Abstract
Purpose:
To investigate receptor and post-receptor function in KCNV2 retinopathy (cone dystrophy with supernormal rod ERG), using the pupillary light reflex (PLR) and the electroretinogram (ERG).
Methods:
Two unrelated patients (1 male, 1 female) with molecularly confirmed KCNV2 retinopathy underwent full-field two-color pupillometry testing in one eye, with monitoring of the stimulated eye by an infrared digital camera. Pupillometry stimuli consisted of 1-sec duration, short-wavelength (465-nm, blue) and long-wavelength (642-nm, red) stimuli. Pupillometry intensity series were performed under both a dark-adapted condition and a light-adapted condition (on a 0.76-log cd-m−2 blue background). The transient PLR, defined as the maximum constriction following flash onset, was measured under all conditions. The melanopsin-mediated sustained constriction was measured 5 to 7 sec following flash offset for the highest flash luminance presented in the dark. Both patients were also tested in one eye with the full-field ERG, including a dark-adapted intensity series and ISCEV-standard stimuli.
Results:
Dark-adapted PLRs were markedly attenuated or extinguished for low luminance stimuli, but the responses to higher luminance blue stimuli were within normal limits. Light-adapted PLRs to blue stimuli were generally within normal limits, exceeding the responses to photopically matched red stimuli. Thus, light-adapted responses were consistent with either rod- or S-cone mediation of the PLR. Melanopsin-mediated sustained PLRs were within normal limits. ERG showed the characteristic findings previously reported in this condition. Cone-mediated ERG responses were markedly decreased in amplitude. Rod-mediated ERG responses were absent for low luminance stimuli (−3 log cd-s-m−2), but had normal amplitude for stimuli of −2 log cd-s-m−2 and above (although none were “supernormal”). The b-wave for dark-adapted ISCEV standard 0.01 stimulus was markedly delayed, whereas the b-wave timing was generally normal for higher flash luminances.
Conclusions:
The abnormalities measured by pupillometry have a similar pattern to the outer-retinal abnormalities measured by ERG in KCNV2 retinopathy. These findings, as well as the normal sustained PLR suggests that inner-retinal function may be preserved in KCNV2 retinopathy and highlights the potential for therapies designed to restore outer-retinal function in these individuals.
Keywords: KCNV2, KCNV2 retinopathy, cone dystrophy with supernormal rod ERG, pupillometry, pupillary light reflex
Introduction
Autosomal recessive mutations in the KCNV2 gene result in an inherited retinal degeneration (IRD)[1] that has been referred to as “cone dystrophy with supernormal rod ERG”[2] and “supernormal and delayed rod ERG syndrome”.[3] KCNV2 retinopathy[4, 5] is associated with unique full-field electroretinogram (ERG) abnormalities. Light-adapted ERG responses tend to be considerably reduced and delayed, consistent with other cone dystrophies. The dark-adapted ERGs, however, are unusual in that the ERGs elicited by low luminance stimuli are non-detectable, whereas the responses to high luminance stimuli are either normal or larger than normal (“super-normal”).[6–8]
Symptoms attributable to loss of cone function, including central vision reduction, are noted in the first few years of life in this retinal dystrophy.[6, 7, 9–11] Visual acuity commonly stabilizes around 20/200 Snellen by the 3rd decade, with very few adult patients documented to have significantly worse acuity.[7] A mix of other cone-related symptoms are often present, including photoaversion, color vision deficiency, and nystagmus.[5–7, 9, 10, 12] Nyctalopia, a rod-related symptom, is a common finding, but it is not reported in all cases, and it is often described as mild.[5, 7, 8, 10, 12] The cone ERG can become progressively attenuated over time, more so than the rod ERG, but ERG progression has not been observed consistently.[7, 10, 13]
The KCNV2 gene codes for a potassium channel protein, Kv8.2, which localizes to the cone and rod inner segments.[1] This protein does not form functional channels by itself, and it is therefore called a “modifier/silent” voltage gated potassium channel protein.[1, 14] The Kv8.2 protein combines with a Kv2 protein in heteromeric protein complexes.[15] The presence of Kv8.2 modulates the function of the Kv2 protein complex, causing a change in the “activation range” of the photoreceptor inner segment potassium channels. In a patient with KCNV2 mutations, the absence of Kv8.2 (or the presence of a less-functional version of the protein) would modulate the cell’s resting potential and activation range, and this is presumably the cause of at least some of the unusual KCNV2 retinopathy ERG findings. Precisely how this change leads to the dysfunction and degeneration seen in the KCNV2 retinopathy phenotype is still not fully understood. Gayet-Primo et al. (2018) and Hart et al. (2019) both offer thorough and recent discussions of the anticipated effects of KCNV2 mutations on photoreceptor function.[14, 16]
Although photoreceptor dysfunction has been well-documented in KCNV2 retinopathy, much less attention has been paid to potential inner-retinal abnormalities. A useful approach to evaluating the effects of outer-retinal dysfunction on the inner-retina is to record the response of the pupil to flashes of light (the pupillary light reflex; PLR). The pupillary response is largely mediated by a subset of retinal ganglion cells (RGCs) that are intrinsically photosensitive (ipRGCs), as they express the photopigment melanopsin. In addition to being intrinsically photosensitive, ipRGCs receive signals from rod and cone photoreceptors. Thus, the response of the pupil to a flash of light can be complex, in that it can be rod-, cone-, or melanopsin-mediated. The contribution of these three photoreceptor classes can be evaluated by manipulating the stimulus and adaptation characteristics under which the PLR is recorded. To our knowledge, the extent to which the PLR is affected in KCNV2 retinopathy is currently unknown. Also unknown is whether the unusual rod function measured by ERG (non-detectable low luminance responses, with large or super-normal high luminance responses) is also apparent in the pupil response. Thus, the primary purpose of this study was to evaluate PLRs mediated by the rod, cone, and melanopsin pathways as indices of outer- and inner-retinal function in patients with KCNV2 retinopathy.
Methods
Subjects
Patient 1 was a 30 year-old male from a consanguineous family of Pakistani descent. He had complaints of moderate photoaversion, moderate color deficiency, and reduced central vision. The color and central vision had been stable all his life, while the photoaversion had worsened between his teens and 20’s. He felt that his vision was better in the dark than in the light, but he did admit both to slow dark-adaptation and to some difficulty while walking under the dimmest conditions. His best-corrected visual acuity (BCVA) at age 16 had been 20/70–2 OD and 20/70–1 OS. At all subsequent visits it had been in the 20/100 to 20/200 range. His BCVA at the study visit was 10/60–2 OD and OS (Feinbloom Low Vision chart), with a highly myopic refraction of −13.00+1.25×100 OD and −13.00+1.25×081 OS. Goldmann visual fields at this visit showed normal peripheral boundaries and central scotomas to the III4e target in each eye. Fundus exam showed a grossly normal macula and retinal vessels in each eye, with likely myopia-related changes of peripapillary atrophy and a prominent choroidal pattern throughout the fundus. His optic nerve heads were somewhat anomalous, with horizontal elongation of the discs and a mildly pale appearance.
Patient 2 was a 24-year-old female of Iranian descent with poor central and color vision, as well as nyctalopia, and moderate photoaversion, all of which were stable. She had a history of congenital nystagmus that resolved around 4 years of age. Her BCVA at the study visit was 10/100+1 OD and 10/80 OS (Feinbloom chart), with a myopic refraction of −4.00 sphere OD and −3.00 sphere OS. BCVA had been around 20/200 in each eye at all prior visits back to age 7. Goldmann visual fields at the study visit showed normal peripheral boundaries and central scotomas to the V4e target in each eye. Fundus exam showed “bull’s eye” type atrophy in the macula of both eyes and normal retinal vessels. Peripapillary atrophy was present in each eye, and both optic nerves had mild temporal pallor.
In addition to the two KCNV2 retinopathy patients, 6 age-similar, visually-normal control subjects (mean age 30.2 years, standard deviation of 4.4 years) also participated. The visually-normal control subjects had no history of disease, normal color vision (Oculus Heidelberg Multi-Color Anomaloscope), and ETDRS best-corrected visual acuity of 0 log MAR or better. Informed consent was obtained from all subjects before their participation. Procedures adhered to the tenets of the Declaration of Helsinki, and the protocol was approved by the Western and University of Illinois at Chicago IRBs.
Genotyping
Patient 1 was genotyped by the Casey Eye Institute Diagnostic Laboratory at the Oregon Health & Science University. His brother and more distant relatives were affected as well. Patient 1 was found to be homozygous for a four base deletion Lys3 del4AACA (c.8_11delAACA) of the KCNV2 gene. Patient 2 was genotyped by the John and Marcia Carver Nonprofit Genetic Testing Laboratory. She was found to be compound heterozygous for two mutations: Glu219Stop (c.655G>T) and Pro296 del2cCA (c.887_888 delCA). Only the Pro296 del2cCA variant was found in her unaffected mother.
Full-field ERG
All subjects underwent a dark-adapted full-field ERG in one pharmacologically dilated eye (tropicamide 1% and phenylephrine 2.5%). Included in the dark-adapted testing were three ISCEV standard responses (0.01, 3.0, and 10.0 cd-s-m−2), plus a dim 0.001 cd-s-m−2 stimulus. The light-adapted ISCEV standard 30-Hz flicker and single-flash 3.0 cd-s-m−2 responses were tested as well.[17] ERG stimuli were generated by a Diagnosys ColorDome ganzfeld (Diagnosys LLC, Lowell, MA, USA) and recorded using conventional techniques using a fiber DTL-plus recording electrode (Diagnosys LLC, Lowell, MA) as the positive electrode.
Pupillometry
All subjects underwent full-field pupillometry testing according to methods that have been described in detail elsewhere.[18–20] The untested eye was patched. After 10 minutes of dark adaptation, a pupillometry luminance intensity series was performed, with 1-sec stimuli ranging from −4 log (photopic) cd-m−2 to 2.65 log cd-m−2, using both long-wavelength (642-nm, “red”) and short-wavelength (465-nm, “blue”) stimuli. A light-adapted intensity series utilizing both colors was also performed, with a 0.78-log cd-m−2 blue rod-suppressing background and stimuli ranging from −1 to 2.65 log cd-m−2. The PLR was normalized by the mean pupil size during the 1-sec pre-stimulus period. Transient response amplitudes were measured for each stimulus as the maximal pupillary constriction shortly after stimulus onset. A sustained melanopsin-mediated pupil response was measured as the median pupil size between 5 and 7 seconds after offset of the dark-adapted 2.65 log cd-m−2 blue stimulus.[18, 19]
Results
Full-field Electroretinogram
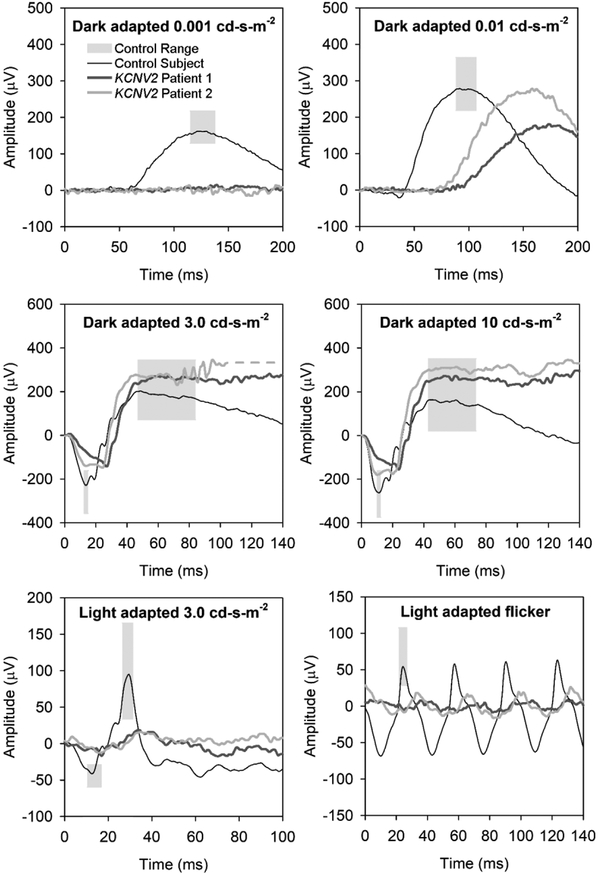
Figure 1 shows the full-field ERG responses from patients 1 (OD) and 2 (OD). The waveforms were highly similar for the two subjects, showing several features common to KCNV2 retinopathy. To the low luminance 0.001 cd-s-m−2 stimulus, both patients had non-detectable responses. The dark-adapted rod-isolated (0.01 cd-s-m−2) response amplitude was within normal limits in patient 2 and mildly sub-normal in patient 1. However, the implicit time (time to peak) of the b-wave was delayed well beyond the control range for both patients. Also, delays of the latency (time of onset) of the b-waves were grossly evident. For the dark-adapted combined-response stimuli (3.0 and 10.0 cd-s-m−2), a-waves had an elongated, squared shape, with amplitudes near the lower limit of normal; b-wave amplitudes to these stimuli were within normal limits (neither sub-normal, nor super-normal). Under the light-adapted 3.0 cd-s-m−2 condition, both the a-wave and b-wave single flash responses were reduced in amplitude, and the b-waves were delayed. Similarly, the trough-to-peak amplitude was reduced to below half the lower limit of controls, and the peak time was delayed for the 3.0 cd-s-m−2 30-Hz flicker (lower right panel).
Figure 1.
Full-field electroretinogram in KCNV2 retinopathy patient 1 (dark gray traces) and 2 (medium gray traces). A representative control trace is shown in black, and light gray boxes represent the a- and b-wave control ranges for amplitude and implicit time (time to peak amplitude). The dark-adapted rod-isolated responses are shown in the top two panels, with a very dim flash (0.001 cd-s-m−2) response in the upper left panel and the ISCEV-standard rod-isolated stimulus (0.01 cd-s-m−2) in the upper right panel. The middle panels show dark-adapted ISCEV-standard combined (rod and cone) responses, with the 3.0 cd-s-m−2 response in the middle left and the 10.0 “maximal” response in the middle-right panel. The ISCEV standard light-adapted cone-isolated responses are shown in the bottom panels, with the 3.0 cd-s-m−2 single-flash response in the lower left panel and the 30-Hz flicker in the lower right panel.
Pupillometry
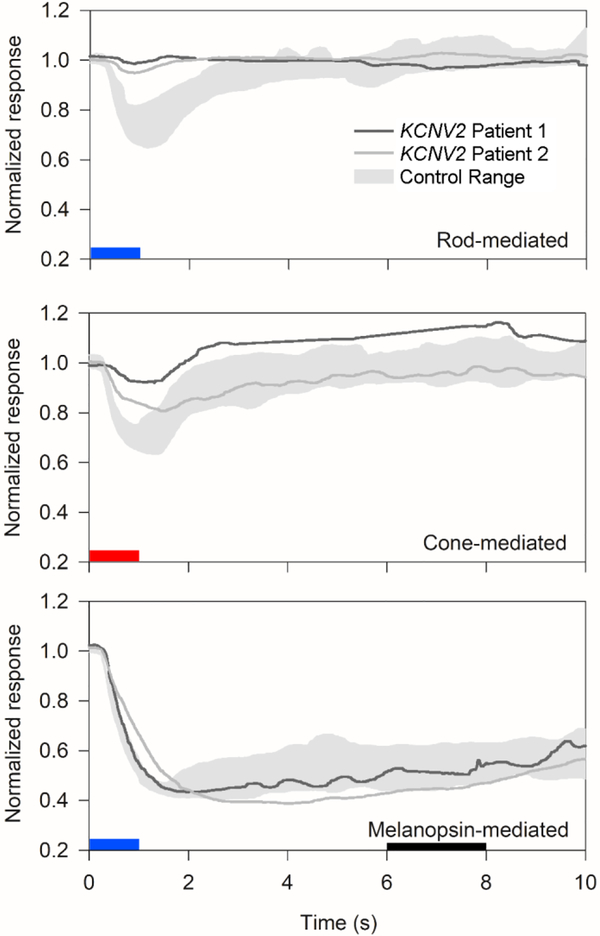
Figure 2 shows the PLR under three commonly recorded[18, 19] conditions: rod-mediated, cone-mediated and melanopsin-mediated for patient 1 (OD) and patient 2 (OS). The dark-adapted rod-mediated stimulus (−3-log cd-m−2, short-wavelength) elicited a robust transient constriction in normal subjects, but only very small responses from the two KCNV2 retinopathy patients. The PLR elicited by the light-adapted, cone-mediated stimulus (1-log cd-m−2 long-wavelength on a 0.78-log cd-m−2 short-wavelength background) was reduced for patient 1 and mildly subnormal for patient 2. The melanopsin-mediated sustained response was within normal limits for both patients.
Figure 2.
Pupillary light reflex to rod-, cone-, and melanopsin-mediated stimuli. As in Figure 1, dark gray traces represent patient 1, medium gray traces represent patient 2, and light gray zones represent control range for each stimulus. The pupillary light reflex is plotted as a proportion of baseline pupillary diameter (y-axis) versus time after onset of a 1-second full-field stimulus, represented by blue (for short-wavelength stimulus) or red (for long-wavelength stimulus) boxes. The top panel shows the rod-isolated transient response to a dim short-wavelength stimulus. The middle panel shows the nominally cone-isolated response to a long-wavelength stimulus on a rod-suppressing short-wavelength background light. The bottom panel shows the melanopsin-mediated sustained response, measured at 5–7 seconds after stimulus offset (represented by a black box).
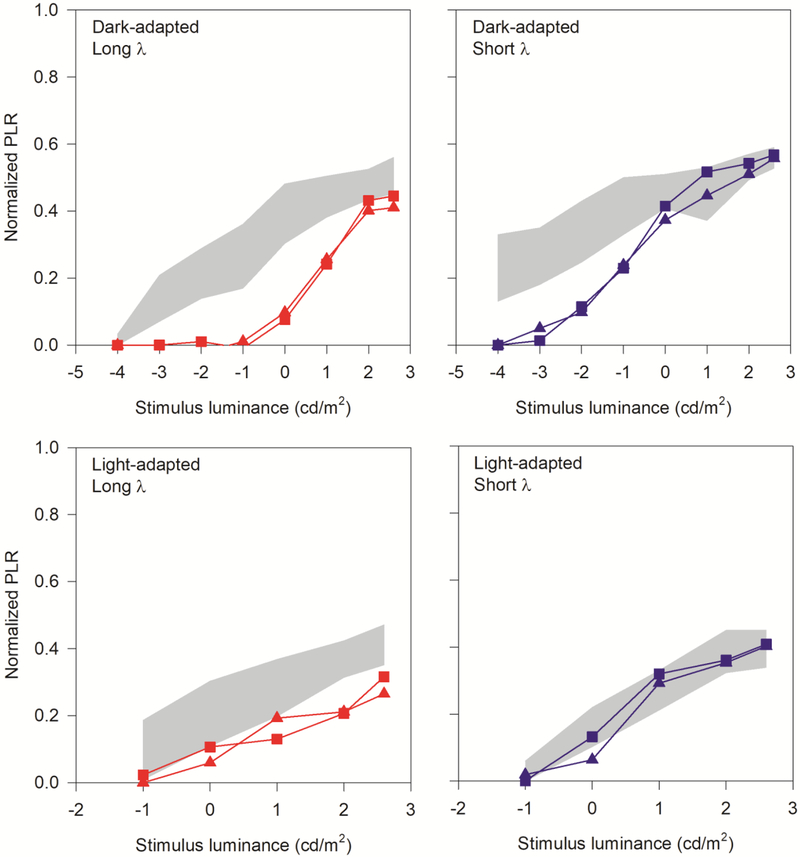
Figure 3 shows the transient PLR as a function of log flash luminance for patient 1 (squares) and patient 2 (triangles). The range of normal for the 6 control subjects is marked by the gray regions. The top row shows the PLRs obtained under dark-adapted conditions for long wavelength (red; left panel) and short wavelength (blue; right panel) stimuli. The bottom row shows the PLRs obtained under light-adapted conditions for long wavelength (red; left panel) and short wavelength (blue; right panel) stimuli. The PLRs for the two patients were highly similar to one-another across all conditions. The dark-adapted long-wavelength stimulus was non-detectable for the four lowest flash luminances. The PLRs were recordable, but reduced for moderate flash luminance (0 and 1 log cd/m2), and slightly subnormal for the two highest flash luminances. Overall, the patients’ functions appear to be shifted rightward by approximately 3 log units relative to controls. Likewise, the dark-adapted short-wavelength stimulus (upper right panel) was non-detectable (or markedly attenuated) for the two lowest flash luminances. The PLRs were recordable, but reduced for moderate flash luminance (−2 and −1 log cd/m2), and within the normal range for the highest flash luminances. The PLR versus luminance function measured with the short-wavelength stimuli appeared to be shifted rightward for the patients compared to the controls by 2–3 log units. Of note, super-normal PLRs were not observed for any of the dark-adapted stimuli. Light-adapted responses (lower two panels of Figure 3) were generally reduced at all luminance levels for the long wavelength stimulus (lower left panel), whereas the short-wavelength responses were generally within the lower range of normal (lower right panel).
Figure 3.
Pupillary light reflex luminance series under dark-adapted (top row) and light adapted (bottom row, short-wavelength background) conditions, with red (long-wavelength) stimuli in the right column, and blue (short-wavelength) stimuli in the right column. Control ranges for pupil constriction to each stimulus are shown as gray shaded areas on each graph. KCNV2 retinopathy patients 1 and 2 are represented by squares and triangles, respectively.
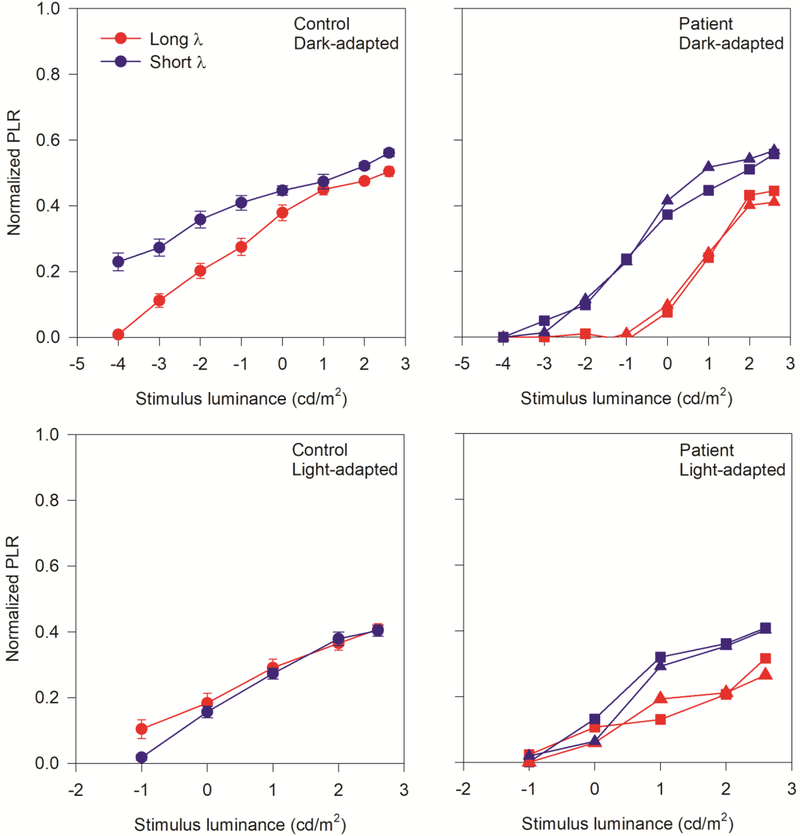
Further analysis was performed by re-plotting the short and long wavelength PLR data of Fig. 3 together in Fig. 4. This figure allows direct comparisons of the PLRs measured with long- and short-wavelength stimuli for the controls and KCNV retinopathy patients. As in Figure 3, the dark-adapted PLRs are shown in the top panels, and the light-adapted PLRs are in the bottom panels. In the dark-adapted conditions, the control subjects (upper left panel) had larger PLRs for the short-wavelength stimulus compared to the long-wavelength stimulus for the same luminance (photopic units), at low flash luminance levels. Large differences in PLR for the two wavelengths under dark-adapted conditions are consistent with mediation by the rod pathway. For high luminance flashes, the control PLRs were nearly equivalent for the long- and short-wavelength stimuli, consistent with mediation by the cone pathway. Likewise, the PLRs elicited by the two wavelengths were approximately equivalent under the light-adapted (presumably cone-mediated) condition for the controls (lower left). The pattern of PLR responses observed for the two KCNV2 retinopathy patients differed considerably from that of the controls. For both KCNV2 retinopathy patients, there was no measurable PLR for low luminance stimuli. For stimulus luminance levels great enough to elicit a PLR, there was substantial differences between the short- and long-wavelength stimuli, with PLRs larger for the short-wavelength (blue) than the long wavelength (red). For the highest flash luminances, differences between the PLRs elicited by short- and long-wavelengths remained, suggesting that the cone pathway may not fully mediate the response to both the short- and long-wavelengths for the KCNV2 retinopathy patients. Similarly, there were clear differences between the PLRs elicited by high luminance short- and long-wavelength stimuli under photopic conditions (lower right panel). This is in marked contrast to the control data, where photopically matched short- and long-wavelength stimuli elicit similar PLRs under photopic conditions. Table 1 summarizes the ERG findings common to KCNV2 retinopathy patients alongside the PLR findings from this report.
Figure 4.
Pupillary light reflex luminance series under dark-adapted (top row) and light adapted (bottom row) conditions. Responses to red (long-wavelength) and blue (short-wavelength) stimuli are shown in each panel. Control means with standard error in brackets are shown in the left column, and the two KCNV2 retinopathy patients are shown in the right column. Control subjects are represented by circles, and patients 1 and 2 are represented by squares and triangles, respectively.
Table 1.
Summary of full-field electroretinogram (ERG) and full-field pupillary light reflex (PLR) findings in KCNV2 retinopathy
| Test stimulus | Predominant photoreceptor | KCNV2 retinopathy findings (relative to control ranges) | |
|---|---|---|---|
| ERG | LA 0.5 log (3.0 cd-s-m2)* and 30-Hz flicker* | Cone system | Moderate-marked decrease |
| DA −3 log (0.001 cd-s-m2) | Rod system | Non-detectable | |
| DA −2 log (0.01 cd-s-m2)* | Rod system | Variably decreased, markedly delayed | |
| DA 0.5 log (3.0 cd-s-m2)* and 1.0 log (10.0 cd-s-m2) | Rod+cone systems | a-wave near lower limit of normal, "rhomboid" shape; b-wave upper range of normal or supernormal | |
| PLR | LA 1 log (10.0 cd-m2) LW transient | Cone system | Moderate decrease |
| DA −3 log (0.001 cd-m2) SW transient | Rod system | Nearly non-detectable | |
| DA 2.65 log (450 cd-m2) SW transient | Rod+cone systems | Amplitude within normal limits (delayed in Patient 2) | |
| DA 2.65 log (450 cd-m2) SW sustained | Melanopsin | Amplitude within normal limits |
Abbreviations: ERG = full-field electroretinogram; LA = light-adapted; DA = dark-adapted; SW = short wavelength (~blue); LW = long-wavelength (~red); PLR = pupillary light reflex;
ISCEV-standard minimum protocol
Discussion
In this paper, we demonstrate for the first time pupillometric measurements in patients with KCNV2 retinopathy. We found detectable pupillary responses for moderate to high luminance stimuli, including responses to high-luminance short-wavelength stimuli that were within normal limits. The sustained pupillary responses were within normal limits, indicating that ipRGC function is preserved. The pupillometry results were to a degree analogous to the ERG results, suggesting that at least some of the abnormalities due to outer-retinal dysfunction are also apparent at a more proximal location in the visual system. As with the ERGs in these two patients, there were no “super-normal” pupil responses, even to higher luminance stimuli under dark adapted conditions.
Our KCNV2 retinopathy patients had non-specific atrophic changes in the macula, mild disc pallor, as well as additional fundus changes that can be attributed to high myopia. Macular changes on examination and fundus autofluorescence (FAF) tend to be subtle in the first decades,[7–9, 12, 13] whereas many older patients can have atrophic foveal lesions with corresponding hypo-AF.[7, 12] Progression of macular changes has been documented longitudinally.[7, 13, 21] These macular findings are all consistent with cone dystrophy, but none have been found to be specific to KCNV2 retinopathy.[7, 11]
The ERG findings in KCNV2 retinopathy have been well documented in prior studies.[3, 5, 7, 10] Many patients may not display the characteristic “supernormal” dark-adapted b-wave.[7, 9] The diagnosis, therefore, may often be missed if examiners are unaware of other clues that can be found in ERG responses to standard stimuli.[6, 9] Both of our subjects demonstrated ERG findings (Figure 1) that are common for this condition. Dark-adapted findings included non-detectable responses to very dim stimuli, much delayed responses to the standard rod-isolated stimulus, a squared-off a-wave to combined response stimuli,[7, 10] and larger than expected (but not supernormal) combined response b-waves, given that their a-waves were at the lower limit of normal.[22] Light-adapted ERG responses were considerably reduced in amplitude. As others have suggested, the very delayed rod-isolated ERG (in the presence of markedly reduced cone responses) may be the best marker of this condition among the findings on an ISCEV standard full-field ERG.[6] Non-standard ERG stimuli in the form of a dark-adapted intensity series can make the phenotype more readily apparent, especially the relatively rapid increase in b-wave amplitude with increasing stimulus intensity.[3, 5, 7, 10, 13, 23]
For low luminance, dark-adapted pupillometry stimuli, an increase in luminance of around 2–3 log units was required to achieve similar transient pupillary light reflexes in KCNV2 retinopathy patients compared to controls. This is consistent with both psychophysical and ERG studies of rod-mediated thresholds in this condition, which have been reported to be elevated by 1 to 3 log units.[2, 3, 5, 8, 24, 25] In spite of the attenuated (or extinguished) rod-mediated PLRs for low luminance stimuli, high luminance stimuli elicited responses that were in the normal range. This is consistent with the “gated” mechanism that has been used to describe similar findings in the dark-adapted ERG intensity series, in which the amount of light needed to elicit a response is abnormally high, but the b-wave rapidly approaches (and may even exceed) the normative range with increasing stimulus intensity.[2, 7, 10]
The mildly decreased light-adapted PLRs measured with the long-wavelength stimuli found in this study are within the spectrum of previous psychophysical measures, which ranged from mildly elevated to 2 log units elevated relative to controls.[2, 5, 13, 25] Thus, the cone-mediated PLR findings appear to be more similar to the psychophysical thresholds than to the substantially reduced light-adapted ERG responses found in our two patients and in others.[7] Light-adapted pupillary responses in the KCNV2 retinopathy patients showed greater responses to blue than to red. The two possible mediators for the greater response to photopically matched blue than red are either rods or S-cones. If rods are mediating these signals, it could simply be that cones are sufficiently dysfunctional and allow the residual rod signal to mediate the pupillary response. A more interesting explanation could be that KCNV2 retinopathy rods may not be sufficiently saturated by the background light, due, for example, to the modulation of their inner segment potassium channel function.
Alternatively, S-cones could have an unusually large influence on the light-adapted pupillary response. Several investigations have found primarily protan-deutan errors on color testing,[2, 5, 7, 8, 11, 21] and the S-cone ERG has been reported to be relatively spared compared to the photopic single white flash and 30-Hz ERG amplitudes.[7, 8] Some investigators have therefore suggested that S-cones may be relatively spared versus L- and M-cones in KCNV2 retinopathy.[2, 11, 21] Stockman et al. (2014), on the other hand, found similar temporal acuity losses for rods, S-cones and L-cones in KCNV2 retinopathy, which might suggest that S-cones are not spared.[24] An intriguing recent finding from a fluorescence microscopy study is that Kv2.2, which is present in cone inner segments, but not in rods, shows significantly lower expression in human S-cones than in L/M-cones.[14] This finding provides a potential mechanism for a difference in response between S-cones and L/M-cones to loss or dysfunction of Kv8.2 (the KCNV2 protein that interacts with Kv2.2 or Kv2.1).
A limitation of this study is the small sample size (N=2). A larger sample could potentially find aspects of the pupil responses that do not fit those that were consistent between the two study subjects. For example, both of our patients had severe mutations, with likely complete loss of Kv8.2 protein function, while patients with milder missense mutations could have greater pupillary light reflex amplitudes. However, keeping in mind that several of the unique full-field ERG findings in KCNV2 retinopathy are quite consistent across larger case series, it is reasonable to anticipate that the findings on our two subjects would most likely be consistently observed in a larger sample. Even with these limitations, we can make some statements on the utility of pupillometry as an objective functional measure in KCNV2 retinopathy. The high luminance dark-adapted transient responses and all photopic transient responses were detectable, although some were decreased from normal. As a consequence, the PLR recorded in response to high luminance flashes could potentially be used for safety and efficacy monitoring in treatment trials. Further, the melanopsin-mediated response can provide a measure of optic nerve function in these patients.
Funding:
The Pangere Family Foundation, Gary, Indiana (GAF), National Institutes of Health research grant P30EY001792 (core grant), an unrestricted departmental grant and a Dolly Green Scholar award (JM) from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: All of the authors (Frederick T. Collison, Jason C. Park, Gerald A. Fishman, Edwin M. Stone, J. Jason McAnany) declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Wu H, Cowing JA, Michaelides M, et al. (2006) Mutations in the gene KCNV2 encoding a voltage-gated potassium channel subunit cause “cone dystrophy with supernormal rod electroretinogram” in humans. Am J Hum Genet 79:574–579. 10.1086/507568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaelides M, Holder GE, Webster AR, et al. (2005) A detailed phenotypic study of “cone dystrophy with supernormal rod ERG.” Br J Ophthalmol 89:332–339. 10.1136/bjo.2004.050567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood DC, Cideciyan AV, Halevy DA, Jacobson SG (1996) Sites of disease action in a retinal dystrophy with supernormal and delayed rod electroretinogram b-waves. Vision Res 36:889–901 [DOI] [PubMed] [Google Scholar]
- 4.Sergouniotis PI, Holder GE, Robson AG, et al. (2012) High-resolution optical coherence tomography imaging in KCNV2 retinopathy. Br J Ophthalmol 96:213–217. 10.1136/bjo.2011.203638 [DOI] [PubMed] [Google Scholar]
- 5.Zobor D, Kohl S, Wissinger B, et al. (2012) Rod and Cone Function in Patients with KCNV2 Retinopathy. PLOS ONE 7:e46762 10.1371/journal.pone.0046762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelinger L, Wissinger B, Eli D, et al. (2013) Cone dystrophy with supernormal rod response: novel KCNV2 mutations in an underdiagnosed phenotype. Ophthalmology 120:2338–2343. 10.1016/j.ophtha.2013.03.031 [DOI] [PubMed] [Google Scholar]
- 7.Robson AG, Webster AR, Michaelides M, et al. (2010) “Cone dystrophy with supernormal rod electroretinogram”: a comprehensive genotype/phenotype study including fundus autofluorescence and extensive electrophysiology. Retina 30:51–62. 10.1097/IAE.0b013e3181bfe24e [DOI] [PubMed] [Google Scholar]
- 8.Gouras P, Eggers HM, MacKay CJ (1983) Cone dystrophy, nyctalopia, and supernormal rod responses. A new retinal degeneration. Arch Ophthalmol 101:718–724 [DOI] [PubMed] [Google Scholar]
- 9.Khan AO, Alrashed M, Alkuraya FS (2012) “Cone dystrophy with supranormal rod response” in children. Br J Ophthalmol 96:422–426. 10.1136/bjophthalmol-2011-300271 [DOI] [PubMed] [Google Scholar]
- 10.Vincent A, Wright T, Garcia-Sanchez Y, et al. (2013) Phenotypic Characteristics Including In Vivo Cone Photoreceptor Mosaic in KCNV2-Related “Cone Dystrophy with Supernormal Rod Electroretinogram.” Invest Ophthalmol Vis Sci 54:898–908. 10.1167/iovs.12-10971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiagalingam S, McGee TL, Weleber RG, et al. (2007) Novel mutations in the KCNV2 gene in patients with cone dystrophy and a supernormal rod electroretinogram. Ophthalmic Genet 28:135–142. 10.1080/13816810701503681 [DOI] [PubMed] [Google Scholar]
- 12.Ben Salah S, Kamei S, Sénéćhal A, et al. (2008) Novel KCNV2 mutations in cone dystrophy with supernormal rod electroretinogram. Am J Ophthalmol 145:1099–1106. 10.1016/j.ajo.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 13.Friedburg C, Wissinger B, Schambeck M, et al. (2011) Long-Term Follow-Up of the Human Phenotype in Three Siblings with Cone Dystrophy Associated with a Homozygous p.G461R Mutation of KCNV2. Invest Ophthalmol Vis Sci 52:8621–8629. 10.1167/iovs.11-8187 [DOI] [PubMed] [Google Scholar]
- 14.Gayet-Primo J, Yaeger DB, Khanjian RA, Puthussery T (2018) Heteromeric KV2/KV8.2 Channels Mediate Delayed Rectifier Potassium Currents in Primate Photoreceptors. J Neurosci 38:3414–3427. 10.1523/JNEUROSCI.2440-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czirják G, Tóth ZE, Enyedi P (2007) Characterization of the heteromeric potassium channel formed by kv2.1 and the retinal subunit kv8.2 in Xenopus oocytes. J Neurophysiol 98:1213–1222. 10.1152/jn.00493.2007 [DOI] [PubMed] [Google Scholar]
- 16.Hart NS, Mountford JK, Voigt V, et al. (2019) The Role of the Voltage-Gated Potassium Channel Proteins Kv8.2 and Kv2.1 in Vision and Retinal Disease: Insights from the Study of Mouse Gene Knock-Out Mutations. eNeuro 6:. 10.1523/ENEURO.0032-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCulloch DL, Marmor MF, Brigell MG, et al. (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12. 10.1007/s10633-014-9473-7 [DOI] [PubMed] [Google Scholar]
- 18.Park JC, Moura AL, Raza AS, et al. (2011) Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci 52:6624–6635. 10.1167/iovs.11-7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JC, McAnany JJ (2015) Effect of stimulus size and luminance on the rod-, cone-, and melanopsin-mediated pupillary light reflex. J Vis 15:. 10.1167/15.3.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collison FT, Park JC, Fishman GA, et al. (2015) Full-Field Pupillary Light Responses, Luminance Thresholds, and Light Discomfort Thresholds in CEP290 Leber Congenital Amaurosis Patients. Invest Ophthalmol Vis Sci 56:7130–7136. 10.1167/iovs.15-17467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wissinger B, Dangel S, Jägle H, et al. (2008) Cone Dystrophy with Supernormal Rod Response Is Strictly Associated with Mutations in KCNV2. Invest Ophthalmol Vis Sci 49:751–757. 10.1167/iovs.07-0471 [DOI] [PubMed] [Google Scholar]
- 22.Khan AO (2013) Recognizing the KCNV2-related retinal phenotype. Ophthalmology 120:e79–80. 10.1016/j.ophtha.2013.07.030 [DOI] [PubMed] [Google Scholar]
- 23.Fujinami K, Tsunoda K, Nakamura N, et al. (2013) Molecular characteristics of four Japanese cases with KCNV2 retinopathy: Report of novel disease-causing variants. Mol Vis 19:1580–1590 [PMC free article] [PubMed] [Google Scholar]
- 24.Stockman A, Henning GB, Michaelides M, et al. (2014) Cone dystrophy with “supernormal” rod ERG: psychophysical testing shows comparable rod and cone temporal sensitivity losses with no gain in rod function. Invest Ophthalmol Vis Sci 55:832–840. 10.1167/iovs.13-12919 [DOI] [PubMed] [Google Scholar]
- 25.Tanimoto N, Usui T, Ichibe M, et al. (2005) PIII and derived PII analysis in a patient with retinal dysfunction with supernormal scotopic ERG. Doc Ophthalmol 110:219–226. 10.1007/s10633-005-7861-8 [DOI] [PubMed] [Google Scholar]