Abstract
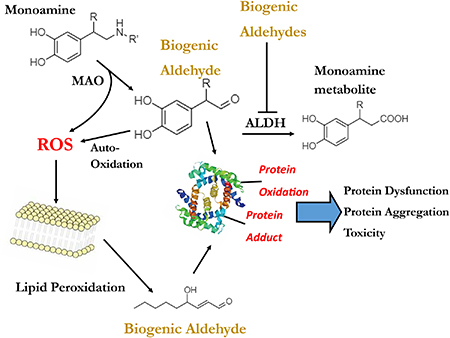
Oxidative decomposition of several biomolecules produces reactive aldehydes. Monoamine neurotransmitters are enzymatically converted to aldehydes via monoamine oxidase followed by further metabolism such as carbonyl oxidation/reduction. Elevated levels of aldehyde intermediates are implicated as factors in several pathological conditions, including Parkinson’s disease. The biogenic aldehydes produced from dopamine, norepinephrine and serotonin are known to be toxic, generate reactive oxygen species and/or cause aggregation of proteins such as α-synuclein. Polyunsaturated lipids undergo oxidative decomposition to produce biogenic aldehydes, including 4-hydroxy-2-nonenal and malondialdehyde. These lipid aldehydes, some including an α,β-unsaturated carbonyl, target important proteins such as α-synuclein, proteasome degradation and G-protein-coupled signaling. Overproduction of biogenic aldehydes is a hypothesized factor in neurodegeneration; preventing their formation or scavenging may provide means for neuroprotection.
Keywords: DOPAL, Parkinson’s disease, aldehydes, ROS, lipid peroxidation, DOPEGAL
Graphical Abstract
1. Introduction
Spontaneous or enzymatic oxidation of various biomolecules produces aldehydes in the human body. Enzymatic oxidation of monoamine and indoleamine neurotransmitters yields reactive biogenic aldehydes. The oxidative decomposition of lipids (i.e., lipid peroxidation) likewise produces reactive biogenic aldehydes, some with α,β-unsaturated carbonyls. Cellular defenses of carbonyl metabolism include redundant enzymes, specifically, isoforms of aldehyde dehydrogenases, and compensatory systems, namely, aldehyde reductases. Overwhelming or impairing aldehyde metabolism yields aberrant levels of such reactive species and is predicted to contribute to or exacerbate human disorders/degenerative conditions [1–4]. Given this, targeting the production or mitigating levels via scavengers of such biogenic aldehydes is predicted to be a therapeutic means to disease.
2. Oxidation of monoamine and indoleamine neurotransmitters
2.1. Monoamine oxidase metabolism can produce reactive aldehydes
Monoamine oxidase (MAO) metabolizes primary amines, such as the neurotransmitters norepinephrine (NE), epinephrine (EPI), serotonin (5-HT), and dopamine (DA), to produce a reactive biogenic aldehyde. MAO has two isoforms: MAO-A and MAO-B. Both isoforms can metabolize all four neurotransmitters and are located throughout the body, though MAO-A is present in the main production sites of NE, EPI, and DA: the locus coeruleus (LC), the rostral ventral lateral medulla (RVLM), and substantia nigra (SN), respectively [5]. MAO-B is present mostly in glia and significantly contributes to monoamine metabolism of DA [6, 7]. A 1952 review by Blaschko proposed that the aldehydes produced from the metabolism of indoleamines and catecholamines by amine oxidases were toxic to the cells they were produced in [8]. This suggestion has evolved into the “catecholaldehyde hypothesis” which proposes that the buildup of toxic aldehyde metabolites of neurotransmitters is a significant contributor to the pathogenesis of Parkinson’s disease (PD) and potentially other neurodegenerative diseases that involve the loss of catecholamine neurons [9–12].
The aldehyde product of DA, 3,4-dihydroxyphenylacetaldehyde (DOPAL), is especially implicated in this toxicity. Other reactive aldehyde products include 3,4dihydroxyphenylglycoaldehyde (DOPEGAL) from NE and EPI, and 5-hydroxyindoleacetaldehyde (5-HIAL) from 5-HT [5, 13]. Though there is much less known about DOPEGAL and 5-HIAL compared to DOPAL, it has been reported that both DOPEGAL and 5-HIAL are more reactive and toxic [5, 14]. The loss of NE, EPI, 5-HT, and DA neurons have all been implicated in PD-related pathology [5]. Loss of DA neurons in the SN and NE neurons in the LC is linked to both motor and non-motor symptoms in PD [5].
2.2. DOPEGAL, the aldehyde product of norepinephrine and epinephrine
NE is synthesized in the LC and is used as a neurotransmitter that promotes alertness in the central nervous system (CNS) [15]. In the sympathetic nervous system, it is used as a hormone in the blood stream [15]. EPI is formed in the RVLM and is used as a neurotransmitter in the CNS and as the primary hormone secreted by the adrenal medulla [5]. In the sympathetic nervous system, EPI is used as an excitatory modulator that increases blood flow, heart rate, and blood sugar.
The aldehyde product of both NE and EPI, DOPEGAL, is toxic to the cells it is produced in. Levels as low as 6μM have been shown to kill PC-12 cells, and sympathetic ganglion cells undergo apoptosis when exposed to DOPEGAL [5, 16]. It is possible that higher concentrations could induce necrosis [5, 17]. A proposed mechanism of toxicity to explain the apoptosis observed after DOPEGAL exposure involves the permeability transition (PT) pore on the inner mitochondrial membrane. Induction of the PT pore causes the release of factors such as cytochrome c, which activates a downstream caspase cascade that triggers apoptosis [5, 18–20]. When in the presence of Ca2+, concentrations of DOPEGAL as low as 6μM have been found to induce the PT pore [5, 21]. The PT pore activation mechanism requires a reactive chemical species. When under oxidative stress, DOPEGAL produces a free radical, fulfilling this requirement [5, 21]. DOPEGAL also induces cytosolic Ca2+; apoptosis is associated with Ca2+ dysregulation [5, 22].
2.3. 5-HIAL, the aldehyde product of 5HT
5-HT is synthesized in serotonergic terminals where it is used as a neurotransmitter in the CNS. 5-HT is implicated in many behaviors, such as feeding, affective disorders, sleep-wake cycles, motor system control, and reward [23]. 5-HT is enzymatically metabolized to the aldehyde 5-HIAL. A potential mechanism of toxicity of 5-HIAL is the oligomerization of α-synuclein (aSyn), and these oligomers are hypothesized to be involved in PD pathogenesis [13]. 5-HIAL generated in situ via 5-HT oligomerized aSyn in vitro, in PC12 cells, which could be blocked via addition of an MAO inhibitor [13].
2.4. DOPAL, the aldehyde product of DA
2.4.1. DOPAL is toxic to dopaminergic cells
DA is synthesized in the SN, ventral tegmental area, and hypothalamus. It is used as a neurotransmitter in the CNS [24]. DArgic pathways include the nigrostriatal pathway, which is involved in motor control, and the mesolimbic pathway, which is involved in reinforcement and reward [24–26]. The aldehyde product of DA is DOPAL. DOPAL has been found to be toxic to DArgic cells at 7μM, which is not far from in vivo concentrations of about 2μM [27]. This could suggest that altering DOPAL metabolism even slightly can raise DOPAL to toxic levels [27].
2.4.2. DOPAL induces the PT pore in the presence of Ca2+
DOPAL, like DOPEGAL, has been found to induce the mitochondrial PT pore in the presence of Ca2+ in concentrations as low as 125 nM [5, 28]. The generation of a reactive chemical species is necessary for induction of the mitochondrial PT pore. It is claimed DOPAL generates a hydroxyl radical when under conditions of oxidative stress; however, the generation of this free hydroxyl radical does not occur for DOPEGAL [5, 29]. DOPAL and DOPEGAL could trigger induction independent of the free radicals produced, although the mechanistic target is not known. The production of free radicals when under oxidative stress may exacerbate this issue, or could be the primary cause of induction, it is not known.
2.4.3. DOPAL oligomerizes α-synuclein
DOPAL, like 5-HIAL, oligomerizes aSyn [13, 30]. Werner-Allen et al. demonstrated a unique chemical mechanism for this oligomerization via isoindole cross-linking in vitro [31]. DOPAL binds covalently to the N-terminal lysine residues, potentially by Schiff-base and Michaeladdition adducts, which stabilizes the oligomer [32–35]. A large or small oligomer can form from this DOPAL interaction. The oxidation of DOPAL forms reactive oxygen species (ROS) which subsequently leads to the oxidation of the methionine residues on aSyn [32]. When all four possible methionine residues are oxidized there is a reduction in the formation of large oligomers, which are more toxic than the small oligomers. This suggests that the methionine residues play a role in the neurotoxicity of DOPAL-aSyn interactions [32]. Furthermore, DOPAL has been shown to stimulate aSyn binding to tropomyosin receptor kinase B (TrkB), leading to interference with neurotrophic activities, thereby increasing the susceptibility of neurons to degeneration [36].
2.4.4. DOPAL produces reactive oxygen species and modifies proteins
The metabolism of DA by MAO generates DOPAL but also hydrogen peroxide, which can generate a toxic hydroxyl radical via Fenton chemistry to damage proteins, DNA and lipids [37]. In addition, DOPAL can auto-oxidize or be enzymatically oxidized to a reactive quinone, producing ROS such as superoxide anion [34, 38, 39]. It was proposed that DOPAL reacts with proteins through a Schiff base; however, recent evidence suggests a mechanism for protein modification involving Schiff base formation followed by oxidative rearrangement to an indoletype linkage [39–41]. Such a hypothesis may explain the following: 1) observed stability of the DOPAL adduct; 2) addition of sodium cyanoborohydride or antioxidants slows down or prevents protein modification by DOPAL; 3) reaction of DOPAL with proteins produces ROS [41].
2.5. Metabolism of biogenic aldehydes from monoamine and indoleamine neurotransmitters
2.5.1. Aldehydes are metabolized by aldehyde dehydrogenase
Typically, toxic aldehydes are metabolized by aldehyde dehydrogenase (ALDH). 5-HIAL is oxidized to 5-hydroxyindole acetic acid (5-HIAA) by ALDH [42]. DOPAL can undergo carbonyl oxidation to 3,4-dihydroxyphenylacetic acid (DOPAC) by ALDH or reduction to the alcohol 3,4-dihydroxyphenylethanol (DOPET) by aldose reductase (AR) [43]. DOPEGAL is typically metabolized by AR to 3,4-dihydroxyphenylglycol (DHPG) and ALDH to 3,4-dihydroxymandelic acid (DHMA) [43]. A decrease in ALDH activity is linked to PD-like pathology and behavior [44–46].
2.5.2. Aldehyde scavengers and DOPAL
Carnosine, a β-alanyl-histidine dipeptide, is found in the brain and myocardium in millimolar concentrations and may represent a novel scavenger of biogenic aldehydes such as DOPAL and DOPEGAL. Recently, carnosine was shown block formation of catecholaldehyde protein adducts after NE exposure in isolated human cardiac mitochondria, and unlike GSH, form stable conjugates with DOPAL [47]. In addition, hydralazine has been proposed as a means to capture and detoxify biogenic aldehydes [41]. N-acetyl cysteine has also been shown to prevent oxidation of DA as well as block DOPAL from reacting with proteins, perhaps via an antioxidant mechanism [12, 39].
3. Oxidative decomposition of lipids
3.1. Lipid peroxidation produces lipid aldehydes
Another type of biogenic aldehyde implicated in disease are lipid aldehydes formed via lipid peroxidation. This process is generally initiated by free radicals [48, 49]. These free radicals are increased in the presence of oxidative stress, which is thought to play a role in PD. Further mechanistic detail of lipid peroxidation and the formation of lipid aldehydes has been previously described [50, 51]. The products of lipid peroxidation are lipid aldehydes, including 4-hydroxy2-nonenal (4-HNE), malondialdehyde (MDA), and acrolein. Increases in lipid peroxidation products have been found in the brains of PD patients [52]. These lipid peroxidation products are often used as markers for oxidative stress, however the precise mechanism in which lipid aldehydes contribute to the disease etiology of PD is not well understood [53]. Lipid aldehydes have been implicated in a variety of other diseases. This includes other neurodegenerative disorders – Alzheimer’s disease, amyotrophic lateral sclerosis, and Huntington’s disease - as well as neuropsychiatric disorders, cancer, diabetic-complications, and liver disease [48, 49, 54].
3.2. The brain is susceptible to lipid peroxidation
Increases in lipid peroxidation products are seen in models of PD including exposure to rotenone, 6-OHDA, and MPTP [55–57]. This can be likened to the increase of lipid peroxidation seen in PD brains post mortem. The brain and especially DArgic neurons are more susceptible to lipid peroxidation, which is due in part to the brain’s high energy demand requiring a large amount of oxygen and mitochondria [58]. Oxygen in the brain can then aid in the autoxidation of catecholamines and catecholaldehydes leading to produce superoxide [38]. Furthermore, areas of the brain have increased levels of iron.[59]. This iron can be used in the Fenton reaction to oxidize hydrogen peroxide to the hydroxyl radical and a hydroxyl anion. Both the hydroxyl radical and superoxide can lead to the initiation of lipid peroxidation. In healthy individuals the brain maintains homeostasis through antioxidant enzymes which work to prevent damage done by oxidative stress. Glutathione peroxidase (GPX) is one of these important detoxifying enzymes. Both GPX-1 and GPX-4 have been implicated in PD etiology. Whether this is due to a loss of enzyme activity or an upregulation of activity in response to oxidative stress is still unclear. GPX-4 expression has been found to be decreased in PD brains post mortem, yet found to be increased when normalized to cell-count [60]. More research is required, especially regarding GPX-4.
3.3. Lipid peroxidation is implicated in PD
Since most cases of PD are idiopathic, the exact cause for oxidative stress and increase in lipid peroxidation is not well understood. However, there has been progress in terms of how these lipid aldehydes contribute to the disease etiology of PD. 4-HNE has been shown to interact with aSyn as well as interfere with dopamine metabolism by inhibiting aldehyde dehydrogenase [40, 61]. Furthermore, 4-HNE has been found to alter regulator of G-protein signaling 4 (RGS4) activity which is important for regulating G-protein coupled receptors, and is implicated as a potential therapeutic target for PD [62].
It has recently been found that 4-HNE not only reacts with aSyn but also leads to the release of pathogenic aSyn [63]. 4-HNE has been shown to interfere with proteasome degradation and lysosomal function and is able to trigger extracellular vesicle release with intact protein [63, 64]. Through treating primary neurons with 4-HNE and analyzing the extracellular vesicles secreted, an overall increase of aSyn and oligomeric aSyn in the 4-HNE-treated neurons was determined [63]. While aSyn is most likely a protein important for mitochondrial function, its accumulation is a pathogenic hallmark of PD [61]. This process is one way in which lipid peroxidation products contribute to the disease etiology of PD. In addition, there may be an interplay or toxic synergy between the lipid peroxidation aldehydes and catecholaldehydes. Work in previous years found that both 4-HNE and MDA potently inhibit ALDH and/or AR metabolism of DOPAL, contributing to increases in the level of this biogenic catecholaldehyde, as discussed in 2.5.1 [40, 65–67]. As a result, neurotoxicity via lipid aldehydes such as 4-HNE and MDA may be augmented as the result of their ability to impede the carbonyl oxidation/reduction step for the metabolism of DA and other monoamines.
4. Comparison of biogenic aldehyde reactivity
The various biogenic aldehydes formed can vary in terms of origin (e.g., lipid) and tissue/cell type location as described above but also demonstrate diversity of reactivity for rate and target nucleophile. The monoamine-derived aldehydes may primarily target amines, such as Lys or Arg while the α,β-unsaturated aldehydes derived from lipids may primarily react with Cys/thiols. Although there may be several exceptions, under physiologic conditions, the reaction for monoamine-derived aldehydes such as DOPAL initially involve Schiff base chemistry followed by rearrangement or condensation while the α,β-unsaturated carbonyls (e.g., 4-HNE) modify Cys/thiols by Michael-type addition.[31, 33, 41, 68, 69] Reactivity rates may vary or be difficult to measure given instability of the intermediate, such as observed for DOPEGAL which appears to degrade spontaneously and quickly.[47] A rate constant was measured for DOPAL modification of Lys/primary amine (2 M−1 min−1) and found to be 20 to 30-fold greater than that for the reaction of 4-HNE with Lys/primary amine.[41, 70] However, 4-HNE and α,βunsaturated carbonyls rapidly react with Cys/thiols (>1 M−1 s−1) while DOPAL does not. [70]
5. Summary
The oxidative decomposition of biomolecules, such as neurotransmitters and lipids, produces a range of aldehyde-containing intermediates that vary in location (e.g., tissue/cell type, subcellular location) and reactivity. Normal, physiologic processes produce these species at levels controllable by carbonyl-metabolizing enzymes, and it is the aberrant and chronic overproduction of biogenic aldehydes that is hypothesized as an initiating factor for pathogenic events relevant to neurodegenerative disease. Mechanisms for cellular injury include formation of ROS, mitochondrial toxicity, modification of protein targets critical for survival of neurons and aggregation/cross-linking of aSyn. Knowledge of such pathways for toxicity may yield biomarkers of early pathogenic events and therapeutic targets for neuroprotection.
Acknowledgements.
This work was supported by NIH grants R01 ES029035, R21 AG057006 and T32 GM067795.
Abbreviations
- 4-HNE
4-hydroxynonenal
- 5-HIAA
5-hydroxyindole acetic acid
- 5-HIAL
5-hydroxyindoleacetaldehyde
- 5-HT
serotonin
- 6-OHDA
6-hydroxydopamine
- ALDH
aldehyde dehydrogenase
- AR
aldose reductase
- aSyn
α-synuclein
- CNS
central nervous system
- DA
dopamine
- DArgic
dopaminergic
- DHMA
3,4-dihydroxymandelic acid
- DHPG
3,4-dihydroxyphenylglycol
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- DOPEGAL
3,4-dihydroxyphenylglycoaldehyde
- DOPET
3,4-dihydroxyphenylethanol
- EPI
epinephrine
- GPX
glutathione peroxidase
- LC
locus coeruleus
- MAO
monoamine oxidase
- MDA
malondialdehyde
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NE
norepinephrine
- PD
Parkinson’s Disease
- PT
permeability transition
- PUFA
polyunsaturated fatty acids
- RGS4
regulator of G-protein signaling 4
- ROS
reactive oxygen species
- RVLM
rostral ventral lateral medulla
- SN
substantia nigra
- TrkB
tropomyosin receptor kinase B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: The authors declare no conflict of interest.
References
- [1].Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O’Donnell KC, Barnhill L, Casida JE, Cockburn M, Sagasti A, Stahl MC, Maidment NT, Ritz B, and Bronstein JM, “Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease,” Proc Natl Acad Sci U S A, vol. 110, no. 2, pp. 636–41, January 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, and Bronstein JM, “Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease,” Neurology, vol. 82, no. 5, pp. 419–26, February 4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ritz BR, Paul KC, and Bronstein JM, “Of Pesticides and Men: a California Story of Genes and Environment in Parkinson’s Disease,” Curr Environ Health Rep, vol. 3, no. 1, pp. 40–52, March, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A, and Goldstein DS, “Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease,” Chem Res Toxicol, vol. 27, no. 8, pp. 1359–61, August 18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, and Zahm DS, “Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases,” Neurotoxicology, vol. 25, no. 1–2, pp. 101–115, 2004/01//, 2004. [DOI] [PubMed] [Google Scholar]
- [6].Di Monte DA, DeLanney LE, Irwin I, Royland JE, Chan P, Jakowec MW, and Langston JW, “Monoamine oxidase-dependent metabolism of dopamine in the striatum and substantia nigra of L-DOPA-treated monkeys,” Brain Res, vol. 738, no. 1, pp. 53–9, October 28, 1996. [DOI] [PubMed] [Google Scholar]
- [7].Glover V, Sandler M, Owen F, and Riley GJ, “Dopamine is a monoamine oxidase B substrate in man,” Nature, vol. 265, no. 5589, pp. 80–1, January 6, 1977. [DOI] [PubMed] [Google Scholar]
- [8].Blaschko H, “Amine oxidase and amine metabolism,” Pharmacol Rev, vol. 4, no. 4, pp. 415–58, December, 1952. [PubMed] [Google Scholar]
- [9].Goldstein DS, Kopin IJ, and Sharabi Y, “Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders,” Pharmacol Ther, vol. 144, no. 3, pp. 268–82, December, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goldstein DS, Jinsmaa Y, Sullivan P, Holmes C, Kopin IJ, and Sharabi Y, “Comparison of Monoamine Oxidase Inhibitors in Decreasing Production of the Autotoxic Dopamine Metabolite 3,4-Dihydroxyphenylacetaldehyde in PC12 Cells,” The Journal of pharmacology and experimental therapeutics, vol. 356, no. 2, pp. 483–492, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goldstein DS, Jinsmaa Y, Sullivan P, Holmes C, Kopin IJ, and Sharabi Y, “3,4Dihydroxyphenylethanol (Hydroxytyrosol) Mitigates the Increase in Spontaneous Oxidation of Dopamine During Monoamine Oxidase Inhibition in PC12 Cells,” Neurochemical research, vol. 41, no. 9, pp. 2173–2178, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goldstein DS, Jinsmaa Y, Sullivan P, and Sharabi Y, “N-Acetylcysteine Prevents the Increase in Spontaneous Oxidation of Dopamine During Monoamine Oxidase Inhibition in PC12 Cells,” Neurochemical Research, vol. 42, no. 11, pp. 3289–3295, 2017/11/01, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jinsmaa Y, Cooney A, Sullivan P, Sharabi Y, and Goldstein DS, “The serotonin aldehyde, 5-HIAL, oligomerizes alpha-synuclein,” Neurosci Lett, vol. 590, pp. 134–7, March 17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burke WJ, Chung HD, and Li SW, “Quantitation of 3,4-Dihydroxyphenylacetaldehyde and 3,4-Dihydroxyphenylglycolaldehyde, the Monoamine Oxidase Metabolites of Dopamine and Noradrenaline, in Human Tissues by Microcolumn High-Performance Liquid Chromatography,” Analytical Biochemistry, vol. 273, no. 1, pp. 111–116, 1999/08/15/, 1999. [DOI] [PubMed] [Google Scholar]
- [15].Sara SJ, and Bouret S, “Orienting and reorienting: the locus coeruleus mediates cognition through arousal,” Neuron, vol. 76, no. 1, pp. 130–41, October 4, 2012. [DOI] [PubMed] [Google Scholar]
- [16].Burke WJ, Schmitt CA, Gillespie KN, and Li SW, “Norepinephrine transmitter metabolite is a selective cell death messenger in differentiated rat pheochromocytoma cells,” Brain Research, vol. 722, no. 1, pp. 232–235, 1996/05/25/, 1996. [DOI] [PubMed] [Google Scholar]
- [17].Burke WJ, Li SW, Zahm DS, Macarthur H, Kolo LL, Westfall TC, Anwar M, Glickstein SB, and Ruggiero DA, “Catecholamine monoamine oxidase a metabolite in adrenergic neurons is cytotoxic in vivo,” Brain Research, vol. 891, no. 1, pp. 218–227, 2001/02/09/, 2001. [DOI] [PubMed] [Google Scholar]
- [18].Scarlett JL, and Murphy MP, “Release of apoptogenic proteins from the mitochondrial intermembrane space during the mitochondrial permeability transition,” FEBS Letters, vol. 418, no. 3, pp. 282–286, 1997. [DOI] [PubMed] [Google Scholar]
- [19].Kluck RM, Bossy-Wetzel E, Green DR, and Newmeyer DD, “The Release of Cytochrome c from Mitochondria: A Primary Site for Bcl-2 Regulation of Apoptosis,” Science, vol. 275, no. 5303, pp. 1132–1136, 1997. [DOI] [PubMed] [Google Scholar]
- [20].Wallace DC, “Mitochondrial Diseases in Man and Mouse,” Science, vol. 283, no. 5407, pp. 1482–1488, 1999. [DOI] [PubMed] [Google Scholar]
- [21].Burke WJ, Kristal BS, Yu BP, Li SW, and Lin T-S, “Norepinephrine transmitter metabolite generates free radicals and activates mitochondrial permeability transition: a mechanism for DOPEGAL-induced apoptosis,” Brain Research, vol. 787, no. 2, pp. 328332, 1998/03/23/, 1998. [DOI] [PubMed] [Google Scholar]
- [22].Miyamoto S, Howes AL, Adams JW, Dorn GW, and Brown JH, “Ca2+ Dysregulation Induces Mitochondrial Depolarization and Apoptosis: ROLE OF Na+/Ca2+ EXCHANGER AND AKT,” Journal of Biological Chemistry, vol. 280, no. 46, pp. 38505–38512, November 18, 2005, 2005. [DOI] [PubMed] [Google Scholar]
- [23].Best J, Nijhout HF, and Reed M, “Serotonin synthesis, release and reuptake in terminals: a mathematical model,” Theor Biol Med Model, vol. 7, pp. 34, August 19, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McHugh PC, and Buckley DA, “Chapter Eleven - The Structure and Function of the Dopamine Transporter and its Role in CNS Diseases,” Vitamins & Hormones, Litwack G, ed., pp. 339–369: Academic Press, 2015. [DOI] [PubMed] [Google Scholar]
- [25].Adinoff B, “Neurobiologic processes in drug reward and addiction,” Harv Rev Psychiatry, vol. 12, no. 6, pp. 305–20, Nov-Dec, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luo SX, and Huang EJ, “Dopaminergic Neurons and Brain Reward Pathways: From Neurogenesis to Circuit Assembly,” Am J Pathol, vol. 186, no. 3, pp. 478–88, March, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schamp JH, and Doorn JA, “CHAPTER 4 Dopamine Metabolism and the Generation of a Reactive Aldehyde,” Oxidative Stress and Redox Signalling in Parkinson’s Disease, pp. 97–115: The Royal Society of Chemistry, 2017. [Google Scholar]
- [28].Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, and Burke WJ, “Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria,” Free Radical Biology and Medicine, vol. 30, no. 8, pp. 924–931, 2001/04/15/, 2001. [DOI] [PubMed] [Google Scholar]
- [29].Li SW, Lin TS, Minteer S, and Burke WJ, “3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson’s disease pathogenesis,” Brain Res Mol Brain Res, vol. 93, no. 1, pp. 1–7, September 10, 2001. [DOI] [PubMed] [Google Scholar]
- [30].Jinsmaa Y, Sullivan P, Sharabi Y, and Goldstein DS, “DOPAL is transmissible to and oligomerizes alpha-synuclein in human glial cells,” Autonomic neuroscience : basic & clinical, vol. 194, pp. 46–51, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[31].Werner-Allen JW, Monti S, DuMond JF, Levine RL, and Bax A, “Isoindole Linkages Provide a Pathway for DOPAL-Mediated Cross-Linking of alpha-Synuclein,” Biochemistry, vol. 57, no. 9, pp. 1462–1474, March 6, 2018.The authors identified a unique dicatechol DOPAL-aSyn adduct using isotopic labeling and mass spectrometry analysis in vitro. While Lys residues on aSyn have been found to result in Schiff base and Michael addition products, this is the first indication of a DOPAL-based cross-linked product.
- *[32].Carmo-Goncalves P, do Nascimento LA, Cortines JR, Eliezer D, Romao L, and Follmer C, “Exploring the role of methionine residues on the oligomerization and neurotoxic properties of DOPAL-modified alpha-synuclein,” Biochem Biophys Res Commun, September 21, 2018.The authors showed that oxidation of Met residues in aSyn prevents DOPAL-aSyn oligomers from forming, implicating Met residues in the formation and/or modulation of DOPAL derived aSyn neurotoxic species. While DOPAL is generally known to react with Lys residues in aSyn, this is one of the first studies exploring the role of Met residues.
- [33].Follmer C, Coelho-Cerqueira E, Yatabe-Franco DY, Araujo GD, Pinheiro AS, Domont GB, and Eliezer D, “Oligomerization and Membrane-binding Properties of Covalent Adducts Formed by the Interaction of alpha-Synuclein with the Toxic Dopamine Metabolite 3,4-Dihydroxyphenylacetaldehyde (DOPAL),” J Biol Chem, vol. 290, no. 46, pp. 27660–79, November 13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[34].Jinsmaa Y, Sharabi Y, Sullivan P, Isonaka R, and Goldstein DS, “3,4-Dihydroxyphenylacetaldehyde-Induced Protein Modifications and Their Mitigation by NAcetylcysteine,” The Journal of pharmacology and experimental therapeutics, vol. 366, no. 1, pp. 113–124, 2018.The authors show that DOPAL induced protein-quinone adduct formation of several proteins that play a role in catecholaminergic neurodegeneration. They also saw oligomerization of these proteins in the presence of DOPAL. Furthermore, Nacetylcysteine was able to inhibit or prevent these protein modification by DOPAL.
- *[35].Lima VA, do Nascimento LA, Eliezer D, and Follmer C, “Role of Parkinson’s Disease-Linked Mutations and N-Terminal Acetylation on the Oligomerization of alphaSynuclein Induced by 3,4-Dihydroxyphenylacetaldehyde,” ACS Chem Neurosci, November 5, 2018.The authors show that N-terminal acetylation of aSyn alters the formation of DOPALinduced aSyn oligomers. Wild type acetylation decreased the formation of oligomers, while N-terminal acetylation of PD-linked mutations increased the formation of oligomers. In addition, they showed that the affinity of aSyn for membranes plays a role in the formation of DOPAL-aSyn oligomers.
- *[36].Kang SS, Zhang Z, Liu X, Manfredsson FP, Benskey MJ, Cao X, Xu J, Sun YE, and Ye K, “TrkB neurotrophic activities are blocked by alpha-synuclein, triggering dopaminergic cell death in Parkinson’s disease,” Proc Natl Acad Sci U S A, vol. 114, no. 40, pp. 10773–10778, October 3, 2017.The authors show that aSyn interacts with TrkB and thereby interfers with neurotrophic signaling. Furthermore, they showed that DOPAL promotes this interaction, while an MAO inhibitor prevents the interaction of aSyn and TrkB. This study implicates a novel role of DOPAL in leading to dopaminergic cell death.
- [37].Linert W, and Jameson GN, “Redox reactions of neurotransmitters possibly involved in the progression of Parkinson’s Disease,” Journal of inorganic biochemistry, vol. 79, no. 1–4, pp. 319–326, 2000/04//, 2000. [DOI] [PubMed] [Google Scholar]
- [38].Anderson DG, Mariappan SV, Buettner GR, and Doorn JA, “Oxidation of 3,4dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone,” J Biol Chem, vol. 286, no. 30, pp. 26978–86, July 29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[39].Anderson DG, Florang VR, Schamp JH, Buettner GR, and Doorn JA, “Antioxidant-Mediated Modulation of Protein Reactivity for 3,4-Dihydroxyphenylacetaldehyde, a Toxic Dopamine Metabolite,” Chem Res Toxicol, vol. 29, no. 7, pp. 1098–107, July 18, 2016.It was proposed that DOPAL reacts with proteins via Schiff base chemistry. The authors provide evidence that Lys modification by DOPAL involves Schiff base formation, an oxidative step of catechol oxidation, and rearrangement to an indole-structure.
- [40].Rees JN, Florang VR, Anderson DG, and Doorn JA, “Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate,” Chem Res Toxicol, vol. 20, no. 10, pp. 1536–42, October, 2007. [DOI] [PubMed] [Google Scholar]
- [41].Rees JN, Florang VR, Eckert LL, and Doorn JA, “Protein reactivity of 3,4dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol,” Chem Res Toxicol, vol. 22, no. 7, pp. 1256–63, July, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Squires LN, Jakubowski JA, Stuart JN, Rubakhin SS, Hatcher NG, Kim WS, Chen K, Shih JC, Seif I, and Sweedler JV, “Serotonin catabolism and the formation and fate of 5-hydroxyindole thiazolidine carboxylic acid,” J Biol Chem, vol. 281, no. 19, pp. 13463–70, May 12, 2006. [DOI] [PubMed] [Google Scholar]
- [43].Eisenhofer G, Kopin IJ, and Goldstein DS, “Catecholamine metabolism: a contemporary view with implications for physiology and medicine,” Pharmacol Rev, vol. 56, no. 3, pp. 331–49, September, 2004. [DOI] [PubMed] [Google Scholar]
- [44].Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, and Sharabi Y, “Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease,” J Neurochem, vol. 126, no. 5, pp. 591–603, September, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wey MC, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, and Strong R, “Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease,” PLoS One, vol. 7, no. 2, pp. e31522, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Marchitti SA, Deitrich RA, and Vasiliou V, “Neurotoxicity and Metabolism of the Catecholamine-Derived 3,4-Dihydroxyphenylacetaldehyde and 3,4Dihydroxyphenylglycolaldehyde: The Role of Aldehyde Dehydrogenase,” Pharmacological Reviews, vol. 59, no. 2, pp. 125, 2007. [DOI] [PubMed] [Google Scholar]
- *[47].Nelson MM, Builta ZJ, Monroe TB, Doorn JA, and Anderson EJ, “Biochemical characterization of the catecholaldehyde reactivity of L-carnosine and its therapeutic potential in human myocardium,” Amino Acids, September 6, 2018.The authors show that in isolated mitochondria, carnosine decreased the amount of catechol-modified protein adducts. They also demonstrate that carnosine reacted directly with DOPAL in a time-dependent manner and was more rapid than the reaction of carnosine with 4-HNE. Carnosine has previously been shown to detoxify lipid-derived aldehydes, and this is the first study with evidence to support that carnosine scavenges catecholaldehydes as well.
- [48].Perluigi M, Coccia R, and Butterfield DA, “4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies,” Antioxid Redox Signal, vol. 17, no. 11, pp. 1590–609, December 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ayala A, Munoz MF, and Arguelles S, “Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal,” Oxid Med Cell Longev, vol. 2014, pp. 360438, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zielinski ZA, and Pratt DA, “Lipid Peroxidation: Kinetics, Mechanisms, and Products,” J Org Chem, vol. 82, no. 6, pp. 2817–2825, March 17, 2017. [DOI] [PubMed] [Google Scholar]
- [51].Esterbauer H, Schaur RJ, and Zollner H, “Chemistry and biochemistry of 4hydroxynonenal, malonaldehyde and related aldehydes,” Free Radical Biology and Medicine, vol. 11, no. 1, pp. 81–128, 1991/01/01/, 1991. [DOI] [PubMed] [Google Scholar]
- [52].Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, and Mizuno Y, “Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease,” Proceedings of the National Academy of Sciences of the United States of America, vol. 93, no. 7, pp. 2696–2701, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Miletic J, Drakulic D, Pejic S, Petkovic M, Ilic TV, Miljkovic M, Stefanovic A, Prostran M, and Stojanov M, “Prooxidant-antioxidant balance, advanced oxidation protein products and lipid peroxidation in Serbian patients with Parkinson’s disease,” Int J Neurosci, vol. 128, no. 7, pp. 600–607, July, 2018. [DOI] [PubMed] [Google Scholar]
- [54].Romano A, Serviddio G, Calcagnini S, Villani R, Giudetti AM, Cassano T, and Gaetani S, “Linking lipid peroxidation and neuropsychiatric disorders: focus on 4-hydroxy2-nonenal,” Free Radic Biol Med, vol. 111, pp. 281–293, October, 2017. [DOI] [PubMed] [Google Scholar]
- [55].Wu C-R, Tsai C-W, Chang S-W, Lin C-Y, Huang L-C, and Tsai C-W, “Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction,” Chemico-Biological Interactions, vol. 225, pp. 40–46, 2015/01/05/, 2015. [DOI] [PubMed] [Google Scholar]
- [56].Tyagi RK, Bisht R, Pant J, Kumar P, Majeed AB, and Prakash A, “Possible role of GABA-B receptor modulation in MPTP induced Parkinson’s disease in rats,” Exp Toxicol Pathol, vol. 67, no. 2, pp. 211–7, February, 2015. [DOI] [PubMed] [Google Scholar]
- [57].Aksoy D, Solmaz V, Cavusoglu T, Meral A, Ates U, and Erbas O, “Neuroprotective Effects of Eexenatide in a Rotenone-Induced Rat Model of Parkinson’s Disease,” Am J Med Sci, vol. 354, no. 3, pp. 319–324, September, 2017. [DOI] [PubMed] [Google Scholar]
- [58].Shichiri M, “The role of lipid peroxidation in neurological disorders,” Journal of Clinical Biochemistry and Nutrition, vol. 54, no. 3, pp. 151–160, 04/09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zecca L, Youdim MBH, Riederer P, Connor JR, and Crichton RR, “Iron, brain ageing and neurodegenerative disorders,” Nature Reviews Neuroscience, vol. 5, pp. 863, 11/01/online, 2004. [DOI] [PubMed] [Google Scholar]
- [60].Bellinger FP, Bellinger MT, Seale LA, Takemoto AS, Raman AV, Miki T, Manning-Bog AB, Berry MJ, White LR, and Ross GW, “Glutathione Peroxidase 4 is associated with Neuromelanin in Substantia Nigra and Dystrophic Axons in Putamen of Parkinson’s brain,” Mol Neurodegener, vol. 6, no. 1, pp. 8, January 21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Plotegher N, and Bubacco L, “Lysines, Achilles’ heel in alpha-synuclein conversion to a deadly neuronal endotoxin,” Ageing Res Rev, vol. 26, pp. 62–71, March, 2016. [DOI] [PubMed] [Google Scholar]
- [62].Monroy CA, Doorn JA, and Roman DL, “Modification and functional inhibition of regulator of G-protein signaling 4 (RGS4) by 4-hydroxy-2-nonenal,” Chem Res Toxicol, vol. 26, no. 12, pp. 1832–9, December 16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[63].Zhang S, Eitan E, Wu TY, and Mattson MP, “Intercellular transfer of pathogenic alpha-synuclein by extracellular vesicles is induced by the lipid peroxidation product 4hydroxynonenal,” Neurobiol Aging, vol. 61, pp. 52–65, January, 2018.4-HNE was shown to induce the release of aSyn from extracellular vesicles in primary neurons further implicating lipid peroxidation in Parkinson’s disease. The involvement of lipid peroxidation products in this process was previously unknown.
- [64].Rajapakse D, Curtis T, Chen M, and Xu H, “Zinc Protects Oxidative Stress-Induced RPE Death by Reducing Mitochondrial Damage and Preventing Lysosome Rupture,” Oxidative Medicine and Cellular Longevity, vol. 2017, pp. 6926485, 11/14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Doorn JA, Florang VR, Schamp JH, and Vanle BC, “Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons,” Parkinsonism Relat Disord, vol. 20 Suppl 1, pp. S73–5, January, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Florang VR, Rees JN, Brogden NK, Anderson DG, Hurley TD, and Doorn JA, “Inhibition of the oxidative metabolism of 3,4-dihydroxyphenylacetaldehyde, a reactive intermediate of dopamine metabolism, by 4-hydroxy-2-nonenal,” Neurotoxicology, vol. 28, no. 1, pp. 76–82, January, 2007. [DOI] [PubMed] [Google Scholar]
- [67].Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, and Doorn JA, “Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate,” Chem Res Toxicol, vol. 22, no. 5, pp. 835–41, May, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Werner-Allen JW, DuMond JF, Levine RL, and Bax A, “Toxic Dopamine Metabolite DOPAL Forms an Unexpected Dicatechol Pyrrole Adduct with Lysines of αSynuclein,” Angewandte Chemie (International ed. in English), vol. 55, no. 26, pp. 73747378, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Spickett CM, “The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis,” Redox Biol, vol. 1, pp. 145–52, January 21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Doorn JA, and Petersen DR, “Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal,” Chem Res Toxicol, vol. 15, no. 11, pp. 1445–50, November, 2002. [DOI] [PubMed] [Google Scholar]


