Abstract
Background:
Rates of non-medical use of opioids, and opioid use disorders (OUD) have been rising throughout North America. Methadone and buprenorphine/naloxone are the recommended first-line treatment options for OUD in Canada. Most studies to date have been conducted among heroin users, in controlled settings, and using similar strict dosing schedules (i.e., daily witnessed ingestion) despite buprenorphine/naloxone’s superior safety profile, which allows a more flexible take-home dosing schedule. This study was designed to assess the relative effectiveness of buprenorphine/naloxone- and methadone-based models of opioid agonist therapy (OAT) for the treatment of prescription opioid use disorder (POUD) in routine clinical care.
Methods:
OPTIMA is a multicenter, open-label, pragmatic, randomized, two-arm, non-inferiority, 24-week study comparing the relative effectiveness of buprenorphine/naloxone (provided via flexible take-home doses) to methadone (provided via daily witnessed ingestion) models of OAT for the treatment of POUD. Approximately 276 non-pregnant adults meeting DSM-5 criteria for OUD, currently not in OAT, will be randomized across 7 Canadian sites. The primary outcome is reduction of non-medical opioid use, measured by bi-weekly urine drug screens during the 24-week study period. Secondary outcomes include treatment retention and satisfaction, safety, medication adherence, and patient engagement.
Discussion:
The OPTIMA study is the first randomized clinical trial to compare the relative effectiveness of buprenorphine/naloxone (flexible take-home doses) versus methadone (daily witnessed ingestion) models of OAT for POUD in real-world clinical settings. This study will generate urgently needed evidence towards treatment options to guide the health system response to the ongoing opioid crisis.
Keywords: opioid use disorder, methadone, buprenorphine/naloxone, prescription opioids, opioid agonist therapy, Canada, fentanyl
1. Background
Non-medical use prescription opioids (PO) has substantially increased in most industrialized nations in recent years, particularly in North America. Adverse consequences associated with POs, including accidental overdose deaths, morbidity and societal costs have commonly increased in parallel [1–4]. Although the United States (U.S.) reports higher overall levels of PO consumption, PO use rates in Canada has increased at a steeper rate (98% vs. 185%) in the past decade [3, 4]. Available data suggest that opioid use disorder (OUD) attributable to PO constitute the third-highest burden of disease attributable to substance use in Canada, after alcohol and tobacco [3]. As a result, the opioid “crisis” has become a national public health priority, further underlining the urgent need for an evidence-based continuum of care [1, 2, 4, 5].
Methadone or buprenorphine/naloxone (BUP-NX)-based opioid agonist therapy (OAT) are the recommended first-line options for the treatment of OUD in Canada [6]. Well-established evidence demonstrates that OAT is effective in reducing illicit opioid use and retaining patients in treatment [7, 8], as well as in reducing drug-related mortality and involvement in criminal activity. OAT has also been shown to decrease the overall risk of HIV and hepatitis C (HCV) infection and improve HIV and HCV treatment outcomes [9–13]. Unfortunately, access and adherence to long-term OAT is associated with several patient and system related challenges that undermine potential clinical and population benefits [14]. For instance, some studies indicate that less than half of individuals starting OAT are retained in treatment at 6 months [14], highlighting the need to better understand factors that shape OAT outcomes, to adapt models of care and to disseminate best practices accordingly [15, 16].
Despite the growing population of individuals with OUD attributed to PO, research to date on the efficacy of OAT for OUD has largely been conducted among (long-term) heroin users [4, 7, 8]. Thus, the extent to which results from these studies can be extrapolated to individuals with OUD attributable to PO (hereafter, POUD) is unclear. Moreover, studies comparing BUP-NX- to methadone- based regimens have all used similarly strict dosing schedules (i.e., daily witness ingestion) despite BUP-NX’s superior safety profile and reduced risk of overdose in the case of diversion [7, 8], which makes it amenable for early take-home dosing. While in other jurisdictions (e.g., U.S., France) BUP-NX is typically provided via earlier take-home dosing, adoption of this practice has been slow in Canada. In addition, research comparing outcomes under these two models of care (i.e., methadone in daily witnessed ingestion versus buprenorphine in early take-home dosing) is scant [17], further hindering clinician’s and patient’s ability to make fully informed decisions.
In response to the urgent need to generate practice-based evidence to help inform the response to the Canadian opioid crisis, the “Optimizing Patient Centered-care: A Pragmatic Randomized Control Trial Comparing Models of Care in the Management of Prescription Opioid Misuse” (OPTIMA) study was developed [18] to evaluate the effectiveness and safety of methadone maintenance treatment (MMT) and BUP-NX models of care within a Canadian practice-based framework. Our primary hypothesis is that delivery of BUP-NX under flexible take-home dosing will be non-inferior to MMT for the management of POUD.
2. Study design
OPTIMA is the first clinical trial of the Canadian Research Initiative in Substance Misuse (CRISM), a pan-Canadian research collaboration focusing on evidence-based interventions for substance use disorders funded by the Canadian Institutes of Health Research, and partially modeled after the US National Institute on Drug Abuse’s Clinical Trial Network (NIDA CTN). CRISM’s mandate is twofold: (1) to foster relevant and innovative research studies on substance misuse; and (2) to effectively translate and mobilize evidence from rigorous scientific studies into more effective (prevention and treatment) interventions, health system improvements, and policies at different levels across Canada. The CRISM initiative consists of four nodes, each representing a Canadian region (Quebec/Maritimes, Ontario, Prairies and British Columbia), across which the OPTIMA study will be conducted.
2.1. Overview of study design
The OPTIMA study is a Canadian-wide multicenter, open-label, pragmatic, randomized, two-arm, 24-week study comparing the effectiveness and safety of methadone versus BUP-NX models of care for the treatment of POUD. In contrast to double-blinded efficacy studies designed to maximize internal validity under ideal service delivery conditions, pragmatic trials incorporate, rather than eliminate or standardize, diversity in presenting patient characteristics (e.g., co-morbidities) and local service delivery parameters into the study design. All of these features are designed to enhance external validity of study results and in so doing, provide evidence to influence routine health services and policies [19, 20].
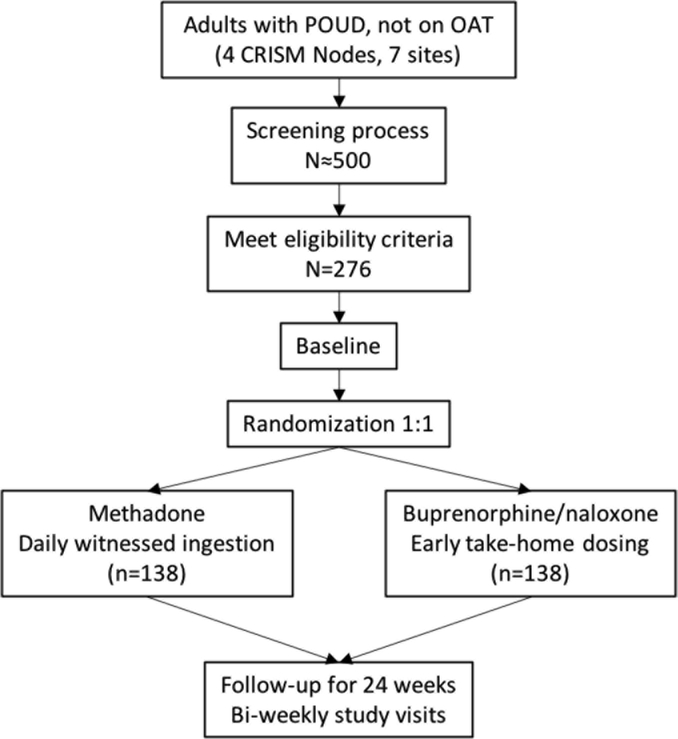
The study schema is presented in Figure 1. Interested individuals will be recruited from either existing patient population at the sites, offsite locations (e.g. withdrawal management facilities, hospital) or through self-referral. Interested individuals will approach study staff by contacting sites (in person or telephone) or will be approached by study staff in clinic settings. They will initially undergo a pre-screening assessment to determine general eligibility. Study candidates will be then invited to complete inform consent procedures. Once written consent is voluntarily given, participants will undergo screening evaluations (Table 1) to confirm eligibility, which will be completed as promptly as possible, but may last up to 28 days. Eligible participants will be then randomly assigned on a 1:1 ratio to either: (a) BUP-NX provided via flexible take-home dosing, once deemed clinically stable by the study physician, and in accordance to local guidelines; or (b) methadone provided via initial daily witnessed ingestion as per local guidelines.
Figure 1. Study schema.
POUD, prescription opioid use disorder. OAT, opioid agonist therapy. CRISM, Canadian Research Initiative in Substance Misuse.
Table 1.
Schedule of study procedures and assessments
| Assessment | Frequency |
|---|---|
| GENERAL | |
| Verbal consent and pre-screening questionnaire | Pre-SCR |
| Informed Consent Form | SCR |
| Demographics | SCR |
| Inclusion/Exclusion criteria | SCR |
| Medical Release Form | SCR |
| Locator Form | BSL, then at each study visit |
| Randomization | BSL |
| SAFETY and MEDICAL ASSESSMENTS | |
| DSM-5 Diagnostic Criteria for OUD | SCR |
| Medical and Psychiatric History | SCR |
| Physical Exam & Vital Signs | SCR |
| Pregnancy and Birth Control Assessment | SCR, BSL, W 4, 8, 12, 16, 20, EOT |
| Concomitant medications | SCR, BSL, then at each study visit |
| Adverse Events and Serious Adverse Events | BSL, then at each study visit |
| Clinical Opiate Withdrawal Scale | BSL, W 2, 4, 6 |
| EFFICACY and OTHER ASSESSMENTS | |
| Urine Drug Screen (UDS) | SCR, BSL, then every 2 weeks |
| Assigned OAT - Pharmacy Abstraction | BSL, then at each study visit |
| Assigned OAT - Self-report | BSL, then at each study visit |
| Client Satisfaction Questionnaire-8 | W 4, 12, EOT |
| Treatment Entry Questionnaire | BSL |
| Health Care Climate Questionnaire | W4, 12, EOT |
| Control Preference Scale | BSL |
| CollaboRATE questionnaire | BSL, then at each study visit |
| Health Service Utilization form | BSL, W 4, 8, 12, 16, 20, EOT |
| EuroQol-5D-3L/5L | BSL, W 4, 8, 12, 16, 20, EOT |
| Health Utilities Index - III | BSL, W 4, 8, 12, 16, 20, EOT |
| Brief Pain Inventory - SF | BSL, W 6, 12, 18, EOT |
| Timeline Follow Back | BSL, then at each study visit |
| ASI-Self Report Form | SCR and EOT |
| Beck Depression Inventory - II | BSL, W 12, EOT |
| Beck Anxiety Inventory | BSL, W 12, EOT |
| Kessler Psychological Distress Scale 10 | BSL, W 12, EOT |
| Risk Behaviour Survey | BSL, W 12, EOT |
| Brief Substance Craving Scale | BSL, W 2, 4, 8, 12, 16, 20, EOT |
| TREATMENT | |
| Assigned OAT - Dosing | BSL, then W 1 to 24 |
| Medical Management | BSL, then as clinically needed |
SCR, Screening. BSL, Baseline. EOT, End of Treatment. W, week.
Randomization will be accomplished using a stratified permuted block design at the site level and by presence of lifetime heroin use, with blocks of varying sizes. This strategy will allow relative balance across the two study arms across recruitment sites, while decreasing the likelihood of predictability of treatment assignment. Randomization will be centralized and managed by the Data and Statistics Center (DSC) in a blind-fashion, and using the RedCap℠ web-based electronic data capture system. Participants will be initiated onto MMT or BUP-NX following provincial OAT guidelines and Health Canada approved product monographs for each medication, within 14 days of randomization (see Study Interventions). Once OAT is initiated, participants will be followed for 24 weeks, with study visits conducted every 2 weeks. As the participant approaches the end of the intervention period, the research team will ensure arrangements for continuation of treatment, as needed, and as locally appropriate. The trial design and protocol are registered in ClinicalTrials.gov ( NCT03033732).
2.2. Study objectives
The primary objective is to evaluate the relative effectiveness of BUP-NX model of care in reducing illicit opioid use during the 24-week intervention period compared to MMT. Secondary objectives are to evaluate: 1) retention in treatment, 2) safety of the study interventions, 3) medication adherence, 4) treatment satisfaction, and 5) patient engagement. Exploratory objectives include the assessment of the use of other illicit substances and changes in drug-related problems, quality of life and cost-effectiveness of the study treatments, psychological functioning, pain and risk behaviours.
2.3. Study sites
Sites for this study were selected based on their availability of clinicians with relevant OAT expertise and patient population (i.e., sufficient number of individuals with POUD to meet enrollment targets), access to a pharmacy that dispenses BUP-NX and daily witness ingestion of methadone, capacity to participate in clinical trials, and absence of competing studies. A total of seven sites across Canada, including hospital- and community-based clinics were selected to ensure diversity and representativeness of the population under study. The selected sites are: The Rapid Access Addiction Clinic (Vancouver, BC); the Opioid Dependency Program Clinic (Edmonton, AL); the Opioid Dependency Program Clinic (Calgary, AL); the Centre for Addictions and Mental Health (Toronto, ON); the Ontario Addiction Treatment Centre (Sudbury, ON); the Centre Hospitalier de l’Université de Montréal (Montréal, QC); and the Centre de Recherche et d’Aide pour Narcomane (Montréal, QC).
2.4. Study population
Consistent with a pragmatic study approach, the OPTIMA study seeks to enrol a diverse and representative sample of individuals with POUD and who may benefit from OAT across Canada. As such, the OPTIMA trial will enrol non-pregnant adults (18–64 years) who meet DSM-V criteria for OUD primarily attributable to PO (i.e., prescription opioids, fentanyl), not on stable OAT in the 4 weeks prior, without concurrent chronic pain requiring ongoing opioid therapy, and without any medical or mental condition that precludes safe participation in the study or ability to provide fully informed consent. Complete eligibility criteria are shown in Table 2.
Table 2.
Study eligibility criteria
| Inclusion Criteria | |
|---|---|
| Be aged between 18 and 64 years of age, inclusively; | |
| Be diagnosed with prescription opioid use disorder (moderate to severe, as defined by the DSM-5 criteria) which requires opioid agonist therapy as per the discretion of the physician; | |
If female:
|
|
| Be willing to be randomized to 24 weeks of either methadone or buprenorphine/naloxone adapted model of care, and to be followed for the duration of the trial; | |
| Be able to provide written informed consent; | |
| Be willing to comply with study procedures; | |
| Be able to communicate in English or French. | |
| Exclusion Criteria | |
| Any disabling medical condition, as assessed by medical history, physical exam, vital signs and/or laboratory assessments that, in the opinion of the study physician, precludes safe participation in the study or the ability to provide fully informed consent; | |
| Any disabling, unstable or acute mental condition that in the opinion of the study physician precludes safe participation in the study or ability to provide fully informed consent; | |
| Heroin reported as the most frequently used opioid in the past 30 days; | |
| Methadone or buprenorphine/naloxone use as maintenance OAT in the four weeks prior to screening; | |
| Pain of sufficient severity as to require ongoing pain management with opioids; | |
| History of a severe adverse event, hypersensitivity reaction, or allergic reaction to either methadone or buprenorphine/naloxone; | |
| Pregnancy, nursing, or planning to become pregnant during the study period; | |
| Current or past use of an investigational drug in another study in the last 30 days, confirmed via self-report; | |
| Pending legal action or other reasons in the opinion of the study physician that might prevent completion of the study; | |
| Presence of a substance use disorder that, in the opinion of the study physician, precludes safe participation in the study (e.g. unstable or severe alcohol use disorder, unstable or severe benzodiazepine use disorder); | |
| Current treatment with medications that may interact with either methadone or buprenorphine/naloxone (e.g. clonazepam, benzodiazepines) OR anticipation that the patient may need to initiate pharmacological treatment during the trial that is deemed unsafe by the study physician or could prevent study completion. | |
3. Study treatments (interventions)
Dosing and administration of both study treatments will follow national and provincial guidelines for the management of OUD and Health Canada-approved product monographs [6, 21–26]. Below, a brief general description of these two models of OAT is presented.
3.1. Methadone
The initial methadone dose is determined based on recent opioid use and level of tolerance, not to exceed 30 mg in the first day. Doses should be slowly titrated (i.e., 5–10 mg/day every 3–5 or more days) to target elimination of withdrawal symptoms, cessation of opioid craving and use, while avoiding opioid toxicity. Most patients will achieve stabilization on maintenance doses of 60 to 120 mg/day, although higher doses may be necessary for some individuals. Methadone will be initially provided via daily witnessed ingestion. Take home doses may be allowed for socially and clinically stable patients on a case-by-case basis at their care provider’s discretion, and according to local guidelines, usually not before 2–3 months of daily supervised administration.
3.2. Buprenorphine/Naloxone
BUP-NX induction should be initiated when there are signs of at least moderate opioid withdrawal (e.g., clinical opioid withdrawal scale (COWS) scores ≥ 12). The most common starting dose of BUPNX is 4 mg/1 mg. If withdrawal symptoms are not controlled after 1–3 hours, additional doses may be administered up to a total of 12 mg/3 mg in the first day. In following days, titration should continue as needed up to a maximum daily dose of 24 mg/6 mg of buprenorphine/naloxone. Take-home doses will be allowed as soon as the participant is clinically and socially stable as per product monograph and national guidelines for the management of OUD [6, 26], and at the discretion of the study physician. Specific protocol procedures include: witnessed ingestion of the first dose by a health care provider, a minimum of 1-week carries two weeks after treatment initiation, and 2-week carries four weeks after treatment initiation, except in specific situations that compromise participant’s safety and/or adequate storage of medication.
3.3. Medical Management
Participants in both arms will receive medical management by trained study physicians at each clinical site, in accordance with regular standard of care. During these visits, physicians are expected to routinely review recent drug and alcohol use and adherence to OAT, as well as to support efforts to reduce drug use or achieve/remain abstinent. Clinical visits will be independent of research visits and will continue regardless of ongoing study participation.
4. Assessments
4.1. Primary outcome measure
The primary outcome measure will be suppression of illicit opioid use, as evidenced by the overall percent of urine drug screens (UDS) that are negative for opioids (excluding assigned OAT or respective metabolites) from weeks 2 to 24. UDS will be collected prior to treatment initiation, and bi-weekly thereafter (Table 1). Missing urines will be considered positive for opioids.
Urines specimens will be collected using Heath Canada-approved Rapid Response™ Multi-Drug one-step Test Panel temperature-controlled urine drug test cups and analyzed for the presence of opiates, including morphine, oxycodone, fentanyl, methadone and its metabolite (EDDP), tramadol, buprenorphine, as well as for other common drugs used for non-medical purposes or respective metabolites (e.g., cocaine, benzodiazepines, THC). In addition, single test strips for both hydromorphone and 6-monoacetylmorphine (6-MAM) will be used.
4.2. Secondary outcome measures
Secondary outcome measures will include: (1) retention on assigned OAT, defined as having both a) an active prescription for the assigned OAT at week 24, and b) a positive UDS result for the assigned OAT at week 24; (2) medication adherence, measured as the proportion of assigned medication doses received over the 24-week study period; (3) safety, measured by adverse and serious adverse events (AEs and SAEs); (4) participant satisfaction with assigned OAT, assessed by the Client Satisfaction Questionnaire-8 (CSQ-8) [27]; and (5) participant engagement, measured by a battery of brief assessment tools that evaluate participant’s needs, preferences and involvement in the decision-making process: the Treatment Entry Questionnaire [28], Health Care Climate Questionnaire [29], and the Control Preference Scale [30] and the CollaboRATE questionnaire [31].
4.3. Exploratory outcome measures
Exploratory outcome measures will include: (1) cost-effectiveness and quality of life, assessed by the EuroQoL-5D (EQ-5D) [32, 33] Health Utilities Index III (HUI3) [34] instruments, as well as information on health service utilization; (2) pain, assessed by the Brief Pain Inventory- Short Form (BPI-SF) [35]; (3) use of other substances, as evidenced by a combination of UDS and self-report, using the Timeline Follow-Back (TLFB) [36, 37]; (4) changes in drug related problems, as indicated by score changes in the Addiction Severity Index (ASI)-self report assessment between screening and week 24 [38]; (5) psychological functioning, assessed by the Beck Depression Inventory – Second Edition (BDI-II) [39], the Beck Anxiety Inventory (BAI) [40], and the Kessler Psychological Distress Scale (K10) [41]; (6) drug and sex-related HIV and viral hepatitis risks, assessed by the Risk Behavior Survey (RBS) [42]; (7) proportion of participants who initiate taper, assessed by dosage information collected through pharmacy record abstraction and by self-report; (8) opioid craving, assessed with the Brief Substance Craving Scale (BSCS) [43]; and (9) opioid withdrawal, assessed with the Clinical Opiate Withdrawal Scale (COWS) [44].
4.4. Other assessments
Socio-demographics and information on overdose and addiction treatment exposures will be collected at screening. Information on HIV and Hepatitis C and B serology status will be collected at screening (or abstracted from medical records when available). Pregnancy and birth control will be assessed at screening, and every 4 weeks thereafter in female participants of childbearing potential. Information on the use of prescription (including opioids) and over-the-counter medications, as well as utilization of addiction-related services will be collected at screening and at every study visit throughout the study. The complete schedule of assessments is presented in Table 1.
5. Data analysis
Analyses will adhere to recommendations of the Extension of the CONSORT 2010 Statement for the reporting of non-inferiority and equivalence randomized trials [45]. All the proposed analyses will be conducted under the “intention-to-treat” (ITT) principle. A “switch equals failure” approach will be used, where participants who discontinue their assigned medication for any reason (including switches to the other arm) are classified as failures (i.e., UDS from the time a participant discontinue his/her assigned OAT will be considered as positive for opioids) [46, 47]. To assess the robustness of the findings, a sensitivity analyses using a “per-protocol” (PP) analysis approach will also be conducted, where non-adherent and/or non-compliant participants will be excluded from the analysis.
5.1. Primary endpoint
The primary endpoint is opioid use, as assessed by the overall proportion of opioid-free UDS, which will be conducted bi-weekly after treatment initiation for 24 weeks (i.e., 12 UDS in total). Missing UDS results will be considered as positive for opioids. Although a proportion is computed for each participant, the mean of these proportions will be use for the non-inferiority comparison of BUP-NX to MMT (current Canadian standard of care for OAT). The non-inferiority margin was set at 15%, following the results of a literature review [48], and relevant expert consultations. The choice of this margin reflected our willingness to accept a decreased effectiveness of BUP-NX in return of a superior safety profile and greater ease of administration of BUP-NX over methadone. The mean difference between the BUP-NX and methadone arms along with its 95% confidence interval (CI) will be calculated. Non-inferiority will be demonstrated if the lower limit of the 2-side 95% CI for the mean difference lies above −15%.
5.2. Secondary endpoints
All the analyses for the secondary endpoints except for the safety endpoint will use ‘superiority’ hypotheses. In general, if use of formal testing to assess differences between arms is needed, the following methods will be used: for binomial response variables, chi-square tests and logistic regression; for continuous variables, t-tests and linear regression, or nonparametric methods if data follow a non-normal distribution. Two-sided tests will be performed with a α-level of 5%.
5.3. Safety analysis
The number and percentage of participants experiencing each specific AE will be tabulated by severity and by relationship to treatment regimen. For the calculations in these tables, each participant’s AE will be counted once under the maximum severity or the strongest recorded causal relationship to study product. For each arm, all AEs will be grouped by body system and a p-value and confidence interval for the relative risk (comparing both arms) of each AE will be calculated, as well as the difference in rates between treatment arms and its confidence interval.
6. Sample size and power calculation
The sample size calculation for the non-inferiority evaluation of the difference in mean percentages of opioid-free UDS over 24 weeks for BUP-NX compared to MMT was based on the following assumptions, drawn both from previous studies [7, 8, 48] and input from addiction medicine experts within the CRISM network: an expected mean of 75% opioid-free UDS during the 24-week intervention period in the methadone arm and 67.5% in the BUP-NX arm. Under the above assumptions, and using a common standard deviation of 25%, a 1-sided α-level of 0.05, 80% power, a non-inferiority margin of 15%, and 1:1 allocation ratio, approximately 276 participants would be needed (138 per arm). With a conservative estimate of a 45% screening failure rate, it was estimated that approximately 500 individuals would need to be screened to achieve this sample size. The sample size calculation was performed using the R software, version 3.3.1 (TrialSize 1.3 package). Once a third of the participants have completed the 24-week study period, the Data and Safety Monitoring Board (DSMB) will conduct a blinded interim analysis of aggregate data to evaluate the accuracy of the variance parameter initially used for sample size calculation and of compliance parameters to protocol procedures (e.g., proportion of participants who discontinue assigned treatment), and eventually recommend on sample size re-estimation, following U.S. Food and Drug Administration (FDA) guidance [49].
7. Interim safety and efficacy analyses
An independent DSMB will examine accumulating data (e.g., every 6 months) to ensure participants’ safety and to determine that the scientific goals of the study are being met. Recommendations of study termination or modification may be provided for poor accrual/recruitment, adherence/product use, retention, and/or clear evidence of serious safety issues (e.g., excess of AEs or SAEs in one of the arms). Given the relatively short duration of the trial and that both study arms are considered standard treatments for OUD, no formal interim efficacy analysis is planned. The interim analysis for sample size re-estimation is described above, under the Sample size and power calculation section.
8. Ancillary studies
To further leverage the core scientific knowledge gained in the OPTIMA trial, several ancillary studies will be conducted alongside the main trial. The participants may or may not choose to take part in any or all of these ancillary studies without affecting participation in the main study.
8.1. Health economics analysis
The cost-effectiveness of BUP-NX vs. MMT will be evaluated by trial-based, as well as model-based analysis. A societal perspective that incorporates the direct and indirect costs of treatment will be adopted for all cost analysis, including costs of criminal activity and lost productivity, along with medication and inpatient/outpatient care.
8.2. Pharmacogenomics study
Participants will have the option to participate in a pharmacogenomics ancillary study that will involve collection of a blood sample and certain outcome data collected as part of the main study. The main objective of this study is to determine if treatment response is affected by genetic variation in drug targets (i.e., Opioid Receptor Mu 1 [OPRM1]) or drug metabolizing enzymes (e.g. CYP2B6, OPRD1, CYP2C19, CYP3A4 and UGT2B7). A second objective is to investigate the potential association of these gene variants with phenotypes associated with prescription opioid use disorders.
8.3. Persons Who Use Drugs’ experiences in clinical trials
This ancillary study will involve longitudinal semi-structured qualitative interviews with key groups of individuals to generate knowledge and understanding of how study (e.g., eligibility criteria, design and protocol requirements), individual (e.g., sociodemographic), social (e.g., drug use networks) and structural (e.g., criminalization of substance use) characteristics impact study recruitment, retention, adherence and other study outcomes (e.g., generalizability). Fifty-six OPTIMA participants (14 at each of four sites; seven from each study arm) and 16 OPTIMA study physicians (from each of the same 4 sites) will participate in this ancillary study. OPTIMA participants will complete two interviews, one following baseline and randomization (baseline interview) and one following study completion or study/intervention withdrawal (exit interview).
8.4. Sexual dysfunction study
The main objectives of this ancillary study is to determine the relative effects of BUP-NX and methadone on sexual function in both men and women, and the potential impacts of OAT-sexual dysfunction on study outcomes. Self-report questionnaires evaluating sexual function (Female Sexual Function Index [FSI] [50] and International Index of Erectile Function [IIEF] [51] for women and men, respectively), satisfaction (Global Measure of Sexual Satisfaction) [52], and distress (Sexual Distress Scale [SDS]) [53] will be administered to consenting participants at baseline and every 4 weeks for the 24-week intervention period.
8.5. Take-home naloxone study
This ancillary study will evaluate the awareness and uptake of take-home naloxone (THN) programs among participants enrolled in OPTIMA trial. The study will involve a one-time cross-sectional survey administered during the screening visit. The main objective of this study is to assess the reach of THN programs among individuals with prescription opioid use disorders in Canada and investigate inter-provincial differences.
9. Current status of the study
As of January 2018, the OPTIMA study has received ethical approval from the Research Ethics Boards of each participating clinical sites. Five sites have begun enrollment, with the remaining two sites scheduled to start in February 2018. A total of twenty-four participants have been enrolled, and of these, fourteen have been randomized.
10. Discussion
The OPTIMA trial is the first national study of CRISM and was conceived in response to the escalating public health opioid crisis in Canada. Over the course of OPTIMA preparation, the increasing contamination of the drug supply with illicitly manufactured fentanyl (IMF) and related analogs has further worsened the overdose epidemic, as demonstrated by the almost 3,000 opioid-related deaths in 2016 across Canada, and estimated 4,000 in 2017 [54].
In this context, the comparative effectiveness pragmatic trial of BUP-NX and MMT Canadian models of care proposed by OPTIMA is particularly timely and relevant. In Canada, both medications are available for the treatment of OUD in outpatient community-based settings. Due to their different safety profiles, methadone usually requires daily witnessed ingestion in a pharmacy setting, while BUP-NX allows for early take-home doses upon achieving clinical stability, which has the potential to reduce access barriers, a critical need given the state of the opioid-related overdose epidemic. However, clinical trials comparing BUP-NX and methadone have usually assessed similar daily witnessed ingestion dosing strict dosing schedules, and therefore the evidence base on the relative effectiveness, safety and acceptability of these two models of care (i.e., methadone in daily witness ingestion and buprenorphine in flexible take home doses) is limited [17]. In addition, very few studies have compared these two medications among the growing population of individuals with OUD primarily attributed to prescription opioids. Even less is known about how these medications, and respective models of care may perform among individuals who have been exposed (intentionally or inadvertently) to fentanyl. These knowledge gaps create a huge clinical dilemma for providers and patients, underscoring the urgent need to assess the effectiveness, acceptability and safety of these two models of care work in real-life clinical practice settings and among the diverse population of individuals with prescription opioid use disorder.
The choice of our primary outcome provides an assessment of the overall effectiveness of the model of care (i.e., combination of tolerability, adherence, retention and efficacy of the drug), as well as reflects a clinically meaningful outcome for both health care providers and patients (i.e., reduction in use). The evaluation of secondary outcomes (i.e., retention in treatment, safety, treatment satisfaction and patient engagement, cost-effectiveness), as well as the inclusion of a number of ancillary studies, will further contribute to our understanding of the relative effectiveness and acceptability of the two interventions, providing valuable information for provision of health and social services and informing evidence-based clinical practice and systems of care. The variety of outcomes that will be assessed will also allow for identification of specific aspects of OUD that may be better targeted by the two available OAT approaches. This may also be an opportunity to further explore whether certain subpopulations are more likely to respond positively to one of these models of care.
Among the limitations of the study, the open-label design may potentially introduce bias. However, the efficacy of both medications in double-blind controlled studies is known. Further, the main goal of OPTIMA, as a pragmatic clinical trial, is to compare BUP-NX and MMT as they are delivered in real-life clinical settings (e.g., early take-home doses vs. daily witnessed ingestion) in Canada to enhance the external validity of the findings. Second, the study would potentially benefit from a longer duration of follow-up and larger sample size (including for the assessment of heterogeneity between sites), which was not feasible due to costs constraints. The relative small sample size will limit the comparisons of different delivery models for each medication (e.g., varying schedules for supervised BUP-NX ingestion), or the conduct of sufficiently powered subgroup analyses. Finally, the pragmatic approach imposes a limited time for research procedures, which hinders a more thorough examination of certain outcomes such as mental health.
Despite these limitations, OPTIMA will be among the first studies to compare the relative effectiveness of BUP-NX and MMT models of care for the treatment of OUD, including the comparison of supervised vs. take-home strategies [17]. We believe that information derived from this study will provide valuable information to further our understanding on the relative effectiveness, safety and acceptability of each model of care. In addition, given that in Canada both treatments are delivered through primary care, findings from this study may also be of interest for other jurisdictions willing to expand access to low-threshold community-based OAT. Above and beyond the critical data that OPTIMA will provide, this pan-Canadian, multisite study will mobilize and strengthen a collaborative network of clinicians, researchers and various stakeholders to work towards an evidence-based response to the ongoing devastating opioid crisis in North America.
Acknowledgements
The authors thank the study participants for their contributions to the research, as well as current and past researchers and staff. We would specifically like to thank Benoît Mâsse, Jill Fikowski, Aïssata Sako, Katrina Blommaert, Emma Garrod, Denise Adams, José Trigo, Amel Zertal, Nirupa Goel, and Farihah Ali for their research and administrative assistance.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR) through the Canadian Research Initiative in Substance Misuse (CRISM; grant numbers CIS-144301, CIS-144302, CIS-144303, CIS-144304). The 4 nodes of CRISM received independent funding through a CIHR priority-driven initiative (grant numbers SMN-139148, SMN-139149, SMN-139150, SMN-139151). MES is supported by Michael Smith Foundation for Health Research (MSFHR) and CIHR fellowship awards. KA is supported by an Embedded Clinician Researcher Salary Award from CIHR. EW is supported in part by a Tier 1 Canada Research Chair in Inner City Medicine. BF is supported in part by an Addiction Psychiatry Chair, Department of Psychiatry, University of Toronto. DJA is supported by a clinical researcher career award from the Fonds de Recherche du Québec—Santé (FRQS).
Footnotes
Clinical Trial Registration: NCT03033732.
11. References
- [1].Fischer B, Rehm J, Tyndall M, Effective Canadian policy to reduce harms from prescription opioids: learning from past failures, Cmaj 188(17–18) (2016) 1240–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Socias ME, Ahamad K, An urgent call to increase access to evidence-based opioid agonist therapy for prescription opioid use disorders, Cmaj 188(17–18) (2016) 1208–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fischer B, Gooch J, Goldman B, Kurdyak P, Rehm J, Non-medical prescription opioid use, prescription opioid-related harms and public health in Canada: An update 5 years later, Canadian Journal of Public Health-Revue Canadienne De Sante Publique 105(2) (2014) E146–E149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC, The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction, Annu Rev Public Health 36 (2015) 559–74. [DOI] [PubMed] [Google Scholar]
- [5].Socias ME, Volkow N, Wood E, Adopting the ‘cascade of care’ framework: an opportunity to close the implementation gap in addiction care?, Addiction 111(12) (2016) 2079–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].A Guideline for the Clinical Management of Opioid Use Disorder, British Columbia Centre on Substance Use (BCCSU), Vancouver, BC, Canada, 2017. [Google Scholar]
- [7].Mattick RP, Breen C, Kimber J, Davoli M, Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence, Cochrane Database of Systematic Reviews (2) (2014). [Google Scholar]
- [8].Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N, Opioid agonist treatment for pharmaceutical opioid dependent people, Cochrane Database Syst Rev (5) (2016) CD011117. [DOI] [PubMed] [Google Scholar]
- [9].MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, Degenhardt L, Hickman M, Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis, BMJ 345 (2012) e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perlman DC, Jordan AE, Uuskula A, Huong DT, Masson CL, Schackman BR, Des Jarlais DC, An international perspective on using opioid substitution treatment to improve hepatitis C prevention and care for people who inject drugs: Structural barriers and public health potential, Int J Drug Policy 26(11) (2015) 1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, Cunningham CO, Sullivan LE, Vergara-Rodriguez P, Fiellin DA, Cajina A, Botsko M, Nandi V, Gourevitch MN, Finkelstein R, Collaborative B, HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study, J Acquir Immune Defic Syndr 56 Suppl 1 (2011) S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nosyk B, Min JE, Colley G, Lima VD, Yip B, Milloy MJ, Wood E, Montaner JS, The causal effect of opioid substitution treatment on HAART medication refill adherence, AIDS 29(8) (2015) 965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R, Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies, BMJ 357 (2017) j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C, Retention in medication-assisted treatment for opiate dependence: A systematic review, J Addict Dis 35(1) (2016) 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Teruya C, Schwartz RP, Mitchell SG, Hasson AL, Thomas C, Buoncristiani SH, Hser Y-I, Wiest K, Cohen AJ, Glick N, Jacobs P, McLaughlin P, Ling W, Patient Perspectives on Buprenorphine/Naloxone: A Qualitative Study of Retention During the Starting Treatment with Agonist Replacement Therapies (START) Study, Journal of Psychoactive Drugs 46(5) (2014) 412–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yarborough BJ, Stumbo SP, McCarty D, Mertens J, Weisner C, Green CA, Methadone, buprenorphine and preferences for opioid agonist treatment: A qualitative analysis, Drug Alcohol Depend 160 (2016) 112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saulle R, Vecchi S, Gowing L, Supervised dosing with a long-acting opioid medication in the management of opioid dependence, Cochrane Database Syst Rev 4 (2017) CD011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Canadian Research Initiative in Substance Misuse (CRISM). http://www.cihrirsc.gc.ca/e/44597.html. (Accessed August 18 2017).
- [19].Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, Tunis S, Bergel E, Harvey I, Magid DJ, Chalkidou K, A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers, Journal of Clinical Epidemiology 62(5) (2009) 464–475. [DOI] [PubMed] [Google Scholar]
- [20].Schwartz D, Lellouch J, EXPLANATORY AND PRAGMATIC ATTITUDES IN THERAPEUTICAL TRIALS, Journal of Chronic Diseases 20(8) (1967) 637-&. [DOI] [PubMed] [Google Scholar]
- [21].Alberta Methadone Maintenance Treatment Standards and Guidelines for Dependence, College of Physicians and Surgeons of Alberta, Edmonton, AB, 2014. [Google Scholar]
- [22].Buprenorphine/Naloxone for Opioid Dependence - Clinical Practice Guideline, Centre for Addiction and Mental health, Toronto, ON, 2011. [Google Scholar]
- [23].Methadone Maintenance Treatment Program Standards and Clinical Guidelines, The College of Physicians and Surgeons of Ontario, Toronto, ON, 2011. [Google Scholar]
- [24].La buprénorphine dans Le traitement de la dépendance aux opioïdes, Collège des médecins du Québec, Montreal, QC, 2009. [Google Scholar]
- [25].Utilisation de la méthadone dans le traitement de la toxicomanie aux opiacés, Collège des médecins du Québec, Montreal, QC, 1999. [Google Scholar]
- [26].Bruneau J, Ahamad K, Goyer ME, Poulin G, Selby P, Fischer B, Wild TC, Wood E, C.C.R.I.i.S. Misuse, Management of opioid use disorders: a national clinical practice guideline, Cmaj 190(9) (2018) E247–E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].De Wilde EF, Hendriks VM, The Client Satisfaction Questionnaire: psychometric properties in a Dutch addict population, Eur Addict Res 11(4) (2005) 157–62. [DOI] [PubMed] [Google Scholar]
- [28].Wild C, Treatment Entry Questionnaire (TEQ) Version 1.0, Users Guide, Toronto, Ontario, Canada: Addiction Research Foundation; (1999). [Google Scholar]
- [29].Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL, Autonomous regulation and long-term medication adherence in adult outpatients, Health Psychology 17(3) (1998) 269. [DOI] [PubMed] [Google Scholar]
- [30].Degner LF, Sloan JA, Venkatesh P, The Control Preferences Scale, The Canadian journal of nursing research= Revue canadienne de recherche en sciences infirmieres 29(3) (1996) 21–43. [PubMed] [Google Scholar]
- [31].Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G, The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process, J Med Internet Res 16(1) (2014) e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X, Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L), Qual Life Res 20(10) (2011) 1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nosyk B, Sun H, Guh DP, Oviedo-Joekes E, Marsh DC, Brissette S, Schechter MT, Anis AH, The quality of eight health status measures were compared for chronic opioid dependence, J Clin Epidemiol 63(10) (2010) 1132–44. [DOI] [PubMed] [Google Scholar]
- [34].Furlong WJ, Feeny DH, Torrance GW, Barr RD, The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies, Ann Med 33(5) (2001) 375–84. [DOI] [PubMed] [Google Scholar]
- [35].Cleeland CS, Ryan KM, Pain assessment: global use of the Brief Pain Inventory, Ann Acad Med Singapore 23(2) (1994) 129–38. [PubMed] [Google Scholar]
- [36].Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P, The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties, J Consult Clin Psychol 68(1) (2000) 134–44. [DOI] [PubMed] [Google Scholar]
- [37].Robinson SM, Sobell LC, Sobell MB, Leo GI, Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use, Psychol Addict Behav 28(1) (2014) 154–62. [DOI] [PubMed] [Google Scholar]
- [38].Cacciola JS, Mclellan AT, Alterman AI, Mulvaney FD, A comparison of a self-administered ASI with the standard ASI interview, Problems of Drug Dependence, 1997, Proceedings of the 59th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc. NIH Publication no. 98± 4305 (1998). [Google Scholar]
- [39].Beck AT, Steer RA, Brown GK, Manual for the beck depression inventory-II, San Antonio, TX: Psychological Corporation; 1 (1996) 82. [Google Scholar]
- [40].Beck AT, Epstein N, Brown G, Steer RA, An inventory for measuring clinical anxiety: Psychometric properties, Journal of Consulting and Clinical Psychology (56) (1988) 893–897. [DOI] [PubMed] [Google Scholar]
- [41].Kessler RC, Andrews G, Colpe, e. al, Short screening scales to monitor population prevalences and trends in non-specific psychological distress, Psychological Medicine 32 (2002) 893–897. [DOI] [PubMed] [Google Scholar]
- [42].National Institute on Drug Abuse, Risk Behavior Assessment, 3rd ed., NIDA Community Research Branch, Rockville, MD, 1993. [Google Scholar]
- [43].Somoza E, Dyrenforth S, Goldsmith J, Mezinskis J, Cohen M, In search of a universal drug craving scale, Paper presented at the Annual Meeting of the American Psychiatric Association, Miami Florida (1995). [Google Scholar]
- [44].Wesson DR, Ling W, The Clinical Opiate Withdrawal Scale, Journal Of Psychoactive Drugs 35(2) (2003). [DOI] [PubMed] [Google Scholar]
- [45].Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, Group C, Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement, Jama 308(24) (2012) 2594–2604. [DOI] [PubMed] [Google Scholar]
- [46].Morden JP, Lambert PC, Latimer N, Abrams KR, Wailoo AJ, Assessing methods for dealing with treatment switching in randomised controlled trials: a simulation study, BMC Medical Research Methodology 11(4) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hill A, Demasi R, Discordant conclusions from HIV clinical trials--an evaluation of efficacy endpoints, Antiviral Therapy 10(3) (2005) 367–374. [PubMed] [Google Scholar]
- [48].Carrieri PM, Michel L, Lions C, Cohen J, Vray M, Mora M, Marcellin F, Spire B, Morel A, Roux P, Methaville Study G, Methadone induction in primary care for opioid dependence: a pragmatic randomized trial (ANRS Methaville), PLoS One 9(11) (2014) e112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Guidance for Industry. Adaptive Design Clinical Trials for Drugs and Biologics, Food and Drug Administration, U.S. Department of Health and Human Services, February 2010. [Google Scholar]
- [50].Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D’Agostino R Jr., The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function, J Sex Marital Ther 26(2) (2000) 191–208. [DOI] [PubMed] [Google Scholar]
- [51].Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A, The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction, Urology 49(6) (1997) 822–30. [DOI] [PubMed] [Google Scholar]
- [52].Lawrance K-A, Byers ES, Sexual satisfaction in long-term heterosexual relationships: The interpersonal exchange model of sexual satisfaction, Personal Relationships 2(4) (1995) 267–285. [Google Scholar]
- [53].Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J, The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal distress in women, J Sex Marital Ther 28(4) (2002) 317–30. [DOI] [PubMed] [Google Scholar]
- [54].National report: Apparent opioid-related deaths in Canada (January 2016 to March 2017), 2017. https://www.canada.ca/en/public-health/services/publications/healthy-living/apparent-opioid-related-deaths-report-2016.html#fn1. (Accessed September 14 2017).