Abstract
Background:
In people with HIV on antiretroviral therapy (ART), the relationship between HIV-specific immune responses and measures of HIV persistence is uncertain.
Methods:
We evaluated 101 individuals on suppressive ART in the AIDS Clinical Trials Group A5321 cohort. Cell-associated (CA) HIV DNA and RNA levels and HIV antibody concentrations and avidity to Env/p24 were measured longitudinally at years 1, 4 and 6–15 after ART initiation. Plasma HIV RNA by single copy assay and T cell responses (IFN-γ ELISPOT) against multiple HIV antigens were measured at the last time point.
Results:
HIV antibody levels declined significantly with increasing time on ART (19%/year between year 1 and 4). HIV antibody levels correlated with T cell responses to HIV Pol (r=0.28, p=0.014) and to Nef/Tat/Rev (r=0.34; p=0.002). HIV antibody and T cell responses were positively associated with HIV DNA levels; for example, at the last time point (median 7 years on ART), r=0.35 for antibody levels and HIV DNA (p<0.001); r=0.23 for Nef/Tat/Rev-specific T cell responses and HIV DNA (p=0.03). Neither antibody nor T cell responses correlated with cell-associated HIV RNA or plasma RNA by single copy assay.
Conclusions:
In individuals on long-term ART, HIV-specific antibody and T cell responses correlate with each other and with HIV DNA levels. The positive correlation between HIV immune responses and HIV DNA implies that the immune system is sensing, but not clearing, infected cells, perhaps because of immune dysfunction. Measuring immune responses to HIV antigens may provide insight into the impact of reservoir-reducing strategies.
Keywords: HIV antibodies, HIV persistence, HIV T cell responses, HIV reservoir
INTRODUCTION
In people with HIV, antiretroviral therapy (ART) suppresses plasma HIV RNA to levels below the limits of detection of commercial assays1 but a reservoir of latently-infected cells persists that leads to HIV rebound if ART is stopped.2 A measure of the latently-infected cell population in people on ART is cell-associated HIV DNA which is comprised of intact and defective proviruses. Because defective proviruses rapidly accumulate soon after HIV acquisition, only a small fraction of proviruses is intact3 and potentially replication-competent; as a result, HIV DNA is considered to be a maximal measure of the HIV reservoir. Latently-infected HIV DNA-containing cells have previously been thought to not express antigen and, therefore, to be invisible to the immune system. There are recent data, however, indicating that intact and defective HIV proviruses are transcribed leading to the possibility of intermittent antigen expression in people on ART.4
HIV-specific immune responses usually decline after ART is initiated because of decreasing levels of viral antigen.5,6 For example, over the first one to two years of ART, HIV-specific CD8 T cell responses declined by median 52% per year (half-life 49.8 weeks, based on 5 individuals).7
However, if there is intermittent antigen expression and recognition, HIV antibody and T-cell responses may be elicited and maintained. Understanding the longitudinal relationship between HIV-specific immune responses and measures of HIV persistence may provide insight into whether the immune response continues to sense infected cells in people on ART. In addition, if HIV-specific immune responses correlate with the number of infected cells during ART, tracking these responses may provide information on the impact of interventions designed to stimulate virus expression or reduce HIV reservoirs.8–10 To understand the relationship between HIV-specific antibody responses and measures of HIV persistence, we conducted a longitudinal study in a large cohort of participants on long-term suppressive ART.
METHODS
Study population
We evaluated participants with chronic HIV infection who initiated ART in AIDS Clinical Trials Group (ACTG) studies and had subsequent follow-up specimens while continuing to receive ART (ACTG studies A5001 and A532111). Participants had HIV RNA <50 copies/mL by commercial assays at or before week 48 of ART and at all subsequent time points and no reported ART interruptions. Informed consent was obtained from participants and human experimentation guidelines of the US Department of Health and Human Services were followed in the conduct of clinical research.
Virologic assays
Cell-associated (CA) HIV DNA and unspliced HIV RNA levels (using quantitative PCR targeting HIV pol) in peripheral blood mononuclear cells (PBMC) were measured longitudinally prior to initiation of ART and on-ART years 1, 4 and (for some participants) years 6–15 using previously described methods.11,12 Primers and probes used for qPCR of HIV DNA, CA-RNA and plasma HIV RNA were identical. Testing of the cellular housekeeping gene, IPO8, served as an internal control for mRNA recovery. Results were expressed as copies/million CD4+T-cells normalized for %CD4+T-cells in blood. Plasma HIV RNA by single copy assay (SCA) was measured on samples from the last on-ART time point using a previously reported method.13
HIV Antibody Assays
HIV antibodies were measured at years 1, 4 and, for some participants, years 6–15 after ART initiation using assays that have been previously described.8 Less-sensitive (LS) and Avidity-modified VITROS® HIV 1+2 were used to measure antibodies against HIV envelope (Env) and p24 (Gag).8 Limiting Antigen (Lag)-Avidity EIA was performed as previously described.14 The assays for HIV antibodies used in this study are FDA-cleared diagnostic tests that provide reproducible clinical measurements over time and across assay lots; the performance characteristics have been published.15,16
T cell responses measured by IFN-γ ELISPOT
HIV interferon-gamma ELISPOT assays were performed as previously described and reported on samples from the last on-ART time point.9 In brief, Multiscreen IP 96-well plates (Millipore) were coated with 0.5 ug/mL of anti-interferon-gamma antibody (clone 1-D1K, MAbtech) in phosphate-buffered saline and incubated overnight. Plates were washed, peripheral blood mononuclear cells were added at 2×105 cells per well and HIV peptide pools (10 ug/ml/peptide) and phytohemaggultinin (2ug/mL) were added. Plates were incubated overnight, washed and secondary antibody was added (clone 7-B6–1, Mabtech) and incubated for 1 hour. Plates were developed with Streptavidin-ALP (MAbtech) and developed with Color Development buffer (Bio-Rad). Plates were washed, dried overnight and spots were counted.
Statistical Analysis
Rank-based correlations (Spearman) and rank-sum test were used. Results below assay limits were assigned the lowest rank. Within-participant change per year between years 1 to 4 on ART in log10-transformed measures of HIV antibody were estimated with participant-specific linear regression models. Changes over time were compared against the null hypothesis of no change using the Wilcoxon signed-rank test.
RESULTS
Study Population
A total of 101 participants who initiated ART underwent longitudinal testing of HIV antibodies as well as measurements of HIV DNA and CA-RNA. The median age at ART initiation was 39 years; 21% were female. Participants had a median of 7 years on ART (minimum, maximum: 4, 15) at the time of the last sample collection. Median pre-ART CD4+ and CD8+ T-cell counts were 290 cells/mm3 and 792 cells/mm3, respectively. At the time of the last blood collection, median CD4+ and CD8+ T-cell counts were 681 cells/mm3 and 699 cells/mm3, respectively. Median pre-ART HIV RNA was 4.6 log10 copies/mL. All participants had plasma HIV RNA levels <50 copies/mL at all time points at or after week 48 of ART. Additional details regarding the cohort have been previously published.11
HIV Antibodies Decline during Long-term ART
There was a strong correlation between antibody level (as measured by signal to cutoff ratio) and avidity at each on-treatment time point (all r≥0.95, p<0.001; Supplemental Figure 1, Supplemental Digital Content).
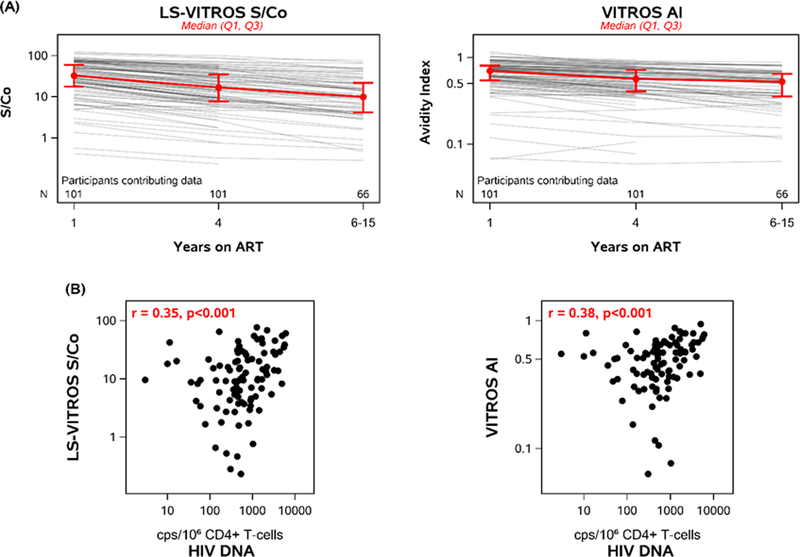
HIV antibodies declined significantly with increasing time on ART (Figure 1A): between years 1 and 4 on ART, antibody levels declined by median 19%/year and avidity declined by median 5.6%/year (p-values<0.001). Ninety-nine percent of participants had a negative antibody level slope and 96% had a negative avidity slope. Participants with higher HIV antibody levels and avidity at year 1 of ART had higher levels and avidity at year 4 of ART (r=0.97 and 0.96, respectively; p <0.001) (Supplemental Figure 2, Supplemental Digital Content 2).
Figure 1. HIV antibody levels and avidity decline with time on antiretroviral treatment and correlate with HIV DNA levels.
Lines correspond to longitudinal antibody responses for each participant (n=101). Red lines represent the median and interquartile range. Antibody levels were measured using the LS-Vitros Signal/Cut-off (S/Co) ratio. Antibody avidity was measured by the Vitros Avidity Index (AI). A) LS-VITROS S/Co and AI results over time on antiretroviral therapy. Between years 1 and 4 on ART, antibody levels declined by median 19%/year (1.23-fold/year) and avidity declined by median 5.6%/year (1.06-fold/year) (p-values<0.001). B) Correlation of LS-VITROS and AI with HIV DNA/106 CD4+T cells at the last longitudinal time point (n=96).
HIV Antibodies and Pre-ART Characteristics
At year 1 of ART, higher HIV antibody levels and avidity were modestly correlated with older age (r=0.23 and r=0.23, p <0.05, respectively) but not participant sex (p≥0.37). Of the 101 participants, 21% were female; this number may be too low to assess differences in antibody responses by sex. There were no significant correlations observed between HIV antibody level or avidity at year 1 on ART with pre-ART plasma HIV RNA, pre-ART HIV DNA, pre-ART CA-RNA, pre-ART CD4 cell count or pre-ART CD4:CD8 ratio (all p-values ≥0.08).
HIV Antibody Responses Correlate with T cell responses directed against Nef/Tat/Rev and Pol
HIV ELISPOT assays were performed at the last time point after ART initiation (median of 7 years) and compared to antibody levels and avidity at the same time point (Figure 2). HIV antibody and avidity correlated with T cell responses to all HIV peptides (r=0.27, p=0.02 and r=0.27, p=0.02, respectively). HIV antibody levels and avidity also correlated with T cell responses to HIV Pol (r=0.28, p=0.014 and r=0.25, p=0.024, respectively) and to Nef/Tat/Rev (r=0.34, p=0.002 and r=0.34, p=0.002). There were no correlations between HIV antibody and T cell responses to HIV Gag or Env, or to CMV and EBV controls.
Figure 2. HIV antibody levels and avidity correlate with HIV antigen-specific T cell responses.
Each data point represents the antibody measurements or the number of antigen-specific IFN-γ spot forming T cell responses for each participant. Shown are correlations between IFN-γ -producing HIV-specific T-cell responses and LS-VITROS or AI measurements of antibody. Top panels are T cell responses to Pol, middle panels are responses to Nef/Tat/Rev, and bottom panels are T cell responses to total HIV.
HIV Antibody and T cell Responses on ART correlate with HIV DNA
At year 1 of ART, HIV antibody level and avidity were positively correlated with HIV DNA (r=0.24 and 0.27, respectively; p<0.02). Similarly, at year 4 on ART, both HIV antibody measures were correlated with HIV DNA (r=0.31 and 0.36, p ≤0.002, respectively). At the last time point (median of 7 years on ART), antibody level and avidity continued to be correlated with HIV DNA levels (r=0.35 and 0.38, respectively, p <0.001; Figure 1B). Similarly, at the last time point, Nef/Tat/Rev-specific T cell responses correlated with HIV DNA levels (r=0.23, p=0.03), as previously reported.9
HIV antibody levels and avidity were not significantly correlated with CA-RNA (measured at years 1 and 4; p ≥0.24) and were not significantly correlated with plasma HIV RNA by SCA (measured at year 4–15). Similar to the results for HIV antibodies, at the last time point (as previously reported9), T cell responses did not correlate with CA-RNA or plasma HIV RNA by SCA.
DISCUSSION
In this longitudinal study, we found that HIV antibody levels and avidity declined significantly during ART. HIV antibody levels correlated with T cell responses to HIV Pol and Nef/Tat/Rev. Despite many years of ART, during which plasma HIV RNA was consistently suppressed to <50 copies/mL, we found that HIV antibody and T cell responses were positively correlated with HIV DNA levels. The positive correlation between HIV immune responses and HIV DNA implies that the immune system is sensing, but not clearing, infected cells, perhaps because of functional defects in immune responses or resistance of infected cells to elimination (both of which have been described in previous studies in ART-treated individuals17,18.)
Before initiation of ART, viral replication and antigen production induce HIV-specific antibody and T cell responses. The decline in immune responses after initiation of ART is expected, given the reduced levels of HIV production in persons on suppressive ART. In fact, the latently-infected cell population that persists in people on long-term ART has been thought to be invisible to the immune system. Some HIV-infected cells, however, are releasing intact viral particles that can be detected in plasma by single-copy assay1 and recent findings that defective HIV proviruses are transcribed provides another avenue by which antigen expression may occur in people on ART.4 Our findings that HIV antibody responses and our previous report that T cell responses9 correlate with HIV DNA levels supports the hypothesis that there is intermittent antigen production during ART19 that maintain immune responses against the virus.
A strength of this study is that participants had documented sustained suppression of plasma HIV RNA for many years after treatment initiation, allowing us to assess the relationship between immune responses and HIV persistence without potential confounding that may result from transient detectable viremia or virologic failure. The finding that antibody levels and avidity – and the previous finding that HIV-specific T cell responses -- are correlated with HIV DNA, but not CA-RNA or low level plasma viremia, was unexpected. One potential explanation is that the number of infected cells, as measured by HIV DNA, reflects the likelihood of intermittent antigen production and induction of immune responses better than CA-RNA because of blocks in nuclear export or translation. Another possibility is that blood levels of CA-RNA and HIV RNA by SCA may not reflect cumulative in vivo antigenicity, perhaps because of fluctuations in RNA levels over time, varying copy numbers per cell and/or antigen production in lymphoid or other compartments.
The current findings should be considered in light of the results of a previous study performed on the same set of samples, which reported a direct correlation between HIV-specific T cell responses and HIV DNA.9 The correlation was unique to T cell responses directed against Nef, with no correlations detected for T cell responses to other gene products, including Gag and Env. In the current study, there was a correlation between antibody responses to Gag/Env and HIV DNA (we did not measure antibody responses to Nef). Gag/Env-specific antibody responses correlated strongly with Nef-specific T cell responses, but not with Gag- or Env-specific T cell responses. We propose two potential explanations: 1) Nef-mediated immune-evasion through MHC class I down-regulation20 may prevent T cells, but not B cells, from sensing late gene products, such as Gag and Env, produced by infected HIV DNA-positive cells; 2) long-term antigen exposure and paucity of CD8 cells to clear HIV-infected cells in the B cell follicle may drive a differential B and T-cell immune response.21 Further study is needed to distinguish among these and other possibilities.
HIV antibodies have been proposed as a method to monitor HIV persistence in people on ART.22 Here we show that there are significant declines in antibody levels and avidity in almost all persons on ART, likely because of the loss of stimulating antigens.22 One limitation is that we were not able to test pre-ART specimens for antibody; as a result, we cannot assess changes in these measures during the first year of ART. However, a cross-sectional analysis of a separate cohort by our group supports the finding that antibodies correlate with measures of HIV persistence during treatment.8 In addition, quantitative HIV-specific antibodies were recently reported to correlate with HIV DNA levels in children on ART,10 leading the authors to similarly propose their utility for monitoring HIV persistence.
Another study limitation is that the cohort started ART at a lower CD4 cell count (median 290/mm3) than is typical currently and with regimens that include older medications. This CD4 cell count nadir may be lower than current values, but there is still a substantial proportion of people in the US who have a CD4 cell count <200/mm3 at time of diagnosis (about 25% in a recent study).23 In terms of ART regimen, approximately 50% of study participants received a non-nucleoside reverse transcriptase inhibitor, 30% received a protease inhibitor and 20% received an integrase inhibitor.11 Even though integrase inhibitors are used more widely now than in the past, differences in regimen are unlikely to affect immune responses in people with sustained virologic suppression, as in our cohort.
In conclusion, in persons on long-term ART, HIV-specific antibody and T-cell responses correlate with each other and with HIV DNA levels. The positive correlation between HIV immune responses and HIV DNA suggests that the immune system may be periodically sensing, but not efficiently clearing, infected cells, perhaps because of immune dysfunction. Sensing of infected cells by immune responses suggests that tracking these measures may be a method of assessing the impact of novel reservoir-reducing strategies.
Supplementary Material
Supplemental Figure 1. HIV antibody levels (as measured by LS-VITROS S/Co) and antibody avidity (as measured by Vitros Avidity Index or AI) correlate over time on ART. Data points correspond to the LS-VITROS and AI measurements for each participant at 1 year (n=101), 4 years (n=101) and 6–15 years (n=66) after ART initiation
Supplemental Figure 2. Participants with higher HIV antibody responses at year 1 of treatment have higher levels at year 4 of treatment. Data points correspond to the antibody levels (as measured by LS-VITROS S/Co) and antibody avidity (as measured by Vitros Avidity Index or AI) for each participant at 1 year and 4 years (n=101)
Acknowledgements
We would like to thank all the members of the ACTG A5321 team. We would also like to express our sincere appreciation to the study participants, the ALLRT team who established the original cohort, study staff and the sites who enrolled participants, NIAID and NIMH, and the ACTG.
We thank Paul Contestable and Ortho Clinical Diagnostics for supplying reagents.
We greatly appreciate Delaney Taylor’s assistance in preparing the manuscript.
Conflicts of Interest and Source of Funding: MPB receives ongoing funding from Ortho Clinical Diagnostics, Inc., provided to Blood Systems Research Institute, to enable ongoing evaluations of their respective assays. RTG’s institution has received educational grants from Gilead, Viiv, Janssen, Theratechnologies and Merck; RTG has served on scientific advisory boards for Merck, Gilead and Theratechnologies. JJE is an ad hoc consultant to Merck, Gilead Sciences, Janssen and ViiV Healthcare and receives contract support from Gilead Sciences, Janssen and ViiV Healthcare for work unrelated to this study. RBJ serves on the scientific advisory board of Abbvie Inc. For the remaining authors, none were declared. This study received grant support from AI – 68634 (Statistical and Data Management Center), UM1 – A1 – 26617, AI – 131798,AI – 68636 (ACTG), and R01- AI – 144994. Research was also supported as part of the amfAR Institute for HIV Cure Research, with funding from amfAR grant number 109301.
Footnotes
This work was presented at Conference on Retroviruses and Opportunistic Infections (CROI), Seattle 2017 and Boston 2018.
References
- 1.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS pathogens. 2007;3(4):e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature medicine. 2003;9(6):727–728. [DOI] [PubMed] [Google Scholar]
- 3.Bruner KM, Murray AJ, Pollack RA, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nature medicine. 2016;22(9):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imamichi H, Dewar RL, Adelsberger JW, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(31):8783–8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogg GS, Jin X, Bonhoeffer S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. Journal of virology. 1999;73(1):797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RB, Walker BD. HIV-specific CD8(+) T cells and HIV eradication. The Journal of clinical investigation. 2016;126(2):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casazza JP, Betts MR, Picker LJ, Koup RA. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. Journal of virology. 2001;75(14):6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating SM, Pilcher CD, Jain V, et al. HIV Antibody Level as a Marker of HIV Persistence and Low-Level Viral Replication. The Journal of infectious diseases. 2017;216(1):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas AS, Jones KL, Gandhi RT, et al. T-cell responses targeting HIV Nef uniquely correlate with infected cell frequencies after long-term antiretroviral therapy. PLoS pathogens. 2017;13(9):e1006629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus M, Henderson J, Gautam A, et al. Quantitative HIV-1 antibodies correlate with plasma HIV-1 RNA and cell-associated DNA levels in children on ART. Clinical Infectious Diseases. 2018:ciy753–ciy753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi RT, McMahon DK, Bosch RJ, et al. Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS pathogens. 2017;13(4):e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong F, Aga E, Cillo AR, et al. Novel Assays for Measurement of Total Cell-Associated HIV-1 DNA and RNA. Journal of clinical microbiology. 2016;54(4):902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cillo AR, Vagratian D, Bedison MA, et al. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. Journal of clinical microbiology. 2014;52(11):3944–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duong YT, Qiu M, De AK, et al. Detection of Recent HIV-1 Infection Using a New Limiting-Antigen Avidity Assay: Potential for HIV-1 Incidence Estimates and Avidity Maturation Studies. PloS one. 2012;7(3):e33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evaluation of Ortho Avidity-VITROS ECi. http://www.incidence-estimation.org/media/webcontent/2015/02/21/CEPHIA_Ortho_VITROS_Avidity.pdf. Published February 11, 2015. Accessed March 26, 2019.
- 16.Evaluation of Ortho Less Sensitive (LS)-VITROS ECi. http://www.incidence-estimation.org/media/webcontent/2015/02/19/CEPHIA-Ortho-LS-VITROS.pdf. Published February 11, 2015. Accessed March 26, 2019.
- 17.Trabattoni D, Piconi S, Biasin M, et al. Granule-dependent mechanisms of lysis are defective in CD8 T cells of HIV-infected, antiretroviral therapy-treated individuals. Aids. 2004;18(6):859–869. [DOI] [PubMed] [Google Scholar]
- 18.Huang SH, Ren Y, Thomas AS, et al. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. The Journal of clinical investigation. 2018;128(2):876–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferdin J, Goricar K, Dolzan V, et al. Viral protein Nef is detected in plasma of half of HIV-infected adults with undetectable plasma HIV RNA. PloS one. 2018;13(1):e0191613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391(6665):397–401. [DOI] [PubMed] [Google Scholar]
- 21.Bronnimann MP, Skinner PJ, Connick E. The B-Cell Follicle in HIV Infection: Barrier to a Cure. Frontiers in immunology. 2018;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananworanich J, Puthanakit T, Suntarattiwong P, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28(7):1015–1020. [DOI] [PubMed] [Google Scholar]
- 23.Hall HI, Tang T, Espinoza L. Late Diagnosis of HIV Infection in Metropolitan Areas of the United States and Puerto Rico. AIDS and behavior. 2016;20(5):967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. HIV antibody levels (as measured by LS-VITROS S/Co) and antibody avidity (as measured by Vitros Avidity Index or AI) correlate over time on ART. Data points correspond to the LS-VITROS and AI measurements for each participant at 1 year (n=101), 4 years (n=101) and 6–15 years (n=66) after ART initiation
Supplemental Figure 2. Participants with higher HIV antibody responses at year 1 of treatment have higher levels at year 4 of treatment. Data points correspond to the antibody levels (as measured by LS-VITROS S/Co) and antibody avidity (as measured by Vitros Avidity Index or AI) for each participant at 1 year and 4 years (n=101)