Abstract
Purpose of review:
Age and metabolic disorders result in the accumulation of advanced glycation endproducts (AGEs), oxidative stress and inflammation, which cumulatively cause a decline in skeletal health. Bone becomes increasingly vulnerable to fractures and its regenerative capacity diminishes under such conditions. With a rapidly aging population in the United States and the global increase in diabetes, efficacious, multi-dimensional therapies that can treat or prevent skeletal diseases associated with metabolic dysfunction and inflammatory disorders are acutely needed.
Recent findings:
Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) is a key regulator of nutrient intake, glucose metabolism, insulin production and adipogenesis. Recent studies suggest a pivotal role for CaMKK2 in bone metabolism, fracture healing, and inflammation.
Summary:
Aside from rekindling previous concepts of CaMKK2 as a potent regulator of whole-body energy homeostasis, this review emphasizes CaMKK2 as a potential therapeutic target to treat skeletal diseases that underlie metabolic conditions and inflammation.
Keywords: CaMKK2, Diabetes, Diabetic Osteopathy, Skeletal Disease, Fracture Healing
Introduction
One in two adults in the United States suffer from one or more musculoskeletal disorders, with collective treatment costs exceeding $882.5 billion annually.[1] As the population ages, these costs will only continue to increase. The most common musculoskeletal conditions, such as osteoporosis and osteoarthritis, result from an age-related decline in bone and joint health or occur secondary to metabolic disorders, sports-related injuries, inflammatory diseases, and/or their corresponding drug therapies. Whereas no cures exist for osteoarthritis, osteoporosis has been successfully treated by inhibiting osteoclast (OC)-mediated bone resorption using bisphosphonates, such as Zoledronic acid, or using Denosumab, a monoclonal antibody against receptor activator of nuclear factor kappa-B ligand (RANKL).[2] Although these treatments halt bone resorption and preserve bone mass, their long-term use have deleterious effects on bone strength.[3, 4] In contrast, there are only two approved bone anabolic therapies —Teriparatide and Abaloparatide — available to stimulate bone formation and restore the lost bone mass.[5] Both of these agents are fragments of recombinant human parathyroid hormone (PTH) or PTH-related peptide (PTHrP).[5] Thus, these current treatments for bone diseases are one-dimensional in that they target either bone formation or bone resorption. Recent studies indicate that combinations of anabolic and anti-resorptive therapies provide long-term benefits.[6] Notably, Evenity (romosozumab), a monoclonal antibody against sclerostin that stimulates bone formation and inhibits resorption, was recently approved for in women with a history of osteoporotic fracture or are intolerant to other osteoporosis therapies.[7] Furthermore, our ability to heal from bone fractures diminishes with age, and co-morbidities such as diabetes further impair bone healing. No therapies are available to combat diabetic osteopathy and recent evidence indicates that some anti-hyperglycemia therapies are detrimental to bone remodeling and may even contribute to fragility fractures.[8] To reverse these trends, we need new therapeutic strategies that promote bone anabolism and accelerate healing. However, we must also address the underlying disturbances in the whole-body metabolism that cause inflammation and other pathologies that detrimentally affect bone health. Thus, there is clearly a need for smarter multi-dimensional therapies that can treat and/or prevent skeletal diseases that arise from metabolic dysfunction, aging and inflammation.
Whole-body energy homeostasis involves central regulation of feeding behavior and energy expenditure in response to the peripheral metabolic environments. Nutrient, endocrine, and neuronal factors drive transitions between cellular anabolic and catabolic processes primarily via adenosine monophosphate (AMP)-activated protein kinase (AMPK), a heterotrimeric kinase that modulates multiple aspects of energy balance including nutrient intake, glucose homeostasis, and energy expenditure.[9] Peripheral tissues such as the liver, pancreas and adipose, sense and respond to changes in circulating levels of glucose and other nutrients. During times of nutrient shortage, the pancreas secretes glucagon to stimulate the breakdown of macronutrients and storage molecules into fuel for energy production via glycolysis, oxidative phosphorylation (OXPHOS) or beta-oxidation. In times of nutrient abundance, pancreatic beta cells and adipocytes secrete insulin and leptin respectively, to stimulate the uptake and storage of available glucose and lipids by the liver and other peripheral tissues. Excessive nutrient intake, sedentary lifestyle and aging contribute to accumulation of excess adipose tissue and disproportionate levels of leptin, resistin and pro-inflammatory cytokines that contribute to low-grade systemic inflammation, resulting in obesity-induced insulin resistance. As cells are unable to use insulin and effectively absorb glucose, it builds up in circulation. Hyperglycemia generates imbalances in cellular metabolism and energy homeostasis at peripheral tissues and increases the risk of cardiovascular disease, nerve damage, retinopathy and nephropathy. [10]
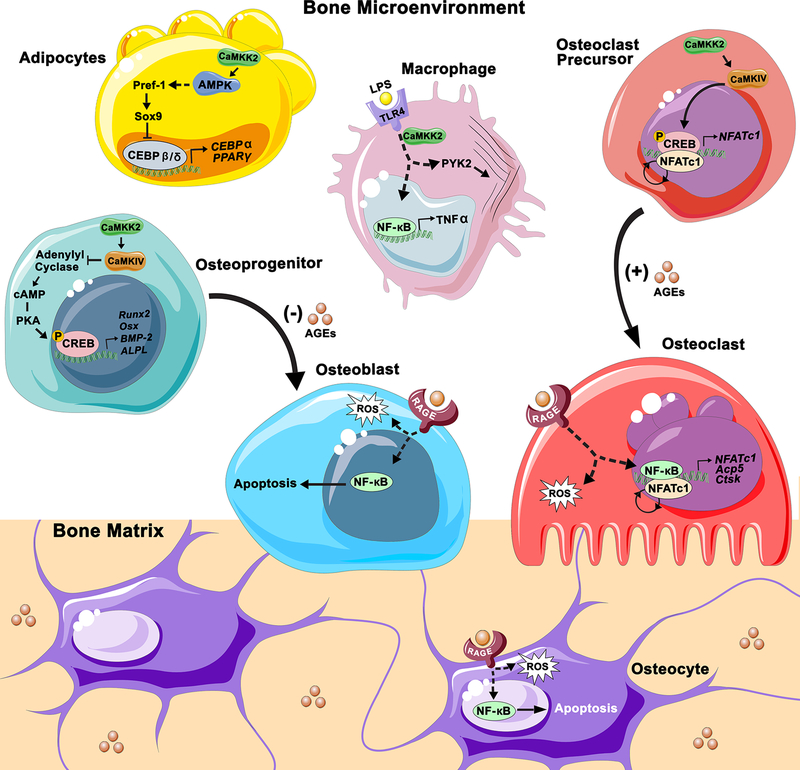
Chronic hyperglycemia is detrimental to skeletal health, and diabetics possess a greater risk for fractures and impaired healing capability.[11] Bone quality and strength are maintained through homeostatic bone remodeling, however changes in cellular metabolism and inflammatory signaling can disrupt the balance between osteoblast (OB)-mediated bone formation and OC-mediated bone resorption over time. Aging and diabetes, disturb the balance of bone remodeling by inducing oxidative stress and inflammation, and ultimately affecting the quantity and quality of the bone formed. As we age, long-lived proteins such as collagen in the connective tissue are susceptible to glycation by glucose and other simple sugars to form advanced glycation endproducts (AGEs). Chronic hyperglycemia accelerates AGE accumulation, which compromises bone material properties by inducing non-enzymatic crosslinks within the collagen matrix.[12] AGEs also increase the production of reactive oxygen species (ROS), which bind to and activate the AGE-specific receptor (RAGE), a multi-ligand receptor implicated in the pathogenesis of many age-related diseases and diabetes.[13] Oxidative stress also plays a role in the regulation of pro-inflammatory and apoptotic signaling. RANKL induces RAGE expression in OCs and AGE-RAGE-mediated production of ROS promotes OC differentiation. In other cell types such as OBs and osteocytes, AGE-RAGE signaling disrupts normal mitochondria function and induces apoptosis through a nuclear factor kappa B (NFκB)-mitogen-activated protein kinase (MAPK)-mediated mechanism (Figure 1).[12]
Figure 1.
The role of CaMKK2 and advanced glycation endproducts (AGEs) within the bone microenvironment. In pre-adipocytes, CaMKK2 inhibits differentiation by maintaining Pref-1 and Sox9 expression via the activation of AMPK. Sox9 inhibits transcription of early adipogenic transcription factors CEBPβ and CEBPδ, which in turn reduce expression of CEBPα and PPARγ. Thus, inhibition of CaMKK2 would relieve Sox9 mediated repression of adipogenic gene transcription to promote adipogenesis. In osteoprogenitors, CaMKK2-CaMKIV signaling antagonizes the cAMP-PKA pathway as a checkpoint mechanism during MSC differentiation to OBs. Under homeostatic conditions, the CaMKK2-CaMKIV signaling cascade inhibits the production cAMP by adenylyl cyclase (AC), which is required for activation of PKA and the subsequent phosphorylation of CREB, a transcriptional activator of osteogenic gene expression. Inhibition of CaMKK2 would relieve this inhibition of PKA signaling to promote OB differentiation. In OC precursors CaMKK2-CaMKIV signaling leads to phosphorylation of CREB and upregulation and autoamplification of NFATc1 to drive OC differentiation. Additionally, in macrophages CaMKK2 regulates TLR4 mediated cytoskeletal rearrangement and cytokine production through an unknown mechanism. Additionally, accumulation of AGEs in bone tissue can exert pressures on bone cell differentiation and survival. AGEs inhibit osteogenic differentiation and, in OBs and osteocytes, AGE-RAGE signaling stimulates production of ROS and promotes expression of pro-apoptotic factors through activation of NFκB. On the other hand, AGE-RAGE mediated activation of NFκB promotes OC differentiation and survival.
In recent years, bone has emerged as an important endocrine organ with the ability to regulate organismal glucose and insulin homeostasis. OB-derived osteocalcin, in its undercarboxylated form, functions as a hormone that promotes secretion of insulin from pancreas and adiponectin from adipose tissue. Insulin, in turn, acts on OBs to increase their differentiation and production of osteocalcin. Insulin also indirectly promotes OC differentiation by stimulating the production of receptor activator of NFκB ligand (RANKL) by OBs. OCs decarboxylate osteocalcin embedded within the bone matrix, and the resulting undercarboxylated osteocalcin is released into circulation, forming a positive feedback loop. Recent work indicates that changes in OB metabolism is sufficient to affect whole-body glucose levels.[14]
In this review, we briefly discuss the skeletal complications associated with obesity and diabetes, and the detrimental effects elicited by hyperglycemia and related chronic inflammation on bone. Additionally, we discuss findings that identify Ca2+/calmodulin (CaM)-dependent protein kinase kinase 2 (CaMKK2), a potent regulator of whole-body energy metabolism with additional roles in bone remodeling and inflammation, as a novel therapeutic target to promote bone anabolism and regeneration, thereby reducing the risk of fragility fractures.
CaMKK2 in Metabolism and Bone
Calcium (Ca2+) serves as a universal second messenger that mediates a wide range of cellular functions in response to external stimuli. Free intracellular Ca2+ levels are tightly regulated at nanomolar concentrations. Ca2+ enters the cytosol through ligand- or voltage-gated ion channels at the plasma membrane, or upon release from internal stores such as the endoplasmic reticulum. Transitory increases in intracellular Ca2+ are detected by its ubiquitous high-affinity receptor, calmodulin (CaM).[15, 16] Ca2+/CaM signaling amplifies the downstream effects of many hormones, inflammatory agents and metabolites, in part through the activation of the Ca2+/CaM-dependent protein kinase (CaMK) signaling cascade. Binding of Ca2+/CaM exposes a critical Thr on the activation loop in all CaMKs, which allows for their activation through autophosphorylation in case of CaMKK1 and CaMKK2, which then phosphorylate the downstream kinases CaMKI and CaMKIV.[17, 18] CaMKK2 is uniquely capable of Ca2+/CaM-independent autonomous activity that is tightly regulated by cyclin-dependent kinase (CDK5) and glycogen synthase kinase (GSK3β). [19, 20] This allows CaMKK2 to respond to stimuli other than Ca2+ and activate novel substrates. Indeed, CaMKK2 (not CaMKK1) serves as an upstream activator of the AMPK, a key regulator of cellular energy balance.[21] The CaMKK2-AMPK pathway regulates many physiological processes, such as hypothalamic feeding behavior, hepatic gluconeogenesis, adipocyte differentiation and macroautophagy. Recent studies indicate roles for CaMKK2 in hepatocellular carcinoma, inflammation and bone remodeling through AMPK-independent mechanisms.[22, 23]
CaMKK2 regulates hypothalamic control of appetite, hepatic glucose production and insulin secretion. CaMKK2 is highly expressed in the arcuate nucleus (ARC) of the hypothalamus where it regulates the expression of neuropeptide Y (NPY) and agouti-related protein (AgRP) in response to ghrelin feedback. Mice lacking CaMKK2 express lower levels of NPY, which interrupts the appetite feedback loop and contributes to protection from HFD-induced weight gain.[24] The role of CaMKK2 in whole-body energy homeostasis extends beyond the central nervous system, to glucose and fat metabolism at peripheral metabolic tissues. The liver acts as a metabolic hub regulating metabolites in the blood and coordinating the storage and release of glucose and fatty acids (FAs) in response to insulin and other hormones.[25] CaMKK2 phosphorylates HDAC5 in hepatocytes to drive expression of gluconeogenic enzymes. Loss of endogenous CaMKK2 in the liver is sufficient to lower blood glucose and improve glucose tolerance in HFD fed mice. Furthermore, primary hepatocytes lacking CaMKK2 exhibit suppressed gluconeogenesis, and increased de novo lipogenesis and FA oxidation.[26] Pharmacological inhibition of CaMKK2 reverses hepatic steatosis and attenuates many of the hallmarks of non-alcoholic fatty liver disease (NAFLD).[27] CaMKK2 also appears to regulate insulin production in response to glucose signaling in β cells of the pancreas. Loss of CaMKK2 increases insulin secretion and its levels in circulation; however, it is important to note plasma insulin levels of CaMKK2-null mice are still within normal physiological levels.[28] Thus, CaMKK2 is a potent regulator of glucose metabolism and whole-body energy homeostasis.
Expression of CaMKK2 is cell-type and tissue-specific, making it an appealing target to develop pharmacological agents that block its activity. The most effective inhibitor of CaMKK2 activity is STO-609 (7-Oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate), a cell permeable compound that antagonizes ATP binding. Although STO-609 can inhibit downstream CaMKs, its potency for other kinases is much lower, as a 100-fold higher concentration is needed to inhibit them. Moreover, STO-609 is a highly selective inhibitor of CaMKK2, as its Ki value for CaMKK2 is 5-fold lower than that for CaMKK1.[29, 30] The compound is primarily metabolized by the liver, and there are no signs of hepatic or renal toxicity at even high doses (300 μg/mL).[27] In-vivo, STO-609 has been used to inhibit CaMKK2 in mouse liver, pancreas, adipose, and skeleton.[24, 28, 31, 32]
Recent studies suggest a pivotal role for CaMKK2 in the bone. Homeostatic bone remodeling relies on a balance between the numbers of bone resorbing OCs and bone matrix producing OBs. Osteocytes regulate the remodeling process through the secretion of RANKL and the Wnt-inhibitor Sclerostin (SOST). OBs also secrete factors that regulate OC activity, such as macrophage colony stimulating factor (M-CSF) and RANKL.[33] The formation of multinucleated OCs is primarily driven by RANKL induced expression of nuclear factor of activated T cells c1 (NFATc1), a master regulator of OC differentiation. Binding of RANKL to RANK leads to transient increases in calcium and activation of Ca2+-dependent Ser/Thr phosphatase calcineurin, which dephosphorylates and enables the nuclear translocation of NFATc1.[34] Furthermore, CaMKK2-CaMKIV signaling activates cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) which is necessary for the transcriptional activation and autoamplification of NFATc1 (Figure 1). In addition, the CaMKK2-CaMKIV cascade antagonizes the cAMP-protein kinase A (PKA) pathway, an inhibitor of NFATc1. Thus, the Ca2+/CaMKK2/CaMKIV pathway plays a positive role in OC differentiation. Indeed, the Camkk2−/− mice possess fewer multinuclear OCs, and bone marrow (BM)-derived monocytes (BMMs) from these mice yield fewer OCs in vitro. Moreover, acute inhibition of CaMKK2 using its selective cell permeable inhibitor STO-609 diminishes OC formation and reduces expression of phospho-CREB, NFATc1 and tartarate-resistant acid phosphatase (TRAP) in wild-type (WT)-derived BMMs.[35] Treatment with STO-609 attenuates OC formation in vivo to protect mice from ovariectomy (OVX)-induced bone loss. [35]
Conversely, a number of signaling pathways influence the differentiation of mesenchymal stem cells (MSCs) to OBs, including those of bone morphogenic protein (BMP), Wnt-β-catenin and PTH. For example, activation of the PTH receptor activates cAMP-PKA signaling, which in turn phosphorylates CREB to drive osteogenic gene expression. In contrast to its role in OCs, CaMKK2, through its regulation of CaMKIV, acts as a checkpoint mechanism during OB differentiation by inhibiting cAMP-PKA signaling. Indeed, genetic ablation of CaMKK2 or its pharmacological inhibition enhances the levels of phosphorylated forms of PKA and CREB, as well as those of osteogenic genes such as BMP-2 and RunX-2, the master regulator of OB differentiation (Figure 1). This accelerates OB formation and increases mineralization in vitro.[35] Indeed, Camkk2−/− mice possess more cuboidal OBs, and higher bone mass. Moreover, the bones of CaMKK2 null mice have superior microarchitecture and mechanical strength relative to age-matched WT mice.[32] Pharmacological inhibition of CaMKK2 stimulates bone formation and reverses age-associated loss of bone mass and strength. [32] Together, these studies demonstrate that blocking CaMKK2 causes a shift from catabolic processes to bone anabolism to reverse skeletal aging and improve bone strength.
Metabolic Dysfunction and Diabetic Osteopathy
In the United States, obesity and diabetes are increasingly prevalent due to physical inactivity and poor eating habits. More than one third of the adults in the United States are obese and at risk of developing type 2 diabetes mellitus (T2DM), contributing to $237 billion in medical expenses annually.[36, 37] Although they may possess normal bone mineral density (BMD), the destructive effects of chronic hyperglycemia and systemic inflammation compromise bone quality and thereby, increase the risk of fractures in patients with T2DM.[38–40]
Hyperglycemia accelerates the accumulation of AGEs and increases expression of the AGE receptor RAGE in multiple tissues, including bone.[41, 42] The AGE-RAGE axis stimulates the production of ROS and inflammatory cytokines.[43] In bone, activation of RAGE not only suppresses osteoblastogenesis, but also promotes the apoptosis of OBs and osteocytes. [44, 45] Recent evidence suggests that AGEs further constrain OB-mediated bone formation by increasing expression of SOST by osteocytes.[44] Together, these events impair the healing properties of the bone. We still have much to learn about the pathophysiology of diabetes, and its effects on the bone microenvironment and bone cell metabolism.
Thiazolidinediones (TZDs) such as Rosaglitazone are a common class of therapeutics for T2DM that increase insulin sensitivity and glucose uptake in peripheral tissues through activation of peroxisome proliferator-activated receptor-gamma (PPARγ).[46] However, PPARγ steers MSCs away from osteogenesis and towards adipogenesis. Rosiglitazone treatment increases marrow adiposity and decreases BMD in mice.[47] Human studies indicate that whereas TZDs are effective in controlling blood glucose levels in diabetics, they also increase bone fracture risk.[48, 8] Under homeostatic conditions, CaMKK2-AMPK signaling inhibits the differentiation of preadipocytes by maintaining expression of preadipocyte factor 1 (Pref-1) and Sox 9, a transcriptional repressor of genes associated with adipogenesis (Figure 1). Inhibition of CaMKK2 depletes the preadipocyte pool and protects mice from diet-induced obesity.[31] Another common anti-hyperglycemia medication, metformin, is an insulin sensitizing biguanide that reduces hepatic glucose production and increases glucose uptake by muscle and other peripheral tissues to lower circulating glucose levels. Metformin inhibits mitochondrial respiration at the respiratory chain complex I to deplete cellular ATP and alter gluconeogenic flux via allosteric regulation of key enzymes. As the AMP/ATP ratio rises, AMP inhibits fructose 1, 6-bisphoshpatase and promotes activation of AMPK via the upstream liver kinase B1 (LKB1) to suppress glucose production.[49, 50] Activation of AMPK enhances autophagy, which promotes OB differentiation and mineralization in culture.[51] Furthermore, metformin inhibits osteoclastogensis by decreasing the production of RANKL and increasing the production of its decoy receptor osteoprotegerin (OPG) by OBs, and conferring protection from OVX-induced bone loss in rats.[52] Whether metformin can protect bone health and prevent fractures in diabetic patients is unclear at the present time.[53] On the other hand, CaMKK2 activity drives gene expression of gluconeogenic enzymes independent of AMPK signaling, making the kinase a potent regulator of glucose production in the liver. [26] Thus, CaMKK2, with its AMPK-dependent and independent regulation of whole-body glucose metabolism, insulin production by pancreatic β cells and bone mass accrual, emerges as an attractive therapeutic target to combat hyperglycemia while preserving the overall health of the skeleton.
Aging and Inflammation
Chronic inflammation is a hallmark of metabolic disorders and aging. Imbalances in whole-body energy homeostasis lead to accumulation of excess adipose tissue and altered adipokine production. Adipose tissue regulates whole-body metabolism through the production of hormones such as leptin and adiponectin that influence nutrient intake and insulin sensitivity. As adipocytes increase in number and size they outgrow the blood supply, generating a hypoxic environment that increases production of interleukin-6 (IL-6) and other factors that recruit pro-inflammatory M1 macrophages.[54] Overproduction of pro-inflammatory cytokines, such as IL-6, IL-1β and tumor necrosis factor α (TNFα) contributes to the pathophysiology of many age-related and autoimmune disorders, including type 1 diabetes (T1D) as well as metabolic disorders such as T2D.[55, 56]
Chronic inflammation is commonly associated with systemic bone loss and diminished healing capacity. Inflammatory diseases generate an imbalance between the catabolic and anabolic processes of bone remodeling, such that OC-mediated bone resorption outpaces OB-mediated synthesis. Pro-inflammatory cytokines induce expression of RANKL to promote OC differentiation. In particular, TNFα directly hinders OB differentiation, and the production of type I collagen (COL1) and osteocalcin, through the activation of the transcription factor NFκB.[57] Interestingly, TNFα stimulates OBs to produce IL-6 and other pro-inflammatory cytokines to increase OC formation and bone resorption, creating a vicious cycle of bone destruction.[58]
Non-steroidal anti-inflammatory drugs (NSAIDs) or exogenous glucocorticoids are attractive treatments for autoimmune and inflammatory conditions due to their immunosuppressive and anti-inflammatory properties. Although the exact mechanism remains unclear, the long-term use of NSAIDs has adverse effects on bone metabolism and healing.[59, 60] On the other hand, the prolonged use of glucocorticoids leads to secondary osteoporosis by promoting the survival of OCs while suppressing that of OBs, osteoprogenitors and osteocytes. Subsequently, rapid bone loss and even osteonecrosis occur as OB and osteocyte populations decline while OC-mediated bone resorption increases.[61] Glucocorticoids induce osteocyte apoptosis through the activation of protein-tyrosine kinase 2-beta/focal adhesion kinase 2 (Pyk2), which plays a role in cytoskeletal reorganization and cell detachment. Induction of osteocyte apoptosis with dexamethasone increases Pyk2 phosphorylation and activation, whereas knocking down Pyk2 expression in osteocytes reduces their detachment and apoptosis in vitro.[62]
As a multifunctional kinase, the role of CaMKK2 extends beyond bone and glucose metabolism to inflammation. Indeed, CaMKK2 mediates cytokine release in BM-derived macrophages (BMMs) in response to stimulation by toll-like receptor 4 (TLR4)/integrin signaling. Consequently, the ablation or inhibition of CaMKK2 in BMMs is associated with anti-inflammatory properties. CaMKK2-null BMMs display diminished cytokine release following stimulation with TLR4. These BMMs also exhibit impaired spreading and phagocytosis due to decreased activation of Pyk2, a member of the focal adhesion kinase (FAK) family.[63] Furthermore, Camkk2−/− mice possess fewer M1 macrophages in their visceral adipose tissue, indicating protection from obesity-induced inflammation. The combined effects of CaMKK2 inhibition on bone remodeling and macrophage-mediated inflammation suggest that CaMKK2 is an effective therapeutic target to protect bone from damaging chronic inflammation.
Bone Healing and CaMKK2
Most bone fractures completely heal naturally, but are associated with substantial healing time that may lead to immobility and loss of productivity. Long bone fractures with intramedullary stabilization take approximately 3 to 6 months to completely heal in healthy individuals. Smoking and comorbidities, such as diabetes, can impair the natural process of bone healing and extend patient suffering. [64, 11] Approximately 5–10% of all fractures result in delayed healing or non-union as a consequence of chronic inflammation, or other underlying pathophysiology, that alters the molecular and cellular processes necessary for bone tissue regeneration, further contributing to disability.[65, 66] There are no therapeutic strategies to promote efficient bone repair and accelerated healing.
Patients with diabetes are more likely to experience delayed bone healing or nonunion, as systemic inflammation and AGE accumulation within the bone microenvironment disrupt the normal bone fracture healing mechanisms. AGE-RAGE signaling leads to overproduction of ROS and activation of NFκB, which inhibits OB differentiation and promotes OC differentiation.[67] Furthermore, activation of NFκB in OBs and osteocytes upregulates pro-inflammatory and pro-apoptotic gene expression.[43] Most fractures heal via a combination of endochondral and intramembranous ossification, wherein a cartilaginous scaffold forms around the fracture site and is enveloped by intramembranous bone formation at the callus periphery. The calcified bone matrix of the callus is remodeled overtime to bridge the fracture gap and restore the newly formed bone to its natural anatomical shape. Whereas acute inflammation following injury is crucial for proper bone healing, chronic inflammation results in insufficient angiogenesis and stem cell senescence, essentially delaying the natural healing response.[68] [69]
Similar to embryonic skeletal formation, chondrocytes within the central “soft” callus adjacent to the fracture gap proliferate and undergo hypertrophy to produce calcified cartilage that forms a template for new bone formation by OBs. Indian hedgehog (IHH) and PTH related protein (PTHrP) signaling form a feedback loop essential for endochondral ossification, long bone development, and maintenance of the growth plate. IHH is a morphogen primarily expressed by pre-hypertrophic chondrocytes. It stimulates the expression of PTHrP to drive chondrocyte and OB differentiation. If levels of PTHrP drop below a critical level then chondrocytes will undergo hypertrophy and mineralize. [69,70] During fracture healing, IHH is expressed in the callus by pre-hypertrophic chondrocytes and perichondrial OBs, where it enhances cartilage deposition, neovascularization, and osteogenesis. Disruption of IHH signaling has deleterious effects on endochondral bone healing.[70] Chronic inflammation, such as that associated with T2DM, suppresses IHH signaling through TNFα. The importance of IHH in fracture healing was recently demonstrated in a mouse model of diabetes mellitus. Delayed bone healing in these mice was associated with chronic inflammation-mediated downregulation of IHH in skeletal stem cells and progenitors. Administration of recombinant IHH into these mice restored the osteogenic potential of skeletal stem cells and rescued bone healing. [69]
Recent studies indicate CaMKK2 inhibition to be an effective strategy to accelerate bone fracture healing and improve bone strength following injury. [71] Pharmacological inhibition of CaMKK2 with STO-609 enhances the influx of osteo-chondro progenitors and accelerates endochondral ossification within the fracture callus. Notably, CaMKK2 inhibition does not disturb the normal acute inflammatory responses during fracture healing. Instead, treatment with STO-609 promotes the influx of periosteal MSCs and osteoprogenitors into the fracture callus, advancing the formation of cartilage and new bone. Further, CaMKK2 inhibition upregulates IHH mRNA just 3 days post-fracture, thereby stimulating IHH signaling via Patched 1 (ptch1), a patched family hedgehog receptor, and downstream target Gli1 in the fracture callus within 7 days post-fracture. This boost in IHH signaling accelerates cartilage formation and chondrocyte hypertrophy within the fracture callus. Moreover, treatment with STO-609 enhances the early influx of osterix-expressing osteoprogenitors and OB-mediated expression of vascular endothelial growth factor (VEGF), a key angiogenic factor within the callus, resulting in the early stimulation of primary bone formation at the callus. This acceleration of endochondral ossification by day 7 and bony callus formation by day 14 post-fracture ultimately results in a faster bridging of the fracture gap, imparting greater torsional strength to the fracture site by 4 weeks post-fracture. Thus, by accelerating key early molecular events, CaMKK2 inhibition potentiates a 20% acceleration of the bone healing process. [71]
Perspectives and Concluding Remarks
Whole-body energy homeostasis is complex and involves the coordination of central and peripheral metabolic environments. Imbalances in glucose and fat metabolism due to aging or disease result in rapid accumulation of deleterious byproducts including AGEs and ROS within metabolically active tissues, including the skeleton. [43] AGEs promote cellular oxidative stress and weaken the material properties of bone through non-enzymatic crosslinking of collagen fibers in the bone matrix.[42] Inflammation and oxidative stress act as selective pressures within the bone microenvironment that favor OC mediated bone resorption and reduce OB production of new bone matrix. Furthermore, low-grade systemic inflammation diminishes the regenerative capacity of progenitor cell populations in bone tissue. [67] [69] Thus, metabolic diseases such as T2DM reduce bone quality, increase the likelihood of fractures and impair bone healing.
CaMKK2 has cell-intrinsic roles in a variety of metabolically active tissues. CaMKK2 is a potent regulator of whole-body glucose and fat metabolism. Recent work reveals an important role for CaMKK2 in bone metabolism. Deletion or inhibition of CaMKK2 favors OB differentiation by relieving its inhibition of the cAMP-PKA signaling pathway. Conversely, CaMKK2-CaMKIV signaling promotes NFATc1 gene expression and OC differentiation, and therefore blocking CaMKK2 suppresses OCs. [35] Pharmacological inhibition of CaMKK2 with STO-609 promotes bone anabolism and protects from age-related and OVX-induced osteoporosis. [35, 32] Although the exact mechanism is unknown, CaMKK2 also regulates IHH expression during the early stages of bone healing. CaMKK2 inhibition accelerates bone healing by enhancing the influx of osteoprogenitors and elevating IHH at the fracture site to drive endochondral ossification and osteogenesis. [71] Taken together, CaMKK2 inhibition offers a multidimensional therapeutic strategy to treat and/or prevent skeletal diseases and injuries that arise from aging or imbalances in whole-body energy metabolism and the associated chronic inflammation.
Grant Support:
This work was supported by NAIMS/NIH R01 AR068332 to US. JN was supported through a Comprehensive Musculoskeletal T32 Training Program from NIAMS/NIH (AR065971).
Footnotes
Disclosures: Authors of this manuscript state that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
**Of major importance
- 1.Yelin EH, Cisternas M . Annual All-cause and Incremental Direct Costs for All Musculoskeletal Diseases in Current and 2014 Dollars, United States 1996–2014. The Burden of Musculoskeletal Diseases in the United States 2017. [Google Scholar]
- 2.Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA et al. Denosumab or Zoledronic Acid in Postmenopausal Women With Osteoporosis Previously Treated With Oral Bisphosphonates. J Clin Endocrinol Metab. 2016;101(8):3163–70. doi: 10.1210/jc.2016-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK et al. Osteoporosis: A Review of Treatment Options. P T. 2018;43(2):92–104. [PMC free article] [PubMed] [Google Scholar]
- 4.Jin A, Cobb J, Hansen U, Bhattacharya R, Reinhard C, Vo N et al. The effect of long-term bisphosphonate therapy on trabecular bone strength and microcrack density. Bone Joint Res. 2017;6(10):602–9. doi: 10.1302/2046-3758.610.BJR-2016-0321.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller PD, Hattersley G, Lau E, Fitzpatrick LA, Harris AG, Williams GC et al. Bone mineral density response rates are greater in patients treated with abaloparatide compared with those treated with placebo or teriparatide: Results from the ACTIVE phase 3 trial. Bone. 2018;120:137–40. doi: 10.1016/j.bone.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Cosman F, Eriksen EF, Recknor C, Miller PD, Guanabens N, Kasperk C et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):503–11. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 7.Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K et al. FRAME Study: The Foundation Effect of Building Bone With 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab. J Bone Miner Res. 2018;33(7):1219–26. doi: 10.1002/jbmr.3427. [DOI] [PubMed] [Google Scholar]
- 8.Dormuth CR, Carney G, Carleton B, Bassett K, Wright JM. Thiazolidinediones and fractures in men and women. Arch Intern Med. 2009;169(15):1395–402. doi: 10.1001/archinternmed.2009.214. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36(1):14–20. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Fan Y, Wei F, Lang Y, Liu Y. Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos Int. 2016;27(1):219–28. doi: 10.1007/s00198-015-3279-7. [DOI] [PubMed] [Google Scholar]
- 12.Miranda C, Giner M, Montoya MJ, Vazquez MA, Miranda MJ, Perez-Cano R. Influence of high glucose and advanced glycation end-products (ages) levels in human osteoblast-like cells gene expression. BMC Musculoskelet Disord. 2016;17:377. doi: 10.1186/s12891-016-1228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **13.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–29. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study uggests that the skeleton is capable of regulating systemic glucose homeostasis.
- 14.Dirckx N, Tower RJ, Mercken EM, Vangoitsenhoven R, Moreau-Triby C, Breugelmans T et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J Clin Invest. 2018;128(3):1087–105. doi: 10.1172/JCI97794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carafoli E Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A. 2002;99(3):1115–22. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Marcelo KL, Means AR, York B. The Ca(2+)/Calmodulin/CaMKK2 Axis: Nature’s Metabolic CaMshaft. Trends Endocrinol Metab. 2016;27(10):706–18. doi: 10.1016/j.tem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racioppi L, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J Biol Chem. 2012;287(38):31658–65. doi: 10.1074/jbc.R112.356485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green MF, Scott JW, Steel R, Oakhill JS, Kemp BE, Means AR. Ca2+/Calmodulin-dependent protein kinase kinase beta is regulated by multisite phosphorylation. J Biol Chem. 2011;286(32):28066–79. doi: 10.1074/jbc.M111.251504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokumitsu H, Hatano N, Fujimoto T, Yurimoto S, Kobayashi R. Generation of autonomous activity of Ca(2+)/calmodulin-dependent protein kinase kinase beta by autophosphorylation. Biochemistry. 2011;50(38):8193–201. doi: 10.1021/bi201005g. [DOI] [PubMed] [Google Scholar]
- 21.Green MF, Anderson KA, Means AR. Characterization of the CaMKKbeta-AMPK signaling complex. Cell Signal. 2011;23(12):2005–12. doi: 10.1016/j.cellsig.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dadwal UC, Chang ES, Sankar U. Androgen Receptor-CaMKK2 Axis in Prostate Cancer and Bone Microenvironment . Front Endocrinol (Lausanne). 2018;9:335. doi: 10.3389/fendo.2018.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin F, Marcelo KL, Rajapakshe K, Coarfa C, Dean A, Wilganowski N et al. The camKK2/camKIV relay is an essential regulator of hepatic cancer. Hepatology. 2015;62(2):505–20. doi: 10.1002/hep.27832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7(5):377–88. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Rui L Energy metabolism in the liver. Compr Physiol. 2014;4(1):177–97. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson KA, Lin F, Ribar TJ, Stevens RD, Muehlbauer MJ, Newgard CB et al. Deletion of CaMKK2 from the liver lowers blood glucose and improves whole-body glucose tolerance in the mouse. Mol Endocrinol. 2012;26(2):281–91. doi: 10.1210/me.2011-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.York B, Li F, Lin F, Marcelo KL, Mao J, Dean A et al. Pharmacological inhibition of CaMKK2 with the selective antagonist STO-609 regresses NAFLD. Sci Rep. 2017;7(1):11793. doi: 10.1038/s41598-017-12139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcelo KL, Ribar T, Means CR, Tsimelzon A, Stevens RD, Ilkayeva O et al. Research Resource: Roles for Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CaMKK2) in Systems Metabolism. Mol Endocrinol. 2016;30(5):557–72. doi: 10.1210/me.2016-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, Kobayashi R. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J Biol Chem. 2002;277(18):15813–8. doi: 10.1074/jbc.M201075200. [DOI] [PubMed] [Google Scholar]
- 30.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Lin F, Ribar TJ, Means AR. The Ca2+/calmodulin-dependent protein kinase kinase, CaMKK2, inhibits preadipocyte differentiation. Endocrinology. 2011;152(10):3668–79. doi: 10.1210/en.2011-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pritchard ZJ, Cary RL, Yang C, Novack DV, Voor MJ, Sankar U. Inhibition of CaMKK2 reverses age-associated decline in bone mass. Bone. 2015;75:120–7. doi: 10.1016/j.bone.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 34.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. [DOI] [PubMed] [Google Scholar]
- 35.Cary RL, Waddell S, Racioppi L, Long F, Novack DV, Voor MJ et al. Inhibition of Ca(2)(+)/calmodulin-dependent protein kinase kinase 2 stimulates osteoblast formation and inhibits osteoclast differentiation. J Bone Miner Res. 2013;28(7):1599–610. doi: 10.1002/jbmr.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Diabetes A. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–28. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maimoun L, Mura T, Leprieur E, Avignon A, Mariano-Goulart D, Sultan A. Impact of obesity on bone mass throughout adult life: Influence of gender and severity of obesity. Bone. 2016;90:23–30. doi: 10.1016/j.bone.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 39.King LK, March L, Anandacoomarasamy A. Obesity & osteoarthritis. Indian J Med Res. 2013;138:185–93. [PMC free article] [PubMed] [Google Scholar]
- 40.Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. 2011;96(6):1654–63. doi: 10.1210/jc.2011-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Son M, Chung WJ, Oh S, Ahn H, Choi CH, Hong S et al. Age dependent accumulation patterns of advanced glycation end product receptor (RAGE) ligands and binding intensities between RAGE and its ligands differ in the liver, kidney, and skeletal muscle. Immun Ageing. 2017;14:12. doi: 10.1186/s12979-017-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautieri A, Passini FS, Silvan U, Guizar-Sicairos M, Carimati G, Volpi P et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017;59:95–108. doi: 10.1016/j.matbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Cannizzaro L, Rossoni G, Savi F, Altomare A, Marinello C, Saethang T et al. Regulatory landscape of AGE-RAGE-oxidative stress axis and its modulation by PPARgamma activation in high fructose diet-induced metabolic syndrome. Nutr Metab (Lond). 2017;14:5. doi: 10.1186/s12986-016-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015;461(2):193–9. doi: 10.1016/j.bbrc.2015.02.091. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka K, Yamaguchi T, Kaji H, Kanazawa I, Sugimoto T. Advanced glycation end products suppress osteoblastic differentiation of stromal cells by activating endoplasmic reticulum stress. Biochem Biophys Res Commun. 2013;438(3):463–7. doi: 10.1016/j.bbrc.2013.07.126. [DOI] [PubMed] [Google Scholar]
- 46.Davidson MB. Thiazolidinediones. N Engl J Med. 2005;352(2):205–7; author reply −7. doi: 10.1056/NEJM200501133520222. [DOI] [PubMed] [Google Scholar]
- 47.Broulik PD, Sefc L, Haluzik M. Effect of PPAR-gamma agonist rosiglitazone on bone mineral density and serum adipokines in C57BL/6 male mice. Folia Biol (Praha). 2011;57(4):133–8. [PubMed] [Google Scholar]
- 48.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180(1):32–9. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci(Lond) . 2012;122(6):253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Su J, Sun W, Cai L, Deng Z. AMP-activated protein kinase stimulates osteoblast differentiation and mineralization through autophagy induction. Int J Mol Med. 2018;41(5):2535–44. doi: 10.3892/ijmm.2018.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mai QG, Zhang ZM, Xu S, Lu M, Zhou RP, Zhao L et al. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J Cell Biochem. 2011;112(10):2902–9. doi: 10.1002/jcb.23206. [DOI] [PubMed] [Google Scholar]
- 53.McCarthy AD, Cortizo AM, Sedlinsky C. Metformin revisited: Does this regulator of AMP-activated protein kinase secondarily affect bone metabolism and prevent diabetic osteopathy. World J Diabetes. 2016;7(6):122–33. doi: 10.4239/wjd.v7.i6.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11(3):234–50. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 56.Tsiotra PC, Boutati E, Dimitriadis G, Raptis SA. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. Biomed Res Int. 2013;2013:487081. doi: 10.1155/2013/487081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hughes FJ, Turner W, Belibasakis G, Martuscelli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol 2000. 2006;41:48–72. doi: 10.1111/j.1600-0757.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 58.Confalone E, D’Alessio G, Furia A. IL-6 Induction by TNFalpha and IL-1beta in an Osteoblast-Like Cell Line. Int J Biomed Sci. 2010;6(2):135–40. [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Martinez O, De Luna-Bertos E, Ramos-Torrecillas J, Manzano-Moreno FJ, Ruiz C. Repercussions of NSAIDS drugs on bone tissue: the osteoblast. Life Sci. 2015;123:72–7. doi: 10.1016/j.lfs.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Bhattacharyya T, Levin R, Vrahas MS, Solomon DH. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53(3):364–7. doi: 10.1002/art.21170. [DOI] [PubMed] [Google Scholar]
- 61.Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015;1(1):e000014. doi: 10.1136/rmdopen-2014-000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plotkin LI, Manolagas SC, Bellido T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival. Evidence for inside-out signaling leading to anoikis. J Biol Chem. 2007;282(33):24120–30. doi: 10.1074/jbc.M611435200. [DOI] [PubMed] [Google Scholar]
- 63.Racioppi L, Noeldner PK, Lin F, Arvai S, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J Biol Chem. 2012;287(14):11579–91. doi: 10.1074/jbc.M111.336032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med. 2015;32(9):1134–42. doi: 10.1111/dme.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45 Suppl 2:S3–7. doi: 10.1016/j.injury.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Griffin XL. Low intensity pulsed ultrasound for fractures of the tibial shaft. BMJ. 2016;355:i5652. doi: 10.1136/bmj.i5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim HS, Nam ST, Mun SH, Lee SK, Kim HW, Park YH et al. DJ-1 controls bone homeostasis through the regulation of osteoclast differentiation. Nat Commun. 2017;8(1):1519. doi: 10.1038/s41467-017-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okonkwo UA, DiPietro LA. Diabetes and Wound Angiogenesis . Int J Mol Sci. 2017;18(7). doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **69.Tevlin R, Seo EY, Marecic O, McArdle A, Tong X, Zimdahl B et al. Pharmacological rescue of diabetic skeletal stem cell niches. Sci Transl Med. 2017;9(372). doi: 10.1126/scitranslmed.aag2809. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates a potential mechanism for type 2 diabetes to impair the skeletal stem cell response during bone fracture healing.
- **70.Lin YC, Roffler SR, Yan YT, Yang RB. Disruption of Scube2 Impairs Endochondral Bone Formation. J Bone Miner Res. 2015;30(7):1255–67. doi: 10.1002/jbmr.2451. [DOI] [PubMed] [Google Scholar]; This study reveals inhibition of CaMKK2 promotes efficient bone fracture healing through modulation of IHH.
- 71.Williams JN, Kambrath AV, Patel RB, Kang KS, Mevel E, Li Y et al. Inhibition of CaMKK2 Enhances Fracture Healing by Stimulating Indian Hedgehog Signaling and Accelerating Endochondral Ossification. J Bone Miner Res. 2018;33(5):930–44. doi: 10.1002/jbmr.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]