Abstract
Objectives:
To determine trajectories of glycemic control and BMI z-score in a large pediatric sample with type 1 diabetes (T1D) over a 38-year period, and to evaluate sex differences and temporal changes in the prevalence of these trajectories.
Methods:
We conducted a longitudinal, retrospective study of 7002 2–18 year olds with T1D followed between 1978 and 2016 at a single center. Group-based modeling was used to identify trajectories for hemoglobin A1c (HbA1c) and BMI z-score. Multinomial logistic regression identified predictors of membership to less favorable glycemic trajectories.
Results:
Group-based modeling yielded 5 HbA1c trajectories. 86% of the sample fell within 3 trajectories that were largely stable across childhood and adolescence, and 14% fell within 2 trajectories characterized by marked deterioration beginning in pre-adolescence. Girls were more likely to be in the HbA1c trajectory with the highest starting HbA1c and significant deterioration during adolescence, and in the highest two BMI z-score trajectories. Patients with non-white race had the highest odds of belonging to a less favorable HbA1c trajectory. Prevalence of the High Stable HbA1c trajectory decreased and prevalence of the Low Stable HbA1c trajectory increased over the study period.
Conclusions:
A minority of youth with T1D experienced deterioration of glycemic control during adolescence. Girls were more likely to belong to the worst HbA1c trajectory and to BMI z-score trajectories in the overweight/obese range, which may increase cardiometabolic risk. Addressing racial/ethnic disparities in glycemic control should remain a priority. Advances in T1D management correlated with favorable shifts in HbA1c trajectory prevalence.
Keywords: Body Mass Index, Diabetes Mellitus, Type 1, Health Status Disparities, Glycated Hemoglobin A, Models, Statistical
INTRODUCTION
Most children and adolescents with T1D in the United States (US) do not meet the recommended HbA1c target of <7.5% (58mmol/mol)1–4, which increases their risk of developing diabetes-related complications5–8. Recent studies have reported that girls with T1D have worse metabolic control and complications than boys with T1D9,10, and higher BMI z-scores than boys with T1D and girls without T1D9. Women with T1D have a significantly higher relative risk of cardiovascular disease (CVD) compared to men with T1D, when each are compared to their non-diabetic counterparts11. What remains poorly understood is when this divergence in risk by sex begins and what is driving it. But data suggest that adolescence may be a critical time period during which sex differences in CVD risk factors emerge, particularly glycemic control and obesity.
Deterioration of glycemic control for youth with T1D during adolescence has been frequently reported12,13, and potential contributors to poor glucose control during puberty include a physiologic decrease in insulin sensitivity, increased autonomy in diabetes management, and psychosocial changes2,12. However, the premise that all adolescents will experience worsened glycemic control is an imprecise overgeneralization, and may contribute to missed opportunities to identify and treat the highest-risk youth. Several studies have used longitudinal group-based modeling to identify subgroups of children with T1D that follow distinct trajectories of glycemic control during adolescence, and all have, surprisingly, reported that only the minority of young people with T1D experience deteriorating glycemic control during adolescence14–17.
Previous studies have aimed to determine if membership to unique trajectory groups could be predicted by psychosocial factors, behavioral markers of self-care, healthcare utilization, and/or clinical variables (e.g. total daily insulin dose)15,16,18,19. Remaining gaps in understanding include whether trajectories of glycemic control differ by sex, how these trajectory groups have changed over time with treatment advances in insulin delivery and glucose monitoring, and how the obesity epidemic of the past several decades may have affected glycemic control trajectories.
Here we present a large, longitudinal, retrospective cohort study of children and adolescents (2–18 years old) with T1D for at least 1 year, seen at the Barbara Davis Center for Diabetes (BDC) in Colorado between 1978 and 2016. Our primary objective was to use group-based modeling to identify trajectories of HbA1c and BMI z-score. Secondary objectives were to determine characteristics that could predict membership to each trajectory group, to explore sex differences in HbA1c and BMI z-score trajectories across childhood and adolescence, and to examine how the prevalence of glycemic and BMI z-score trajectory groups has changed over time. We hypothesized that the majority of youth would not experience a significant deterioration in glycemic control during adolescence; that girls would be more likely than boys to belong to clinically unfavorable HbA1c trajectories; that the prevalence of favorable HbA1c trajectories has increased over the study period, mirroring improved diabetes care; and that the obesity epidemic would result in increasing prevalence of higher BMI z-score trajectories.
METHODS
Study population and design
We performed a longitudinal, retrospective cohort study of children and adolescents seen at the BDC between 1978 and 2016, using electronic medical records. Inclusion criteria were age between 2 and 18 years, a diagnosis of T1D for at least 1 year, and contribution of at least 1 HbA1c or BMI z-score measure. T1D was defined by the clinically accepted criteria at the time of the patient’s diagnosis. Patients meeting these criteria were identified using unique identifiers through queries of 2 electronic medical record systems. Individuals were excluded if sex was not recorded, or if there were no HbA1c or BMI z-score data available. Data during the first year of diabetes diagnosis were excluded because of known weight changes and wide HbA1c fluctuations around the time of diagnosis and during the “honeymoon period”20,21. Data cleaning consisted of an assessment of plausibility for each value (e.g. was a weight physiologically possible, based on that patient’s trends). Race/ethnicity groups were defined as non-Hispanic White, Hispanic, Black, or Other. Inconsistencies in how race/ethnicity was reported over the 38-year study period and the small number of patients falling into other racial categories necessitated that the “other” group be formed, which included patients who did not indicate Hispanic ethnicity, and who identified as American Indian/Alaskan Native, Asian, Native Hawaiian/Other Pacific Islander, Other, or where race was unknown or not reported. Insulin pump use was recorded in the ‘insulin delivery’ field of the medical record, and patients with any recorded pump use at any visit were categorized as “ever pump users”. This study was approved by the Colorado Multiple Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
Outcome Variables
HbA1c and BMI z-score were the primary outcomes of interest. HbA1c values and BMI z-scores were averaged for each child per year of age from all visits within that year (e.g. the mean of all HbA1c values for all visits for a particular child at 2 years of age was used as that child’s 2 year old HbA1c). For earlier time points where HbA1 was reported (before the more specific HbA1c became routine), we used the formula derived by Krolewski et al. to convert HbA1 to HbA1c: HbA1c = [HbA1 – 0.14] / 1.2322.
BMI z-scores by age and sex were calculated using the Centers for Disease Control and Prevention’s (CDC) SAS program, which uses CDC 2000 Growth Charts (ages 2–20) as reference data23.
Statistical Analysis
SAS version 9.4 (SAS Institute Inc., Cary, NC, US) was used for all analyses.
Group-Based Trajectory Modeling
We used group-based trajectory modeling (GBTM), adapted from the original approach described by Nagin24,25, and its recent application by Schwandt et al.17, to identify subgroups within the dataset that followed distinct trajectories for HbA1c and BMI z-score. GBTM is a semi-parametric technique applied to longitudinal data to define groups that follow similar patterns for a specified outcome variable over time26. This method allows for unbiased estimates in longitudinal modeling even when there are missing data as is the case, for example, when patients are diagnosed with T1D at different ages, and therefore some patients have data available at younger ages and others do not. Further, it allows for unbiased estimates when some data are missing after diagnosis, such as when patients are no longer followed at the clinic and do not contribute data through the entire time period.
GBTM allows for unbiased estimates as long as missing data (e.g. those patients leaving the clinic prior to the age of 18 or having sparse data) are missing completely at random (MCAR). To assess the pattern of missing data in our sample, we compared group-based trajectory assignments from a restricted cohort that had more complete data to those of the entire cohort (see Figure S1, Supporting Information). The restricted cohort included patients who established care at the BDC within a year of diagnosis who were followed through age 17 or 18, and who had at least 8 HbA1c measures. As in the overall cohort, data from within the first 12 months of diagnosis were excluded. Only 2093 patients (out of 6987 patients with HbA1c data) met these criteria, which is not unexpected considering the migration of individuals into and out of communities and the fractured healthcare system in the US. Utilizing this limited sample would restrict the power to examine differences by time period and subgroups by sex and race/ethnicity and would bias results toward patients diagnosed with T1D at younger ages. So, agreement in group trajectory assignments between this restricted cohort and the entire cohort was determined using the weighted Cohen’s kappa statistic (κ = 0.84, 95% CI 0.83–0.86), where values between 0.81 and 1.00 are considered “almost perfect agreement”27. Given the similarity between the two cohorts, missing data in the entire cohort was assumed to be MCAR. We therefore present results from the entire sample.
The decision to include individuals with even 1 HbA1c or BMI z-score measure, as opposed to 2 or 3 or more aligns with the guiding document on growth curve modeling by Curran et al., which states that a minimum of 3 measures per person is recommended for a “sizeable portion of the cases”, but acknowledges that some proportion of the sample may only contribute 1 or 2 measures28. In our dataset, 87% of individuals contributed at least 3 HbA1c measures and 86% of individuals contributed at least 3 BMI z-score measures. Thus, we included all individuals with 1 or more HbA1c or BMI z-score measures.
To generate group trajectories using the SAS application PROC TRAJ, the optimal number of groups was chosen based on model fit (lower Bayes information criterion [BIC]), a minimum percent membership in each group (≥5%), and consideration of clinical context. Once the number of groups was chosen, the best polynomial fit for each group (e.g. linear, cubic, quadratic) was determined by BIC. Individuals were assigned a single trajectory based on the probability of group membership. To generate sex-specific HbA1c and BMI z-score group trajectories, data were first stratified by sex, then PROC TRAJ was run independently for each sex.
Time Periods by Year of Type 1 Diabetes Diagnosis
Patients were divided into 3 time periods based on their year of T1D diagnosis (1978–1995, 1996–2005, and 2006–2016). 1996 was chosen as a defining year based on significant clinical practice changes that were being adopted at the BDC at this time as a result of the Diabetes Control and Complications Trial (DCCT). The remaining 20 years of data were split approximately evenly to create the other two time periods.
Weight Categories
We defined weight categories according to BMI percentiles for age and sex and the corresponding z-scores as follows: underweight (BMI<5th%ile, z-score < −1.64), normal weight (BMI 5th through 84th%ile, z-score −1.64 to < 1.04), overweight (BMI 85th through 94th%ile, z-score 1.04 to < 1.64), and obese (BMI ≥95th%ile, z-score ≥1.64).
Description, Comparison, and Prevalence of Trajectory Groups
We examined characteristics that could distinguish HbA1c trajectory groups overall, and distinguish each HbA1c trajectory group from the ideal Low Stable group as a reference, using likelihood ratio chi-square tests for categorical variables and univariate ANOVA using PROC GLM for continuous variables (where p <0.05 was considered statistically significant and <0.001 was considered highly statistically significant). We then compared characteristics of specific HbA1c trajectory pairs using multinomial logistic regression expressed as odds ratios with 95% confidence intervals. Additionally, the prevalence of each HbA1c and BMI z-score trajectory group was determined for the 3 diabetes onset periods (1978–1995, 1996–2005, and 2006–2016), and changes in the prevalence of trajectory groups across time periods were assessed using ANOVA.
RESULTS
Sample Characteristics
The final sample represented 7002 unique children and adolescents who had a diagnosis of T1D for at least 1 year and had at least 1 visit with either HbA1c or BMI z-score measured. 6987 individuals had at least 1 HbA1c measurement, and contributed a total of 107367 HbA1c measurements, with 6064 (87%) contributing at least 3 years of data. The median number of HbA1c measures per person was 12 (Interquartile Range (IQR) 5–23). The median time interval followed for HbA1c measures was 3.9 years (IQR 1.5–7.2). 6897 individuals had at least 1 BMI z-score measurement and contributed a total of 106736 BMI z-score measurements, with 5941 (86%) contributing at least 3 years of data. The median number of BMI z-score measures per person was 11 (IQR 5–22). The median time interval followed for BMI z-score was 3.8 years (IQR 1.4–7).
47% of the sample was female and 53% was male. 73% identified as non-Hispanic White, 8% as Hispanic, 3% as Black, and 12% were classified as Other. Within the Other category, 63% were documented as “unknown”, 12% were documented as “other”, 15% were labeled “more than one race”, 5% were American Indian/Alaskan Native, 5% were Asian, and 0.4% were Native Hawaiian/Other Pacific Islander. The mean age at onset of T1D was 8.3 years for females and 8.5 years for males. 21% of individuals were diagnosed with T1D before 1996, 36% between 1996–2005, and 43% between 2006–2016. 9.6% of all visits were for 2–6 year olds, 29.2% for 7–11 year olds, and 61.2% for 12–18 year olds. Any insulin pump use was significantly lower among minority patients compared to those who were non-Hispanic white, in the 2 most recent time periods, when pump use became prevalent. Between 1978–1995, pump use was 10%, 18%, and 25% in black, Hispanic, and non-Hispanic white patients, respectively (likelihood ratio chi-square p=0.05). Between 1996 and 2005, pump use was 16%, 24%, and 51% in black, Hispanic, and non-Hispanic white patients, respectively (p <0.0001). And, between 2006–2016, pump use was 29% in black patients, 36% in Hispanic patients, and 65% in non-Hispanic white patients (p <0.0001). The percentage of visits for which insulin delivery information was missing was 19%, 1%, and 5% for the 1978–1995, 1996–2005, and 2006–2016 time periods, respectively. Because pumps were not widely adopted at the BDC until 1996, the majority of visits before 1996 for which insulin delivery was “missing” were very likely to be by injection, with no documentation of insulin delivery provided since injection was the assumed method prior to pump use.
Group-Based Trajectory Modeling
HbA1c Trajectories
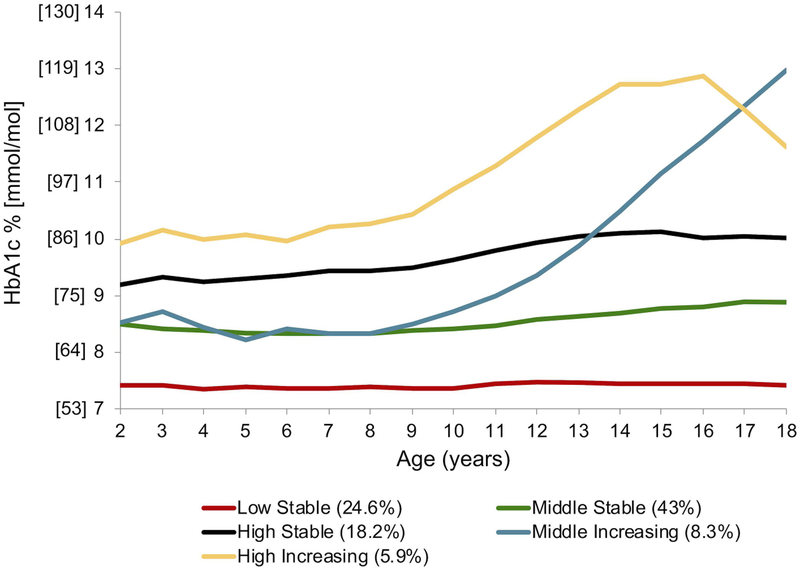
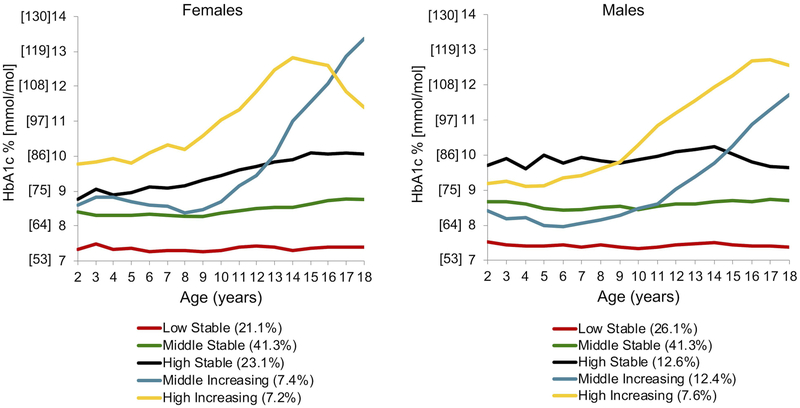
Group-based modeling for HbA1c from ages 2 through 18 yielded 5 distinct trajectory groups (Figure 1a): Low Stable (24.6% of the sample), Middle Stable (43%), High Stable (18.2%), Middle Increasing (8.3%), and High Increasing (5.9%). “Low”, “Middle”, and “High” refer to where each trajectory begins on the y-axis (i.e. the relative HbA1c value at age 2). “Stable” refers to trajectories with relative constancy of HbA1c values across the age range and “Increasing” denotes trajectories with a significant increase in HbA1c at some point across the age range. We tested for sex as a “risk factor” in the overall HbA1c trajectory group model, and found that girls were more likely to be in the High Increasing trajectory group than boys (p=0.0008). We then stratified the data by sex and generated HbA1c trajectory groups independently for each sex (Figure 2a). Independent trajectory models (i.e. girls vs. boys) cannot be compared statistically. However, visually, HbA1c trajectory groups were similar for boys and girls, and were most closely aligned for the Low, Middle, and High Stable groups. The Middle Increasing HbA1c trajectory demonstrated a faster rate of rise from ages 10 through 18 for females compared to males. Further, the High Increasing trajectory peaked earlier for females (age 14) than it did for males (age 16–17).
Figure 1a.
HbA1c trajectory groups for all 2–18 year olds seen at the Barbara Davis Center in Colorado between 1978–2016, with type 1 diabetes for ≥ 1 year, and at least 1 HbA1c measure (n=6987). HbA1c values were averaged for each child per year of age from all visits within that year. Trajectory names: “Low”, “Middle”, and “High” refer to where each trajectory begins on the y-axis (i.e. the relative HbA1c value at age 2). “Stable” refers to trajectories with relative constancy of HbA1c values across the age range and “Increasing” denotes trajectories with a significant increase in HbA1c at some point across the age range.
Figure 2a.
Sex-specific HbA1c trajectory groups. After stratifying the data by sex, trajectories were created and plotted independently for females (n=3281) and males (n=3706). This represents all 2–18 year olds seen at the Barbara Davis Center in Colorado between 1978–2016, with type 1 diabetes for ≥ 1 year, and at least 1 HbA1c measure. Trajectory names: “Low”, “Middle”, and “High” refer to where each trajectory begins on the y-axis (i.e. the relative HbA1c value at age 2). “Stable” refers to trajectories with relative constancy of HbA1c values across the age range and “Increasing” denotes trajectories with a significant increase in HbA1c at some point across the age range.
Table 1 shows select characteristics by HbA1c trajectory group for the overall sample. Characteristics that were statistically significantly different across all 5 HbA1c trajectory groups were age at T1D onset (p< 0.0001), sex (p 0.02), race (p <0.001), average HbA1c (p <0.0001), and average BMI z-score (p <0.0001).
Table 1.
Characteristics by HbA1c trajectory group
| N- | Low Stable n=1721 24.6% | Middle Stable n=3002 43% | HighStable n=1273 18.2% | Middle Increasing n=582 8.3% | High Increasing n=409 5.9% | p-value |
|---|---|---|---|---|---|---|
|
Age at onset
(y) (LSM +/− SE) |
9.4 ± 0.1 | 7.9** ± 0.1 | 8.1** ± 0.1 | 8.8*± 0.2 | 8.8*± 0.2 | <0.0001 |
| Sex (% female) | 45.3 | 45.9 | 48.7 | 49.8 | 52.6* | 0.02 |
| Other | 10.69 | 12.76 | 10.76 | 11.68 | 13.94 | |
|
HbA1c
(mmol/mol) (LSM +/− SE) |
56 ± 0.2 | 71** ± 0.1 | 85** ± 0.2 | 93** ± 0.3 | 108** ± 0.4 | <0.0001 |
|
HbA1c (%) (LSM +/− SE) |
7.3 ± 0.02 | 8.6** ± 0.01 | 10.0** ± 0.02 | 10.6** ± 0.03 | 12.0** ± 0.03 | <0.0001 |
|
BMI
z-score (+/− SE) |
0.37 ± 0.02 | 0.45*± 0.02 | 0.53** ± 0.02 | 0.52** ± 0.03 | 0.43 ± 0.04 | <0.0001 |
Likelihood ratio chi-square was used for categorical variables and univariate ANOVA for continuous variables, for all 2–18 year olds seen at the Barbara Davis Center in Colorado between 1978–2016, with Type 1 diabetes for ≥ 1 year, and at least 1 HbA1c measure (N=6987).
LSM: least squares mean; SE: standard error
p-value column indicates significance for the overall group comparison
= p<0.05,
= p< 0.001 indicates significance for each trajectory group compared to the clinically ideal Low Stable trajectory group
Next, for every characteristic, each HbA1c trajectory group was compared to the Low Stable HbA1c trajectory group using multinomial logistic regression (Table 2). Data are presented as odds ratios with 95% confidence intervals, in which the odds describe the risk of being in the worse HbA1c trajectory group compared to the clinically ideal Low Stable group across multiple variables. For every 1 year increase in age at onset of T1D, there was a 2–7% decreased risk of belonging to any of the less favorable trajectories compared to the Low stable group. (E.g. for every year older at the age of onset, an individual was 7% less likely (OR 0.93 [0.91–0.94]) to be in the Middle Stable group than the Low Stable group). Patients diagnosed between 1996–2005, compared to those diagnosed between 1978–1995 had a 28–56% decreased odds of being in a less favorable HbA1c trajectory group and patients diagnosed from 2006–2016 vs. 1978–1995 had a 47–73% decreased odds of being in a less favorable HbA1c trajectory group. Being female was associated with a 29% increased odds of being in the High Increasing group vs. the Low Stable group. Being a member of the highest BMI z-score group (overweight/obese) compared to the lower 4 groups, was associated with a 32, 63, and 58% increased odds of being a member of the less favorable HbA1c trajectory group for Middle Stable vs. Low Stable, High Stable vs. Low Stable, and Middle Increasing vs. Low Stable comparisons, respectively. The odds of being in the High Increasing vs. Low Stable comparison were not statistically significant for this BMI variable. Individuals who were Black, Hispanic, or Other vs. White were at significantly greater odds of being in the less favorable HbA1c trajectory group across all comparisons. This ranged from a minimum of a 27% increased odds of being in the Middle Stable vs. Low Stable group for individuals who were “Other” race vs. White, to a 1222% (greater than 12x) increased odds of being in the High Increasing group vs. Low Stable group for individuals who were Black vs. White. When the model was adjusted for insulin pump use (any use of pump ever vs. never), pump use was strongly and significantly related to a lower risk of membership to an unfavorable HbA1c trajectory (data not shown). However, it did not substantially change the relationship of race or any other variable with HbA1c trajectory group. Because pump use was uncommon in the first time period studied, we did not include this variable in our main model (Table 2).
Table 2.
Comparison of HbA1c trajectory pairs.
| Odds Ratio (95% CI) | Middle Stable vs. Low Stable
|
High Stable vs. Low Stable
|
Middle Increasing vs. Low Stable
|
High Increasing vs. Low Stable
|
|---|---|---|---|---|
|
Age at onset (per 1y increase) |
0.93 (0.91–0.94) | 0.95 (0.93–0.96) | 0.98 (0.96–1.00) | 0.97 (0.94–1.00) |
| 2006+ vs. 1978–1995 | 0.53 (0.44–0.64) | 0.27 (0.22–0.33) | 0.34 (0.26–0.44) | 0.49 (0.35–0.67) |
| Female vs. Male | 0.97 (0.86–1.09) | 1.05 (0.90–1.22) | 1.12 (0.93–1.36) | 1.29 (1.03–1.62) |
| BMIz group 5 (highest) vs. BMIz groups 1–4 | 1.32 (1.08–1.61) | 1.63 (1.30–2.05) | 1.58 (1.19–2.09) | 1.13 (0.80–1.59) |
| Other vs. White | 1.27 (1.05–1.54) | 1.31 (1.03–1.67) | 1.35 (1.00–1.84) | 2.19 (1.56–3.05) |
Comparisons were made using multinomial logistic regression, for all 2–18 year olds seen at the Barbara Davis Center in Colorado between 1978–2016, with Type 1 diabetes for ≥ 1 year, and at least 1 HbA1c measure (n=6987). For each column, odds ratios represent the odds of being in the clinically worse HbA1c trajectory group compared to the ideal Low Stable group. Each row has been adjusted for every other row variable (e.g. Age at onset has been adjusted for Time period of diagnosis, Sex, BMI z-score Trajectory 5 vs. 1–4, and Race).
BMI z-score Trajectories
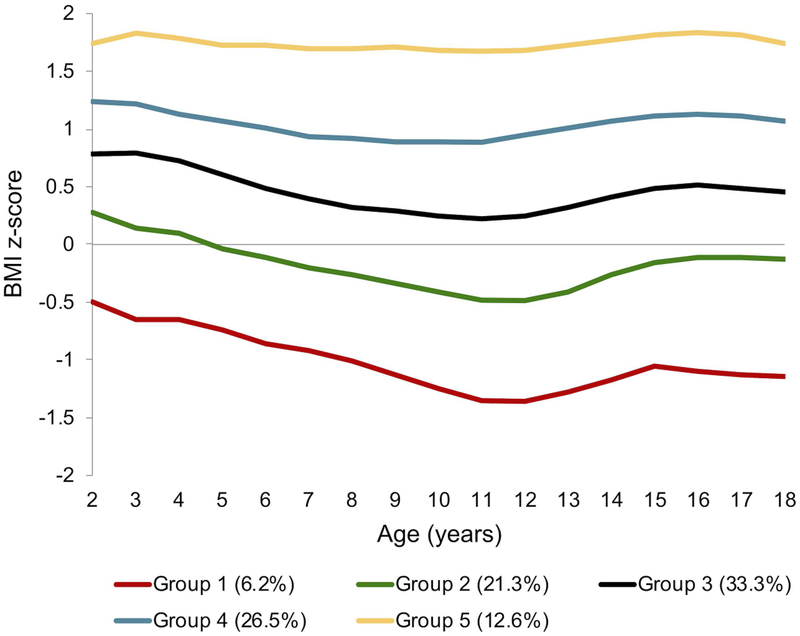
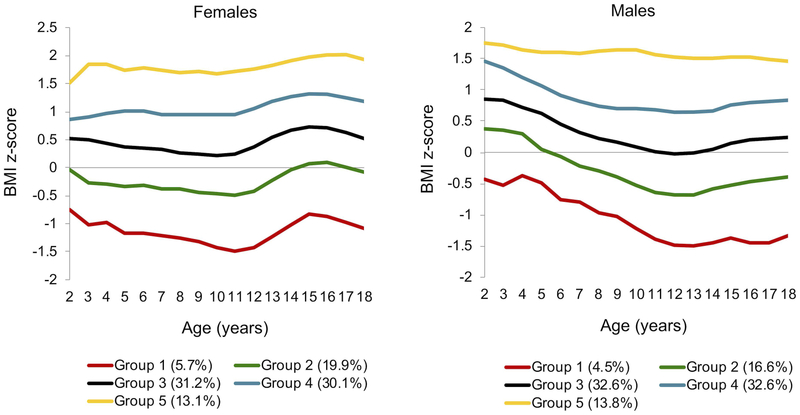
Group-based modeling for BMI z-score from ages 2 through 18 also yielded 5 unique trajectories (Figure 1b): Group 1 (6.2% of the sample), Group 2 (21.3%), Group 3 (33.3%), Group 4 (26.5%), and Group 5 (12.6%). Trajectory groups 1 through 3 represented youth with a normal weight status and made up 60.8% of the total sample. Trajectory group 4 (26.5%) contained patients of both normal and overweight status. And, trajectory group 5 (12.6%) contained patients who were either overweight or obese. We tested for sex as a “risk factor” in the overall BMI z-score trajectory group model, and found that girls were significantly more likely than boys to be in Groups 4 (p=0.0002) and 5 (p<0.0001). We then stratified the data by sex and generated BMI z-score trajectory groups independently for each sex (Figure 2b). Again, stratified trajectory groups cannot be compared statistically, but they can be explored visually. Girls had lower BMI z-scores than boys in early childhood. However, between age 11 and 15, girls demonstrated a clear upward inflection in BMI z-score in all groups, exceeding that of the boys within every trajectory. Boys showed a less prominent rise in BMI z-score in Groups 1–4 between ages 13 and 15. This difference in BMI z-score by sex appeared to be diminishing in all 5 trajectories by age 18.
Figure 1b.
BMI z-score trajectory groups for all 2–18 year olds seen at the Barbara Davis Center in Colorado between 1978–2016, with type 1 diabetes for ≥ 1 year, and at least 1 BMI z-score measure (n=6897). BMI z-scores were averaged for each child per year of age from all visits within that year. Weight categories are defined as follows: underweight (BMI z-score < −1.64), normal weight (BMI z-score −1.64 to < 1.04), overweight (BMI z-score 1.04 to < 1.64), and obese (BMI z-score ≥1.64).
Figure 2b.
Sex-specific BMI z-score trajectory groups. After stratifying the data by sex, trajectories were created and plotted independently for females (n=3242) and males (n=3655). This represents all 2–18 year olds seen at the Barbara Davis Center in Colorado between 1978–2016, with type 1 diabetes for ≥ 1 year, and at least 1 BMI z-score measure. Weight categories are defined as follows: underweight (BMI z-score < −1.64), normal weight (BMI z-score −1.64 to < 1.04), overweight (BMI z-score 1.04 to < 1.64), and obese (BMI z-score ≥1.64).
Prevalence of Trajectory Groups over Time
HbA1c Trajectory Group Prevalence
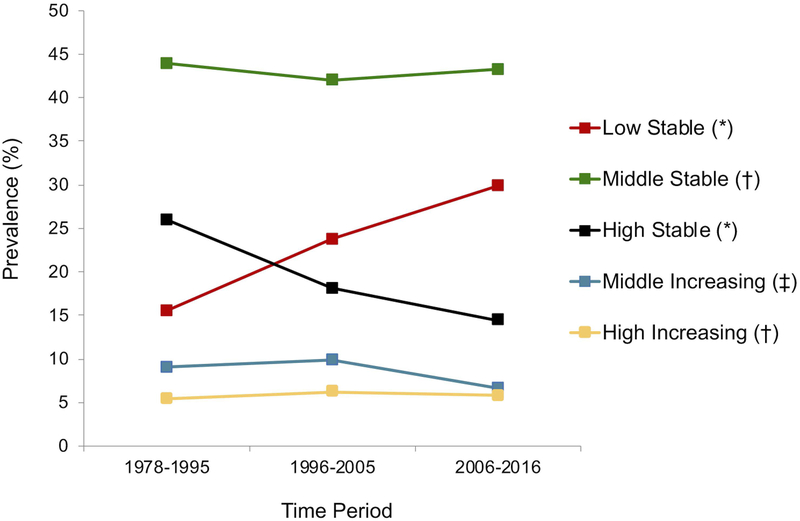
Examining changes in prevalence of HbA1c trajectory groups over time (Figure 3a), we found that the percentage of children and adolescents belonging to the ideal Low Stable HbA1c trajectory has steadily increased, from 15.6% (1978–1995), to 23.8% (1996–2005), to 29.9% (2006–2016), with p<0.001 between each time period. Conversely, the percentage of children and adolescents belonging to the less favorable High Stable HbA1c trajectory has steadily decreased over time: 26% (1978–1995), 18.1% (1996–2005), and 14.4% (2006–2016), with p<0.001 between each time period. The prevalence of the Middle Stable HbA1c trajectory group (between 42–44%) and High Increasing HbA1c trajectory group (between 5–6%) has not changed significantly over time (p>0.05 between each time period). Lastly, the prevalence of the Middle Increasing HbA1c trajectory group did not change between the earlier 2 time periods (p>0.05), but decreased from 1978–1995 and 1996–2005 to 2006–2016 (p=0.01 and p<0.001, respectively).
Figure 3a.
Prevalence of HbA1c trajectories by diabetes onset time period for all 2–18 year olds seen at the Barbara Davis Center (BDC) in Colorado between 1978–2016, with type 1 diabetes for ≥ 1 year, and at least 1 HbA1c measure (n=6987). 1996 marked significant clinical practice changes at the BDC as a result of the Diabetes Control and Complications Trial. Data from 1996–2016 were split about evenly to create the 1996–2005 and 2006–2016 time periods.
* = p<0.001 for T1 vs. T2, T2 vs. T3, and T1 vs. T3
‡ = p<0.01 for T1 vs. T3 and T2 vs. T3
§ = p < 0.05 for T1 vs. T2 and T1 vs. T3
† = no significant differences between any two time points
BMI z-score Trajectory Group Prevalence
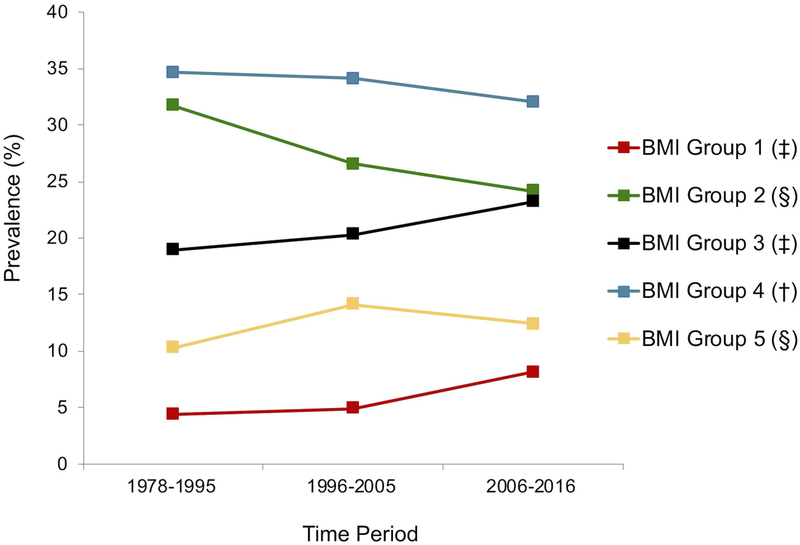
Looking at changes in prevalence of BMI z-score trajectory groups over the same 3 time periods (Figure 3b), there have been statistically significant increases in membership to BMI z-score groups 1, 3, and 5, a decrease in membership to BMI z-score group 2, and stability in BMI z-score group 4.
Figure 3b.
Prevalence of BMI z-score trajectory groups by diabetes onset time period for all 2–18 year olds seen at the Barbara Davis Center (BDC) in Colorado between 1978–2016, with type 1 diabetes for ≥ 1 year, and at least 1 BMI z-score measure (n=6897). 1996 marked significant clinical practice changes at the BDC as a result of the Diabetes Control and Complications Trial. Data from 1996–2016 were split about evenly to create the 1996–2005 and 2006–2016 time periods.
* = p<0.001 for T1 vs. T2, T2 vs. T3, and T1 vs. T3
‡ = p<0.01 for T1 vs. T3 and T2 vs. T3
§ = p < 0.05 for T1 vs. T2 and T1 vs. T3
† = no significant differences between any two time points
DISCUSSION
This is one of the longest published studies of HbA1c and BMI z-score trajectories among youth with T1D. Previous studies examining trajectories of glycemic control over childhood and adolescence, including the T1D Exchange13, have used mean HbA1c for age, which is vulnerable to the influence of extreme values, exaggerates the prevalence of deterioration over adolescence, and masks important subgroup differences. Others29 have attempted to identify predictors of glycemic control at a single age, which provides less robust information about when to intervene on significant predictors to prevent deterioration. In contrast, the GBTM approach used in this study is powerful in its ability to model patterns from longitudinal data that allow for early identification of the highest risk children. Unlike previous GBTM studies in T1D, including a large German/Austrian cohort17,18,30, children as young as 2 years of age were represented in our sample, which allowed for a more comprehensive evaluation of glycemic control and BMI z-score patterns across childhood and adolescence. This may be particularly relevant given the overall trend of earlier T1D onset31. Additionally, unlike many GBTM studies, we examined changes in the prevalence of HbA1c and BMI z-score trajectory groups over 4 decades, providing context for the impact of concurrent trends like the obesity epidemic and the significant advances in T1D management.
One potential limitation of our study, compared to other GBTM studies, is that we had more heterogeneity in the amount of data contributed by each person. This was a consequence of the variation in the age of onset of T1D, and the shortage of population-based healthcare registries in US. As an academic specialty center for T1D, our findings might not be generalizable to all settings. However, the BDC provides care to all children regardless of insurance status, and serves a high proportion of all children with T1D throughout the Rocky Mountain region. Our study included data from 2 electronic medical record systems, which increases the risk for inconsistencies in data collection and entry. BMI z-score for assessment of longitudinal weight status provides only an indirect measurement of adiposity, and it is excess adiposity that is most relevant to cardiometabolic disease risk. We also recognize that BMI z-score is now considered to be a statistically poor measure to describe adiposity for children and adolescents with severe obesity32. However, because only a very small proportion of individuals in our dataset had severe obesity, we used BMI z-scores instead of BMI as a percentage of the 95th%ile. Use of BMI z-scores also allows for easier comparisons to be made between our results and other studies of BMI z-score trajectories among children with T1D30. Finally, the retrospective nature of this study increases the risk of unmeasured confounders or mediators, which may be particularly relevant to the finding of significant racial/ethnic disparities in HbA1c trajectory membership.
Our results demonstrate significant racial/ethnic disparities, where minorities had strikingly higher odds of belonging to the least favorable HbA1c trajectory groups. The effect of race/ethnicity on HbA1c membership was stronger than any other measured variable in our model. And, while there was a strong relationship between race and insulin pump use (with non-Hispanic whites having significantly higher rates of pump use than any minority), and pump use was protective against membership to an unfavorable HbA1c trajectory, adjusting for pump use did not significantly change the relationship between race and HbA1c trajectory. Race/ethnicity is likely a surrogate for other important unmeasured variables, which might include biopsychosocial factors like genetic risk, provider and family perceptions of treatment intensity, or access to care33,34. Racial/ethnic disparities in insulin pump use, complications, and treatment outcomes have been widely reported in youth with T1D35,36, including recently in the SEARCH for Diabetes in Youth study37, and there is a clear need for novel strategies to close this gap.
There have been significant advances in T1D treatment over the time period studied including adoption of the DCCT’s intensive insulin approach, increased utilization of insulin pumps, and the advent of continuous glucose monitoring. In our multinomial logistic regression model, we not surprisingly found that a more recent diagnosis date predicted membership to a more favorable HbA1c trajectory group.
And, consistent with these advances, we saw favorable shifts in the prevalence of the High Stable and Low Stable HbA1c trajectory groups. However, these improvements in clinical care have not decreased the prevalence of the smallest, but highest risk group of youth that start with the poorest glycemic control, experience significant deterioration during adolescence, and disproportionately represent females and racial/ethnic minorities.
We did not see a linear increase in BMI (by prevalence) over the 3 time periods studied, which is inconsistent with what has been reported by some38, and with the US childhood obesity epidemic39. Others have similarly reported stability in the prevalence of overweight/obesity in youth with T1D over a more recent time period40. The High Stable HbA1c trajectory group, which decreased in prevalence over the study period, had the highest mean BMI z-scores, and the Low Stable HbA1c trajectory group, which increased in prevalence, had the lowest mean BMI z-scores, which could partially explain these results. The relationship between BMI and glycemic control is complex, particularly during adolescence. Higher insulin doses are often required during this period, which could contribute to weight gain41. And, adolescents may omit insulin intentionally or accidentally, which can result in hyperglycemia and resultant weight loss42.
The divergence seen in BMI z-score by sex (females > males) during adolescence is consistent with trajectory findings recently reported in a German Austrian cohort by Prinz et al.30. The consequences of excess weight in adolescent females with T1D were recently evaluated by Castro-Correia et al. who reported higher prevalence of dyslipidemia and hypertension among females with T1D who were overweight/obese compared to normal weight43. To minimize cardiometabolic risk, an intensive approach to weight management should be considered for girls with T1D who are overweight or obese during pre-adolescence, taking into account the complex relationship with glycemic control and insulin omission as noted above. The significant differences by sex in the overall HbA1c trajectory group model (Table 1), and, in the multinomial logistic regression (Table 2), are consistent with a previous cross-sectional study of sex differences in HbA1c and BMI z-score9.
Findings from the present study challenge the notion that most children with T1D experience deterioration of glycemic control during adolescence, and present an initial set of risk factors that may help to predict membership to subgroups that are at highest risk for suboptimal clinical outcomes during adolescence. Essential next steps include investigation of variables that help to explain the sex and racial/ethnic disparities found in this study. Future work may include model extension to investigate novel predictors of HbA1c or BMI z-score trajectories in clinical cohorts (e.g. fear of hypoglycemia, mental health co-morbidities). Broader applications include examining trajectory-specific effects of an exposure, intervention, or policy44,45, or evaluating an intervention or policy based on its ability to move individuals from a less favorable to a more favorable trajectory group. Finally, future GBTM studies would ideally examine cohorts across childhood and adulthood, where longer-term clinical outcomes can be assessed.
Supplementary Material
HbA1c trajectory groups for the restricted cohort (n= 2093). This sample included individuals who established care at the Barbara Davis Center in Colorado between 1978 and 2016, within 1 year of diagnosis, were followed through age 17 or 18, and who had at least 8 HbA1c measures. As in the broader cohort, data from within the first 12 months of diagnosis was excluded because of expected fluctuations in weight and glycemic control. Trajectory names: "Low", "Middle", and "High" refer to where each trajectory begins on the y-axis (i.e. the relative HbA1c value at age 2). "Stable" refers to trajectories with relative constancy of HbA1c values across the age range and "Increasing" denotes trajectories with a significant increase in HbA1c at some point across the age range.
Funding
J.M. funding: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [grant 6T32DK007658 (Krebs)]
J.S. funding: This study was supported by the National Heart, Lung, and Blood Institute [grant R01 HL113029], and the American Diabetes Association [grant 7–13-CD-10 (Snell-Bergeon)]
The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Abbreviations:
- BDC
Barbara Davis Center
- BIC
Bayes information criterion
- CDC
Center for Disease Control and Prevention
- CVD
cardiovascular disease
- DCCT
Diabetes Control and Complications Trial
- GBTM
group-based trajectory modeling
- HbA1c
hemoglobin A1c
- MCAR
missing completely at random
- T1D
type 1 diabetes
- US
United States
Footnotes
Data accessibility
The datasets created or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
J.M. and J.S. have no conflicts of interest to disclose
Contributor Information
Jaime M. Moore, University of Colorado School of Medicine; Department of Pediatrics, Section of Nutrition, Denver CO, US, 80045
Janet K. Snell-Bergeon, University of Colorado School of Medicine; Departments of Pediatrics and Epidemiology, Denver CO, US, 80045
References
- 1.Alman AC, Talton JW, Wadwa RP, et al. Cardiovascular health in adolescents with type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes. 2014;15(7):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook A. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couper JJ, Haller MJ, Ziegler AG, Knip M, Ludvigsson J, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2014. Phases of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2014;15 Suppl 20:18–25. [DOI] [PubMed] [Google Scholar]
- 4.Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125(2):177–188. [DOI] [PubMed] [Google Scholar]
- 6.Anderzen J, Samuelsson U, Gudbjornsdottir S, Hanberger L, Akesson K. Teenagers with poor metabolic control already have a higher risk of microvascular complications as young adults. J Diabetes Complications. 2016;30(3):533–536. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 9.Brown TL, Maahs DM, Bishop FK, Snell-Bergeon JK, Wadwa RP. Influences of gender on cardiovascular disease risk factors in adolescents with and without type 1 diabetes. Int J Pediatr Endocrinol. 2016;2016:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuelsson U, Anderzen J, Gudbjornsdottir S, Steineck I, Akesson K, Hanberger L. Teenage girls with type 1 diabetes have poorer metabolic control than boys and face more complications in early adulthood. J Diabetes Complications. 2016;30(5):917–922. [DOI] [PubMed] [Google Scholar]
- 11.Huxley RR, Peters SAE, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. The Lancet Diabetes & Endocrinology. 2015;3(3):198–206. [DOI] [PubMed] [Google Scholar]
- 12.Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010;22(4):405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 14.Helgeson VS, Snyder PR, Seltman H, Escobar O, Becker D, Siminerio L. Brief report: trajectories of glycemic control over early to middle adolescence. J Pediatr Psychol. 2010;35(10):1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King PS, Berg CA, Butner J, et al. Longitudinal trajectories of metabolic control across adolescence: associations with parental involvement, adolescents’ psychosocial maturity, and health care utilization. J Adolesc Health. 2012;50(5):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luyckx K, Seiffge-Krenke I. Continuity and change in glycemic control trajectories from adolescence to emerging adulthood: relationships with family climate and self-concept in type 1 diabetes. Diabetes Care. 2009;32(5):797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwandt A, Hermann JM, Rosenbauer J, et al. Longitudinal Trajectories of Metabolic Control From Childhood to Young Adulthood in Type 1 Diabetes From a Large German/Austrian Registry: A Group-Based Modeling Approach. Diabetes Care. 2017;40(3):309–316. [DOI] [PubMed] [Google Scholar]
- 18.Helgeson VS, Vaughn AK, Seltman H, Orchard T, Libman I, Becker D. Featured Article: Trajectories of Glycemic Control Over Adolescence and Emerging Adulthood: An 11-Year Longitudinal Study of Youth With Type 1 Diabetes. J Pediatr Psychol. 2018;43(1):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohan JM, Rausch JR, Pendley JS, et al. Identification and prediction of group-based glycemic control trajectories during the transition to adolescence. Health Psychol. 2014;33(10):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonolleda M, Murillo M, Vazquez F, Bel J, Vives-Pi M. Remission Phase in Paediatric Type 1 Diabetes: New Understanding and Emerging Biomarkers. Horm Res Paediatr. 2017;88(5):307–315. [DOI] [PubMed] [Google Scholar]
- 21.Gregg B, Connor CG, Ruedy KJ, et al. Body mass index changes in youth in the first year after type 1 diabetes diagnosis. J Pediatr. 2015;166(5):1265–1269.e1261. [DOI] [PubMed] [Google Scholar]
- 22.Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;332(19):1251–1255. [DOI] [PubMed] [Google Scholar]
- 23.A SAS Program for the 2000 Growth Charts (ages 0 to <20 years) [computer program]. Centers for Disease Control and Prevention. [Google Scholar]
- 24.Nagin D, Tremblay RE. Trajectories of boys’ physical aggression, opposition, and hyperactivity on the path to physically violent and nonviolent juvenile delinquency. Child Dev. 1999;70(5):1181–1196. [DOI] [PubMed] [Google Scholar]
- 25.Nagin DS. Group-Based Modeling of Development. Harvard University Press; 2005. [Google Scholar]
- 26.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 27.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 28.Curran PJ, Obeidat K, Losardo D. Twelve Frequently Asked Questions About Growth Curve Modeling. J Cogn Dev. 2010;11(2):121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell MS, Schatz DA, Chen V, et al. A contrast between children and adolescents with excellent and poor control: the T1D Exchange clinic registry experience. Pediatr Diabetes. 2014;15(2):110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinz N, Schwandt A, Becker M, et al. Trajectories of Body Mass Index from Childhood to Young Adulthood among Patients with Type 1 Diabetes-A Longitudinal Group-Based Modeling Approach Based on the DPV Registry. J Pediatr. 2018;201:78–85.e74. [DOI] [PubMed] [Google Scholar]
- 31.Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet. 2000;355(9207):873–876. [PubMed] [Google Scholar]
- 32.Freedman DS, Butte NF, Taveras EM, et al. BMI z-Scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity (Silver Spring). 2017;25(4):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coulon SJ, Velasco-Gonzalez C, Scribner R, et al. Racial differences in neighborhood disadvantage, inflammation and metabolic control in black and white pediatric type 1 diabetes patients. Pediatr Diabetes. 2017;18(2):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pihoker C, Badaru A, Anderson A, et al. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study. Diabetes Care. 2013;36(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paris CA, Imperatore G, Klingensmith G, et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155(2):183–189.e181. [DOI] [PubMed] [Google Scholar]
- 36.Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahkoska AR, Shay CM, Crandell J, et al. Association of Race and Ethnicity With Glycemic Control and Hemoglobin A1c Levels in Youth With Type 1 Diabetes. JAMA network open. 2018;1(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DuBose SN, Hermann JM, Tamborlane WV, et al. Obesity in Youth with Type 1 Diabetes in Germany, Austria, and the United States. J Pediatr. 2015;167(3):627–632 e621–624. [DOI] [PubMed] [Google Scholar]
- 39.Skinner AC RS, Skelton JA, Perrin EM, Armstrong SC. . Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018;141(3):e20173459 Pediatrics. 2018;142(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baskaran C, Volkening LK, Diaz M, Laffel LM. A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the Diabetes Control and Complications Trial. Pediatr Diabetes. 2015;16(4):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care. 2001;24(10):1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wisting L, Froisland DH, Skrivarhaug T, Dahl-Jorgensen K, Ro O. Disturbed eating behavior and omission of insulin in adolescents receiving intensified insulin treatment: a nationwide population-based study. Diabetes Care. 2013;36(11):3382–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro-Correia C, Santos-Silva R, Pinheiro M, Costa C, Fontoura M. Metabolic risk factors in adolescent girls with type 1 diabetes. J Pediatr Endocrinol Metab. 2018;31(6):631–635. [DOI] [PubMed] [Google Scholar]
- 44.Haviland A, Nagin DS, Rosenbaum PR. Combining propensity score matching and group-based trajectory analysis in an observational study. Psychol Methods. 2007;12(3):247–267. [DOI] [PubMed] [Google Scholar]
- 45.Haviland A, Nagin DS, Rosenbaum PR, Tremblay RE. Combining group-based trajectory modeling and propensity score matching for causal inferences in nonexperimental longitudinal data. Dev Psychol. 2008;44(2):422–436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HbA1c trajectory groups for the restricted cohort (n= 2093). This sample included individuals who established care at the Barbara Davis Center in Colorado between 1978 and 2016, within 1 year of diagnosis, were followed through age 17 or 18, and who had at least 8 HbA1c measures. As in the broader cohort, data from within the first 12 months of diagnosis was excluded because of expected fluctuations in weight and glycemic control. Trajectory names: "Low", "Middle", and "High" refer to where each trajectory begins on the y-axis (i.e. the relative HbA1c value at age 2). "Stable" refers to trajectories with relative constancy of HbA1c values across the age range and "Increasing" denotes trajectories with a significant increase in HbA1c at some point across the age range.