Abstract
Pentachlorophenol (PCP) and dichlorodiphenyltrichloroethane (DDT), are organochlorine environmental contaminants found in human blood at very significant levels (as high as 5 µM for PCP and 260 nM for DDT). Cancers of the blood (lymphoma and myeloma) and kidney as well as others have been associated with exposure to these contaminants. Interleukin 1 beta (IL-1β) is a pro-inflammatory cytokine and is involved in stimulating cell proliferation. High levels of IL-1β are associated with inflammatory diseases and tumor progression. Previous studies showed that PCP and DDT at certain concentrations were able to stimulate secretion of IL-1β. This study shows that the increased secretion of IL-1β seen with both contaminants is due to compound-induced increases in production of this cytokine. Increased production began within 6 h of exposure to PCP and continued to increase out to 24 h. DDT-induced stimulation of IL-1β appeared to be maximal after 6 h of exposure and then diminished by 24 h. The increases seen in IL-1β production stimulated by PCP appear to be at least partially due to compound-induced increases in IL-1β mRNA. Although DDT caused increased production of IL-1β, it did not appear to cause consistent increases in its mRNA. PCP-and DDT-induced increases in IL-1β production were dependent primarily on the p38 MAPK pathway. These results indicate that both PCP and DDT are able to increase IL1-β production in a p38 MAPK dependent manner, which may have the potential to influence chronic inflammation.
Keywords: Immune cells, Pentachlorophenol, Dichlorodiphenyltrichloroethane, Interleukin 1β, MAP kinases, mRNA
SHORT ABSTRACT
Production of IL-1β stimulated by PCP began within 6 h of exposure and continued to increase out to 24 h and DDT-induced stimulation appeared to be maximal after 6 h of exposure. Increases in IL-1β production by PCP are partially due to compound-induced increases in IL-1β mRNA. PCP-and DDT-induced increases in IL-1β production were dependent primarily on the p38 MAPK pathway. These results indicate that PCP and DDT increase IL1-β production and have the potential to influence chronic inflammation.
INTRODUCTION
Interleukin 1 beta (IL-1β) is a pro-inflammatory cytokine that has the ability to influence numerous cell types and contributes to several pathologies. Its roles include recruiting adaptive immune cells such as neutrophils, assisting with tissue repair, and stimulating the production of other cytokines. (Sahoo et al., 2011; Cooper et al., 2001). IL-1β plays a role in regulating local and systematic inflammatory responses. High levels of IL-1β can influence tumor growth by causing chronic inflammation. (Landskron et al., 2014). The presence of high IL-1β levels in the body without an infection present are connected to cardiovascular disease, systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis and multiple sclerosis to name a few (Dinarello, 1996; Braddock and Quinn, 2004; Dinarello, 2004). Several types of immune cells (Monocytes, T lymphocytes and NK cells) produce IL-1β and agents that alter their ability to synthesize IL-1β could lead to inadequate immune responsiveness or chronic inflammation. (Braddock and Quinn, 2004; Dinarello, 2004; Shi & Palmer, 2011).
Pentachlorophenol (PCP) is a chlorinated aromatic compound, it has been found in herbicides, insecticides, fungicides, and antifouling paint (Cirelli, 1978). PCP is use as a preservative to treat wooden utility poles and log cabins (Cline, et.al, 1989). Previous studies found that sawmill workers that were exposed to chlorophenols, as well as some of their children, developed leukemia, lymphoma and brain cancer (Cooper and Jones, 2008; Demers et al., 2006). Individuals can be exposed to PCP by absorption through skin, inhaling, and consumption of contaminated food or water. It has been reported in sediment and mussel tissues from freshwater lakes (Brown et al., 2005). PCP was found in human blood with serum levels ranging from 0.26 −5 µM in individuals who lived in PCP-treated log homes (Cline et al., 1989) and 0.15 µM in individuals with no known exposures (Cline et al., 1989; Uhl et al., 1986). Another organochlorine contaminant, 4, 4′-Dichlorodiphenyltrichloroethane (4, 4′-DDT), is used as a pesticide to kill insects, mainly mosquitoes. Although banned in the United States it is still used in developing countries to control insects that spread diseases such as malaria (Rengam 2013; Davis, 2006; WHO 2006). The lipophilic character of DDT leads to bioaccumulation and biomagnification in the food chain leading to high accumulation in human tissue (Koepke et al.,2004; Kodavanti and Loganathan, 2017). Like PCP, DDT has been found in human bodily fluid (breast milk, and plasma or serum) and has been detected at measurable levels in blood samples within the human population. DDT exposure has also been associated with a number of cancers (Eskenazi, et al., 2009; Harada et al., 2016).
Previous studies show PCP having the ability to increase the secretion of IL-1β from peripheral blood lymphocytes and monocytes at concentrations that have been measured in human blood. This PCP-induced increase in IL-1β secretion from immune cells utilized the ERK 1/2 and p38 MAPK signaling pathways (Martin and Whalen, 2017). As seen with PCP, exposures to DDT also induced increased secretion of IL-1β from human peripheral blood immune cells (Martin and Whalen, 2017).
It is important to determine if PCP and DDT are able to increase the secretion of IL-1β by simply causing the cell to release a store of already existing cytokine or if they are stimulating immune cells to increase their production of this potent pro-inflammatory molecule. This study examines both intracellular and secreted levels of IL-1β from the same cells in response to exposures to PCP and DDT. If secretion is increased and intracellular levels remain unchanged or increase then increased production of IL-β has occurred. Concentrations of PCP and DDT that increased levels in cytokine production were further investigated to discover whether this was caused by increases in the mRNA for IL-1β. Additionally, signaling pathways known to be involved in cellular production of each of the cytokines were investigated for potential roles in any PCP-induced or DDT-induced increases in IL-1β. These included, mitogen activated protein kinases (MAPKs) and p 38 protein kinase (Gaestel et al., 2009).
MATERIALS AND METHODS
Preparation of peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated from leukocyte filters (PALL-RCPL or FLEX) obtained from the Red Cross Blood Bank (Nashville, TN) as described in Meyer et al., 2005. Leukocytes were obtained from the filters by back-flushing the filters with an elution medium (sterile PBS containing 5 mM disodium EDTA and 2.5% [w/v] sucrose) and then collecting the eluent. The eluent was layered onto Lymphosep® (1.077g/mL) and centrifuged at 1200g for 30 min. Following centrifuging and washing, the cells were layered on bovine calf serum for platelet removal. The cells were then suspended in RPMI-1640 complete medium, which consisted of RPMI-1640 supplemented with 10% heat-inactivated BCS, 2 mM L-glutamine and 50 U penicillin G with 50 μg streptomycin/mL.
Chemical Preparation
DDT and PCP were purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions were prepared as 100 mM solutions in Dimethyl sulfoxide (DMSO). Desired concentrations were prepared of either DDT or PCP by dilution of the desired stock into cell culture media.
Inhibitor Preparation
Enzyme inhibitors were purchased from Fischer Scientific (Pittsburgh, PA). The stock solution for each inhibitor was a 50 mM solution in dimethyl sulfoxide (DMSO). MEK 1/2 pathway inhibitor (PD98059), p38 inhibitor (SB202190) were prepared by dilution of the stocks.
Cell lysis and collection of supernatants for production studies
PBMCs were treated with 5–0.05 µM PCP and 2.5–0.025 µM DDT or control for 10 min, 1 h, 6 h or 24 h. Following the treatments, the cells were centrifuged, supernatants collected, and the cell pellets lysed using 133 µL of lysis buffer (Active motif, Carlsbad, CA) per 3–4 million cells. The cell lysates were stored frozen at −80 o C up to the point when they were run on SDS-PAGE. All controls and PCP/DDT-exposed cells for a given experimental set-up were from an individual donor. Each of the experimental set-ups was repeated a minimum of four times using cells from different donors. For the pathway inhibitor experiments, PBMCs were treated with enzyme inhibitors 1h prior to adding PCP at concentrations of 5, 2.5, and 1 μM for 24 h or DDT at concentrations of 0.1, 0.05, and 0.025 μM for 6 h. After the cells were incubated, the cells were pelleted, and supernatants were obtained and stored at −80 o C until assaying for the IL-1β cytokine and the pellet was lysed for future analysis by western blot.
IL-1β Secretion Assay
Levels of secreted IL-1β were measured using the BD OptEIA™ Human IL-1β enzyme-linked immunosorbent assay (ELISA) kit (BD-Pharmingen, San Diego, CA). This kit is highly selective for IL-1β and does not cross react with other proteins including other cytokines. A 96-well micro well plate, designed for ELISA assays (Fisher, Pittsburgh, PA), was coated with a capture antibody for IL-1β that was diluted in coating buffer. The ELISA plate was incubated with the capture antibody overnight at 4 ˚C. After the incubation, the capture antibody was removed and blocking solution (PBS and bovine calf serum) was added to each well and incubated at room temperature for 1h. Following the blocking step, cell supernatants and IL-1β standards were added to the plate and incubated for 2 h at room temperature. Detection antibody linked to horseradish peroxidase (HRP) was then added followed by substrate. The reaction was stopped by addition of 1 M phosphoric acid and absorbance was measured at 450 nm on a Thermo Labsystems Multiskan MCC/340 plate reader (Fisher Scientific).
Western blot
Cell lysates were run on 10% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred to a PVDF (polyvinylidene difluoride) membrane. The PVDF was immunoblotted with specific primary antibodies: IL-1β (Cell Signaling Technology, Danvers, MA), and actin (Sigma). Antibodies were visualized using the UVP Imager. The density of each protein band was determined by densitometric analysis using the UVP image analysis software. The samples from all the experimental set-ups were run on a separate gel/blot. A given experimental set-up had its own internal control. Differences in protein expression were determined relative to the internal control, which provided quantitation by evaluating whether a given treatment altered these proteins relative to untreated cells. β-Actin levels were determined for each condition to verify that equal amounts of protein were loaded. In addition, the density of each protein band was normalized to β-actin to correct for minor differences in protein loading among the lanes.
RNA Isolation and RT-qPCR
PBMCs (2–4 million cells) were extracted with RNeasy Mini Kit (Qiagen, Venlo, Netherlands). RNA concentrations were measured with a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE). PCR primers were designed using Primer Express 2.0 (Applied Biosystems, Foster City, CA). All RT-qPCR assays were conducted using QuantiTect SYBR Green RT-PCR kit (Qiagen). Reactions were done in 20 µL containing 50 ng of total RNA and 0.4 µM of each primer. Thermal cycles contained one cycle of pre-incubation at 50∘C for 10 minutes and 95∘C for 15 minutes, 35 cycles of amplification (95∘C for 15 seconds and 60∘C for 60 seconds). Primers were validated by a melting curve analysis. For standard curve analysis, an RNA pool was made, serial diluted, and measured again with spectrophotometer.
Statistical Analysis
Statistical analysis of the data was done using ANOVA and the Student’s t test. Data were initially compared within a given experimental setup by ANOVA. A significant ANOVA evaluation was followed by pair wise analysis of control versus exposed data using Student’s t test, a p value of less than 0.05 was considered significant.
RESULTS
Effects of exposures to PCP on the Production (Secretion + Intracellular Levels) of IL-1β.
10 min exposure:
The effects of 10 min exposures to PCP on intracellular and secreted levels of IL-1β were examined in PBMCs from 4 separate donors. If there is an increase in secretion and no accompanying decrease in the intracellular level of IL-1β (or an increase in the intracellular level), this indicates that there is an increase in production of the cytokine. There were no consistent increases in the production of IL-1β after 10 min exposures to PCP (data not shown).
1h exposure:
Similar to what was seen at 10 min., there were no consistent increases in the production of IL-1β (secretion + intracellular levels) when cells from 4 separate donors were exposure to PCP for 1 h. Data not shown.
6 h exposure:
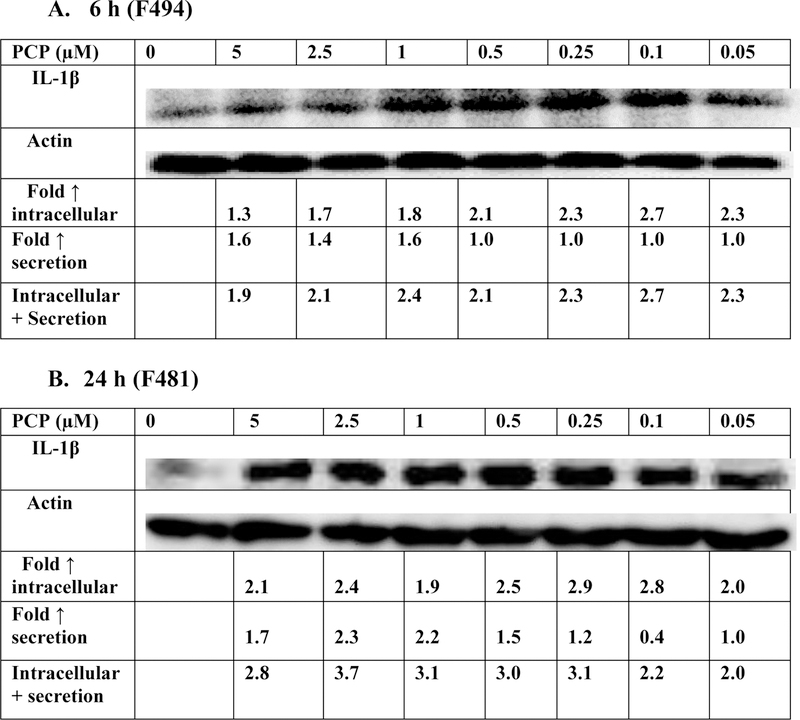
The effects of 6 h exposures to PCP on production of IL-1β are shown in Figure 1A and Table 1 (PBMCs from 4 separate donors). Figure 1 shows the data from the cells from a representative experiment (donor F494) where there were increases in production of IL-1β of approximately 2 fold at every level of PCP to which the cells were exposed. There were consistent increases in the production of IL-1β (secretion + intracellular levels) after 6-hours of exposure to PCP in cells from every donor at multiple PCP concentrations (Table 1). These results indicate that PCP stimulates IL-1β production in PBMCs within 6 hours of exposure.
Figure 1.
Effects of exposures to PCP on IL-1β production in PBMCs. A) 6 h exposure to 0–5 µM PCP. The blot is from a representative experiment (F494) with accompanying secretion data. An increase in secretion or in intracellular level is a number greater than 1; a decrease in secretion compared to the control is a number less than 1. The control is arbitrarily set at 1. A combined fold increase (secretion + intracellular) greater than 1 indicates an increase in production. Changes in fold production for 3 additional experiments are given in Table 1. B) 24 h exposure to 0–5 µM PCP. The blot is from a representative experiment (F481) with accompanying secretion data. Changes in fold production for 3 additional experiments are given in Table 1.
Table 1:
Effects of PCP exposures on production of IL-1β in PBMCS after 6 h and 24 h
| Fold increase in production IL-1 β | ||||||||
|---|---|---|---|---|---|---|---|---|
| [PCP] µM | 5 | 2.5 | 1 | 0.5 | 0.25 | 0.1 | 0.05 | |
| Exposure time | donor | |||||||
| 6h | F490 | 2.4 | 2.3 | 1.6 | 1.8 | 1.8 | 1.8 | NI |
| F492 | 1.5 | 1.4 | 1.1 | 2.3 | 2.5 | 2.2 | NI | |
| F493 | 1.7 | 1.7 | 1.4 | 1.1 | NI | NI | NI | |
| 24 h | F444 | 5.1 | 2.8 | 2.0 | 2.0 | 2.3 | 2.6 | 2.5 |
| F451 | 3.3 | 4.1 | 1.6 | NI | 1.4 | 1.3 | NI | |
| F485 | 3.2 | 4.0 | 2.3 | 1.2 | 1.3 | 1.1 | 1.4 | |
NI indicates no significant increase ( Fold increase ≤1)
24 h exposure:
When PBMCs were exposed to PCP for 24 h, there was an increase in production of IL-1β. For a representative experiment (Figure 1B, donor F481), increases of approximately 2–4 fold were seen at every PCP exposure level (0.05–5 µM). Table 1 summarizes the effects of PCP on IL-1β production in cells from 3 additional donors. Cells from all donors showed increases in the production of IL-1β after 24-hours of exposure to PCP.
Effects of 2 h, 6 h, and 24 h exposures to PCP on IL-1β mRNA levels in PBMCs
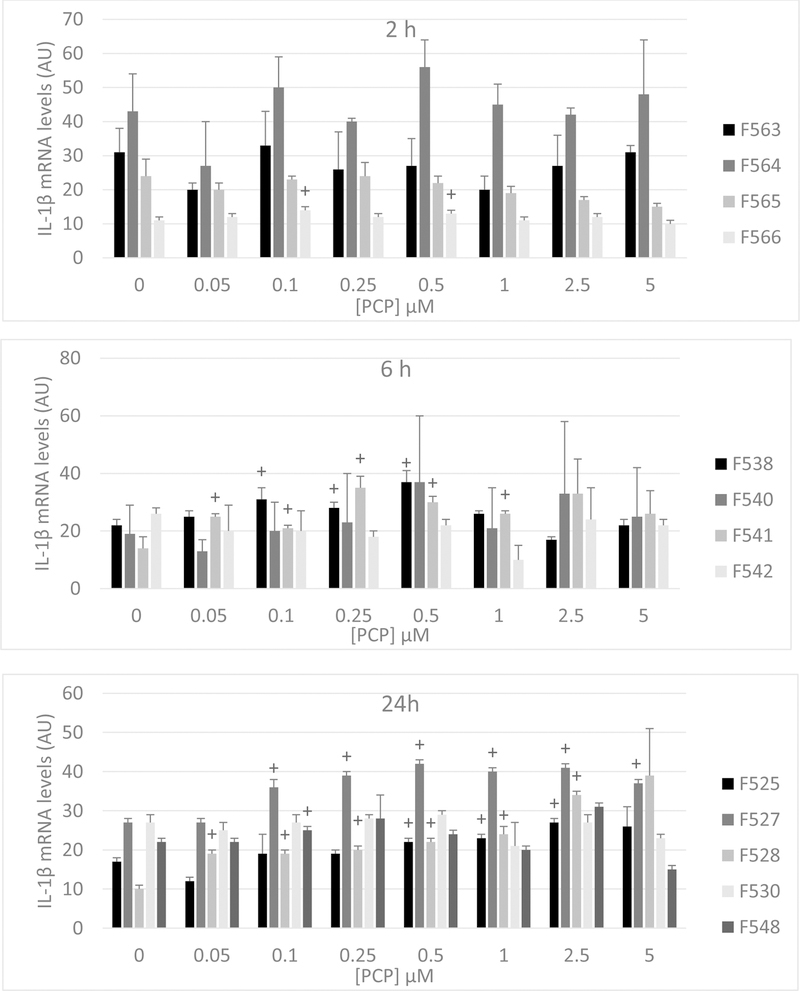
Figure 2 shows the levels of IL-1β mRNA in PBMCs exposed to PCP concentrations ranging from 0.05 µM-5 µM for 2 h, 6 h, and 24 h. Exposure to PCP for 2 h caused no notable changes in the levels of IL-1β mRNA in cells from any of the 4 donors tested. After a 6 h exposure, cells from 2 donors (F538 and F541) showed PCP-induced increases in IL-1β mRNA while cells from the other 2 donors showed no increases (Figure 2). For cells from donor F538 fold increases ranged between 1.3–1.7 and in those from F541 fold increase ranged between 1.5–2.5. When cells were exposed to PCP for 24 h, there were PCP-induced increases in IL-1β mRNA seen in 4 of the 5 donors tested (Figure 2). Thus, it appears that PCP may lead to increases in IL-1β production, at least in part, by increasing the levels of IL-1β mRNA.
Figure 2:
Effects of 2 h, 6 h, and 24 h exposures to PCP on IL-1β mRNA levels in PBMCs. Data are from cells isolated from 4 different donors for the 2 h and 6 h exposures and from 5 different donors for 24 h exposures (individual donor designation is indicated in the figure). Values are the mean ±S.D. of triplicate determinations. Statistically significant increases (P<0.05) in mRNA compared to the control are indicated by (+).
AU=Arbitrary Units
Effects of exposures to PCP on the production of IL-1β in PBMCs when the p44/42 and p38 signaling pathways are inhibited
Inhibition of p44/42 pathway by PD98059
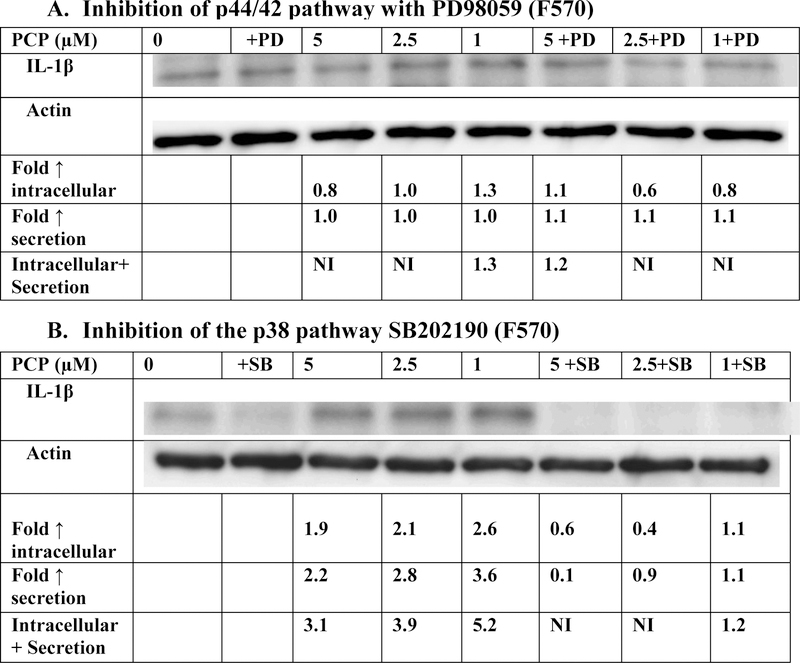
Figure 3A (representative experiment) and Table 2 show the effects of 24 h exposures to 1, 2.5, and 5 μM PCP on IL-1β production in PBMCs when the p44/42 MAP kinase pathway was inhibited with PD98059. When the inhibitor was present, PBMCs from 2 of the 4 donors continued to show PCP-stimulated production of IL-1β. For instance, when cells from donor F569 were exposed to 1, 2.5, and 5 μM PCP in the absence of PD98059 inhibitor there were 1.2, 2.6, and 2.9 fold increases in IL-1β production. When the inhibitor was present those same PCP exposures caused 1.7, 3.2, and 5.3 fold increases at 1, 2.5, 5 µM exposures to PCP (Table 2). However, in cells from donor F582 increases in IL-1β production were 6.5, 6.0, and 6.2 fold at 1, 2.5, and 5µM PCP in the absence of inhibitor but were decreased to nothing, 1.8 and 1.2 fold, respectively, when the inhibitor was present (Table 2). Overall, the results indicate that the p44/42 pathway is utilized by PCP to stimulate IL-1β production in cells from some donors.
Figure 3.
Effects of the inhibition of selected signaling pathways on IL-1β production in PBMCs exposed to PCP for 24 h. A) MEK inhibitor (PD98059). Data is from a representative experiment (F570). Data from additional experiments are given in Table 3; B) p38 inhibitor (SB202190) Data is from a representative experiment (F570). Data from additional experiments are given in Table 2. An increase in secretion or in intracellular level is a number greater than 1 a decrease in secretion compared to the control is a number less than 1. The control is arbitrarily set at 1. Cells treated with inhibitor are compared to control cells treated with inhibitor. A combined fold increase (secretion + intracellular) greater than 1 indicates an increase in production.
NI indicates no significant increase in production ( Fold increase ≤1)
Table 2:
Effects of PCP exposure on the production of IL-1β in PBMCS when the p44/42 or p38 signaling pathways are inhibited.
| Fold increase in production IL-1 β | |||||||
|---|---|---|---|---|---|---|---|
| [PCP] µM | 5 | 2.5 | 1 | 5+I | 2.5+I | 1+I | |
| Inhibitor (I) | donor | ||||||
| PD98059 | F569 | 2.9 | 2.6 | 1.2 | 5.3 | 3.2 | 1.7 |
| F571 | 1.8 | NI | NI | 1.4 | 1.1 | NI | |
| F582 | 6.2 | 6.0 | 6.5 | 1.2 | 1.8 | NI | |
| SB202190 | F571 | NI | 1.4 | NI | NI | NI | NI |
| F582 | 1.4 | 1.5 | 1.2 | NI | NI | NI | |
| F598 | 4.4 | 6.7 | 5.2 | NI | NI | NI | |
NI indicates no significant increase ( Fold increase ≤1)
Inhibition of p38 pathway by SB202190
The effect of p38 MAPK pathway inhibition on PCP-induced increases in IL-1β production from PBMCs are shown in Figure 3B (representative experiment) and Table 2. All donors consistently showed lowered PCP-induced IL-1β production in the presence of SB202190. For example, donor F598 showed 5.2, 6.7, and 4.4 fold increases when PBMCs were exposed to 1, 2.5 and 5 µM PCP in the absence of the p38 inhibitor. When the inhibitor was present, those same PCP exposures caused no measurable increases in IL-1β production. These data indicate that PCP utilizes the p38 pathway to stimulate IL-1β production in PBMCs.
Effects of DDT Exposures on the Production (Secretion + Intracellular Levels) of IL-1β
1h exposure:
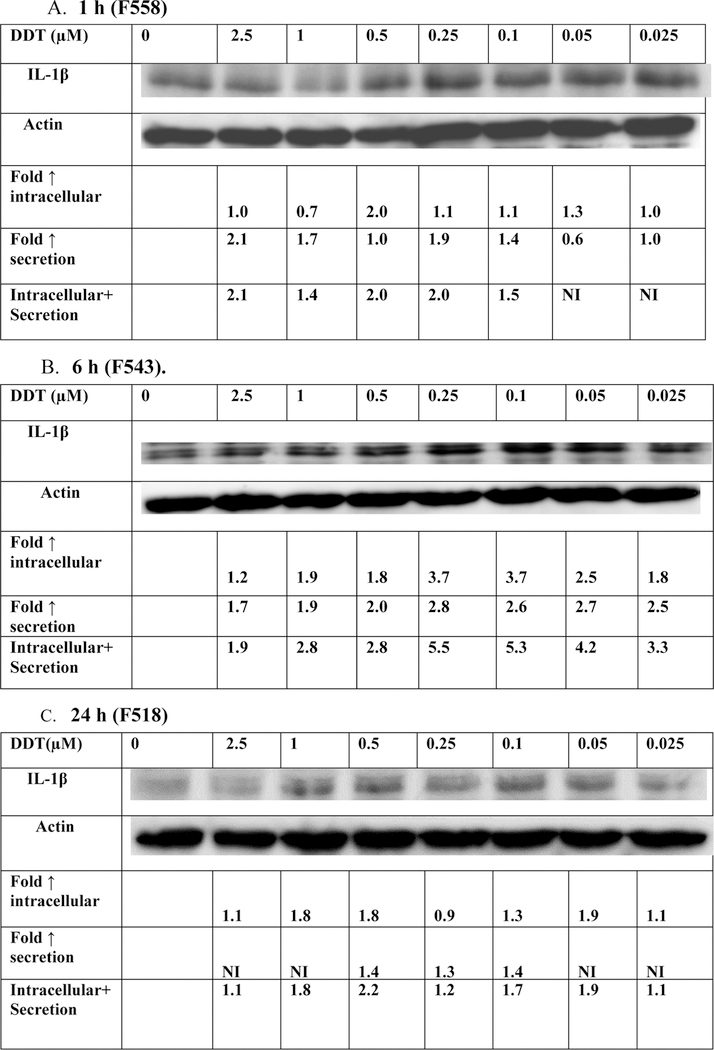
When PBMCs were exposed to DDT (0.025 −2.5 µM) for 1 h, there were increases in production of IL-1β in cells from 4 donors tested (Figure 4A and Table 3). For example the cells from donor F558 showed increases of 2.1, 1.4, 2.0, 2.0, and 1.5 fold at the 2.5, 1, 0.5,0.25, and 0.1 µM DDT concentrations (Figure 4A).
Figure 4.
Effects of exposures to DDT on IL-1 β production in PBMCs. A) 1 h exposure to 0–2.5 µM DDT. The blot is from a representative experiment (F558) with accompanying secretion data. B) 6 h exposure to 0–2.5 µM DDT. The blot is from a representative experiment (F543) with accompanying secretion data. C) 24 h exposure to 0–2.5 µM DDT. The blot is from a representative experiment (F518) with accompanying secretion data. An increase in secretion or in intracellular level is a number greater than 1; a decrease in secretion compared to the control is a number less than 1. The control is arbitrarily set at 1. A combined fold increase (secretion + intracellular) greater than 1 indicates an increase in production. Changes in fold production for 3 additional experiments at each length of exposure are given in Table 3.
NI indicates no significant increase ( Fold increase ≤1)
Table 3:
Effects of DDT exposures on production of IL-1β in PBMCS after 1 h, 6 h, and 24 h
| Fold increase in production IL-1 β | ||||||||
|---|---|---|---|---|---|---|---|---|
| [DDT] µM | 2.5 | 1 | 0.5 | 0.25 | 0.1 | 0.05 | 0.025 | |
| Exposure time | donor | |||||||
| 1h | F557 | 1.8 | 1.6 | 4.9 | 8.4 | 7.4 | 6.2 | 5.8 |
| F560 | 1.8 | 1.1 | 1.5 | 1.3 | 1.3 | 1.1 | 1.2 | |
| F561 | NI | NI | NI | 2.1 | NI | 2.3 | 2.4 | |
| 6 h | F535 | NI | NI | 1.8 | 2.6 | 2.0 | 3.8 | 3.3 |
| F536 | 1.3 | NI | 1.2 | 1.4 | 1.3 | 1.2 | NI | |
| F545 | NI | 1.1 | 1.1 | NI | 1.1 | 1.2 | 1.3 | |
| 24 h | F517 | NI | NI | NI | NI | NI | 1.3 | NI |
| F522 | 2.8 | 4.0 | 5.0 | 4.0 | 3.6 | 2.5 | 2.9 | |
| F523 | 1.5 | NI | NI | NI | NI | NI | NI | |
NI indicates no significant increase ( Fold increase ≤1)
6 h exposure:
Exposures of PBMCs from 4 separate donors to DDT for 6 h on production (intracellular and secreted levels) of IL-1β were examined. Figure 4B and Table 3 shows increases in production occurred in all donors at five or more concentrations or DDT. F543 shows fold increases ranging from 1.9–5.5 (Figure 4B). These data indicate that there are consistent increases in the production of IL-1β within 6 h of exposure to multiple concentrations of DDT.
24 h exposures:
The effects of 24 h exposures to DDT on production of IL-1β were examined in PBMCs from 4 separate donors are shown in Figure 4C and Table 3. Cell from all donors showed DDT-induced increases in IL-1β production at one or more concentration. Figure 4C shows that there were increases of 1.1, 1.8, 2.2, 1.2, 1.7, 1.9 and 1.1 fold at concentration of 2.5, 1, 0.5, 0.25, 0.1, 0.05, and 0.25 µM DDT. However, the increases were seen at fewer total DDT concentrations overall after 24 h than were seen after 1 h and 6 h of exposure.
Effects of 2 h and 6 h exposures to DDT on IL-1β mRNA levels in PBMCs
Figure 5 shows IL-1β mRNA expression in PBMCs from 4 separate donors exposed to DDT concentrations ranging from 0.025–2.5 µM for 2 h and 6 h. There are not consistent increases in IL-1β mRNA after either length of exposure. In contrast to the effects seen with PCP, it does not appear that the DDT-induced increases in IL-1β production can be accounted for by increases in IL-1β mRNA.
Figure 5:
Effects of 2 h and 6 h exposures to DDT on IL-1β mRNA levels in PBMCs. Data are from cells isolated from 4 different donors (individual donor designation is indicated in the figure). Values are the mean ±S.D. of triplicate determinations. Statistically significant increases (P<0.05) in mRNA compared to the control are indicated by (+).
AU=Arbitrary Units
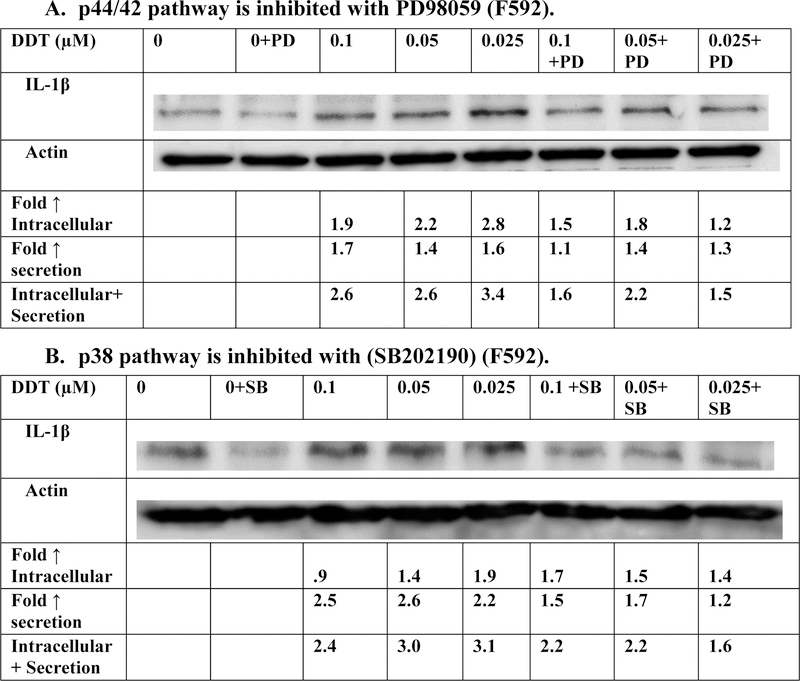
Effects of exposures to DDT on the production of IL-1β in PBMCs when the p44/42 and p38 signaling pathways are inhibited
Inhibition of p44/42 pathway by PD98059
The effects of 6 h exposures to 0.025, 0.05, and 0.1 μM DDT on production of IL-1β from PBMCs where p44/42 MAPK pathway function had been inhibited with PD98059 are shown in Figure 6A and Table 4. Cells exposed to DDT when the inhibitor was present showed diminished DDT-stimulated production of IL-1β in three out of four donors. Overall, the results indicate that the p44/42 MAPK pathway may be being utilized to some extent by DDT to lead to increases in IL-1β production in the PBMCs.
Figure 6.
Effects of the inhibition of selected signaling pathways on IL-1β production in PBMCs exposed to DDT for 6 h. A) MEK inhibitor (PD98059). Data is from a representative experiment (F592). Data from additional experiments are given in Table 4; B) p38 inhibitor (SB202190) Data is from a representative experiment (F592). Data from additional experiments are given in Table 4. An increase in secretion or in intracellular level is a number greater than 1 a decrease in secretion compared to the control is a number less than 1. The control is arbitrarily set at 1. Cells treated with inhibitor are compared to control cells treated with inhibitor. A combined fold increase (secretion + intracellular) greater than 1 indicates an increase in production.
NI represents not applicable with no significant increase ( Fold increase ≤1)
Table 4:
Effects of DDT exposure on the production of IL-1β in PBMCS when the p44/42 or p38 signaling pathways are inhibited.
| Fold increase in production IL-1 β | |||||||
|---|---|---|---|---|---|---|---|
| [DDT] µM | 0.1 | 0.05 | 1 | 0.1+I | 0.05+I | 0.025+I | |
| Inhibitor (I) | donor | ||||||
| PD98059 | F590 | 1.2 | NI | NI | 1.2 | NI | 1.2 |
| F591 | 5.9 | 3.8 | 8.1 | 4.2 | 4.5 | 3.9 | |
| F593 | 2.7 | 2.4 | 3.6 | 2.1 | 2.0 | 3.1 | |
| SB202190 | F591 | 3.5 | 1.8 | 2.6 | 1.7 | 1.3 | 1.6 |
| F593 | 2.4 | 3.0 | 3.0 | 2.2 | 2.2 | 1.9 | |
| F595 | NI | 1.1 | NI | 1.2 | 1.4 | 1.1 | |
NI indicates no significant increase ( Fold increase ≤1)
Inhibition of p38 pathway by SB202190
The effect of p38 MAPK pathway inhibition on DDT-induced increases in IL-1β production from PBMCs (6 h exposures) are shown in Figure 6B and Table 4. Three out of 4 donors consistently showed lowered DDT-induced IL-1β production in the presence of SB202190. For example, donor F591 showed 2.6, 1.8, and 3.5 fold increases in IL-1β production when PBMCs were exposed to 0.025, 0.05 and 0.1 µM DDT in the absence of the p38 inhibitor. When the inhibitor was present, those same DDT exposures caused 1.6, 1.3, and 1.8 fold increases. This indicates that the p38 pathway is likely being utilized by DDT to stimulate IL-1β production.
DISCUSSION
IL-1β can significantly contribute to a number of diseases when its levels are elevated in the absence of infection or injury, producing a state of chronic inflammation. Tumor growth, rheumatoid arthritis, Crohn’s disease, and multiple sclerosis are some of the pathologies associated with chronic inflammation (Landskron et al., 2014; Choy and Panayi, 2001; Dinarello, 2011; Rossi et al., 2012; Elaraj et al., 2006; Jin et al., 1997; Lewis and Varghese, 2006; Voronov et al., 2002; Rubin et al., 2012; Shamesh et al., 2002; Straub et al., 2016). PCP is an environmental contaminant found in human blood with serum levels ranging as high as 5 μM (Cline et al., 1989; Uhl et al., 1986). PCP exposure has been associated with a number of malignancies in humans including cancers of the blood (lymphoma and myeloma) and kidney (Demers et al., 2006; Cooper & Jones, 2008). DDT is another organochlorine compound that continues to contaminate the environment, primarily due to its past and current use as an insecticide. Although DDT usage is banned in many industrialized countries, due to its destructive and intense effects on the environment, it is still used in developing countries for public health purposes (Turusov et al., 2002; Loganathan, 2016). DDT has been discovered in serum at levels as high as 23, 169 ng/g of lipid (approximately 260 nM) (Koepke et al., 2004; Trejo-Acevedo et al., 2009). Both PCP and DDT have also been shown to increase IL-1β secretion from human immune cells (Martin and Whalen,2017). However, it was not possible to tell from those studies whether PCP and DDT were simply increasing the release of pre-existing IL-1β or if they were able to stimulate immune cells to produce these cytokines. The later effect would have greater capacity to induce sustained inflammation than would the former. Thus, studies were carried out to determine if either PCP or DDT were able to stimulate production of IL-1β. If PCP and DDT are only stimulating the release of IL-1β that already exists in the cell, then there will be a limit to their ability to increase the levels of IL-1β secreted by immune cells. On the other hand, if PCP or DDT stimulate production of IL-1β by immune cells this could provide sustained elevation of this cytokine and lead to chronic inflammation.
The results indicated that PCP exposures stimulated IL-1β production after 6 h and these compound-induced increases maintained and intensified out to 24 h. Results after 6 h showed that PCP increased IL-1β production in all donors at one or more concentrations. After 24 h PCP-stimulated IL-1β production occurred consistently in all donors at PCP concentrations of 0.1–5 µM. DDT also induced increases in IL-1β production, however unlike PCP, DDT seemed to maximally stimulate the production IL-1β at earlier time points (1 h and 6 h) rather than after 24 h of exposure. Significant increases that were seen in IL-1β production from PCP and DDT exposures could be due to increased translation/transcription, mRNA stability, protein stability or a combination of these factors. Thus, studies were carried out to investigate the effects of each contaminant on the levels of IL-1β mRNA in PBMCs. It appears that PCP-induced increases in production of IL-1β can at least be partially attributed to compound-stimulated increases in the mRNA for the cytokine. While, DDT caused consistent increases in IL-1β production, it did not produce consistent increases in the mRNA for IL-1β within the 6 h time frame. Thus, it appears that PCP is able to stimulate increased IL-1β production at least in part by increasing its mRNA. In contrast, the increase in production of IL-1β stimulated by DDT does not appear to be the result of increases in the mRNA for IL-1β. Thus, further studies examining the role of contaminant-stimulated increases in translation or decreases in protein degradation are warranted.
The roles of certain signaling pathways in PCP and DDT-induced increases in production of IL-1β in PBMCs were also investigated. The mitogen activated protein kinase (MAPK) pathways, p44/42 and p38 are known to be involved in regulating the production of IL-1β cytokines in immune cells (Gaestel et al., 2009). The results of these studies indicated that PCP may utilize p44/42 pathway in some donors to increase IL-1β production. PCP appears to consistently rely upon the p38 pathway to produce increased production of IL-1β. The roles of these same pathways in DDT-induced increases in production of IL-1β were also studied. As was seen with PCP, DDT may utilize p44/42 pathway to a certain extent in increasing IL-1β production. Like PCP, DDT appears to require the p38 pathway to stimulate IL-1β production. These results can be compared with those seen with the organotin contaminant, TBT. TBT like PCP and DDT appears to predominantly rely on the p38 pathway, and has some requirement for the p44/42 pathway in order to stimulate IL-1β production in immune cells (Brown et. al, 2018). Additionally, activation of p38 by DDT and its metabolites leads to altered gene expression in a human embryonic kidney epithelial cell line and in an endometrial cell line (Frigo et al., 2004;) and apoptosis in cultured rat sertoli cells (Song, et al., 2011)
Other contaminants such as the brominated flame retardant hexabromocyclododecane (HBCD) have been shown to increase the secretion of IL-1β from human immune cells (Anisuzzaman and Whalen, 2016). Organotin contaminants, tributylin (TBT) and dibutyltin (DBT) also increase IL-1β secretion from human lymphocytes and monocytes (Brown and Whalen, 2015; Brown et al.,2017). Additionally, TBT can significantly increase the production of IL-1β in lymphocytes. Production begins to increase after 6 h exposure and continued to increase after 24h exposure (Brown et. al, 2018). TBT’s ability to alter production can be partially attributed to increases in mRNA for IL-1β (Brown et al., 2018). Due to the importance of IL-1β in normal immune function and in the development of chronic inflammation leading to certain disease states such as rheumatoid arthritis, it is important to understand the role of the array of contaminants that humans are exposed to in altering appropriate levels of these proteins.
In summary, PCP caused increased production of IL-1β after 6 h exposures and the increases continued and intensified out to 24 h. In contrast, DDT caused maximal IL-1β production within 6 h, which then appeared to diminish between 6 and 24 h. PCP-stimulated increases in mRNA for IL-1β appear to at least partially explain its ability to increase IL-1β production, while this does not appear to be the case for DDT. Furthermore, results indicate that the p38 MAPK pathway appears to be required for both PCP-and DDT-induced increases in IL-1β production. The p44/p42 pathway is also utilized by each compound to increase IL-1β production, but not in cells from all donors.
ACKNOWLEDGEMENT
Grant U54CA163066 from the National Institutes of Health
REFERENCES
- Anisuzzaman S, Whalen MM 2016. Tetrabromobisphenol A and hexabromocyclododecane alter secretion of IL-1β from human immune cells. Journal of Immunotoxicology 13: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddock M, Quinn A 2004. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat. Rev. Drug Discov 3:1–10. [DOI] [PubMed] [Google Scholar]
- Brown B, Loganathan BG, Owen D 2005. Chlorophenol concentrations in sediment and mussel tissues from selected locations in Kentucky Lake. Chrysalis, Murray State University Undergraduate Research Journal 1: 5–10. [Google Scholar]
- Brown S, Whalen M 2015. Tributyltin alters secretion of interleukin 1 beta from human immune cells. J Appl Toxicol 35: 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Tehrani S, Whalen MM 2017. Dibutyltin-induced alterations of interleukin 1beta secretion from human immune cells. Journal of Applied Toxicology 37: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Hamza N, Boules M, Wang X, Whalen MM 2018. Production of interleukin 1 beta and interleukin 6 in human lymphocytes is stimulated by tributyltin. Archives of Toxicology 92:2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E and Panayi G 2001. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. New England Journal of Medicine, 344: 907–916. [DOI] [PubMed] [Google Scholar]
- Cirelli DP 1978. Patterns of pentachlorophenol usage in the United States of America-an overview. In: Rao KR., editor. Pentachlorophenol, chemistry, pharmacology, and environmental toxicology Plenum Press; New York, NY: p. 13–18. 10.1002/jat.1367 [DOI] [Google Scholar]
- Cline RE, Hill RH, Phillips DL, Needham LL 1989. Pentachlorophenol measurements in body fluids of people in log homes and workplaces. Arch. Environ. Contam. Toxicol 18:475–481. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Jones S 2008. Pentachlorophenol and Cancer Risk: Focusing the Lens on Specific Chlorophenols and Contaminants. Environmental Health Perspectives,116: 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Ponnappan A, Mehta V, Wewers MD, Caligiun M 2001. Interleukin-1β co-stimulates interferon-γ production by human natural killer cells. European Journal of Immunology 31, 792–801. [DOI] [PubMed] [Google Scholar]
- Davis MP 2006. With DDT Spraying in Malaysia Can Show the World How to Control Dengue; 21st Century Science and Technology, Summer 2006. pp 53–60.
- Demers PA, Davies HW, Friesen MC, Hertzman C, Ostry A, Hershler R, et al. 2006. Cancer and occupational exposure to pentachlorophenol and tetrachlorophenol. Cancer Causes Control 17:749–758. [DOI] [PubMed] [Google Scholar]
- Dinarello CA 1996. Biologic basis for interleukin-1 in disease. Blood 87:2095–2147. [PubMed] [Google Scholar]
- Dinarello CA 2004. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr. Opin. Pharmacol 4:378–385. [DOI] [PubMed] [Google Scholar]
- Dinarello C 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM, Alexander HR. 2006. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clinical Cancer Research 12: 1088–1096 [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevier J, Rosas LG, Anderson HA, Bornman MS, Bouwman H, Chen A, Cohn BA, deJager C, Henshel DS, Leipzig F, Leipzig JS, Lorenz EC, Snedeker SM, Stapleton D 2009. The Pine River Statement: Human Health Consequences of DDT Use. Environ. Health Perspec 117:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigo DE, Tang Y, Beckman BS, Scandurro AB, Alam J, Burow ME, McLachlan JA 2004. Mechanism of AP-1-mediated gene expression by select organochlorines through the p38 MAPK pathway. Carcinogenesis 25:249–261. [DOI] [PubMed] [Google Scholar]
- Gaestel M, Kotlyarov A and Kracht M 2009. Targeting innate immunity protein kinase signaling in inflammation. Nature Reviews Drug Discovery, 8: 480–499. [DOI] [PubMed] [Google Scholar]
- Harada T, Takeda M, Kojima S, & Tomiyama N (2016). Toxicity and Carcinogenicity of Dichlorodiphenyltrichloroethane (DDT). Toxicological Research,32: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guidae A, Hastings HM, Andres J, Turkel G, Polverini PJ, Goldberg ID, Rosen EM. 1997. Expression of interleukin-1β in human breast carcinoma. Cancer 80: 421–434. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Loganathan BG, 2017. Organohalogen Pollutants and Human Health. In: Quah SR and Cockerham WC (eds.) The International Encyclopedia of Public Health, 2nd edition. vol. 5, pp. 359–366. Oxford: Academic Press. [Google Scholar]
- Koepke R, Warner M, Petreas M, Cabria A, Danis R, Hernandez-Avila M, & Eskenazi B (2004). Serum DDT and DDE levels in pregnant women of Chiapas. Mexico. Arch. Environ. Health 59:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA “Chronic Inflammation and Cytokines in the Tumor Microenvironment,” Journal of Immunology Research, vol. 2014, Article ID 149185, 19 pages, 2014 10.1155/2014/149185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A, Varghese S. 2006. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. Journal of Translational Medicine 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan BG 2016. Persistent organic chemicals in the Pacific Basin countries: An overview. In. Persistent Organic Chemicals in the Environment: Status and trends in the Pacific Basin Countries I. Eds. Loganathan BG., Khim JS, Kodavanti PR, Masunaga S ACS Symposium Series Vol. 1243 American Chemical Society and Oxford University Press; Pp 1–15. [Google Scholar]
- Martin T and Whalen M (2017). Exposures to the environmental toxicants pentachlorophenol (PCP) and dichlorodiphenyltrichloroethane (DDT) modify secretion of interleukin 1-beta (IL-1β) from human immune cells. Archives of Toxicology 91:1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, Weinauer F, Schmitz J, Illert WE. 2005. Filter Buffy Coats (FBC): A source of peripheral blood leukocytes recovered from leukocyte depletion filters. Journal of Immunological Methods 307: 150–166. [DOI] [PubMed] [Google Scholar]
- Rengam SV 2013. Banned but Still in Use. Letters. PAN Asia and the Pacific February 1, 2013. [Google Scholar]
- Rossi S, Furlan R, De Chiara V, Motta C, Studer V, Mori F, Musella A, Bergami A, Muzio L, Bernardi G, Battistini L, Martino G and Centonze D (2012). Interleukin-1β causes synaptic hyperexcitability in multiple sclerosis. Annals of Neurology 71: 76–83. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Shaker A, Levin MS 2012. Chronic intestinal inflammation:inflammatory bowel disease and colitis-associated colon cancer . Front. Immunol 3: Article 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo M, Ceballos-Olvera I,delBarrio L and Re F 2011. Role of the Inflammasome, IL-1β, and IL-18 in Bacterial Infections The Scientific World JOURNAL 11: 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamash S, Reichert F, & Rotshenker S (2002). The cytokine network of Wallerian degeneration: Tumor Necrosis Factor-α, Interleukin-1α, and Interleukin-1β. J. Neurosci; 22:3052–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, & Pamer EG (2011). Monocyte recruitment during infection and inflammation. Nature Reviews. Immunology 11: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Shi Y, Yu H, Wang Y, Yang K 2011. p,p’-Dichlorodiphenoxydichloroethylene induced apoptosis of Sertoli cells through oxidative stress-mediated p38 MAPK and mitochondrial pathway. Toxicol. Lett 202: 55–60. [DOI] [PubMed] [Google Scholar]
- Straub RH, Schradin C 2016. Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public Health 2016: 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo-Acevedo A, Diaz-Barriga F, Carrizales L, Dominguez G, Costilla R, Ize-Lema I, Yarto-Ramirez M, Gavilan-Garcia A, Mejia-Saavedra JJ, & Perez-Maldonado IN 2009. Exposure assessment of persistent organic pollutants and metals in Mexican children. Chemosphere; 74:974–980. [DOI] [PubMed] [Google Scholar]
- Turusov V, Rakitsky V, & Tomatis L 2002. Dicholordiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environmental Health Perspectives 110:125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl S, Schmid P, & Schlatter C (1986). Pharmacokinetics of pentachlorophenol in man. Arch. Toxicol; 58:182–186. [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. 2002. IL-1 is required for tumor invasiveness and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America 100: 2645–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]