Abstract
Hypervirulent Klebsiella pneumoniae (hvKp) is an underrecognized pathotype of K. pneumoniae since the majority of cases have occurred in East Asia. However, hvKp is a public health treat due to its ability to infect healthy individuals, ongoing dissemination, and acquisition of resistance determinants. hvKp-directed antivirulence therapy is appealing since it has the potential to minimize resistance selection. The discovery that aerobactin is a critical hvKp-specific virulence factor has made its biosynthetic enzymes attractive targets for the development of small molecule inhibitors. However, identification of addition high value, targets is needed to enable a robust countermeasure program for this evolving superbug.
Keywords: aerobactin, antimicrobial resistance, hypervirulent Klebsiella pneumoniae, antivirulence therapy, drug screen, siderophores
The ongoing antimicrobial crisis has dictated the need to consider treatment approaches for infections that minimize the selection of multi and extensively drug resistant strains. Antivirulence therapy, which targets bacterial factors that inhibit the virulence of a pathogen at the site of infection without affecting viability, has been promoted as a means for decreasing the emergence of resistance 1. An underappreciated caveat of antivirulence therapy is that resistance selection is more likely to occur at sites of sub-therapeutic drug concentrations such as mucosal and epithelial sites of colonization or in the environment 2. An ideal antivirulence target would be one that contributes to fitness during infection but that is not required for colonization or environmental survival. Such targets include factors that are required to obtain or produce essential nutrients critical for microbial growth and survival within extraintestinal, nutrient-constrained sites of infection, but that are non-essential in nutrient-accessible settings such as the gastrointestinal tract or sewage. Microbial iron acquisition systems would seem to meet these criteria. We propose that hypervirulent Klebsiella pneumoniae (hvKp), an emerging public health threat, may be an ideal and important human pathogen with which we can explore the utility of such an antivirulence approach and, ultimately, that management of hvKp infections could greatly benefit from this type of novel antimicrobial strategy.
Two pathotypes of Klebsiella pneumoniae are circulating across the globe. Classical K. pneumoniae (cKp) are responsible for the majority of K. pneumoniae infections in Western countries; these usually occur in the healthcare setting in patients with co-morbidities or barrier disruptions due to indwelling devices or surgery. cKp strains have gained notoriety for acquiring an increasing number of antimicrobial resistance determinants, recently highlighted by the death of a women infected with a pan drug resistant strain. By contrast, hvKp’s initial evolutionary path was acquiring an increasing number of virulence determinants. hvKp was first reported from the Asian Pacific Rim in 1986, but hvKp is now being increasingly recognized outside of Asia. Cardinal clinical features include the ability to cause organ or life-threatening infection in otherwise healthy hosts, community-acquisition, and the propensity for infecting multiple sites or subsequent metastatic spread. Liver abscess is most commonly described, but infection can occur in virtually every body site, including a variety of non-hepatic abscesses and non-compartmentalized infections such as meningitis, endophthalmitis, necrotizing fasciitis, osteomyelitis, and primary bacteremia 3.
The hypervirulent phenotype of hvKp is conferred by the acquisition of a large virulence plasmid and perhaps integrated chromosomal elements. The identification of the complete repertoire of virulence genes is incomplete. Biomarkers present on the virulence plasmid have been shown to most accurately differentiate hvKp from cKp strains 4. Initially, hvKp isolates were antimicrobial susceptible, but resistance is increasing by acquisition of conjugal plasmids or transposons containing resistance determinants, or by cKp strains acquiring the hvKp virulence plasmid 5. The confluence of extreme drug resistance (XDR) and hypervirulence is a lethal combination 5. The evolution of XDR hvKp strains portends a medical crisis.
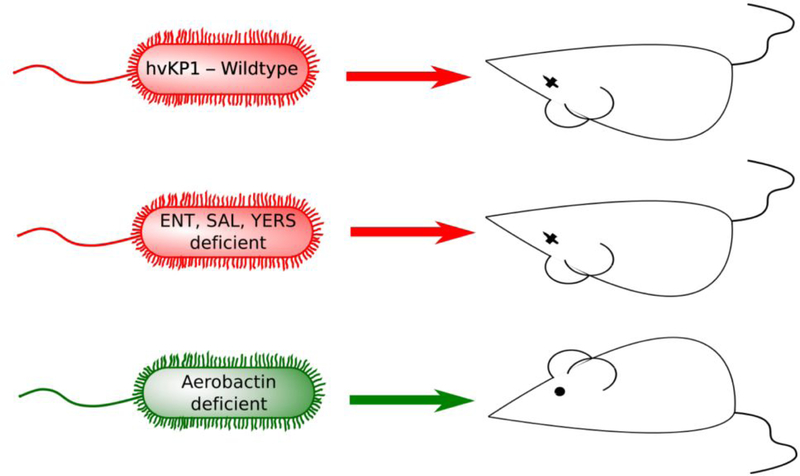
One of the defined virulence traits for hvKp is the ability to produce significantly higher levels of the iron acquisition molecules (siderophores) than cKp strains 6. Although hvKp can produce four siderophores (enterobactin, salmochelin, yersiniabactin, and aerobactin), aerobactin accounts for greater than 90% of siderophore production 6. This correlation of aerobactin with virulence was noted in the 1980s 7; however, recent data have directly demonstrated the critical role that aerobactin, but not the other siderophores, plays in hvKp growth/survival ex vivo and for the extreme virulence of the hvKp in vivo 8 (Figure 1). Isogenic mutants of different siderophore biosynthetic pathways were constructed in multiple hvKp strains. Whereas iuc strains that were mutated for the ability to produce aerobactin were unable to grow in human ascites fluid or human serum, mutations in any of the three remaining siderophores had no effect on cell growth. Indeed, a mutant strain that could only produce aerobactin and none of the other three siderophores showed no growth defect. More critically, these same strains were probed in mouse pneumonia and systemic infection models where, similarly, only aerobactin played a role in the virulent phenotype of hvKp. Finally, the genes responsible for aerobactin production are present on the hvKp virulence plasmid and appear to be hvKp specific 4. Therefore, we hypothesized that aerobactin production of hvKp could make an ideal antivirulence target.
Figure 1.
Aerobactin is a critical virulence factor for hypervirulent K. pneumoniae. The inability to produce aerobactin, but not enterobactin, salmochelin, and yersiniabactin, significantly decreased the virulence of the hypervirulent K. pneumoniae strain hvKP1 in outbred CD1 mice after either pulmonary or subcutaneous challenge. ENT- enterobactin, SAL – salmochelin, YERS – yersiniabactin.
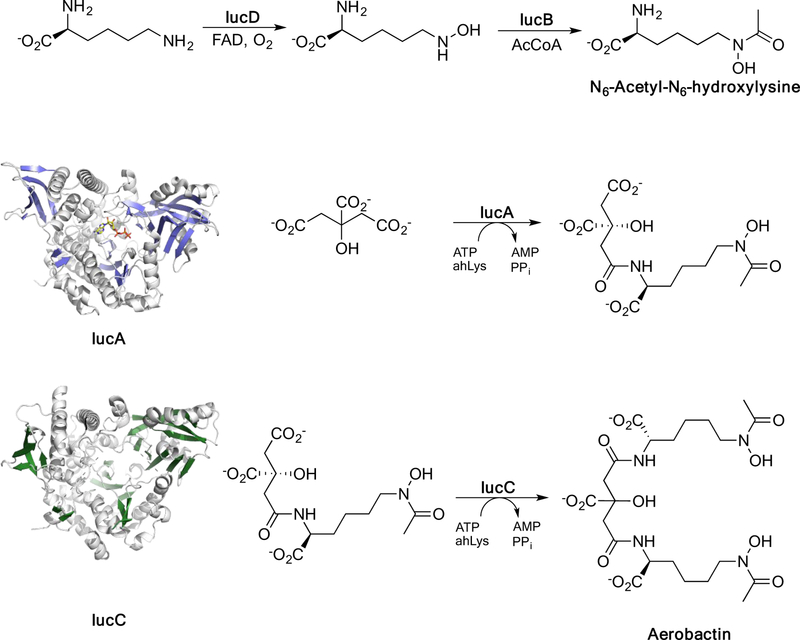
Aerobactin is a hydroxamate-based siderophore that is biosynthesized through the activity of four proteins, IucABCD (Figure 2). First, N6-acetyl-N6-hydroxylysine (ahLys) is produced through the sequential activities of the hydroxylase IucD and acetyltransferase IucB. Two molecules of ahLys are then attached to the primary carboxylates of citrate. First, the aerobactin synthetase IucA stereospecifically adds one ahLys molecule to a primary carboxylate of citrate. The second ahLys molecule is subsequently added by a second aerobactin synthetase IucC. IucA and IucC belong to a family of ATP dependent ligases that are involved in the production of numerous siderophores including the virulence related siderophores petrobactin of B. anthracis and staphyloferrin of S. aureus. Distinguishing these siderophores from the alternate common pathway for siderophore biosynthesis, these carboxylate- and hydroxamate-based siderophores have been referred to as NRPS-Independent Siderophores (NISs) 9. Aerobactin biosynthesis has been reconstituted biochemically confirming the biosynthetic pathway and achieved via heterologous expression of all four biosynthetic genes in E. coli. Despite the overall shared structural fold of IucA and IucC and the striking similarity of their catalytic reactions, the two enzymes share only 24 % sequence identity 10.
Figure 2.
Aerobactin biosynthetic pathway. Aerobactin is produced from two molecules of lysine and citrate that are combined through the sequential activities of IucD, IucB, IucA, and IucC. Crystal structures have been determined for NIS synthetases, IucA and IucC.
Having characterized aerobactin biosynthesis biochemically and structurally, we adapted a high throughput biochemical screen (HTS) to identify compounds that could inhibit aerobactin production 11. This screen uses inorganic pyrophosphatase to convert product pyrophosphate to phosphate that forms a complex with molybdate and malachite green. The assay is robust and orthogonal assays exist to further probe hit compounds. An initial screen with this assay identified several inhibitors that showed micromolar IC50 values in both primary and secondary assays. However, the best of the compounds also possessed signs of aggregation-dependent inhibition including a dependence of inhibition on pre-incubation time, enzyme concentration, and potential nonspecificity at higher concentrations. Although the identification of small molecule inhibitors of aerobactin synthesis that possess the qualities needed to proceed with pre-clinical studies is in early stages, the development of a validated HTS will enable this process to move forward. An advantage of this antivirulence approach is that aerobactin synthesis proteins are new targets, therefore inhibitors will be unaffected by pre-existing antimicrobial resistance determinants.
An alternative antivirulence target for hvKp would be capsular polysaccharide. Increased capsular production, mediated the capsule regulatory genes rmpA and/or rmpA2 is an hvKp-specific virulence trait. Although, the number of capsule types that exist for hvKp, which is ever-increasing as cKp stains acquire hvKp virulence genes, makes capsule biosynthetic proteins untenable targets, transport proteins remain a consideration. However, capsule is almost certainly a critical survival factor within the gastrointestinal tract or in the environment outside of the human host. Thereby it would be predicted that there would be an increased risk for the selection of capsule transport protein inhibitors resistant mutants in these settings. Non-antivirulence, capsule directed therapeutic considerations for hvKp also include passive immunization and phage therapy. For both approaches the number of hvKp capsule types pose a challenge as do scientific and regulatory concerns for phage therapy 12.
A final concern raised by the emergence of hvKp is its potential to be a source of genetic material for other Enterobacteriaceae during their co-habitation in animal and environmental reservoirs. hvKp already has demonstrated the ability to transfer its virulence plasmid, which included the hvKp specific aerobactin biosynthetic operon, to cKp, with disastrous results 5. The prospect of this occurring with other Enterobacteriaceae would compound present concerns. Therefore, it is our opinion that development of a new class of antimicrobials active against hvKp is critical. Aerobactin synthesis proteins are the most promising antivirulence target identified to date for hvKp. Additional studies towards the development of small molecule inhibitors of aerobactin synthesis are warranted as are an ongoing search for addition high value, hvKp specific targets.
Acknowledgment:
This work was supported by NIH 1R21AI088318 (Dr. Russo) and the Department of Veterans Affairs VA Merit Review (1I01BX000984) (Dr. Russo) and NIH 1R01AI116998 (Dr. Gulick). The funders had no role in the decision to publish or the preparation of this manuscript.
Abbreviations Used:
- hvKp
hypervirulent Klebsiella pneumoniae
- cKp
classical Klebsiella pneumoniae
- XDR
extreme drug resistance
- NISs
NRPS-Independent Siderophores
- NRPS
nonribosomal peptide-synthetase
- HTS
high throughput screen
- ENT
enterobactin
- SAL
salmochelin
- YERSIN
yersiniabactin
References and Notes:
- 1.Maura D; Ballok AE; Rahme LG, Considerations and caveats in anti-virulence drug development. Curr Opin Microbiol 2016, 33, 41–46. DOI 10.1016/j.mib.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo TA; Spellberg B; Johnson JR, Important complexities of the antivirulence target paradigm: A novel ostensibly resistance-avoiding approach for treating infections. J Infect Dis 2016, 213 (6), 901–3.DOI 10.1093/infdis/jiv533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sellick JA; Russo TA, Getting hypervirulent Klebsiella pneumoniae on the radar screen. Current opinion in infectious diseases 2018. DOI 10.1097/qco.0000000000000464 [DOI] [PubMed] [Google Scholar]
- 4.Russo TA; Olson R; Fang CT; Stoesser N; Miller M; MacDonald U; Hutson A; Barker JH; La Hoz RM; Johnson JR, Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol 2018, 56 (9).DOI 10.1128/jcm.00776-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu D; Dong N; Zheng Z; Lin D; Huang M; Wang L; Chan EW; Shu L; Yu J; Zhang R; Chen S, A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis 2017. DOI 10.1016/s1473-3099(17)30489-9 [DOI] [PubMed] [Google Scholar]
- 6.Russo TA; Olson R; Macdonald U; Metzger D; Maltese LM; Drake EJ; Gulick AM, Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun 2014, 82 (6), 2356–67.DOI 10.1128/iai.01667-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nassif X; Sansonetti PJ, Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun 1986, 54 (3), 603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo TA; Olson R; MacDonald U; Beanan J; Davidson BA, Aerobactin, but not yersiniabactin, salmochelin and enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infection and Immunity 2015, 83 (8), 3325–3333.DOI 10.1128/IAI.00430-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll CS; Moore MM, Ironing out siderophore biosynthesis: a review of non-ribosomal peptide synthetase (NRPS)-independent siderophore synthetases. Critical reviews in biochemistry and molecular biology 2018, 53 (4), 356–381.DOI 10.1080/10409238.2018.1476449 [DOI] [PubMed] [Google Scholar]
- 10.Bailey DC; Alexander E; Rice MR; Drake EJ; Mydy LS; Aldrich CC; Gulick AM, Structural and functional delineation of aerobactin biosynthesis in hypervirulent Klebsiella pneumoniae. J Biol Chem 2018, 293 (20), 7841–7852.DOI 10.1074/jbc.RA118.002798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey DC; Buckley BP; Chernov MV; Gulick AM, Development of a high-throughput biochemical assay to screen for inhibitors of aerobactin synthetase IucA. SLAS discovery : advancing life sciences R & D 2018, 23 (10), 1070–1082.DOI 10.1177/2472555218787140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelfrene E; Willebrand E; Cavaleiro Sanches A; Sebris Z; Cavaleri M, Bacteriophage therapy: a regulatory perspective. The Journal of antimicrobial chemotherapy 2016, 71 (8), 2071–4.DOI 10.1093/jac/dkw083 [DOI] [PubMed] [Google Scholar]