Abstract
Background and Objective:
To identify and characterize subgroups of adolescents with type 1 diabetes (T1D) and elevated hemoglobin A1c (HbA1c) who share patterns in their continuous glucose monitoring (CGM) data as ‘dysglycemia phenotypes.’
Methods:
Data were analyzed from the Flexible Lifestyles Empowering Change randomized trial. Adolescents with T1D (13-16 years, duration>1 year, HbA1c 8-13% (64-119 mmol/mol) wore blinded CGM at baseline for 7-days. Participants were clustered based on eight CGM metrics measuring hypoglycemia, hyperglycemia, and glycemic variability. Clusters were characterized by their baseline features and 18-month changes in HbA1c using adjusted mixed effects models. For comparison, participants were stratified by baseline HbA1c (≤/>9.0% (75 mmol/mol)).
Results:
The study sample included 234 adolescents (49.8% female, age 14.8±1.1, duration 6.4±3.7 years, HbA1c 9.6±1.2% (81±13 mmol/mol)). Three Dysglycemia Clusters were identified with significant differences across all CGM metrics (p<0.001). Dysglycemia Cluster 3 (n=40, 17.1%) showed severe hypoglycemia and glycemic variability with moderate hyperglycemia and had a lower baseline HbA1c than Clusters 1 and 2 (p<0.001). This cluster showed increases in HbA1c over 18-mo (p-for-interaction=0.006). No other baseline characteristics were associated with Dysglycemia Clusters. High HbA1c was associated with lower pump use, greater insulin doses, more frequent blood glucose monitoring, lower motivation, and lower adherence to diabetes self-management (all p<0.05).
Conclusions:
There are subgroups of adolescents with T1D for which glycemic control is challenged by different aspects of dysglycemia. Enhanced understanding of demographic, behavioral, and clinical characteristics that contribute to CGM-derived dysglycemia phenotypes may reveal strategies to improve treatment.
Keywords: type 1 diabetes, continuous glucose monitoring, adolescent, hypoglycemia
Introduction
While hemoglobin A1c (HbA1c) is the gold standard for measuring intermediate-term glycemic control, continuous glucose monitoring (CGM) data captures transient glucose fluctuations to various thresholds of hypoglycemia and hyperglycemia, as well as overall glycemic variability in the daytime and overnight.1,2 These features of dysglycemia represent distinct clinical issues for individuals with type 1 diabetes which may be amenable to different self-management and medication adjustments.1 They also confer independent risk for short and long-term complications of type 1 diabetes.1-4 Recently, CGM data have also been used to provide validated metrics such as time in range5,6 or average glucose exposure over a shorter period of time, also referred to as the Glucose Management Indicator.7 CGM as a source of patient data thus offers the opportunity to understand patterns of glycemia that are not necessarily represented by HbA1c and inform an individualized approach to type 1 diabetes management for decreased patient burden and better outcomes.1
The most effective strategy to both leverage the depth and integrate the breath of information that CGM offers remains unclear, especially in light of the rapidly increasing uptake of CGM.8 This step is critical to inform tailored approaches to diabetes care. We focused on young individuals with type 1 diabetes and suboptimal glycemic control as it is measured by HbA1c because this population is in great need for improved clinical strategies.8,9 Our objective was to use longitudinal CGM data from adolescents with type 1 diabetes and elevated HbA1c >65 mmol/mol (8.0%) to identify clinically-relevant subgroups sharing multifacteted patterns in hypoglycemia, hyperglycemia, and glycemic variability as distinct ‘dysglycemia phenotypes’. These comprehensive dysglycemia phenotypes could be used to characterize glycemic control across the population in a more nuanced, patient-oriented manner compared to HbA1c and inform the development of future interventions.2
To follow best practices and maximize relevance to future research, we used a combination of CGM metrics consistent with Advanced Technologies & Treatments for Diabetes (ATTD) Congress consensus statement to standardize the reporting of CGM variables in clinical and epidemiologic research.10 Given significant skews in the distribution of key CGM metrics across the sample that are important to clinical care, namely hypo- and hyperglycemia, it was important to identify a statistical method that would retain information from data at the extremes of the distribution. We chose a neural-network approach to clustering and grouped individuals based on their placement on a self-organzing map (SOM) constructed from eight CGM metrics selected to be maximally clinically-relevant.11 The SOM is a machine learning technique that is robust to different distributions of data when uncovering underlying clusters.12 We then tested for differences in the baseline sociodemographic, clinical, and pyschosocial correlates of each Dysglycemia Cluster and 18-month changes in HbA1c.
Methods
Study Sample
Data were analyzed from the baseline visit of the Flexible Lifestyles Empowering Change randomized trial (FLEX) (ClinicalTrials.gov identifier: NCT01286350). FLEX was a randomized clinical trial testing an adaptive, 18-month intervention including behavioral skills and problem solving for youth with type 1 diabetes, with respect to HbA1c (primary outcome), glycemic variability, CVD risk factors, health-related quality of life, and cost effectiveness.13 The study was conducted at two pediatric endocrinology diabetes clinics in Colorado and Ohio, USA, with institutional review board approval for ethical conduct of human subjects research at each institution and at the coordinating center located in North Carolina.
Inclusion Criteria
FLEX enrolled 258 adolescents with type 1 diabetes who were instructed to wear a blinded CGM for 7 days at baseline.14 Participants were recruited from 05/0½014 to 04/04/2016.14 Eligible participants were youth ages 13-16 years with type 1 diabetes for ≥1 year, literacy in English, HbA1c 64-119 mmol/mol (8.0-13.0%), and ≥1 primary caregiver with no other serious medical conditions or pregnancy. All participants and their caregivers provided informed assent and consent, respectively. Detailed considerations of the FLEX design and baseline participant characteristics have been described elsewhere.14
Participants were excluded from the present analyses if they reported a severe hypoglycemic event (an episode of hypoglycemia requiring external aid) during the study week (n=0) or if ≥24 hours of CGM data were missing at the baseline visit (n=24).
Measures
All data collection was standardized as per FLEX study protocol and are described in detail elsewhere.14
Continuous Glucose Monitoring
A blinded CGM [iPro®2 Professional CGM; Medtronic Diabetes, Northridge, CA; median absolute relative difference: 11.1%] was worn for a 7-day period to measure interstitial glucose levels in real time throughout the day and night. At the baseline visit, study participants inserted the iPro®2 CGM system with the Enlite™ sensor into abdominal subcutaneous adipose tissue. Participants were carefully instructed on the use and maintenance of the CGM and advised to calibrate the sensor before eating and before bed with an iPro2 compatible glucometer (OneTouch® Ultra® 2). The Enlite™ sensor measured interstitial glucose level every 5 minutes within the 3-147 mmol/mol (40-400 mg/dL) range. On the last day of the CGM wear week, participants were reminded to send the devices back using a pre-paid box/envelope. CGM data were downloaded with CareLink iPro® System and uploaded to the coordinating center for data processing. As part of blinding, no communication from the device was available to participants.
Laboratory data
A central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA, USA) provided oversight and conducted all assays. At all timepoints, HbA1c was measured in whole blood using an automated nonporous ion exchange HPLC system (model G-7; Tosoh Bioscience).
Clinical Measures
Height was measured using a stadiometer, and weight was measured to the nearest 0.1 kg using an electronic scale. Body mass index (BMI, weight (kg) / height (m)2) was calculated and then converted to an age- and sex-specific and BMI z-score (BMIz) according to the Centers for Disease Control and Prevention growth charts.15
Questionnaires
Standardized questionnaires were used to collect self-reported data including race/ethnicity, highest level of parental education, duration of type 1 diabetes, insulin delivery method (pump versus multiple daily injections (MDI)), and previous CGM use. Self-reported race and ethnicity was classified as non-Hispanic white, non-Hispanic Black, Black, and other including Asian/Pacific Islander, Native American, or unknown. Motivation and Intention were measured by a validated questionnaire adapted for relevance to type 1 diabetes self-management.16,17 The Social Problem Solving Inventory – Revised: Short (SPSI-R:S) was used to assess adolescents’ cognitive, affective, and behavioral abilities to resolve problems in everyday living.18 Diabetes adherence over the past 3 months was measured with the Diabetes Self-Management Profile – Self Report (DSMP-SR).19. Depressive symptoms were assessed using the Centers for Epidemiologic Study – Depression Scale (CES-D).20 Health-related quality of life was assessed with the Pediatric Quality of Life Inventory™ – Generic Core Scales (PedsQL™ Generic).21 Fear of hypoglycemia was assessed by the Hypoglycemia Fear Survey (HFS) 22. Adolescent-reported diabetes-related family conflict was measured with the Diabetes Family Conflict Scale (DFCS).23
Statistical Analysis
CGM Data Selection of Variables and Pre-processing
All CGM-variables were calculated for the 7-day wear time and were stratified by day (6:00 AM – 11:59 PM) and night (12:00 AM – 5:59 AM).10 First, a subset of eight CGM features recommended by the ATTD Congress as key metrics to assess glycemic control, reported by day and night, were selected for a total of sixteen variables (see Supplementary Material; Section 1).10 The justification for using CGM measures stratified by time block was two-fold. First, from a clinical and behavioral perspective, patterns in dysglycemia in the daytime versus overnight represent distinct phenomena and may carry specific implications for future intervention. For example, frequent hypoglycemia overnight is a distinct clinical issue from frequent hypoglycemia during the daytime, particularly in the youth and adolescent age range, and may be associated with different risk factors. In addition, CGM metrics by day and night were not found to be highly colinear from a statistical perspective. The variables were pruned to remove highly correlated variables, biological redundancy, and degrees of freedom (Supplementary Figure S1).24 The remaining eight CGM input metrics were selected to comprehensively characterize features of dysglycemia in the day and nighttime: area-over-curve (AOC) of hypoglycemia (level 1; 3.9 mmol/L (70 mg/dL)), incidence of hypoglycemia (level 1; 3.9 mmol/L (70 mg/dL)) lasting 15 minutes or longer, area-under-curve (AUC) of hyperglycemia range (level 2; 13.9 mmol/L (250 mg/dL)), and glycemic variability as coefficient of variation (CV) (Supplementary Table S1). Of note, time in range was not included due to multicollinearity with the AUC 250 mg/dL metric (r= −0.80, p<0.0001). All variables were left continuous and standardized to be expressed on the same scale. To facilitate clinical interpretation, clusters were also characterized by percent of time spent in hypoglycemic (<3.9 mmol/L (70 mg/dL)) and hyperglycemic (13.9 mmol/L (250 mg/dL)) ranges, using the same threshold as the AOC and AUC measures, as well as time in range (3.9-10 mmol/L (70-180 mg/dL)).
Clustering Methods
The selection of SOM as a clustering algorithm and an in-depth description of the methods are deferred to the Supplementary Material; Section 2. Briefly, the SOM is a neural network11 that serves as a model-based clustering method (Supplementary Figure S2).24,25 The a priori justification for selecting a neural network-based clustering approach was that it does not rely on strong assumptions about the underlying data such as the distributional assumption of multivariate normality or symmetry.12 For measures of hypoglycemia and hyperglycemia, some individuals never experienced time below or above the threshold, resulting in severely skewed distributions resistant to transformation. The ability of the SOM to accommodate skewed input data12 and capture information in the tails of the distribution was considered critical to understanding the range of dysglycemia in the sample. Finally, SOMs have strong visualization attributes to understanding complex, multivariate relationships and improve the validity of unsupervised learning.25,26
FLEX participants were mapped based on their eight CGM measures to a 5×5 square grid SOM with a Gaussian neighborhood function using the Package ‘SOMBrero’ in R version 3.4.2.27 The dimensions of the SOM were selected based on the total sample size 24. 1000 iterations (approximately 4.3 cycles through the full data) were run to ensure the shape of the grid stabilized. Input data was randomized to produce a more reliable neighborhood structure. Additional analyses observed stability of the map across testing and training partitions. The final map was run on the full dataset to maximize statistical power.
The SOM was randomly initialized and re-run 10 times on the full data to check for consistency in parameters and quality criteria (see Supplementary Table S2). The best out of 10 maps were selected based on the lowest quantization error, a measure of the average Euclidian distance between a participant’s CGM measures and the codebook vector of their assigned unit (Supplementary Material; Section 3). A hierarchical clustering algorithm was applied to the codebook vectors of the final map units using the function superclass in the SOMbrero package.28 The NbClust package in R guided the selection of the final number of clusters, with minimum and maximum number of clusters set to 1 and 10, respectively.29 Clusters from the SOM were validated for internal validity, stability, and fidelity to the original data (Supplementary Material; Section 4.)
Baseline Characterization and Associations with Longitudinal Clinical Outcomes
The baseline correlates of each cluster were summarized using descriptive statistics. Skewed variables were assessed using non-parametric tests. Overall-tests of difference were carried out using ANOVA and chi-squared tests or Kruskal-Wallis and Fisher’s exact tests, where appropriate. Pairwise comparisons were performed via unpaired t-tests or Dunn’s test. To discern the significance of Dysglycemia Clusters versus subgroups defined by HbA1c, FLEX participants were also stratified by baseline HbA1c: (≤ or >75 mmol/mol (8.0%)) and described in terms of their baseline characteristics. Significance differences across baseline HbA1c groups were tested using chi-squared tests and unpaired t-tests.
Mixed effect regression analysis was used to determine whether observed dysglycemia clusters show differential changes in HbA1c over 18-months. A main effect was fit for visit and cluster and a visit*cluster interaction term. Participants were treated as random effects to take into account the repeated measures. All models were adjusted for randomization status and site. Post-hoc comparisons by cluster were performed within each mixed model analysis and the effects were examined at each longitudinal timepoint in the FLEX study. Descriptive statistics and multilevel modeling (PROC MIXED) were conducted in SAS 9.4 (SAS Institute, Cary, NC).
Additional Statistical Considerations
SOM has been used previously to cluster small datasets, outperforming k-means on data of similar dimensions to the FLEX data 30. P-values were evaluated at the 0.05 significance level and were not adjusted for multiple comparisons in the exploratory analysis.
Results
The final study sample included 234 adolescents with type 1 diabetes. Participants were 76.1% non-Hispanic white and 50.0% female with mean age 14.8±1.1 years and mean diabetes duration was 6.4±3.7 years (Table 1). Mean HbA1c was 81±13 mmol/mol (9.6±1.2%). Participants had blood glucose readings for a median of 160.0 hours (IQR 24.8) or approximately 6.7 days.
Table 1.
Baseline Characteristics of FLEX Participants Overall and by Dysglycemia Cluster
| Dysglycemia Cluster | |||||
|---|---|---|---|---|---|
| Baseline characteristics, n (%) or mean (SD) |
All (n=234) | Dysglycemia Cluster 1 (n=141, 60.3%) |
Dysglycemia Cluster 2 (n=53, 22.7%) |
Dysglycemia Cluster 3 (n=40, 17.1%) |
p-value |
| Sociodemographic Characteristics | |||||
| Age (years) | 14.8 (1.1) | 14.8 (1.1) | 14.9 (1.2) | 15.0 (1.2) | 0.60 |
| Female sex | 117 (50.0) | 68 (48.2) | 30 (56.6) | 19 (47.5) | 0.55 |
| Non-Hispanic White† | 178 (76.1) | 104 (73.8) | 42 (79.3) | 32 (80.0) | 0.59 |
| Parental Education | 0.16 | ||||
| Graduate degree | 43 (18.5) | 22 (15.8) | 10 (18.9) | 11 (27.5) | |
| College Degree | 67 (20.9) | 54 (38.9) | 21 (39.6) | 21 (52.5) | |
| Some College | 67 (28.9) | 44 (31.7) | 17 (32.1) | 6 (15.0) | |
| High School or less | 26 (11.2) | 19 (13.7) | 5 (9.4) | 2 (5.0) | |
| Private Health Insurance | 164 (70.1) | 105 (74.5) | 35 (66.0) | 24 (60.0) | 0.16 |
| Single adult home | 30 (13.1) | 17 (12.4) | 8 (15.1) | 5 (12.8) | 0.88 |
| Clinical Characteristics | |||||
| Duration of diabetes (years) | 6.4 (3.7) | 6.5 (3.8) | 6.4 (3.5) | 6.3 (3.8) | 0.96 |
| HbA1c (mmol/mol) | 81 (5) | 85 (14) | 81 (11) | 72 (9)** | <0.001* |
| HbA1c (%) | 9.6 (1.2) | 9.9 (1.3) | 9.6 (1.0) | 8.7 (0.8)** | <0.001* |
| HbA1c above 9.0% [75 mmol/mol] | 156 (66.7) | 104 (73.8) | 38 (71.7) | 14 (35.0)** | <0.001* |
| Insulin Regimen | 0.64 | ||||
| Multiple daily injection | 68 (29.2) | 38 (27.1) | 18 (34.0) | 12 (30.0) | |
| Pump | 165 (70.8) | 102 (72.9) | 35 (66.0) | 28 (70.0) | |
| Insulin Dose, total (units/kg) | 0.98 (0.33) | 1.01 (0.36) | 0.92 (0.24) | 0.95 (0.35) | 0.23 |
| Average frequency of self-monitoring blood glucose, daily | 2.2 (0.8) | 2.1 (0.8) | 2.3 (0.9) | 2.1 (0.7) | 0.47 |
| BMI z-score | 0.71 (0.91) | 0.70 (0.92) | 0.78 (0.88) | 0.71 (0.95) | 0.86 |
| Weight Status | 0.94 | ||||
| Under- or normal weight | 143 (61.1) | 88 (62.4) | 30 (56.6) | 25 (62.5) | |
| Overweight | 56 (23.9) | 32 (22.7) | 14 (26.4) | 10 (25.0) | |
| Obese | 35 (15.0) | 21 (14.9) | 9 (17.0) | 5 (12.5) | |
| Psychosocial Characteristics | |||||
| Motivation‡ | 7.6 (1.6) | 7.7 (1.4) | 7.7 (1.7) | 7.5 (1.8) | 0.76 |
| Intention‡ | 9.1 (1.0) | 9.2 (0.9) | 8.9 (1.1) | 8.9 (1.1) | 0.22 |
| Problem solving§ | 105.6 (13.0) | 106.2 (12.5) | 105.5 (14.3) | 103.5 (13.1) | 0.50 |
| Adherence to Diabetes self-management∥ | 55.2 (11.6) | 55.5 (11.9) | 53.3 (9.7) | 56.7 (12.8) | 0.33 |
| Depression symptoms¶ | 9.1 (8.4) | 8.6 (7.6) | 9.8 (10.0) | 10.1 (8.7) | 0.47 |
| Quality of life# | 81.0 (12.4) | 81.7 (12.0) | 79 (12.7) | 80.6 (13.3) | 0.50 |
| Fear of hypoglycemia†† | |||||
| Maintain High BG | 1.2 (0.9) | 1.2 (0.9) | 1.2 (0.8) | 1.2 (0.8) | 0.99 |
| Helplessness/Worry | 1.1 (0.6) | 1.1 (0.6) | 1.1 (0.7) | 1.1 (0.4) | 0.81 |
| Worry about negative social consequences | 1.1 (0.7) | 1.1 (0.8) | 1.1 (0.7) | 1.1 (0.6) | 0.93 |
| Diabetes Family Conflict‡‡ | 1.4 (0.3) | 1.4 (0.4) | 1.2 (0.3) | 1.4 (0.3) | 0.57 |
Abbreviations: SD – standard deviation. CGM – continuous glucose monitoring. HbA1c – hemoglobin A1c. BMI z-score – body mass index z-score.
For dyslgycemia clusters, p-values are from Chi squared or fisher exact test for categorical variables, and ANOVA or Kruskal-Wallis Test for continuous variables. For baseline HbA1c, p-values are from unpaired t-tests.
Denotes significance test of overall difference (p<0.05).
Denotes significant difference in unpaired, pairwise t-tests (p<0.05), compared to Dysglycemia Cluster 1.
Non-Hispanic white race/ethnicity versus non-Hispanic Black, Black, and other including Asian/Pacific Islander, Native American, or unknown.
Motivation and Intention were measured by a validated questionnaire adapted for relevance to type 1 diabetes self-management.
The Social Problem Solving Inventory – Revised: Short (SPSI-R:S); higher score indicates higher ability to resolve problems in everyday living.
Diabetes Self-Management Profile – Self Report (DSMP-SR); higher score indicates higher adherence.
Centers for Epidemiologic Study – Depression Scale (CES-D); higher score indicates increased depressive symptoms.
Pediatric Quality of Life Inventory™ – Generic Core Scales; higher score indicates higher quality of life
Hypoglycemia Fear Survey (HFS); fear of hypoglycemia measured in three domains: behaviors used to keep blood glucose high to prevent hypoglycemia (Maintain High BG), worry about helplessness (Worry/Helplessness), and worry about social consequences associated with hypoglycemia (Worry/Social Consequences); higher scores indicate greater fear.
Diabetes Family Conflict Scale (DFCS); higher score indicates higher conflict.
Figure 1A visualizes the 5×5 SOM grid, where individuals with similar CGM measures are assigned to proximal map units. Further visualizations are available in Supplementary Figure S3. Three clusters were identified, capturing areas of the map that were similar to each other with regards to the 8 CGM metrics (Figure 1B). All CGM metrics showed significantly different means and medians across clusters (p<0.001) (Table 2, Figure 1C). Cluster 1 comprised 141 individuals (60.3%) and showed severe daytime hyperglycemia with low exposure to and incidence of hypoglycemia relative to other clusters. Cluster 1 also showed the lowest glycemic variability (mean (SD) daytime and nightime CV: 35.5% (6.4%) and 35.7% (10.7%), respectively). Cluster 2 comprised 53 indiviudals (22.7%) and showed severe hyperglycemia, particularly overnight, with moderate hypoglycemia (median (IQR) daytime episodes: 4 (3)) and moderate variablity. Cluster 3 comprised 40 individuals (17.1%) and showed moderate hyperglycemia with the highest measures of hypoglycemia exposure and incidence relative to the other clusters (median (IQR) daytime episodes over the 7 days: 8 (5.5)). This group also showed the highest glycemic variability in the daytime and overnight (mean daytime and nightime CV: 4.1% (7.0%) and 51.7% (12.9%), respectively).
Figure 1: Use of a Self-Organizing Map (SOM) trained by 7-day continuous glucose monitoring (CGM) data to identify Dysglycemia Clusters at baseline of the FLEX trial (n=234).
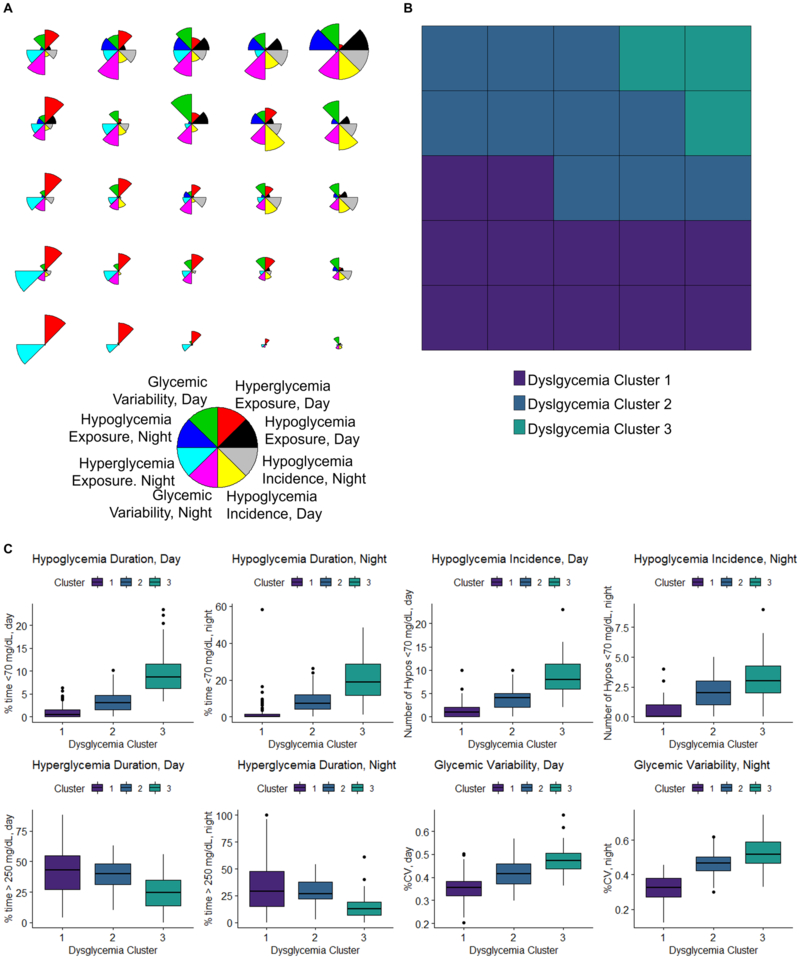
The clustering is carried out using a two-level approach, where the dataset is first clustered onto the units SOM and then the units SOM is clustered. A 5×5 SOM with 25 map units and a 3-cluster solution were selected. All CGM-variables were calculated for the 7-day wear time and were stratified by day (6:00 AM – 11:59 PM) and night (12:00 AM – 5:59 AM). Panel A: Radar plots showing the integrated CGM profile of each of the 25 units on the 5×5 SOM, as determined by the individuals assigned to that region. Each input CGM variable is represented by a different color in the radar. Input CGM variables were defined as follows: Hypoglycemia Exposure: area-over-the-curve of 3.9 mmol/L (70 mg/dL), Hypoglycemia Incidence: average number of hypoglycemic (<3.9 mmol/L (70 mg/dL)) episodes lasting 15 or more minutes, Hyperglycemia Exposure: area-under-the-curve of 13.9 mmol/L (250 mg/dL), and Glycemic Variability: %CV. Panel B: The SOM colored by Dysglycemia Cluster assignments. Each unit was assigned to a Dyslgycemia Cluster. Dysglycemia Cluster assignments (Cluster 1, Cluster 2, and Cluster 3) are shown by colored boxes. Panel C: CGM measures of hypoglycemia, hyperglycemia, and glycemic variability across the 3 Dysglycemia Clusters. To aid in clinical interpretation of hypoglycemia and hyperglycemia exposure, the percent of time are depicted in place of the area-over-the-curve and area-under-the-curve measures that were used to construct the SOM. Data represents 7-days of blinded CGM wear. All p<0.001. Hypoglycemia Exposure is depicted as percent of time <3.9 mmol/L (70 mg/dL). Hypoglycemia Incidence is depicted as average number of hypoglycemic (<3.9 mmol/L (70 mg/dL)) episodes lasting 15 or more minutes. Hyperglycemia Exposure is depicted as percent of time >13.9 mmol/L (250 mg/dL). Glycemic Variability is depicted as %CV. Abbreviations: CV – coefficient of variation.
Table 2.
Input CGM Metrics at Baseline, Overall and by Dysglycemia Cluster measured over 7 days
| CGM Metrics, mean (SD), or median (IQR) |
All (n=234) | Dysglycemia Cluster 1 (n=141, 60.3%) |
Dysglycemia Cluster 2 (n=53, 22.7%) |
Dysglycemia Cluster 3 (n=40, 17.1%) |
p-value |
|---|---|---|---|---|---|
| Hypoglycemia Exposure | |||||
| AOC 3.9 mmol/L (70 mg/dL), Day† | 0.15 (0.52) | 0.03 (0.14) | 0.39 (0.35)** | 1.2 (1.1)** | <0.0001* |
| AOC 3.9 mmol/L (70 mg/dL), Night† | 0.11 (1.31) | 0.00 (0.11) | 0.78 (1.47)** | 3.5 (4.0)** | <0.0001* |
| Percent of time below 3.9 mmol/L (70 mg/dL)‡, %, Day† | 1.5 (4.0) | 0.5 (1.5) | 3.0 (3.2)** | 8.7 (5.6)** | <0.0001* |
| Percent of time below 3.9 mmol/L (70 mg/dL)‡, %, Night† | 1.8 (8.5) | 0.0 (1.4) | 7.3 (7.7)** | 18.9 (17.2)** | <0.0001* |
| Hypoglycemia Incidence | |||||
| Episodes<3.9 mmol/L (70 mg/dL) for 15+ minutes, Day† | 2 (5) | 1 (2) | 4 (3)** | 8 (5.5)** | <0.0001* |
| Episodes<3.9 mmol/L (70 mg/dL) for 15+ minutes, Night† | 1 (2) | 0 (1) | 2 (2)** | 3 (2.5)** | <0.0001 |
| Hyperglycemia Exposure | |||||
| AUC 13.9 mmol/L (250 mg/dL), Day† | 26.9 (21.1) | 29.9 (27.4) | 29.5 (17.6) | 13.7 (17.5)** | <0.0001* |
| AUC 13.9 mmol/L (250 mg/dL), Night† | 13.0 (21.1) | 13.3 (21.8) | 17.4 (16.6) | 4.5 (11.6)** | <0.0001* |
| Percent of time above 13.9 mmol/L (250 mg/dL)‡, Day† | 38.1 (25.8) | 43.0 (27.9) | 39.5 (17.2) | 24.3 (20.9)** | <0.0001* |
| Percent of time above 13.9 mmol/L (250 mg/dL)‡, Night† | 23.8 (28.0) | 29.2 (32.9) | 26.7 (15.7) | 12.5 (13.1)** | <0.0001* |
| Glycemic Variability | |||||
| Coefficient of Variation, %, Day | 39.8 (7.4) | 35.5 (6.4) | 41.4 (8.7)** | 47.1 (7.0)** | <0.0001* |
| Coefficient of Variation %, Night | 38.8 (11.9) | 32.7 (10.7) | 46.6 (7.8)** | 51.7 (12.9)** | <0.0001* |
| Time in Rangea | |||||
| Percent of time 3.9-10 mmol/L (70-180 mg/dL)), day | 32.0 (20.7) | 30.4 (13.9) | 31.9 (13.8) | 43.7 (17.2)** | <0.0001* |
| Percent of time 3.9-10 mmol/L (70-180 mg/dL)), night | 38.3 (26.6) | 36.1 (20.9) | 39.5 (20.9) | 47.5 (15.9)** | 0.0014* |
Abbreviations: SD- standard deviation. IQR- Interquartile range. AOC – area over the curve. AUC - area under the curve.
Denotes significance test of overall difference from ANOVA or Kruskal-Wallis test (p<0.05).
Denotes significant difference in unpaired, pairwise t-test or Dunn’s test (p<0.05), compared to Dysglycemia Cluster 1.
Data were right-skewed and are reported as median (interquartile range). P-value from Kruskal-Wallis test. There were no missing data.
To aid in clinical interpretation of hypoglycemia and hyperglycemia exposure, the percent of time is provided to address the same threshold as the area-over-the-curve and area-under-the-curve measures. For additional clinical context, time in range is provided but was not used as an input variable for CGM clusters.
Mean baseline HbA1c was highest in Cluster 1 (85±14 mmol/mol (9.9±1.1%)) and lowest in Cluster 3 (72±9 mmol/mol (8.7%±0.8%)). In pairwise comparisons, Cluster 3 showed significant differences from Clusters 1 and 2 (p<0.001), but Clusters 1 and 2 did not show significant differences from each other (p=0.07). No other baseline characteristics were significantly different across clusters. There were differences in the correlates of subgroups defined by baseline HbA1c. Compared to participants with HbA1c ≤ 75 mmol/mol (9.0%) at baseline, participants with a high HbA1c showed lower insulin pump use (p=0.2), greater insulin doses (p=0.03), a higher frequency of blood glucose monitoring (p=0.004), lower motivation (p=0.03), and adherence to diabetes self-management (p=0.003).
HbA1c measures over 18-months were significantly different across clusters, adjusted for study site and randomization (p-for-interaction=0.006; Figure 2, Supplementary Table S5). Dysglycemia Clusters 1 and 2 showed stable mean HbA1c, while Dysglycemia Cluster 3 showed significant increases over the 18-month study period (mean baseline HbA1c: 71 mmol/mol (8.7%); mean HbA1c at 18-month visit: 81 mmol/mol (9.6%). There were no signifiant differences in mean HbA1c level at the 18-month visit (p=0.71). CGM metrics at the 18-month visit for each cluster are depicted in Supplementary Table S6.
Figure 2: Longitudinal Hemoglobin A1c (HbA1c) outcomes of FLEX Participants by Dysglycemia Cluster, adjusted for FLEX study site and randomization assignment (p-for-interaction = 0.006).
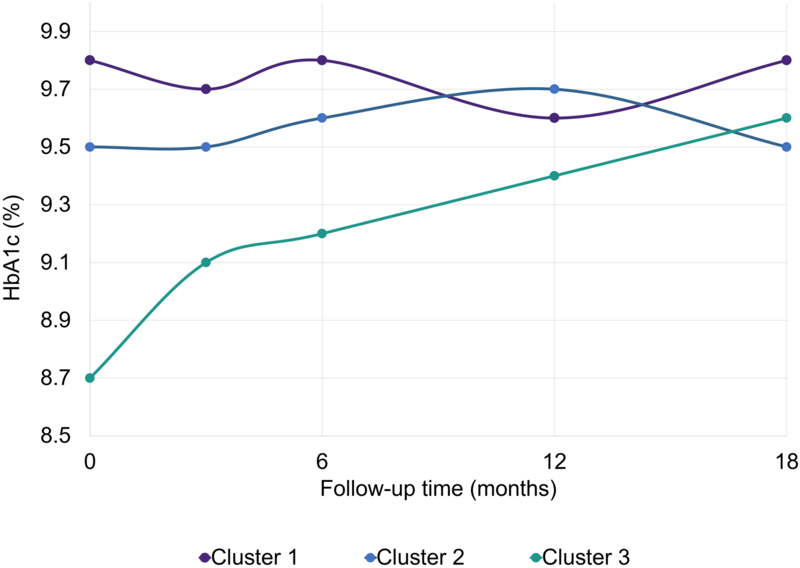
The p-for-interaction represents Type 3 Test of Fixed Effects for time point × cluster interaction term. Missing data— Baseline: n=0; 3-month HbA1c: n= 10; 6-month HbA1c: n= 14; 12-month HbA1c: n=20; 18-month HbA1c: n=16. p-values for each cluster*timepoint estimate (Cluster 1 vs Cluster 2, Cluster 1 vs Cluster 3): Baseline (p=0.89, p<0.0001); 3-month (p=0.23, p=0.08); 6-month (p=0.49, p=0.14), 18-month (p=0.03, p=0.85). Abbreviations: HbA1c – hemoglobin A1c.
Discussion
Using 7-day blinded CGM data from 234 adolescents with type 1 diabetes and elevated HbA1c, we identified three distinct, clinically-meaningful clusters sharing phenotypes defined by different exposure to and incidence of hypoglycemia, exposure to hyperglycemia, and glycemic variability. All eight CGM metrics were significantly different across clusters and can thus considered to be relevant for the clustering definition. Subgroups showed differences in baseline and longitudinal HbA1c. However, there were no other significant differences in baseline characteristics according to dysglycemia cluster. These results reinforce the concept that adolescents with type 1 diabetes and elevated HbA1c do not show homogenous patterns in CGM-measures of blood glucose dynamics; this analytic approach can help refine understanding of dysglycemia patterns to better identify interventions. Interestingly, different patterns in dysglycemia are not explained by the individual sociodemographic, clinical, or psychosocial characteristics that typically drive treatment recommendations with regards to HbA1c.
To our knowledge, there is limited data available for comparison because the majority of existing CGM data collected in comparable age ranges are from adolescents with lower HbA1c levels.31 Patterns in dysglycemia across clusters are consistent with other CGM studies suggesting that a positive association between glycemic variability and the risk for hypoglycemia.32
A previous cluster analysis using 3-days of data from self-monitoring blood glucose values provided evidence for distinct glycemic profiles among a small sample of adults with type 1 diabetes.33 Although all FLEX participants had elevated HbA1c as per inclusion criteria, we found similar evidence for the existence of subgroups typified by specific blood glucose dynamics. The striking differences in CGM measures suggest that these distinct ‘phenotypes’ are comprised of adolescents who struggle with different aspects of their blood glucose control. For example, individuals in Cluster 1 were typified by hyperglycemia with fewer episodes of hypoglycemia and less pronounced variability, especially overnight, while individuals in Cluster 3 experienced less hyperglycemia but a median of 8 episodes of hypoglycemia per week with severe variability in the daytime and nighttime (mean CV: 47% and 52%, respectively). Measures of variability in the latter group greatly exceeded the CV threshold of 36% that has previously been proposed to indicate ‘unstable’ glycemia and increased risk for hypoglycemia.32
In the analysis to identify potential patient-related drivers of the clusters, there were no significant differences in the sociodemographic, clinical, or psychosocial measures across Dysglycemia Clusters. One possible reason for the lack of statistically significant correlates is the small sample size which may limit statistical power. We explored the clinical utility of a 2-cluster solution to detect differences but failed to identify significant correlates to distinguish the two subgroups (Supplementary Material, Section 6, Tables S7-S9).
Another interpretation of the data is that a broad range of demographic, clinical, or psychosocial characteristics do not drive the specific blood glucose issues that may be challenging overall glycemic control among adolescents with type 1 diabetes and elevated HbA1c. It is particularly interesting that the risk factors of poor glycemic control as it is measured by HbA1c do not appear to be risk factors for poor glycemic control as it manifests as membership in a Dysglycemia Cluster. Within the FLEX sample, participants with a high baseline HbA1c showed lower insulin pump use, greater insulin doses, a higher frequency of blood glucose monitoring, and lower motivation and adherence to diabetes self-management; none of these associations emerged as correlates of Cluster membership. Other well-studied associations of suboptimal HbA1c measures in this age range were not replicated as differences across subgroups, including nonwhite race,34 lower measures of socioeconomic position,35 and poorer psychosocial well-being.35 More work is needed to understand the drivers of dysglycemia phenotypes, including significant behavioral mediators or patterns that can be addressed clinically such as omitted or ill-timed boluses with regards to meal initiation.
There are several points of clinical relevance for the findings. Because the extraction of key clinical metrics from longitudinal CGM data emulates the process of patient care where these measures are used to identify specific issues,2 this study offers proof-of-principle for how CGM data may be consolidated and used to identify the subgroups of patients within a specific population of individuals with T1D that are be recognizable to care providers as intuitive clinical phenotypes. Such subgroup identification compliments the use of CGM data in individuals to measure time in range5,6 or average glucose exposure over a shorter period of time.7 CGM-derived subgroups may also act as prognostic phenotypes with different longitudinal trends in key clinical outcomes such as HbA1c. With increasing availability of CGM data as well as documentation of treatment regime and other outcomes in electronic health records, this work may in the future offer an emerging platform to pool data across one or more clinics to test how CGM clusters function as predictive or prescriptive phenotypes for treatment recommendations.
Outside of the clinic, the results may be used towards the development of effective interventions for this at-risk and challenging adolescent population.8,13 Although main analysis of the FLEX intervention did not show improvements in HbA1c at 18-months,13 a three-way interaction term between cluster, FLEX intervention randomization assignment, and timepoint was tested in exploratory longitudinal analyses; it was not statistically significant. It is possible that approaches to diabetes management in the heterogenous adolescent population are maximally effective as a set of interventions tailored to specific issues of dysglycemia, which can then be targeted towards phenotypes that are expected to maximally benefit. For example, Cluster 3 was the only subgroup to show an increase in HbA1c over 18-months; this subgroup also had the highest hypoglycemia and variability at baseline and may represent a previously-proposed sequela of recurrent hypoglycemia and overcorrection that leads to worsened glycemic control over time.36 Therefore, this group may benefit from specific efforts addressing frequent hypoglycemia and its overcorrection early in adolescence. By contrast, interventions focused on increasing insulin doses may be salient for Cluster 1, who spends most of the time in hyperglycemic ranges with low variability, rendering hypoglycemia counseling less immediately relevant.
A further aspect of clinical significance is the presumed differential risk for acute and chronic diabetes complications across clusters. Aside from well-established risk associated with hyperglycemia,9 the high degree of glycemic variability noted in Clusters 2 and 3 may confer additional, independent risk for micro- and macrovascular complications, including cardiovascular disease.3,4 Cluster 3’s pattern of hypoglycemia may contribute to the development of defective symptomatic responses, positioning these individuals at an increased risk for severe hypoglycemia.37
The analysis has several limitations. Self-organizing maps are difficult to validate. The SOM analysis was repeated to check for consistency, and resulting clusters were assessed for stability and validity against other clustering algorithms on the raw data. Clusters showed stability in cross-validation studies with preservation of patterns in dysglycemia (Supplementary Table S3, Supplementary Figure S4). The results may be affected by the selection of the CGM metrics used to train the SOM. We explored dysglycemia clustering derived from a set of 16- and 24- CGM metrics and found that the recommended number of clusters and clustering solutions were not significantly impacted by additional CGM metrics, although the projection quality of the SOM was reduced (Supplementary Table S4). In addition, the SOM clusters were compared to clusters derived directly from the data.25 Although the assumptions of the hierarchical clustering algorithm are not met using the input data, we found similar clusters with both algorithms (Supplementary Figure S5, Supplementary Figure S6). Together, the results suggest that the SOM clusters demonstrate internal validity, stability, and accurately represented clustering structure present in the raw data.
Additional limitations include availability of CGM data spanning 7 days versus the 14 days recommended for optimal data analysis;10 7 days of data may not be representative of long-term deglycation. The small sample size may be underpowered to detect differences between clusters. The inclusion and exclusion criteria of the FLEX trial limit generalizability, particularly for adolescents with lower HbA1c levels. In the present analysis, we constrained CGM metrics to be consistent with standardized practices of CGM reporting.10 However, additional measures of glycemic variability such as mean amplitude of glycemic excursion (MAGE) and mean of daily differences (MODD) might help to further delineate subgroups. Future work may also explore how deep learning can be used to extract hidden layers of the CGM data and explore clusters based on those hidden layers.38
Despite the aforementioned limitations, here, we elucidated dysglycemia phenotypes among a sample of adolescents with type 1 diabetes and suboptimal glycemic control, a population with great need for future interventions in which CGM data has only recently become available to help.8,13 CGM metrics were selected to be consistent with best research practices,10 and a clustering algorithm was selected to leverage information from the tails of the distribution to understand underlying cluster structure in the data.12 The analytic approach is distinct from but compliments ongoing work to model CGM data via temporal analysis with regards to the shape of the curve/aspects of glycemic variability,39,40 and it may be applied to CGM data from variable durations of wear-time. In full, the study represents a novel use of CGM data towards broadening the concept of glycemic control from HbA1c to understanding a multifaceted profile that includes glycemic excursions and overall variability. Understanding of these subgroups is crucial to pave the way for targeted interventions to optimize dysglycemia and the associated clinical outcomes in type 1 diabetes.
In conclusion, among adolescents with type 1 diabetes and elevated HbA1c, CGM data may be pooled and analyzed to uncover subgroups displaying distinct dysglycemia phenotypes, for which glycemic control is challenged by different patterns in hypoglycemia, hyperglycemia, and glycemic variability. More work is needed to understand the risk factors for glycemic control as it is represented from CGM data by dysglycemia phenotypes for future development of phenotype-specific interventions to improve glycemic control.
Supplementary Material
Acknowledgements
The FLEX Study is indebted to the many youth and their families whose participation made this study possible. This study was supported by NIH/ NIDDK (1UC4DK101132) and Helmsley Charitable Trust. ARK is supported by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number F30DK113728. DMM is supported by P30DK116074. MRK, CN, and JBB are supported by funding from NC Tracs (the CTSA at UNC): UL1TR002489.
Role of the Funding Source: The sponsor of the FLEX study was represented on the FLEX study steering committee (Christine M. Hunter, PhD, NIDDK) and as part of this committee contributed to the collaborative development of the FLEX study design and oversight of its execution. The sponsor was not directly involved in this secondary analysis of the FLEX data.
Footnotes
Dualities of Interest: DMM has consulted for Abbott, the Helmsley Charitable Trust, Sanofi, and Eli Lilly and has served on an advisory board for Insulet. EJM-D has consulted for Helmsley Charitable Trust. All other authors declare no conflict of interest.
Data Availability: The data that support the findings of this study are available from the FLEX Study Executive Committee, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the FLEX Study Executive Committee.
References
- 1.Wright LA-C, Hirsch IB. Metrics beyond hemoglobin A1c in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes technology & therapeutics. 2017;19(S2):S-16–S-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? Journal of diabetes science and technology. 2008;2(6):1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilpatrick ES, Rigby AS, Atkin SL. HbA1c variability and the risk of microvascular complications in type 1 diabetes: data from the DCCT. Diabetes care. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigersky RA, McMahon C. The Relationship of Hemoglobin A1C to Time-in-Range in Patients with Diabetes. Diabetes technology & therapeutics. 2018. [DOI] [PubMed] [Google Scholar]
- 6.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes care. 2018;41(11):2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes technology & therapeutics. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMeglio LA, Acerini CL, Codner E, et al. Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatric diabetes. 2018. [DOI] [PubMed] [Google Scholar]
- 10.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohonen T The self-organizing map. Proceedings of the IEEE. 1990;78(9):1464–1480. [Google Scholar]
- 12.Kiang MY, Kumar A. An evaluation of self-organizing map networks as a robust alternative to factor analysis in data mining applications. Information Systems Research. 2001;12(2):177–194. [Google Scholar]
- 13.Mayer-Davis EJ, Maahs DM, Seid M, et al. Efficacy of the Flexible Lifestyles Empowering Change intervention on metabolic and psychosocial outcomes in adolescents with type 1 diabetes (FLEX): a randomised controlled trial. The Lancet Child & Adolescent Health. 2018;2(9):635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kichler JC, Seid M, Crandell J, et al. The Flexible Lifestyle Empowering Change (FLEX) intervention for self-management in adolescents with type 1 diabetes: Trial design and baseline characteristics. Contemporary clinical trials. 2018;66:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000(314):1–27. [PubMed] [Google Scholar]
- 16.Seid M, D’Amico EJ, Varni JW, et al. The In Vivo Adherence Intervention For at Risk Adolescents With Asthma: Report of a Randomized Pilot Trial. JPediatrPsychol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller WR, Johnson WR. A natural language screening measure for motivation to change. AddictBehav. 2008;33(9):1177–1182. [DOI] [PubMed] [Google Scholar]
- 18.D’Zurilla TJ, Nezu AM, Maydeu-Olivares A. Social problem-solving inventory--revised (SPSI-R). Multi-Health Systems; 2002. [Google Scholar]
- 19.Wysocki T, Buckloh LM, Antal H, Lochrie A, Taylor A. Validation of a self-report version of the diabetes self-management profile. PediatrDiabetes. 2012;13(5):438–443. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LD. The CES-D scale: a self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 21.Varni J, Seid M, Kurtin P. The PedsQL’4.0: Reliability and validity of the Pediatric Quality of Life Inventory 4.0 version. Med Care. 2001. [DOI] [PubMed] [Google Scholar]
- 22.Shepard JA, Vajda K, Nyer M, Clarke W, Gonder-Frederick L. Understanding the construct of fear of hypoglycemia in pediatric type 1 diabetes. Journal of pediatric psychology. 2014;39(10):1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised Diabetes Family Conflict Scale. Diabetes Care. 2007;30(7):1764–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottrell M, Olteanu M, Rossi F, Villa-Vialaneix N. Theoretical and applied aspects of the self-organizing maps Advances in Self-Organizing Maps and Learning Vector Quantization: Springer; 2016:3–26. [Google Scholar]
- 25.Vesanto J, Alhoniemi E. Clustering of the self-organizing map. IEEE Transactions on neural networks. 2000;11(3):586–600. [DOI] [PubMed] [Google Scholar]
- 26.Liao TW. Clustering of time series data—a survey. Pattern recognition. 2005;38(11):1857–1874. [Google Scholar]
- 27.Boelaert J, Bendhaiba L, Olteanu M, Villa-Vialaneix N. SOMbrero: an r package for numeric and non-numeric self-organizing maps Advances in Self-Organizing Maps and Learning Vector Quantization: Springer; 2014:219–228. [Google Scholar]
- 28.Olteanu M, Villa-Vialaneix N. Using SOMbrero for clustering and visualizing graphs. Journal de la Société Française de Statistique. 2015;156(3):95–119. [Google Scholar]
- 29.Charrad M, Ghazzali N, Boiteau V, Niknafs A, Charrad MM. Package ‘NbClust’. Journal of Statistical Software. 2014;61:1–36. [Google Scholar]
- 30.Bação F, Lobo V, Painho M. Self-organizing maps as substitutes for k-means clustering. Paper presented at: International Conference on Computational Science 2005. [Google Scholar]
- 31.DeSalvo DJ, Miller KM, Hermann JM, et al. Continuous Glucose Monitoring (CGM) and Glycemic Control Among Youth with Type 1 Diabetes (T1D): International comparison from the T1D Exchange and DPV Initiative. Pediatric Diabetes. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40(7):832–838. [DOI] [PubMed] [Google Scholar]
- 33.Takita M, Matsumoto S, Noguchi H, et al. Cluster analysis of self-monitoring blood glucose assessments in clinical islet cell transplantation for type 1 diabetes. Diabetes care. 2011;34(8):1799–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. The Journal of pediatrics. 2009;155(5):668–672. e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan K, Loar R, Anderson BJ, Heptulla RA. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. The Journal of pediatrics. 2006;149(4):526–531. [DOI] [PubMed] [Google Scholar]
- 36.Pinhas-Hamiel O, Hamiel U, Levy-Shraga Y. Eating disorders in adolescents with type 1 diabetes: Challenges in diagnosis and treatment. World J Diabetes. 2015;6(3):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cryer PE, Arbeláez AM. Hypoglycemia in diabetes. Textbook of Diabetes. 2017:513–533. [Google Scholar]
- 38.Yang B, Fu X, Sidiropoulos ND, Hong M. Towards k-means-friendly spaces: Simultaneous deep learning and clustering. arXiv preprint arXiv:161004794. 2016. [Google Scholar]
- 39.Hall H, Perelman D, Breschi A, et al. Glucotypes reveal new patterns of glucose dysregulation. PLoS biology. 2018;16(7):e2005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fico G, Hernández L, Cancela J, et al. Exploring the frequency domain of continuous glucose monitoring signals to improve characterization of glucose variability and of diabetic profiles. Journal of diabetes science and technology. 2017;11(4):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


