Abstract
Background
Food-related quality of life (FRQoL) evaluates the impact of diet, eating behaviors, and food-related anxiety on a person’s quality of life. This is the first study to evaluate FRQoL in inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), two illnesses where food and diet are of importance.
Methods
One hundred seventy-five participants (80 IBS, 95 IBD) participated in the study by completing measures evaluating FRQoL, psychological distress, and health-related quality of life. Primary analyses evaluated differences in FRQoL between IBD and IBS patients. Secondary analyses compared differences based on remission status, dietary use, and dietary consultation, as well as evaluated potential predictors of FRQoL.
Results
IBD patients in remission report the highest FRQoL (IBD-remission: 91.2 (26.5) vs. IBD-active: 67.7 (19.6) and IBS-active: 67.6 (18.3), p<.001). Using more dietary treatments is associated with decreased FRQoL for IBS (r = −0.23, p<.05) and IBD patients (r= −0.31, p<.01). IBS patients are more likely to use dietary treatments than IBD (IBS = 81% vs. IBD = 64%, p < .01), with self-directed diets being the most commonly used approach. Symptom severity is the strongest predictor of FRQoL in both groups (IBD: R2 = .27, p < .01; IBS: R2 = .23, p < .001).
Conclusion
FRQoL is a unique construct for IBD and IBS patients that can be influenced by several clinical and dietary factors, including number of diets and type of diet used, depending on the diagnosis. Thus, FRQoL should be considered when working with both IBD and IBS patients.
Keywords: Inflammatory Bowel Disease, Irritable Bowel Syndrome, Diet, Quality of Life, Outcomes Research
Introduction
Inflammatory Bowel Disease (IBD), including Crohn’s Disease (CD), Ulcerative Colitis (UC), Indeterminate Colitis (IC), and Irritable Bowel Syndrome (IBS) are two of the most common chronic gastrointestinal (GI) illnesses seen in behavioral medicine practice [1,2]. IBD and IBS exhibit similar symptoms including abdominal pain, altered bowel habits, nausea, and fatigue despite major differences in pathophysiological origin, treatment, and disease course [3–5]. Their chronic nature and illness symptom-burden can impact psychosocial functioning [6,7]. Health-related quality of life (HRQoL), or the social, emotional, physical, and financial impact of an illness, is widely studied in IBD and IBS [8–13]. Many studies show these patients report significant degradations in HRQoL and increased psychological distress due to their illness [13–16], as well as stigmatization and social withdrawal [17–19]. Food consumption and eating behaviors are additional areas of concern that may have an impact on HRQoL [20,21].
Several dietary treatments exist for IBD and IBS including the specific carbohydrate diet [22,23], low FODMAP diet [24,25], food allergy/intolerance guided elimination diets [26,27], and elemental formulas [28]. Patients are extremely interested in diet, specifically its impact on disease activity and symptoms [29]. Data on dietary intervention efficacy and the role of foods in IBD and IBS onset and symptom exacerbation are mixed [22,24,30,27,25,31]. However, most patients believe certain foods make symptoms worse [32], and many will restrict their diets either independently or with clinician guidance. Using dietary restriction may help patients feel more in control over their illness [33,34]; however, the more severe the dietary restriction the greater chance for impact across HRQoL domains [23,21].
Food-related quality of life (FRQoL) is a similar construct to HRQoL, but assesses the specific impact of diet, eating behaviors, and food-related anxiety on a person’s HRQoL. Given the emerging interest in dietary treatments for the IBD and IBS population and potential impact diets can have on quality of life, evaluating FRQoL is likely an important area for patients and clinicians to be aware of. However, no research exists in FRQoL in IBD or IBS with exception of a recently validated, 29-item measure to assess FRQoL in patients with IBD [35]. The study identified themes associated with eating, socialization, need for control, and food-related anxiety. Currently, there are no studies evaluating FRQoL in IBS, or comparing the two groups for differences.
The present study aims to evaluate FRQoL in IBD and IBS and assess differences between the two disease groups. Secondary aims are to examine relationships between FRQoL, disease-specific HRQoL, dietary treatment use, and psychological distress. We hypothesized while both IBS and IBD patients will have alterations in FRQoL, the magnitude of changes in IBD and IBS patients will differ. We also hypothesized higher FRQoL will be associated with increased disease-specific HRQoL, lower disease activity, as well as decreased dietary treatment use and psychological distress.
Methods
Sample Size
A priori power analysis for planned statistics using G*Power[36] indicated a minimum necessary sample size of 128 participants (64 per group for t-tests; hypothesis 1) with 0.5 effect size, 0.8 power and alpha error at 0.05. For hypothesis 2, a sample size of 26 is needed for correlational tests, and sample of 20 is necessary for each regression analysis with the same power and alpha error requirements.
Participants and Recruitment
Individuals aged 18-70 with confirmed IBD or IBS diagnosis for a minimum of 3 months were recruited from two outpatient university-based gastroenterology practices.
Electronic medical record documentation of accepted endoscopic, histologic, and/or imaging findings confirmed IBD;[37] Rome III criteria documentation confirmed IBS [38]. Additional patients with self-reported IBD or IBS diagnosis were recruited via ResearchMatch.org, and social media (Facebook, Twitter). ResearchMatch.org is a website that matches individuals interested in research with opportunities in their area of interest. The recruitment source distribution was as follows: University-based clinics (IBD = 23.2%, IBS = 16.5%), ResearchMatch (IBD = 42.1%; IBS = 67.1%), and “other” recruitment methods (IBD = 7.4%; IBS = 16.5%).
Prior to study item administration, IBS patients completed the Rome III bowel questionnaire. Those who did not report abdominal pain or discomfort coinciding with altered bowel habits for a minimum of three months were excluded. For IBD, patients completed diagnosis (CD, UC, IC) and method of diagnosis (e.g. colonoscopy with biopsy, radiological imaging such as MRI or CT scan, or laboratory tests) as screeners.
Assessments
Structured surveys captured demographic and clinical data including age, gender, race, ethnicity, community environment (rural or urban), current city/town population, marital status, education level, employment status, household income, and recruitment source.
The clinical information included disease duration (years), age at symptom onset (years), current (at study) medications, current diet use for disease, current diet duration (months), and self-report of seeing a dietitian for treatment of their disease currently or in the past (Yes/No). Current dietary types included low/high fiber focused, grain/carbohydrate focused, dairy focused, low-FODMAP, and self-directed elimination diets. Current diet adherence (“How well would you say you are able to follow your dietary treatment(s) as you should?”) and efficacy (“How well would you say your dietary treatment(s) help your symptoms?”) was rated on a 0 (not at all) to 10 (excellent) scale. The partial Harvey Bradshaw Index (HBI) [39] measured disease activity for CD patients and Simple Clinical Colitis Activity Index (SCCAI) [40,41] for UC/IC; HBI ≤ 4 [42] and SCCAI ≤ 3 [40] equated to remission status. Patients currently prescribed a biologic and/or corticosteroid were classified as “more severe IBD.” IBS type was classified as diarrhea (D), constipation (C), mixed (M) per Rome III Diagnostic Criteria [43,38], The validated IBS Symptom Severity Scale measured IBS severity with a cutoff score for remission set to 75 [44].
The participants also all filled out the following validated questionnaires:
Food-Related Quality of Life in IBD Questionnaire (Fr-QOL-29)
Fr-QOL-29 [35] is a 29-item self-report measure of impact of food restriction/dietary changes on several daily living domains, validated for IBD patients. Items are rated on a 5-point Likert scale from 1 (Strongly Agree) to 5 (Strongly Disagree). For IBS patients, “IBS” replaced the acronym “IBD.” Basic reliability statistics evaluated the modified version to confirm internal consistency remained (Cronbach α IBS = 0.95, IBD = 0.96). Higher scores denote better FRQoL with a maximum score of 145.
National Institute of Health Patient-Reported Outcomes Measurement Information System (NIH-PROMIS) Emotional Distress–Anxiety and Depression
NIH PROMIS Anxiety and Depression scales [45] assess for self-reported psychological distress. The anxiety scale measures fear, worry, and symptoms associated with arousal [45]. The depression scale measures negative mood, decreased affect, and social cognition [45]. Each scale has 8 items rated on a 5-point Likert scale from 1 (Never) to 5 (Always). Higher scores indicate greater anxiety and depression. The NIH PROMIS scales demonstrate excellent reliability and validity [46].
IBS Quality of Life Scale (IBS-QOL)
IBS-QOL [47] is a 34-item measure used to assess impact of IBS and its treatment on HRQoL. As it focusses on bowel habits, IBS-QOL is used for patients with IBS and IBD for comparative purposes [48–50]. Jones et al. found the IBS-QOL to be correlated with the Inflammatory Bowel Disease Questionnaire [51] (r = −.83, P < .001), used to assess HRQoL in individuals with IBD [50,51]. Considering IBS-QOL previously assessed and compared IBD and IBS patients, the current study used IBS-QOL to evaluate HRQoL across diseases. Items on IBS-QOL are evaluated on a 5-point Likert scale from 1 (Not at All) to 5 (Extremely/A Great Deal). Maximum total score is 100, with higher scores indicating better disease-specific HRQoL. The IBS-QOL demonstrates reliability and validity in IBS and IBD samples [52,47,53].
Statistical Analyses
Analyses were conducted using IBM SPSS v24 for Macintosh (Chicago, IL). All continuous variables were checked for normality using measures of skewness and kurtosis with a cutoff of 2.0 and results determined necessity for non-parametric tests for some variables. Descriptive statistics evaluated all variables using mean, median, standard deviation (SD), or range, as appropriate. Due to potentially heterogeneous recruitment samples (e.g. online versus clinic) all variables were analyzed using the appropriate statistical test (t-Test or Mann-Whitney U for continuous variables, Chi Square for categorical variables) to determine between group differences for recruitment sources as a whole, then separately for each diagnosis. The main a-priori comparison chosen was between IBD and IBS. Secondary a-priori planned analyses included: 1) comparing patients in remission versus those with active disease determined by accepted cutoff scores for each symptom severity scale, 2) comparison between users and non-users of diet categories and dietitian consultation, and 3) evaluating potential FRQoL predictors.
Independent samples t-tests or one-way ANOVA with Tukey post-hoc test assessed differences in normally distributed continuous variables; chi-square or Fisher exact tests assessed categorical variables with frequency data, as appropriate. Non-normality distributed data were analyzed using nonparametric Mann-Whitney U. Effect sizes for clinical significance were reported as Cohen’s d interpreted with accepted score cut-offs [54] (≤ 0.2 = small, 0.3 - 0.5 = medium, ≥ 0.6 = large). Statistical significance was set to P < .01 (Bonferroni correction) to control for Type 1 error in detected differences.
For secondary analyses, we first grouped current diets by primary dietary item being modified in order to assess impact of each modification separately and to determine whether FQoL differs across various diet types: Fiber Focused (low or high), Grain/Carbohydrate Focused (Specific Carbohydrate Diet, Gluten Free, Paleo), Dairy Focused (Vegan, Vegetarian, Dairy Free, Paleo), Self-Directed (Self-Directed Elimination, Other), and low-FODMAP. Next, patients were categorized as those using multiple diets (>1) at time of study, a single dietary treatment, or no diet and analyses repeated.
For IBD, HBI and SCCAI scores were converted to standardized Z scores for a uniform symptom severity measure. A series of univariate analysis of covariance (ANCOVA) assessed differences in FRQoL by diet use while adjusting for symptom severity (covariate). Estimated marginal means are reported with Bonferroni adjustment. Pearson’s or Spearman’s correlations determined relationships between FRQoL and demographic and disease variables, as appropriate.
Two separate hierarchical multiple regression analyses evaluated significant FRQoL predictors by diagnosis. Considering food intake may worsen symptoms in active disease for both IBD and IBS, we predicted this may be confounder. For both diseases, symptom severity was entered at step one of the regression to control for this variable. Our clinical observations from treating patients with both IBS and IBD led to some predictions as to what additional variables could affect FRQoL and/or be predictors of it: In terms of diet, we predicted diet type and number of diets (more restrictive diets and a higher number of different diets used concomitantly are harder to implement, which may create more food related anxiety and thereby lead to decreased FRQoL), diet duration (longer duration of diets may lead to adaptation, especially in terms of better handling of social situations around food, potentially increasing FRQoL), diet efficacy (diets perceived as more efficacious in improving symptoms may increase FRQoL), diet compliance (higher compliance with diet may require compliance in social situations and may be harder to implement, thereby resulting in lower FRQoL), instructions from a dietitian (expert help may ease the burden of gathering accurate knowledge in implementing diets and may thereby improve FRQoL) may be potential predictors. In terms of psychological variables, we predicted baseline anxiety and depression could also be a cause of food related anxiety and potentially impact FRQoL. Therefore, diet variables were entered at step two, and any relevant psychological variables at step three into the regression analyses. Adjusted R2, standardized Beta weights (β), and standard error are reported for each model.
Compliance with Ethical Standards
The IRBs of XXXXX (IRB #00202950) and YYYYY (IRB #16041206) approved the study. All participants provided informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
Study Sample
A total of 265 individuals consented to the study with 253 completing all study questions (95% completion rate). Of completed surveys, 78 were excluded from data analysis for one or more following reasons: 7 did not pass Rome III criteria, 27 did not pass IBD screening questions, and 61 did not pass other screening items (e.g. co-morbid GI illness). The final sample consisted of 175 patients (80 IBS, 95 IBD; 69% inclusion rate). We did not observe significant differences in FRQoL, demographic, or clinic variables by recruitment source for the entire sample aside from geographic area, which demonstrated participants recruited in clinic were more likely to be living in an urban area (p <.01). This finding was likely due to the clinic recruitment sources in Chicago. When assessing differences by diagnosis, we found no significant differences for IBS patients recruited online versus in clinic; patients recruited online did report higher levels of anxiety but below the corrected cutoff for statistical significance (Online Mean= 18.34±7.68 vs. Clinic Mean= 17.04±6.68, p=.034). For IBD, online participants tended to use dietary therapy for longer duration, but again this difference fell below the corrected cutoff for significance (p=.024). Therefore, participants from all recruitment sources were pooled for analysis. Subject demographic and clinical data are presented in Tables 1 and 2.
Table 1.
Demographic Characteristics of Study Sample
| IBD (N = 95) | IBS (N = 80) | |
|---|---|---|
| Age at Diagnosisa | 27.6 (13.0) | 31.8 (12.4) |
| Age at Symptom Onseta | 23.4 (12.9) | 24.8 (12.7) |
| Current Agea | 41.9 (14.2) | 42.7 (13.9) |
| Gender | ||
| Male | 23.2% (22)* | 8.9% (7) |
| Female | 76.8% (73) | 91.1% (73) |
| Race | ||
| Non-Hispanic White | 89.5% (85) | 89.9% (71) |
| African-American/Black | 4.2% (4) | 2.5% (2) |
| Latino(a), Hispanic | 0% | 3.8% (3) |
| Asian American | 2.1% (2) | 0% |
| Other | 4.2% (4) | 3.8% (3) |
| College Educated | 73.7% (70) | 82.3% (66) |
| Married or in Committed Relationship | 51.6% (49) | 57.1% (44) |
| Urban Community | 84.2% (80) | 82.3% (65) |
| Household Income > $50,000 per year | 72.1% (68) | 73.5% (50) |
| Employed (Full or Part Time) | 73.4% (69) | 70.9% (56) |
| Recruitment Source | ||
| Online | 52.6% (50) | 83.6% (66) |
| Clinic | 47.4% (45) | 16.5% (13) |
Reported as mean (SD) years.
P < .01
Table 2.
Clinical Characteristics of Study Sample by Diagnosis
| IBD (N=95) | IBS (N=80) | |
|---|---|---|
| Diagnosis Years | 13.7 (10.3) | 10.0 (9.0) |
| HBI (CD Only)a | 4.4 (3.7) | - |
| SCCAI (UC Only)a | 3.4 (2.8) | - |
| IBS-SSS | - | 221.7 (73.2) |
| In Remissionb | 61% (58)* | 0% |
| Currently Using Medication | 85% (81) | 76% (61) |
| Biologics | 50% (47) | - |
| Corticosteroids | 18% (17) | - |
| Immunomodulators | 26% (25) | - |
| Probiotics | - | 24% (19) |
| Antispasmodics | - | 14% (11) |
| Laxatives | - | 24% (19) |
| Other | 44% (34) | 33% (26) |
| Currently Using Diet | 64% (61) | 81% (65)* |
| Single Diet Use | 32% (30) | 44% (36) |
| Multiple Diet Use | 33% (31) | 36% (29) |
| Dietitian Visit | 41% (39)* | 35% (20) |
P < .01
IBD diagnosis is presented as Crohn’s Disease (CD) and ulcerative colitis (UC) due to different symptom severity scales used.
Remission status based on accepted cutoff scores for Harvey Bradshaw Index (HBI), Simple Colitis Clinical Activity Index (SCCAI), and IBS-Symptom Severity Scale (IBS-SSS).
No significant differences existed between IBS and IBD patients for demographic data except for gender, with more male IBD subjects (IBD = 23.2% vs. IBS = 8.8%, p=.01). IBS patients were also more likely to have been recruited online (p<.001). Contrary to the Fr-QOL-29 validation study, no significant differences in FRQoL existed by gender (p=.22) [35]. No significant differences existed between IBD and IBS for anxiety (p=.17), depression (p=.08), or HRQoL (p=.03). As expected, IBS and IBD patients with higher anxiety and depression reported poorer HRQoL (IBS: r= −0.53, −0.58; IBD r= −0.47, −0.40; all p<.001). No IBS patients met clinical remission criteria based on accepted IBS-SSS cutoff likely due to the fact the initial study screening required all IBS subjects to meet Rome-III criteria. Approximately half of IBD patients were in remission based on HBI or SSCAI cutoff scores.
Food-Related Quality of Life for IBD and IBS Patients
IBD patients reported better FRQoL than IBS patients (IBD = 82.0 (26.6); IBS = 69.0 (18.8), p<.001; Figure 1), with a medium effect size (d = 0.56). When evaluated by disease status (active vs. remission), IBD patients in remission demonstrated higher FRQoL than IBD and IBS patients with active disease (IBD-remission: 91.2 (26.5) vs. IBD-active: 67.7 (19.6) and IBS-active: 67.6 (18.3), p<.001); IBD and IBS patients with active disease did not differ in FRQoL. Both effect sizes were large (d=1.0). Patients with more severe symptoms reported poorer FRQoL (IBD, r = −0.48, IBS, r = −0.46, both p < .01). Approximately half the IBD group was classified as severe and reported poorer FRQoL compared to those classified with less severe IBD (76.9 (26.5) vs. 88.4 (25.5), p = .03). However, this did not reach statistical significance set for this study after multiple comparison adjustments.
Figure 1.
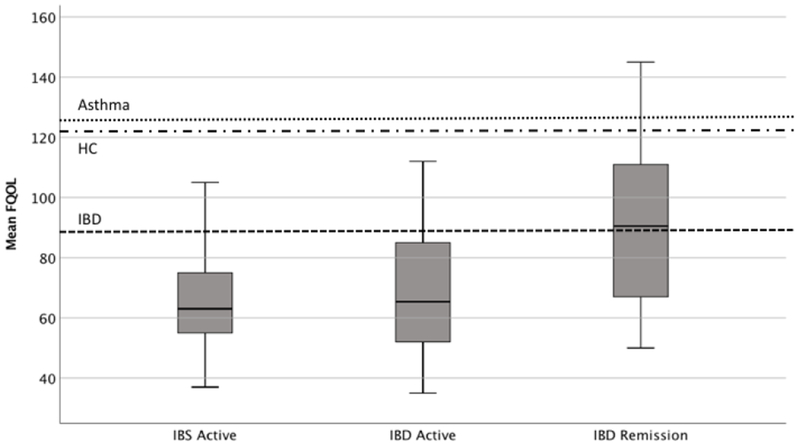
Boxplot for Mean FRQoL Score by Diagnosis and Remission Status with Comparative Groups
Notes: Dashed lines represent mean FRQoL score for Asthma, Healthy Controls (HC) and IBD patients in original Fr-QOL-29 validation study.[35]
Some unexpected differences were found for psychological variables. Poorer FRQoL was associated with lower HRQoL for IBS (r = 0.67, p < .001) and IBD (r = 0.72, p < .001) patients, as anticipated. However, the relationship between FRQoL and anxiety differed for IBS and IBD. Specifically, IBD patients with poorer FRQoL reported more anxiety (r = −0.27, p < .01), while IBS patients did not demonstrate this association (r = −0.21, p=.06). Depression showed no relationship with FRQoL for either IBD (r = −0.19) or IBS (r = −0.18).
Dietary Treatment Use and FRQoL in IBD and IBS Patients
Overall, IBS patients were more likely to use dietary treatments (Table 2). Interestingly, self-directed dietary therapy was most used by patients in this study, rather than adherence to a well-described diet (Table 3). Concurrent multiple diet use occurred in 33% of IBD and 36% of IBS patients at time of study; the maximum simultaneous diets used was three in 11% of subjects. The more diets a patient used, the poorer FRQoL for IBS (r = −0.23, p<.05) and IBD patients (r= −0.31, p<.01). Using any dietary treatment was only potentially detrimental for FRQoL in IBD patients (Table 3). Fiber-focused, grain/carbohydrate-focused, and self-directed diets reduced FRQoL in IBD; and grain/carbohydrate focused diets reduced FRQoL in IBS but was above statistical significance level set when adjusting for multiple comparisons. When re-categorized by no diet, single diet, or multiple diet use no differences existed between IBD and IBS patients for number of dietary interventions used (χ2 = 5.68, p =.06). When controlling for symptom severity, only being on multiple diets remained a significant factor in reduced FRQoL in IBD patients (Table 4). No significant differences in FRQoL was seen with diet type or number in IBS patients.
Table 3.
FRQoL for Dietary Treatment and Dietitian Use by Diagnosis
| IBD FRQoLa | IBS FRQoLa | |||||
|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | |
| Any Current Diet | 75.6 (23.2), 59 | 92.5 (28.7), 36 | .002 | 67.0 (16.9), 65 | 77.2 (24.2), 15 | .06 |
| Low or High Fiber Focused | 68.1 (22.4), 16 | 84.8 (26.6), 79 | .02 | 68.6 (21.4), 19 | 69.1 (18.0), 61 | .92 |
| Grain/Carbohydrate Focused | 71.0 (19.2), 19 | 84.8 (27.6), 76 | .02 | 58.0 (9.5), 7 | 70.1 (19.2), 73 | .02 |
| Dairy Focused | 77.7 (24.6), 20 | 83.1 (27.1), 75 | .43 | 65.3 (15.4), 15 | 70.0 (19.5), 65 | .38 |
| Low FODMAP | 77.3 (35.6), 4 | 82.2 (26.4), 91 | .72 | 62.8 (14.9), 13 | 70.2 (19.3), 67 | .20 |
| Self-Directed Elimination | 75.2 (23.3), 41 | 87.2 (27.9), 54 | .03 | 67.1 (18.0), 44 | 71.3 (19.7), 36 | .33 |
| Seen by a Dietitian | 78.9 (26.4), 39 | 84.1 (26.7), 56 | .35 | 64.1 (15.2), 19 | 69.8 (19.6), 38 | .27 |
Mean(SD), N presented.
Table 4.
Univariate Analysis of Covariance for Differences in FRQoL in IBD and IBS by Diet Use Adjusted for Symptom Severity
| IBD | IBS | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Meanadj | F | P | Mean (SD) | Meanadj | F | P | |
| Fiber Focused Diet | 2.41 | .12 | 0.17 | .69 | ||||
| Yes | 68.1 (22.4) | 73.5 | 63.9 (21.4) | 65.9 | ||||
| No | 84.4 (26.6) | 83.7 | 68.6 (17.5) | 68.1 | ||||
| Grain/carbohydrate | 3.15 | .08 | 1.81 | .18 | ||||
| Focused Diet | 71.0 (19.2) | 73.5 | 57.5 (10.3) | 59.1 | ||||
| Yes | 84.8 (27.6) | 84.1 | 68.7 (18.8) | 68.6 | ||||
| No | ||||||||
| Dairy Focused Diet | 0.34 | .56 | 3.70 | .06 | ||||
| Yes | 80.8 (27.7) | 79.3 | 57.9 (12.9) | 58.1 | ||||
| No | 82.3 (26.5) | 82.7 | 69.4 (18.7) | 69.3 | ||||
| Self-Directed | 2.39 | .13 | 0.66 | .42 | ||||
| Elimination Diet | 75.2 (23.3) | 77.7 | 64.5 (16.2) | 65.6 | ||||
| Yes | 87.2 (27.9) | 85.3 | 70.1 (19.9) | 69.2 | ||||
| No | ||||||||
| Low FODMAP | 0.27 | .60 | 1.46 | .23 | ||||
| Yes | 77.3 (35.6) | 76.0 | 61.1 (14.8) | 62.2 | ||||
| No | 82.2 (26.4) | 82.3 | 69.1 (18.9) | 68.8 | ||||
| Number of Diets | 4.55 | .01 | 1.80 | .18 | ||||
| No Diet | 92.7 (28.3) | 90.2 | 75.7 (22.5) | 73.2 | ||||
| 1 Diet | 81.0 (24.7) | 82.0 | 67.3 (14.7) | 68.1 | ||||
| Multiple Diets | 71.3 (22.1) | 73.1 | 61.9 (17.0) | 62.7 | ||||
Interestingly, diet adherence, duration, and self-rated efficacy were not associated with FRQoL. Less than half of subjects reported ever meeting with a dietitian regarding dietary treatment for their disease. IBD patients were more likely to have met with a dietitian (IBD = 41%, IBS = 32%, p=−.02). However, meeting with a dietitian did not translate to improved FRQoL in either IBD (p = 0.35) or IBS patients (p = −.27).
Predictors of FRQoL in IBD and IBS
Hierarchical regression analyses evaluated FRQoL predictors in IBD and IBS. Based prior analyses, two clinical and two dietary variables were potential independent predictors in the IBD group; and one clinical and one dietary variable were potential independent predictors in IBS (Table 5). HRQoL was excluded from the regression models due to high levels of multicollinearity (r ≥ 0.70) with FRQoL. For IBS and IBD, increased symptom severity was the greatest predictor of poorer FRQoL (explaining 22% of variance in the IBD group results, 19% of variance in the IBS group). Even though symptom severity was important, it still did not account for majority of the variance in FRQoL. Using multiple diets was an additional potential contributing factor to poorer FRQoL in IBD. For IBS, only using a dairy-focused diet had some trend to predict poorer FRQoL.
Table 5.
Hierarchical Linear Regression for Predictors of FRQoL in IBD and IBS
| R2adj | β | SE | P | |
|---|---|---|---|---|
| IBD | ||||
| Model 1 | .22 | .0001 | ||
| Symptom Severitya | −.48 | 2.3 | ||
| Model 2 | .26 | .038 | ||
| Symptom Severitya | −.44 | 2.3 | .0001 | |
| Number Current Dietsb | −.18 | .80 | .064 | |
| Grain/carbohydrate Focused Dietc | −.11 | 6.2 | .267 | |
| Model 3 | .27 | .129 | ||
| Symptom Severitya | −.41 | 2.3 | .0001 | |
| Number Current Dietsb | −.15 | .81 | .122 | |
| Grain/carbohydrate Focused Dietc | −.12 | 6.2 | .199 | |
| Anxietyd | −.14 | .34 | .129 | |
| IBS | ||||
| R2adj | β | SE | P | |
| Model 1 | .19 | .0001 | ||
| Symptom Severitya | −.46 | .03 | ||
| Model 2 | .23 | .06 | ||
| Symptom Severitya | −.45 | .03 | .0001 | |
| Dairy Focused Dietc | −.23 | 5.8 | .06 | |
Symptom Severity: standardized Z Score of HBI or SCCAI (IBD), IBS-SSS (IBS)
Scale: 0=None, 1=Single, 2=Multiple
Scale: 0=No, 1=Yes
Anxiety: NIH PROMIS Anxiety total score
Discussion
This is the first study to evaluate FRQoL, using a disease-specific measure, in IBD and IBS patients and suggests FRQoL is a unique and potentially important patient reported outcome for these common chronic gastrointestinal diseases. Our finding that IBD and IBS patients differ in FRQoL (despite similar HRQoL) underscores the FrQOL-29’s ability to capture distinct aspects of food-related well-being (or lack thereof), not assessed by general HRQoL measures. Further support FRQoL is a unique construct stems from our findings anxiety is only weakly associated with FRQoL in IBD, and depression does not correlate with FRQoL in either diagnosis; whereas anxiety and depression both moderately correlate with HRQoL in IBD and IBS patients in prior studies [55,56] and in the present sample set (as expected). In our study, when contrasted to the FrQOL-29 validation sample, IBD and IBS patients with active disease exhibited the poorest FRQoL when compared with literature reported healthy controls, asthma patients, and all IBD patients in general [35]. Hence, symptom severity appears to play an important role in FRQoL. But notably, the majority of differences between patients in FRQoL (i.e. close to 80% of variance) remained still unexplained by symptom severity. While not significant predictors of FRQoL, dietary variables did have a significant relationship. Dietary use as a compensatory behavior in response to symptoms and the subsequent impact on FRQoL is plausible.
FRQoL has not yet been specifically evaluated in other gastrointestinal illnesses, such as celiac disease and eosinophilic esophagitis (EoE) , so no direct comparison can be made. However, some comparisons can be made using HRQoL data that touched on food-related concerns. Like in celiac disease [57], IBD and IBS patients with more severe symptoms reported poorer FRQoL. In some studies, once celiac patients are adherent to gluten-free (GF) diets HRQoL increases or returns to the equivalent of the general population [57,58] - a finding we did not see in our study. However, other research indicates patients on the GF diet may still experience psychosocial issues despite symptom reduction [59]. Therefore, it is important to note although symptom reduction may have positive benefits on HRQoL and partially in FRQoL, patients may still experience negative impacts when modifying diets, likely due to efforts required to implement and continue dietary therapy.
Interestingly, one-third of our participants reported using more than one diet concurrently, often without dietitian direction. IBS patients use dietary treatment more than IBD, but are not more likely to use multiple concurrent diets. One reason may be there are several efficacious treatment options for IBD [60,37]. Given the “functional” nature of IBS, treatment options can be more limited and often target symptoms as opposed to underlying brain-gut axis dysregulation. As such, IBS patients may increasingly seek dietary therapy to feel in control of symptoms [34]. Moreover, dietary treatment use in IBD and IBS appears to be independent of the diet’s effectiveness, which may reflect patient’s desire to actively participate in treatment and have some control over their illness. As opposed to celiac disease or EoE, where food triggers are more obvious, when the multiplicity of foods that may trigger symptoms are taken into account and when dietary intervention in IBD and IBS has varying results, it is not surprising perceived dietary efficacy had no impact on FRQoL in our dataset [22,24,30].
FRQoL did not appear to be influenced by diet type after controlling for symptom severity. FRQoL and food-related challenges that accompany IBD and IBS, such as difficulty identifying trigger foods and instability in food intolerances, can be independent of a diet itself. Therefore, impacts on FRQoL may not be fully captured by a specific diet alone and probably depend on psychological factors other than diet itself. Future studies should work to capture additional psychological aspects (beyond anxiety and depression) of food-related experience in IBD and IBS patients. Perhaps it is most interesting to note most IBD and IBS patients report trying a self-directed diet rather than a pre-specified or medically prescribed diet in our sample. Self-directed diets may involve eliminating multiple foods, and could cause further difficulties given the extra effort required [63–65] and potentially impact FRQoL, psychosocial functioning, and overall HRQoL. Our study did not measure the number eliminated foods with self-directed diets, which future research should address.
In our dataset, meeting with a dietitian (currently or in past) did not influence FRQoL. Several factors may have influenced patients’ responses. First, our study did not consider visit frequency, and those who met with a dietitian for more visits may have a different result than those who only met one time. Our data does not address whether a patient met with a GI-specific registered dietitian, a general registered dietitian, or another clinician providing dietary advice (e.g. nutritionist). It is expected a registered dietitian has a specific certification, whereas a nutritionist or another clinician providing dietary advice may not, which may impact the quality of advice received. Further, a registered dietitian specializing in GI illnesses may have extensive experience in IBD and IBS-related diets, and may be able to offer more support than a general dietitian. Future studies should evaluate advice quality provided to patients, provider types and provider training background in more detail to assess if differences exist.
While this study presents novel findings about FRQoL, there are limitations to consider. Our study recruited from online and clinic sources. While diagnoses are confirmed in clinic samples, we cannot definitively verify diagnosis in online patients, even though screening questions were used for inclusion to obtain as uniform a patient group as possible. In addition, there may be differences between patients recruited online and in clinic [50]. However, online recruitment use is gaining in popularity and acceptability, including in IBD studies, [17,66] and we did not observe significant differences by recruitment source. Our sample is primarily non-Hispanic white, middle-to-upper class socioeconomic status, college educated, and living in an urban setting. Caution should be used when applying findings to other cultural groups. The inherent nature of self-report questionnaires lends results to measurement error including fatigue, straight-line answering, and the Hawthorne effect; the online survey anonymity may mitigate some of these. We used Rome-III criteria for IBS sample inclusion, as this study was conceived and conducted prior to Rome IV publication. These results may change in IBS patients meeting Rome IV criteria. While the statistical significance set for the study analyses was stringent (P < .01) to correct for potential Type I error that can occur when performing multiple comparisons, it is certainly possible Type II errors could occur with our sample size, especially for findings that fell just below the significance set for the current study and would otherwise be considered significant. Lastly, IBS patients in this study were symptomatic and not in remission using the IBS-SSS to assess symptom severity. Future studies should also focus on clinical populations with IBS patients in remission.
In summary, the present study identifies FRQoL as an important patient outcome that may capture independent aspects of patient well-being (or lack thereof) not readily apparent in HRQoL measures in IBD and IBS. As research into dietary treatment efficacy in IBD and IBS expands, studies should incorporate FRQoL as an outcome measure to understand dietary treatment and patient well-being relationships. Clinicians treating these patients should evaluate FRQoL in addition to HRQoL, since dietary treatments are widely practiced by IBD and IBS patients without much input from medical professionals.
Supplementary Material
Acknowledgements:
The authors thank Rawan Abbasi for support with study coordination.
Funding: Livia Guadagnoli is supported by a training grant through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), USA (1T32DK101363). Ece Mutlu is supported by NIDDK grant (R01CA204808). Tiffany Taft is supported by NIDDK grant (R01DK079902)
Abbreviations:
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- CD
Crohn’s disease
- UC
ulcerative colitis
- IC
indeterminate colitis
- FRQoL
food-related quality of life
- SD
standard deviation
Footnotes
Conflicts of Interest: Tiffany Taft: ongoing speaker for Abbvie for physician education programs; Bethany Doerfler: speaker for Alakos, Allergan, Nutricia North America, and is a member of the Speakers Bureau for Nutricia North America. All other authors have nothing to disclose.
Compliance with Ethical Standards
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Ballou S, & Keefer L (2017). Psychological Interventions for Irritable Bowel Syndrome and Inflammatory Bowel Diseases. [Clinical and Systematic Reviews]. Clinical And Translational Gastroenterology, 8, e214, doi: 10.1038/ctg.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. (2012). Burden of Gastrointestinal Disease in the United States: 2012 Update. Gastroenterology, 143(5), 1179–1187.e1173, doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chey WD, Kurlander J, & Eswaran S (2015). Irritable bowel syndrome: a clinical review. JAMA, 313(9), 949–958, doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YZ, & Li YY (2014). Inflammatory bowel disease: pathogenesis. World J Gastroenterol, 20(1), 91–99, doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbara G, Cremon C, & Stanghellini V (2014). Inflammatory bowel disease and irritable bowel syndrome: similarities and differences. Curr Opin Gastroenterol, 30(4), 352–358, doi: 10.1097/mog.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 6.Ballou S, Bedell A, & Keefer L (2015). Psychosocial impact of irritable bowel syndrome: A brief review. World Journal of Gastrointestinal Pathophysiology, 6(4), 120–123, doi: 10.4291/wjgp.v6.i4.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casati J, & Toner BB (2000). Psychosocial aspects of inflammatory bowel disease. Biomed Pharmacother, 54(7), 388–393, doi: 10.1016/s0753-3322(01)80006-8. [DOI] [PubMed] [Google Scholar]
- 8.Amouretti M, Le Pen C, Gaudin AF, Bommelaer G, Frexinos J, Ruszniewski P, et al. (2006). Impact of irritable bowel syndrome (IBS) on health-related quality of life (HRQOL). Gastroenterol Clin Biol, 30(2), 241–246. [DOI] [PubMed] [Google Scholar]
- 9.Blondel-Kucharski F, Chircop C, Marquis P, Cortot A, Baron F, Gendre JP, et al. (2001). Health-related quality of life in Crohn’s disease: a prospective longitudinal study in 231 patients. Am J Gastroenterol, 96(10), 2915–2920, doi: 10.1111/j.1572-0241.2001.4681_b.x. [DOI] [PubMed] [Google Scholar]
- 10.Casellas F, Vivancos JL, Sampedro M, & Malagelada JR (2005). Relevance of the phenotypic characteristics of Crohn’s disease in patient perception of health-related quality of life. Am J Gastroenterol, 100(12), 2737–2742, doi: 10.1111/j.1572-0241.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 11.Casellas F, Lopez-Vivancos J, Badia X, Vilaseca J, & Malagelada JR (2000). Impact of surgery for Crohn’s disease on health-related quality of life. Am J Gastroenterol, 95(1), 177–182, doi: 10.1111/j.1572-0241.2000.01681.x. [DOI] [PubMed] [Google Scholar]
- 12.De Gucht V (2015). Illness perceptions mediate the relationship between bowel symptom severity and health-related quality of life in IBS patients. Qual Life Res, 24(8), 1845–1856, doi: 10.1007/s11136-015-0932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, & Creed F (2002). Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn’s disease. Am J Gastroenterol, 97(8), 1994–1999, doi: 10.1111/j.1572-0241.2002.05842.x. [DOI] [PubMed] [Google Scholar]
- 14.Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, et al. (2005). Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care, 11(1 Suppl), S17–26. [PubMed] [Google Scholar]
- 15.Koloski NA, Boyce PM, Jones MP, & Talley NJ (2012). What level of IBS symptoms drives impairment in health-related quality of life in community subjects with irritable bowel syndrome? Are current IBS symptom thresholds clinically meaningful? Qual Life Res, 21(5), 829–836, doi: 10.1007/s11136-011-9985-5. [DOI] [PubMed] [Google Scholar]
- 16.Daniel JM (2002). Young adults’ perceptions of living with chronic inflammatory bowel disease. Gastroenterol Nurs, 25(3), 83–94. [DOI] [PubMed] [Google Scholar]
- 17.Taft TH, Keefer L, Leonhard C, & Nealon-Woods M (2009). Impact of perceived stigma on inflammatory bowel disease patient outcomes. Inflamm Bowel Dis, 15(8), 1224–1232, doi: 10.1002/ibd.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taft TH, Keefer L, Artz C, Bratten J, & Jones MP (2011). Perceptions of illness stigma in patients with inflammatory bowel disease and irritable bowel syndrome. Qual Life Res, 20(9), 1391–1399, doi: 10.1007/s11136-011-9883-x. [DOI] [PubMed] [Google Scholar]
- 19.Taft TH, Ballou S, & Keefer L (2013). A preliminary evaluation of internalized stigma and stigma resistance in inflammatory bowel disease. J Health Psychol, 18(4), 451–460, doi: 10.1177/1359105312446768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohn L, Storsrud S, Tornblom H, Bengtsson U, & Simren M (2013). Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol, 108(5), 634–641, doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson U, & Draper A (2009). The social aspects of food and nutrition. J Hum Nutr Diet, 22(2), 87–88, doi: 10.1111/j.1365-277X.2009.00951.x. [DOI] [PubMed] [Google Scholar]
- 22.Kakodkar S, Farooqui AJ, Mikolaitis SL, & Mutlu EA (2015). The Specific Carbohydrate Diet for Inflammatory Bowel Disease: A Case Series. J Acad Nutr Diet, 115(8), 1226–1232, doi: 10.1016/j.jand.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Burgis JC, Nguyen K, Park KT, & Cox K (2016). Response to strict and liberalized specific carbohydrate diet in pediatric Crohn’s disease. World J Gastroenterol, 22(6), 2111–2117, doi: 10.3748/wjg.v22.i6.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staudacher HM, Irving PM, Lomer MC, & Whelan K (2014). Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat Rev Gastroenterol Hepatol, 11(4), 256–266, doi: 10.1038/nrgastro.2013.259. [DOI] [PubMed] [Google Scholar]
- 25.Marsh A, Eslick EM, & Eslick GD (2016). Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr, 55(3), 897–906, doi: 10.1007/s00394-015-0922-1. [DOI] [PubMed] [Google Scholar]
- 26.Drisko J, Bischoff B, Hall M, & McCallum R (2006). Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J Am Coll Nutr, 25(6), 514–522. [DOI] [PubMed] [Google Scholar]
- 27.Moayyedi P, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. (2015). The Effect of Dietary Intervention on Irritable Bowel Syndrome: A Systematic Review. Clin Transl Gastroenterol, 6, e107, doi: 10.1038/ctg.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomer MC, Gourgey R, & Whelan K (2014). Current practice in relation to nutritional assessment and dietary management of enteral nutrition in adults with Crohn’s disease. J Hum Nutr Diet, 27 Suppl 2, 28–35, doi: 10.1111/jhn.12133. [DOI] [PubMed] [Google Scholar]
- 29.Lomer MC, Hart AL, Verjee A, Daly A, Solomon J, & McLaughlin J (2017). What are the dietary treatment research priorities for inflammatory bowel disease? A short report based on a priority setting partnership with the James Lind Alliance. J Hum Nutr Diet, 30(6), 709–713, doi: 10.1111/jhn.12494. [DOI] [PubMed] [Google Scholar]
- 30.Obih C, Wahbeh G, Lee D, Braly K, Giefer M, Shaffer ML, et al. (2016). Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition, 32(4), 418–425, doi: 10.1016/j.nut.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Triggs CM, Munday K, Hu R, Fraser AG, Gearry RB, Barclay ML, et al. (2010). Dietary factors in chronic inflammation: food tolerances and intolerances of a New Zealand Caucasian Crohn’s disease population. Mutat Res, 690(1-2), 123–138, doi: 10.1016/j.mrfmmm.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Cohen AB, Lee D, Long MD, Kappelman MD, Martin CF, Sandler RS, et al. (2013). Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci, 58(5), 1322–1328, doi: 10.1007/s10620-012-2373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper JM, Collier J, James V, & Hawkey CJ (2010). Beliefs about personal control and self-management in 30-40 year olds living with Inflammatory Bowel Disease: a qualitative study. Int J Nurs Stud, 47(12), 1500–1509, doi: 10.1016/j.ijnurstu.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson AE, Fletcher PC, & Schneider MA (2007). Seeking control through the determination of diet: a qualitative investigation of women with irritable bowel syndrome and inflammatory bowel disease. Clin Nurse Spec, 21(3), 152–160, doi: 10.1097/01.NUR.0000270015.97457.9c. [DOI] [PubMed] [Google Scholar]
- 35.Hughes LD, King L, Morgan M, Ayis S, Direkze N, Lomer MC, et al. (2016). Food-related Quality of Life in Inflammatory Bowel Disease: Development and Validation of a Questionnaire. J Crohns Colitis, 10(2), 194–201, doi: 10.1093/ecco-jcc/jjv192. [DOI] [PubMed] [Google Scholar]
- 36.Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein CN, Fried M, Krabshuis J, Cohen H, Eliakim R, Fedail S, et al. (2010). World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflammatory bowel diseases, 16(1), 112–124. [DOI] [PubMed] [Google Scholar]
- 38.Drossman DA (2006). The functional gastrointestinal disorders and the Rome III process. Gastroenterology, 130(5), 1377–1390, doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Harvey RF, & Bradshaw MJ (1980). Measuring Crohn’s disease activity. Lancet, 1(8178), 1134–1135. [DOI] [PubMed] [Google Scholar]
- 40.Marin-Jimenez I, Nos P, Domenech E, Riestra S, Gisbert JP, Calvet X, et al. (2016). Diagnostic Performance of the Simple Clinical Colitis Activity Index Self-Administered Online at Home by Patients With Ulcerative Colitis: CRONICA-UC Study. Am J Gastroenterol, 111(2), 261–268, doi: 10.1038/ajg.2015.403. [DOI] [PubMed] [Google Scholar]
- 41.Walmsley R, Ayres R, Pounder R, & Allan R (1998). A simple clinical colitis activity index. Gut, 43(1), 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vermeire S, Schreiber S, Sandborn WJ, Dubois C, & Rutgeerts P (2010). Correlation Between the Crohn’s Disease Activity and Harvey-Bradshaw Indices in Assessing Crohn’s Disease Severity. Clinical Gastroenterology and Hepatology, 8(4), 357–363, doi: 10.1016/i.cgh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Drossman DA (2006). Rome III: the new criteria. Chin J Dig Dis, 7(4), 181–185, doi: 10.1111/j.1443-9573.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 44.Francis CY, Morris J, & Whorwell PJ (1997). The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther, 11(2), 395–402. [DOI] [PubMed] [Google Scholar]
- 45.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, & Cella D (2011). Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment, 18(3), 263–283, doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, et al. (2016). Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. Journal of Clinical Epidemiology, 73, 119–127, doi: 10.1016/Mclinepi.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patrick DL, Drossman DA, Frederick IO, DiCesare J, & Puder KL (1998). Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci, 43(2), 400–411. [DOI] [PubMed] [Google Scholar]
- 48.Drossman D, Morris CB, Hu Y, Toner BB, Diamant N, Whitehead WE, et al. (2007). Characterization of health related quality of life (HRQOL) for patients with functional bowel disorder (FBD) and its response to treatment. Am J Gastroenterol, 102(7), 1442–1453, doi: 10.1111/j.1572-0241.2007.01283.x. [DOI] [PubMed] [Google Scholar]
- 49.Andrae DA, Patrick DL, Drossman DA, & Covington PS (2013). Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health and Quality of Life Outcomes, 11, 208–208, doi: 10.1186/1477-7525-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones MP, Bratten J, & Keefer L (2007). Quality of Life in Patients With Inflammatory Bowel Disease and Irritable Bowel Syndrome Differs Between Subjects Recruited from Clinic or the Internet. Am J Gastroenterol, 102(10), 2232–2237. [DOI] [PubMed] [Google Scholar]
- 51.Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. (1989). A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology, 96(3), 804–810. [PubMed] [Google Scholar]
- 52.Jones MP, Bratten J, & Keefer L (2007). Quality of life in patients with inflammatory bowel disease and irritable bowel syndrome differs between subjects recruited from clinic or the internet. Am J Gastroenterol, 102(10), 2232–2237, doi: 10.1111/j.1572-0241.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- 53.Jones MP, Wessinger S, & Crowell MD (2006). Coping strategies and interpersonal support in patients with irritable bowel syndrome and inflammatory bowel disease. Clin Gastroenterol Hepatol, 4(4), 474–481, doi: 10.1016/j.cgh.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences (2ed.). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- 55.Lee E-H, Kwon O, Hahm KB, Kim W, Kim JI, Cheung DY, et al. (2016). Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health and Quality of Life Outcomes, 14, 22, doi: 10.1186/s12955-016-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vidal A, Gomez-Gil E, Sans M, Portella MJ, Salamero M, Pique JM, et al. (2008). Health-related quality of life in inflammatory bowel disease patients: the role of psychopathology and personality. Inflamm Bowel Dis, 14(7), 977–983, doi: 10.1002/ibd.20388. [DOI] [PubMed] [Google Scholar]
- 57.Casellas F, Rodrigo L, Vivancos JL, Riestra S, Pantiga C, Baudet JS, et al. (2008). Factors that impact health-related quality of life in adults with celiac disease: A multicenter study. World Journal of Gastroenterology : WJG, 14(1), 46–52, doi: 10.3748/wjg.14.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norstrom F, Lindholm L, Sandstrom O, Nordyke K, & Ivarsson A (2011). Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol, 11, 118, doi: 10.1186/1471-230x-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauser W, Gold J, Stein J, Caspary WF, & Stallmach A (2006). Health-related quality of life in adult coeliac disease in Germany: results of a national survey. Eur J Gastroenterol Hepatol, 18(7), 747–754, doi: 10.1097/01.meg.0000221855.19201.e8. [DOI] [PubMed] [Google Scholar]
- 60.Dassopoulos T, Sultan S, Falck-Ytter YT, Inadomi JM, & Hanauer SB (2013). American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology, 145(6), 1464–1478 e1461–1465, doi: 10.1053/j.gastro.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 61.Maagaard L, Ankersen DV, Vegh Z, Burisch J, Jensen L, Pedersen N, et al. (2016). Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World Journal of Gastroenterology, 22(15), 4009–4019, doi: 10.3748/wjg.v22.i15.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prince AC, Myers CE, Joyce T, Irving P, Lomer M, & Whelan K (2016). Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis, 22(5), 1129–1136, doi: 10.1097/mib.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 63.Wolf WA, Jerath MR, Sperry SLW, Shaheen NJ, & Dellon ES (2014). Dietary Elimination Therapy is an Effective Option for Adults with Eosinophilic Esophagitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 12(8), 1272–1279, doi: 10.1016/j.cgh.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, & Hirano I (2012). Elimination Diet Effectively Treats Eosinophilic Esophagitis in Adults; Food Reintroduction Identifies Causative Factors. Gastroenterology, 142(7), 1451–1459.e1451, doi: 10.1053/i.gastro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Menard-Katcher P, Marks KL, Liacouras CA, Spergel JM, Yang YX, & Falk GW (2013). The natural history of eosinophilic oesophagitis in the transition from childhood to adulthood. Aliment Pharmacol Ther, 37(1), 114–121, doi: 10.1111/apt.12119. [DOI] [PubMed] [Google Scholar]
- 66.Knowles SR, Gass C, & Macrae F (2013). Illness perceptions in IBD influence psychological status, sexual health and satisfaction, body image and relational functioning: A preliminary exploration using Structural Equation Modeling. Journal of Crohn’s and Colitis, 7(9), e344–e350, doi: 10.1016/j.crohns.2013.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


