Abstract
Background:
Many men with HIV express fertility intentions and nearly half have HIV-uninfected sexual partners. We measured partner pregnancy among a cohort of men accessing antiretroviral therapy (ART) in Uganda.
Methods:
Self-reported partner pregnancy incidence and bloodwork (CD4, HIV-RNA) were collected quarterly. Interviewer-administered questionnaires assessed men’s sexual and reproductive health annually and repeated at time of reported pregnancy (2011–2015). We measured partner pregnancy incidence overall, by pregnancy intention, and by reported partner HIV-serostatus. We assessed viral suppression (≤400 copies/mL) during the peri-conception period. Cox proportional hazard regression with repeated events identified predictors of partner pregnancy.
Results:
Among 189 men, baseline median age was 39.9 years [IQR:34.7,47.0], years on ART was 3.9 [IQR:0.0,5.1], and 51% were virally suppressed. Over 530.2 person-years of follow-up, 63 men reported 85 partner pregnancies (incidence=16.0/100 person-years); 45% with HIV-serodifferent partners. By three years of follow-up, 30% of men reported a partner pregnancy, with no difference by partner HIV-serostatus (p=0.75). 69% of pregnancies were intended, 18% wanted but mis-timed, and 8% unwanted. 78% of men were virally suppressed prior to pregnancy report. Men who were younger (aHR:0.94/year;95%CI:0.89–0.99), had incomplete primary education (aHR:2.95;95%CI:1.36–6.40), and reported fertility desires (aHR:2.25;95%CI:1.04–4.85) had higher probability of partner pregnancy.
Conclusion:
A high incidence of intended partner pregnancy highlights the need to address men’s reproductive goals within HIV care. Nearly half of pregnancy partners were at-risk for HIV and one-quarter of men were not virally suppressed during peri-conception. Safer conception care provides opportunity to support men’s health and reproductive goals, while preventing HIV transmission to women and infants.
Keywords: HIV, men, antiretroviral therapy (ART), safer conception, pregnancy, Uganda
INTRODUCTION
Globally, millions of men and women living with HIV want to have children and an estimated half have HIV-uninfected sexual partners1–6. For the majority, achieving pregnancy involves condomless sex, presenting risks of HIV transmission to uninfected partners and infants. To achieve global 90–90–90 goals and eliminate perinatal HIV transmission, comprehensive HIV treatment and prevention strategies are needed that address the reproductive rights and desires of HIV-affected men and women1.
A range of safer conception strategies create opportunities to support individuals and couples to meet reproductive goals with minimal HIV risk1. These strategies include sustained use of antiretroviral therapy with viral suppression by the partner living with HIV and/or pre-exposure prophylaxis (PrEP) by the HIV-uninfected partner, which effectively eliminate HIV transmission risk during condomless sex7–11. Treatment for sexually transmitted infections may further reduce HIV risk1. For HIV-serodifferent couples in which the female partner is living with HIV, male medical circumcision and home insemination reduce sexual HIV-transmission while allowing for conception1. For men living with HIV (MLWH), sperm washing with insemination offers additional options when such services are available and accessible12,13.
While increasing attention has focused on improving access to safer conception care for women14–16, less is known about the needs of men. In Uganda2,3,5 and globally1, approximately half of MLWH have HIV-uninfected partners17 and an estimated 30–50% of MLWH desire children. Yet globally, heterosexual men are poorly engaged in HIV care18 and largely absent from sexual and reproductive health programming19. Men are less likely than women to engage in HIV care, initiate antiretroviral therapy, and achieve a suppressed HIV viral load18,20, which compromises their health and survival outcomes and exposes potential pregnancy partners to HIV. Constructs of masculinity contribute to men’s control of many relationship and reproductive decisions21, and accordingly women report limited power within sexual relationships22. Such gender and power inequities contribute to strong gender normative expectations for childbearing, regardless of HIV status and risk21,23. Despite these gender dynamics, safer conception interventions have primarily focused on women15,16. Previous work in Uganda highlights that MLWH are eager to discuss their reproductive goals with providers1,23,24; however, providers are unlikely to initiate such discussions in part because little is known about men’s reproductive health needs25.
Given men’s often dominant role in couple decision-making regarding reproductive goals, plans, and practice, including uptake of HIV prevention strategies1,24, new approaches are required to inform safer conception services that include men. To inform the design and implementation of such services, we measured partner pregnancy incidence, intention, and predictors among MLWH enrolled in HIV care in rural Uganda.
METHODS
Study design and participants
This study was conducted in Mbarara District, a rural setting (population 418,200) located approximately 265 kilometres southwest of Kampala, Uganda. In Uganda, HIV prevalence among adult males (aged 15–64 years) is 4.7% compared to 7.6% among adult females20. The estimated total fertility rate in Mbarara is 7.0 children per woman and the regional adult HIV prevalence is 7.9%, amongst the highest in the country20.
Study participants were enrolled in the Uganda AIDS Rural Treatment Outcomes (UARTO) prospective cohort study between 2005–2012 with follow-up until September 2015. Treatment-naïve men and women living with HIV were recruited from the HIV treatment clinic at Mbarara Regional Referral Hospital, which offers comprehensive HIV care at no-cost to patients. Clients who were ≥18 years old and living within 60km of the clinic were eligible to enroll in this cohort.
All participants provided voluntary written informed consent. Study procedures were approved by the Institutional Ethics Review Boards of Mbarara University of Science and Technology (Uganda), Partners Human Research Committee at Massachusetts General Hospital (Boston, USA), and Simon Fraser University (Burnaby, Canada). Consistent with national guidelines, approvals were also obtained from the Uganda National Council for Science and Technology and the Research Secretariat in the Office of the President.
Procedures
All cohort participants completed phlebotomy (CD4 cells/mm3, HIV-RNA) and interviewer-administered questionnaires, detailing mental and physical health, behavior, and pregnancy incidence (self or partner), quarterly. Annual questionnaires assessed socio-demographics.
In October 2011, the Reproductive Health Component study of the cohort was initiated to assess additional sexual and reproductive health outcomes, relationship dynamics, fertility desires, and attitudes and feelings about reported pregnancies. Between 2011 and 2015, all male participants completed the Reproductive Health Component questionnaire annually. Every three months (quarterly) male participants were asked about partner pregnancy. For men reporting a new pregnancy at any quarterly assessment, the Reproductive Health Component questionnaire was then repeated, thus potentially preceding the next scheduled annual questionnaire. Procedures for female UARTO participants are detailed elsewhere26.
Questionnaires were developed by Ugandan and global experts in HIV and sexual and reproductive health. Questionnaires were translated from English into Runyankole, the dominant local language, and then back-translated into English. Questionnaires (available in English and Runyankole) were administered by bilingual interviewers who had completed extensive training in survey administration. Consistent with research site standards, participants were given a small honorarium for their participation and reimbursed for transportation costs at each study visit.
Inclusion and exclusion criteria
Starting in 2005, 235 men and 524 women living with HIV were enrolled in the parent cohort study. This analysis of partner pregnancy incidence includes male participants who completed the Reproductive Health Component questionnaire at least once (considered the ‘baseline’ visit) beginning October 2011 with follow-up to September 2015. All men, regardless of reported sexual activity, were included in the partner pregnancy analysis. We excluded one participant who reported a vasectomy prior to study enrolment.
Measures
Primary outcomes
At each quarterly study visit, men reported on partner pregnancies and partnership characteristics among up to four sexual partners in the previous year or since the previous visit. For each reported partner pregnancy, men were asked to report the pregnancy outcome. Among reported livebirths, men were asked about infant HIV testing and status within one year. While relying on men’s report of partner pregnancy is likely to underestimate true pregnancy incidence, we identified this as the best option to assess partner pregnancy because we were (1) concerned about limiting partner pregnancy data to the small proportion of couples who are able to present to care together; and (2) interested in understanding partner pregnancy incidence from the perspective of men living with HIV.
Partner pregnancy incidence rate was computed using person-time methods. For time-to-event analyses, we analyzed the time to first reported pregnancy. For those with an event (i.e., partner pregnancy), the event time was based on the date that the participant first reported a pregnant partner. For those who did not have a pregnant partner during the course of follow-up, person-time was censored at the last UARTO study visit. For both those with events and those censored, the start time was first completion of the Reproductive Health Component questionnaire. Thus, time-to was calculated as the number of months from first completion of the Reproductive Health Component questionnaire through to either first report of pregnancy or last study visit.
The pregnancy partner’s HIV-serostatus was assessed at first report of pregnancy, as reported by the male partner in response to a question about their partner’s HIV status at the last sexual encounter. We identified pregnancies within HIV-seroconcordant (i.e., HIV-positive pregnancy partner) or HIV-serodifferent (HIV-negative or HIV-status unknown pregnancy partner) partnerships. For four participants missing data on pregnancy partner’s HIV status, we extrapolated a partner HIV status by assessing relationship and partner HIV status at the visits immediately prior to and after the visit where a pregnancy was reported. All reported partner pregnancies were assumed to be fathered by the male participant reporting them.
We assessed the proportion of pregnancies where the male index partner had laboratory confirmation of viral suppression (HIV-RNA ≤400 copies/mL) during the peri-conception period, defined as viral suppression at the closest study visit prior to first pregnancy report. For participants reporting partner pregnancy at ART initiation, we assumed an unsuppressed viral load.
At first report of pregnancy, we assessed the participant’s attitudes and feelings about the pregnancy27 (i.e., “Thinking back to just before she got pregnant, how did you feel about her becoming pregnant?”) as well as his thoughts of his partner’s attitudes and feelings. A pregnancy was considered ‘intended’ if the participant reported “I wanted to get pregnant sooner” or “I wanted to get pregnant then”; considered ‘mistimed’ if he reported “I wanted to get pregnant later”; or ‘unwanted’ if he reported “I did not want her to be pregnant then or at any time in the future”. Both mistimed and unwanted pregnancies were classified as unintended (vs. intended) pregnancies28. We also assessed whether the participant (or his partner) was “trying” to get pregnant (Yes vs No) and how happy he felt when he found out that his partner was pregnant (5-point Likert scale from ‘Very Unhappy’ to ‘Very Happy’).
Covariates
We examined the association of incident partner pregnancy with baseline and time-updated variables, including socio-demographic characteristics (age, marital status, employment, education, and household income), sexual and reproductive history (number of children fathered, fertility desire, number of sexual partners in the 12 months before interview, HIV-serostatus disclosure to primary sexual partner, and knowledge of partner’s HIV-serostatus), and HIV clinical history confirmed via clinical chart review and laboratory data (time on ART, CD4 at ART initiation, most recent CD4, and most recent viral load). Socio-economic status was assessed using the Filmer-Pritchett Asset Index, which estimates wealth based on asset ownership and housing characteristics, with higher scores indicative of greater wealth29.
Statistical analysis
We compared baseline characteristics of men who did and did not report partner pregnancy using Pearson’s chi-squared test or Fisher’s exact test for categorical variables and Wilcoxon’s Rank Sum test for continuous variables.
We used Kaplan-Meier methods to measure time to first partner pregnancy overall and by pregnancy partner HIV-serostatus. Log rank test assessed differences.
Cumulative incidence of partner pregnancies was calculated as the total number of partner pregnancies reported over the follow-up period (including multiple unique pregnancies per male participant) by person-years of follow-up. No person-time was eliminated from the denominator while his partner was pregnant given that some men reported (or may have had) multiple partners and that we had incomplete data on the length of each pregnancy.
Generalized estimating equation (GEE) Poisson models were used to calculate partner pregnancy incidence rates (per 100 person-years) with 95% confidence intervals. We used modified Poisson GEE methods accounting for repeated measures to assess and compare the proportion of all pregnancies occurring within HIV-seroconcordant and HIV-serodifferent partnerships where the male index partner was virally suppressed.
Cox Proportional Hazards regression was used to produce unadjusted estimates of the association between baseline covariates and hazard of partner pregnancy. A multivariable model investigated independent baseline and time-updated predictors of partner pregnancy. Time-updated variables included: Asset Index, HIV-serostatus disclosure, knowledge of primary partner’s HIV status, primary partner’s HIV status, fertility desire, CD4, and viral suppression. For the latter three variables, we imputed missing data with the last observation (within 12 months of the visit) carried forward. For time-updated viral load, only values reported within 14 months of the first pregnancy report visit were included. We selected 14 months since viral load testing is typically performed annually in most clinical settings in sub-Saharan Africa, and allowing a 2-month window around a scheduled study visit.
The final model was conducted using a backwards stepwise elimination technique, whereby the least significant variable was dropped until the final model had the optimum (minimum) AIC while maintaining covariates with type III p-values <0.2030.
RESULTS
Overall, 189 MLWH completed the Reproductive Health Component questionnaire at least once, and were included in this analysis (i.e., 189/235; 80% of all men enrolled in UARTO). Men included in this analysis (n=189) were more likely to be married (56% vs 33%; p=0.005) and have a higher Asset Index (median scores of −0.20 vs −1.10; p=0.005) at UARTO enrollment, compared with men who were excluded (n=46). We detected no differences between groups in terms of age, education, employment status, income, or number of children.
Baseline characteristics
At first completion of the Reproductive Health Component questionnaire (i.e. baseline), median age was 39.9 years [IQR: 34.7–47.0], 93% of men were employed, 49% had not completed primary education, median monthly household income was 170,000 UGX (~$99 USD) [IQR: 80,000–300,000], and a median Asset Index score of −0.2 [IQR: −1.2, 1.5]. Median number of prior livebirths was 4 [IQR: 2–6] and 33% reported desiring a child now or in the future (i.e., fertility desire). Most (77%) were married and 19% reported two or more sexual partners in the 12 months prior to interview. Of men with a sexual partner, 88% had disclosed their HIV-serostatus to their partner and 80% knew their partner’s HIV-serostatus. Median years since HIV diagnosis was 4.9 [IQR: 2.5–6.4], median years on ART was 3.9 [IQR: 0–5.1], and median CD4 at ART initiation was 175 [IQR 86–277]. Overall 51% of men were virally suppressed, including 90% of those on ART for ≥3 months (Table 1).
Table 1.
Baseline characteristics of men living with HIV and receiving ART, overall and by partner pregnancy after study enrollment, Uganda (n=189)
| Variable | Overall (n=189) n (%) Median [IQR] |
Total n | Partner pregnancy after study enrollment | p-value | |
|---|---|---|---|---|---|
| Yes (n=63) n (%) or Median [IQR] |
No (n=126) n (%) or Median [IQR] |
||||
| Age, years | 39.9 [34.7, 47.0] | 189 | 36.7 [32.1, 41.9] | 42.5 [36.4, 48.7] | <0.001 |
| Currently married | 140 (77%) | 183 | 54 (90%) | 86 (70%) | 0.003 |
| Employed | 171 (93%) | 183 | 55 (92%) | 116 (94%) | 0.532 |
| Education: < Primary 7 (vs. ≥ P7) | 93 (49%) | 189 | 40 (63%) | 53 (42%) | 0.008 |
| Monthly household income (UGX) | 170,000 [80,000, 300,000] |
152 | 200,000 [80,000, 330,000] |
150,000 [85,000, 300,000] |
0.396 |
| Filmer-Pritchett Asset Index | −0.2 [−1.2, 1.5] | 180 | −0.5 [−1.3, 1.2] | −0.1 [−1.1, 1.6] | 0.219 |
| Number of children fathereda | 4 [2, 6] | 183 | 4 [2, 5.5] | 4 [2, 6] | 0.229 |
| Fertility Desireb | 173 | 0.010 | |||
| Sexual partners in previous 12 months | 188 | 0.065 | |||
| HIV disclosure to primary partnerc | 143 (88%) | 162 | 56 (97%) | 87 (84%) | 0.020 |
| Know partner’s HIV statusc | 135 (80%) | 169 | 53 (88%) | 82 (75%) | 0.046 |
| Time on ART, years | 3.9 [0, 5.1] | 189 | 1.9 [0, 4.1] | 4.1 [0, 5.1] | 0.005 |
| CD4 at ART initiation (cells/mm3) | 175 [86, 277] | 152 | 203 [111, 292] | 164 [75,255] | 0.078 |
| Most recent CD4 (cells/mm3) | 319 [235, 424] | 186 | 299 [243, 393] | 328 [230, 443] | 0.452 |
| Most recent HIV-RNA suppressed (≤400 copies/mL)d | 74 (51%) | 144 | 23 (45%) | 51 (55%) | 0.298 |
| Most recent HIV-RNA suppressed (≤400 copies/mL) among men on ART for ≥3 months | 74 (90%) | 82 | 23 (96%) | 51 (88%) | 0.426 |
Notes:
Excludes current pregnancies;
Excludes n=16 participants with missing responses because they reported a partner pregnancy at baseline and did not report on fertility desires;
Of those who report having a primary sexual partner;
Of n=45 men with missing VL data at baseline, all were on ART with a minimum duration of use of 1.5 years. Given that among men with non-missing VL data, 90% who had been on ART for ≥3 months had HIV-RNA suppression, the true proportion of men with HIV-RNA suppression at baseline overall is likely higher than the 51% reported here.
Several baseline characteristics (including younger age, being married, lower education, fertility desire, HIV disclosure, knowledge of partner’s HIV status, and fewer years on ART) were associated (p<0.05) with reporting a partner pregnancy after study enrollment.
Partner pregnancy incidence
Among 189 men followed over 530.2 person-years (PYs), 63 men (33%) reported at least one partner pregnancy. Of these, 46 (73%) reported one, 12 (19%) reported two, and five (8%) reported three incident partner pregnancies, totaling 85 pregnancies (partner pregnancy incidence rate=16.0 per 100 PYs; 95% CI: 13.0–19.8).
The 85 pregnancies resulted in 62 live births (73%). Among the 62 livebirths, 17 participants (27%) reported knowing the infant’s HIV testing status. Among these 17 infants, only 11 men knew the test results and reported that the infant tested HIV-negative. Thus, of 62 livebirths, 18% of infants were known to be HIV-negative while the HIV status of the remaining 82% was unknown by the male pregnancy partner.
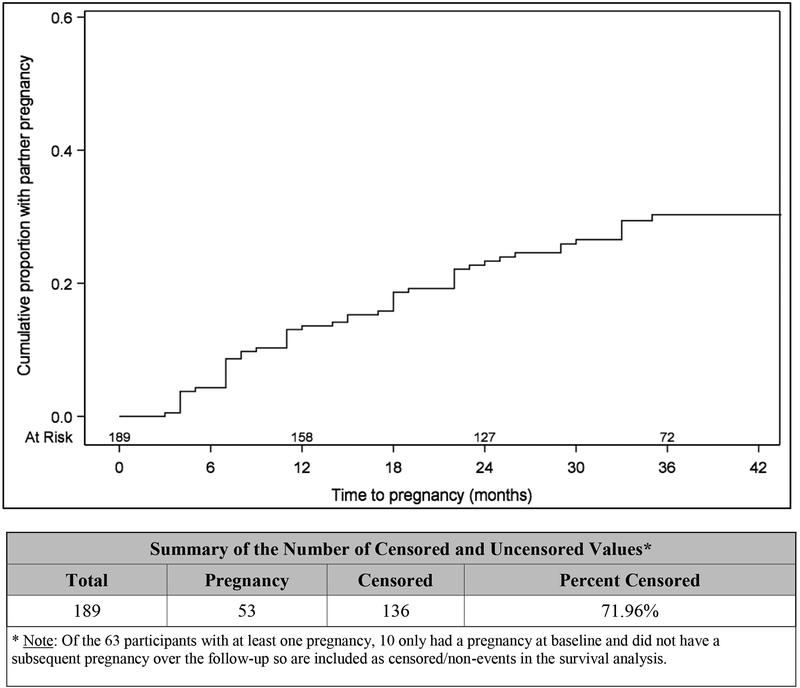
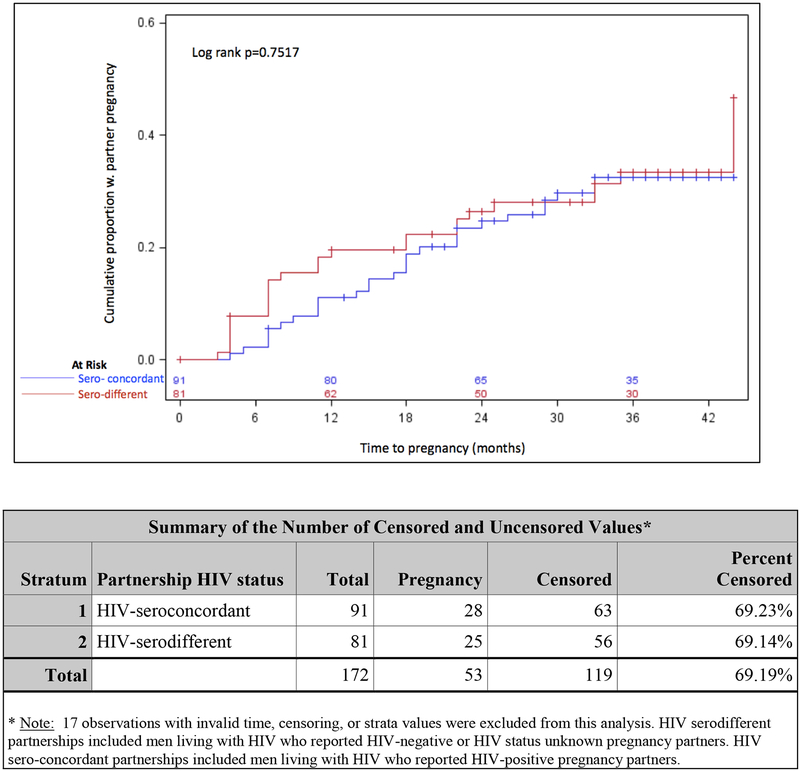
By one, two, and three years post-first completion of the reproductive health questionnaire, the cumulative probability of partner pregnancy was 14%, 23%, and 30%, respectively. No difference in time to first pregnancy was detected by pregnancy partner HIV-serostatus (p=0.75) (Figures 1a, 1b).
Figure 1a.
Probability of partner pregnancy over time reported by men living with HIV on ART, Uganda
Figure 1b.
Probability of partner pregnancy over time reported by men living with HIV on ART by partnership HIV status (HIV-seroconcordant vs HIV-serodifferent), Uganda
Pregnancy partner HIV-serostatus and viral suppression
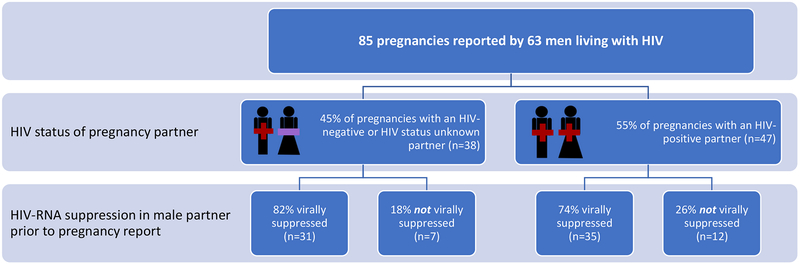
Of 85 pregnancies, 38 (45%) occurred with an HIV-serodifferent partner (including 24/38 with a known HIV-negative partner), while 47 (55%) occurred with an HIV-seroconcordant partner. The male partner was not virally suppressed at the study visit prior to pregnancy report in 19 (22%) of all pregnancies, including 7 (18%) and 12 (26%) of pregnancies that occurred with an HIV-serodifferent or HIV-seroconcordant partner, respectively (p=0.46) (Figure 2).
Figure 2.
Male partner viral suppression (HIV-RNA ≤ 400 copies/mL) prior to report of pregnancy, by pregnancy partner HIV sero-status, Uganda
Of the 19 pregnancies where the male partner was not virally suppressed during the peri-conception period, 10 were reported at UARTO enrollment and assumed to be not virally suppressed (given that cohort enrollment coincided with ART initiation, as per the study design). For the remaining 9 pregnancies, median viral load of men who were not virally suppressed was 4.38 log10 copies/mL [IQR: 4.12–5.52] and median time between viral load assessment and first report of pregnancy was 3.82 months [IQR: 3.68–4.31]. For all virally suppressed participants, median time between viral load assessment and first report of pregnancy was 7.33 months [IQR: 3.78–10.86].
Attitudes and feelings about the pregnancy
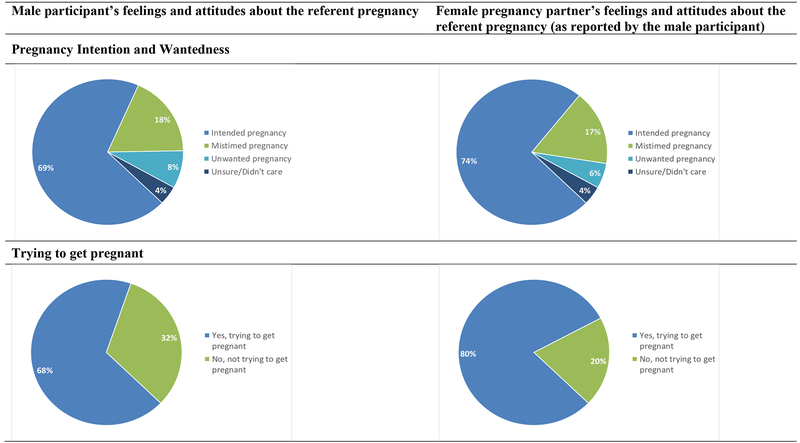
Of 72 pregnancies with non-missing responses, 50 (69%) were reported as intended, 13 (18%) were mis-timed, and 6 (8%) were unwanted (the remaining 3 (4%) “didn’t care”). Similarly, 53 (74%) of men reported that his partner intended the pregnancy, 12 (17%) that his partner mistimed the pregnancy, and 4 (6%) that his partner did not want the pregnancy. For 49 (68%) of the pregnancies, men reported “trying” for pregnancy and for 56 (80%), men reported that his partner was trying for pregnancy (Figure 3). For 55 (77%) of the pregnancies, men reported that they were very happy (n=30; 42%) or happy (n=25; 35%) when they found out about their partner’s pregnancy. The remaining 17 (24%) were not sure (n=4; 6%), unhappy (n=6; 8%), or very unhappy (n=7; 10%).
Figure 3.
Pregnancy intention and wantedness at first report of pregnancy (n=72 partner pregnancies among men living with HIV)
Feelings about the pregnancy strongly correlated with reports about pregnancy intention. Of men reporting that the pregnancy was intended, 44/50 (88%) were happy or very happy when they found out about the pregnancy, compared with 8/13 (62%) of men reporting a mistimed pregnancy, and 1/6 (17%) of men reporting an unwanted pregnancy.
Predictors of partner pregnancy
Factors associated with increased risk of partner pregnancy included younger age of the male partner, being married, having a primary school education or less, and reporting a desire for a child (i.e., fertility desire) at the study visit prior to pregnancy report.
In the adjusted model, younger age (aHR 0.94 per year, 95% CI: 0.89–0.99), having not completed primary education (aHR 2.95, 95%CI: 1.36–6.40), and reporting fertility desires at a study visit prior to first pregnancy report (aHR: 2.25, 95%CI: 1.04–4.85) predicted increased probability of partner pregnancy. No HIV-related treatment factors predicted partner pregnancy (Table 2).
Table 2.
Cox Proportional Hazards regression of baseline and time-updated factors associated with partner pregnancy among men living with HIV and on ART in Mbarara, Uganda (n=181)
| Variable | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI) |
p-value |
|---|---|---|---|---|
| Age at baseline (per year) | 0.93 (0.90, 0.96) | <0.001 | 0.94 (0.89, 0.99) | 0.012 |
| Currently married | 2.40 (1.02, 5.62) | 0.044 | Not selected | |
| Employed | 0.67 (0.24, 1.87) | 0.445 | 0.33 (0.08, 1.47) | 0.146 |
| Education: Incomplete primary school | 1.89 (1.08, 3.29) | 0.025 | 2.95 (1.36, 6.40) | 0.006 |
| Asset index, time-updated | 1.06 (0.91, 1.25) | 0.451 | 1.14 (0.96, 1.35) | 0.137 |
| Number of children fathered | Not selected | |||
| Desire for a child at previous visit, time-updated | 0.003 | 0.039 | ||
| Sexual partners in previous 12 months | Not selected | |||
| Disclosed HIV status to partner, time-updated | 0.68 (0.21, 2.21) | 0.516 | Not selected | |
| Know partner’s HIV status prior to pregnancy report, time-updated | 0.96 (0.38, 2.44) | 0.924 | 2.38 (0.77, 7.40) | 0.134 |
| Partner’s HIV status, time-updated | 0.665 | Not selected | ||
| Time on ART (per year), at baseline | 0.89 (0.80, 1.00) | 0.045 | Not selected | |
| CD4 per 50 cells/mm3, time-updated | 0.99 (0.91,1.07) | 0.703 | Not selected | |
| VL suppressed (≤400 copies/mL), time-updated | 0.80 (0.37, 1.76) | 0.585 | Not selected |
Note: Baseline refers to entry into the Reproductive Health Component study.
DISCUSSION
This is among the first studies to assess partner pregnancy incidence among men living with HIV and accessing ART in an HIV-endemic setting. We found a high incidence of partner pregnancy (16.0 per 100 person-years), a majority of which (≥ 69%) were reported as intended or perceived as intended by both pregnancy partners. Nearly one-third of men reported at least one partner pregnancy within three years of follow-up, nearly half of which occurred with HIV-seronegative or unknown serostatus partners (i.e., HIV sero-different partnerships). In almost one-quarter of pregnancies, men were not virally suppressed in the visit prior to pregnancy report. The observed high prevalence of intended pregnancy and sub-optimal viral suppression in the peri-conception period underscore a need to address the reproductive goals of men who have sex with women, within HIV care.
Our reported partner pregnancy incidence is higher than the 10.3 per 100 person-year incidence rate reported in the Partners PrEP study which included 1,785 HIV-uninfected female partners of MLWH in Kenya and Uganda31 and is nearly double that reported by women living with HIV from this same cohort (9.40 per 100 woman-years)32. Reasons for this difference may have stemmed from our capture of pregnancies among up to 4 sexual partners (19% of men reported two or more sexual partners at baseline) as well as our capture of partner pregnancy from men enrolled in an observational cohort study, rather than a controlled clinical trial. Despite a higher observed pregnancy incidence than previous studies, our estimates likely underestimate true partner pregnancy incidence given expected under-reporting of partner pregnancy due to men’s lower awareness of early stage pregnancies and stigma towards people living with HIV having children. True partner pregnancy incidence among MLWH is, however, likely lower than the general population of Uganda (where the general fertility rate is 189 per 1,000 women aged 15–44)33 given known behavioral and biological effects of HIV and/or ART use on male infertility34.
ART-mediated HIV-RNA suppression effectively eliminates HIV transmission during condomless sex9. In this cohort of men who knew their HIV-serostatus, were engaged in HIV care, and on ART, 22% had detectable HIV-RNA in the study visit prior to pregnancy report. Sub-optimal viral suppression during the peri-conception period presents risks to the health of MLWH and their female partners. For HIV-negative female partners, recent data suggests that such risks are particularly elevated during pregnancy and postpartum when HIV acquisition probability per condomless sex act increases significantly35. In 26% of pregnancies with a female HIV-seroconcordant partner, the male partner was not virally suppressed during the periconception period. In 3/12 of these pregnancies, the male partner had been on ART for ≥ 6 months, highlighting the importance of viral load monitoring to ensure that HIV-seroconcordant couples are also optimally supported on ART prior to conception attempts.
Sixteen men (25% of all men who reported partner pregnancy) reported a pregnant partner at ART initiation. Furthermore, among those men accessing ART, less time on ART was associated with reporting a partner pregnancy. These findings highlight the importance of counselling all MLWH early and often about their reproductive goals and offering support to help them achieve desired pregnancy as safely as possible, to maximize their own health, as well as that of their partners and families24. Offering reproductive health counselling at each stage of the HIV cascade of care, including at diagnosis, may help normalize such discussions such that both men and their providers become more comfortable routinely discussing reproductive goals. Counselling should include simple safer conception messages about delaying condomless sex until HIV-RNA suppression is achieved or ≥6 months on ART, consistent with national HIV treatment guidelines36.
Given that 20% of men reported not knowing their primary partner’s HIV status, there remains a need to encourage partner HIV testing (individually or within couples-based testing programs) and offering ART if she is living with HIV or PrEP if she is HIV-negative. Moreover, considering that 67% of men reported not desiring a child at baseline and nearly one-quarter of pregnancies were mistimed or unwanted, there is a clear need for integrated family planning services that include men within HIV testing and treatment programs. Across these efforts, additional provider training on initiating discussions about reproductive goals with their male clients is necessary.
Qualitative data from this site23 and elsewhere37 reveal that men may prioritize achieving reproductive goals before disclosing their HIV status or initiating ART, to maximize reproductive options. Thus, unambiguous messaging is needed regarding the survival and HIV prevention benefits of ART initiation, including the option of realizing fatherhood goals without risking HIV transmission to their partner or child. In the context of growing recognition of Undetectable=Untransmittable (#UequalsU)38 and gender gaps whereby more women initiate ART and suppress viral load thus offering HIV prevention benefits to male partners, but not vice versa39, it is critical that these messages permeate beyond the clinic and into the community to promote uptake of HIV testing and early linkage and engagement in HIV treatment and prevention. Such work focusing on MLWH who have sex with women remains rare, but essential.
At baseline, one-third of men reported wanting to have a child now or in the future. While there is a relationship between fertility desire and actual fertility, a sizable proportion of men do not express fertility desires, but subsequently report a partner pregnancy. Our findings suggest, however, that after the male partner is aware of a pregnancy, he is likely to identify that pregnancy as intended, with only 8% of pregnancies reported as unwanted. These findings deviate from research among women living with HIV, whereby an estimated 50–86% of pregnancies are reported unintended after the pregnancy is established1,32. Reasons for this difference have not been fully elucidated, although previous work has shown that MLWH report higher fertility desire6 and less stigma when expressing fertility goals than women40, both of which may contribute to a larger proportion of partner pregnancies described as intended and wanted.
Most men reported having disclosed their HIV-serostatus to his pregnancy partner. While this prevalence of HIV status disclosure is likely inflated due to social desirability reporting biases23, it nonetheless suggests a tremendous opportunity for male-inclusive safer conception programming in this setting. Many safer conception strategies require (i.e., home insemination, condomless sex timed to peak fertility) or at least benefit (i.e., adherence to ART or PrEP) from couple’s mutual disclosure of HIV status. In our cohort, the conditions to support couples-based, male-inclusive programming are evident.
Nearly three-quarters of reported pregnancies ended in a livebirth. This finding is likely an over-estimate given that men may not be aware of partner pregnancies that end within the first trimester. Men’s awareness of infant HIV testing and serostatus outcomes was low; only 18% of men knew the HIV testing results of their infants (all HIV-negative). These data provide additional evidence for the need to actively engage men in counselling and promote family health during peri-conception (i.e., being virally suppressed before conception attempts, HIV-serostatus disclosure, couples-based HIV testing), antenatal (i.e., supporting clinical and social aspects of maternal care, promoting ART adherence), and postnatal (i.e., supporting maternal ART/PrEP adherence, infant HIV testing and care, infant feeding choices, and retention in care) periods, to improve maternal, partner, and infant outcomes.
Younger age, less education, and reports of fertility desire independently predicted incident partner pregnancy, consistent with findings among women living with HIV and HIV-uninfected populations32. All MLWH should receive routine counselling regarding reproductive goals; however, these data suggest that younger men and those who express fertility desires are key populations for this counseling. The association with lower education highlights the need for innovative reproductive health messaging initiatives, to support and engage men with lower literacy. Our team23 and others41 have adapted safer conception messaging into visuals and vignettes, interpretable by lower-literacy populations. Additional tools are warranted. Notably, no partnership-related characteristics or HIV-related clinical factors predicted incident partner pregnancy.
Strengths and Limitations
We enrolled men living with HIV rather than couples and did not attempt to trace pregnancy partners; we were thus unable to conduct pregnancy testing in the female partner. Consequently, limitations of this study include reliance on self-report of partner pregnancy and the probable under-estimation of partner pregnancy incidence and over-estimation of the proportion of pregnancies ending in livebirth. Self-report of other key variables (pregnancy intention, partner HIV-serostatus, disclosure to partner) is subject to social desirability reporting bias, but likely reflects participants’ perceptions of HIV risk. Although we employed a standard approach to assessing intention after pregnancy was established28, this may have yielded an overestimate of intended pregnancy. For disclosure analyses, we assumed that the ‘pregnancy partner’ was the ‘primary sexual partner’. This was the case for all but four reported pregnancies. In only one of these cases, however, disclosure status differed between the primary and pregnancy partners. Among participants assessed to be virally suppressed during the peri-conception period, half of the viral load assessments occurred >7 months before the pregnancy report, presenting risk of misclassification bias. We may have therefore over-estimated the proportion of pregnancies where the male partner was virally suppressed. Moving forward, safer conception counselling programs might consider adapting viral load monitoring guidelines to offer testing more regularly for those who may benefit from additional support while trying to conceive. Relatedly, our assessment of HIV-RNA suppression in the male partner prior to pregnancy by partner HIV-serostatus report yielded small cell sizes, subject to low precision of estimates. Our study inclusion criteria (e.g., living within 60km of the HIV clinic) may have contributed to an overestimation of the reported viral suppression rate compared to that expected in the general population. In general, this analysis was conducted among MLWH who had overcome practical and structural barriers to HIV testing and initiating ART. Thus, these results may not be generalizable to MLWH who are not engaged in HIV care.
Our analysis provides a longitudinal assessment of partner pregnancy incidence in a large cohort of men initiated on ART in rural Uganda, where both HIV prevalence and fertility rates are high20. As we struggle to engage and retain men into HIV care42,43, these findings should inform the design and implementation of HIV prevention programming that acknowledges and supports the reproductive goals of men. In November 2016, we initiated a pilot safer conception program aimed at engaging MLWH in Mbarara. Preliminary findings suggest acceptability and feasibility of the program44.
The evidence supporting the need, demand, and feasibility for safer conception services, and potential benefits, is now extensive1. Excellent safer conception guidelines are available to support adoption into routine clinical care45–47, including a global consensus statement on safer conception care co-written by experts in HIV and reproductive health1. However, there remains insufficient action on converting evidence into practice in Uganda and elsewhere. Given the high pregnancy incidence and high rate of viral non-suppression observed here, we must act now to integrate the services that we know will prevent HIV transmission and support the reproductive goals and rights of women and men living with or affected by HIV.
CONCLUSIONS
Safer conception approaches can extend the reach of HIV prevention initiatives to meet the needs of the millions of HIV-affected individuals and couples who desire children. Such efforts can minimize risks to maternal, partner, and infant health while helping to normalize sex, pregnancy, and family building in the context of HIV. By normalizing reproductive desires of women and men, we can support global efforts to increase HIV testing, linkage and engagement in care, towards meeting the 90–90–90 goals to end AIDS by 2030 and eliminating perinatal transmission48. Proactively including heterosexual men living with HIV in this effort is long overdue.
Acknowledgements
We gratefully acknowledge the many contributors to this study including men living with HIV and their families, the UARTO Reproductive Health Component research team, and the ISS clinic physicians and staff.
Sources of support
We gratefully acknowledge our funders: NICHD (R21-HD069194), NIMH (R01-MH54907, K23-MH095655, K24-MH87227), NIH P30-AI027763, and the Canada-Sub Saharan Africa (CANSSA) HIV/AIDS Network.
Footnotes
Meetings where some of these data have been previously presented
Some of these data have been previously presented at the International AIDS Conference (AIDS2016) in Durban, South Africa. July 18–22, 2016 [Oral Poster Abstract THPDC0106].
Competing interests
All authors declare no competing interests.
Contributor Information
Jerome Kabakyenga, Email: jkabakyenga@gmail.com.
Mwebesa Bwana, Email: mwebesa_bwana@yahoo.com.
Francis Bajunirwe, Email: fbaj@yahoo.com.
Winnie Muyindike, Email: wmuyindike@gmail.com.
Kara Bennett, Email: karabstat@gmail.com.
Annet Kembabazi, Email: akembabazi2005@yahoo.com.
Jessica E. Haberer, Email: JHABERER@partners.org.
Yap Boum, Email: yap.boum2@gmail.com.
Jeffrey N. Martin, Email: Martin@psg.ucsf.edu.
Peter W. Hunt, Email: peter.hunt@ucsf.edu.
David R. Bangsberg, Email: bangsber@ohsu.edu.
Lynn T. Matthews, Email: LTMATTHEWS@mgh.harvard.edu.
REFERENCES
- 1.Matthews LT, Beyeza-Kashesya J, Cooke I, et al. Consensus statement: Supporting Safer Conception and Pregnancy For Men And Women Living with and Affected by HIV. AIDS Behav. 2018;22(6):1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyeza-Kashesya J, Ekstrom AM, Kaharuza F, Mirembe F, Neema S, Kulane A. My partner wants a child: a cross-sectional study of the determinants of the desire for children among mutually disclosed sero-discordant couples receiving care in Uganda. BMC Public Health. 2010;10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Homsy J, Bunnell R, Moore D, et al. Reproductive intentions and outcomes among women on antiretroviral therapy in rural Uganda: a prospective cohort study. PLoS ONE. 2009;4(1):e4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myer L, Morroni C, Rebe K. Prevalence and determinants of fertility intentions of HIV-infected women and men receiving antiretroviral therapy in South Africa. AIDS Patient Care STDS. 2007;21(4):278–285. [DOI] [PubMed] [Google Scholar]
- 5.Nakayiwa S, Abang B, Packel L, et al. Desire for children and pregnancy risk behavior among HIV-infected men and women in Uganda. AIDS Behav. 2006;10(4 Suppl):S95–104. [DOI] [PubMed] [Google Scholar]
- 6.Nattabi B, Li J, Thompson SC, Orach CG, Earnest J. A systematic review of factors influencing fertility desires and intentions among people living with HIV/AIDS: implications for policy and service delivery. AIDS Behav. 2009;13(5):949–968. [DOI] [PubMed] [Google Scholar]
- 7.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–181. [DOI] [PubMed] [Google Scholar]
- 10.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. 2012;367(5):423–434. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375(9):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollock LC, Weber S, Kaida A, Matthews LT, Seidman DL. HIV-affected couples and individuals who desire children should be offered options for safer conception. J Int AIDS Soc. 2017;20(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zafer M, Horvath H, Mmeje O, et al. Effectiveness of semen washing to prevent human immunodeficiency virus (HIV) transmission and assist pregnancy in HIV-discordant couples: a systematic review and meta-analysis. Fertil Steril. 2016;105(3):645–655 e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz SR, Bassett J, Holmes CB, et al. Client uptake of safer conception strategies: implementation outcomes from the Sakh’umndeni Safer Conception Clinic in South Africa. J Int AIDS Soc. 2017;20(Suppl 1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner GJ, Linnemayr S, Goggin K, et al. Prevalence and Correlates of Use of Safer Conception Methods in a Prospective Cohort of Ugandan HIV-Affected Couples with Fertility Intentions. AIDS Behav. 2017;21(8):2479–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goggin K, Hurley EA, Beyeza-Kashesya J, et al. Study protocol of “Our Choice”: a randomized controlled trial of the integration of safer conception counseling to transform HIV family planning services in Uganda. Implement Sci. 2018;13(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyawo O, de Walque D, Ford N, Gakii G, Lester RT, Mills EJ. HIV status in discordant couples in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(11):770–777. [DOI] [PubMed] [Google Scholar]
- 18.Auld AF, Shiraishi RW, Mbofana F, et al. Lower Levels of Antiretroviral Therapy Enrollment Among Men with HIV Compared with Women - 12 Countries, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64(46):1281–1286. [DOI] [PubMed] [Google Scholar]
- 19.UNAIDS. Male engagement in the HIV response—a Platform for Action. 2016; http://www.unaids.org/sites/default/files/media_asset/JC2848_en.pdf. Accessed May 1 2017.
- 20.UPHIA. Uganda Population-based HIV impact assesment UPHIA 2016–2017: Summary sheet of preliminary findings. 2017; http://www.afro.who.int/sites/default/files/2017-08/UPHIA%20Uganda%20factsheet.pdf. Accessed April 8, 2017.
- 21.Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med. 2000;50(10):1385–1401. [DOI] [PubMed] [Google Scholar]
- 22.Blanc AK. The effect of power in sexual relationships on sexual and reproductive health: an examination of the evidence. Stud Fam Plann. 2001;32(3):189–213. [DOI] [PubMed] [Google Scholar]
- 23.Matthews LT, Burns BF, Bajunirwe F, et al. Beyond HIV-serodiscordance: Partnership communication dynamics that affect engagement in safer conception care. PLoS One. 2017;12(9):e0183131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khidir H, Psaros C, Greener L, et al. Developing a Safer Conception Intervention for Men Living with HIV in South Africa. AIDS Behav. 2018;22(6):1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews LT, Bajunirwe F, Kastner J, et al. “I Always Worry about What Might Happen Ahead”: Implementing Safer Conception Services in the Current Environment of Reproductive Counseling for HIV-Affected Men and Women in Uganda. Biomed Res Int. 2016;2016:4195762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieves CI, Kaida A, Seage GR 3rd, et al. The influence of partnership on contraceptive use among HIV-infected women accessing antiretroviral therapy in rural Uganda. Contraception. 2015;92(2):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers MM, Ahluwalia IB, Melvin CL. The pregnancy risk assessment monitoring system (PRAMS). J Womens Health. 1998;7(7):799–801. [DOI] [PubMed] [Google Scholar]
- 28.Santelli J, Rochat R, Hatfield-Timajchy K, et al. The measurement and meaning of unintended pregnancy. Perspect Sex Reprod Health. 2003;35(2):94–101. [DOI] [PubMed] [Google Scholar]
- 29.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. [DOI] [PubMed] [Google Scholar]
- 30.Lima VD, Geller J, Bangsberg DR, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21(9):1175–1183. [DOI] [PubMed] [Google Scholar]
- 31.Mugo NR, Hong T, Celum C, et al. Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA. 2014;312(4):362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaida A, Matthews LT, Kanters S, et al. Incidence and predictors of pregnancy among a cohort of HIV-positive women initiating antiretroviral therapy in Mbarara, Uganda. PLoS One. 2013;8(5):e63411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uganda Bureau of Statistcs (UBOS) and ICF. Uganda Demographic and Health Survey 2016: Key Indicators Report. 2017; https://www.ubos.org/onlinefiles/uploads/ubos/pdf%20documents/Uganda_DHS_2016_KIR.pdf. Accessed January 19, 2019, 2019.
- 34.Pilatz A, Discher T, Lochnit G, et al. Semen quality in HIV patients under stable antiretroviral therapy is impaired compared to WHO 2010 reference values and on sperm proteome level. AIDS. 2014;28(6):875–880. [DOI] [PubMed] [Google Scholar]
- 35.Thomson KA, Hughes J, Baeten JM, et al. Increased Risk of Female HIV-1 Acquisition Throughout Pregnancy and Postpartum: A Prospective Per-coital Act Analysis Among Women with HIV-1 Infected Partners. J Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ministry of Health Republic of Uganda. Consolidated guidelines for prevention and treatment of HIV in Uganda. . 2016; http://library.health.go.ug/publications/service-delivery-diseases-control-prevention-communicable-diseases/hivaids/consolidated. Accessed October 1 2017, 2017.
- 37.Matthews LT, Crankshaw T, Giddy J, et al. Reproductive decision-making and periconception practices among HIV-positive men and women attending HIV services in Durban, South Africa. AIDS Behav. 2013;17(2):461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prevention Access Campaign. Risk of sexual transmission of HIV from a person living with HIV who has an undetectable viral laod: Messaging primer and consensus statement. 2016. https://www.preventionaccess.org/consensus. Accessed August 23, 2018.
- 39.Vandormael A, Akullian AN, Dobra A, de Oliveira T, Tanser1 F. Sharp decline in male HIV incidence in a rural South African population (2004–2015). CROI; 2018; March 4-7, 2018, 2018; Boston, MA. [Google Scholar]
- 40.Cooper D, Harries J, Myer L, Orner P, Bracken H, Zweigenthal V. “Life is still going on”: reproductive intentions among HIV-positive women and men in South Africa. Soc Sci Med. 2007;65(2):274–283. [DOI] [PubMed] [Google Scholar]
- 41.Brown J, Njoroge B, Akama E, et al. A Novel Safer Conception Counseling Toolkit for the Prevention of HIV: A Mixed-Methods Evaluation in Kisumu, Kenya. AIDS Educ Prev. 2016;28(6):524–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adeyeye AO, Stirratt MJ, Burns DN. Engaging men in HIV treatment and prevention. Lancet. 2018;392(10162):P2334–2335. [DOI] [PubMed] [Google Scholar]
- 43.UNAIDS. Reaching out to men and boys: Addressing a blind spot in the response to HIV. 2017; http://www.unaids.org/sites/default/files/media_asset/blind_spot_en.pdf. Accessed November 1, 2017.
- 44.Young CR, Bwana M, Hock RS, et al. Implementation of a safer conception program for HIV-affected men and women in Uganda. AIDS 2018; July 23–27, 2018, 2018; Amsterdam, Netherlands. [Google Scholar]
- 45.Davies N, Ashford G, Bekker LG, et al. Guidelines to support HIV-affected individuals and couples to achieve pregnancy safely: Update 2018. South Afr J HIV Med. 2018;19(1):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fakoya A, Lamba H, Mackie N, et al. British HIV Association, BASHH and FSRH guidelines for the management of the sexual and reproductive health of people living with HIV infection 2008. HIV Med. 2008;9(9):681–720. [DOI] [PubMed] [Google Scholar]
- 47.Loutfy M, Kennedy VL, Poliquin V, et al. No. 354-Canadian HIV Pregnancy Planning Guidelines. J Obstet Gynaecol Can. 2018;40(1):94–114. [DOI] [PubMed] [Google Scholar]
- 48.UNAIDS. 90–90–90: An ambitious treatment target to help end the AIDS epidemic. 2014; http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. Accessed September 14, 2015.