Abstract
The olfactory epithelium (OE) is the peripheral organ for the sense of smell, housing primary sensory neurons that project axons from the nose to the brain. Due the presence of a basal stem cell niche, the adult mammalian OE is a dynamic tissue capable of replacing neurons following their loss. Nonetheless, certain conditions, such as blunt head trauma, can result in persistent olfactory loss, thought to be due to shearing of olfactory nerve filaments at the skull base, degeneration, and failures in proper regeneration/reinnervation. The identification of new treatment strategies aimed at preventing degeneration of olfactory neurons is, therefore, needed. In considering potential therapies, we have focused on N-acetylcysteine (NAC), a glutathione substrate shown to be neuroprotective, with a record of safe clinical use. Here, we have tested the use of NAC in an animal model of olfactory degeneration. Administered acutely, we found that NAC (100 mg/kg, twice daily) resulted in a reduction of olfactory neuronal loss from the OE of the nose following surgical ablation of the olfactory bulb. At one week post-lesion, we identified 54 ± 8.1 mature neurons per 0.5 mm epithelium in NAC-treated animals versus 28 ± 4.2 in vehicle-treated controls (p=0.02). Further, in an olfactory cell culture model we have identified significant alterations in the expression of several genes involved in oxidative stress pathways following NAC exposure. Our results provide evidence supporting the potential therapeutic utility for NAC acutely following head trauma-induced olfactory loss.
Keywords: olfaction, oxidative stress, n-acetylcysteine, neurons, regeneration
INTRODUCTION
Although there are multiple mechanisms potentially involved in neuronal cell death pathways, oxidative damage and mitochondrial perturbations are common features (Raff et al., 1993; Koliatsos VE, 1999; Angelova and Abramov, 2018). Moreover, neuronal degeneration occurring due to loss of target-derived trophic support has also been shown to be associated with oxidative stress pathways (Ferrari et al., 1995; Al-Abdulla and Martin, 1998). Not surprisingly, therapeutic strategies aimed at preventing neuronal degeneration, due to injury or disease, have focused attention on compounds that can block oxidative damage (Sun et al., 2003; Hart et al., 2004). A critical neuronal pool that can degenerate following blunt head trauma or concussion, likely secondary to denervation and loss of trophic support, is the olfactory sensory neuron (OSN) population, projecting as the first cranial nerve from the nasal cavity to the brain. Head injuries can cause rapid and permanent anosmia, for which treatments are currently unavailable (Schofield et al., 2014). In the present study, we have directed attention towards this problem using a mouse model and in vitro assays.
While the olfactory epithelium (OE) in the nose is a site of ongoing neurogenesis, due to basal stem and progenitor cells (Graziadei and Graziadei, 1979; Calof and Chikaraishi, 1989; Huard et al., 1998; Leung et al., 2007; Fletcher et al., 2011; Goldstein et al., 2015; Fletcher et al., 2017; Schwob et al., 2017), its reparative potential may not be unlimited (Choi and Goldstein, 2018). Evidence suggests that cumulative damage may lead to neurogenic exhaustion in situations such as an age-related olfactory decline, termed presbyosmia (Doty et al., 1984; Hoffman et al., 1998; Murphy et al., 2002; Schubert et al., 2009). Alternatively, head trauma-induced anosmia may arise due to excessive degeneration, failed re-innervation by OSNs projecting from the OE to the olfactory bulbs of the brain, or central injuries involving degeneration in cortical areas such as orbitofrontal cortex (Holbrook et al., 2005; Holbrook and Leopold, 2006; Caminiti et al., 2013). Given the potential contribution of oxidative stress to OSN loss in these scenarios, strategies for neuronal preservation aimed at addressing oxidative damage appear warranted.
Here, we have focused on testing n-acetylcysteine (NAC) for preventing OSN degeneration. NAC has broad antioxidant properties: it acts as a cysteine donor to maintain glutathione levels, functions as a free radical scavenger, and protects the cytochrome oxidase complex in mitochondria from damage (Cooper and Kristal, 1997; Moncada, 2000). In peripheral nerve degeneration studies and culture models, NAC has shown marked neuroprotective activity (Mayer and Noble, 1994; Ferrari et al., 1995). NAC has also been studied for protection of cochlear cells from noise-induced cell death (Kopke et al., 2007). Finally, it is a particularly attractive compound because it has a track record of clinical safety and is inexpensive (Hoffer et al., 2013).
In a mouse model of acutely induced OSN degeneration, our results demonstrate that NAC administration can ameliorate loss of mature OSNs, identifiable by expression of olfactory marker protein (OMP). Additionally, in vitro experiments identify gene expression changes in NAC-treated olfactory basal cell cultures, revealing potential unexplored roles for oxidative stress pathway proteins, such a cytoglobin, in injury-activated olfactory progenitors.
MATERIALS AND METHODS
Animals
Adult C57BL/6 mice, weighing 20–30 grams (The Jackson Laboratory, Bar Harbor, Maine), were treated following a protocol approved by University of Miami Institutional Animal Care and Use Committee in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Olfactory bulbectomy model and NAC treatment
Unilateral olfactory bulbectomy was performed under deep anesthesia via ketamine (60–70 mg/kg ) and xylazine (10 mg/kg), intraperitoneal (IP). A skin incision was made to expose the calvarium. A craniotomy was performed to expose the right olfactory bulb (Fig. 1) using a powered drill. The dura was incised sharply and a small suction was used to gently apsirate the olfactory bulb. A piece of Surgicel® (Ethicon, Somerville, New Jersey) was placed for hemostasis, and the skin incision was closed. Systemic treatment with NAC (100 mg/kg, twice daily), based on similar dosage used in a rat peripheral nerve injury model (Hart et al., 2004), or an equivalent volume of phosphate buffered saline (PBS) vehicle (control group) was initiated within 30 minutes after the surgical procedure. NAC powder (Sigma Aldrich, St. Louis, MO) was dissolved in PBS, to a concentration of 5 mg/ml, pH was adjusted to ~ 7.4, and the solution was sterile filtered before administration. Buprenorphine was given post-operative day one for analgesia. The procedure was well tolerated, with no mortalities.
Figure 1.
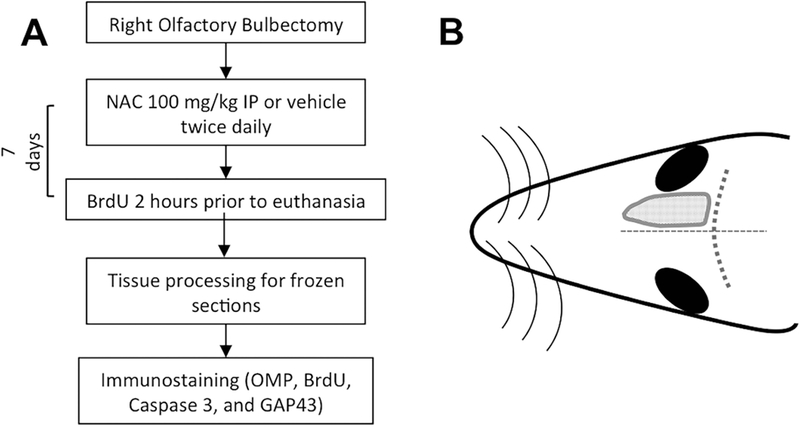
Experimental design. (A) Timeline of in vivo experiments. (B) Schematic drawing of the dorsal region of a mouse head. The gray dotted area represents the location of the right olfactory bulb that was resected for the experiment. The dotted lines indicate sutures used for orientation. NAC, N-acetylcysteine; NS, normal saline; OMP, Olfactory Marker Protein; BrdU, 5’-Bromodeoxyuridine; GAP43, Growth associated protein 43 kD.
Tissue processing
One week following bulbectomy, mice were given 5’-bromodeoxyuridine (BrdU, 50 mg/kg IP, Sigma) 2 hours prior to sacrifice under deep anesthesia via perfusion using PBS and followed by 4% paraformaldehyde. Nasal tissue was isolated and postfixed for 2 hours, then processed with 0.25 M EDTA and 30% sucrose in PBS for 72 hours on a rocker at 4 °C. Samples were placed in O.C.T. compound (VWR, Radnor, PA) under vacuum for 1–2 hours, frozen in liquid nitrogen, then cryosectioned at 10 μm and collected on Superfrost Plus slides (VWR). Slides were stored at – 20 °C.
Immunohistochemistry
Slides were rinsed in PBS for 5 minutes, and dehydrated/rehydrated in a graded series of ethanol for 1 minute each at 70%, 95%, 100%, 95%, 70%, followed by PBS for 5 minutes. Blocking was performed using 5% normal goat serum (Vector Laboratories, Burlingame, CA), 4% bovine serum albumin (Sigma Aldrich), 5% nonfat dried milk (Bio Rad, Hercules, CA) and 0.3% Triton X-100 (Sigma) in PBS for 30 minutes. Primary antibodies are listed (Table 1) and were incubated overnight in blocking solution at 4 °C. For BrdU, heat mediated antigen retrieval was performed using Citrate buffer pH 6.0 in a steamer, before staining. Slides were rinsed in PBS 3 times for 5 minutes each, and incubated with appropriate secondary antibodies (Alexa 594 anti-mouse or anti-rabbit, Jackson ImmunoResearch, West Grove, PA) for 45 minutes. Slides were rinsed and coverslipped with Vectashield containing DAPI (Vector Labs).
Table 1.
Antibody reagents used in the present study. OSNs=Olfactory Sensory Neurons; RRIDs=Research Resource Identifiers.
| Antiboy | Cells labeled | Source | RRIDs |
|---|---|---|---|
| OMP | Mature OSNs | Santa Cruz sc-365818 |
AB_10842164 |
| GAP-43 | Immature OSNs | Abcam #ab16053 |
AB_443303 |
| Caspase 3 | Apoptotic cells | Cell Signaling Technology #9662 |
AB_1083921 |
| BrdU | Mitotically active cells | BD Biosciences Cat# B44 |
AB_2313824 |
Image analysis
For quantification, images were acquired on an epifluorescent microscope (Olympus) using a 20X objective from comparable regions of the nasal cavity at three different anterior-posterior levels. Using slides from 4–6 animals per condition, images were opened in Image J (NIH) and counts were performed on 500 μm mucosal length per region (septum and medial tubinates I-IV). For each antibody, labeling was compared between NAC-treated or vehicle-treated bulbectomized samples. Sections from different, non-adjacent slides were selected; Abercrombie correction was not necessary. Statistical analysis using unpaired t-test with Welch’s correction, for data with a normal distribution, was performed in Prism 7 (Graphpad). P<0.05 was considered significant. Representative images were also acquired using a confocal microscope (Zeiss LSM710) and 10 μm z-stack images were assembled in ImageJ for Figure 2.
Figure 2.
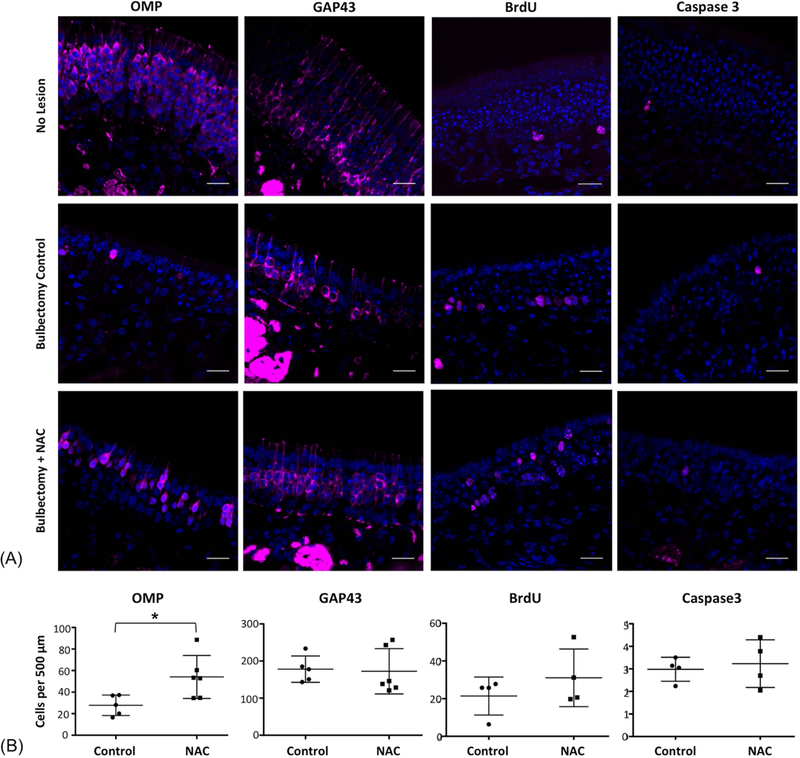
Immunohistochemical analysis of NAC-treated mouse OE following unilateral bulbectomy. Sections were stained to label mature neurons (OMP); immature neurons (GAP43), proliferative cells (BrdU) or apoptosis (Caspase 3). NAC treatment resulted in an increase in OMP (+) neurons. NAC, N-acetylcysteine; NS, normal saline; OMP, Olfactory Marker Protein; BrdU, 5’-bromodeoxyuridine; GAP43, Growth associated protein 43 kD. Nuclei are counterstained with DAPI (blue). N=4–6 animals per condition,* indicates p=0.02, unpaired t test. Bar=20 μm.
Olfactory cell culture and in vitro testing of NAC
We utilized an established globose basal cell culture model (Goldstein et al., 2016), with minor modification. Olfactory tissue was harvested from mice 7–10 days after administration of methimazole to induce olfactory lesion (Bergman et al., 2002). Basal cells were isolated from dissociated OE based on immunomagnetic selection with antibody to c-KIT (eBiosciences 17–1171: RRID:AB_469430), as described (Goldstein et al., 2016). Cells were washed with Hank’s buffered salt solution (HBSS) and plated onto gelatin-coated culture dishes in a 6-well plate. Three replicates were performed, each prepared from individual mice. Cells were incubated in growth medium (NeuroCult NSC, 20 ng/ml EGF, 10 ng/ml FGF2, 2 μg/ml heparin, 10 μM Y27632, all from Stemcell Techologies, Vancouver). RepSox (25 μM, Stemcell Techologies), a selective inhibitor of the Alk5 TGFβ family receptor, was included overnight and then removed, to promote basal cell expansion. Medium was changed every 2 days, without replacing Y27632 or Repsox. Cells were split 1:1 once they reached 80% confluence, after 5–7 days. Three replicates were incubated with L-NAC (5 μg/ml, Sigma, St. Louis) and exposed to stabilized hydrogen peroxide (750 μM, Sigma), three controls were treated identically except NAC was excluded. RNA was collected after 3 hours and purified via column isolation (Quick-RNA MicroPrep kit, Zymo Research, Irvine, CA). On column DNase digestion was performed.
RT-qPCR
The RT2 First Strand Kit was used for cDNA synthesis (Qiagen, Germantown, MD). RT-qPCR arrays were then performed in triplicate for NAC-treated versus control groups, using the Oxidative Stress Pathway and Antioxidant Defense array (#PAMM-065Z, Qiagen) following manufacturer instructions.
CT values were uploaded to the manufacturer’s analytic software for calculation of fold changes in gene expression and for statistical analyses. The p-values were calculated based on a Student’s t-test of the replicate 2^(-Delta CT) values and graphed on a volcano plot (Fig. 3C). Twenty genes with lowest p-values (<0.08) were included to create a heatmap (Fig. 3D). Six genes achieving statistical significance (P<0.05) were selected for further study.
Figure 3.
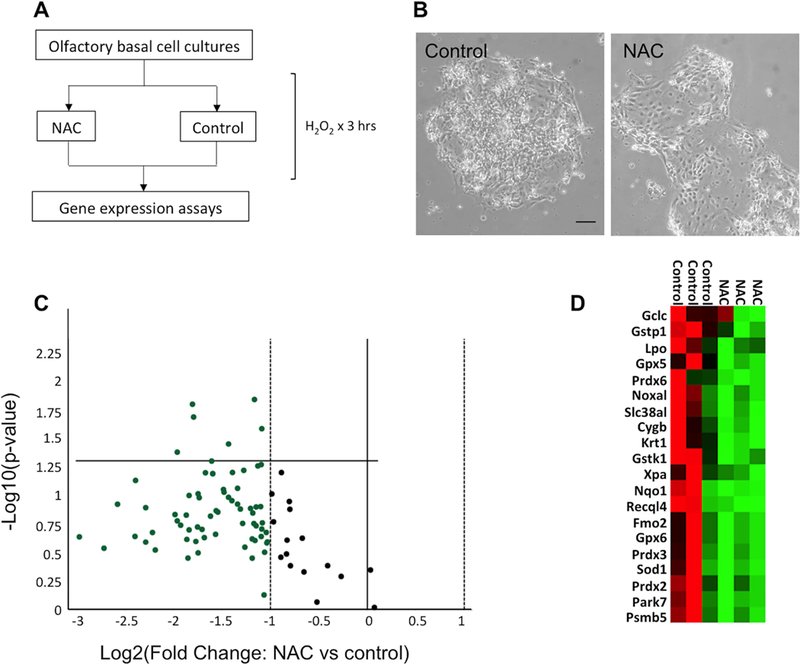
NAC treatment in olfactory cell cultures resulted in decreased expression of oxidative stress pathway genes. (A) Schematic representation of in vitro experiments. (B) Effects of oxidative stress on primary cultures of regenerating OE cells; irregular morphology, floating detached cells and debris are more apparent in control peroxide-treated cultures; bar=50 μm. (C) Volcano plot of the expression of genes involved in oxidative stress pathway, showing down-regulation of many transcripts (green) in response to NAC treatment. (D) Heat map depiction of gene expression changes, listing the 20 transcripts from triplicate experiments found to have lowest p-value on statistical analyses; red represents increased expression, green represents decreased expression.
RESULTS
NAC treatment following olfactory bulbectomy in mice
The mouse olfactory bulbectomy model was used to induce the rapid, coordinated retrograde degeneration of mature olfactory sensory neurons (OSNs) (Schwob et al., 1992). Removal of the bulb results in disruption of the mature OSNs projecting their axons from the olfactory epithelium (OE) in the nose to synapse in the glomerular layer of the olfactory bulb. As a consequence, the mature OSNs, expressing olfactory marker protein (OMP), degenerate and die within approximately a week (Carr and Farbman, 1993). We reasoned that the precise and reproducible nature of the bulbectomy model permits the testing of agents that might be useful for preventing OSN degeneration following damage to, or disconnection from, their central target (Fig. 1 schematic).
Unilateral olfactory bulbectomy was performed. Mice were then treated with systemic administration of NAC or vehicle (n=4–6 mice per group), delivered intraperitoneally (IP) twice daily for 1 week. The one-week time point was chosen for initial analysis, to determine if there are any differences in the NAC-treated OE after the initial wave of cell death that normally occurs in the first few days after bulbectomy. After euthanasia and tissue processing, immunohistochemical study was performed using a panel of cell type-specific markers (Table 1). We used antibody to OMP to label mature OSNs, anti-GAP43 to label immature OSNs, anti-BrdU to label acutely mitotic cells, and antibody to activated Caspase 3 to label apoptotic cells (Fig. 2). The vehicle-treated bulbectomized OE is characterized by few remaining OMP (+) cells, increased numbers of new, immature GAP43 (+) neurons, evidence of ongoing cell death marked by Caspase labeling, and an ongoing proliferative response by globose basal progenitor cells (Schwob et al., 1992), producing new neurons (Fig. 2A). Quantification of immunohistochemical labeling (Fig. 2B) indicated that the OE of mice treated with systemic NAC administration following bulbectomy contained more mature OMP (+) neurons per 0.5 mm epithelium (54 ± 8.1) than vehicle-treated controls (28 ± 4.2, SEM, p=0.02, t test). Quantification of other markers was not statistically significant. In addition, the contralateral un-operated OE (i.e. “No lesion” row in Fig. 2A) served as an internal control; there were no differences observed in labeling of un-lesioned OE in vehicle-treated versus NAC-treated mice (not shown).
Mice were treated with the thymidine analog BrdU 2 hours prior to perfusion, to label acutely proliferative cells passing through S-phase. With this protocol, the majority of cells incorporating BrdU in the OE are globose basal cells (Schwob et al., 1992; Goldstein and Schwob, 1996; Weiler and Farbman, 1997). The globose basal cells are a heterogenous population of stem and progenitor cells, and their proliferation in response to olfactory bulbectomy is well characterized, accounting for the neurogenic response to the death of OSNs. Here, there was no significant change in apoptosis or proliferation, suggesting that the homeostatic mechanisms regulating ongoing turnover in the bulbectomized environment are not markedly altered by NAC. However, increased numbers of OMP (+) cells were noted. These results suggest that acute NAC treatment likely promotes the survival of some mature OSNs following removal of their synaptic target.
NAC treatment of regenerating olfactory epithelial cells in culture
We next sought to identify gene expression changes in OE cells in a culture model in response to NAC exposure. In the adult OE, the c-Kit receptor has been shown to be expressed selectively on neurogenic globose basal stem cells (Goldstein et al., 2015; Goss et al., 2016; Fletcher et al., 2017). Therefore, we have utilized an established culture protocol in which adult OE cells are dissociated and the c-KIT (+) population is purified by immunomagnetic selection (Goldstein et al., 2016).
Here, we modified the protocol by treating only briefly with RepSox, a selective TGFβ family inhibitor, to permit neuron differentiation along with basal cell proliferation (Fig. 3A,B). Triplicate culture preparations, each harvested from individual mice (i.e. biologic replicates) were allowed to expand in vitro and were then stressed with addition of hydrogen peroxide either in the presence or absence of NAC. Using a gene expression array approach, we then assessed changes in olfactory cells in response to NAC exposure (Fig. 3). We found that levels of a large number of oxidative stress pathway transcripts were down-regulated in NAC-treated cultures. Despite expected variation among samples, changes in several transcripts had low p-values by t-test (Fig. 3B,C). The most significantly down-regulated transcripts (p<0.05) included Cytoglobin (Cygb), −3.9 fold; Glutathione peroxidase 5 (Gpx5), −3.5 fold; Glutathione S-transferase kappa 1(Gstk1), −2.1 fold; Glutathione S-transferase, pi 1 (Gstp1), −2.2 fold; Keratin 1 (Krt1), −3.5 fold; and Neutrophil cytosolic factor 2 (Ncf2), −2.7 fold. We interpret the down-regulation of oxidative damage-induced pathway genes as evidence for the antioxidant effects of NAC treatment, i.e. NAC appeared to protect cells from the oxidative insult, thereby blunting the expression of the damage-response genes. Taken together, these results demonstrate that NAC can function to potentiate oxidative stress responses specifically in OE cells, which may account for a neuroprotective effect observed in our in vivo model.
DISCUSSION
The present study sought to determine if exposure to NAC could promote survival or recovery of OSNs following experimental damage or injury, using a mouse model. Clinically, damage induced by head trauma is a common cause of olfactory sensory loss, lacking effective treatment options (Holbrook et al., 2005; Schofield et al., 2014; Choi and Goldstein, 2018). Identification of an agent with neuroprotective activity, that could be administered acutely following injury, may provide a rational basis for a new treatment approach for trauma-induced anosmia. Our data showed that NAC treatment in vivo had a neuroprotective effect after experimental injury, resulting in more OMP (+) OSNs identifiable in the OE of NAC-treated animals compared to controls, at one week after injury. Additionally, gene expression assays in cultured OE cells indicated that NAC treatment altered the expression of several genes involved in oxidative stress or repair pathways.
In considering potential drug candidates for treating traumatic olfactory loss, NAC is an attractive compound, given its reported efficacy at preventing axotomy-induced peripheral nerve degeneration (Hart et al., 2004). Since the coup-contrecoup injury induced by blunt head trauma can cause shearing or disruption of the delicate olfactory nerve filaments connecting the OE of the nose to the olfactory bulbs of the brain, axotomy and resulting degeneration of mature OSNs, housed in the OE, likely occurs in trauma-induced anosmia. Additional central injuries may, of course, also be relevant. However, we reasoned that compounds with acute efficacy for preventing axotomy-induced degeneration are of highest interest. While the basal cells in the OE can produce new OSNs following axotomy, reinnervation of the olfactory bulb by these cells is likely impaired in cases in which the entire projection has degenerated, leaving no remaining connections for the new axons to follow, and/or inducing reactive gliosis intracranially. Therefore, we hypothesize that the preservation of any OSNs following injury may not only maintain connectivity of the OE-bulb circuit, but also may prevent the development of barriers to reinnervation by nascent OSNs replacing degenerated cells.
Mechanistically, NAC functions as a glutathione substrate, providing antioxidant activity and mitochondrial protection. NAC acts as a cysteine donor to maintain glutathione levels, can function as a free radical scavenger, and can protect the cytochrome oxidase complex in mitochondria from damage (Cooper and Kristal, 1997; Moncada, 2000). In vitro, NAC protects neurons, glia and other cell types from a variety of toxic insults, including deprivation of trophic support or exposure to inflammatory mediators such as TNF-alpha (Mayer and Noble, 1994; Ferrari et al., 1995). Research in other neuronal tissues supports directly the ability of NAC to prevent neuronal degeneration in vivo. In models of peripheral nerve injury, dorsal root ganglion neuron survival was increased using systemic NAC therapy following sciatic nerve section in a rat model (Hart et al., 2004). Finally, NAC has a long record of clinical safety, used for inhaled mucolytic effects and for hepatocyte protection following acetaminophen overdose. Recently, oral NAC was found to be safe and efficacious when administered acutely after blast-induced traumatic brain injury, measuring cognitive and vestibular outcomes (Hoffer et al., 2013).
We tested NAC in vivo in a mouse model using olfactory bulbectomy to cause acute coordinated denervation and degeneration of the ipsilateral mature OSNs. While removal of the bulb differs from a human clinical coup-contrecoup injury, this model is well-established, reproducible, causes axonal retrograde degeneration, and consequences are easily measurable in OE tissue sections. For instance, the mouse bulbectomy model has been reported for testing of a jun-N-terminal Kinase (JNK) pathway inhibitor for cell death prevention (Gangadhar et al., 2008). Furthermore, testing drug treatment following short survival interval directly assesses any impacts on the acute degeneration of OSNs. Our findings that more OMP (+) mature OSNs are present in NAC treated animals 1 week post-bulbectomy, with minimal changes in proliferation, GAP43 or caspase expression, are consistent with the interpretation that NAC protects some mature neurons from degeneration. Examination of tissues at differing time points following injury may reveal changes in cell death or proliferation, depending on timing. However, these results provide evidence that NAC may be useful for testing treatment of post-head trauma olfactory loss, if given acutely.
Additionally, profiling oxidative stress-response genes in our culture assays identified candidate transcripts warranting further study in the damaged-induced regenerative response in the OE. Of particular interest, cytoglobin is a highly conserved heme-containing protein expressed in a variety of tissues, including muscle and brain (Mammen et al., 2006). In skeletal muscle, which is capable of remodeling and regeneration in response to damage, cytoglobin expression has been found to be induced by oxidative stress and to play a role specifically in activated progenitor cells, influencing survival, proliferation, and differentiation (Singh et al., 2014). Adult myogenic progenitors and the neurogenic progenitors in the adult OE appear to share analogous regulatory mechanisms, such as basic helix-loop-helix genes, and the culture model utilized here is enriched for OE basal progenitors. Identifying mechanisms promoting the survival or expansion of OE basal progenitors may provide further insights into new strategies for treating certain forms of olfactory loss.
In summary, our results demonstrate that NAC protects OSNs from degeneration in an animal model of trauma-induced injury. Also, profiling gene expression changes in NAC-treated olfactory cell cultures identifies changes in oxidative stress-response genes. We conclude that NAC may be a useful candidate warranting further study for acute treatment of head trauma-induced olfactory loss.
Acknowledgements:
This work was supported by grants from the NIH (K08 DC013556) and a Clinician Scientist Development Award from the Triological Society/American College of Surgeons (B.J.G.)
REFERENCES
- Al-Abdulla NA, Martin LJ. 1998. Apoptosis of retrogradely degenerating neurons occurs in association with the accumulation of perikaryal mitochondria and oxidative damage to the nucleus. Am J Pathol 153:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova PR, Abramov AY. 2018. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett 592:692–702. [DOI] [PubMed] [Google Scholar]
- Bergman U, Ostergren A, Gustafson AL, Brittebo B. 2002. Differential effects of olfactory toxicants on olfactory regeneration. Arch Toxicol 76:104–112. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. 1989. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron 3:115–127. [DOI] [PubMed] [Google Scholar]
- Caminiti F, Ciurleo R, Bramanti P, Marino S. 2013. Persistent anosmia in a traumatic brain injury patient: role of orbitofrontal cortex. Brain Inj 27:1715–1718. [DOI] [PubMed] [Google Scholar]
- Carr VM, Farbman AI. 1993. The dynamics of cell death in the olfactory epithelium. Exp Neurol 124:308–314. [DOI] [PubMed] [Google Scholar]
- Choi R, Goldstein BJ. 2018. Olfactory epithelium: Cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig Otolaryngol 3:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Kristal BS. 1997. Multiple roles of glutathione in the central nervous system. Biol Chem 378:793–802. [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. 1984. Smell identification ability: changes with age. Science 226:1441–1443. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Yan CY, Greene LA. 1995. N-acetylcysteine (D- and L-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci 15:2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, Das D, Gadye L, Street KN, Baudhuin A, Wagner A, Cole MB, Flores Q, Choi YG, Yosef N, Purdom E, Dudoit S, Risso D, Ngai J. 2017. Deconstructing Olfactory Stem Cell Trajectories at Single-Cell Resolution. Cell Stem Cell 20:817–830 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, Prasol MS, Estrada J, Baudhuin A, Vranizan K, Choi YG, Ngai J. 2011. p63 regulates olfactory stem cell self-renewal and differentiation. Neuron 72:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadhar NM, Firestein SJ, Stockwell BR. 2008. A novel role for jun N-terminal kinase signaling in olfactory sensory neuronal death. Mol Cell Neurosci 38:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Goss GM, Choi R, Saur D, Seidler B, Hare JM, Chaudhari N. 2016. Contribution of Polycomb group proteins to olfactory basal stem cell self-renewal in a novel c-KIT+ culture model and in vivo. Development 143:4394–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Goss GM, Hatzistergos KE, Rangel EB, Seidler B, Saur D, Hare JM. 2015. Adult c-Kit(+) progenitor cells are necessary for maintenance and regeneration of olfactory neurons. J Comp Neurol 523:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Schwob JE. 1996. Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci 16:4005–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss GM, Chaudhari N, Hare JM, Nwojo R, Seidler B, Saur D, Goldstein BJ. 2016. Differentiation potential of individual olfactory c-Kit+ progenitors determined via multicolor lineage tracing. Dev Neurobiol 76:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei GA, Graziadei PP. 1979. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol 8:197–213. [DOI] [PubMed] [Google Scholar]
- Hart AM, Terenghi G, Kellerth JO, Wiberg M. 2004. Sensory neuroprotection, mitochondrial preservation, and therapeutic potential of N-acetyl-cysteine after nerve injury. Neuroscience 125:91–101. [DOI] [PubMed] [Google Scholar]
- Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B. 2013. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One 8:e54163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HJ, Ishii EK, MacTurk RH. 1998. Age-related changes in the prevalence of smell/taste problems among the United States adult population. Results of the 1994 disability supplement to the National Health Interview Survey (NHIS). Ann N Y Acad Sci 855:716–722. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Leopold DA. 2006. An updated review of clinical olfaction. Curr Opin Otolaryngol Head Neck Surg 14:23–28. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Leopold DA, Schwob JE. 2005. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope 115:2144–2154. [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. 1998. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol 400:469–486. [PubMed] [Google Scholar]
- Koliatsos VE RR. 1999. Cell death and diseases of the nervous system. Totowa, New Jersey: Humana Press. [Google Scholar]
- Kopke RD, Jackson RL, Coleman JK, Liu J, Bielefeld EC, Balough BJ. 2007. NAC for noise: from the bench top to the clinic. Hear Res 226:114–125. [DOI] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. 2007. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci 10:720–726. [DOI] [PubMed] [Google Scholar]
- Mammen PP, Shelton JM, Ye Q, Kanatous SB, McGrath AJ, Richardson JA, Garry DJ. 2006. Cytoglobin is a stress-responsive hemoprotein expressed in the developing and adult brain. J Histochem Cytochem 54:1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Noble M. 1994. N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sci U S A 91:7496–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S 2000. Nitric oxide and cell respiration: physiology and pathology. Verh K Acad Geneeskd Belg 62:171–179; discussion 179–181. [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. 2002. Prevalence of olfactory impairment in older adults. JAMA 288:2307–2312. [DOI] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. 1993. Programmed cell death and the control of cell survival: lessons from the nervous system. Science 262:695–700. [DOI] [PubMed] [Google Scholar]
- Schofield P, Moore T, Gardner A. 2014. Traumatic brain injury and olfaction: a systematic review. Frontiers in Neurology 5:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Murphy C, Huang GH, Klein BE, Klein R, Nieto FJ, Pankow JS, Tweed TS. 2009. Olfactory impairment in adults. Ann N Y Acad Sci 1170:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN, Hewitt Coleman J. 2017. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J Comp Neurol 525:1034–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Szumowski KE, Stasky AA. 1992. Olfactory sensory neurons are trophically dependent on the olfactory bulb for their prolonged survival. J Neurosci 12:3896–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Canseco DC, Manda SM, Shelton JM, Chirumamilla RR, Goetsch SC, Ye Q, Gerard RD, Schneider JW, Richardson JA, Rothermel BA, Mammen PP. 2014. Cytoglobin modulates myogenic progenitor cell viability and muscle regeneration. Proc Natl Acad Sci U S A 111:E129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. 2003. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A 100:3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. 1997. Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci 17:3610–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]


