercise Study (ODES): design and objectives. Control Clin Trials 1993; 14: 229–43. [DOI] [PubMed] [Google Scholar]
- [106].Torjesen PA, Birkeland KI, Anderssen SA, Hjermann I, Holme I, Urdal P. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care 1997; 20: 26–31. [DOI] [PubMed] [Google Scholar]
- [107].Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011; 364: 1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011; 365: 1969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. BMJ 2014; 349: g4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 2014; 312: 1218–26. [DOI] [PubMed] [Google Scholar]
- [111].Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP- 1). Diabetologia 2006; 49: 289–97. [DOI] [PubMed] [Google Scholar]
- [112].Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tsuzaki K et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health 2011; 11: 40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care 2014; 37: S14–S80. [DOI] [PubMed] [Google Scholar]
- [114].Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda ) 2013; 28: 330–58. [DOI] [PubMed] [Google Scholar]
- [115].Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow- up study. Lancet Diabetes Endocrinol 2014; 2: 474–80. [DOI] [PubMed] [Google Scholar]
- [116].Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care 2009; 32: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].The DECODE study group. Is fasting glucose sufficient to define diabetes? Epidemiological data from 20 European studies. Diabetologia 1999; 42: 647–54. [DOI] [PubMed] [Google Scholar]
- [118].Zhuo X, Zhang P, Selvin E, Hoerger TJ, Ackermann RT, Li R et al. Alternative HbA1c cutoffs to identify high-risk adults for diabetes prevention: a cost-effectiveness perspective. Am J Prev Med 2012; 42: 374–81. [DOI] [PubMed] [Google Scholar]
- [119].Zhuo X, Zhang P, Kahn HS, Gregg EW. Cost-effectiveness of alternative thresholds of the fasting plasma glucose test to identify the target population for type 2 diabetes prevention in adults aged >/=45 years. Diabetes Care 2013; 36: 3992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


