Abstract
Background/Aims:
Improving care and treatment for persons infected with hepatitis C virus (HCV) can reduce HCV-related morbidity and mortality. Our primary objective was to examine the HCV care continuum among patients receiving care at five Federally Qualified Health Centers (FQHCs) in Philadelphia, PA where a testing and linkage to care program had been established.
Methods:
Among the five FQHCs, one served a homeless population, two served public housing residents, one served a majority Hispanic population, and the last, a “test and treat” site, also provided HCV treatment to patients. We analyzed data from electronic health records of patients tested for HCV antibody from 2012–2016 and calculated the percentage of patients across nine steps of the HCV care continuum ranging from diagnosis to cure. We further explored factors associated with successful patient navigation through two steps of the continuum using multivariable logistic regression.
Results:
Of 885 chronically infected patients, 92.2% received their RNA positive result, 82.7% were referred to an HCV provider, 69.4% were medically evaluated by the provider, 55.3% underwent liver disease staging, 15.0% initiated treatment, 12.0% completed treatment, 8.7% were assessed for sustained virologic response (SVR), and 8.0% achieved SVR. Regression results revealed that test and treat site patients were significantly more likely to be medically evaluated (aOR=2.76; 95% CI=1.82, 4.17) and undergo liver disease staging (aOR=1.92, 95% CI=1.02, 2.86) than patients at the other FQHCs combined.
Conclusions:
In this U.S. urban setting, over two-thirds of HCV-infected patients were linked to care. Although treatment uptake was low overall, it was highest at the test and treat site. Scaling up treatment services in HCV testing settings will be vital to improve the HCV care continuum.
Keywords: HCV testing, federally qualified health centers, HCV treatment
INTRODUCTION
Hepatitis C virus (HCV) infection, the most common chronic blood-borne infection in the United States, poses a growing public health concern. Based on national data for 2003–2010, approximately 3.2 million persons in the general U.S. non-institutionalized population were estimated to be infected with HCV [1]. Including populations typically excluded from national surveys such as those who are homeless and incarcerated, the number of persons with HCV infection was estimated to be as high as 3.5 million in 2013 [2]. Furthermore, from 2010 to 2016, reported cases of acute HCV infection in the United States have more than tripled [3].
HCV infection disproportionately affects persons born 1945–1965 (i.e., baby boomers), underserved populations, and persons who inject drugs (PWID) [2,4,5]. Cause for concern is two-fold. First, because infection with HCV is primarily asymptomatic, undiagnosed baby boomers are experiencing the downstream effects of the disease decades later. Recent analyses have revealed an increase in two critical HCV-related outcomes, cirrhosis and hepatocellular carcinoma [6,7]. Second, injection drug use (IDU), the predominant risk for HCV transmission, has increased in concert with the nation’s opioid epidemic [8]. Moreover, among persons successfully treated and cured, active drug injection poses a major risk of re-infection [9].
Since the development of direct acting antiviral drugs (DAAs) that offer curative treatment to persons diagnosed with HCV infection, several studies have identified important gaps in the HCV care continuum, that is the steps along the pathway from identifying persons with active infection through to cure. National estimates suggest that in 2013, among the estimated 3.2–3.5 million persons in the United States with chronic HCV infection, roughly 50% were aware of their diagnosis, up to 38% were referred to an HCV provider, between 20% and 27% received confirmatory HCV RNA testing, 17% underwent a liver biopsy, but only 7%−16% were treated, and less than 10% achieved sustained virologic response (SVR) (i.e., cure) [10,11].
While more recent data among select populations suggest improvements in the HCV care continuum, results vary. For example, among chronically infected formerly incarcerated individuals in one study, 57% were referred to an HCV provider, but only 36% were medically evaluated and only 10% initiated treatment [12]. A study among homeless veterans found that those who were homeless were more likely to be identified and medically evaluated by an HCV provider, but were less likely to initiate HCV treatment, compared to veterans who were not homeless [13]. Better outcomes were found among opioid users undergoing substance abuse treatment, in which 71% of persons with chronic HCV infection initiated treatment [14]. In addition, a study in a Baltimore sexually transmitted infections clinic found high linkage to care rates among persons who were referred to offsite specialists, with the assistance of patient navigation [15].
With the development of more effective curative treatments for HCV infection, progress toward the goal of eliminating hepatitis C as a public health threat by 2030 could be achieved by improving the care continuum for persons with chronic HCV infection [16]. To add to our understanding of care and treatment among underserved populations, we examined the HCV care continuum for patients engaged in care in one major urban center in the United States. We also sought to identify factors associated with successful navigation of these patients through two key steps (medical evaluation by a provider and liver disease staging) of the HCV care continuum. These two steps were selected because we had sufficient numbers of patients at each to conduct our analyses and previous data have shown that each step is a critical drop off point for patients in the care continuum [10,11]. At the same time, each step is an important point for staff intervention (e.g., patient navigation) to prevent drop off.
MATERIALS AND METHODS
In October 2012, the National Nurse-Led Care Consortium (NNCC), a membership organization that advances nurse-led care, collaborated with its parent company Public Health Management Corporation (PHMC), which runs a health network of five Federally Qualified Health Centers (FQHCs) in Philadelphia, PA, to implement universal one-time HCV testing and linkage to care in the FQHCs. The testing and linkage to care project employed a previously reported model that included strategies such as medical assistant-initiated testing of patients aged 18 years or older and automated prompts for the provider in the electronic health record [17]. The project also utilized HCV reflex testing technology, in which the laboratory automatically conducted HCV RNA testing on all specimens that were seropositive for HCV antibody. Universal HIV testing, performed annually, was added in September 2013 [18]. Each clinic provided medical care to a distinct patient population: Mary Howard Health Center treated an exclusively homeless population; Congreso Health Center served a Hispanic population; Rising Sun Health Center and Health Connection provided primary care to residents of nearby public housing facilities; and the PHMC Care Clinic provided primary care to all patients and specialty care to patients who were infected with HCV as well as those who were co-infected with HIV. The PHMC Care Clinic served as a “test and treat” site, which offered universal screening and employed primary care providers who were trained to treat both HCV and HIV infections. The other four FQHCs were distinct from the PHMC Care Clinic in that treatment was provided offsite.
Data Collection
From October 2012 through June 2016, demographic and clinical data, including HCV antibody and RNA testing data, were collected from the electronic health records of patients from the five clinics, supplemented with more detailed chart review by a trained data abstractor. For patients with chronic HCV infection as determined by a positive HCV RNA test result during October 2012-June 2016, additional data on linkage to care and treatment outcomes were obtained, and data collection was extended through June 2017.
The HCV care continuum included the following nine steps: HCV RNA positive test result, receipt of HCV RNA positive test result, referral to a provider trained to treat HCV, medical evaluation by a provider trained to treat HCV, liver disease staging, initiation of HCV treatment, completion of HCV treatment, assessment of SVR, and achieving SVR (i.e., cure). Progress through the HCV care continuum was tracked three ways: retrospective chart review, reports from the Linkage to Care Coordinator and HCV Treatment Coordinator, or specialist consultation notes scanned into the patient chart. Documentation (ICD-9 or ICD-10 code for HCV) in the patient’s chart, either at the time of their HCV RNA test or at any visit after the HCV RNA test, was used to determine if a patient received their HCV RNA positive test result. In addition, progress reports in the patient chart were used to confirm that the patient received their HCV RNA test result.
Referrals to an HCV provider were made for all patients who received a positive HCV RNA test result. In our study, HCV providers were defined as primary care providers trained to treat HCV infection at the PHMC Care Clinic or specialists with a gastroenterology or hepatology practice affiliated with a Philadelphia hospital at the other four FQHCs. Thus, patients at the PHMC Care Clinic were referred to an onsite provider, while patients at the other four clinics were referred offsite to hospital-based practices that were typically close to the clinic, although in some cases, patients preferred to see a specialist closer to their home. All referrals were confirmed by progress notes in the electronic health record and location of the specialist practice was clearly labeled in the medical chart for patients referred offsite.
A review of the medical chart was used to determine if the patient was medically evaluated at the PHMC Care Clinic and also confirmed by progress notes in the electronic health record. Reports from the Linkage to Care Coordinator and specialist consultation notes were used to determine if a patient was evaluated by an offsite HCV provider. Specialist consultation notes were scanned into patient charts and used to identify the date of medical evaluation. Liver disease staging was assessed by liver fibrosis panel, liver biopsy, liver ultrasound, or Fibroscan, and indicated in the medical chart or consultation note. Although a liver ultrasound is not typically used for staging, it was performed on a small number of patients early in the study.
Treatment initiation, treatment completion, treatment outcome, and SVR data were obtained through retrospective chart review (extracted monthly) or from the HCV Treatment Coordinator (via weekly reports). Quantitative HCV RNA test results were automatically uploaded from the lab to the medical chart for patients treated onsite at the PHMC Care Clinic or reported in the consultation note that was scanned into the medical chart for patients treated offsite. In 2016, a list comprised of the names of patients who were referred offsite, as documented in the electronic health record, was sent to each specialist practice. The specialist office was asked to provide dates of the patient’s first appointment, whether they attended, liver disease staging test result, and dates of treatment initiation, treatment completion, and SVR. SVR was defined as undetectable HCV RNA at least 3 months after treatment completion as indicated in the patient’s medical chart or the scanned consultation note. The IRB of the Public Health Management Company (PHMC) approved the study October 28, 2016; amended approval was granted February 7, 2017.
Statistical Analysis
Of all patients from the five FQHCs who tested positive for HCV antibody and were tested for HCV RNA, we calculated the percentage of those who were chronically infected at each of nine steps across the HCV care continuum. One important objective for this analysis was to see how our results would compare with previously reported estimates and whether we would find improvement in patient progression along the continuum. We further compared patients served at the test and treat site (PHMC Care Clinic) to those served at the other four clinics (test and linkage to care sites) combined across the HCV care continuum. We then performed multivariable logistic regression to identify factors associated with two steps along the HCV care continuum: (1) medical evaluation by an HCV provider among patients with a positive HCV RNA test result; and (2) liver disease staging among those medically evaluated by an HCV provider. A backward logistic regression approach was used to identify covariates for models, using the p-value cut point of <0.10. Additional covariates were included based on prior evidence of an association with our outcomes and included sex, birth cohort, race/ethnicity, whether the patient was seen at the PHMC Care Clinic or was a patient at one of the other FQHCs, whether the patient had a known history of injection drug use, incarceration, or homelessness, and year of diagnosis with chronic infection (i.e., positive HCV RNA test result). We used the variance inflation factor (VIF) cutoff value of 10 to indicate collinearity among covariates of interest. In our analyses, all VIF values were less than 2.0, suggesting no collinearity. Cases with missing values were excluded from the analysis. In addition, we calculated the percentage of patients treated within one year of their HCV RNA positive test and compared these patients to those treated more than one year after their HCV RNA positive test by year of follow-up (2012–2016). Finally, we calculated the cumulative number of patients treated by treatment outcome (assessed for SVR and achieved SVR) and year of follow-up (2012–2017). All statistical analyses were performed in Stata 14.2 (College Station, Texas; 2015).
RESULTS
Patient Characteristics and HCV Testing
Among 25,853 patients visiting the five FQHCs between October 1, 2012 and June 30, 2016, a total of 14,790 (57.2%) unique patients were tested for HCV antibody, of which 8.9% (n=1,323) patients tested positive (Table 1). Of these, 96.1% (n=1,272) were tested for HCV RNA and 66.9% (n=885) were positive indicating chronic infection, for an overall prevalence of 6.0% of those tested for HCV antibody. By clinic, HCV antibody prevalence was highest among patients from the Mary Howard Health Center (15.0%) and the PHMC Care Clinic (17.8%). This same pattern was found among patients with chronic HCV infection and was 11.1% at the Mary Howard Health Center and 12.5% at the PHMC Care Clinic. Although all clinics had higher numbers of non-Hispanic black compared to non-Hispanic white and Hispanic patients, the highest prevalence of HCV antibody and chronic infection was observed among non-Hispanic white patients (26.9% and 18.5%, respectively). Compared with females, males had a higher prevalence of HCV antibody and chronic infection, though little difference was found between patients born from 1945–1965 and those born in other years.
Table 1.
Number and prevalence of patients tested for hepatitis C virus (HCV) antibody and identified as HCV antibody positive and HCV RNA positive (chronically infected), by health center and demographic characteristics at five Federally Qualified Health Centers, Philadelphia PA, 2012–2016
| Characteristic | HCV Antibody Tested | HCV Antibody Positive | HCV RNA Positive* | |||
|---|---|---|---|---|---|---|
| N | n | Prevalence† | n | Prevalence¶ | Overall prevalence among patients tested for HCV antibody | |
| Total | 14790 | 1323 | 8.9 | 885 | 66.9 | 6.0 |
| Health center | ||||||
| Mary Howard Health Center | 2491 | 374 | 15.0 | 276 | 73.8 | 11.1 |
| Public Health Management Corporation (PHMC) Care Clinic | 3573 | 637 | 17.8 | 448 | 70.3 | 12.5 |
| Health Connection | 2662 | 118 | 4.4 | 68 | 57.6 | 2.6 |
| Congreso Health Center | 2816 | 119 | 4.2 | 54 | 45.4 | 1.9 |
| Rising Sun Health Center | 3248 | 75 | 2.3 | 39 | 52.0 | 1.2 |
| PHMC Care Clinic patient | ||||||
| No | 11217 | 686 | 6.1 | 437 | 63.7 | 3.9 |
| Yes | 3573 | 637 | 17.8 | 448 | 70.3 | 12.5 |
| Sex | ||||||
| Male | 7308 | 898 | 12.3 | 652 | 72.6 | 8.9 |
| Female | 7482 | 425 | 5.7 | 233 | 54.8 | 3.1 |
| Race/ethnicity | ||||||
| Non-Hispanic Black | 7800 | 634 | 8.1 | 441 | 69.6 | 5.7 |
| Non-Hispanic White | 1403 | 377 | 26.9 | 259 | 68.7 | 18.5 |
| Hispanic | 4094 | 223 | 5.4 | 125 | 56.1 | 3.1 |
| Other/Missing | 1493 | 89 | 6.0 | 60 | 67.4 | 4.0 |
| Birth cohort 1945–1965 | ||||||
| No | 7070 | 591 | 8.4 | 382 | 64.6 | 5.4 |
| Yes | 7525 | 732 | 9.5 | 503 | 68.7 | 6.7 |
| Missing | 195 | 0 | 0.0 | 0 | 0.0 | 0.0 |
Patients with chronic HCV infection
Of patients tested for HCV antibody
Of patients with a positive HCV antibody test result
Note: With implementation of reflex testing, the proportion of HCV antibody positive patients who had confirmatory HCV RNA testing was over 94% at each site: n=363 (97.1%) at Mary Howard Health Center, n=609 (95.6%) at Public Health Management Corporation Care Clinic, n=111 (94.1%) at Health Connection, n=116 (97.5%) at Congreso Health Center, and n=73 (97.3%) at Rising Sun Health Center.
The proportion of patients who tested HCV antibody positive and received confirmatory HCV RNA testing was over 94% at each clinic (see footnote, Table 1). However, the prevalence of HCV RNA among patients testing antibody positive varied across the health centers. For example, 70.3% and 73.8% of HCV antibody positive patients tested HCV RNA positive at the PMHC Care Clinic and Mary Howard Health Center, respectively, compared with the Congreso Health Center where only 45.4% of those who had a positive HCV antibody test result also had a positive HCV RNA test result (Table 1).
HCV Care Continuum
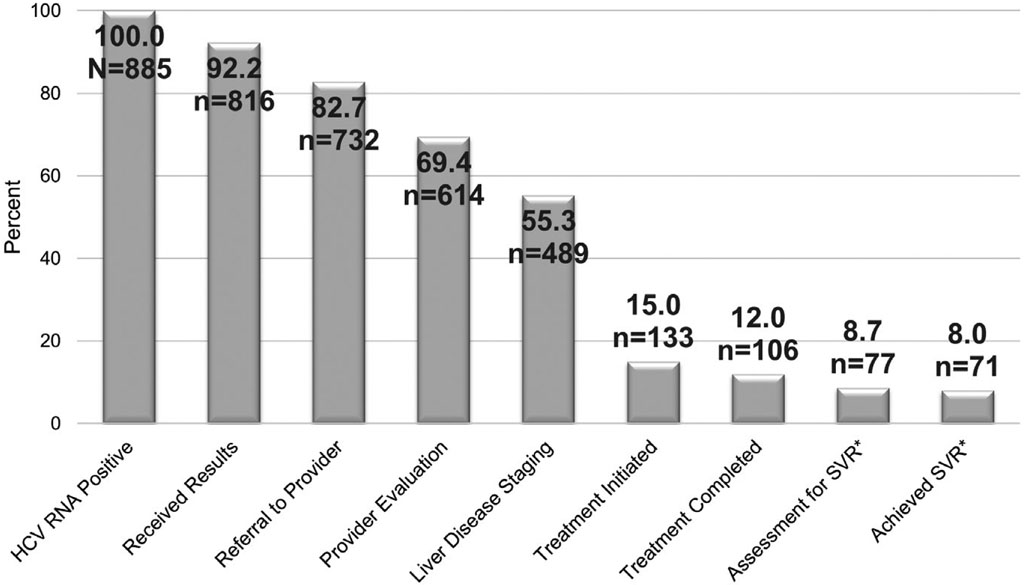
The HCV care continuum is summarized in the Figure. Of the 885 patients chronically infected with HCV, 92.2% received their RNA positive result, 82.7% were referred to an HCV provider, and an HCV provider medically evaluated 69.4%. In addition, of the total number chronically infected patients, 55.3% underwent liver disease staging, 15.0% initiated treatment, 12.0% completed treatment, 8.7% were assessed for SVR, and 8.0% achieved SVR (i.e., cure).
Figure.
Hepatitis C virus (HCV) care continuum for patients with chronic HCV infection at five Federally Qualified Health Centers, Philadelphia PA, 2012–2016.
*Sustained virologic response
Progression along the HCV care continuum was most successful for patients treated by primary care providers at the PHMC Care Clinic (Table 2). Of the 448 chronically infected PHMC Care Clinic patients, 80.8% were medically evaluated by an HCV provider, compared with 57.7% of patients at the other four clinics combined. In addition, of chronically infected patients who underwent liver disease staging, 67.4% of the PHMC Care Clinic patients initiated treatment compared with 42.8% of patients at the other four clinics. Further, of patients who were chronically infected, 13.4% of patients seen at the PHMC Care Clinic achieved SVR, while only 2.5% of patients achieved SVR at the other four clinics combined. Notably, across all five clinics, we identified 33 chronically infected patients who were prescribed treatment, but there was no documentation that these patients were treated.
Table 2.
Hepatitis C virus (HCV) care continuum for patients with chronic HCV infection by clinic at five Federally Qualified Health Centers, Philadelphia PA, 2012–2016
| Clinic | HCV RNA positive | Received results | Referral to provider | HCV provider evaluation | Liver disease staging | Treatment initiated | Treatment completed | Assessed for sustained virologic response | Achieved sustained virologic response |
|---|---|---|---|---|---|---|---|---|---|
| n | n (% of HCV RNA Positive) | n (% of HCV RNA Positive) | n (% of HCV RNA Positive) | n (% of HCV RNA Positive) | n (% of HCV RNA Positive) | n (% of HCV RNA Positive) | n (% of HCV RNA Positive) | n (% of HCV RNA Positive) | |
| Patients served at Public Health Management Corporation (PHMC) Care Clinic | 448 | 418 (93.3) | 401 (89.5) | 362 (80.8) | 302 (67.4) | 96 (21.4) | 79 (17.6) | 64 (14.3) | 60 (13.4) |
| Patients served at one of the other four clinics* | 437 | 398 (91.1) | 331 (75.7) | 252 (57.7) | 187 (42.8) | 37 (8.5) | 27 (6.2) | 13 (3.0) | 11 (2.5) |
Other clinics in the network include: Mary Howard Health Center, Health Connection, Congreso Health Center, and Rising Sun Health Center.
Multivariable Results
In addition to progression of patients along the care continuum, we wanted to assess whether certain factors facilitated successful navigation through two of the steps. Results from multivariable regression analyses demonstrated that patients from the PHMC Care Clinic (adjusted odds ratio [aOR]=2.76; 95% CI=1.82, 4.17) vs. patients at the other four clinics and persons born 1945–1965 (aOR=1.76; 95% CI=1.17, 2.62) vs. those born in other years were significantly more likely to be medically evaluated (Table 3). However, patients who were tested during July 2014-June 2015 (aOR=0.45; 95% CI=0.22, 0.89) and July 2015-June 2016 (aOR=0.46; 95% CI=0.23, 0.92) were significantly less likely to be medically evaluated than those who were tested during October 2012-June 2013. Among those medically evaluated by an HCV provider, PHMC Care Clinic patients (aOR=1.92; 95% CI=1.02, 2.86) were significantly more likely than patients at the other four clinics to undergo liver disease staging. In addition, patients who were tested during July 2013-June 2014 (aOR=0.30; 95% CI=0.12–0.71) were significantly less likely to undergo liver disease staging than those who were tested during October 2012-June 2013.
Table 3.
Logistic regression results for the effects of selected patient characteristics on medical evaluation by a hepatitis C virus (HCV) provider and on liver disease staging of those medically evaluated among patients with chronic HCV infection at five Federally Qualified Health Centers, Philadelphia PA, 2012–2016
| Characteristic | n (% of 885 RNA positive) | Medically evaluated by HCV provider | Liver disease staging among medically evaluated patients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||
| Public Health Management Corporation Care Clinic patient | |||||||||
| No | 437 (49.4) | ref | ref | ref | ref | ||||
| Yes | 448 (50.6) | 3.09 | 2.28, 4.18 | 2.76 | 1.82, 4.17 | 1.75 | 1.18, 2.61 | 1.92 | 1.02, 2.86 |
| Sex | |||||||||
| Male | 652 (73.7) | ref | ref | ref | ref | ||||
| Female | 233 (26.3) | 1.01 | 0.73, 1.40 | 1.03 | 0.68, 1.56 | 1.42 | 0.88, 2.29 | 1.99 | 0.99,3.15 |
| Race/Ethnicity | |||||||||
| Non-Hispanic Black | 441 (49.8) | ref | ref | ref | ref | ||||
| Non-Hispanic White | 259 (29.3) | 0.91 | 0.65, 1.27 | 0.96 | 0.62, 1.49 | 0.71 | 0.45, 1.13 | 0.64 | 0.35, 1.15 |
| Hispanic | 125 (14.1) | 0.71 | 0.47, 1.08 | 0.85 | 0.50, 1.42 | 0.75 | 0.41, 1.39 | 1.21 | 0.58, 2.55 |
| Birth cohort 1945–1965 | |||||||||
| No | 382 (43.2) | ref | ref | ref | ref | ||||
| Yes | 503 (56.8) | 1.64 | 1.23, 2.19 | 1.76 | 1.17, 2.62 | 1.32 | 0.88, 1.96 | 1.25 | 0.85, 2.26 |
| History of injection drug use | |||||||||
| No | 684 (77.3) | ref | ref | ref | ref | ||||
| Yes | 201 (22.7) | 1.14 | 0.81, 1.59 | 1.17 | 0.79, 1.73 | 0.76 | 0.48, 1.21 | 0.67 | 0.38, 1.11 |
| History of homelessness | |||||||||
| No | 496 (56.0) | ref | ref | ref | ref | ||||
| Yes | 389 (44.0) | 0.55 | 0.41, 0.73 | 0.76 | 0.50, 1.14 | 0.79 | 0.53, 1.18 | 1.08 | 0.58, 1.67 |
| History of incarceration | |||||||||
| No | 684 (77.3) | ref | ref | ref | ref | ||||
| Yes | 201 (22.7) | 1.15 | 0.81, 1.63 | 1.17 | 0.77, 1.81 | 1.43 | 0.87, 2.35 | 1.63 | 0.90, 2.86 |
| Year of HCV RNA test | |||||||||
| 10/2012 – 6/2013 | 112 (12.7) | ref | ref | ||||||
| 7/2013–6/2014 | 273 (30.8) | 0.51 | 0.29, 0.90 | 0.51 | 0.26, 1.01 | 0.30 | 0.14, 0.64 | 0.30 | 0.12, 0.71 |
| 7/2014–6/2015 | 235 (26.6) | 0.39 | 0.22, 0.69 | 0.45 | 0.22, 0.89 | 0.45 | 0.20, 1.00 | 0.50 | 0.20, 1.25 |
| 7/2015–6/2016 | 265 (29.9) | 0.32 | 0.18, 0.56 | 0.46 | 0.23, 0.92 | 0.45 | 0.20, 0.98 | 0.51 | 0.20, 1.28 |
OR=Odds ratio
CI=Confidence interval
aOR=Adjusted odds ratio; each model includes all 8 characteristics listed in the table as independent variables.
Treatment Initiation and Outcomes by Year of Follow-up
Table 4 shows the cumulative number of chronically infected patients who initiated treatment and their outcomes each year from 2012–2016. Over the four-year study period, we found an increase in the percentage of patients (from 5.4% to 12.8%) who initiated treatment within 1 year of their first positive HCV RNA test. While the absolute number of chronically infected patients served at the five clinics who initiated and completed treatment approximately doubled each year, the number of chronically infected patients in need of treatment increased six-fold from June 2013 (n=112) to June 2017 (n=795). Among patients who initiated treatment, completion rates were more than 84% across all time periods and achievement of SVR among those who were assessed for SVR increased over time from 75% in the first time-year to 100% in the most recent time-year.
Table 4.
Treatment initiation and outcomes by year of follow-up among patients with chronic hepatitis C virus (HCV) infection at five Federally Qualified Health Centers, Philadelphia PA, 2012–2016
| Year of follow-up | Diagnosed HCV RNA positive during year of follow-up* | Treated within one calendar year of RNA positive test | Treated >one year after RNA positive test | Untreated as of June 2017 | Cumulative HCV RNA positive patients by year of follow-up¶ | Initiated treatment during year of follow-up | Treatment completed | Assessed for sustained virologic response** | Achieved sustained virologic response |
|---|---|---|---|---|---|---|---|---|---|
| N | n (%) | n (%) | n (%) | N | n (%) | n (%) | n (%) | n (%) | |
| 10/2012 – 6/2013 | 112 | 6 (5.4) | 13 (11.6) | 92 (82.1) | 112 | 4 (4.4) | 4(100.0) | 4(100.0) | 3 (75.0) |
| 7/2013–6/2014 | 273 | 11 (4.0) | 26 (9.5) | 236 (86.4) | 382 | 9 (2.4) | 9 (100.0) | 7 (77.8) | 6 (85.7) |
| 7/2014–6/2015 | 235 | 21 (8.9) | 20 (8.5) | 194 (82.6) | 608 | 17 (2.8) | 15 (88.2) | 14 (93.3) | 12 (85.7) |
| 7/2015–6/2016 | 265 | 34 (12.8) | 2 (0.8)† | 230 (86.7)† | 857 | 64 (7.5) | 54 (84.4) | 42 (77.8) | 39 (92.9) |
| 7/2016–6/2017 | Not included | -- | -- | -- | 795 | 41 (5.2) | 26 (90.0)‖ | 12 (46.2) | 12 (100.0) |
| Total | 885 | 72 (8.1) | 61 (6.9) | 752 (85.0) | 885 | 135 (15.3)§ | 108 (87.8)# | 79 (73.1) | 72 (91.1) |
First positive test at the health center during the study period; patients may have had prior testing at the health center or with an outside provider.
Data collection ended June 2017, thus ascertainment of treatment initiation among patients tested in this period is incomplete and represents a minimum estimate.
Cumulative number of known HCV RNA positive patients without prior sustained virologic response during year of follow-up based on first positive HCV RNA test at the health center during the study period; patients may have had prior testing at the health center or with an outside provider.
Total number of patients who initiated treatment during the designated follow-up year (n=135) includes two patients who failed treatment but were retreated during the study period.
Denominator (n=29) adjusted to exclude 12 (29.3%) patients treated in that year who remained on therapy as of July 2017 and could not be evaluated for completion.
Denominator (n=123) adjusted to exclude 12 (8.9%) patients treated in that year who remained on therapy as of July 2017 and could not be evaluated for completion.
Excluding patients lost to follow-up and/or pending laboratory evaluation of treatment outcome. During the final period 12 (46%) patients who completed treatment in that year had not yet reached 12 weeks post-completion for SVR evaluation by June 2017.
DISCUSSION
The purpose of our study was to evaluate the HCV care continuum among patients engaged in care at five FQHCs and determine factors associated with successful navigation through the HCV care continuum after implementation of an HCV testing and linkage to care program. We found that more than two-thirds of patients diagnosed with chronic HCV infection were successfully linked to care, signaling major improvements for this step in the continuum compared with previous reports [10,11]. In fact, our current results show improvement over those found in prior year analyses of the same patient population [17,18]. Although we found that almost 70% of patients with chronic HCV infection were seen by an HCV provider and over one-half of these patients underwent liver disease staging, treatment uptake remained low. Among the 885 chronically infected patients, only 12% of patients completed treatment and only 8% of patients achieved SVR by the end of June 2017. One possible reason for the low treatment uptake is that medical providers did not submit prior authorization for patients who did not meet treatment eligibility criteria from the insurance companies, which typically required a diagnosis of advanced fibrosis. Another reason could be that some patients were prescribed treatment, but then denied treatment by the insurance company. As previously mentioned, we identified 33 chronically infected patients who were prescribed treatment, but we had no documentation that these patients were treated. It is certainly possible that some or all of these patients were denied treatment by their insurer. When patients did complete treatment, 94% of those assessed were cured, highlighting the importance of linking HCV-infected patients to appropriate treatment.
So how might we explain our results? We demonstrated significant differences in successful patient navigation through steps of the HCV care continuum by clinic. Consistently, the best linkage to care and treatment outcomes were found at the health network’s test and treat site, the PHMC Care Clinic. Results from our multivariable analysis confirmed our bivariate results; PHMC Care Clinic patients were significantly more likely to be evaluated by an HCV provider and to undergo liver disease staging than patients at the other four clinics combined. Our multivariable results further revealed that patients who were tested in later years were significantly less likely to be evaluated by an HCV provider and to undergo liver disease staging than those tested in the first year (October 2012-June 2013). It is unclear how to interpret these findings as we would expect patients tested in later years to be more likely to be evaluated and assessed for treatment than those tested in earlier years. One possible explanation is that more than four years of data may be required to determine whether this is an anomaly or whether such a trend would hold. Another possible reason for these findings could be missing data primarily from the FQHCs that had to refer patients offsite for treatment.
Despite this finding, our results overall suggest that building the capacity of primary care providers to treat HCV infection in a setting where routine HCV testing has been implemented can substantially improve the HCV care continuum. Primary advantages include treatment by a trusted medical provider and bypassing steps of the HCV care continuum that could require multiple clinic visits. For example, patients at the PHMC Care Clinic were able to be tested for both HCV antibody and HCV RNA, and if found to be chronically infected had the opportunity to discuss the results with a provider and be assessed for treatment at the same appointment. Providing on-site HCV care and treatment is particularly important for patients from underserved populations who may be reluctant to seek care or may encounter structural barriers that impede their ability to see a specialist, such as not having transportation or a flexible work schedule [19,20]. It is also noteworthy that reflex testing was performed at all five FQHCs which resulted in an average of more than 96% of patients who tested positive for HCV antibody to be tested for HCV RNA at the same time. This proportion is in sharp contrast to estimates reported from one national study that found only 20%−23% of persons with chronic HCV infection underwent confirmatory HCV RNA testing, and in another national study only 27% had a confirmatory HCV RNA test [10,11]. A study that examined the HCV continuum of care among residents in Philadelphia found that only 22% of persons who tested positive for HCV antibody received confirmatory HCV RNA testing [21].
Nonetheless, barriers to treatment remain. While the number of patients treated, and their outcomes improved consistently over time, the overall number of patients treated among those eligible for treatment was lower (15.3%) than expected, though similar to those seen nationally and locally during the time that direct acting agents went to market in early 2014 [10,11,21]. The reasons for this are many, but one major barrier to treatment in Philadelphia and elsewhere has been the high cost of DAAs. Also, in 2014, insurance companies required a sobriety window of up to a year for persons seeking treatment. While this requirement has subsequently been eliminated in Pennsylvania, other restrictions remain. For example, the Pennsylvania state government recently approved treatment for all patients with HCV infection, regardless of fibrosis stage for those on Medicaid. However, managed care organizations (MCOs) and fee for service (FFS) care still require documentation of liver damage [22].
It was encouraging that the absolute number of patients who were treated increased each year. From October 2012 through June 2013, only 4 patients initiated and completed treatment and 3 achieved SVR. However, by July 2015-June 2016, 64 patients initiated treatment, 54 completed treatment, 42 were assessed for SVR, and 39 achieved SVR by the end of data collection. We also found an increase in the number of patients treated within a year of testing. This increase is not surprising since DAAs were not widely available in 2013 and even in 2014. The improvement in the number of patients initiating treatment and the higher cure rates can certainly be attributed in part to the simplification of treatment and the efficacy of the new medications [23,24,25]. We expect to see continued improvement as new therapeutics with even shorter treatment duration are released [26,27,28,29].
Our study is not without limitations. The results might not generalize to other settings, as testing was not completed for all patients visiting the FQHCs likely due to various structural factors such as time constraints. Moreover, the patient population served in these urban FQHCs may differ from other patient populations, including those served by other FQHCs. We also saw variability across the five FQHCs in the prevalence of chronic infection (HCV RNA positive) among patients who were HCV antibody positive. Given that the percentage of HCV antibody positive patients who received HCV RNA confirmatory testing was greater than 94% at each FQHC, it is unlikely that this variation is due to missing data. It is more likely reflective of the positive predictive value of the HCV antibody test, which generally performs better among populations with a high prevalence of HCV infection compared to those with a low prevalence [30].
Another limitation is that we did not have information on the outcome for roughly 25% of the patients who completed treatment. We worked closely with specialist offices to try and remedy the problem for patients seen and treated offsite. We believe a combination of factors contributed to this situation, including that treatment for some patients was not assessed by the clinic; the provider did not receive a record of it; or in some cases patients who completed treatment did not return to be assessed for SVR. Consequently, the number of patients achieving SVR is likely higher than what was reported.
Further, because the clinics served populations at increased risk of HCV infection (e.g. persons who inject drugs), it is possible that some chronically infected patients identified through the testing program were infected recently and subsequently cleared the virus. Although the testing protocol did not require repeat HCV RNA testing for chronically infected patients, some patients were tested more than once, however the findings did not reveal any discordant RNA results or suspected acute cases of HCV infection among these patients. Finally, barriers to treatment in our patient population and setting might not be the same as those in other U.S. cities, as insurance plans and regulations vary across states and jurisdictions.
In summary, our results show that the best linkage to care results, from diagnosis to cure, were among patients at the PHMC Care Clinic, which was the network’s test and treat site. Although the percentage of patients initiating treatment in these clinics was low overall, the HCV care landscape is improving and treatment uptake has increased. Scaling up treatment services in settings where HCV testing is offered may help to improve the HCV care continuum. Expanding the capacity of primary care providers to treat is feasible given the simplicity of treatment and the availability of supportive resources during training and treatment. In fact, as of April 2016, providers at the Mary Howard Health Center and the Health Connection began treating patients diagnosed with chronic HCV infection, creating two additional test and treat sites in the health network. Since the training, an onsite primary care provider trained to treat HCV has evaluated 54 patients from these two clinics, 49 were assessed for HCV treatment, and three initiated treatment.
ACKNOWLEDGEMENTS
The authors thank the medical staff and patients at Mary Howard Health Center, PHMC Care Clinic, Health Connection, Congreso Health Center, and Rising Sun Health Center for making this study possible. The authors also thank Grace Lee from the National Nurse-Led Care Consortium for extracting the data from the electronic health records. Finally, the authors would like to thank Kristine Gonnella from the National Nurse-Led Care Consortium for programmatic support and advocating for the extension of the project.
Financial Support: This study was funded by the Centers for Disease Control and Prevention (CDC) hepatitis C virus grant (CDC-RFA-PS12–1209PPHF12) and Frontlines of Communities in the United States, a program of Gilead Sciences, Inc. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC or Gilead Sciences, Inc. Catelyn Coyle was supported by the NIH Training Grant T32AI102623.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/hep.30501
Conflict of Interest: None of the authors have conflicts of interest to disclose.
REFERENCES
- [1].Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014. March 4;160(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Edlin BR, Eckhardt BJ, Shu MA, et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015. November;62(5):1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].CDC. Viral hepatitis surveillance—United States, 2016. Atlanta, GA: US Department of Health and Human Services, CDC; 2018. [Google Scholar]
- [4].Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012. August 17;61(RR-4):1–32. [PubMed] [Google Scholar]
- [5].Campbell CA, Canary L, Smith N, Teshale E, et al. State HCV incidence and policies related to HCV preventive and treatment services for persons who inject drugs - United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2017. May 12;66(18):465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016. May 1;122(9):1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lu M, Li J, Rupp LB, Zhou Y, et al. Changing trends in complications of chronic hepatitis C. Liver Int. 2017. June 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Falade-Nwulia O, Sulkowski MS, Merkow A, et al. Understanding and addressing hepatitis C reinfection in the oral direct acting antiviral era. J Viral Hepatol. 2018. January 6. doi: 10.1111/jvh.12859. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Holmberg SD, Spradling PR, Moorman AC, et al. Hepatitis C in the United States. N Engl J Med. 2013. May 16;368(20):1859–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yehia BR, Schranz AJ, Umscheid CA, et al. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014. July 2;9(7):e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hawks L, Norton BL, Cunningham CO, et al. The hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016. June;23(6):473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Noska AJ, Belperio PS, Loomis TP, et al. Engagement in the hepatitis C care cascade among homeless veterans, 2015. Public Health Rep. 2017. Mar-Apr;132(2):136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brown JL, Gause NK, Lewis D, et al. Examination of the hepatitis C Virus care continuum among individuals with an opioid use disorder in substance use treatment. J Subst Abuse Treat. 2017. May;76:77–80. [DOI] [PubMed] [Google Scholar]
- [15].Falade-Nwulia O, Mehta SH, Lasola J, et al. Public health clinic-based hepatitis C testing and linkage to care in Baltimore. J Viral Hepat. 2016. May;23(5):366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].National Academies of Sciences, Engineering, and Medicine. 2016. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report. Washington, DC: The National Academies Press; 10.17226/23407 [DOI] [PubMed] [Google Scholar]
- [17].Coyle C, Viner K, Hughes E, et al. Identification and linkage to care of HCV-infected persons in five health centers - Philadelphia, Pennsylvania, 2012–2014. MMWR Morb Mortal Wkly Rep. 2015. May 8;64(17):459–63. [PMC free article] [PubMed] [Google Scholar]
- [18].Coyle C, Kwakwa H. Dual-routine HCV/HIV testing: seroprevalence and linkage to care in four community health centers in Philadelphia, Pennsylvania. Public Health Rep. 2016. Jan-Feb;131 Suppl 1:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bass SB, Jessop A, Maurer L, et al. Mapping the barriers and facilitators of HCV treatment initiation in methadone maintenance therapy patients: implications for intervention development. J Health Commun. 2018;23(1):177–127. [DOI] [PubMed] [Google Scholar]
- [20].Ahmed SM, Lemkau JP, Nealeigh N, Mann B. Barriers to healthcare access in a non-elderly urban poor American population. Health Soc Care Community. 2001. November;9(6):445–453. [DOI] [PubMed] [Google Scholar]
- [21].Viner K, Kuncio D, Newbern EC, et al. The continuum of hepatitis C testing and care. Hepatology. 2015. March;61(3):783–9. [DOI] [PubMed] [Google Scholar]
- [22].National Viral Hepatitis Roundtable. Hepatitis C: The state of Medicaid access report card [PDF File]. Presented at: The Liver Meeting, 68th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Washington DC, October 20–24, 2017. Retrieved from https://stateofhepc.org/report/. [Google Scholar]
- [23].Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and Sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. [DOI] [PubMed] [Google Scholar]
- [24].Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with Daclatasvir plus Sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. Effectiveness of Simeprevir plus Sofosbuvir, with or without Ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology. 2016. February;150(2):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Foster GR, Foster GR, Afdhai N, et all. Sofosbuvir and Velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. [DOI] [PubMed] [Google Scholar]
- [27].Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, et al. Sofosbuvir and Velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015. December 31;373(27):2599–607. [DOI] [PubMed] [Google Scholar]
- [28].Asselah T, Kowdley KV, Zadeikis N, et al. Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 weeks in patients with HCV genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2017. September 22. [DOI] [PubMed] [Google Scholar]
- [29].Jacobson IM, Lawitz E, Gane EJ, et al. Efficacy of 8 weeks of Sofosbuvir, Velpatasvir, and Voxilaprevir in patients with chronic HCV infection: 2 Phase 3 randomized Trials. Gastroenterology. 2017. July;153(1):113–122. [DOI] [PubMed] [Google Scholar]
- [30].Moorman A, Drobeniuc J, Kamili S. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012. J Clin Virol. 2017; 89: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]