Abstract
Background:
In the United States, incidence and mortality rates of hepatocellular carcinoma (HCC) are increasing in older individuals. Chronic infection with hepatitis C virus (HCV) and hepatitis B virus (HBV) are important causes of HCC; however, the contribution of viral hepatitis to recent trends in HCC incidence among older Americans is unclear.
Methods:
We used data from the Surveillance, Epidemiology and End Results-Medicare linkage (SEER-Medicare) for years 2001–2013 to identify HCC cases among individuals aged ≥66 years and Medicare files to assess the HCV and HBV status of HCC cases. Age-standardized incidence rates of HCV-attributable, HBV-attributable and HCV/HBV-unrelated HCC were estimated overall, and by age group, sex, and race/ethnicity. We also calculated annual percent changes (APCs) in HCC incidence.
Results:
During 2001–2013, 15,300 HCC cases occurred in this population. Overall HCC rates increased 43% from 16.3 to 23.3/100,000 (APC=3.40%/year), while HCV-attributable HCC rates almost doubled from 4.2 to 8.2/100,000 (APC=5.62%/year). HCC rates increased more slowly for HBV-attributable HCC (1.3 to 1.8/100,000; APC=3.17%/year) and HCV/HBV-unrelated HCC (11.3 to 14.1/100,000, APC=2.35%/year). The proportion of HCC cases with evidence of HCV infection increased from 25.7% in 2001–2004 to 32.3% in 2011–2013, while the proportion with HBV remained stable at 8%. In 2013, higher rates for both HCV- and HBV-attributable HCC were seen in individuals ages 66–75 years, men and individuals of Asian ancestry.
Conclusions:
Among Americans aged ≥66 years, HCC rates increased rapidly during 2001–2013. HCV-attributable cases contributed substantially to this increase, however, rates of HBV-attributable and HCV/HBV-unrelated HCC also rose during this period.
Keywords: Liver Cancer, Hepatitis, Trends, Medicare, Epidemiology
Precis:
Among Americans aged ≥66 years, HCC rates increased rapidly during 2001–2013. HCV-attributable cases contributed substantially to this increase, however, rates of HBV-attributable and HCV/HBV-unrelated HCC also rose during this period.
Introduction
In the United States, liver cancer incidence and mortality rates increased substantially during 2003–2012.1 Nearly three-quarters of all liver cancer diagnoses are hepatocellular carcinomas (HCCs).2 During 2000–2010, temporal trends in HCC rates diverged by age group, with stable rates among 35–49-year-olds, but strong increases among 50–64 and ≥65-year-olds.2 If current trends continue, it has been projected that U.S. HCC rates among ≥65-year-olds will increase 5.9%/year through 2030.3
Chronic infection with hepatitis C virus (HCV) is an important driver of these rising liver cancer rates.1, 2 HCV is associated with a 60-fold increase in HCC risk4 and caused an estimated 31% of U.S. liver cancer cases in 2015.5 Available data do not support a precise determination of HCV prevalence in the U.S., however, a recent estimate is that 2.5 to 4.7 million U.S. residents have chronic hepatitis C.6 As HCV infection rates were high in the 1960s-1980s,7 and HCC risk rises with increasing duration of HCV infection, rates of liver cancer due to HCV are climbing over time.1, 3, 8
Chronic infection with hepatitis B virus (HBV) is another important cause of liver cancer worldwide, increasing risk 20-fold, and causing an estimated 9% of cases in the US.4, 5 In 2012, an estimated 850,000 people were living with chronic HBV in the US, with the highest prevalence reported among foreign-born Asians.9 In addition to HCV and HBV, diabetes/obesity/metabolic syndrome and excessive alcohol consumption are also associated with liver cancer, and may have also contributed to rising rates over time.10
In the current study, we assessed HCC rates with data from Surveillance, Epidemiology and End Results (SEER)-Medicare11 among Americans aged ≥66 years, an age range that included 44% of U.S. HCC cases in 2015.12 We assessed trends in HCC rates, estimated HCV and HBV prevalence among HCC cases, and disaggregated HCC time trends by viral hepatitis status, sex, age group and race/ethnicity during 2001–2013.
Methods
Study Population
This analysis was carried out with data from the SEER-Medicare dataset, a linkage of two population-based data sources – SEER cancer registries and a database of U.S. Medicare enrollees.11 In the U.S., Medicare is the federally-funded health insurance program that primarily covers people aged ≥65 years. SEER is a set of cancer registries funded by the National Cancer Institute, and includes information on tumor characteristics, patient demographics and survival. SEER-Medicare includes Medicare claims during 1991–2014 for all cancer cases diagnosed during 1991–2013 as well as a 5% random sample of all Medicare enrollees from SEER areas.
Hepatocellular carcinoma cases
Invasive HCC cases that were diagnosed as a first primary cancer during 2001–2013 were identified by cancer registries and defined based on International Classification of Diseases for Oncology, 3rd edition (ICD-O3), by site (C220) and histology (8170–8175) codes. The analysis was restricted to cases among individuals aged 66–99 years at diagnosis who had ≥12 months of Medicare coverage, including both part A (inpatient) and part B (health care provider) coverage outside of a health maintenance organization (HMO), prior to cancer diagnosis and at least one Medicare claim.
We ascertained infection with HCV or HBV from Medicare files for the period from 60 months before until 12 months after cancer diagnosis based on these ICD-9 diagnosis codes – HCV (including codes for acute HCV given the high probability of progression to chronic HCV13): 070.41, 070.44, 070.51, 070.54, 070.7, 070.70, 070.71, or V02.62; and HBV (limited to codes for chronic HBV): 070.22, 070.23, 070.32, 070.33, 070.42, 070.52 or V02.61 (Supplemental table 1). A positive HCV or HBV diagnosis required one inpatient claim or two physician or outpatient claims filed at least 30 days apart. No HCC cases were classified as HCV or HBV-infected based on diagnoses recorded on or after the date of death. Claims related to the following conditions were also ascertained: diabetes mellitus (250.0–250.9), alcohol-related liver disorders (571.0, 571.1, 571.2, 571.3, and V11.3, 571.6 in the presence of 291, 303, or 305.0), and rare genetic disorders (i.e., tyrosinemia [270.2], alpha-1 antitrypsin deficiency [273.4], hemochromatosis [275.0], Wilson disease [275.1] and porphyrias [277.1]).
Five Percent Random Population Sample
A 5% random sample of all Medicare recipients (those with and without cancer diagnoses) was ascertained for each calendar year, restricting to enrollees who on July 1 of that year were aged 66–99 years and had ≥12 months of Medicare coverage, including both part A and part B coverage outside of an HMO, and at least one claim.
Statistical Analysis
The proportions of HCC cases that were HCV-attributable, HBV-attributable and both HCV- and HBV-attributable were assessed by calendar period of HCC diagnosis (2001–04, 2005–07, 2008–10, 2011–13), sex, age at diagnosis (66–75, 76–85, 86–99-year-olds), and race/ethnicity (non-Hispanic white [i.e., white], non-Hispanic black [i.e., black], Asian, Hispanic, other/unknown). We consider cases with evidence for HBV or HCV infection as attributable to those infections based on prior analyses showing that the prevalence of these viruses among HCC cases is nearly identical to the fraction of HCC cases caused by these viruses.4, 14
HCC incidence rates were estimated by dividing the number of HCC diagnoses by person-time from the 5% random sample, which was multiplied by 20 to represent the full Medicare population. Age-standardized rates were based on the 2000 U.S. population age distribution. Temporal trends were assessed separately for HCV-attributable, HBV-attributable and HCV/HBV-unrelated HCC cases, and stratified by sex, age group, and race/ethnicity during 2001–2013. Joinpoint regression was used to identify statistically significant changes in the slope of time trends in incidence rates (based on single year data) and to estimate annual percent changes (APCs) in rates in each group.15 For display purposes, we aggregated rates across 2 or 3-year periods in figures.
To assess the extent to which SEER-Medicare rates represent rates from the full SEER population, HCC rates overall were compared to those for the full SEER population (aged ≥66 years) of included registries (i.e., not restricting to eligible Medicare recipients).12 Further, trends by HCV and HBV status were estimated requiring only one physician or outpatient claim for a diagnosis of HCV or HBV infection.
Results
During 2001–2013, 15,300 cases of invasive HCC were diagnosed among eligible Medicare recipients (Table 1). HCC cases were more likely to be male than female, though a larger fraction of HCV-attributable cases occurred among women (39%), than HBV-attributable (29%) and HCV/HBV-unrelated cases (31%). The age distribution of HCV-attributable and HBV-attributable HCC cases was similar; in contrast, a smaller fraction of HCV/HBV-unrelated cases were 66–75, and a larger fraction were ≥86 years old. The largest fraction of HCV-attributable (54%) and HCV/HBV-unrelated (76%) HCC cases occurred among whites, while the largest fraction of HBV-attributable HCC cases occurred among Asians (53%). Claims for diabetes were most common among HCV/HBV-unrelated (59%) HCC cases, and claims for alcohol-related liver disorders were most common among HCV-attributable cases (31%). HCC-related genetic disorders (2.7%) were uncommon across case groups.
Table 1.
Characteristics of hepatocellular carcinoma cases by hepatitis C and B viral status in SEER-Medicare, 2001–2013
| All HCC Cases | HCV-attributable HCC Cases* | HBV-attributable HCC Cases* | HCV/HBV-unrelated HCC Cases | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Total | 15,300 | 100.0 | 4,518 | 29.5 | 1,253 | 8.2 | 10,071 | 65.8 |
| Sex | ||||||||
| Male | 10,262 | 67.1 | 2,744 | 60.7 | 892 | 71.2 | 7,002 | 69.5 |
| Female | 5,038 | 32.9 | 1,774 | 39.3 | 361 | 28.8 | 3,069 | 30.5 |
| Age group, years | ||||||||
| 66–75 | 8,006 | 52.3 | 2,888 | 63.9 | 821 | 65.5 | 4,656 | 46.2 |
| 76–85 | 6,039 | 39.5 | 1,448 | 32.0 | 385 | 30.7 | 4,376 | 43.5 |
| 86+ | 1,255 | 8.2 | 182 | 4.0 | 47 | 3.8 | 1,039 | 10.3 |
| Race/ethnicity | ||||||||
| White | 10,259 | 67.1 | 2,443 | 54.1 | 281 | 22.4 | 7,676 | 76.2 |
| Black | 1,317 | 8.6 | 694 | 15.4 | 64 | 5.1 | 598 | 5.9 |
| Asian | 1,984 | 13.0 | 835 | 18.5 | 668 | 53.3 | 754 | 7.5 |
| Hispanic | 713 | 4.7 | 214 | 4.7 | 29 | 2.3 | 488 | 4.8 |
| Other/unknown | 1,027 | 6.7 | 332 | 7.3 | 211 | 16.8 | 555 | 5.5 |
| Diabetes Mellitus | ||||||||
| Yes | 8,560 | 55.9 | 2,278 | 50.4 | 633 | 50.5 | 5,939 | 59.0 |
| No | 6,740 | 44.1 | 2,240 | 49.6 | 620 | 49.5 | 4,132 | 41.0 |
| Alcohol-related liver disorders | ||||||||
| Yes | 3,811 | 24.9 | 1,401 | 31.0 | 284 | 22.7 | 2,281 | 22.6 |
| No | 11,489 | 75.1 | 3,117 | 69.0 | 969 | 77.3 | 7,790 | 77.4 |
| Rare Genetic Disorders | ||||||||
| Yes | 408 | 2.7 | 103 | 2.3 | 21 | 1.7 | 295 | 2.9 |
| No | 14,892 | 97.3 | 4,415 | 97.7 | 1,232 | 98.3 | 9,776 | 97.1 |
HCC: hepatocellular carcinoma; HCV: hepatitis C virus, HBV: hepatitis B virus
HCV-attributable and HBV-attributable HCC cases were not mutually exclusive. 542 HCC cases were coinfected with HBV and HCV
The overall HCV prevalence among HCC cases was 29.5% (n=4,518) and HBV was 8.2% (n=1,253) – 542 (3.5%) of these cases occurred among individuals who were infected with both HCV and HBV. HCV prevalence in HCC cases increased from 25.7% in 2001–2004 to 32.3% in 2011–2013, while HBV prevalence remained stable at 8% (Figure 1a). These prevalence estimates are not mutually exclusive; individuals infected with both HBV and HCV are included in the prevalence estimates for both viruses. The prevalence of co-infection was 3.9% in 2001–2004 and 3.5% in 2011–2013. HCV prevalence among HCC cases increased in both men and women, with a higher HCV prevalence among women than among men (36.7% vs. 30.3% in 2011–2013). HCV prevalence among HCC cases increased over time among 66–75 (32.2% in 2001–2004; 39.9% in 2011–13) and 76–85-year-olds (20.1% vs. 25.9%), but declined in the most recent period among ≥86-year-olds (17.5% in 2008–2010; 14.4% in 2011–2013) (Figure 1b). Temporal changes in HCV and HBV prevalence differed by race/ethnicity. HCV prevalence increased across time periods among white (20.4% in 2001–2004, 26.7% in 2011–2013), black (40.3% vs. 63.1%) and Hispanic (26.0% vs. 30.3%) HCC cases, and remained relatively stable among Asian (40.9% vs. 41.1%) and other/unknown race/ethnicity (31.7% vs. 33.9%) HCC cases. There were no notable time trends in HBV prevalence among HCC cases across age and racial/ethnic groups; however, HBV prevalence among HCC cases was far higher among Asians (34.9% in 2011–2013) and those of other/unknown race/ethnicities (21.1%) than among white (2.7%), black (4.9%) or Hispanic (5.4%) HCC cases.
Figure 1.
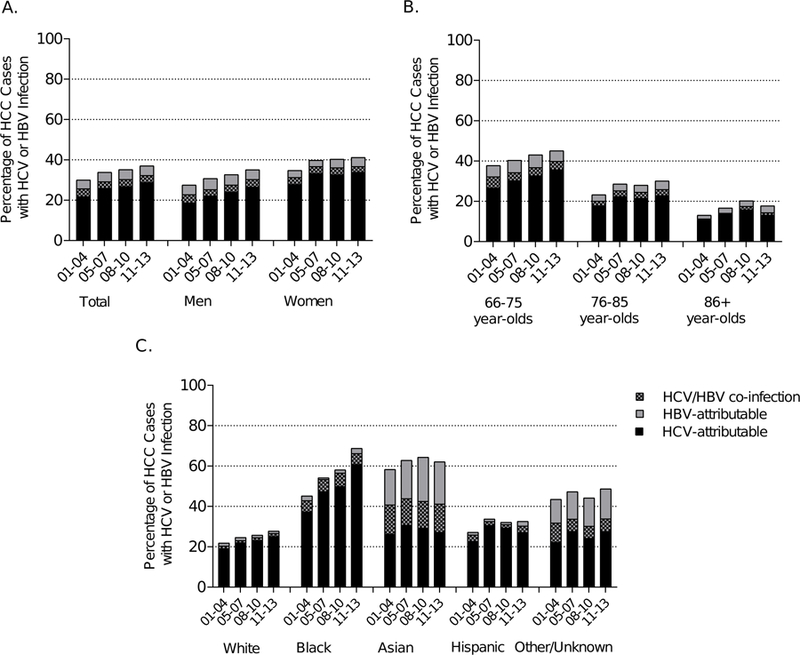
Prevalence of HCV and HBV infection among hepatocellular carcinoma cases in SEER-Medicare, 2001–2013 by A) sex, B) age and C) race/ethnicity. Black bars indicate the fraction of cases with HCV, gray bars indicate the fraction of cases with HBV and checkered bar indicate the fraction of cases with HCV/HBV coinfection.
Age-standardized rates of HCC increased from 16.3/100,000 in 2001 to 23.3/100,000 in 2013 (APC=3.40%/year) (Figure 2, Table 2). Though the annual increase in incidence rates was most rapid for HCV-attributable HCC (APC=5.62%/year), rates of HBV-attributable HCC (APC=3.17%/year) and HCV/HBV-unrelated HCC (APC=2.35%/year) also increased significantly over time. The absolute increase in age-standardized HCC rates between 2001 and 2013 was largest for HCV-attributable cases (4.01/100,000) and HCV/HBV-unrelated cases (2.80/100,000), and smaller for HBV-related cases (0.46/100,000).
Figure 2.
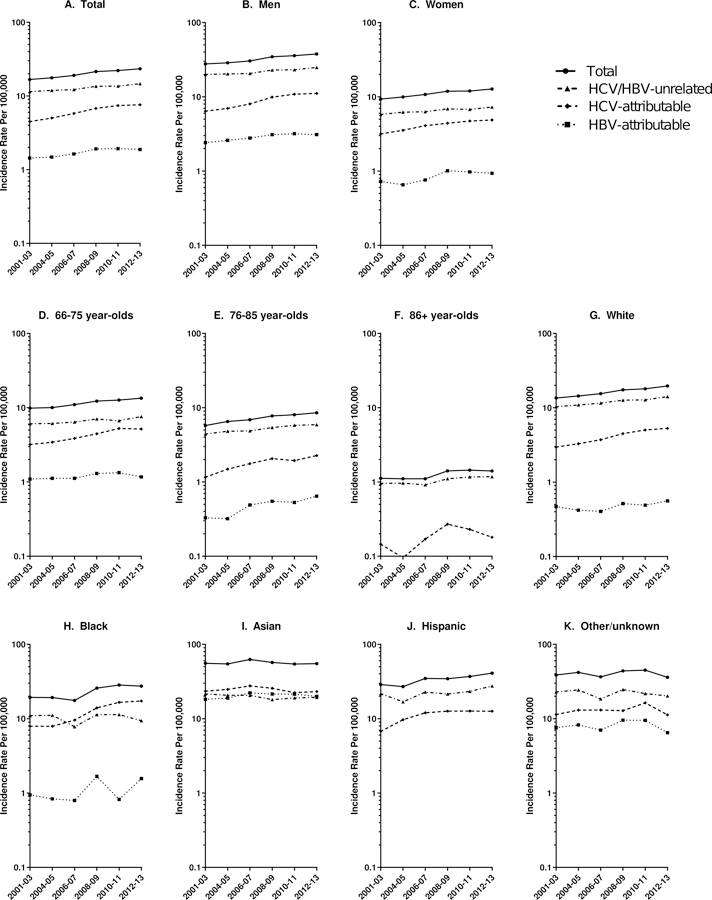
Age-standardized hepatocellular incidence rates in SEER-Medicare by sex, age and race/ethnicity, 2001–2013. Circles represent overall HCC rates, triangles represent HCV/HBV-unrelated HCC rates, diamonds represent HCV-attributable HCC rates and squares represent HBV-attributable HCC rates. Of note, rates of HCV-attributable and HBV-attributable HCC are not mutually exclusive.
Table 2.
Annual percent changes in age-standardized hepatocellular carcinoma incidence rates by hepatitis C and B viral status in SEER-Medicare, 2001–2013
| All HCC Cases | HCV-attributable HCC Cases | HBV-attributable HCC Cases | HCV/HBV-unrelated HCC Cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic | 2001 Rate | 2013 Rate | APC (95% CI) | 2001 Rate | 2013 Rate | APC (95% CI) | 2001 Rate | 2013 Rate | APC (95% CI) | 2001 Rate | 2013 Rate | APC (95% CI) |
| Total | 16.3 | 23.3 | 3.40 (2.80–4.01) | 4.19 | 8.20 | 5.62 (4.50–6.76) | 1.32 | 1.78 | 3.17 (1.66–4.71) | 11.3 | 14.1 | 2.35 (1.55–3.16) |
| Sex | ||||||||||||
| Male | 27.7 | 36.6 | 3.16 (2.50–3.82) | 6.34 | 11.9 | 6.11 (4.55–7.68) | 2.00 | 2.93 | 2.86 (1.00–4.76) | 20.1 | 23.2 | 2.05 (1.09–3.01) |
| Female | 8.77 | 13.4 | 3.14 (2.19–4.11) | 2.69 | 5.26 | 4.37 (2.82–5.95) | 0.82 | 0.91 | 3.39 (1.38–5.45) | 5.68 | 7.55 | 2.18 (1.26–3.10) |
| Age group, years | ||||||||||||
| 66–75 | 9.92 | 13.6 | 3.20 (2.42–3.99) | 3.11 | 5.66 | 5.43 (4.09–6.78) | 1.16 | 1.13 | 1.48 (−0.46–3.46) | 6.12 | 7.37 | 1.96 (0.84–3.09) |
| 76–85 | 5.26 | 8.44 | 3.86 (3.17–4.55) | 0.93 | 2.38 | 6.24 (4.22–8.31) | ~ | 0.61 | 6.54 (2.95–10.3) | 4.25 | 5.70 | 2.90 (2.22–3.59) |
| 86+ | 1.11 | 1.25 | 2.76 (0.91–4.65) | ~ | 0.16 | 4.45† (−0.56–9.72) | ~ | ~ | n.e. | 0.96 | 1.05 | 2.21 (0.39–4.06) |
| Race/ethnicity | ||||||||||||
| White | 12.8 | 19.7 | 3.69 (3.16–4.22) | 2.58 | 5.80 | 6.30 (5.09–7.53) | 0.52 | 0.58 | 2.14 (0.01–4.31) | 9.98 | 13.6 | 2.92 (2.18–3.66) |
| Black | 21.6 | 28.1 | 4.21 (1.91–6.56) | 8.93 | 19.4 | 9.04 (6.30–11.9) | ~ | ~ | 3.73 (−3.44–11.4) | 12.4 | 8.04 | −0.87 (−4.00–2.35) |
| Asian | 62.2 | 54.8 | −0.35 (−1.84–1.15) | 25.1 | 23.7 | −0.61 (−2.17–0.97) | 16.9 | 19.5 | 1.06 (−1.21–3.38) | 26.0 | 20.2 | −1.52 (−3.48–0.48) |
| Hispanic | 32.2 | 39.1 | 3.62 (0.99–6.31) | ~ | 14.3 | 6.01 (0.68–11.6) | ~ | ~ | 6.76 (−2.05–16.4) | 25.2 | 24.9 | 2.37 (−0.46–5.28) |
| Other/unknown | 29.5 | 34.6 | 0.05 (−2.47–2.64) | ~ | 10.4 | 0.77 (−3.24–4.95) | ~ | 4.84 | 0.48 (−3.84–5.01) | 18.6 | 20.7 | −0.99 (−3.80–1.90) |
HCC: hepatocellular carcinoma; HCV: hepatitis C virus, HBV: hepatitis B virus, APC: annual percent change, CI: confidence interval
Statistically significant APCs are denoted in bold text.
APCs are shown for the entire 2001–2013; however, a statistically significant joinpoint for APC was detected for HCV-attributable HCC rates among 86+ year-olds (2001–04: −17.6%/year; 2004–08: 30.1%/year; 2008–13: −7.71%/year) and for HBV-attributable HCC rates among females (2001–04: −8.53%years; 2004–09: 11.0%/year; 2009–13: −2.73%/year).
Incidence rates with <11 cases were suppressed.
HCC rates increased 3.16%/year among men and 3.14%/year among women; however, HCC rates were much higher among men than women in 2013 (36.6 vs. 13.4/100,000 in 2013; Table 2; Figure 2). HCC rates increased across age groups (66–75: 3.20%/year; 76–85: 3.86%/year; 86+: 2.76%/year), and were highest among 66–75-year-olds. Rates of HCV/HBV-unrelated HCC increased significantly in each age group. HCV-attributable HCC rates increased significantly among 66–75 and 76–85-year-olds, and HBV-attributable HCC increased significantly over time among 76–85-year-olds, though few cases occurred in ≥86-year-olds.
Age-standardized HCC rates varied substantially by race/ethnicity – in 2013, rates were nearly three times higher among Asians (54.8/100,000) than among whites (19.7/100,000; Table 2; Figure 2). Overall HCC rates increased significantly over time among whites (APC=3.69%/year), blacks (APC=4.21%/year) and Hispanics (APC=3.62%/year), but not among Asians or those of other/unknown race/ethnicities. In 2013, Asians also had the highest rates of HCV-attributable (23.7/100,000) and HBV-attributable HCC (19.5/100,000), while rates of HCV/HBV-unrelated HCC were highest among Hispanics (24.9/100,000). HCV-attributable HCC rates increased significantly among whites, blacks and Hispanics, while HBV-attributable HCC rates and HCV/HBV-unrelated HCC rates increased significantly only among whites. Rates of HCV-attributable and HBV-attributable HCC were stable among Asians.
In the full SEER population, 41% of all HCC cases were aged ≥66 years during 2001–2013 (Supplemental Figure 1). Comparing HCC rates in SEER-Medicare to those in the full SEER population, the SEER-Medicare rates were 10–14% lower, however, the APCs were consistent (SEER-Medicare; 3.40%/year; full SEER population, 3.29%/year; Supplemental Figure 2).
In a sensitivity analysis requiring only one inpatient, physician or outpatient claim for a diagnosis of HCV or HBV, HCV prevalence was 35.6% (n=5,440) and HBV prevalence was 11.4% (n=1,744). Increases in prevalence were largely driven by an increase in HCV/HBV-coinfected HCC cases (n=1,102). Additionally, APCs were consistent with those estimated in the primary analysis (HCV-attributable: 5.54%/year, HBV-attributable: 3.48%/year, HCV/HBV-unrelated: 2.15%/year).
Discussion
During 2001–2013, overall HCC rates among Americans of Medicare age increased 3.4%/year, with the proportion of HCC cases attributable to HCV infection increasing from 26% to 32%. The rates of HCV-attributable HCC increased strongly overall (5.6%/year) and in most demographic subgroups, contributing substantially to the increase in total HCC rates over time. Rates of HBV-attributable HCC increased significantly over time (3.2%/year); however, absolute rates remained low. Rates of HCC unrelated to viral hepatitis also increased over time (2.4%/year), indicating that HCC driven by other causes unrelated to viral hepatitis have also contributed substantially to increasing overall trends.
Chronic infection with either HCV or HBV can lead to inflammation of the liver with resulting development of fibrosis and cirrhosis in some infected individuals.16 This process results in liver cells that are abnormal and at substantial risk for progressing to cancer.8 In 2011–2013, we estimated that 32% of HCC cases were HCV-attributable and 8% were HBV-attributable, similar to estimates published by the Global Burden of Disease group for the US in 2015 (31% and 9%, respectively).5
In the U.S., HCV incidence was high in the 1960s-1980s, likely spread through injection drug use and receipt of infected blood products prior to HCV screening of the blood supply.16 As a result, Americans born during 1945–1965 (i.e., “baby boomers”) have a higher prevalence of chronic HCV infection than other birth cohorts.7 Prolonged chronic HCV infection in this aging birth cohort has resulted in particularly high liver cancer rates. Only the oldest baby boomers (i.e., 1945–1947 birth cohorts) are included in the current study, therefore, rising HCC rates in our study largely reflect earlier birth cohorts.
Given the higher HCV prevalence among baby boomers, we might anticipate that rates of HCV-associated HCC in the Medicare population will continue to increase in coming years. However, that trend might be blunted by the availability of more effective therapy for chronic HCV.17 Until recently, treatment consisted of pegylated-interferon-α plus ribavirin, which produced a virological cure (‘sustained virological response”) in only ~50% of patients overall and an even lower proportion of individuals with advanced fibrosis or cirrhosis (i.e., those at highest risk of HCC). In 2011, first generation direct anti-viral agents (DAAs) for HCV were introduced and, in 2014, highly effective DAA regimens pushed sustained virological response rates over 90%. Successful treatment of chronic HCV markedly reduces the risk of developing HCC,18 however, many infected individuals are unaware that they have chronic HCV.19, 20 Future rates of HCV-associated HCC in the Medicare population will be affected by both the higher HCV prevalence among baby boomers, and success in identifying and treating chronic HCV in this population.
The prevalence of chronic HBV in the US was low (0.3% in 2007–2012) and did not change significantly during 1999–2012.21 Current therapies for chronic HBV are generally not curative, however, in observational studies, antiviral therapy in patients with immune‐active chronic hepatitis B has been found to reduce the risk of HCC by ~50%.22–24 Current initial therapy recommendations for chronic HBV include pegylated-interferon-α, entecavir, tenofovir disoproxil fumarate or tenofovir alafenamide.23 However, even with effective treatment, HCC may still develop.25 Efforts are underway to develop new anti-viral agents for HBV infection.22, 24 Of note, though universal vaccination against HBV infections is recommended in the U.S., 26, 27 it cannot impact HBV-attributable HCC occurring among persons who are already chronic HBV carriers.
Prior studies have reported on rising liver cancer rates,1, 2 however, we have presented rates disaggregated based on the HBV and HCV status of cases, providing insight into the etiologic drivers of the rising rates. The rate of HCV-attributable HCC nearly doubled during 2001–2013, increasing 4–9%/year among men and women, 66–85-year-olds and whites, blacks and Hispanics. In contrast, HCV-attributable HCC rates were stable among Asians and those of other/unknown race/ethnicity. Though rates of HBV-attributable HCC also increased significantly, the APCs were generally lower than those for HCV-attributable HCC, and HCV-attributable HCC rates were 3.5-times higher than HBV-attributable HCC rates in 2013. Of note, HBV-attributable HCC rates among Asians, the group with the highest rates, remained stable over time.
HCC rates unrelated to HCV or HBV infection increased overall, among both men and women, and in all age groups. These increases appear to be more notable among white and Hispanic populations. Non-viral causes of HCC that may be contributing to rising HCC rates include alcohol consumption, obesity, diabetes and non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of metabolic syndrome.10 The prevalence of alcohol abuse in the U.S increased in all age groups. between 2001–02 and 2012–1328 and there have been steady increases in the prevalence of obesity (37.0% of adults aged ≥60 in 2011–14), type II diabetes (25.2% of ≥65-year-olds in 2015), and NAFLD (31% of adults aged 20–74 in 2011–12).29–32 Associations between these risk factors and liver cancer are much weaker than those noted for HCV and HBV, therefore, the presence of one of these conditions among HCC cases cannot be inferred as causal.33 Nonetheless, alcohol abuse, obesity, diabetes and NAFLD are far more common in the U.S. than chronic viral hepatitis. It should be noted that these non-viral risk factors may act synergistically with HCV and HBV infection,16 thereby further contributing to HCC cases attributable to viral hepatitis in this analysis.
The main strength of this study was the use of linked datasets to distinguish between HCV-attributable, HBV-attributable and HCV/HBV-unrelated HCC cases. With the exception of one older study that focused on HCC rates in the 1990s,34 the current study is the only one to our knowledge that has estimated HCC incidence trends by viral hepatitis status. A recent study reported HCC mortality trends by viral status; however, this study relied on hepatitis information reported on death certificates,35 which is known to be vastly underreported, as a prior study estimated that only 31% of all liver cancer deaths among chronic HCV-infected individuals listed HCV on the death certificate.36 In addition, SEER-Medicare is a large, nationally-representative resource, and we have shown that HCC trends based on Medicare recipients closely represent rates from the same SEER cancer registries.
The main limitation of our study was the use of medical claims, which do not capture HBV or HCV status with complete sensitivity or specificity and do not capture infection duration. To enhance specificity, we classified HCC cases as HBV or HCV positive based on one inpatient claim or two physician or outpatient claims filed at least 30 days apart. An additional limitation of our study is the lack of information on body mass index, cigarette smoking, and average alcohol consumption. We had limited power to detect significant changes in rates in some groups due to the small number of HCC cases. Finally, the age range of this analysis was restricted to Americans aged ≥66 years, and; thus, it did not capture the birth cohort with the highest HCV prevalence in the U.S. The prevalence of HCV among HCC cases is likely higher among Americans who were in their 40s and 50s during the study period, and HCV-attributable HCC rates for this birth cohort likely increased more rapidly than reported here. However, it is important to note that 41% of HCC cases diagnosed in SEER during 2001–13 were aged ≥66 years; thus, the Medicare-aged population captures a substantial fraction of HCC cases in the U.S.
In summary, among Americans aged ≥66 years-old, HCC rates increased rapidly during 2001–2013. HCV-attributable HCCs contributed substantially to this observed increase, with a smaller contribution by HBV-attributable HCCs. Interventions to treat and prevent viral hepatitis infections will affect future HCC rates among Medicare-aged Americans. Non-viral-related HCC cases will likely continue to increase in the U.S., largely due to sustained increases in obesity and metabolic syndrome-related conditions. Interventions focused on modifiable risk factors for HCC, such as promoting health body weight, and reducing alcohol intake and cigarette smoking, could also have an impact on future HCC rates.
Supplementary Material
Supplemental Figure 1. Number of incident HCC cases in the full SEER population by age group and calendar period. Gray bars represent cases diagnosed in <56-year-olds, the purple bars represent cases diagnosed among 56–65-year-olds, and the blue bars represent cases diagnosed among ≥66-year-olds. SEER registries included in this analysis are: San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose, Los Angeles, Rural Georgia, Greater California, Kentucky, Louisiana and New Jersey.
Supplemental Figure 2. Comparison of age-standardized HCC incidence rates and annual percent changes in rates between SEER-Medicare (solid line) and the full SEER population (dashed line). Rates in both populations were limited to people aged ≥66 years. SEER registries included in this analysis are: San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose, Los Angeles, Rural Georgia, Greater California, Kentucky, Louisiana and New Jersey.
Acknowledgements
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement # U58DP003862–01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The authors would also like to acknowledge the statistical support of Ms. Winnie Ricker and Mr. Nathan Appel (IMS, Inc.).
This work was funded by the Intramural Research Program of the National Cancer Institute.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016;122: 1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109: 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34: 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122: 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Liver Cancer Collaboration. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol 2017;3: 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015;62: 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazo M, Nwankwo C, Daya NR, et al. Confluence of Epidemics of Hepatitis C, Diabetes, Obesity, and Chronic Kidney Disease in the United States Population. Clin Gastroenterol Hepatol 2017;15: 1957–1964 e1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JK, Lemon SM, McGivern DR. How do persistent infections with hepatitis C virus cause liver cancer? Current Opinion in Virology 2015;14: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services. Hepatitis B Basic Information Available from URL: https://www.hhs.gov/hepatitis/learn-about-viral-hepatitis/hepatitis-b-basics/index.html [accessed February 5, 2019].
- 10.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011;15: 223–243, vii-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40: IV-3–18. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute, Surveillance Research Program. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2017 Sub (2000–2015) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2016 Counties, released April 2018, based on the November 2017 submission.
- 13.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis 2005;9: 383–398, vi. [DOI] [PubMed] [Google Scholar]
- 14.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol 2013;108: 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19: 335–351. [DOI] [PubMed] [Google Scholar]
- 16.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142: 1264–1273 e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter W, Connelly S, Struble K. Reinventing HCV Treatment: Past and Future Perspectives. J Clin Pharmacol 2017;57: 287–296. [DOI] [PubMed] [Google Scholar]
- 18.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology 2016;64: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med 2013;368: 1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology 2012;55: 1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology 2016;63: 388–397. [DOI] [PubMed] [Google Scholar]
- 22.Emery JS, Feld JJ. Treatment of hepatitis B virus with combination therapy now and in the future. Best Pract Res Clin Gastroenterol 2017;31: 347–355. [DOI] [PubMed] [Google Scholar]
- 23.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67: 1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63: 261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varbobitis I, Papatheodoridis GV. The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy. Clin Mol Hepatol 2016;22: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006;55: 1–33; quiz CE31–34. [PubMed] [Google Scholar]
- 27.Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54: 1–31. [PubMed] [Google Scholar]
- 28.Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74: 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stokes A, Preston SH. The contribution of rising adiposity to the increasing prevalence of diabetes in the United States. Prev Med 2017;101: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief 2015: 1–8. [PubMed]
- 31.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017 Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services, 2017. [Google Scholar]
- 32.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015;41: 65–76. [DOI] [PubMed] [Google Scholar]
- 33.Campbell PT, Newton CC, Freedman ND, et al. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults. Cancer Res 2016;76: 6076–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology 2004;127: 1372–1380. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Li AA, Perumpail BJ, et al. Changing Trends in Etiology- and Ethnicity-Based Annual Mortality Rates of Cirrhosis and Hepatocellular Carcinoma in the United States. Hepatology 2018. [DOI] [PMC free article] [PubMed]
- 36.Mahajan R, Xing J, Liu SJ, et al. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006–2010. Clin Infect Dis 2014;58: 1055–1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Number of incident HCC cases in the full SEER population by age group and calendar period. Gray bars represent cases diagnosed in <56-year-olds, the purple bars represent cases diagnosed among 56–65-year-olds, and the blue bars represent cases diagnosed among ≥66-year-olds. SEER registries included in this analysis are: San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose, Los Angeles, Rural Georgia, Greater California, Kentucky, Louisiana and New Jersey.
Supplemental Figure 2. Comparison of age-standardized HCC incidence rates and annual percent changes in rates between SEER-Medicare (solid line) and the full SEER population (dashed line). Rates in both populations were limited to people aged ≥66 years. SEER registries included in this analysis are: San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose, Los Angeles, Rural Georgia, Greater California, Kentucky, Louisiana and New Jersey.


