Abstract
The term priority map is commonly used to describe a map of the visual scene in which objects and locations are represented by their attentional priority, which itself is a combination of low-level salience and top-down control. The aim of this review is to examine how such a map may be represented at the neuronal level. We propose that there is not a single, common map in the brain, but that a number of cortical areas work together to generate the resultant behavior. Specifically, we suggest that the lateral intraparietal area (LIP) of posterior parietal cortex provides a simple representation of attentional priority, which remaps across saccades, so that there is an apparent allocentric map in a region with retinocentric encoding scheme. We propose that the frontal eye field (FEF) of prefrontal cortex receives the responses from LIP, but can suppress them to control the flow of eye movement behavior, and that the intermediate layers of the superior colliculus (SCi) reflect the final saccade goal. Together, these areas function to guide eye movements and may play a similar role in allocating covert visual attention.
Introduction
The concept of a priority map is derived from the saliency map models of Itti, Koch and colleagues [1], which were aimed at modeling shifts of visual attention. These models were primarily driven by low-level salience, with a modicum of top-down inputs. Attention was allocated, in a winner-take-all manner, to the peak of the map, which was then inhibited so that attention could move on to the next highest point. However, a whole host of factors influence the allocation of attention, so we [2] and others [3,4] prefer to use the term priority map to describe the map that ultimately is used to guide eye movements and covert visual attention. We define a priority map as a map of the visual scene, in which activity is driven by low-level salience and by a range top-down influences, such as task rules and goals, experience, expectations and saccade plans (Fig. 1). Below, we will describe regions of the non-human primate brain thought to act as priority maps and propose a mechanism for how these areas work together to guide saccades and the allocation of covert attention.
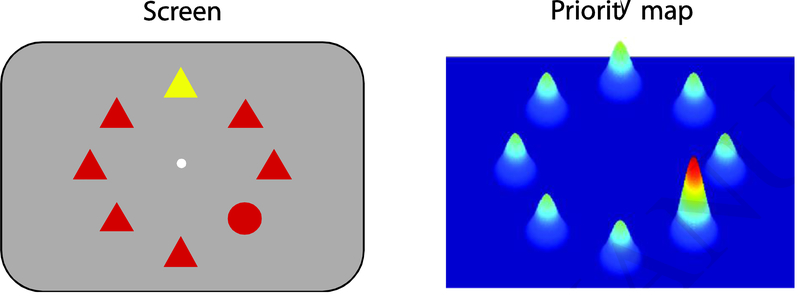
Figure 1.
A hypothetical priority map (right panel) in response to an array of stimuli (left panel) in which the subject is asked to find a circle. Each stimulus is represented by a response. The popout (yellow triangle) is represented by a slightly higher response due to its low level salience and the circle is represented by a much higher response as this represents the goal of the task, i.e. a top down input. Note that top down influences are not limited to task rules and goals; in natural behavior they can be driven by influences such as experience, saccade plans, memories and expectations.
Brain areas involved in priority map processing
A number of cortical and subcortical areas of the brain have been proposed as priority maps. These include the lateral intraparietal area (LIP) of posterior parietal cortex, the frontal eye field (FEF) of prefrontal cortex, the superior colliculus (SC) and several visual cortical areas [5–8, 9*, 10]. Given that our definition of a priority map is one in which the activity is primarily driven by attentional priority and which is involved in the allocation of covert attention and the guidance of eye movements, we think it unlikely that visual areas can act as true priority maps because their responses are primarily driven by stimulus features. However, it is clear that many visual areas include enhanced responses to salience within the feature space that they encode [5,6,10], so it may not be inappropriate to call them saliency maps. Indeed, the term saliency map is still used in several contexts, so it is worth differentiating them here. Some use the term to describe what we have called a priority map, that is a map that integrates both top-down and bottom-up inputs to guide attention and eye movements [8]. However, the term has also been used to illustrate the way visual area V1 [5] and the superficial layers of the superior colliculus [9*,11] preferentially respond to salient stimuli. Recent work has noted that the salience response emerges in the superficial layers of the superior colliculus before it emerges in V1 [11], which may be a result of the evolution of attentional behaviors in animals without a neocortex [12*, but see 13].
LIP, FEF and the intermediate layers of the superior colliculus (which we shall abbreviate as SCi) seem to fit the profile for a priority map. Each has responses that are modulated by low-level salience [14–16] and a variety of top-down factors [17*, 18–23, 24**]. In addition to being driven by the right sort of factors, LIP, FEF and SCi are involved in both the guidance of eye movements and covert attention [17*, 25–30].
The priority map for saccade goal selection
Behaviorally, we tend to think of a priority map as the final common map for guiding attention [31,32]. This is because behavioral studies can only manipulate external factors and then interpret the resulting behavior. As noted above, however, at least three brain areas in the non-human primate have been proposed as priority maps. This has led to questions about the underlying hypothesis [33*], such as: if there are multiple maps, which map is used to allocate attention or guide saccades? Here, we propose a way for these areas to work in concert to act, effectively, as a single priority map.
To elucidate the roles played by each area in more natural behavior, we refer to data from a series of studies using free viewing visual search tasks. In our work, we have used a visual foraging task, in which multiple target stimuli are presented among distractors, but only one target stimulus will give the animal a reward. This leads each animal to visually forage among the stimuli, until it finds the reward-bearing target. By aligning the stimuli so that one stimulus is in the recorded neuron’s receptive field when the animal is fixating another stimulus, data can be collected across multiple eye movements within a single trial and a representation of all aspects of the map can be built up within and across trials. Using this task, we have identified a number of differences between LIP and FEF, which allow us to identify the different roles they play in behavior.
Responses in LIP seem to make up a simple priority map. In the visual foraging task, the majority of neurons respond more to a target than to a distractor and the remaining neurons tend to not have task-related responses [34]. When a saccade is to be made into a neurons’ receptive field, many neurons show a burst of activity leading up to the saccade, suggesting that they get top-down feedback indicating the goal of the upcoming saccade. The task-responsive neurons also tend to show a reduced response to a target, if that target had been looked at earlier in the trial and did not give a reward [34]. This is similar to the inhibition of return incorporated into saliency map models [1], but is not as effective: neuronal responses are only slightly reduced. Most importantly, once the array of stimuli has appeared, responses stay fairly consistent during each fixation and are remapped after each saccade. We use the term remapped to describe the shifting of responses within LIP, analogous to the shifting of the image on the retina after an eye movement, but that occurs before the information could reach LIP from the retina. In the visual foraging task, LIP robustly reflects the identity of the stimulus in neurons’ receptive fields 20 ms after a saccade [35]. This means that, except for a brief period immediately after an eye movement, the representation of the entire visual field is present in LIP at all times during the task. Thus, LIP activity seems to be a simple priority map: most neurons have responses that are driven by the task demands and they respond the same way throughout the trial and across saccades.
FEF activity in this task is subtlety different. The majority of neurons in FEF respond like priority map neurons, i.e. they preferentially respond to targets more than to distractors and have reduced responses to previously fixated targets. However, unlike LIP neurons, the FEF responses get suppressed while animals make purposeful fixations [36]. Approximately 150 ms after the eyes stop moving, the responses of these neurons drop down to below baseline levels. If one thinks of FEF as creating a map to identify saccade goals, then the suppression of the map makes sense: when the animal wants to keep its eyes stable, it removes any activity that could lead to the generation of a saccade. The neuronal responses reactivate about 150 ms before the next saccade, which could allow for the sort of rise-to-threshold decision-making process that has been hypothesized to occur in FEF [8], and it would do so on a saccade by saccade basis. For short fixations, the activity remains elevated throughout the fixation, just as it does in LIP. We hypothesize that while LIP provides a priority map at all times, the modulations in FEF allow the animal to control the timing and flow of saccades. When FEF inhibition is released, it uses the input from LIP to make the decision about where to look. This is consistent with other studies that have examined FEF activity in free viewing behavior of natural stimuli and which have found responses that primarily represent the goal of the next saccade [37–39].
Based on these results, we suggest that during natural visual behavior, in which eye movements are made 2–3 times per second, LIP acts as the default priority map, combining top-down and bottom-up inputs. When things change, whether externally or based on internal goals, the activity in LIP will change [40]. The priority map in FEF, on the other hand, can choose to use the activity from LIP to generate an eye movement or can remain silent to keep the eyes stable. We hypothesize that SCi would reflect the output of FEF and is unlikely to be active during stable fixation. Indeed, we expect that SCi is likely to represent the winner-take-all aspect of the priority map process, with activity that primarily identifies where and when the next saccade will go. Only two studies have examined responses of neurons in SCi during free viewing. In both, activity was almost silent, unless an eye movement was about to go into the neuron’s receptive field [9*, 41].
The priority map for covert spatial attention
Most previous studies have examined activity in LIP, FEF and SCi using tasks in which animals were allowed to make only a single saccade. Unlike the free viewing responses described above, activity in these sorts of tasks is usually elevated throughout the duration of fixation in FEF and SCi [15, 42]. These tasks rely on explicit covert visual attention – if the animals make the wrong eye movement, then they will forfeit their reward – so they must be sure, covertly, that their saccade goal is the correct one before generating the eye movement. Such behavior is relatively uncommon in natural conditions (with the caveat that it can occur in non-human primates with social hierarchy rules that limit eye contact with dominant males). Instead, we tend to use eye movements to probe the visual scene. As such, it is unclear whether the activity in these conditions is different because the system is in a different state, in which covert attention is being explicitly used while the eyes don’t move, or because different neurons become active during this behavior. Whatever the genesis, data from these experiments gives important insight into how the activity in these areas might work as priority maps to guide covert attention.
We suggest that covert attention follows the same process as overt attention, particularly given the automatic allocation of attention at the goal of a saccade [28, 43–45] and the interactions between microsaccades and covert attention [46, 47]. The only exception would be that covert attention is allocated to the peak of a priority map on a moment-by-moment basis. However, understanding this process is hindered because the neural correlates of covert attention [48–50] have recently been dissociated from behaviors that would classically be described as covert attention [51**, 52]. This can lead to different interpretations of what roles areas play, depending on how one defines covert attention. So, for example, pharmacological activation of FEF modifies the attentional modulation of activity in visual areas [53], but the inactivation of SC does not [51**], yet the inactivation of SC has a much greater effect on behavior [30] than the inactivation of FEF [54].
Our hypothesis is that the priority map system is similar for overt and covert attention: LIP activity creates a first level priority map, influenced by both top-down and bottom up factors. FEF, which appears to remain active during periods of explicit covert attention, receives inputs from LIP and feeds back to visual areas to modulate their responses. SC, which likely represents similar activity to FEF and LIP via feedforward networks, seems to be critical in controlling the behavioral use of the activity represented in FEF and LIP. Under this hypothesis, during ongoing visual behavior, when FEF and SC are mostly suppressed, the corollary discharge sent to FEF from SC before an eye movement [55] is likely to drive the pre-saccadic benefit of attention seen at the goal of the saccade [28,43,44].
There are a number of experimental predictions that are born from this hypothesis, but the most obvious is that if FEF and SC are mostly silent during ongoing search, then one might predict that we should not see traditional attentional modulation in visual cortical areas during ongoing visual search. We know that V4 neurons show feature based attention during ongoing search [6,56] and that they have clear modulation when saccades are made to neurons’ receptive fields [57], but whether they show the sort of responses seen in covert attentional tasks [48–50] is not known. It may seem counterintuitive to suggest that these effects are not present in natural viewing behaviors, but we have recently shown that covert attentional modulation is absent while animals perform a change detection task with multiple items [52], yet as soon as they utilize explicit covert attention, the attentional modulation is seen. The presence of feature based attentional modulation during ongoing search could mean that feature based attention is driven by LIP [58], which is active during each fixation, or FEF [18], which is active during short fixations, but it is also possible that it is driven by pre-frontal areas other than FEF [59]. While it is commonly thought that feature based attention is not spatial, it is implemented in a spatial based system, so it is possible that priority maps could play a role in this process.
This review has focused on the process occurring in the non-human primate, given our ability to probe the activity of individual neurons. Functional imaging and various electrophysiological methods have shown somewhat similar activity in putative priority maps in the human [60]. However, it is clear that the human has a more complex network. As such, it is also possible that the control of covert attention is even more complex than in the non-human primate. This possibility is supported by the subtle perceptual differences in the effects of covert attention in the human when driven by different mechanisms [61].
Acknowledgments:
The authors are supported by the National Eye Institute (R01 EY019273 & R01 EY027968).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Itti L, Koch C: A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Res 2000, 40:1489–1506. [DOI] [PubMed] [Google Scholar]
- 2.Zelinsky GJ, Bisley JW: The what, where, and why of priority maps and their interactions with visual working memory. Ann N Y Acad Sci 2015, 1339:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fecteau JH, Munoz DP: Salience, relevance, and firing: A priority map for target selection. Trends Cogn Sci 2006, 10:382–390. [DOI] [PubMed] [Google Scholar]
- 4.Serences JT, Yantis S: Selective visual attention and perceptual coherence. Trends Cogn Sci 2006, 10:38–45. [DOI] [PubMed] [Google Scholar]
- 5.Li Z: A saliency map in primary visual cortex. Trends Cogn Sci 2002, 6:9–16. [DOI] [PubMed] [Google Scholar]
- 6.Mazer JA, Gallant JL: Goal-related activity in v4 during free viewing visual search. Evidence for a ventral stream visual salience map. Neuron 2003, 40:1241–1250. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb J, Balan P, Oristaglio J, Suzuki M: Parietal control of attentional guidance: The significance of sensory, motivational and motor factors. Neurobiol Learn Mem 2009, 91:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purcell BA, Schall JD, Logan GD, Palmeri TJ: From salience to saccades: Multiple-alternative gated stochastic accumulator model of visual search. J Neurosci 2012, 32:3433–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. *.White BJ, Berg DJ, Kan JY, Marino RA, Itti L, Munoz DP: Superior colliculus neurons encode a visual saliency map during free viewing of natural dynamic video. Nat Commun 2017, 8:14263 An interesting study that compares activity in the intermediate and superficial layers of the superior colliculus to a metric of salience during free viewing. It shows the stark difference between the layers in a completely natural behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Zhaoping L, Zhou T, Fang F: Neural activities in v1 create a bottom-up saliency map. Neuron 2012, 73:183–192. [DOI] [PubMed] [Google Scholar]
- 11.White BJ, Kan JY, Levy R, Itti L, Munoz DP: Superior colliculus encodes visual saliency before the primary visual cortex. Proc Natl Acad Sci U S A 2017, 114:9451–9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. *.Krauzlis RJ, Bogadhi AR, Herman JP, Bollimunta A: Selective attention without a neocortex. Cortex 2018, 102:161–175. A facinating review of attention-like behavior and the possible neural underpinings in a number of species that lack a neocortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhaoping L: From the optic tectum to the primary visual cortex: Migration through evolution of the saliency map for exogenous attentional guidance. Curr Opin Neurobiol 2016, 40:94–102. [DOI] [PubMed] [Google Scholar]
- 14.Thompson KG, Hanes DP, Bichot NP, Schall JD: Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 1996, 76:4040–4055. [DOI] [PubMed] [Google Scholar]
- 15.McPeek RM, Keller EL: Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 2002, 88:2019–2034. [DOI] [PubMed] [Google Scholar]
- 16.Arcizet F, Mirpour K, Bisley JW: A pure salience response in posterior parietal cortex. Cereb Cortex 2011, 21:2498–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. *.Buschman TJ, Miller EK: Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations. Neuron 2009, 63:386–396. The authors make a strong argument that activity in FEF correlates with covert attention, as animals move their attention around in a visual search task. They also convincinly show that decoding the locus of attention from FEF activity was generally better when the activity was tied to the underlying LFP phase rather than to external events (such as the saccade). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Desimone R: Feature-based attention in the frontal eye field and area v4 during visual search. Neuron 2011, 70:1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen K, Pare M: Neuronal activity in superior colliculus signals both stimulus identity and saccade goals during visual conjunction search. J Vis 2007, 7:15 11–13. [DOI] [PubMed] [Google Scholar]
- 20.Sugrue LP, Corrado GS, Newsome WT: Matching behavior and the representation of value in the parietal cortex. Science 2004, 304:1782–1787. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Gottlieb J: Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci 2013, 16:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa T, Komatsu H: Condition-dependent and condition-independent target selection in the macaque posterior parietal cortex. J Neurophysiol 2009, 101:721–736. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Wang X, Goldberg ME: A spatially nonselective baseline signal in parietal cortex reflects the probability of a monkey’s success on the current trial. Proc Natl Acad Sci U S A 2014, 111:8967–8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. **.Foley NC, Kelly SP, Mhatre H, Lopes M, Gottlieb J: Parietal neurons encode expected gains in instrumental information. Proc Natl Acad Sci U S A 2017, 114:E3315–E3323. An elegant study that shows that LIP neurons can respond to pure expected gains in information. The authors use a number of clever controls to make the strong argument that that the LIP activity they see representing the information gain is not driven by other factors that LIP activity is known to encode. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson DA, Fuchs AF: Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol 1969, 32:637–648. [DOI] [PubMed] [Google Scholar]
- 26.Robinson DA: Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 1972, 12:1795–1808. [DOI] [PubMed] [Google Scholar]
- 27.Andersen RA, Brotchie PR, Mazzoni P: Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol 1992, 2:840–846. [DOI] [PubMed] [Google Scholar]
- 28.Bisley JW, Goldberg ME: Neuronal activity in the lateral intraparietal area and spatial attention. Science 2003, 299:81–86. [DOI] [PubMed] [Google Scholar]
- 29.Moore T, Fallah M: Control of eye movements and spatial attention. Proc Natl Acad Sci U S A 2001, 98:1273–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovejoy LP, Krauzlis RJ: Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci 2010, 13:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chelazzi L, Estocinova J, Calletti R, Lo Gerfo E, Sani I, Della Libera C, Santandrea E: Altering spatial priority maps via reward-based learning. J Neurosci 2014, 34:8594–8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belopolsky AV: Common priority map for selection history, reward and emotion in the oculomotor system. Perception 2015, 44:920–933. [DOI] [PubMed] [Google Scholar]
- 33. *.Maunsell JHR: Neuronal mechanisms of visual attention. Annual Review of Vision Science 2015, 1:373–391. An excellent primer for people interested in knowing about all the ways attention affects neuronal activity in visual areas and the factors and neural areas that may be driving it. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirpour K, Arcizet F, Ong WS, Bisley JW: Been there, seen that: A neural mechanism for performing efficient visual search. J Neurophysiol 2009, 102:3481–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirpour K, Bisley JW: Remapping, spatial stability, and temporal continuity: From the pre-saccadic to postsaccadic representation of visual space in lip. Cereb Cortex 2016, 26:3183–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirpour K, Bolandnazar Z, Bisley JW: Suppression of frontal eye field neuronal responses with maintained fixation. Proc Natl Acad Sci U S A 2018, 115:804–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes HL, Stevenson IH, Phillips AN, Segraves MA, Kording KP: Saliency and saccade encoding in the frontal eye field during natural scene search. Cereb Cortex 2014, 24:3232–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glaser JI, Wood DK, Lawlor PN, Ramkumar P, Kording KP, Segraves MA: Role of expected reward in frontal eye field during natural scene search. J Neurophysiol 2016, 116:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramkumar P, Lawlor PN, Glaser JI, Wood DK, Phillips AN, Segraves MA, Kording KP: Feature-based attention and spatial selection in frontal eye fields during natural scene search. J Neurophysiol 2016, 116:1328–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlieb JP, Kusunoki M, Goldberg ME: The representation of visual salience in monkey parietal cortex. Nature 1998, 391:481–484. [DOI] [PubMed] [Google Scholar]
- 41.Shen K, Pare M: Predictive saccade target selection in superior colliculus during visual search. J Neurosci 2014, 34:5640–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson KG, Bichot NP, Schall JD: Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol 1997, 77:1046–1050. [DOI] [PubMed] [Google Scholar]
- 43.Kowler E, Anderson E, Dosher B, Blaser E: The role of attention in the programming of saccades. Vision Res 1995, 35:1897–1916. [DOI] [PubMed] [Google Scholar]
- 44.Deubel H, Schneider WX: Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Res 1996, 36:1827–1837. [DOI] [PubMed] [Google Scholar]
- 45.Steinmetz NA, Moore T: Eye movement preparation modulates neuronal responses in area v4 when dissociated from attentional demands. Neuron 2014, 83:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hafed ZM, Clark JJ: Microsaccades as an overt measure of covert attention shifts. Vision Res 2002, 42:2533–2545. [DOI] [PubMed] [Google Scholar]
- 47.Lowet E, Gomes B, Srinivasan K, Zhou H, Schafer RJ, Desimone R: Enhanced neural processing by covert attention only during microsaccades directed toward the attended stimulus. Neuron 2018, 99:207–214 e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moran J, Desimone R: Selective attention gates visual processing in the extrastriate cortex. Science 1985, 229:782–784. [DOI] [PubMed] [Google Scholar]
- 49.Fries P, Reynolds JH, Rorie AE, Desimone R: Modulation of oscillatory neuronal synchronization by selective visual attention. Science 2001, 291:1560–1563. [DOI] [PubMed] [Google Scholar]
- 50.Cohen MR, Maunsell JH: Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 2009, 12:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. **.Zenon A, Krauzlis RJ: Attention deficits without cortical neuronal deficits. Nature 2012, 489:434–437. For almost 30 years, the field had studied the effects of attention in visual cortical areas. In this study, the authors show that inactivation of the superior colliculus has a major effect on covert attention behavior, but the activity in visual cortex remains unchanged. This suggests that attentional activity in visual cortex is not sufficient for the behavioral instantiation of covert attention and has forced many of us to question what role these well studied effects may play in behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arcizet F, Mirpour K, Foster DJ, Bisley JW: Activity in lip, but not v4, matches performance when attention is spread. Cereb Cortex 2018, 28:4195–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noudoost B, Moore T: Control of visual cortical signals by prefrontal dopamine. Nature 2011, 474:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wardak C, Ibos G, Duhamel JR, Olivier E: Contribution of the monkey frontal eye field to covert visual attention. J Neurosci 2006, 26:4228–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sommer MA, Wurtz RH: A pathway in primate brain for internal monitoring of movements. Science 2002, 296:1480–1482. [DOI] [PubMed] [Google Scholar]
- 56.Bichot NP, Rossi AF, Desimone R: Parallel and serial neural mechanisms for visual search in macaque area v4. Science 2005, 308:529–534. [DOI] [PubMed] [Google Scholar]
- 57.Tolias AS, Moore T, Smirnakis SM, Tehovnik EJ, Siapas AG, Schiller PH: Eye movements modulate visual receptive fields of v4 neurons. Neuron 2001, 29:757–767. [DOI] [PubMed] [Google Scholar]
- 58.Ibos G, Freedman DJ: Interaction between spatial and feature attention in posterior parietal cortex. Neuron 2016, 91:931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bichot NP, Heard MT, DeGennaro EM, Desimone R: A source for feature-based attention in the prefrontal cortex. Neuron 2015, 88:832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sprague TC, Itthipuripat S, Vo VA, Serences JT: Dissociable signatures of visual salience and behavioral relevance across attentional priority maps in human cortex. J Neurophysiol 2018, 119:2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrasco M: How visual spatial attention alters perception. Cogn Process 2018, 19:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]