Abstract
As novel metallodrugs continue to emerge, they are evaluated using models, including zebrafish, that offer unique sublethal endpoints. Testing metal-based anticancer compounds with high throughput zebrafish toxicological assays requires analytical methods with the sensitivity to detect these sublethal tissue doses in very small sample masses (e.g. egg mass 100 μg). A robust bioanalytical model, zebrafish embryos coupled with ICPMS for measurement of delivered dose, creates a very effective means for screening metal-based chemotherapeutic agents. In this study we used ICPMS quantitation with the zebrafish embryo assays to detect metal equivalents at multiple response endpoints for two compounds, the chemotherapeutic agent cisplatin and Ru-based prospective metallodrug, PMC79. We hypothesized that cisplatin and PMC79 have different mechanisms for inducing apoptosis and result in similar lesions but different potencies following water-borne exposure. An ICPMS method was developed to detect the metal in waterborne solution and tissue (Detection Limit: 5 parts per trillion for Ru or Pt). The Ru-based compound was more potent (LC50: 7.8 μM) than cisplatin (LC50: 158 μM) and induced disparate lesions. Lethality from cisplatin exposure exhibited a threshold (values >15 mg/L) while no threshold was observed for delayed hatching (LOAEL 3.75 mg/L cisplatin; 8.7 Pt (ng)/organism). The Ru organometallic did not have a threshold for lethality. Cisplatin-induced delayed hatching was investigated further by larval-platinum distribution and found to be preferentially distributed to the chorion. We propose that zebrafish embryo-larval assays coupled with ICPMS serve as a powerful platform to evaluate relative potency and toxic effects of metallodrug candidates.
Keywords: Metallodrugs, Cisplatin, Zebrafish, ICPMS, Ruthenium organometallic compounds, Anticancer agents, Dose/Response, Analytical, Sublethal
Short Abstract.
Anticancer metallodrug evaluation requires a high throughput model, testing potency and toxicity. This study evaluated ICPMS metal equivalent detection coupled with a zebrafish embryo larval assay. We evaluated a popular chemotherapeutic, cisplatin, with a novel ruthenium-based anticancer compound, PMC79. This method allowed comparison of the two compounds showing different uptake, distribution, potency and lesions. We believe this bioanalytical model will serve as a platform for metallodrugs’ evaluation.
Introduction.
The discovery of platinum (Pt)-based antiproliferative compounds has resulted in their widespread use as chemotherapeutics. Originally identified in 1965, cisplatin, a square planar molecule comprised of a single platinum scaffold, is currently considered by the World Health Organization (WHO) as an essential drug for treating the ten most common cancers and other tumors (Rosenberg et al., 1965; WHO, 2016). The broad-spectrum cytotoxicity of cisplatin is due to its electrophilic reactivity upon entering the cell. The reactive molecule has been shown to bind proteins as well as cellular DNA. The DNA binding and subsequent adduct formation result in an apoptotic cascade and cancer cell death (Harrington et al., 2010; Ming et al., 2017).
Cisplatin’s success stimulated the synthesis and investigation of additional metal-based chemotherapeutics. Furthermore, drug resistance to Pt-based molecules has accentuated the need for alternative transition metal scaffolds (Corte-Rodriguez et al., 2015; Hall et al., 2008). Ruthenium (Ru) is an attractive candidate due to the structural diversity that can be achieved. Not only are many Ru compounds stable in multiple oxidation states relevant under physiological conditions, but the preferable octahedral geometry adopted by most of Ru compounds allows the fine tuning of electronic and steric properties (Gasser et al., 2011; Zhang & Sadler, 2017). These characteristics led to the prolific production of Ru-based molecules, some of which like KP1019 and NAMI-A, have promising anticancer capabilities and entered clinical trials (Hartinger et al., 2006; Leijen et al., 2015).
Unfortunately, the rate of development for these compounds does not appear to have matched the rate of potency or efficacy assessment. The zebrafish model has gained popularity for toxicity assessment of drug candidate efficacy because it examines adverse outcome pathways (AOPs) from biochemical to whole organism endpoints, unlike cell culture and cost-limiting use of rodent models. However, the zebrafish model has been limited due to determination of uptake through waterborne treatments, which otherwise would allow for comparison of delivered dosages of higher and lower vertebrates. We propose using the zebrafish model (OECD, 2013) coupled with inductively coupled plasma mass spectrometry (ICPMS). This may allow for expedited bioanalytical screening of critical toxicological endpoints including LC50 and EC50 values, (lethal and effective concentrations for 50% of the population) and the NOAEL and LOAEL (no or lowest observed adverse effect level). In order to refine the method, cisplatin the mechanism of which has been elucidated, and a novel Ru compound, PMC79, were evaluated (Figure 1) (Côrte-Real et al., 2019; Moreira et al., submitted). PMC79 was found to be more cytotoxic than cisplatin in ovarian (A2780) and breast (MCF-7 and MDA-MB-231) human cancer cells. Both compounds induce cell death by apoptosis, however their targets are different. While DNA is the main target for cisplatin, in the case of PMC79, a proteomic study indicated that the proteins that regulate the actin dynamics are the main target for this compound (Moreira et al., submitted). As such, we hypothesize the observed lesions will be similar although the potencies will be different due to their distinct modes of action. We report in this paper that the ICPMS method can be successfully used to determine delivery and uptake of metal-based anticancer drugs, and that PMC79 was more potent than cisplatin and very different lesions were observed in the developing zebrafish.
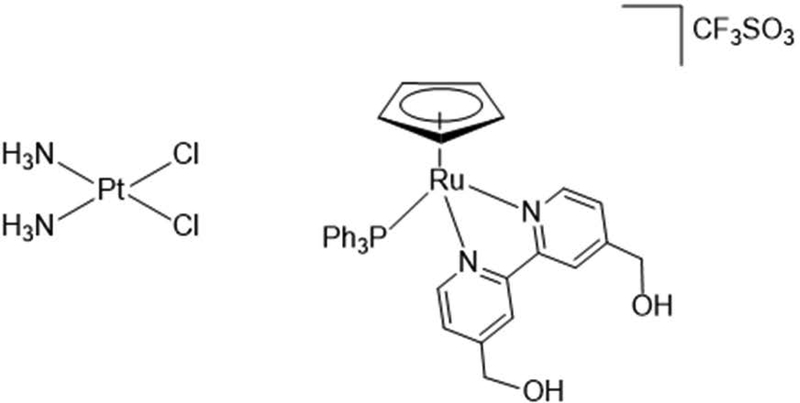
Figure 1:
Chemical structure comparison of platinum-based, square-planar molecule, cisplatin (300.01 molar mass) and the ruthenium-based, piano stool molecule, PMC79 (793.76 molar mass).
Materials and Methods.
Zebrafish Husbandry:
AB strain zebrafish (Danio rerio), obtained from the Zebrafish International Resource Center, were bred and maintained in a recirculating aquatic habitat system on a 14-h light:10-h dark cycle. Fish system water, obtained by carbon/sand filtration of municipal tap water, was maintained at 28°C, < 0.05 ppm nitrite, < 0.2 ppm ammonia, and pH between 7.2 and 7.7. Zebrafish are fed a diet of hatched Artemia cysts, brine shrimp (PentairAES) and a 1:4 ratio of Aquatox Fish Diet flake food (Zeigler Bros, Inc.) and Tetramin (Tetra). The AB strain fish were used for all experiments, and the husbandry protocol (#08–025) was approved by the Rutgers University Animal Care and Facilities Committee.
Experimental Design:
A modified version of the Organization for Economic Cooperation and Development (OECD) Fish Embryo Toxicity Test (FET) Test Number 236 (July 26, 2013) was incorporated in this study (OECD, 2013). AB strain zebrafish male and female were placed in groups for breeding. Fertilized eggs were collected, and randomly sorted into individual glass 3-dram glass vials beginning at 3 hours post fertilization (512-cell stage) (Kimmel et al., 1995). Zebrafish embryos were grown in 500 μl of aerated egg water (60 μg/ml Instant Ocean in DI water) dosed with nominal concentrations of cisplatin (Cas No. 15663-27-1), cis-Diamineplatinum(II) dichloride, Pt(NH3)2Cl2 (≥99.9% trace metals basis purchased from Sigma-Aldrich) or PMC79 ([Ru(η5-Cp)(PPh3)(2,2’-bipy-4,4’-CH2OH)]+, synthesized by us and previously reported (Côrte-Real et al., 2019) (Figure 1). Embryos were observed daily and incubated in darkness at 26°C. Endpoints examined for both cisplatin and PMC79 included delayed hatching, pericardial sac edema, yolk sac edema, as well as embryo death. Observed lesions in zebrafish larvae were obtained with Olympus SZ-PT dissecting microscope equipped with Scion digital camera model CFW-1310C and analyzed with Adobe Photoshop software. Due to delayed hatching, cisplatin treated organisms were manually dechorionated prior to imaging. The dose response studies for cisplatin and PMC79 were repeated twice with 20 embryos per dose. LC50 and confidence interval (CI) values were calculated based on the Litchfield and Wilcoxon method (Litchfield & Wilcoxon, 1949).
Dose Administration:
The cisplatin experiments were conducted as static renewal (every 24 hours) for 5 days due limited physical and chemical stability of cisplatin in solution over 24 hours, at concentrations of 0, 3.75, 7.5, 15, 30, or 60 mg/L. PMC79 was conducted as static non-renewal, due to negligible degradation, for 120 hours. PMC79 was dissolved in pure dimethyl sulfoxide (DMSO) and diluted to a maximum of 0.5% DMSO per dose. Nominal zebrafish dose response concentrations 0, 3.1, 6.2, 9.3, 12.4 mg/L were determined using derivatives of an IC50 value (3.1 mg/L) established following A2780 human ovarian cancer cells after 72 hours. The exposure vials were continuously rocked on shaker platforms at 26°C for the duration of the study to insure constant egg exposure. PMC79 experiments were conducted as static non-renewal.
Solution and Tissue Preparation for ICPMS Analysis:
Cisplatin exposed embryos at 5-day post-fertilization (dpf) were euthanized by placement in −20 °C for 30 minutes. Either upon lethality during the study, or at 5 dpf upon completion of the study, organisms were rinsed 3 times within the vial and subsequently collected and rinsed together with 50 mL of Milli-Q (EMD Millipore) to ensure removal of excess compound. A minimum of 4 organisms were collected at random per dose to form composite samples. Three composite samples per dose per experiment were analyzed. It should be noted that due to a delayed hatching phenomenon, a mixture of unhatched embryos and hatched larvae were collected during composite sampling. No delayed hatching was noted for PMC79. Only hatched viable larvae treated with PMC79 were selected at 5 dpf for analysis and followed the same preparation protocol as cisplatin. All composite samples of cisplatin and PMC79 were drained of any remaining rinse water and digested in a CEM MARS X microwave digester (Matthews, NC) using 0.25 mL of Nitric Acid 67–70%, OmniTrace Ultra, until all organic matter was visibly oxidized (Table 1). The solutions were then diluted to 3.5% nitric acid using MilliQ water. In addition, PMC79 solutions were collected from at least 4 vials per dose to form the composite sample for analysis to confirm exposure dose. Because cisplatin dissolved well into solution and the stability kinetics are well known, ICPMS analysis for Pt equivalents of nominal cisplatin concentrations were not conducted and it was assumed that nominal and measured concentrations in solution would be equivalent (Karbownik et al., 2012; Sewell, 2010).
Table 1.
CEM MARS X Microwave protocol used to digest zebrafish larval samples in 0.25 mL of OmniTrace Ultra 67–70%. Nitric Acid. This cycle was repeated three times or until all organic matter was visibly oxidized.
| Microwave Digestion Protocol for Larval Tissue Mass | ||
|---|---|---|
| Watts | Power | Minutes |
| 300 | 50% | 5 |
| 300 | 75% | 5 |
| 300 | 0% | 5 |
| 300 | 75% | 5 |
Cool down 5 minutes and centrifuge at 2 RPM for 2 minutes between cycles (3 total cycles).
Evaluation of chorionic accumulation:
In order to further investigate the delayed hatching endpoint specific to cisplatin, the Pt distribution between the chorions and the larvae was evaluated. Fertilized embryos were randomly selected at 3 hpf and grouped in four sets of 10 to 15 for exposure to 7.5 mg/L and 15 mg/L cisplatin dissolved in egg water in 10 mL of solution in glass vials. These concentrations were selected due to the high rate of delayed hatching and low rate of perceived mortality. At 5 dpf, the embryos were drained of solution rinsed within the vial three times with 5 mL of Milli-Q water and subsequently collected and rinsed in with 50 mL of Milli-Q water to remove any excess remaining solution. The embryos were manually dechorionated under a dissecting scope using needles. The chorions and the larvae were collected separately per treatment in composite samples of four or more and the remaining water was drained. A Thermo Scientific CL2 Centrifuge was used to pellet the chorions. Samples were prepared for analysis as described above, however, an additional step was included for difficult to dissolve chorions in which 30% trace metal grade hydrogen peroxide was added to achieve a final concentration of 3.5% nitric acid. The chorionic accumulation experiment was repeated twice and T-tests were performed between the larvae and chorion Pt concentrations.
ICPMS Analysis:
Metal content in the samples was quantified via high resolution inductively coupled plasma mass spectrometry (HR-ICP-MS) [Nu Instruments Attom®, UK]. Data was recorded by the Attom software (Attolab v.1) and analyzed with NuQuant by using a seven-point calibration curve of either Ru or Pt. Samples were introduced using an ASX-510 Autosampler [CETAC Technologies]. Several isotopes of Pt and Ru were analyzed in order to minimize isobaric interferences and are listed in the Table 1 below. The limit of detection for tissue and solution ranged from 0.005 to 0.05 parts per billion (ppb), and remained a minimum of one order of magnitude below the treated samples per analytical run. Matrix matched samples (egg water, or digested larvae or chorions) were spiked with 1 ppb of standard and included every 10 samples to monitor for instrument drift. Additional ICPMS operating parameters are listed in Table 2 below. It should be noted that 100Ru has isobaric interferences with strontium oxides, which are formed from the strontium salts contained in the egg water solutions. 192Pt and 99Ru were used for qualitative analysis. The instrument performed 3 technical replicates per sample. Outliers determined to exceed two standard deviations from the mean were removed from analysis.
Table 2.
ICPMS method parameters for analysis of metal equivalents. Pt and Ru isotopes were used to determine concentrations of cisplatin and PMC79, respectively, in digested larval samples and exposure solutions. Sr was included in elemental analysis due to the potential isobaric interference of strontium oxides with isotopes of Ru. Three replicates of these parameters were recorded for each sample. ICPMS: Inductively Coupled Plasma Mass Spectrometry; Pt: Platinum; Ru: Ruthenium; Sr: Strontium.
| ICPMS Method Parameters | |
| Dwell Time per Peak | 4 ms |
| Switch delay/peak (x10 micros) | 2 |
| Number of Sweeps | 350 |
| Number of Cycles | 1 |
| Instrument Resolution | 300 |
| Detection Mode | Attenuated, Single Mass Jump |
| Park Mass | 98.90594 |
| Element (isotopes) | Pt (192, 194, 195, 196), Ru (99, 100, 101, 102) and Sr (84) |
Results.
Analytical Evaluation:
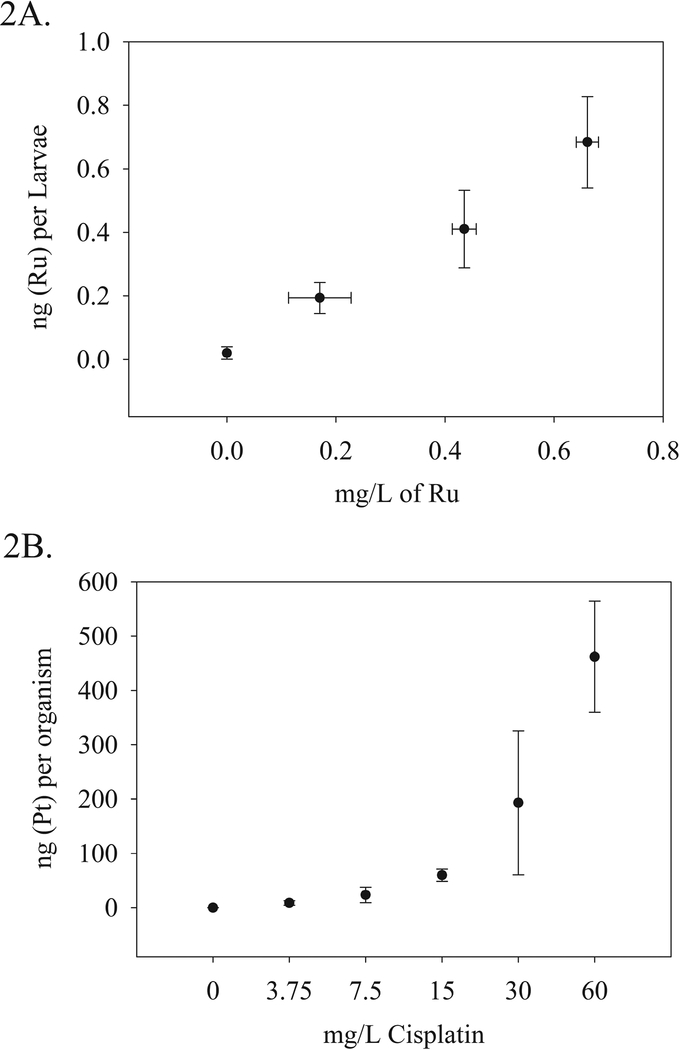
Nominal waterborne concentrations of 0, 3.75, 7.5, 15, 30 and 60 mg/L cisplatin were compared to Pt accumulation in organism (larvae with or without chorion) tissue determined by ICPMS analysis. The nominal waterborne concentrations delivered respective doses of 0.05, 8.7, 23.5, 59.9, 193.2, and 461.9 ng (Pt) per organism with the average embryo mass at approximately 100 μg. The two highest cisplatin doses have larger variability than observed in lower dosages. This may be due to inclusion of viable and nonviable organisms or the delayed hatching phenomenon, as the entire organism was digested with no dechorionation. This was especially relevant in higher doses where higher percentages of delayed hatching were observed. Chorions were included as part of whole organism analysis.
Nominal waterborne concentrations of 0, 3.1, 6.2, 9.2, 12.4 mg/L of PMC79 were analytically determined to contain 0, 0.17, 0.44, 0.66, and 0.76 mg/L of Ru. The delivered tissue doses of these concentrations were only determined for viable larvae and thus were applicable for the first three lowest concentrations of PMC79. Because delayed hatching was not observed, chorions were not included in organism analysis. The respective tissue doses were 0.19, 0.41, and 68 ng (Ru) per larvae. The vehicle control for both the exposure medium and the tissue was below the detection limit (DL) of 0.005 ppb. The relationship between exposure medium (cisplatin or PMC79) and metal equivalent (Pt or Ru) per organism is depicted in Figure 2.
Figure 2:
ICPMS analytical determination of Pt (A) and Ru (B) concentrations in digested tissue samples relative to nominal and analytically determined concentrations of cisplatin and PMC79 compound, respectively. N = 4–6 composite samples per dose. Two experimental replicates were conducted.
Dose Response:
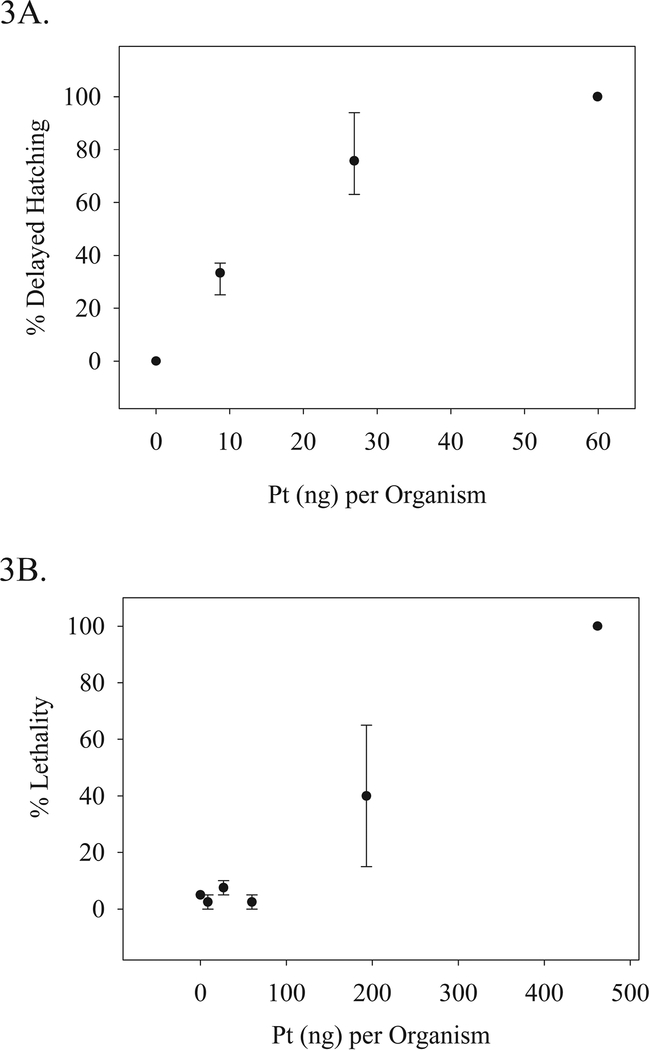
Dose response studies were conducted with the aforementioned nominal waterborne concentrations of cisplatin based on previous literary findings (Kovács et al., 2016). Lethality and a delayed hatching endpoint were analytically evaluated (Figure 3). The lethal concentration for 50% or half the group of test animal (LC50) value was determined to be 31 (95% CI: 21–34) mg/L cisplatin (158.9, [95% CI 105–174] μM) and the corresponding delivered tissue dose was 193.2 (+/− 130) ng (Pt) per organism. The effective concentration for half the population regarding the delayed hatching endpoint (EC50) was determined to be 5 mg/L (25.6 μM) and effective dose for this endpoint (ED50) was determined to be 22 Pt (ng)/organism [95% CI 11–30]. Delayed hatching was observed at all concentrations and thus a NOAEL was not able to be determined. However, the LOAEL value was observed at a nominal concentration of 3.75 mg/L (19.2 μM) and at a delivered dose of 8.7 Pt (ng) per organism (44 pmoles). The lethality curve appeared to have a threshold effect at approximately 60 (ng) per organism, which was not observed in the delayed hatching endpoint. Concentrations beyond this threshold appeared to have much greater variability than treatments below. Several lesions were observed including pericardial hemorrhaging, and spinal curvature (Figure 5).
Figure 3.
Cisplatin Dose Response: A) Percent mean delayed hatching at 5 days post-fertilization (dpf) correlated to the mean Pt equivalents determined per organism, B) Percent mean lethality at 5 dpf correlated to the mean Pt equivalents per organism. Percent means: N = 40 per dose. Pt (ng) per organism: >4 composite samples per dose. Two experimental replicates were conducted; the ranges of which are displayed.
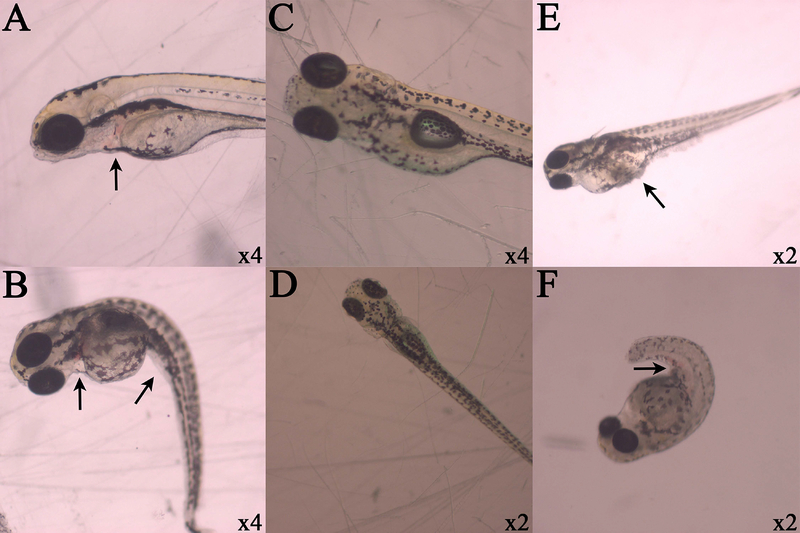
Figure 5.
Observed lesions in zebrafish larvae. Respective magnifications are displayed in the lower right hand corner of each photograph, and black arrows indicate lesions of interest. Cisplatin exposure at 30 mg/L demonstrate pericardial sac hemorrhaging (A and B) and spinal curvature (B) after dechorionation. Control organisms without lesions are centered (C and D). PMC79 exposure at arrows indicate yolk sac edema and protein coagulation and precipitation (E) and hemorrhaging along caudal vein or tail artery (F).
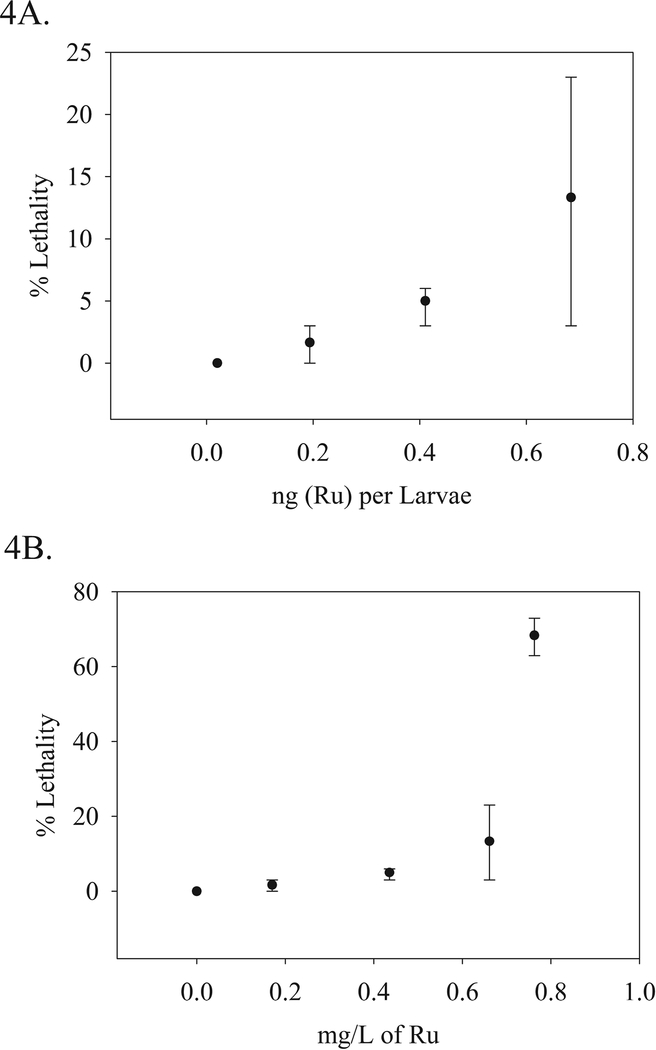
Additionally, dose response studies were conducted with the ICPMS measured waterborne concentrations of PMC79 based on IC50 values (3.1 mg/L) established following A2780 human ovarian cancer cells after 72 hours. Lethality was evaluated, compared to the solution concentrations and delivered tissue dose (Figure 4). The LC50 was determined to be 0.79 (95% CI 0.43–1.20) mg/L Ru (7.8, [95% CI 4.2–11.8] μM). The NOAEL was not determined due to adverse lesions observed at the lowest treated dose. The lesions included hemorrhaging along the caudal vein and tail artery, spinal curvature, and yolk sac edema (Figure 5). The LOAEL was 0.17 mg/L (1.7 μM).
Figure 4.
PMC79 Dose Response: A) Percent mean lethality was correlated to the analytically determined mean Ru equivalents in solution (mg/L). and B) Percent mean lethality at 5 dpf from the same experiment was correlated to the mean Ru equivalents per larvae. Lethality: N = 40 per dose. Ru (mg/L): N = 6 composite samples per dose. Ru (ng) per larvae >4 composite samples per dose. Two experimental replicates were conducted; the ranges of which are displayed.
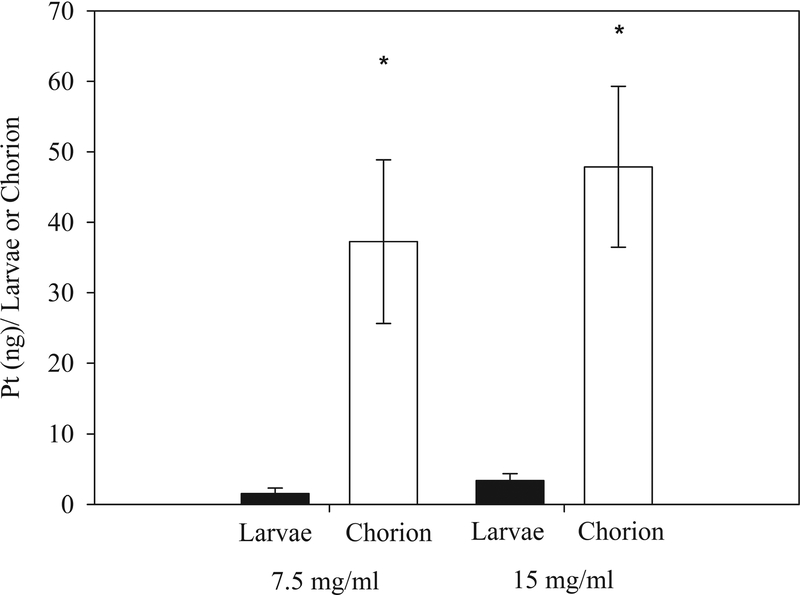
In order to further investigate whether cisplatin levels in the chorion or larvae could be correlated with delayed hatching, embryos with delayed hatching at 5 dpf were dechorionated and both the chorion and larvae were analyzed. Contrary to the discarded chorions of the control larvae, the chorions for the cisplatin treated embryos were intact and rigid. This effect was not observed in PMC79 exposed embryos. The dechorionation results demonstrate that cisplatin preferentially bound to the chorion and much less to the developing organism (Figure 6). Treatment with 7.5 mg/L and 15 mg/L cisplatin were used for these studies because it corresponded to high percent viability coupled with delayed hatching. Doses of 7.5 mg/L resulted in 1.5 Pt (ng) per larvae (7.7 pmoles, 4% of total delivered dose [TDD]) and 37.3 Pt (ng) per the chorion (191 pmoles, 96% TDD). The higher dose of 15 mg/L resulted in 3.4 Pt (ng) per larvae (17.4 pmoles, 7% TDD) and 47.9 Pt (ng) per the chorion (245.6 pmoles, 93% TDD). Once removed from the chorion, the majority of the larvae exposed to 15 mg/L or higher of cisplatin did not respond to tactile stimuli. The embryos hearts were beating and no additional visible lesions were observed.
Figure 6.
Comparison of Pt (ng) present in the larvae and in the chorion after exposure to 7.5 mg/L or 15 mg/L. Composite >3 larvae or chorions per sample; from left to right N = 13, 10, 10, and 11. Mann-Whitney Rank Sum Test P < 0.001 between larvae and chorion for both doses.
Discussion.
We developed an ICPMS technique that has the sensitivity necessary to detect Pt and Ru in zebrafish embryo, larval, or chorionic tissue. Although metals have been detected in zebrafish tissue samples, this study is unique in that it used metal equivalents to calculate the uptake of metallodrugs. This technique determined the tissue dose for the developing zebrafish which can allow for comparisons with dose determined in other model systems. Additionally, dose quantification allows for determination of toxic potencies on a tissue basis for comparison across anti-cancer drugs (Table 3). This bioanalytical assessment used for evaluation and comparison of both cisplatin and PMC79 is applicable to other metal-based compounds. With this assay we determined the individual LC50 and EC50 values, and the NOAEL or LOAEL. Not only were the potencies of the compounds different, (cisplatin LC50: 31 mg/L (158.9 μM), PMC79: 0.79 mg/L (7.8 μM)), but the observed adverse effects of each compound manifested as disparate lesions. Although we hypothesize similar lesions with distinct potencies, both the lesions and potencies were different. This difference provides further evidence that the different modes of action, although apoptotic, result in very different whole organism toxicity.
Table 3.
Determination of solution concentrations and metallodrug update associated with specific toxicological endpoints. LD50 was determined by metal equivalent analysis of Pt and Ru for cisplatin and PMC79, respectively. The LC50 concentrations for PMC79 were analytically determined. However, analytical determination of nominal cisplatin concentrations was not conducted; given the known stability of cisplatin in solution, it was assumed that nominal and measured concentrations in solution would be equivalent. The delayed hatching endpoint for cisplatin exposure was evaluated in terms of EC50/ ED50 and LOAEL. The LOAEL concentrations of PMC79 were analytically determined. The LOAEL included lesions such as hemorrhaging along the caudal vein and tail artery, spinal curvature, and yolk sac edema. All 95% Confidence Intervals (CI) were calculated using the Litchfield Wilcoxon method. LC50/LD50: lethal concentration/dose for 50% of treated organisms; EC50/ED50: effective concentration/dose for 50% of treated organisms; LOAEL: lowest observed adverse effect level.
| Dose Response Data | ||||||
|---|---|---|---|---|---|---|
| Cisplatin | PMC79 | |||||
| Nominal (mg/L) | μM | Pt (ng) / organism | Analytical Ru (mg/L) | μM | Ru (ng) / organism | |
| LC50/LD50: | 31 95% CI: (20.5–34.0) |
158 95% CI: (105–174) |
193 (± 130) | 0.79 95% CI: (0.43–1.20) |
7.8 95% CI: (4.2–11.8) |
NA |
| EC50/ED50: | 5 | 12.5 | 22 95% CI: (11–30) |
NA | NA | NA |
| LOAEL | 3.75 | 15.3 | 8.7 (± 4) | 0.17 (± 0.06) | 1.7 (± 0.6) | 0.19 (± 0.05) |
PMC79 treated larvae had yolk sac edema and no adverse effects on the pericardium were observed. The contrary was observed for cisplatin; no adverse effects were observed on the yolk sac size, but pericardial sac hemorrhaging was present. The organ related alterations indicate different chemical specific organ system toxicity by these two anti-cancer drugs. Additionally, the delayed hatching and intact chorions that were observed for cisplatin were not observed with exposure to the Ru-based compound. Control and PMC79 exposed chorions were completely disintegrated post-hatching. The rigid chorions following cisplatin exposure may be explained by cisplatin electrophilic cross-linking of the chorionic proteins, although this needs to be further investigated (Li et al., 2011). Preliminary studies demonstrated that proteases were less effective in breaking down the cisplatin treated chorions. This cross-linking between proteins would cause rigidity and resistance to protease degradation. Delayed hatching and protease chorion breakdown may be able to be exploited as a biomarker for compounds that rely on a cross-linking mechanism of action.
Additionally, the evaluation of cisplatin distribution between the chorion and the larvae indicated that the majority of the cisplatin (92–96% TDD) was bound to the chorion reducing the amount of drug available to reach the embryo (Figure 6). This was in contrast with PMC79, for which the chorions were degraded and were unable to be collected for analytical evaluation. The chorion may have inhibited Pt delivery to the larvae and may explain the difference in toxicities. However, it is still unclear whether the chorionic accumulation of cisplatin contributes to alternative lesions including the immobility which may be explained by either the potential alteration of the physiochemical properties of the chorion (diminished nutrient and oxygen exchange), or extended confinement. Finally, given the immobility of the dechorionated embryos, one must consider the criteria for considering an organism viable. These questions must be taken into consideration regarding the toxicological and efficacy evaluation of future metal-based compounds using zebrafish embryo larval assays.
During chemotherapeutic treatment, cisplatin is administered intravenously (IV) at a maximum dosage of 100 mg/m2/cycle (Fresenius Kabi USA, 2017). Assuming average body surface area, blood volume and complete absorption and distribution, this would result in approximately 0.03 mg of cisplatin per gram of blood. The concentration found distributed to the larvae determined by the dechorionation experiment (assuming an approximate larval mass of 100 μg) (Hachicho et at., 2015); the concentration of cisplatin per larvae resulted in 0.05 mg/g. The uptake of cisplatin by zebrafish larvae compared with an estimate of human concentrations achieved by IV dosing, suggest that our model can be related to human doses and treatment.
This model differentiates tissue effects and allows for direct comparison of a series of Ru anticancer drugs. Additionally, we have found that it is important to determine the actual dose and not rely on nominal concentrations. Using ICPMS analysis with the zebrafish embryo larval model brought unforeseen insight into the toxicological evaluation of these compounds. The use of zebrafish may help elucidate the mechanism of action of novel metallodrugs and potential non-targeted tissues or biochemical pathways. Currently, we have evaluated six Ru-based anticancer compounds with these techniques (Côrte-Real et al., 2019; Côrte-Real et al., submitted). We believe this model could be a promising asset to high throughput evaluation of metal-based anticancer compounds, especially if coupled with new technologies including robotics to actualize whole organisms and automated chemical delivery systems (Wang et al., 2015; Truong et al., 2016). This becomes increasingly relevant as the literature regarding metallodrugs in modern clinical medicine and biochemical research continues to expand. The advancement of these technologies will increase efficacy, robustness, and reproducibility.
Sponsors.
Brittany F. Karas. NJAES-Rutgers NJ01201, and NIEHS Training Grant T32-ES 007148.
Leonor Côrte-Real. FCT Ph.D. Grant (SFRH/BD/100515/2014) and Fulbright
Brian T. Buckley. NIH-NIEHS P30 ES005022.
Keith R. Cooper. NJAES Project 01202 (W2045), and NIH ES005022.
Andreia Valente. FCT for the projects UID/QUI/00100/2013 and PTDC/QUI-QIN/28662/2017 and for the Investigator FCT2013 (IF/01302/2013, acknowledging as well as POPH and FSE - European Social Fund) and the CEEC Individual 2017 (CEECIND/01974/2017).
Reference List:
- Côrte-Real L, Karas B, Gírio P, Moreno A, Avecilla F, Marques F, … Valente (2019). Unprecedented inhibition of P-gp activity by a novel ruthenium-cyclopentadienyl compound bearing a bipyridine-biotin ligand. European Journal of Medicinal Chemistry, 163, 853–863. doi: 10.1016/j.ejmech.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côrte-Real L, Karas B, Rita Brása AR, Pilona A, Avecillae F, Marques F, … Valente A (2019). Ruthenium-cyclopentadienyl bipyridine-biotin based compounds: synthesis, in vitro cytotoxic evaluation and in vivo toxicity Submitted. [DOI] [PubMed]
- Corte-Rodriguez M, Espina M, Sierra LM, Blanco E, Ames T, Montes-Bayon M, & Sanz-Medel A (2015). Quantitative evaluation of cellular uptake, DNA incorporation and adduct formation in cisplatin sensitive and resistant cell lines: Comparison of different Pt-containing drugs. Biochemical Pharmacology, 98, 69–77. doi: 10.1016/j.bcp.2015.08.112 [DOI] [PubMed] [Google Scholar]
- Fresenius Kabi USA, LLC (2017). Cisplatin - cisplatin injection, solution [package insert]. Lake Zurich, IL. [Google Scholar]
- Gasser G, Ott I, & Metzler-Nolte N (2011). Organometallic anticancer compounds. Journal of Medicinal Chemistry, 54, 3–25. doi: 10.1021/jm100020w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachicho N, Reithel S, Miltner A, Heipieper HJ, Küster E, & Luckenbach T (2015). Body Mass Parameters, Lipid Profiles and Protein Contents of Zebrafish Embryos and Effects of 2,4-Dinitrophenol Exposure. PloS one, 10, e0134755–e0134755. doi: 10.1371/journal.pone.0134755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MD, Okabe M, Shen DW, Liang XJ, & Gottesman MM (2008). The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annual Review of Pharmacology and Toxicology, 48, 495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426 [DOI] [PubMed] [Google Scholar]
- Harrington CF, Le Pla RC, Jones GD, Thomas AL, & Farmer PB (2010). Determination of cisplatin 1,2-intrastrand guanine-guanine DNA adducts in human leukocytes by high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry. Chemical Research in Toxicology, 23, 1313–1321. doi: 10.1021/tx100023c [DOI] [PubMed] [Google Scholar]
- Hartinger CG, Jakupec MA, Keppler BK, Zorbas-Seifried S, Zorbas H, & Kynast B (2006). From bench to bedside - preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A). Journal of Inorganic Biochemistry, 100, 891–904. doi: 10.1016/j.jinorgbio.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Karbownik A, Szałek E, Urjasz H, Głęboka A, Mierzwa E, & Grześkowiak E (2012). The physical and chemical stability of cisplatin (Teva) in concentrate and diluted in sodium chloride 0.9%. Contemporary Oncology, 16, 435–439. doi: 10.5114/wo.2012.31775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, & Schilling TF (1995). Stages of embryonic development of the zebrafish. Developmental Dynamics, 203, 253–310. doi: 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Kovács R, Bakos K, Urbányi B, Kövesi J, Gazsi G, Csepeli A, … Horváth Á (2016). Acute and sub-chronic toxicity of four cytostatic drugs in zebrafish. Environmental Science and Pollution Research International, 23, 14718–14729. doi: 10.1007/s11356-015-5036-z [DOI] [PubMed] [Google Scholar]
- Leijen S, Burgers SA, Baas P, Pluim D, Tibben M, van Werkhoven E, … Schellens JH (2015). Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investigational New Drugs, 33, 201–214. doi: 10.1007/s10637-014-0179-1 [DOI] [PubMed] [Google Scholar]
- Li H, Zhao Y, Phillips HI, Qi Y, Lin TY, Sadler PJ, & O’Connor PB (2011). Mass spectrometry evidence for cisplatin as a protein cross-linking reagent. Analytical Chemistry, 83, 5369–5376. doi: 10.1021/ac200861k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield JT Jr., & Wilcoxon F (1949). A simplified method of evaluating dose-effect experiments. Journal of Pharmacology and Experimental Therapeutics, 96, 99–113. [PubMed] [Google Scholar]
- Ming X, Groehler A. t., Michaelson-Richie ED, Villalta PW, Campbell C, & Tretyakova NY (2017). Mass Spectrometry Based Proteomics Study of Cisplatin-Induced DNA-Protein Cross-Linking in Human Fibrosarcoma (HT1080) Cells. Chemical Research in Toxicology, 30, 980–995. doi: 10.1021/acs.chemrestox.6b00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira T, Francisco R, Comsa E, Duban-Deweer S, Labas V, Teixeira-Gomes AP, Valente A (2019) Polymer “ruthenium-cyclopentadienyl” conjugates - new emerging anti-cancer drugs. Submitted. [DOI] [PubMed]
- OECD. (2013). Test No. 236: Fish Embryo Acute Toxicity (FET) Test OECD Guidelines for the Testing of Chemicals. [Google Scholar]
- Rosenberg B, Van Camp L, & Krigas T (1965). Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature, 205, 698. doi: 10.1038/205698a0 [DOI] [PubMed] [Google Scholar]
- Sewell G (2010). Physical and chemical stability of cisplatin infusions in PVC containers. European Journal of Oncology Pharmacy, 4, 11–13. [DOI] [PubMed] [Google Scholar]
- Truong L, Bugel SM, Chlebowski A, Usenko CY, Simonich MT, Simonich SL, & Tanguay RL (2016). Optimizing multi-dimensional high throughput screening using zebrafish. Reproductive Toxicology, 65, 139–147. doi: 10.1016/j.reprotox.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Rajpurohit SK, Delaspre F, Walker SL, White DT, Ceasrine A, … Mumm JS (2015). First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic beta-cell mass. Elife, 4. doi: 10.7554/eLife.08261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2016). Essential medicines for cancer: WHO recommendations and national priorites. Bull World Health Organization, 94, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, & Sadler PJ (2017). Advances in the design of organometallic anticancer complexes. Journal of Organometallic Chemistry, 839, 5–14. doi: 10.1016/j.jorganchem.2017.03.038 [DOI] [Google Scholar]