Abstract
Objective:
Some individuals with functional neurological disorder (FND) exhibit motor and affective disturbances, along with limbic hyper-reactivity and enhanced motor-limbic connectivity. Given that the multimodal integration network (insula, dorsal cingulate, temporoparietal junction (TPJ)) is implicated in convergent sensorimotor, affective and interoceptive processing, we hypothesized that patients with FND would exhibit altered motor and amygdalar resting-state propagation to this network. Patient-reported symptom severity and clinical outcome were also hypothesized to map onto multimodal integration areas.
Methods:
Between-group differences in primary motor and amygdalar sub-nuclei (laterobasal, centromedial) were examined using graph-theory stepwise functional connectivity (SFC) in 30 patients with motor FND compared to 30 healthy controls. Within-group analyses correlated functional propagation profiles with symptom severity and prospectively collected 6-month outcomes as measured by a Screening for Somatoform Symptoms Conversion Disorder subscale and Patient Health Questionnaire-15 composite score. Findings were cluster-wise corrected for multiple comparisons.
Results:
Compared to controls, patients with FND exhibited increased SFC from motor regions to the bilateral posterior insula, TPJ, middle cingulate cortex, and putamen. From the right laterobasal amygdala, the FND cohort showed enhanced connectivity to the left anterior insula, periaqueductal gray and hypothalamus among other areas. In within-group analyses, symptom severity correlated with enhanced SFC from the left anterior insula to the right anterior insula and TPJ; increased SFC from the left centromedial amygdala to the right anterior insula correlated with clinical improvement. Within-group associations held controlling for depression, anxiety and antidepressant use.
Conclusions:
These neuroimaging findings suggest potential candidate neurocircuit pathways in the pathophysiology of FND.
Keywords: conversion disorder, somatization, psychogenic nonepileptic seizures, functional movement disorders, fMRI
INTRODUCTION
Despite its important role in the development of modern-day neurology and psychiatry, functional neurological disorder (FND, a.k.a. conversion disorder) has been largely neglected by both fields for decades.1,2 However, the reframing of FND as a “rule-in” diagnosis based on neurological signs and semiologic features has catalyzed renewed interest.3,4 Improved diagnostic specificity offers the opportunity to elucidate the pathophysiology of this enigmatic condition, which in turn, could challenge mind-body dualism, reduce patient stigma, and aid the development of neurobiologically-informed treatments.
Several neuroimaging studies in FND cohorts have identified amygdalar abnormalities including hyper-reactivity to affectively-valenced stimuli,5–8 impaired habituation,7 sensitized emotional processing,5 and heightened coupling to motor preparation areas including the supplementary motor area (SMA).6,7,9,10 For example, patients with FND performing an affectively-valenced face viewing task demonstrated increased amygdalar activity and delayed habituation compared to controls.7 These findings were replicated in another FND study that also showed increased dorsal anterior cingulate cortex (ACC), periaqueductal gray (PAG) and SMA activations during emotional processing in patients compared to controls.5 Increased task-based6,7 and resting-state8,11 amygdalar coupling to motor and cognitive control regions has also been described. In addition, cingulo-insular resting-state connectivity to sensorimotor areas is increased in some FND populations,12 and these connectivity relationships correlate with symptom severity.13 While important unanswered questions remain, such as the role of specific amygdalar sub-nuclei, these findings support salience network (amygdala, cingulo-insular, PAG) involvement in the pathophysiology of FND.
Neuroimaging findings related to altered motor preparation, execution, inhibitory control and conceptualization have also been reported in patients with FND.14–16 For example, patients with functional paralysis attempting to move an affected limb exhibited an expanded network of insular, ventrolateral prefrontal cortex, basal ganglia and lingual gyrus activations.17 During the execution of internally generated movements in individuals with functional movement disorders, similar anterior insular and amygdalar hyperactivations were observed.18 Amplified startle responses have also been described in individuals with FND.19 While heightened limbic-paralimbic influence on behavior has been proposed as an important aspect of the pathophysiology of FND,7 the specific pathways through which motor and affective neural systems interact in FND remain poorly understood.
Stepwise functional connectivity (SFC) is a novel graph-theory resting-state functional connectivity approach developed by Sepulcre and colleagues that characterizes the propagation and convergence of functional connectivity across brain networks.20–22 Conventional resting-state methods predominantly characterize the segregation of discrete large-scale networks; SFC is specifically designed to capture not only the segregation of brain networks but also the integration between them, as a proxy of the information flow across neuronal systems. Using this method, sensorimotor systems in healthy subjects have been observed to propagate over a series of functional connectivity relay stations (link-steps) to a core set of multimodal integration areas20,21. The multimodal integration network includes the anterior insula, dorsal ACC/middle cingulate cortex (MCC), ventral premotor cortex, and temporoparietal junction (TPJ). Anterior portions of the multimodal integration network overlap with the salience network, while posterior areas of this network (e.g., TPJ) are implicated in motor intention awareness and action authorship deficits in FND.23–25 Notably, SFC has characterized mechanistic insights in neurologic26 and neuropsychiatric populations.27
In this resting-state neuroimaging study, we examined the functional propagation of primary motor (hand, foot, tongue) and amygdalar (laterobasal, centromedial nuclei) regions in 30 patients with motor FND compared to 30 healthy controls. A SFC interconnector analysis also explored common points of altered functional connectivity between motor and amygdalar areas in patients with FND compared to controls. Complementary within-group approaches investigated correlations between SFC profiles, patient-reported symptom severity, and 6-month clinical outcomes. We have previously characterized salience network structural alterations in this cohort,28,29 and have theorized that cingulo-insular areas contribute to impaired integration of sensorimotor, affective and viscerosomatic information in FND populations.30 Thus, we hypothesized that individuals with motor FND would exhibit altered motor and amygdalar link-step connectivity to the multimodal integration network. We also hypothesized that FND symptom severity and prospectively collected 6-month clinical outcome would correlate with multimodal integration network functional connectivity profiles.
METHODS
Participants and questionnaires
All subjects signed informed consent and the Partners Human Research Committee approved this study. Thirty subjects with motor FND (24 women, 6 men; mean age=40.1±12.9; average illness duration=3.0±3.8 years; 24-right-handed, 6-ambidextrous or left-handed) were recruited from the Massachusetts General Hospital FND Clinic following a “rule-in” FND diagnosis in accord with the Diagnostic and Statistical Manual of Mental Disorders 5th Edition criteria.3 An additional 5 patients were enrolled but excluded following imaging preprocessing (see Supplementary Methods). Given the overlap across the motor FND spectrum,30 we used a transdiagnostic approach that included those with clinically-established functional movement disorders (n=16),31 functional weakness (n=12),32 and documented (n=12) or clinically-established (n=1) psychogenic nonepileptic seizures (PNES).33 Ten of the 30 subjects had mixed motor FND. Exclusion criteria included major neurological comorbidities with magnetic resonance imaging (MRI) abnormalities, epilepsy, poorly controlled medical problems with known central nervous system consequences, active substance dependence, a history of mania or psychosis, and/or active suicidality. Comorbid psychiatric diagnoses as assessed using the Structured Clinical Interview (SCID-I) for DSM-IV-TR were present in 27 of 30 participants. Fourteen patients were on selective serotonin reuptake inhibitors (SSRIs) and/or serotonin-norepinephrine reuptake inhibitors (SNRIs). See Supplementary Table 1 for clinical information. Thirty healthy subjects (22 women, 8 men; mean age=40.0±12.6; 25-right-handed, 5-ambidextrous or left-handed) were recruited through local advertisements, and all screened negative for SCID-I major psychiatric comorbidities (one control met criteria for past depression not-otherwise-specified). Five additional controls were enrolled but excluded due to lifetime major psychiatric comorbidities (n=4) or excess head motion (n=1).
Subjects completed the Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale (SOMS:CD)34 and the Patient Health Questionnaire-15 (PHQ15)35 as patient-reported symptom severity measures within a detailed psychometric battery at enrollment. The SOMS:CD is a 14-item measure of sensorimotor FND symptoms within the past 7 days, with each item scored on a 5-point scale. The PHQ15 is a 15-item measure of somatic complaints within the past 4 weeks, with each item scored on a 3-point scale. These scales were also prospectively completed at 6-month follow-up by 22 of 30 patients (8 were lost to follow-up; interval follow-up=6.1±1.1 months). To reduce multiple comparisons, we constructed a SOMS:CD-PHQ15 composite by averaging the z-scores of the two variables. All subjects also completed the Beck Depression Inventory-II and the Spielberger State-Trait Anxiety Inventory (STAI).
Prior to enrollment, patients were diagnosed with FND and the term “Functional Neurological Disorder” was communicated along with the pertinent motor-subtype.36 FND was presented as common, real, and treatable. Patients were introduced to the www.neurosymptoms.org website and given printed educational materials. Treatments were individualized, emphasizing cognitive behavioral therapy (CBT) and physiotherapy (PT). Fifteen individuals were in psychotherapy at baseline and 8 were newly referred (including one who remained in supportive psychotherapy while also starting CBT). In CBT, patients were encouraged to explore the relationships between functional symptoms, thoughts, behaviors, emotions and psychosocial factors.37 Nine were in PT at baseline, and 5 were newly referred. Physiotherapists were recommended to use the Nielsen et al recommendations.38 Patients did not exclusively receive care at MGH, which limited compliance information.
MRI data acquisition and preprocessing
See Supplementary Methods for data acquisition and preprocessing procedures including scrubbing head motion correction.
Stepwise functional connectivity analyses
SFC methods delineated the functional propagation of specific regions-of-interest (ROIs) across distinct link-steps.20–22 First, individual connectivity matrices were computed. As shown in Fig. 1, the Pearson correlation between the time series of all pairs of cortical-subcortical gray matter voxels were computed. Then, a Fisher transformation was applied to the resulting correlation matrix. After removing negative values due to their controversial interpretation, a false discovery rate correction of p=0.0001 was applied to obtain only the most significant links. Thus, a high-resolution 5142 × 5142 connectivity matrix was obtained for each subject.
Figure 1. Stepwise functional connectivity pipeline used to characterize differences in the integration of motor and amygdalar information between patients with functional neurological disorder (FND) and healthy controls (HC).
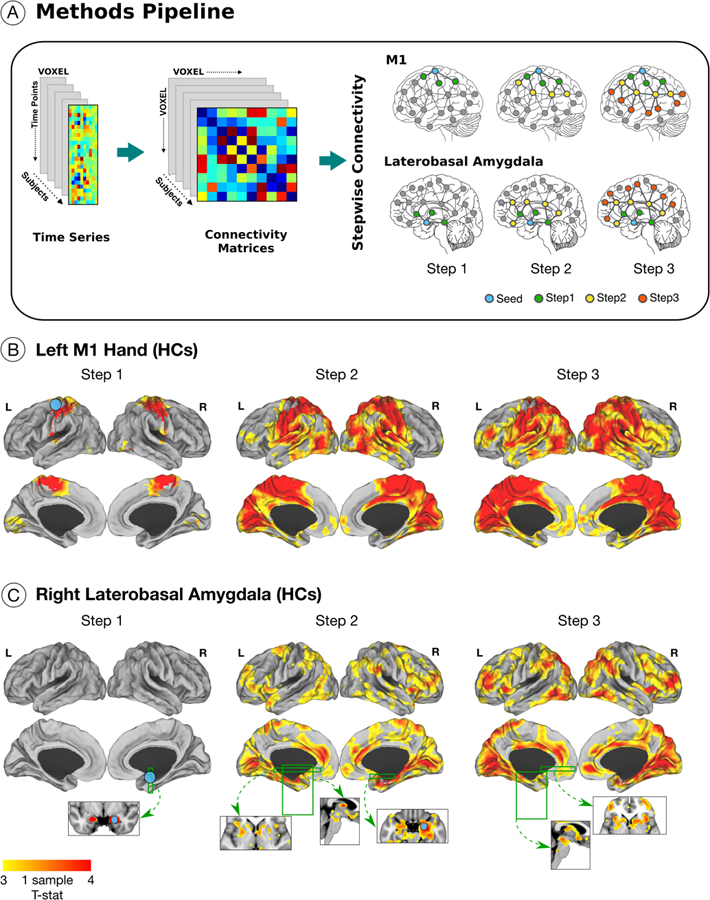
(A) The blood-oxygen-level-dependent time series preprocessed signal was used to create a voxel by voxel connectivity matrix at the individual-subject level. Using motor and amygdalar regions-of-interest (ROIs), we computed the step-link connectivity for steps 1, 2 and 3. Green nodes represent the regions reached by the 1st link-step from the ROI (represented in blue); yellow and red nodes represent 2nd and 3rd link-steps from the ROI. As an illustrative example, panel (B) shows the functional propagation for 30 healthy subjects from the left M1-hand (one sample T-test threshold at p-value 0.01 corrected for multiple comparisons; replication of Sepulcre 2014). Panel (C) shows the functional propagation of the right laterobasal amygdala ROI in healthy subjects; one sample T-test thresholded at p-value 0.01 corrected for multiple comparisons. The 1st link-step shows connectivity to adjacent bilateral amygdalar regions. In the 2nd link-step, connectivity is observed to cingulo-insular salience network regions among other areas; in the 3rd link-step, propagation reaches the periaqueductal gray and hypothalamus. See Supplementary Fig. 1 for delineation of link-step connectivity profiles from other amygdalar sub-nuclei in healthy subjects.
Validated bilateral Montreal Neurological Institute (MNI) voxel-wise hand (±41, −20, 62), foot (±6,−26,76), and tongue (±55,−4,26) primary motor cortex ROIs were chosen.39 In addition, laterobasal and centromedial amygdalar ROIs were extracted from the probabilistic cytoarchitectonic mapping of the human amygdala in the SPM anatomy toolbox consistent with previously validated approaches40 (Fig. 1 and Supplementary Fig. 1). Only 3 link-steps are presented given that healthy subjects reach the multimodal integration network by this stage.20,21
Between-group analyses
Two-class general linear models examined between-group differences in the stepwise functional propagation of motor and amygdalar areas. All analyses (including within-group analyses described below) controlled for age, gender, and handedness (right-handed yes/no). Secondary analyses also adjusted between-group findings for depression and anxiety scores. There were no group-level differences in mean frame displacement (FND: 0.098±0.055; controls: 0.08±0.043; p-value=0.15). Between-group SFC findings were corrected for multiple comparisons using Monte Carlo simulation cluster-wise correction with 10,000 iterations to estimate the probability of false positive clusters with a p-value<0.05.
Exploratory between-group interconnector analysis
While SFC delineates functional connectivity pathways from one region to the rest of the brain, it is not well suited to delineate interconnecting paths across multiple ROIs. Interconnector analysis, another graph-theory network analysis,21,41 restricts the SFC analysis only to those pathways that end in a common target. Following the identification of between-group SFC differences from a priori ROIs (see results), statistically significant motor and amygdalar ROIs were used in a single interconnector analysis to test for common sites of altered functional propagation. For this exploratory general linear model analysis, a right-tailed uncorrected t-test was used to compute group differences between patients and controls. Only clusters larger than 1.080 mm3 with a p<0.05 were reported to minimize Type-I errors.
Within-group correlation with symptom severity
A single-class general linear model investigated the effect of symptom severity (SOMS:CD-PHQ15 composite). There was no significant relationship between individual subject frame displacements and composite scores (Spearman correlation 0.24; p-value=0.2). The motor and amygdalar ROIs used for between-group analyses were also used here (although no significant results were identified). We also performed a data-driven approach to identify all voxels where link-step propagation correlated with SOMS:CD-PHQ15 scores, and the left anterior insula was amongst the most robust areas (Supplementary Methods, Supplementary Fig. 2). Given that we previously characterized inverse correlations between left anterior insular volume and FND symptom severity,28,29 the left anterior insula (−45, 9, −9) was chosen as an additional ROI. Correction for multiple comparisons employed cluster-wise correction using Monte Carlo simulation. Post-hoc analyses evaluated if statistically significant within-group findings held adjusting for baseline: 1) mood and anxiety symptoms (BDI-II and STAI-total scores); 2) SSRI/SNRI use (yes/no); and 3) motor FND subtypes (PNES, functional movement disorders, and functional weakness).
Pilot within-group correlation with 6-month improvement
In the 22 of 30 FND subjects with prospectively collected 6-month follow-up data, a pilot analysis evaluated associations between baseline SFC and clinical outcome. The same motor and amygdalar ROIs were also used here to identify functional propagation profiles correlated with 6-month change in SOMS:CD-PHQ15 scores. The percentage change in the SOMS:CD-PHQ15 composite score was used in a single-class general linear model to study relationships between SFC and clinical outcome (scores were inverted so that higher scores reflected greater improvement). Correction for multiple comparisons applied a cluster-wise correction using a Monte Carlo simulation, and post-hoc analyses adjusted statistically significant findings for baseline: 1) BDI-II and STAI-total scores, and 2) SSRI/SNRI use. Given sample size limitations, we did not adjust for motor subtypes.
RESULTS
Motor findings
Compared to controls, the 1st link-step from left-M1 hand in patients with FND showed increased connectivity to the bilateral pre-and postcentral gyri, SMA, MCC, and superior parietal lobule (Fig. 2). In the 2nd link-step from the left-M1 hand, patients with FND also displayed enhanced propagation to the bilateral posterior insula, TPJ and putamen (in addition to the areas described in the 1st link-step which were also present). In the 3nd link-step from the left-M1 hand, patients with FND compared to controls also showed greater link-step connectivity to bilateral dorsomedial prefrontal areas. Post-hoc analyses adjusting for depression and anxiety showed enhanced propagation from the left-M1 hand to the bilateral dorsal ACC/MCC (in all 3 link-steps), dorsomedial prefrontal cortex (2nd and 3rd link-steps), and right TPJ (2nd and 3rd link-steps) in FND subjects compared to controls.
Figure 2. Altered stepwise functional propagation from primary motor areas in patients with functional neurological disorder (FND).
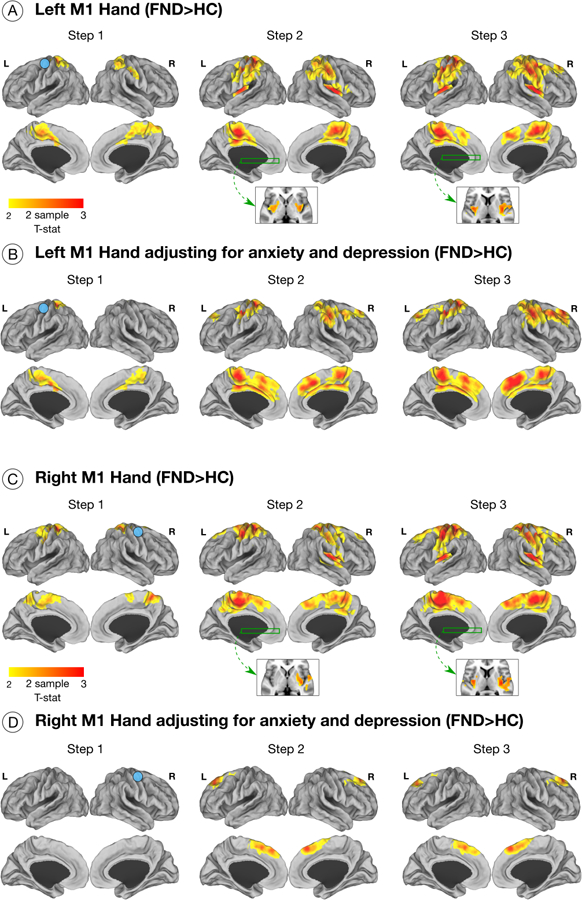
Maps display results of general linear models comparing functional propagation in patients with FND vs. healthy controls (HC). Only results surviving multiple comparisons are shown adjusting for age, gender and handedness. (A) Patients with FND compared to controls exhibited increased 1st link-step connectivity from the left M1-hand (right hand projected in left hemisphere) to bilateral pre-and postcentral gyri, supplementary motor area, middle cingulate cortices, and superior parietal lobule. In the 2nd link-step from the left M1-hand area, patients with FND compared to controls displayed increased connectivity to the bilateral posterior insula, temporoparietal junction (TPJ) and putamen (in addition to the areas found in the 1st-link step). In the 3rd link-step, patients with FND showed greater connectivity in bilateral dorsomedial prefrontal areas in addition to the regions observed in earlier steps. (B) Displays left M1-hand functional propagation differences between patients with FND compared to controls adjusting for depression and anxiety scores. (C-D) Depicts results obtained from the right M1-hand in patients with FND compared to controls, which are similar to those observed for the left M1-hand region.
From the right-M1 hand area, patients with FND showed similar increased SFC profiles across all link-steps to those seen from the left-M1 hand compared to controls (Fig. 2). In post-hoc analyses adjusting for depression and anxiety, only increased link-step connectivity to the bilateral dorsomedial prefrontal cortex was observed in the 2nd and 3rd steps in patients with FND compared to controls.
Originating from the left-M1 foot, the 1st link-step did not show any between-group differences. In the 2nd and 3rd link-steps, patients with FND compared to controls demonstrated similar group-level differences as those described for the left and right M1 hand areas (Supplementary Fig. 3). Group-level differences did not remain significant adjusting for depression and anxiety. No other between-group differences were observed across the 6 motor ROIs.
Amygdala findings
From the right laterobasal amygdala, in the 2nd link-step patients with FND displayed enhanced propagation to the left > right anterior insula, bilateral parahippocampal and fusiform gyri, hippocampus, putamen, pallidum and the PAG compared to controls (Fig. 3). In the 3rd link-step, patients with FND showed increased connectivity to the left parahippocampal and fusiform gyri, PAG and hypothalamus. These between-group findings did not hold adjusting for depression and anxiety scores. There were no other SFC differences across the bilateral amygdala ROIs.
Figure 3. Altered stepwise propagation from the right laterobasal amygdala and an exploratory amygdala – motor interconnector analysis in patients with functional neurological disorder (FND).
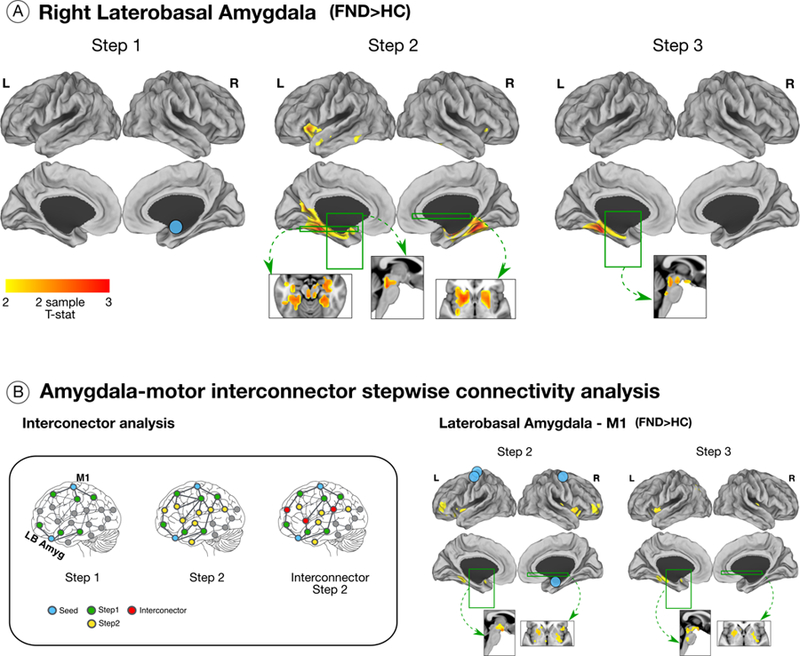
(A) Patients with FND compared to controls showed greater 2nd link-step connectivity from the right laterobasal amygdala to the left > right anterior insula, bilateral parahippocampal and fusiform gyri, hippocampus, putamen, pallidum, and the periaqueductal gray compared to controls. Patients with FND in the 3rd link-step also showed enhanced connectivity to left parahippocampal and fusiform gyri, hypothalamus and the periaqueductal gray compared to controls. These group-level differences in right laterobasal amygdala functional propagation did not remain significant adjusting for depression and anxiety. (B) In the left panel, green nodes represent voxels within one link-step from any of the amygdala or motor regions-of-interest (ROIs, blue nodes). The next link-step from green nodes are displayed in yellow (2nd link-step). The yellow nodes present in the 2nd link-step from both amygdala or motor regions are represented in red. The nodes displayed in red represent the interconnector regions where the propagation of information between different ROIs converge. In the right panel, uncorrected right-tailed T-test results are displayed. Patients with FND compared to controls showed enhanced 2nd link-step interconnector connectivity to bilateral anterior insula, dorsolateral prefrontal cortices, putamen, left fusiform gyrus and hypothalamus. In the 3rd link-step, higher connectivity was observed in left anterior insula, parahippocampal and fusiform gyri, right posterior insula, bilateral putamen, periaqueductal gray and hypothalamus in patients with FND compared to controls.
Exploratory interconnector analysis
When examining common M1 – right laterobasal amygdala functional propagation profiles, patients with FND compared to controls showed increased 2nd link-step connectivity to bilateral anterior insula, dorsolateral prefrontal cortex (dlPFC), putamen, left fusiform gyrus and the hypothalamus (Fig. 3). In the 3rd link-step, patients with FND compared to controls showed greater connectivity to the left anterior insula, parahippocampal and fusiform gyri, right posterior insula, bilateral putamen, PAG and hypothalamus (Fig. 3).
Within-group symptom severity analyses
There were no statistically significant relationships between motor or amygdala SFC profiles and SOMS:CD-PHQ15 scores in patients with FND.
SOMS:CD-PHQ15 scores positively correlated with enhanced left anterior insular 1st link-step connectivity to the right anterior-middle insula, MCC, inferior frontal gyrus, TPJ, bilateral precuneus, and post-central gyri in patients with FND (Fig. 4). In the 2nd link-step, FND symptom severity also positively correlated with increased left anterior insula link-step connectivity to the right anterior-middle insula, inferior frontal gyrus, premotor, SMA, TPJ, bilateral dorsal ACC/MCC, precentral/postcentral gyri, and occipital areas (Fig. 4). The 3rd link-step showed similar findings to the 2nd link-step. Of note, using Cook’s distance there were no outliers.
Figure 4. Insular stepwise functional connectivity correlated with symptom severity in patients with functional neurological disorder (FND).
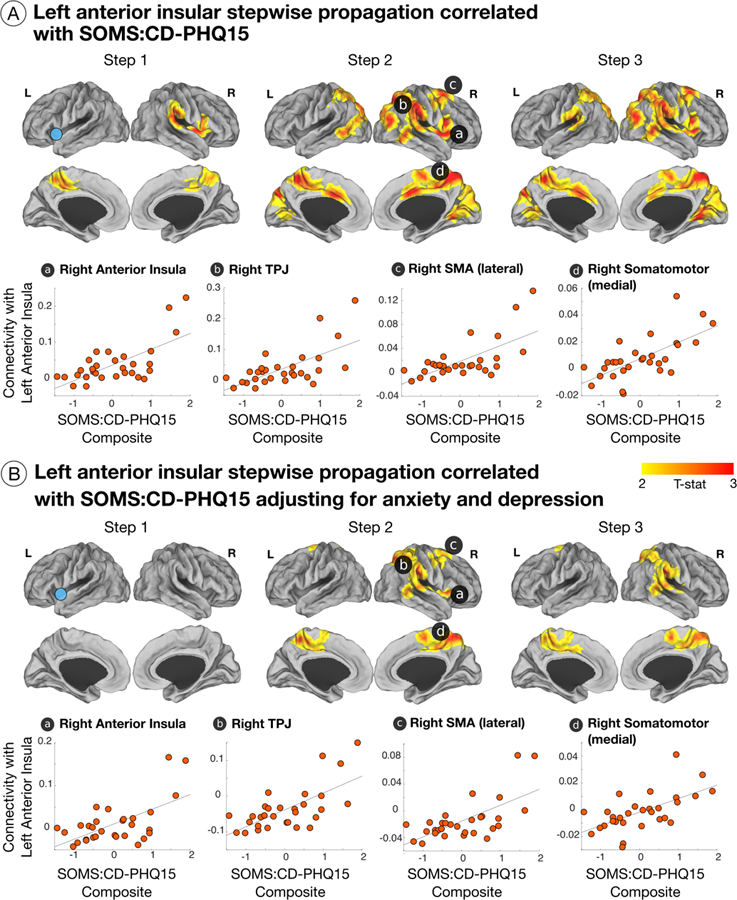
(A) 1st link-step showed a positive correlation between patient-reported symptom severity and increased left anterior insula to right anterior insula, middle cingulate cortex, inferior frontal gyrus, temporoparietal junction (TPJ), bilateral precuneus, and postcentral gyri link-step connectivity. For the 2nd link-step, this correlation included the right anterior insula, inferior frontal gyrus, premotor, supplementary motor area (SMA), TPJ, bilateral dorsal anterior cingulate/middle cingulate cortices, precentral/postcentral gyri, and occipital areas. The 3rd link-step showed similar results to the 2nd link-step. Scatter plots display findings with a T statistic > 4 in the 2nd link-step. (B) Displays the correlations between patient-reported symptom severity and left anterior insula functional propagation profiles adjusting for anxiety and depression.
In post-hoc analyses adjusting separately for depression/anxiety and SSRI/SNRI medication use, correlations between SOMS:CD-PHQ15 scores and left anterior insular enhanced 2nd link-step connectivity to the right anterior insula, TPJ, bilateral pre/post central gyri, and SMA remained statistically significant. Increased 2nd and 3rd link-step propagation to the dorsal ACC/MCC held adjusting for SSRI/SNRI medication use, but not for depression/anxiety. In post-hoc analyses accounting for motor FND-subtypes, FND symptom severity positively correlated with enhanced left anterior insula to right TPJ link-step connectivity across all subgroups; positive correlations between increased left to right anterior insular link-step connectivity and SOMS:CD-PHQ15 scores were most appreciable in those with functional weakness (Supplementary Fig. 4).
Within-group naturalistic 6-month outcome pilot
6-month improvement in SOMS:CD-PHQ15 scores positively correlated with left centromedial amygdalar 1st link-step connectivity to the right anterior insula, dorsal ACC/MCC, putamen and TPJ (Fig. 5). Baseline left centromedial amygdalar 2nd link-step connectivity to the right anterior insula and putamen also predicted 6-month improvement in SOMS:CD-PHQ15 scores. No outliers were identified in this 22-subject cohort using Cook’s distance. A post-hoc stratified analysis (7 most improved, 7 least improved, 8 with medium-range outcomes), showed that the relationship between baseline left centromedial amygdala – right anterior insula 1st link-step stepwise functional connectivity and clinical improvement was driven by increased centromedial amygdala-anterior insula connectivity in the 7 most improved individuals compared to the other subgroups. In analyses adjusting separately for baseline depression/anxiety and antidepressant use, only associations between 1st link-step left centromedial amygdala – right anterior insula connectivity and 6-month improvement remained statistically significant.
Figure 5. Pilot stepwise functional connectivity correlations with 6-month clinical improvement in patients with functional neurological disorder (FND).
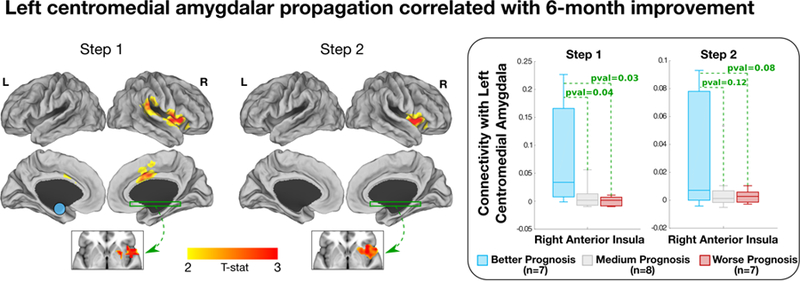
The 1st link-step showed a correlation between better 6-month clinical outcomes and higher left centromedial amygdala connectivity to the right anterior insula, putamen, dorsal anterior cingulate cortex/middle cingulate cortex and TPJ. For the 2nd link-step, improvement positively correlated with left centromedial amygdala connectivity to the right anterior insula and putamen. In additional post-hoc analyses, patients with FND were stratified in three groups: better prognosis (N=7), medium prognosis (N=8) and worse prognosis (N=7). Boxplots show that the relationship between left centromedial amygdalar – right anterior insula 1st link-step stepwise functional connectivity and clinical improvement was driven by increased centromedial amygdala-anterior insula connectivity in the 7 most improved individuals compared to the other sub-groups.
Across the right M1-hand and left M1-foot ROIs, enhanced link-step connectivity to the left inferior temporal gyrus positively correlated with 6-month improvement in SOMS:CD-PHQ15 scores. Only the left M1-foot findings held adjusting for depression/anxiety and antidepressant use (See Supplementary Fig. 5 and Supplementary Fig. 6). No other ROIs showed any statistically significant findings.
DISCUSSION
Consistent with hypotheses, patients with FND compared to controls showed enhanced functional propagation from primary motor areas and amygdalar sub-nuclei to the multimodal integration network. Specifically, patients with FND exhibited increased link-step connectivity between motor regions and the bilateral posterior insula, MCC, TPJ, and putamen. Increased linked-step connectivity from the primary motor cortex to the bilateral MCC and right TPJ remained significant adjusting for group-level depression and anxiety scores. Beginning from the right laterobasal amygdala, the FND cohort also showed enhanced connectivity to the left anterior insula, bilateral parahippocampal/fusiform gyri, hippocampus, basal ganglia, PAG and hypothalamus; these findings, however, did not hold adjusting for depression and anxiety scores. An exploratory interconnector analysis demonstrated that link-steps to the bilateral anterior insula, dlPFC, putamen, PAG, and hypothalamus were altered across motor and amygdalar pathways in FND patients. In within-group analyses, patient-reported symptom severity positively correlated with enhanced link-step connectivity from the left anterior insula to the right anterior insula, TPJ, pre-and post-central gyri, and SMA. In a pilot analysis, increased baseline link-step connectivity from the left centromedial amygdala to the right anterior insula correlated with a more favorable 6-month prognosis. Both symptom severity and outcome related within-group findings held adjusting for depression/anxiety and antidepressant use. Collectively, these findings support aberrant delivery of motor and limbic information to higher-order multimodal integration areas in FND.
Patients with FND have been reported to exhibit an attentional bias for functional motor symptoms,42,43 loss of sensory attenuation,44 and impaired interoceptive accuracy.45 In addition, some individuals with paroxysmal functional symptoms demonstrate heightened somatic and autonomic arousal without the subjective experience of anxiety (“panic without panic”).46 Given the enhanced link-step connectivity from primary motor cortex to posterior insula and MCC observed in this study, along with heightened laterobasal amygdala to bilateral anterior insula SFC, these findings support that impaired interoceptive processing (posterior insula) and altered emotional and self-awareness (anterior insula) may be implicated in the pathophysiology of FND.47 Regarding loss of sensory attenuation previously identified in FND,44 it is possible that enhanced primary motor cortex to posterior insula stepwise connectivity observed in our cohort relates to this phenomenon and warrants further inquiry. Furthermore, the insula and MCC are convergent zones for negative emotional processing, nociception and cognitive control,48 which may shed light on the neural mechanisms driving the multiplicity of sensorimotor, viscerosomatic, affective, and cognitive symptoms frequently endorsed by FND populations. Our findings also suggest that enhanced functional propagation from primary motor cortex to posterior insula, and laterobasal amygdala to anterior insula, may be at the intersection of FND symptoms and negative affect, given that these relationships did not remain significant adjusting for depression and anxiety. Interoceptive awareness tasks, particularly at the intersection of interoception and emotion processing, are needed to clarify the observations of this study.
The finding of enhanced link-step connectivity from the right laterobasal amygdala to the PAG and hypothalamus in patients with FND delineates a potential candidate mechanistic pathway. The laterobasal amygdala receives thalamic sensory afferents, and through interneurons, connects to the centromedial nucleus of the amygdala (the output center projecting to the PAG and hypothalamus).49 In healthy subjects, SFC propagation from the laterobasal amygdala showed strong inter-amygdalar connectivity at the first link-step (Fig. 1). By the 2nd link-step in healthy controls, the laterobasal amygdala connected to the perigenual and dorsal ACC; particularly appreciable in the 3rd link-step was downstream propagation to the PAG and hypothalamus. Given that physical events can precipitate functional neurological symptoms,50 and that some individuals with FND endorse sensory triggers, enhanced laterobasal (sensory) amygdalar link-step functional connectivity in patients with FND suggests a pathway for early sensory-amygdalar influence over the PAG. Notably, the PAG is implicated in pain modulation, homeostasis, autonomic responses, and defensive behaviors (including tonic immobility and freezing).51 PAG hyperactivity has also been identified in patients with FND engaged in emotion processing.5 Increased laterobasal amygdala-PAG connectivity may also relate to sympathetic-parasympathetic imbalances characterized in FND cohorts,52 and enhanced amygdalar–hypothalamic connectivity is consistent with reported links between threat vigilance and salivary cortisol levels in patients with FND.53
Primary motor areas exhibiting enhanced link-step connectivity to the posterior insula, SMA, and putamen is consistent with published reports of heightened connectivity between motor execution and limbic-paralimbic areas in FND populations.7,8,11–13,18 The exploratory interconnector analysis suggests that the bilateral anterior insula, dlPFC, putamen, PAG, and hypothalamus may be common points of altered functional propagation across motor and amygdalar pathways. The dlPFC is implicated in executive control and top-down emotion regulation. Given that dlPFC-related functional alterations have been characterized in several FND studies,8,18,54 non-invasive dlPFC modulation to modify motor-limbic activity trans-synaptically may be a therapeutic intervention warranting study.
In within-group analyses adjusting for depression/anxiety and antidepressant use, a positive correlation was observed between FND symptom severity and enhanced link-step connectivity from the left anterior insula to the right anterior insula, TPJ, SMA and sensorimotor areas. We have previously characterized reduced left anterior insular volume in patients with FND associated with symptom severity.28,29 Our functional propagation findings suggest that while the left anterior insula may be an important node in the pathophysiology of FND, a network perspective incorporating the impact of abnormal integration of information from the left anterior insula to the right anterior insula, TPJ and motor regions sheds additional light on brain-symptom severity relationships. In addition, tasked-based6,55 and resting-state neuroimaging abnormalities23–25 in the TPJ/inferior parietal lobule have been characterized in FND cohorts, and linked to impaired motor attention awareness and self-agency. Enhanced insular-TPJ link-step connectivity correlating with symptom severity in our study, and previously described task-based55 and functional connectivity alterations25 across insular and TPJ areas, suggest that these epicenters both play important roles in promoting altered awareness in individuals with FND.
To date, no published studies have identified functional MRI biomarkers of prognosis in patients with FND. In a pilot analysis, individual differences in link-step connectivity from the left centromedial (output) amygdala to the right anterior insula predicted 6-month improvement. Given that appreciating links between mood, psychosocial stressors and FND symptoms are core components of psychotherapy for FND,37 we speculate that relative increases in baseline connectivity between the centromedial (output) amygdala and anterior insula may be a marker of preserved emotional awareness that potentially aids treatment response. In addition, analyses showed a possible connection between baseline primary motor cortex-left inferior temporal gyrus link-step connectivity and outcome, which may relate to emotional-linguistic processing. Larger scale studies are needed to comprehensively elucidate biomarkers predicting clinical outcomes.
Limitations include modest sample size, psychiatric comorbidities, psychotropic medication use, phenotypic heterogeneity, and sole reliance on patient-reported symptom severity measures. Given that patients with FND commonly have psychiatric comorbidities, future studies with psychiatric controls are needed to clarify our between-group findings, particularly given that some nodes in the neurobiology of FND (e.g. insula, amygdala) may be at the intersection of affective and somatic symptoms. Studies characterizing functional propagation profiles in mood/anxiety, trauma-related disorders and other functional medical syndromes are also needed to better contextualize the findings of this study. In addition, while individuals with FND frequently exhibit mixed symptoms and/or can develop new functional symptoms over the course of their illness, the use of a transdiagnostic research approach remains debated.30,56 In this study, we showed that enhanced left anterior insula to right TPJ link-step connectivity correlated with symptom severity across motor subtypes, while links between FND symptom severity and increased left to right anterior insula connectivity was most appreciable in those with functional weakness. These findings suggest some common pathophysiologic elements across motor FNDs, as well as the potential for distinct neurobiological features. More research is needed to combine patient-report scales with objective measures, as well as combining neuroimaging and questionnaire data with behavioral, neuroendocrine, and autonomic measures. Furthermore, more research is needed to investigate the relationships between primary motor cortex functional propagation profiles and specific FND symptoms (e.g. functional hand weakness vs. functional gait). Serially collected longitudinal neuroimaging data is also needed to contextualize if the findings reported here are disease-related or representative of compensatory mechanisms; additional research is needed to identify neurocircuit nodes that are essential for symptom generation, and those alterations that more closely relate to predisposing vulnerabilities and perpetuating factors. Furthermore, in addition to an important role for the multimodal integration network, altered self-referential processing and contextual appraisal warrant additional research in patients with FND.
In conclusion, we applied network graph-theory approaches to identify enhanced propagation from primary motor cortex and laterobasal amygdala to cortical-subcortical areas implicated in salience, interoception, stress responses and self/emotional awareness. Altered link-step functional connectivity from the left anterior insula to the right anterior insula and TPJ may relate to symptom severity, while centromedial amygdala–anterior insula interactions require more study as a possible outcome biomarker. These findings suggest potential candidate neurocircuit pathways in the pathophysiology of FND.
Supplementary Material
One sample T-test thresholded at p-value 0.01 corrected for multiple comparisons is shown. In the 1st link-step from the left laterobasal amygdala, controls showed propagation to bilateral amygdala regions. In the 2nd link-step, controls exhibited connectivity to bilateral anterior-dorsal anterior cingulate and ventromedial prefrontal cortices, anterior insula, temporal pole, parahippocampus, lateral occipital, anterior hippocampal and periaqueductal gray. In the 3rd link-step, patients also showed propagation to bilateral frontopolar cortices, inferior frontal gyri, precuneus, left dorsolateral prefrontal cortex and hypothalamus in addition to the areas appreciated in earlier link-steps. Beginning from the right centromedial amygdala, controls showed 1st link-step connectivity to adjacent bilateral amygdala areas. In the 2nd link-step, controls exhibited propagation to the bilateral anterior-dorsal anterior cingulate, ventromedial prefrontal, inferior frontal, and dorsolateral prefrontal cortices, supplementary motor area, temporal pole, lateral occipital, putamen, right anterior insula, periaqueductal gray and hypothalamus. In the 3rd link-step, similar findings were appreciated except for the additional spread to medial occipital and left anterior insula areas. The left centromedial amygdala stepwise connectivity profile in healthy subjects was similar to the profile from the right centromedial amygdala.
Brain areas whose stepwise functional connectivity profile correlated with SOMS:CD-PHQ15 composite scores correcting for multiple comparisons included the bilateral anterior insula, inferior frontal gyri, dorsolateral prefrontal, dorsal anterior cingulate, and ventromedial prefrontal cortices, temporoparietal junction, inferior temporal, lateral occipital, amygdala (not within either the laterobasal or centromedial subnuclei), and right temporal pole. The distribution of the impact (how many altered links a region-of-interest (ROI) has summing all results surviving for multiple comparisons across the 1st, 2nd and 3th link-step) vs. all ROIs with statistically significant results shows that the left anterior insula is a highly robust area exhibiting stepwise functional connectivity alterations linked to symptom severity.
Maps displayed a general linear model comparing stepwise propagation in subjects with FND vs. controls. Only results surviving multiple comparisons are shown adjusting for age, gender and handedness. In the 1st link-step, there were no group-level differences. In the 2nd link-step, patients with FND compared to controls showed enhanced link-step connectivity to the right posterior-middle insula, rolandic operculum, putamen, bilateral pre-and postcentral gyri, temporoparietal junction, supplementary motor area, left premotor cortex and right inferior frontal gyrus. In the 3rd link-step, patients with FND compared to controls also showed increased propagation to bilateral posterior insula and putamen areas (in addition to areas appreciated in the 2nd link-step). These between-group findings did not remain significant adjusting for depression and anxiety scores.
In post-hoc analyses adjusting for separate motor subtypes, enhanced link-step connectivity from the left anterior insula to the right temporoparietal junction positively correlated with symptom severity across all subgroups. When adjusting for functional weakness, positive associations between left anterior insula to right anterior insula link-step connectivity did not remain significant, suggesting that the relationship between left to right anterior insula link-step connectivity and symptom severity was particularly driven by those with functional weakness.
In patients with FND, right M1-hand 1st link-step connectivity to bilateral occipital areas and dorsomedial thalamus positively correlated with clinical improvement; this result held adjusting for baseline antidepressant use but did not remain significant controlling for anxiety and depression scores. In the 2nd link step, right M1-hand propagation to the left inferior temporal gyrus also positively correlated with 6-month clinical improvement; this finding did not hold adjusting for depression and anxiety or antidepressant use.
In the 1st link-step, patients with FND showed that enhanced connectivity between the left M1-foot and bilateral inferior temporal, medial occipital and left superior parietal areas correlated with clinical improvement. In the 2nd link-step, left M1-foot connectivity to the bilateral inferior temporal gyri correlated with clinical improvement; left M1-foot connectivity to the left inferior temporal gyrus also correlated with clinical improvement in the 3rd link-step. Adjusting for baseline depression and anxiety scores or antidepressant use, enhanced connectivity between the left M1-foot and the left inferior temporal gyrus remained statistically significant across the 1st and 2nd link-steps.
Supplementary Table 1. Demographic characteristics of patients with functional neurological disorder.
Acknowledgements:
Funding:
D.L.P. was funded by the National Institute of Mental Health Grant K23MH111983–02, Massachusetts General Hospital Physician-Scientist Development Award, and the Sidney R. Baer Jr. Foundation. I.D. was supported by postdoctoral fellowship program from the Basque Country Government. This study was also supported by the NIH shared instrument grant S10RR023043.
Footnotes
Conflicts of Interest
All other authors report no conflicts of interest.
Disclosures:
B.C.D., consultant at Merck, Med Learning Group and Haymarket; royalties from Oxford University Press and Cambridge University Press; on the editorial board of Neuroimage: Clinical, Cortex, Hippocampus, Neurodegenerative Disease Management.
W.C.L., has served on the editorial boards of Epilepsia, Epilepsy & Behavior, Journal of Neurology, Neurosurgery and Psychiatry, and Journal of Neuropsychiatry and Clinical Neurosciences; receives editor’s royalties from the publication of Gates and Rowan’s Nonepileptic Seizures, 3rd ed. (Cambridge University Press, 2010) and 4th ed. (2018); author’s royalties for Taking Control of Your Seizures: Workbook and Therapist Guide (Oxford University Press, 2015); has received research support from the NIH (NINDS 5K23NS45902 [PI]), Rhode Island Hospital, the American Epilepsy Society (AES), the Epilepsy Foundation (EF), Brown University, the Siravo Foundation and the DoD (W81XWH-17–0169 [PI]); serves on the Epilepsy Foundation New England Professional Advisory Board; has received honoraria for the American Academy of Neurology Annual Meeting Annual Course; has served as a clinic development consultant at University of Colorado Denver, Cleveland Clinic, Spectrum Health and Emory University; and has provided medico legal expert testimony.
M.S.K., one-time consultant at Forum Pharmaceuticals; editor for Schizophrenia Research.
D.L.P., received honoraria from Harvard Medical School, the American Academy of Neurology, and the Movement Disorder Society.
References
- 1.Bass C, Peveler R, House A. Somatoform disorders: severe psychiatric illnesses neglected by psychiatrists. Br J Psychiatry 2001;179:11–4. [DOI] [PubMed] [Google Scholar]
- 2.Benbadis SR. The problem of psychogenic symptoms: is the psychiatric community in denial? Epilepsy Behav 2005;6:9–14. [DOI] [PubMed] [Google Scholar]
- 3.Stone J, LaFrance WC Jr., Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry 2010;167:626–7. [DOI] [PubMed] [Google Scholar]
- 4.Espay AJ, Aybek S, Carson A, et al. Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurol 2018;75:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aybek S, Nicholson TR, O’Daly O, et al. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One 2015;10:e0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aybek S, Nicholson TR, Zelaya F, et al. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 2014;71:52–60. [DOI] [PubMed] [Google Scholar]
- 7.Voon V, Brezing C, Gallea C, et al. Emotional stimuli and motor conversion disorder. Brain 2010;133:1526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris LS, To B, Baek K, et al. Disrupted avoidance learning in functional neurological disorder: Implications for harm avoidance theories. Neuroimage Clin 2017;16:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassa T, Sebastian A, Liepert J, et al. Symptom-specific amygdala hyperactivity modulates motor control network in conversion disorder. NeuroImage: Clin 2017;15:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pick S, Goldstein LH, Perez DL, et al. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry 2018 [DOI] [PMC free article] [PubMed]
- 11.Wegrzyk J, Kebets V, Richiardi J, et al. Identifying motor functional neurological disorder using resting-state functional connectivity. Neuroimage Clin 2018;17:163–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Kruijs SJ, Bodde NM, Vaessen MJ, et al. Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2012;83:239–47. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Liu K, Ma X, et al. Altered Functional Connectivity Patterns of the Insular Subregions in Psychogenic Nonepileptic Seizures. Brain Topogr 2015;28:636–45. [DOI] [PubMed] [Google Scholar]
- 14.Voon V, Cavanna AE, Coburn K, et al. Functional Neuroanatomy and Neurophysiology of Functional Neurological Disorders (Conversion Disorder). J Neuropsychiatry Clin Neurosci 2016;28:168–90. [DOI] [PubMed] [Google Scholar]
- 15.Cojan Y, Waber L, Carruzzo A, et al. Motor inhibition in hysterical conversion paralysis. Neuroimage 2009;47:1026–37. [DOI] [PubMed] [Google Scholar]
- 16.de Lange FP, Roelofs K, Toni I. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia 2007;45:2051–8. [DOI] [PubMed] [Google Scholar]
- 17.Stone J, Zeman A, Simonotto E, et al. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom Med 2007;69:961–9. [DOI] [PubMed] [Google Scholar]
- 18.Voon V, Brezing C, Gallea C, et al. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord 2011;26:2396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seignourel PJ, Miller K, Kellison I, et al. Abnormal affective startle modulation in individuals with psychogenic [corrected] movement disorder. Mov Disord 2007;22:1265–71. [DOI] [PubMed] [Google Scholar]
- 20.Sepulcre J Functional streams and cortical integration in the human brain. Neuroscientist 2014;20:499–508. [DOI] [PubMed] [Google Scholar]
- 21.Sepulcre J, Sabuncu MR, Yeo TB, et al. Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci 2012;32:10649–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz-Teran L, Diez I, Ortiz T, et al. Brain circuit-gene expression relationships and neuroplasticity of multisensory cortices in blind children. Proc Natl Acad Sci U S A 2017;114:6830–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology 2010;74:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baek K, Donamayor N, Morris LS, et al. Impaired awareness of motor intention in functional neurological disorder: implications for voluntary and functional movement. Psychol Med 2017;47:1624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer CW, LaFaver K, Ameli R, et al. Impaired self-agency in functional movement disorders: A resting-state fMRI study. Neurology 2016;87:564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sepulcre J, Sabuncu MR, Becker A, et al. In vivo characterization of the early states of the amyloid-beta network. Brain 2013;136:2239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmona S, Hoekzema E, Castellanos FX, et al. Sensation-to-cognition cortical streams in attention-deficit/hyperactivity disorder. Hum Brain Mapp 2015;36:2544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez DL, Williams B, Matin N, et al. Corticolimbic structural alterations linked to health status and trait anxiety in functional neurological disorder. J Neurol Neurosurg Psychiatry 2017;88:1052–59. [DOI] [PubMed] [Google Scholar]
- 29.Perez DL, Matin N, Barsky A, et al. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J Neurol Neurosurg Psychiatry 2017;88:491–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez DL, Dworetzky BA, Dickerson BC, et al. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci 2015;46:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DT, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Advances in Neurology 1995;65:231–57. [PubMed] [Google Scholar]
- 32.Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry 2014;85:180–90. [DOI] [PubMed] [Google Scholar]
- 33.LaFrance WC Jr., Baker GA, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia 2013;54:2005–18. [DOI] [PubMed] [Google Scholar]
- 34.Rief W, Hiller W. A new approach to the assessment of the treatment effects of somatoform disorders. Psychosomatics 2003;44:492–8. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258–66. [DOI] [PubMed] [Google Scholar]
- 36.Perez DL, Williams B, Matin N, et al. Anterior hippocampal grey matter predicts mental health outcome in functional neurological disorders: an exploratory pilot study. J Neurol Neurosurg Psychiatry 2018;89:1221–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology 2011;77:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen G, Stone J, Matthews A, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry 2015;86:1113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckner RL, Krienen FM, Castellanos A, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 2011;106:2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–52. [DOI] [PubMed] [Google Scholar]
- 41.Sepulcre J An OP4 functional stream in the language-related neuroarchitecture. Cereb Cortex 2015;25:658–66. [DOI] [PubMed] [Google Scholar]
- 42.Parees I, Kassavetis P, Saifee TA, et al. ‘Jumping to conclusions’ bias in functional movement disorders. J Neurol Neurosurg Psychiatry 2012;83:460–3. [DOI] [PubMed] [Google Scholar]
- 43.Parees I, Saifee TA, Kassavetis P, et al. Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor. Brain 2012;135:117–23. [DOI] [PubMed] [Google Scholar]
- 44.Parees I, Brown H, Nuruki A, et al. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain 2014;137:2916–21. [DOI] [PubMed] [Google Scholar]
- 45.Ricciardi L, Demartini B, Crucianelli L, et al. Interoceptive awareness in patients with functional neurological symptoms. Biological Psychology 2016;113:68–74. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein LH, Mellers JD. Ictal symptoms of anxiety, avoidance behaviour, and dissociation in patients with dissociative seizures. J Neurol Neurosurg Psychiatry 2006;77:616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- 48.Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 2011;12:154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeDoux J The amygdala. Curr Biol 2007;17:R868–74. [DOI] [PubMed] [Google Scholar]
- 50.Parees I, Kojovic M, Pires C, et al. Physical precipitating factors in functional movement disorders. J Neurol Sci 2014;338:174–7. [DOI] [PubMed] [Google Scholar]
- 51.Roelofs K Freeze for action: neurobiological mechanisms in animal and human freezing. Philos Trans R Soc Lond B Biol Sci 2017;372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurer CW, Liu VD, LaFaver K, et al. Impaired resting vagal tone in patients with functional movement disorders. Parkinsonism Relat Disord 2016;30:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakvis P, Spinhoven P, Roelofs K. Basal cortisol is positively correlated to threat vigilance in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2009;16:558–60. [DOI] [PubMed] [Google Scholar]
- 54.de Lange FP, Toni I, Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia 2010;48:1782–8. [DOI] [PubMed] [Google Scholar]
- 55.Nahab FB, Kundu P, Maurer C, et al. Impaired sense of agency in functional movement disorders: An fMRI study. PLoS One 2017;12:e0172502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanaan RAA, Duncan R, Goldstein LH, et al. Are psychogenic non-epileptic seizures just another symptom of conversion disorder? J Neurol Neurosurg Psychiatry 2017;88:425–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
One sample T-test thresholded at p-value 0.01 corrected for multiple comparisons is shown. In the 1st link-step from the left laterobasal amygdala, controls showed propagation to bilateral amygdala regions. In the 2nd link-step, controls exhibited connectivity to bilateral anterior-dorsal anterior cingulate and ventromedial prefrontal cortices, anterior insula, temporal pole, parahippocampus, lateral occipital, anterior hippocampal and periaqueductal gray. In the 3rd link-step, patients also showed propagation to bilateral frontopolar cortices, inferior frontal gyri, precuneus, left dorsolateral prefrontal cortex and hypothalamus in addition to the areas appreciated in earlier link-steps. Beginning from the right centromedial amygdala, controls showed 1st link-step connectivity to adjacent bilateral amygdala areas. In the 2nd link-step, controls exhibited propagation to the bilateral anterior-dorsal anterior cingulate, ventromedial prefrontal, inferior frontal, and dorsolateral prefrontal cortices, supplementary motor area, temporal pole, lateral occipital, putamen, right anterior insula, periaqueductal gray and hypothalamus. In the 3rd link-step, similar findings were appreciated except for the additional spread to medial occipital and left anterior insula areas. The left centromedial amygdala stepwise connectivity profile in healthy subjects was similar to the profile from the right centromedial amygdala.
Brain areas whose stepwise functional connectivity profile correlated with SOMS:CD-PHQ15 composite scores correcting for multiple comparisons included the bilateral anterior insula, inferior frontal gyri, dorsolateral prefrontal, dorsal anterior cingulate, and ventromedial prefrontal cortices, temporoparietal junction, inferior temporal, lateral occipital, amygdala (not within either the laterobasal or centromedial subnuclei), and right temporal pole. The distribution of the impact (how many altered links a region-of-interest (ROI) has summing all results surviving for multiple comparisons across the 1st, 2nd and 3th link-step) vs. all ROIs with statistically significant results shows that the left anterior insula is a highly robust area exhibiting stepwise functional connectivity alterations linked to symptom severity.
Maps displayed a general linear model comparing stepwise propagation in subjects with FND vs. controls. Only results surviving multiple comparisons are shown adjusting for age, gender and handedness. In the 1st link-step, there were no group-level differences. In the 2nd link-step, patients with FND compared to controls showed enhanced link-step connectivity to the right posterior-middle insula, rolandic operculum, putamen, bilateral pre-and postcentral gyri, temporoparietal junction, supplementary motor area, left premotor cortex and right inferior frontal gyrus. In the 3rd link-step, patients with FND compared to controls also showed increased propagation to bilateral posterior insula and putamen areas (in addition to areas appreciated in the 2nd link-step). These between-group findings did not remain significant adjusting for depression and anxiety scores.
In post-hoc analyses adjusting for separate motor subtypes, enhanced link-step connectivity from the left anterior insula to the right temporoparietal junction positively correlated with symptom severity across all subgroups. When adjusting for functional weakness, positive associations between left anterior insula to right anterior insula link-step connectivity did not remain significant, suggesting that the relationship between left to right anterior insula link-step connectivity and symptom severity was particularly driven by those with functional weakness.
In patients with FND, right M1-hand 1st link-step connectivity to bilateral occipital areas and dorsomedial thalamus positively correlated with clinical improvement; this result held adjusting for baseline antidepressant use but did not remain significant controlling for anxiety and depression scores. In the 2nd link step, right M1-hand propagation to the left inferior temporal gyrus also positively correlated with 6-month clinical improvement; this finding did not hold adjusting for depression and anxiety or antidepressant use.
In the 1st link-step, patients with FND showed that enhanced connectivity between the left M1-foot and bilateral inferior temporal, medial occipital and left superior parietal areas correlated with clinical improvement. In the 2nd link-step, left M1-foot connectivity to the bilateral inferior temporal gyri correlated with clinical improvement; left M1-foot connectivity to the left inferior temporal gyrus also correlated with clinical improvement in the 3rd link-step. Adjusting for baseline depression and anxiety scores or antidepressant use, enhanced connectivity between the left M1-foot and the left inferior temporal gyrus remained statistically significant across the 1st and 2nd link-steps.
Supplementary Table 1. Demographic characteristics of patients with functional neurological disorder.


