Abstract
Breast cancer is a common malignancy with poor prognosis. Cancer cells are heterogeneous and cancer stem cells (CSCs) are primarily responsible for tumor relapse, treatment-resistance and metastasis, so for breast cancer stem cells (BCSCs). Diets are known to be associated with carcinogenesis. Food-derived polyphenols are able to attenuate the formation and virulence of BCSCs, implying that these compounds and their analogs might be promising agents for preventing breast cancer. In the present review, we summarized the origin and surface markers of BCSCs and possible mechanisms responsible for the inhibitory effects of polyphenols on BCSCs. The suppressive effects of common dietary polyphenols against BCSCs, such as curcumin, epigallocatechin gallate (EGCG) and related polyphenolic compounds were further discussed.
Keywords: Dietary polyphenols, breast cancer stem cells, mechanism, differentiation, proliferation
Introduction
Breast cancer is one of the most common malignancies and a primary reason for cancer-related death among women worldwide, and the proportion of breast cancer in newly confirmed cancer cases for women is 29% in the United States (Burnett et al. 2017). Similar to other types of cancers, metastasis, recurrence and drug resistance are leading barriers for breast cancer treatment. Conventional therapy of breast cancer includes chemotherapy (e.g. paclitaxel, docetaxel, adriamycin, cyclophosphamide), radiotherapy and surgery, which show poor prognosis and suffer from long-term side effects. Thus numerous efforts have been made to address these problems.
CSCs are just a minor proportion of cells in a tumor, and accumulating studies have confirmed that CSCs mainly account for tumor metastasis, regrowth and drug resistance (Dean 2009; Yin et al. 2011; Flemming 2015; Iqbal et al. 2016; Liang 2016; Su et al. 2016). Consistently, BCSCs are main reasons of the drug resistance and relapse of breast cancers. Therefore, targeting CSCs combined with differentiated cancer cells has become a promising therapeutic measure to prevent cancers. However, available chemotherapeutic drugs focus on killing differentiated cancer cells, which account for the majority of cells in tumors, rather than the fairly quiescent CSCs, and drugs targeting CSCs remain to be developed (Boman and Wicha 2008). A wide range of evidences show that fruits and vegetables that are rich in various bioactive phytochemicals such as polyphenols decrease the cancer incidence and relapse risk (Demark-Wahnefried et al. 2008; Lanou and Svenson 2010). Interestingly, close to half of currently approved anticancer drugs are either natural phytochemicals (isolated from plants, seeds and so on) or their derivatives (Nobili et al. 2009; Newman and Cragg 2012). Moreover, dietary polyphenols possess prominent effects on inhibiting tumorigenesis and potentiating drug sensitivity of CSCs (Vanden Berghe 2012).
Herein, we focus on discussing the origins and surface markers of BCSCs and mechanisms by which the common dietary polyphenols (curcumin, EGCG, resveratol, quercetin, genistein, and 6-shogaol), and their analogs or nano-formula target BCSCs.
Origins of CSCs/BCSCs
CSCs share the similar special surface tags, self-renewal abilities along with regulatory signaling with normal stem cells. Thus CSCs could spring from the malignant conversion of dormant normal stem cells that have accumulated oncogenic mutations over time, and this increasing mutational burden plays a vital role in tumorigenesis and development of tumor and disrupting proper cell differentiation (Dean, Fojo, and Bates 2005; Yang, Xu, et al. 2017). Besides genetic mutations, other factors such as epithelial mesenchymal transition (EMT) program and epigenetic changes (abnormal methylation, histone modification) may also involve in the transformation of stem cells into CSCs (Garg 2017; Wainwright and Scaffidi 2017).
Another hypothesis for the origin of CSCs is “misplacement of somatic stem cell theory”. It assumes that a handful of embryonal stem cell-like cells exist in the blood or other tissues. If they move at an inappropriate time and/or displaced (suffer from damaging environmental factors), they would turn into CSCs (Justyna Gil, Pesz, and Siadek 2008). Similar theory proposes that stem cell misplaces in the stroma owing to basement membrane lesion, and these misplaced stem cells are the root sources of the invasive tumors (Wang, Li, et al. 2013).
Besides, traditional cancer therapy also induces no-CSCs into CSC-like cells in various cancers such as breast cancer, and the drug-induced CSC-like properties (therapeutic resistance) will lose once chemotherapy is removed, implying that this conversion is reversible and transient (Goldman et al. 2015; Doherty et al. 2016).
BCSCs markers
In order to study the biology of BCSCs, accurately identifying and isolating BCSCs are necessary. Thus far, a series of surface markers have been used for flow cytometry separation of BCSCs. The most widely used surface markers are cluster of differentiation (CD) 44+, CD24−, aldehyde dehydrogenase 1 (ALDH1)+ and CD133+.
CD44 is a multifunctional transmembrane glycoprotein, which binds to hyaluronic acid and components of extracellular matrix (ECM), and is implicated in adhesion between cells and interactions among cells and matrices (Naab, Ricks-Santi, and Khan 2016). CD44 is related to metastasis, cancer invasion and multidrug resistance by interacting with P-glycoprotein (Miletti-Gonzalez et al. 2005; Pokharel et al. 2016. CD24 is a glycosylphosphatidylinositol-anchored membrane protein that is commonly overexpressed in human cancer cells (Ma et al. 2015), which is related to malignancy, chemoresistance, and metastasis of breast cancer cells (Kwon et al. 2015; Ma et al. 2015; Deng et al. 2017). CD44+CD24−/low is a set of markers firstly used for identifying BCSCs, which has been widely used and is reliable to isolate cancer cells with stem cell-like characteristics, highly connected with the malignance of breast cancer (Ma et al. 2014; Wang, Wang, et al. 2017). The stemness of cells expressing CD44+CD24−/low was proven by tumorigenicity tests (tumorigenesis in xenotransplanted mice), colony/mammosphere formation, migration, and invasion assays (Elizabeth Louie et al. 2010; Li, Ma, et al. 2017). However, some breast cancer cell lines do not express CD44+/CD24− (Li, Ma, et al. 2017), implying that additional BCSCs markers should be established and verified.
ALDH1 is another commonly used marker of identifying BCSCs population. ALDH1 is able to convert retinol into retinoic acid, acting as an important detoxifying protease, and suppression of ALDH1 expression decreases the stemness of breast cancer cell lines and BCSCs (Kim et al. 2013). Moreover increased ALDH1 expression is commonly found in malignant breast stem/progenitor cells, which has a positive correlation with poor prognosis (Ginestier et al. 2007). Thus, ALDH1+ maybe a more helpful indicator for forecasting breast cancer metastasis in comparison with CD44+/CD24− (Zhong et al. 2014). Interestingly, 1500 ALDH + cells without the CD44+CD24−/low phenotype were capable of initiating tumors in immunodeficient mouse, while CD44+CD24−/low ALDH – cells did not, suggesting that ALDH1 is an more effective marker to characterize BCSCs compared with CD44+CD24−/low (Ginestier et al. 2007; Liu et al. 2014). In addition, a tiny amount of ALDH1-positive cells also express CD44+CD24−/low marker, which were capable of producing tumors in vivo more frequently (Ginestier et al. 2007; Liu et al. 2014). After examining their ability of self-renewal and tumorigenesis, high CD44/CD24 ratio combined with ALDH1+ were proposed to be alternative markers which were able to identify BCSCs more accurately (Li, Ma, et al. 2017).
CD133, a transmembrane glycoprotein, is also an important surface marker of BCSCs. It was conceived as a specific marker of hematopoietic stem cells at first (Yin et al. 1997; Tume et al. 2016). However, growing evidences show that CD133 is also expressed or hyper-expressed in many cancers including breast cancer (Liu et al. 2013; Jang et al. 2017). Moreover, CD133+ cells in breast carcinoma are often characterized by higher drug tolerance, invasive and metastatic capacity, higher tumor-initiating and self-renewal ability (Liu et al. 2013; Tume et al. 2016; Sansone et al. 2016), suggesting that CD133 is closely related to stemness of BCSCs. In addition, CD133 is up-regulated and facilitates multidrug resistance by enhancing phosphatidylinositol-3-kinase (PI3K)-Akt signaling (Xi et al. 2016). Also, CD133 is involved in tumor metastasis via promoting epithelial-mesenchymal transition (Ding et al. 2014). In terms of the clinical manifestation, people suffering CD133+ mammary neoplasm usually have higher mortalities, poorer overall survival and disease-free survival (Li, Yin, et al. 2017), and nuclear translocation of CD133 may be responsible for poor prognosis of patients with triple-negative breast cancer (Cantile et al. 2013). Thus, CD133+ might be a useful surface marker for identifying BCSCs as well as a helpful indicator for predicting prognosis.
Other markers such as Protein C receptor (PROCR)+, MUC1+/CD24+, CD44+/Vimentin+, CD44+/Osteonectin+, CD24+/CK18+, CD24+/GATA3+, PROCR+/ESA+, and proteosomelow have also been proposed (Engelmann, Shen, and Finn 2008; Vlashi et al. 2009; Park et al. 2010; Pece et al. 2010; Wang, Cai, et al. 2015). Nevertheless, studies on BCSCs markers are still ongoing owing to the fact that BCSCs markers often vary depending on diverse subtypes of breast cancer cells, histologic stages and heterogeneity within tumors (Tsang et al. 2012). In addition, clinical relevance of these identified BCSCs biomarkers remains controversial and requires further studies (Lu et al. 2011).
Signaling pathways regulating BCSCs self-renewal
CSCs share the characteristics with other stem cells such as self-renewal and differentiation. By symmetric or asymmetric cell division, CSCs can repopulate themselves and generate clonal daughter cells (Li et al. 2011). Several key pathways are implicated in regulating BCSCs self-renewal including Wnt/β-catenin, Notch, Hedgehog and Transforming growth factor-β (TGF-β) pathways.
Wnt/β-catenin signaling
Wnt/β-catenin signaling, a highly conserved pathway, plays a vital role in modulating cell propagation and differentiation (Kahn 2014). However, this pathway is commonly deregulated and aberrantly activated during carcinogenesis (Anastas and Moon 2013), which promotes clonal expansion. In breast cancer, this aberrant signaling facilitates selfrenewal and associated properties (metastasis, multi-drug resistance, invasiveness) of BCSCs. Blocking Wnt/β-catenin pathway by knocking down miR-142, a potent effector for activating this signaling, decreased tumor-initiating ability of BCSCs and mammosphere formation by BCSCs (Isobe et al. 2014). Similarly, stemness, self-renewal and proportion of BCSCs were significantly reduced when treated with pyrvinium pamoate, a suppressor of Wnt pathway (Xu et al. 2016). In addition, inhibiting Wnt/β-catenin by Let-7 could make BCSCs more chemosensitive to tamoxifen accompanied by reduced self-renewal and tumorigenic ability (Sun et al. 2018). Thus targeting Wnt/β-catenin signaling would be an effective strategy to suppress BCSCs.
Notch signaling
Notch signaling is associated with cell differentiation, propagation and is indispensible in the development of human organs (Miele 2006). In tumors, the signaling is frequently hyperactive and closely related to self-renewal of CSCs including BCSCs (Bu et al. 2013; Pal et al. 2017). BCSCs bearing ALDH+ express a higher level of Notch-1, a receptor of Notch signaling, which also promotes EMT. However, the promoting effect is inhibited when Notch-1 is suppressed by Psoralidin (Pal et al. 2017). Similarly, both receptors (Notch-1,-2) and ligands of Notch signal are highly expressed in renal CSCs, meanwhile, enforced expression of Notch-1 makes renal CSCs more chemoresistant and upregulatse self-renewal ability, suggesting Notch signaling facilitates self-renewal and stemness of CSCs (Xiao et al. 2017). In addition, disrupting Notch signaling by γ-secretase suppressor in mammary tumor could markedly decrease the number of BCSCs (Mamaeva et al. 2016). In short, Notch signaling is also an effective target for inhibiting BCSCs.
Hedgehog signaling
Hedgehog signaling is critical for morphogenesis during embryonic development (Gritli-Linde et al. 2002; Monkkon and Lewis 2017). However, deregulation of this pathway is associated with carcinogenesis, and the signaling is abnormal and hyperactive in nearly 1/4 human cancers, including mammary cancer (Monkkon and Lewis 2017). Hedgehog signaling is also closely related to self-renewal ability, oncogenicity and stemness of CSCs/BCSCs. Consistently, members in hedgehog signaling were highly expressed in BCSCs (cells bearing CD24+/CD24−/Lin−), and the highly activated hedgehog signaling promoted self-renewal of BCSCs, which was illustrated by increased size and number of mammosphere in vitro and tumor in vivo (Liu et al. 2006). In addition, activation of hedgehog signaling by long non-coding RNAs promoted BCSCs generation, increased the expression of stemness markers (SOX2 and OCT4) and tumorigenicity of BCSCs (Zhou et al. 2016). On the other hand, when hedgehog signaling is suppressed by nitidine chloride (Sun et al. 2016), genistein (Fan et al. 2013), salinomycin (Lu et al. 2015), properties and numbers of BCSCs are also inhibited. Therefore, regulating the abnormal hedgehog signaling is a promising way to fight against BCSCs.
Transforming growth factor-β (TGF-β) signaling
Transforming growth factor-β (TGF-β) signaling is indispensable for proper development of tissues and organs. In breast cancer, this signaling is associated with metastasis (Zhao et al. 2018), migration and invasion (Zhao et al. 2018). TGF-β signaling is frequently activated in BCSCs and contributes to maintaining quiescent state of BCSCs (Tang et al. 2015). In addition, TGF-β signaling was responsible for the augment of EMT and self-renewal of BCSCs induced by paclitaxel, which could be abolished by suppressing TGF-β signaling (Park et al. 2015). Studies focusing on the effects of this signaling on BCSCs are limited, though many studies about promoting effects of the signaling on CSCs self-renewal have been reported in other cancers (Fu, Li, and Hao 2017).
Epigenetic mechanisms associated with BCSCs self-renewal and differentiation
Though analogous to normal stem cells, these pathways mentioned above modulating self-renewal and differentiation are commonly aberrant in CSCs, due to epigenetic and/or genetic alterations. In normal stem cells, promoters of genes regulating cell differentiation usually contain the bivalent marks: H3K4me3 for gene activation and H3K27me3 for gene suppression (Bernstein et al. 2006). When normal stem cells differentiate, the H3K27me3 marker of the lineage specific genes is removed while the H3K4me3 marker of the target genes is retained, initiating a specific differentiation program (Boyer et al. 2006; Sauvageau and Sauvageau 2008). While in CSCs, the genes regulating differentiation are normally suppressed by epigenetic suppression, including aberrant DNA methylation and H3K27 methylation catalyzed by enhancer of zeste homolog 2 (EZH2), the methyltransferase of the PRC2 complex, thereby blocking differentiation and permanently positioning cells to self-renewal (Easwaran et al. 2012). Indeed, EZH2 is commonly overexpressed in many types of cancers (glioblastoma cancer, acute myeloid leukemia, small cell lung cancer and others), which causes blockage of differentiation and is relevant to unfavorable prognosis (Suva et al. 2009; Tanaka et al. 2012; Sato et al. 2013). Meanwhile anti-cancer genes are usually inhibited by abnormal DNA methylation in both leukemia (Rahmani et al. 2018) and solid tumors (Lv et al. 2015; Liang and Weisenberger 2017).
Additionally, microRNAs (miRNAs) are endogenous and non-coding small RNAs with approximately 21–25 nucleotides in lengths. MiRNAs often function as a modulator of gene expression (Bartel 2004; Ha and Kim 2014). Consistently, miRNAs are also dysregulated in cancer cells via epigenetic mechanisms, rendering CSCs to undergo self-renewal instead of differentiation (Tuna, Machado, and Calin 2016). Restoring the expression of miR-34a, an important regulator of stem cell-like characteristics in human pancreatic CSCs, reduces CSCs characteristics including proliferation, self-renewal and EMT (Nalls et al. 2011). Based on their effects on CSCs, there are two categories of miRNAs: pluripotent miRNAs and pro-differentiation miRNAs. Pluripotent miRNAs such as miR-290 and miR-9 favor self-renewal and proliferation of stem cells but attenuate differentiation (Wang, Du, et al. 2013). Pro-differentiation miRNAs (let-7 and miR-470) facilitate differentiation (Wang, Du, et al. 2013). MiRNAs may target Wnt/β-catenin signaling, Notch signaling and other factors to regulate CSCs self-renewal and differentiation. For examples, miRNAs (miR-142 and miR-663) are able to suppress adenomatous poliposis coli (APC), which activate canonical Wnt signaling (Wang and Xu 2010; Isobe et al. 2014). In BCSCs, let-7 miRNAs are decreased, but upregulated during differentiation; it is up-regulation hinders BCSCs proliferation, mammosphere formation, neoplasm growth as well as metastasis in NOD/SCID mice, showing that let-7 suppresses BCSCs self-renewal (Yu et al. 2007). In human BCSCs, the expression of miR-146 is increased (Shimono et al. 2009). The Wnt receptors Frizzled-6 expression levels and low lipoprotein receptor related protein 6 are augmented through downregulation of Zinc Ring finger 3 by miR-146, thereby activating Wnt signaling (Deng et al. 2015). MiRNAs also regulate Notch signaling pathway. MiR-146a, which is upregulated in human BCSCs, activates Notch signaling pathway by targeting Numb, an inhibitor of this pathway (Kuang et al. 2009; Forloni et al. 2014). MiRNA-34a which is downregulated in human breast cancer, is capable of suppressing Notch signaling pathway, thereby inhibiting self-renewal ability, metastatic potential as well as drug resistance of BCSCs (Kang et al. 2015). In addition, Hippo signaling pathway is modulated via miRNAs in breast cancer cells as well. MiRNA-125a indirectly modulates tafazzin, which is a typical effector of Hippo signaling, by targeting leukemia inhibitory factor receptor (LIFR). The miR-125a-LIFR axis could effectively regulate the homeostasis of nonmalignant and malignant breast epithelial stem cells via altering the Hippo signaling (Nandy et al. 2015). Transcription factors required for maintaining pluripotency including Oct4, Sox2, and Nanog could be targeted by miRNAs such as miR-296, thus tilting the balance between self-renewal and differentiation (Tay et al. 2008). Also, miR-590-5p down-regulates Sox2 expression, suppressing BCSCs property (mammosphere formation, tumorigenicity) (Zhou et al. 2017).
Food-derived polyphenols targeting BCSCs
Accumulating studies confirm that dietary polyphenols are promising agents to fight against BCSCs. The inhibitory effects of food-derived polyphenols on BCSCs include decreasing percentage of ALDH + cells, CD44+/CD24− cells or cells bearing other BCSCs markers, and reducing mammosphere formation and tumorigenesis in vivo (Dontu et al. 2003). These reported roles of food-derived polyphenols targeting BCSCs by various mechanisms including blocking pathways enhancing BCSCs self-renewal (Wnt/β-catenin and Hedgehog signaling), altering BCSCs niches (Signal transducer and activator of transcription3(STAT3)-mediated inflammatory signaling, changing growth factors such as hypoxia-inducible factor (HIF) and vascular endothelial growth factor (VEGF), suppressing ATP-binding cassette (ABC) drug transporters (ABCG2, ABCC1 and P-glycoprotein), regulating anti-cancer miRNAs (miR-16, -200c, miR148a), downregulating heat shock protein 27 and heat shock 70kDa protein 5, decreasing estrogen receptor-α36 (ER-α36),and activating intracellular signaling pathways including RAS/ERK and PI3K/AKT pathways, and suppressing adipogenesis and lipid metabolism (Figure 1). The inhibitory effects of dietary polyphenols on BCSCs and potential molecular targets are summarized below (Table 1).
Figure 1.
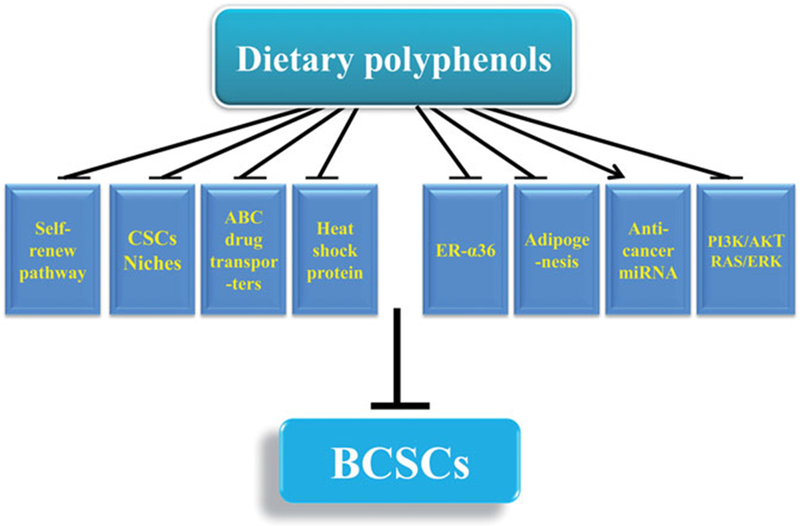
Mechanisms contributing to anti-BCSCSs effects of dietary polyphenols.
Note:  represents inhibition;
represents inhibition;  represents promotion.
represents promotion.
Table 1.
Anti-BCSCs effects of food-derived polyphenols.
| Compound | Polyphenol concentration | Effects on CSCs | Molecular targets | References |
|---|---|---|---|---|
 |
15 μM | inhibit migration, invasion and EMT |
a↑E-cadherin/β-catenin complex; ↓nuclear β-catenin; b↑epithelial markers (E-cadherin, cytokeratin-18 and -19); ↓β-catenin transcriptional targets (cyclin D1, c-Myc, and Slug); ↓EMT-promoting factors (vimentin, MMP-2 and MMP-9) |
(Mukherjee et al., 2014) |
 |
10–40 μM 100 mg/kg/d |
inhibit mammosphere formation; induce autophagy; inhibit tumor growth in vivo; inhibit ALDH + BCSCs tumorigenicity in vivo | ↓β-catenin and cyclin D1 ↑LC3-II, Beclin1 and Atg7 |
(Fu et al. 2014) |
| 50 μM 25 mg/kg/d |
reduce invasive ability inhibit tumor growth | ↑miR-16, -141, -143, and -200c ↑Argonaute2 (Ago2) ↓Zeb1 ↑E-cadherin |
(Hagiwara et al. 2012) | |
| 50-100 μM 22.4 kg/body weight |
reduce mammosphere formation; reduce breast cancer cells viability; induce BCSCs apoptosis; inhibit tumor growth in vivo suppress lipid syntheses | ↓fatty acid synthase ↑pro-apoptotic genes (DAPK2, BNIP3) |
(Pandey et al. 2011) | |
| 50-100 μM | decrease BCSCs proliferation; induce BCSCs apoptosis; down-regulate lipogenesis; inhibit mammosphere formation; | ↓SREBP1 ↑pro-apoptotic genes (DAPK2, BNIP3) ↓SREBP1 downstream genes (ACLY, ACC1 and FAS) | (Pandey et al. 2013) | |
| 25,50 μM | attenuate migrational and invasive ability decrease proportion of BCSCs inhibit mammosphere formation | ↓Cyclin D1and c-Myc ↓MMP-2 and -9 ↓Sox-2 and CD44 ↓p-Akt and p-Stat3 |
(Suh, Kim, and Surh 2018) | |
| Resveratrol analog HS-1793 | 0.6–2.5 μM 1.5 mg/kg |
reduce invasive ability enhance radiosensitivity and apoptosis induced by radiotherapy | ↓Oct4, KLF4 and Sox2 protein ↓VEGF ↓HIF-1α |
(Choi et al. 2016) |
 |
12.5–50 μM | inhibit size and number of mammo-spheres; decrease population of ALDH + cells; suppress EMT and migration; | ↓Hsp27 ↓N-cadherin and twist ↑E-cadherin |
(Li et al. 2011) |
| 12.5-50 μM | repress BCSCs inhibit tumor growth inhibit VM capability | ↓Hsp27 | (Lee et al. 2014) | |
| 20 μM | inhibition migration and population of ALDH + cells | ↓Hsp27 | (Lee et al. 2012) | |
| 0.7 μM | decrease population of CD44+/CD24− cells improve chemosensitivity | ↓nuclear Y-box binding protein 1 ↓P-glycoprotein |
(Li, Zhao, Wang, Yuan, et al. 2018) | |
| 50 μM | decrease population of CD44+/CD24− cells inhibit mammosphere formation inhibit tumor growth | ↓PI3K/Akt/mTOR | (Li, Zhou, Wang, Liu, et al. 2018) | |
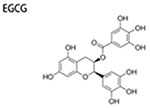 |
10-40 μM | inhibit mammosphere formation; inhibit CD44+/CD24− cells | ↓ER-α36 ↓EGFR |
(Pan et al. 2016) |
| 20–160 μg/mL 16.5 mg/kg |
induce apoptosis of the ALDH + SUM-149 cells; inhibit mammosphere formation; inhibit tumor growth in xenograft mouse model | ↓lymphangiogenesis-promoting genes ↓VEGF-D |
(Mineva et al. 2013) | |
| EGCG analogs | 10,20 μM | inhibit mammosphere formation; inhibition CD44+ high/CD24− low cells | (Chen et al. 2012) | |
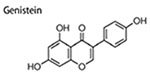 |
0-30 μM 0–50 mg/kg |
inhibit mammosphere formation; decrease ALDH + levels in xenograft tumors; decrease CD44+CD24− population; | ↓Smo ↓Gli1 |
(Fan et al. 2013) |
| 2 μM, and 40 nM | induce differentiation of BCSCs; decrease CD44+/CD24−/ESA+ cells; | ↑differentiated cell markers (E-cadherin, α-smooth muscle actin and Claudin-1 genes) ↓stem cell markers (Fibronectin and Snail); ↑ phospho-Akt308/473 ↑ phospho-ERK1/2 ↑Amphiregulin ↑phospho-β-catenin |
(Liu et al. 2016) | |
| 2 μM, and 40 nM | inhibit adipogenesis inhibit mammosphere formation; | ↑mammary tumor suppressor (PTEN and E-cadherin) ↓Pparγ ↓fatty acid synthase ↑estrogen receptor b |
(Montales et al. 2013) | |
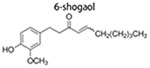 |
2 μM, and 40 nM 1–40 μM |
inhibit mammosphere formation inhibit primary and secondary mammo-sphere formation; decrease CD44+/CD24− cells proportion; |
↑PTEN ↓Cleaved Notch1 ↓Hes1 and Cyclin D1 |
(Montales et al. 2012) (Ray, Vasudevan, and Sengupta 2015) |
| 5-25 μM | inhibit mammosphere formation; decrease CD44 expression of BCSCs; increase chemosensitivity of BCSCs; induce cell necrosis phenomena; | ↓hedgehog ↑p-β-catenin ↓CD44 ↓c-Myc ↓cyclin D1 |
(Wu, Hong, et al. 2015) | |
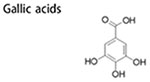 |
– | inhibit metastasis; inhibit tumor growth; inhibit mammosphere formation; suppress cell migration and mobility | ↓PI3K/AKT signaling ↓MAPK/ERK signaling ↓STAT3 signaling |
(Vuong et al. 2016) |
 |
10 μM 20 mg/kg Body Weight |
decrease mesenchymal features; inhibit mammosphere formation; inhibit tumor growth; | ↓TGFβ-SMADs Signal ↑miR-148a ↓DNMT1 and DNMT3a |
(Jiang et al. 2016) |
 |
25, 50 μM 50 mg/kg/d |
inhibit self-renewal and multi-differentiation; reduce BCSCs proportion; inhibit tumor growth; | ↓heat shock 70kDa protein 5 ↓β-catenin/ABCG2 signaling |
(Wang Wang, et al. 2014) |
| 25, 50 μM 50 mg/kg/d |
block self-renewal BCSCs; reduce BCSCs population; induce BCSCs G0/G1 phase arrest; inhibit tumor growth; | ↓DNMT1 methyltransferase ↑WIF1 ↓β-catenin signaling |
(Wang Wang, et al. 2015) | |
| Curcumin + piperine | 5,10 μM | inhibit mammosphere formation; reduce the percent of cells bearing ALDH1+ | ↓Wnt signaling | (Kakarala et al. 2010) |
| Curcumin + Mitomycin C | 5–40 μM 100 mg/kg |
nhibit mammosphere formation; reduce cells expressing CD44+CD24−/low; inhibit tumor growth in vivo | ↓ABCG2 and ABCC1 | (Zhou et al. 2015) |
| Curcumin + EGCG | 10 μM, 10 μM | inhibit mammosphere formation; inhibit invasion; reduce CD44−expressing subpopulation | ↓p-STAT3 ↓nuclear STAT3-NFkB |
(Chung Seyung 2015) |
↓Represents down-regulation;
↑Represents down-regulation.
Abbreviations: ACC1, acetyl CoA carboxylase 1; ALDH, aldehyde dehydrogenase; ACLY, ATP-citrate lyase; BCSCs, breast cancer stem cells; EMT, epithelial mesenchymal transition; EGFR, epidermal growth factor receptor; ER-α36, estrogen receptor-α36; FAS, fatty acid synthase; GSK3β, glycogen synthase kinase 3β; Hsp, Heat shock protein; MMP, metal matrix proteinase; PPARγ, Peroxisome proliferator-activated receptor γ; SREBP1, sterol regulatory element binding proteins1; VEGF, vascular endothelial growth factor.
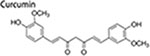
Curcumin
Curcumin is a dietary polyphenol from turmeric with anticarcinogenic effects (Wang et al. 2016; Wang, Hang, et al. 2017. Curcumin is also an effective natural phytochemical inhibiting the proliferation of BCSCs together with suppressing the bulk breast cancer cells (Li and Zhang 2014). Curcumin can reduce CSCs in various cancers by inhibiting signaling pathways promoting self-renewal, particularly the Wnt/β-catenin signaling (Li and Zhang 2014; Wu, Guo, et al. 2015; Yang, Wang, et al. 2017; Zhu, Yang, et al. 2017). Curcumin inhibited BCSCs migration by inhibiting β-catenin nuclear translocation, attenuating the expression of β-catenin transcriptional targets including pro-EMT factors, along with the increased formation of E-cadherin/β-catenin complex and suppressed expression of epithelial markers (Mukherjee et al. 2014). In another study, treatment with curcumin and piperine separately or jointly repress mammosphere formation, reduce the percentage of ALDH1+ cells, and also block secondary/tertiary mammospheres formation by BCSCs. These effects are mediated by suppression of dysregulated Wnt signaling (Kakarala et al. 2010). Additionally, in BT-549 breast cancer cells, curcumin-load POCA4C6 micelles inhibited BCSCs by interruption of androgen receptor signaling and Wnt/β-catenin signaling (Chen, Li, et al. 2017).
Apart from regulating self-renewal pathway of BCSCs, curcumin also attenuates BCSCs by other mechanisms. Generally, BCSCs are resistant to chemotherapy, which is partially caused by ATP-binding cassette (ABC) drug transporters. Curcumin was able to inhibit ABCG2 and ABCC1, thereby decreasing anti-cancer efficiency of chemotherapeutic agents, and combined use of curcumin and Mitomycin C effectively eliminated CD44+CD24−/low cells as well as inhibited BCSCs-derived tumor growth (Zhou et al. 2015). Likewise, MCF-7 cells co-treated by curcumin (10 μM) and EGCG (2 μM) were susceptible to apoptosis and sensitive to doxorubicin treatment (Wang, Chen, et al. 2014).
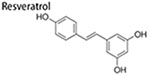
Resveratrol
Resveratrol is one of the most studied polyphenols with anti-cancer effects including suppressing CSCs (Sahin et al. 2016; Chen, Lien, et al. 2017; McCubrey et al. 2017). Resveratrol could preferentially attenuate BCSCs, which was illustrated by suppression of ALDH + cells proportion in SUM159 and MCF-7 cell lines and xenograft breast tumors. Resveratrol also inhibited mammospheres formation and tumorigenicity of BCSCs, and facilitate autophagy at the same time, primarily by means of disrupting Wnt/β-catenin pathway (Fu et al. 2014). In another study, MR-3, a natural methoxylated compound of this polyphenol, was found to suppress invasive ability of breast cancer cells (MCF-7), owing to the blockage of EMT progress by attenuating PI3K/AKT pathway and reducing levels of nuclear β-catenin (Tsai et al. 2013). Additionally, cancer cells commonly show up-regulated expression of adipogenic genes, which may promote energy supply to rapidly growing tumor cells through enhanced β-oxidation and also lipids needed for membrane synthesis (Kuhajda 2000). Hence, suppression of adipogenic gene expression inhibits tumorigenesis of BCSCs. Resveratrol induces apoptosis and inhibits mammosphere formation and xenograft tumor growth of BCSCs (CD24−/CD44+/ESA+), which is associated with suppression of lipogenesis (Pandey et al. 2011). Similarly, key lipogenic genes were upregulated in ductal carcinoma and its cancer stemlike cells, which was disrupted by resveratrol in a xenograft mice model (Pandey et al. 2013). Emerging evidences show that various miRNAs are potential molecule targets to inhibit BCSCs, such as suppression of brain metastasis of BCSCs by miR-7 (Okuda et al. 2013), increasing tumorigenicity of BCSCs by miR-221/222 (Li et al. 2016), inhibiting BCSCs stemness and drug resistance by miR-375 or miR-519d (Okuda et al. 2013; Li et al. 2016; Fu, Li, and Hao 2017; Shindo et al. 2017; Xie et al. 2017). Resveratrol could also promote the expression of anti-cancer miRNAs such as miR-16 and -200c, thus inhibiting BCSCs stemness. Moreover, resveratrol increased the level of Argonaute2, potentiating tumor-suppressive function of miRNAs (Hagiwara et al. 2012). Besides resveratrol is capable of repressing BCSCs through restoring BCSCs niches, including suppressing the secretion of HIF and VEGF, and proliferation of cancer-associated fibroblasts. HIF regulates oxygen homeostasis, which is implicated in drug resistance and the consolidation of cancer microenvironment (Warfel and El-Deiry 2014). HIF promotes VEGF that is indispensable for sustaining tumor growth (Auguste et al. 2005; Liu et al. 2012). HS-1793, a resveratrol analog, reduced the expression of HIF-1a and VEGF under hypoxic conditions in mouse breast cancer FM3A cells, and suppressed hypoxia-induced CSCs properties, including decreased invasive ability along with suppressed levels of stemness markers (Oct4, Klf4 and Sox2 proteins). Furthermore HS-1793 also promoted apoptosis initiated by ionizing radiation in hypoxic FM3A cells (Choi et al. 2016). Cancer-associated fibroblasts, the key component of tumor microenvironment or CSCs niche, contribute to malignancy of cancer cells and self renewal activity of CSCs (Adisetiyo et al. 2014). Resveratrol was able to abolish metastatic and invasive ability, inhibit mammosphere formation of breast cancer cells and decrease BCSCs proportion by suppressing cancer-associated fibroblasts (Suh, Kim, and Surh 2018).
Genistein
Genistein, a dietary isoflavone phytoestrogen mostly found in soybeans, shows promising antitumor effects (Banerjee et al. 2008). Daily consumption of genistein reduces the incidences of breast cancer among women in eastern countries (Iwasaki et al. 2008). Genistein reduces CSCs in mammary cancer (Liu et al. 2016), ovarian cancer (Ning et al. 2014) and gastric cancer (Yu et al. 2014). Genistein inhibited the number and size of mammospheres and decreased the percentage of cells bearing CD44+CD24− in treated MCF-7 cells, and also lowered the proportion of ALDH + cells in xenograft tumors of genistein treated nude mice, which might be due to the suppression of Hedgehog-Gli1 pathway in vivo (Fan et al. 2013). Like resveratrol, genistein could also decrease mammary adipogenesis and upregulate phosphatase and tensin homolog (PTEN) and E-cadherin, both of which are mammary tumor suppressors, in female mice; genistein-treated adipocytes suppressed the formation of MCF-7 mammospheres (Montales et al. 2013). BCSCs are more multi-drug resistant than differentiated cancer cells, a feasible measure to suppress BCSCs is facilitate them differentiation. Genistein (2 μM or 40 nM) could induce the differentiation of BCSCs, confirmed by adverse morphological alteration of mammospheres and upregulated expression of differentiated cell markers in MCF-7 cells, which might attribute to the suppression of PI3K/Akt and MEK/ERK signaling by genistein (Liu et al. 2016). Additionally, genistein inhibited mammospheres formation by MCF-7 and MDA-MB-231 cells partially through suppressing PTEN/PI3K/Akt pathway (Montales et al. 2012).
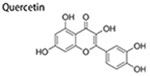
Quercetin
Quercetin, a dietary flavonoid found in many plant foods, displays excellent antitumor activity (Kashyap et al. 2016). Recently, small heat shock proteins 27 (HSP27), a member of a heat shock proteins family, was found to be beneficial to maintain CSCs, and CSCs stemness can be inhibited by downregulation of HSP27 (Lu et al. 2016; Yasuda et al. 2017). Moreover, both Hsp27 and phosphorylated Hsp27 were upregulated in ALDH + BCSCs compared with ALDH-non-BCSCs. Quercetin could act as an inhibitor of Hsp27, thereby decreasing self-renewal of BCSCs and reducing the population of ALDH + cells (Li et al. 2011). Similarly, 20 μM of quercetin displayed a synergistic effect with a low dose of geldanamycin, a Hsp90 inhibitor, in suppression of migration and population of ALDH + BCSCs, further illustrating the synergistic effects of Hsp90 and Hsp27 suppression in inhibition of BCSCs tumorigenesis (Lee et al. 2012). In addition, vascularization of tumors was suppressed by quercetin through targeting epidermal growth factor (EGF)/Hsp27 signaling (Lee et al. 2014). Apart from targeting Hsp27, quercetin was able to effectively reduce number and stemness of BCSCs through blocking PI3K/Akt/mTOR, a pathway regulating self-renewal of CSCs (Li, Zhou, Wang, Liu, et al. 2018). Furthermore, P-glycoprotein pumping drugs out of cells, is also responsible for multidrug resistance of cancer cells, and nuclear translocation of Y-box binding protein 1 contributes to upregulation of P-glycoprotein and stemness of CSCs (Oda et al. 2003; Oda et al. 2007; To et al. 2010). Quercetin could block Y-box binding protein 1 nuclear translocation and down-regulate P-glycoprotein, thus improving chemosensitivity of breast cancer cells and inhibiting proportion of BCSCs (Li, Zhao, Wang, Yuan, et al. 2018).
EGCG
EGCG, the primary bioactive polyphenol in green tea, exhibits potent effects on fighting against cancers, and inhibits CSCs in prostate cancer, lung cancer and others (Fujiki et al. 2017). EGCG also attenuates BSCSs by various mechanisms. STAT3 mediates cytokine-induced signaling, which forms a feedforward circuit with IL6/Nuclear Factor-κB (NF-κB) axis, promoting the tumorigenesis of BCSCs (Iliopoulos, Hirsch, and Struhl 2009; Kise, Kinugasa-Katayama, and Takakura 2016). Hence, targeting STAT3 can inhibit stemness of BCSCs. Consistently, combined treatment of 10 μM EGCG and 10 μM curcumin decreased the formation of mammospheres and CD44-expressing subpopulation, suppressed cell invasiveness of MDAMB-231 and MCF7-HER2 cells. Moreover, Co-treatment with EGCG and curcumin blocked STAT3 phosphorylation, preventing STAT3-mediated inflammatory signaling and suppressing CD44 expression of BCSCs (Chung Seyung 2015). Targeting ER-α36 is also a potential way to eradicate BCSCs. ER-α36, a variant of ERα, is highly expressed in ER− breast cancer cells (Zhang et al. 2011), which is critical to keep stemness of both ER+ and ER− breast cancer cells (Kang et al. 2011; Deng et al. 2014). Accordingly, 20 μM EGCG could effectively decrease the expression of ER-β36, thereby inhibiting tumor sphere growth and reducing CD44+/CD24− cells population of ER− breast cancer cells (Pan et al. 2016). Additionally, EGCG can inhibit BCSCs by blocking lymphangiogenic factor and lymphangiogenesis. VEGF-D is a critical lymphangiogenic factor, which promotes lymphangiogenesis and lymph node metastasis of cancers (Stacker et al. 2014). Lymphatic vessels facilitate tumor metastasis (Li and Li 2015), and lymphatic metastasis is a major form of tumor metastasis. EGCG downregulated expression of VEGF-D which inhibited the lymphangiogenic potential, robustly inhibiting mammosphere formation and neoplasm growth in vivo (Mineva et al. 2013). Octa-acetate derivative of EGCG also displayed inhibiting effects on BCSCs self-renewal, confirmed by reduced mammospheres formation (Chen et al. 2012).
Besides, EGCG could dose-dependently repress Wnt signaling by targeting HMG-box protein 1, a suppressor of Wnt signaling, thus attenuating tumorigenicity and invasiveness of mammary cancer cells (Kim et al. 2006), which suggested that EGCG was capable of inhibiting self-renewal of BCSCs. In addition, EGCG is capable of suppressing CSCs self-renewal in other cancers (Zhu, Jiang, et al. 2017; Fujiki et al. 2018).
6-Shogaol
6-Shogaol, derived from dried ginger, has potent anticancer activity (Warin et al. 2014; Anwar et al. 2016). 6-Shogaol blocks Hedgehog and/or Wnt/β-catenin in BCSCs, which reduces their primary and secondary mammospheres formation and decreasing CD44+CD24−/low cells in mammospheres. Moreover, downregulation of Notch signaling is also involved in anti-BCSCs effects of 6-shogaol (Ray, Vasudevan, and Sengupta 2015). These polyphenolic compounds disrupt hedgehog and Akt/GSK3β signaling pathway, thereby attenuating stemness of BCSCs (Wu, Hong, et al. 2015).
Other dietary polyphenols
Gallic acids, commonly found in fruits (pomegranate, mango and blueberry), have various biological activity (Kosuru et al. 2018). Fermented blueberry juice that is rich in Gallic acids could effectively attenuate self-renewal ability and migration potential of BCSCs by preventing IL6/STAT3 axis, a typical inflammatory signaling (Vuong et al. 2016). Glabridin, a polyphenol extracted from Glycyrrhiza glabra, is a promising anti-cancer agent. Recently, Jiang et al reported that glabridin (10 μM) could potently suppress BCSCs-like properties such as reducing mammospheres formation, ameliorating mesenchymal phenotype and inhibiting tumorigenesis in vivo. These restraining effect ascribed to up-regulation of miR-148a by glabridin, which further inhibited TGFμ-SMADs Signaling, a pathway associated with BCSCs-like characteristics (Jiang et al. 2016). Isoliquiritigenin, a natural polyphenol in licorice, exerts anti-tumor effects in lung cancer (Jung et al. 2014), bladder cancer (Moreno-Londono, Bello-Alvarez, and Pedraza-Chaverri 2017) and breast cancer (Peng et al. 2017). Isoliquiritigenin could make BCSCs more chemosensitive and inhibit BCSCs propagation and self-renewal by directly targeting heat shock 70 kDa protein 5, suppressing β-catenin/ABCG2 axis (Wang, Wang, et al. 2014). Besides isoliquiritigenin could also attenuate BCSCs by epigenetic regulation. Isoliquiritigenin effectively decreased BCSCs proportion, led to BCSCs arrest and blocked tumor growth in vivo by blocking DNA methyl-transferase1, which consequently led to up-regulation of Wnt inhibitory factor by means of promoter demethylation (Wang, Wang, et al. 2015).
Nano-technology to enhance polyphenol bioavailability against BCSCs
Although polyphenols mentioned above display excellent anti-cancer effects, the effectiveness of dietary polyphenols is limited due to their instability and lower bioavailability (Naksuriya et al. 2014). Thus, alternative delivery methods are needed, of which nano-drug delivery system is a good choice. These nano-durgs, also called nano-formulas, are often developed by coating hydrophobic or low bioavailable drugs with nanocarriers, and the size of which are often 10–100 nm, thus enhancing the solubility of hydrophobic chemotherapeutic agents, promoting drug permeation into neoplasm, lowering adverse effects and facilitating targeted delivery of therapeutic agents (Naksuriya et al. 2014; Fatima et al. 2016). Up to now, the reported nano-drugs made of dietary ployphenols targeting BCSCs are mainly curcumin nano-drug, and they are summarized in Table 2. Curcumin nano-medicine (C-SSM) is composed of curcumin coated with a biodegradable and no-toxic wall material (polyethylene glycol-grafted phospholipids), and the surface of the nano-medicine was further conjugated with vasoactive intestinal peptide (VIP), which binds to a receptor commonly found on the surface membranes of cancer cells, thus achieving targeted delivery. Indeed, the nano-medicine displayed lower IC50 value than free drug. This curcumin nano-drug (5 μM) effectively suppressed mammosphere formation, demonstrating that targeting BCSCs by the new nano-formula is promising (Gulcur et al. 2013). In another study, both biocompatible and stable nanoparticle delivery systems for curcumin were developed by embedding curcumin with amphiphilic phosphorylated calixarene POCA4C6. This complex could gradually release curcumin depending on pH with minimal toxicity in vivo. Compared with free curcumin, it was more potent to reduce proliferation, invasive and migrating capacity of BT-549 cancer cells and tumor formation in xenografted mouse, and also reduced the population of CD44+/CD133+ cells (Chen, Li, et al. 2017). Given that CSCs commonly exist in the internal space of tumor, where is difficult for hydrophobic drug to reach owing to the lack of capillaries, specific ligands have been used in nano-drug systems to facilitate their internalization into tumors (Torchilin 2014). Meanwhile, because most of BCSCs highly express CD44, which is a hyaluronic acid, nano anti-BCSCs drugs which could effectively bind to CD44 has been developed. A cholesteryl-hyaluronic acid (CHA) nanogel-drug conjugates embedding curcumin displayed promising effects in suppressing the malignancy of floxuridine-resistant MDA-MB-231 which highly expresses CD44. This novel conjugates were capable of penetrating into mammary tumor effectively and showed more potent cytotoxicity in comparison with free drugs (Wei et al. 2013). Similarly, a more intelligent and effective curcumin delivery system against BCSCs was designed by Yang and colleagues. This co-releasing paclitaxel and curcumin nano-drug could automatically change its surface charge, remove its polyethylene glycol coat to decrease its size during circulation and reaching tumor sites. The new drug could more effectively inhibit mammospheres formation of MCF 7 breast cancer cell line, and reduce CD44+/CD24− and ALDH1+ cancer cells in mammospheres compared with the traditional combination therapy of paclitaxel (Yang, Sun, et al. 2017).
Table 2.
Anti-BCSCs effects of food-derived nano-polyphenols.
| Nano-phytochemicals | Phytochemicals concentration | Effects on CSCs | Molecular targets | References |
|---|---|---|---|---|
| Curcumin-loaded POCA4C6 micelles (CPM) | 3 μM, 5 mg/kg | inhibit proliferation, invasion, migration, tumor spheroid formation; induce apoptosis; | ↓nuclear β-catenin; ↓androgen receptor | (Chen, Li, et al. 2017) |
| co-releasing paclitaxel and curcumin micellar system(PPBV micelles) | 0–2.5 μg/mL 10 mg/kg |
inhibit mammosphere formation; reduce the percent of cells bearing ALDH1+ or CD44+/CD24−; inhibit tumor growth | (Yang, Sun, et al. 2017) | |
| CHA nanogel-drug conjugates embedding curcumin | – | inhibit mammosphere formation; | (Wei et al. 2013) | |
| Curcumin nano-medicine (C-SSM) | 5μM | inhibit mammosphere formation; reduce CD44+CD24−/low cells | (Gulcur et al. 2013) |
↓Represents down-regulation.
Abbreviations: HIF-1α, Hypoxia-inducible factor-1α;VEGF, vascular endothelial growth factor.
Additionally, nano-drugs made of other dietary polyphenols are also reported, although these studies mainly focus on preventing cancer cells rather than CSCs/BCSCs. A nanodrug made by embedding EGCG with polylactic acid-polyethylene glycol was 10 times more effective in inhibiting cancer cells than free EGCG (Siddiqui et al. 2013). A more advanced EGCG nano-fomula was designed later by introducing a special polymer which could specifically recognize prostate membrane antigen, achieving targeted delivery of EGCG. Indeed, the new nano-EGCG displayed better suppressing effects on cancer cells than free drug (Siddiqui et al. 2013). Nano-quercetin was acquired by encapsulating quercetin with lecithin, which was able to improve bioavailability of drug by facilitating drug pass through cell membrane, so lower doses of the naon-drug could effectively induce MCF7 cell apoptosis compared to using quercetin alone (Minaei et al. 2016).
In short, polyphenols not only can be obtained from diets, but also administered through nano drug delivery system to specifically target cancer cells and BCSCs.
Conclusions and perspectives
Food-derived polyphenolic compounds have gained extensive attentions owing to their significant anti-cancer and anti-CSCs effects. Polyphenolic compounds are effective in suppressing the stemness of BCSCs, blocking mammosphere formation and neoplasm growth, as well as suppressing proliferation, migration, invasion and EMT. Polyphenolic compounds are able to inhibit cancer cells and BCSCs through multiple mechanisms, including suppression of Wnt/β-catenin pathway, preventing inflammation along with altering the expression of miRNAs to reduce the stemness of BCSCs. Therefore, polyphenolic compounds are promising in reducing and preventing cancer development, especially breast cancer development. However, several key questions remain to be addressed. Though a number of possible reasons have been identified to account for the antineoplastic functions of polyphenolic compounds, these beneficial effects are likely derived from a key mechanism, which is primarily responsible for the various anti-cancinogenesis and anti-BCSCs effects of polyphenolic compounds. Up to date, this key mechanism explaining the various effects of polyphenolic compounds in preventing/reducing carcinogenesis remain to be established. Mitochondria play a central role on cell fate commitment by orchestrating a series of metabolic events (Siu and Alway, 2005; Alaynick, 2008); mitochondrial malfunction is common and CSCs preferentially rely on glycolytic metabolism, which render cancer cells and CSCs escaping mitochondria-mediated apoptosis (Loureiro et al. 2013; Ju et al. 2014; Shen et al. 2015). Polyphenols, redoxactive compounds, are able to elicit an energy restricted state, which ameliorate mitochondrial dysfunction and induce mitochondrial biogenesis (de Oliveira et al. 2016). Furthermore, polyphenols are able to reprogram metabolic ways of CSCs to oxidative metabolism by modulating mitochondria-related enzyme (hexokinase), which is helpful in suppressing the stemness of CSCs and facilitating mitochondria-mediated apoptosis (Wang, Fan, et al. 2015). Green tea, which is rich in EGCG, suppressed BCSCs proliferation by regulating mitochondrial metabolism, rendering cancer cell more quiescent (Bonuccelli, Sotgia, and Lisanti 2018). Thus improvement in mitochondriogenesis and mitochondrial function due to dietary polyphenols may have a central role in inhibiting cancer cells/CSCs, which needs to be further confirmed in the future. In addition, the most available studies were conducted in cultured cells and in rodents, where high doses of polyphenolic compounds were commonly used. Whether these findings in vitro and in rodents can be translated into humans remain to be tested in vivo. Also, most polyphenolic compounds have low bioavailability and also not quite stable. Thus, how to effectively administer these compounds to humans are another area needing to be further studied. Finally, to accurately quantify the anti-BCSC effects of phytochemicals, BCSCs need to be isolated. Up to now, inconsistency in the definition of BCSCs remains and the biological identity of BCSCs need to be further explored. BCSCs may represent a stage where differentiated cancer cells gain stemness, or they may represent a unique pool of stem-like cells which are the sources of all differentiated cancer cells. The expression of BCSCs markers often vary depending on diverse subtypes of breast cancer, histologic stage and heterogeneity within a tumor (Tsang et al. 2012), which adds further complexity.
Acknowledgments
Funding
This paper was funded by the National Natural Science Foundation of China (Grant No. 31871806) and the National Dairy Industry Technology System-Beijing Innovation Team (Grant No. BAIC06-2018) to Xue-ying Mao, and the National Institutes for Health of USA (R01 HD067449) to Min Du.
Footnotes
Disclosure statement
The authors declare no conflict of interest.
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/bfsn.
References
- Adisetiyo H, Liang MM, Liao CP, Jeong JH, Cohen MB, Roy-Burman P, and Frenkel B. 2014. Dependence of Castration-Resistant prostate cancer (CRPC) Stem cells on CRPC-Associated fibroblasts. Journal of Cellular Physiology 229 (9):1170–1176. doi: 10.1002/jcp.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaynick WA 2008. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion 8 (4):329–337. doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, and Moon RT. 2013. WNT signalling pathways as therapeutic targets in cancer. Nature Reviews Cancer 13 (1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Anwar MS, Yu JQ, Beale P, and Huq F. 2016. 6-Shogaol and mycophenolic acid are seen to act synergistically in combination with platinum drug in killing ovarian cancer cells. European Journal of Cancer 69 (Suppl 1):S18–S18. doi: 10.1016/S0959-8049(16)32634-X. [DOI] [Google Scholar]
- Auguste P, Lemiere S, Larrieu-Lahargue F, and Bikfalvi A. 2005. Molecular mechanisms of tumor vascularization. Critical Reviews in Oncology/Hematology 54 (1):53–61. doi: 10.1016/j.critrevonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Li YW, Wang ZW, and Sarkar FH. 2008. Multi-targeted therapy of cancer by genistein. Cancer Letters 269 (2):226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP 2004. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116 (2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie XH, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. 2006. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125 (2):315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Boman BM, and Wicha MS. 2008. Cancer stem cells: A step toward the cure. Journal of Clinical Oncology 26 (17):2795–2799. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- Bonuccelli G, Sotgia F, and Lisanti MP. 2018. Matcha green tea (MGT) inhibits the propagation of cancer stem cells (CSCs), by targeting mitochondrial metabolism, glycolysis and multiple cell signalling pathways. Aging 10 (8):1867–1883. doi: 10.18632/aging.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. 2006. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441 (7091):349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Bu P, Chen K-Y, Chen JH, Wang L, Walters J, Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, et al. 2013. A microRNA miR-34a-Regulated bimodal switch targets notch in Colon cancer stem cells. Cell Stem Cell 12 (5):602–615. doi: 10.1016/j.stem.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JP, Lim G, Li Y, Shah RB, Lim R, Paholak HJ, McDermott SP, Sun L, Tsume Y, Bai S, et al. 2017. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Letters 394:52–64. doi: 10.1016/j.canlet.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantile M, Collina F, D’Aiuto M, Rinaldo M, Pirozzi G, Borsellino C, Franco R, Botti G, and Di Bonito M. 2013. Nuclear localization of cancer stem cell marker CD133 in triple-negative breast cancer: a case report. Tumori 99 (5):245–250. doi: 10.1700/1377.15325. [DOI] [PubMed] [Google Scholar]
- Chen D, Pamu S, Cui Q, Chan TH, and Dou QP. 2012. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorganic & Medicinal Chemistry 20 (9):3031–3037. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Li L, Zhang X, Liang Y, Pu Z, Wang L, and Mo J. 2017. Curcumin: A calixarene derivative micelle potentiates anti-breast cancer stem cells effects in xenografted, triple-negative breast cancer mouse models. Drug Delivery 24 (1):1470–1481. doi: 10.1080/10717544.2017.1381198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Lien HM, Kao MC, Lo UG, Lin LC, Lin CJ, Chang SJ, Chen CC, Hsieh JT, Lin H., et al. 2017. Sensitization of radioresistant prostate cancer cells by resveratrol isolated from Arachis hypogaea stems. PLoS One 12 (1):e0169204. doi: 10.1371/journal.pone.0169204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Heo K, Park HS, Yang KM, and Jeong MH. 2016. The resveratrol analog HS-1793 enhances radiosensitivity of mouse-derived breast cancer cells under hypoxic conditions. International Journal of Oncology 49 (4):1479–1488. doi: 10.3892/ijo.2016.3647. [DOI] [PubMed] [Google Scholar]
- Chung Seyung VJV 2015. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFkB signaling. Anticancer Research 35 (1):39–46. [PMC free article] [PubMed] [Google Scholar]
- de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, and Nabavi SM. 2016. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochimica et Biophysica Acta 1860 (4):727–745. doi: 10.1016/j.bbagen.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Dean M 2009. ABC transporters, drug resistance, and cancer stem cells. Journal of Mammary Gland Biology and Neoplasia 14 (1):3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, and Bates S. 2005. Tumour stem cells and drug resistance. Nature Reviews Cancer 5 (4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Rock CL, Patrick K, and Byers T. 2008. Lifestyle interventions to reduce cancer risk and improve outcomes. American Family Physician 77 (11):1573–1578. [PubMed] [Google Scholar]
- Deng H, Zhang XT, Wang ML, Zheng HY, Liu LJ, and Wang ZY. 2014. ER-alpha36-mediated rapid estrogen signaling positively regulates ER-positive breast cancer stem/progenitor cells. PLoS One 9 (2):e88034. doi: 10.1371/journal.pone.0088034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XY, Apple S, Zhao H, Song J, Lee M, Luo W, Wu XC, Chung D, Pietras RJ, and Chang HR. 2017. CD24 expression and differential resistance to chemotherapy in triple-negative breast cancer. Oncotarget 8 (24):38294–38308. doi: 10.18632/oncotarget.16203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XZ, Wu B, Xiao K, Kang J, Xie J, Zhang XP, and Fan YB. 2015. MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cellular Physiology and Biochemistry 35 (1):71–82. doi: 10.1159/000369676. [DOI] [PubMed] [Google Scholar]
- Ding Q, Miyazaki Y, Tsukasa K, Matsubara S, Yoshimitsu M, and Takao S. 2014. CD133 facilitates epithelial-mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Molecular Cancer 13 (1):15. doi: 10.1186/1476-4598-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MR, Smigiel JM, Junk DJ, and Jackson MW. 2016. Cancer stem cell plasticity drives therapeutic resistance. Cancers (Basel) 8 (1):8–13. doi: 10.3390/cancers8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, and Wicha MS. 2003. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & Development 17 (10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran H, Johnstone SE, Van Neste L, Ohm J, Mosbruger T, Wang QJ, Aryee MJ, Joyce P, Ahuja N, Weisenberger D, et al. 2012. A DNA hypermethylation module for the stem/progenitor cell signature of cancer. Genome Research 22 (5):837–849. doi: 10.1101/gr.131169.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizabeth Louie SN, Chen J-S, and Schmidt M. 2010. Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation. Breast Cancer Research 12 (6):94–108. doi: 10.1186/bcr2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann K, Shen H, and Finn OJ. 2008. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Research 68 (7):2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- Fan PH, Fan SJ, Wang H, Mao J, Shi Y, Ibrahim MM, Ma W, Yu XT, Hou ZH, Wang B, and Li LH. 2013. Genistein decreases the breast cancer stem-like cell population through hedgehog pathway. Stem Cell Research & Therapy 4 (6):146. doi: 10.1186/scrt357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima MT, Chanchal A, Yavvari PS, Bhagat SD, Gujrati M, Mishra RK, and Srivastava A. 2016. Cell permeating nano-complexes of amphiphilic polyelectrolytes enhance solubility, stability, and anti-cancer efficacy of curcumin. Biomacromolecules 17 (7): 2375–2383. doi: 10.1021/acs.biomac.6b00417. [DOI] [PubMed] [Google Scholar]
- Flemming A 2015. Cancer stem cells: Targeting the root of cancer relapse. Nature Reviews Drug Discovery 14 (3):165–165. doi: 10.1038/nrd4560. [DOI] [PubMed] [Google Scholar]
- Forloni M, Dogra SK, Dong YY, Conte D, Ou JH, Zhu LJ, Deng A, Mahalingam M, Green MR, and Wajapeyee N. 2014. miR-146a promotes the initiation and progression of melanoma by activating notch signaling. Elife 3:e01460. doi: 10.7554/eLife.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Fu L, Xie C, Zuo WS, Liu YS, Zheng MZ, and Yu JM. 2017. miR-375 inhibits cancer stem cell phenotype and tamoxifen resistance by degrading HOXB3 in human ER-positive breast cancer. Oncology Reports 37 (2):1093–1099. doi: 10.3892/or.2017.5360. [DOI] [PubMed] [Google Scholar]
- Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y, Zhu J, and Mi M. 2014. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing wnt/beta-catenin signaling pathway. PLoS One 9 (7):e102535. doi: 10.1371/journal.pone.0102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li H, and Hao XS. 2017. The self-renewal signaling pathways utilized by gastric cancer stem cells. Tumor Biology 39 (4):1–7. doi: 69757710.1177/1010428317697577. [DOI] [PubMed] [Google Scholar]
- Fujiki H, Sueoka E, Rawangkan A, and Suganuma M. 2017. Human cancer stem cells are a target for cancer prevention using (−)-epigallocatechin gallate. Journal of Cancer Research and Clinical Oncology 143 (12): 2401–2412. doi: 10.1007/s00432-017-2515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H, Watanabe T, Sueoka E, Rawangkan A, and Suganuma M. 2018. Cancer prevention with green tea and its principal constituent, EGCG: From early investigations to current focus on human cancer stem cells. Molecular Cell 41(2):73–82.doi: 10.14348/molcells.2018.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M 2017. Epithelial plasticity and cancer stem cells: Major mechanisms of cancer pathogenesis and therapy resistance. World Journal of Stem Cells 9 (8):118–126. doi: 10.4252/wjsc.v9.i8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. 2007. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1 (5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A, Majumder B, Dhawan A, Ravi S, Goldman D, Kohandel M, Majumder PK, and Sengupta S. 2015. Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nature Communications 6:6139. doi: 10.1038/ncomms7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Bei M, Maas R, Zhang XYM, Linde A, and McMahon AP. 2002. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129 (23):5323–5337. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- Gulcur E, Thaqi M, Khaja F, Kuzmis A, and Onyuksel H. 2013. Curcumin in VIP-targeted sterically stabilized phospholipid nanomicelles: A novel therapeutic approach for breast cancer and breast cancer stem cells. Drug Delivery and Translational Research 3 (6): 562–574. doi: 10.1007/s13346-013-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, and Kim VN. 2014. Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology 15 (8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Kosaka N, Yoshioka Y, Takahashi RU, Takeshita F, and Ochiya T. 2012. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Scientific Reports 2:314. doi: 10.1038/srep00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D., Hirsch HA, and Struhl K. 2009. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139 (4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal W, Alkarim S, AlHejin A, Mukhtar H, and Saini KS. 2016. Targeting signal transduction pathways of cancer stem cells for therapeutic opportunities of metastasis. Oncotarget 7(46): 76337–76353. doi: 10.18632/oncotarget.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe T, Hisamori S, Hogan DJ, Zabala M, Hendrickson DG, Dalerba P, Cai S, Scheeren F, Kuo AH, Sikandar SS, et al. 2014. miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. Elife 3:e1977. doi: 10.7554/eLife.01977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Inoue M, Otani T, Sasazuki S, Kurahashi N, Miura T, Yamamoto S, and Tsugane S. 2008. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: A nested case-control study from the Japan public health center-based prospective study group. Journal of Clinical Oncology 26 (10): 1677–1683. doi: 10.1200/JCO.2007.13.9964. [DOI] [PubMed] [Google Scholar]
- Jang JW, Song Y, Kim SH, Kim J, and Seo HR. 2017. Potential mechanisms of CD133 in cancer stem cells. Life Sciences 184:25–29. doi: 10.1016/j.lfs.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Jiang F, Li Y, Mu J, Hu CY, Zhou M, Wang XX, Si L, Ning SL, and Li Z. 2016. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: An epigenetic regulation of miR-148a/SMAd2 signaling. Molecular Carcinogenesis 55(5):929–940. doi: 10.1002/mc.22333. [DOI] [PubMed] [Google Scholar]
- Ju YS, Alexandrov L, Gerstung M, Martincorena I, Stratton M, Campbell PJ, I. B. C. Grp, I. C. M. D. Grp, and I. P. C. Grp. 2014. The landscape of mitochondrial DNA mutations in human cancer. Cancer Research 74(19 Suppl):4322. doi: 10.1158/1538-7445.AM2014-4322. [DOI] [Google Scholar]
- Jung SK, Lee MH, Lim DY, Kim JE, Singh P, Lee SY, Jeong CH, Lim TG, Chen HY, Chi YI, et al. 2014. Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. Journal of Biological Chemistry 289 (52):35839–35848. doi: 10.1016/0002-9610(71)90360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justyna Gil AS, Pesz KA, and Siadek MM. 2008. Cancer stem cells: The theory and perspectives in cancer therapy. Journal of Applied Genetics 49 (2):193–199. doi: 10.1007/BF03195612. [DOI] [PubMed] [Google Scholar]
- Kahn M 2014. Can we safely target the WNT pathway? Nature Reviews Drug Discovery 13 (7):513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, and Wicha MS. 2010. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Research and Treatment 122 (3):777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Guo Y, Zhang X, Meng J, and Wang ZY. 2011. A positive cross-regulation of HER2 and ER-alpha36 controls ALDH1 positive breast cancer cells. The Journal of Steroid Biochemistry and Molecular Biology 127 (3-5):262–268. doi: 10.1016/j.jsbmb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Mao J, Tao YJ, Song B, Ma W, Lu Y, Zhao LJ, Li JZ, Yang BX, and Li LH. 2015. MicroRNA-34a suppresses the breast cancer stem cell-like characteristics by downregulating Notch1 pathway. Cancer Science 106 (6):700–708. doi: 10.1111/cas.12656. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kashyap D, Mittal S, Sak K, Singhal P, and Tuli HS. 2016. Molecular mechanisms of action of quercetin in cancer: Recent advances. Tumor Biology 37 (10):12927–12939. doi: 10.1007/s13277-016-5184-x. [DOI] [PubMed] [Google Scholar]
- Kim JY, Zhang XW, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, and Yee AS. 2006. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells - Requirement of the transcriptional repressor HBP1. Journal of Biological Chemistry 281 (16):10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- Kim RJ, Park JR, Roh KJ, Choi AR, Kim SR, Kim PH, Yu JH, Lee JW, Ahn SH, Gong G, et al. 2013. High aldehyde dehydrogenase activity enhances stem cell features in breast cancer cells by activating hypoxia-inducible factor-2alpha. Cancer Letters 333 (1):18–31. doi: 10.1016/j.canlet.2012.11.026. [DOI] [PubMed] [Google Scholar]
- Kise K, Kinugasa-Katayama Y, and Takakura N. 2016. Tumor microenvironment for cancer stem cells. Advanced Drug Delivery Reviews 99 (Part B):197–205. doi: 10.1016/j.addr.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Kosuru RY, Roy A, Das SK, and Bera S. 2018. Gallic acid and gallates in human health and disease: Do mitochondria hold the key to success? Molecular Nutrition & Food Research 62 (1):699. doi: 10.1002/mnfr.201700699. [DOI] [PubMed] [Google Scholar]
- Kuang W, Tan J, Duan Y, Duan J, Wang W, Jin F, Jin Z, Yuan X, and Liu Y. 2009. Cyclic stretch induced miR-146a upregulation delays C2C12 myogenic differentiation through inhibition of numb. Biochemical and Biophysical Research Communications 378 (2): 259–263. doi: 10.1016/j.bbrc.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP 2000. Fatty-Acid synthase and human cancer: New perspectives on its role in tumor biology. Nutrition 16 (3):202–208. doi: 10.1016/S0899-9007(99)00266-X. [DOI] [PubMed] [Google Scholar]
- Kwon MJ, Han J, Seo JH, Song K, Jeong HM, Choi JS, Kim YJ, Lee SH, Choi YL, and Shin YK. 2015. CD24 overexpression is associated with poor prognosis in Luminal A and TripleNegative breast cancer. PLoS One 10 (10):e0139112. doi: 10.1371/journal.pone.0139112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tume L, Paco K, Ubidia-Incio R, and Moya J. 2016. CD133 in breast cancer cells and in breast cancer stem cells as another target for immunotherapy. Gaceta Mexicana De Oncologia 15 (1):22–30. doi:10.1016/j.gamo.2016.01.003. doi: 10.1016/j.gamo.2016.01.003. [DOI] [Google Scholar]
- Lanou AJ, and Svenson B. 2010. Reduced cancer risk in vegetarians: An analysis of recent reports. Cancer Management and Research 3: 1–8. doi: 10.2147/CMR.S6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Hong HM, Chang YY, and Chang WW. 2012. Inhibition of heat shock protein (Hsp) 27 potentiates the suppressive effect of Hsp90 inhibitors in targeting breast cancer stem-like cells. Biochimie 94 (6):1382–1389. doi: 10.1016/j.biochi.2012.02.034. [DOI] [PubMed] [Google Scholar]
- Lee CH, Wu YT, Hsieh HC, Yu Y, Yu AL, and Chang WW. 2014. Epidermal growth factor/heat shock protein 27 pathway regulates vasculogenic mimicry activity of breast cancer stem/progenitor cells. Biochimie 104:117–126. doi: 10.1016/j.biochi.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Li BL, Lu Y, Wang HH, Han XC, Mao J, Li JZ, Yu LH, Wang B, Fan SJ, Yu XT, and Song B. 2016. miR-22½22 enhance the tumorigenicity of human breast cancer stem cells via modulation of PTEN/Akt pathway. Biomedicine & Pharmacotherapy 79:93–101. doi: 10.1016/j.biopha.2016.01.045. [DOI] [PubMed] [Google Scholar]
- Li S, and Li Q. 2015. Cancer stem cells, lymphangiogenesis, and lymphatic metastasis. Cancer Letters 357 (2):438–447. doi: 10.1016/j.canlet.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Li SZ, Zhao Q, Wang B, Yuan S, Wang XY, and Li K. 2018. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytotherapy Research 32 (8): 1530–1536. doi: 10.1002/ptr.6081. [DOI] [PubMed] [Google Scholar]
- Li W, Ma H, Zhang J, Zhu L, Wang C, and Yang Y. 2017. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Scientific Reports 7 (1): 13856. doi: 10.1038/s41598-017-14364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lui T-T, Wang H-H, and Hong H-M. 2011. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappa B. Breast Cancer Research 13 (5):101–114. doi: 10.1186/bcr3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Zhou N, Wang J, Liu ZJ, Wang XH, Zhang Q, Liu QY, Gao LF, and Wang R. 2018. Quercetin suppresses breast cancer stem cells (CD44(+)/CD24(−)) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sciences 196:56–62. doi: 10.1016/j.lfs.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Li Y, and Zhang T. 2014. Targeting cancer stem cells by curcumin and clinical applications. Cancer Letters 346 (2):197–205. doi: 10.1016/j.canlet.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Li YY, Wicha MS, Schwart SJ, and Sun DX. 2011. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. The Journal of Nutritional Biochemistry 22 (9): 799–806. doi: 10.1016/j.jnutbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yin SC, Zhang L, Liu WG, Chen B, and Xing H. 2017. Clinicopathological characteristics and prognostic value of cancer stem cell marker CD133 in breast cancer: A meta-analysis. OncoTargets and Therapy 10:859–870. doi: 10.2147/OTT.S124733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CJ 2016. Diverse targets of beta-catenin during the epithelial-mesenchymal transition define cancer stem cells and predict disease relapse. Cancer Research 76 (20):6133–6133. doi: 10.1158/0008-5472.CAN-14-3265. [DOI] [PubMed] [Google Scholar]
- Liang GN, and Weisenberger DJ. 2017. DNA methylation aberrancies as a guide for surveillance and treatment of human cancers. Epigenetics 12 (6):416–432. doi: 10.1080/15592294.2017.1311434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, et al. 2014. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports 2 (1): 78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, and Wicha MS. 2006. Hedgehog signaling and bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Research 66 (12):6063–6071.doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, Yao Z, Dong XY, Zhao N, and Liu N. 2013. CD133(+) cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene 32 (5):544–553. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- Liu W, Shen SM, Zhao XY, and Chen GQ. 2012. Targeted genes and interacting proteins of hypoxia inducible factor-1. International Journal of Biochemistry and Molecular Biology 3 (2):165–178. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zou T, Wang S, Chen H, Su D, Fu X, Zhang Q, and Kang X. 2016. Genistein-induced differentiation of breast cancer stem/progenitor cells through a paracrine mechanism. International Journal of Oncology 48 (3):1063–1072. doi: 10.3892/ijo.2016.3351. [DOI] [PubMed] [Google Scholar]
- Loureiro R, Mesquita KA, Oliveira PJ, and Vega-Naredo I. 2013. Mitochondria in cancer stem cells: A target for therapy. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery 7 (2): 102–114. doi: 10.2174/18722148113079990006. [DOI] [PubMed] [Google Scholar]
- Lu KT, Wang BY, Chi WY, Chang-Chien J, Yang JJ, Lee HT, Tzeng YM, and Chang WW. 2016. Ovatodiolide inhibits breast cancer stem/Progenitor cells through SMURF2-Mediated downregulation of Hsp27. Toxins (Basel) 8 (5):235–246. doi: 10.3390/toxins8050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XQ, Xu KJ, Lu HY, Yin YH, Ma CL, Liu YH, Li HX, and Suo ZH. 2011. CD44(+)/CD24(−) cells are transit progenitors and do not determine the molecular subtypes and clinical parameters in breast carcinomas. Ultrastructural Pathology 35 (2):72–78. doi: 10.3109/01913123.2010.544843. [DOI] [PubMed] [Google Scholar]
- Lu Y, Ma W, Mao J, Yu XT, Hou ZH, Fan SJ, Song B, Wang H, Li JZ, Kang L, et al. 2015. Salinomycin exerts anticancer effects on human breast carcinoma MCF-7 cancer stem cells via modulation of hedgehog signaling. Chemico-Biological Interactions 228:100–107. doi: 10.1016/j.cbi.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Lv L, Zhou JY, Lin CW, Hu G, Yi L, Du J, Gao K, and Li XR. 2015. DNA methylation is involved in the aberrant expression of miR-133b in colorectal cancer cells. Oncology Letters 10 (2):907–912. doi: 10.3892/ol.2015.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Li H, Wang H, Shi X, Fan Y, Ding X, Lin C, Zhan Q, Qian H, and Xu B. 2014. Enriched CD44(+)/CD24(−) population drives the aggressive phenotypes presented in triple-negative breast cancer (TNBC). Cancer Letters 353 (2):153–159. doi: 10.1016/j.canlet.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Ma ZL, Chen YP, Song JL, and Wang YQ. 2015. Knockdown of CD24 inhibits proliferation, invasion and sensitizes breast cancer MCF-7 cells to tamoxifen in vitro. European Review for Medical and Pharmacological Sciences 19 (13):2394–2399. [PubMed] [Google Scholar]
- Mamaeva V, Niemi R, Beck M, Ozliseli E, Desai D, Landor S, Gronroos T, Kronqvist P, Pettersen IKN, McCormack E, et al. 2016. Inhibiting notch activity in breast cancer stem cells by glucose functionalized nanoparticles carrying gamma-secretase inhibitors. Molecular Therapy 24 (5):926–936. doi:10.1038/mt.2016.42. doi: 10.1038/mt.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Lertpiriyapong K, Steelman LS, Abrams SL, Yang LV, Murata RM, Rosalen PL, Scalisi A, Neri LM, Cocco L, et al. 2017. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging-Us 9 (6):1477–1536. doi: 10.18632/aging.101250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele L 2006. Notch signaling. Clinical Cancer Research 12 (4):1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- Miletti-Gonzalez KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yang J, Apolito K, Shih WJ, Hait WN, and Rodriguez-Rodriguez L. 2005. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Research 65 (15):6660–6667. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]
- Minaei A, Sabzichi M, Ramezani F, Hamishehkar H, and Samadi N. 2016. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Molecular Biology Reports 43 (2):99–105. doi: 10.1007/s11033-016-3942-x. [DOI] [PubMed] [Google Scholar]
- Mineva ND, Paulson KE, Naber SP, Yee AS, and Sonenshein GE. 2013. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS One 8 (9):e73464. doi: 10.1371/journal.pone.0073464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkkon T, and Lewis MT. 2017. New paradigms for the hedgehog signaling network in mammary gland development and breast cancer. Biochimica Et Biophysica Acta-Reviews on Cancer 1868 (1): 315–332. doi: 10.1016/j.bbcan.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montales MT, Rahal OM, Kang J, Rogers TJ, Prior RL, Wu X, and Simmen RC. 2012. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stemlike/progenitor cells. Carcinogenesis 33 (3):652–660. doi: 10.1093/carcin/bgr317. [DOI] [PubMed] [Google Scholar]
- Montales MT, Rahal OM, Nakatani H, Matsuda T, and Simmen RC. 2013. Repression of mammary adipogenesis by genistein limits mammosphere formation of human MCF-7 cells. Journal of Endocrinology 218 (1):135–149. doi: 10.1530/JOE-12-0520. [DOI] [PubMed] [Google Scholar]
- Moreno-Londono AP, Bello-Alvarez C, and Pedraza-Chaverri J. 2017. Isoliquiritigenin pretreatment attenuates cisplatin induced proximal tubular cells (LLC-PK1) death and enhances the toxicity induced by this drug in bladder cancer T24 cell line. Food and Chemical Toxicology 109:143–154. doi: 10.1016/j.fct.2017.08.047. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Mazumdar M, Chakraborty S, Manna A, Saha S, Khan P, Bhattacharjee P, Guha D, Adhikary A, Mukhjerjee S, and Das T. 2014. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin_b-catenin negative feedback loop. Stem Cell Research & Therapy 5 (5):116–135. doi: 10.1186/scrt506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naab T, Ricks-Santi L, and Khan F. 2016. CD44 expression associated with triple negative breast cancers in african american women without unfavorable outcome. American Journal of Clinical Pathology 146(Suppl 1):295. doi: 10.1093/ajcp/aqw159.066. [DOI] [Google Scholar]
- Naksuriya O, Okonogi S, Schiffelers RM, and Hennink WE. 2014. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 35 (10):3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- Nalls D, Tang SN, Rodova M, Srivastava RK, and Shankar S. 2011. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PLoS One 6 (8):1–12. doi: 10.1371/journal.pone.0024099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandy SB, Arumugam A, Subramani R, Pedroza D, Hernandez K, Saltzstein E, and Lakshmanaswamy R. 2015. MicroRNA-125a influences breast cancer stem cells by targeting leukemia inhibitory factor receptor which regulates the hippo signaling pathway. Oncotarget 6 (19):17366–17378. doi: 10.18632/oncotarget.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, and Cragg GM. 2012. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products 75 (3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning YX, Li QX, Ren KQ, Quan MF, and Cao JG. 2014. 7-difluoromethoxyl-5,4 ‘-di-n-octyl genistein inhibits ovarian cancer stem cell characteristics through the downregulation of FOXM1. Oncology Letters 8 (1):295–300. doi: 10.3892/ol.2014.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, and Capaccioli S. 2009. Natural compounds for cancer treatment and prevention. Pharmacological Research 59 (6):365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Oda Y, Ohishi Y, Basaki Y, Kobayashi H, Hirakawa T, Wake N, Ono M, Nishio K, Kuwano M, and Tsuneyoshi M. 2007. Prognostic implications of the nuclear localization of Y-box-binding protein-1 and CXCR4 expression in ovarian cancer: Their correlation with activated akt, LRP/MVP and P-glycoprotein expression. Cancer Science 98 (7):1020–1026. doi: 10.1111/j.1349-7006.2007.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Ohishi Y, Saito T, Hinoshita E, Uchiumi T, Kinukawa N, Iwamoto Y, Kohno K, Kuwano M, and Tsuneyoshi M. 2003. Nuclear expression of Y-box-binding protein-1 correlates with P-glycoprotein and topoisomerase II alpha expression, and with poor prognosis in synovial sarcoma. The Journal of Pathology 199 (2): 251–258. doi: 10.1002/path.1282. [DOI] [PubMed] [Google Scholar]
- Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK, Mo YY, Iiizumi-Gairani M, Hirota S, Liu Y, et al. 2013. miR-7 suppresses brain metastasis of breast cancer Stem-Like cells by modulating KLF4. Cancer Research 73 (4):1434–1444. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Kolluru V, Chandrasekaran B, Baby BV, Aman M, Suman S, Sirimulla S, Sanders MA, Alatassi H, Ankem MK, and Damodaran C. 2017. Targeting aberrant expression of notch-1 in ALDH(+) cancer stem cells in breast cancer. Molecular Carcinogenesis 56 (3):1127–1136. doi: 10.1002/mc.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Zhao B, Song Z, Han S, and Wang M. 2016. Estrogen receptor-alpha36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. Journal of Pharmacological Sciences 130 (2):85–93. doi: 10.1016/j.jphs.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, Xing F Fukuda K, Hirota S, Sugai T, et al. 2011. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Research and Treatment 130 (2):387–398. doi: 10.1007/s10549-010-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey PR, Xing F, Sharma S, Watabe M, Pai SK, Iiizumi-Gairani M, Fukuda K, Hirota S, Mo YY, and Watabe K. 2013. Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene 32 (42):5111–5122. doi: 10.1038/onc.2012.519. [DOI] [PubMed] [Google Scholar]
- Park SY, Kim MJ, Park SA, Kim JS, Min KN, Kim DK, Lim W, Nam JS, and Sheen YY. 2015. Combinatorial TGF-b attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells. Oncotarget 6 (35): 37526–37543. doi: 10.18632/oncotarget.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Lee HE, Li HL, Shipitsin M, Gelman R, and Polyak K. 2010. Heterogeneity for stem Cell-Related markers according to tumor subtype and histologic stage in breast cancer. Clinical Cancer Research 16 (3):876–887. doi: 10.1158/1078-0432.CCR-09-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, and Di Fiore PP. 2010. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 140 (1):62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Peng F, Tang HL, Liu P, Shen JG, Guan XY, Xie XF, Gao JH, Xiong L, Jia L, Chen JP, and Peng C. 2017. Isoliquiritigenin modulates miR-374a/PTEN/Akt axis to suppress breast cancer tumorigenesis and metastasis. Scientific Reports 7: 9022. doi: 10.1038/s41598-017-08422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokharel D, Padula MP, Lu JF, Jaiswal R, Djordjevic SP, and Bebawy M. 2016. The role of CD44 and ERM proteins in expression and functionality of P-glycoprotein in breast cancer cells. Molecules 21 (3):1–14. doi: 10.3390/molecules21030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani M, Talebi M, Hagh MF, Feizi AAH, and Solali S. 2018. Aberrant DNA methylation of key genes and acute lymphoblastic leukemia. Biomedicine & Pharmacotherapy 97:1493–1500. doi: 10.1016/j.biopha.2017.11.033. [DOI] [PubMed] [Google Scholar]
- Ray A, Vasudevan S, and Sengupta S. 2015. 6-Shogaol inhibits breast cancer cells and stem Cell-Like spheroids by modulation of notch signaling pathway and induction of autophagic cell death. PLoS One 10 (9):e0137614. doi: 10.1371/journal.pone.0137614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Baycu C, Koparal AT, Donmez DB, and Bektur E. 2016. Resveratrol reduces IL-6 and VEGF secretion from co-cultured A549 lung cancer cells and adipose-derived mesenchymal stem cells. Tumor Biology 37 (6):7573–7582. doi: 10.1007/s13277-015-4643-0. [DOI] [PubMed] [Google Scholar]
- Sansone P, Ceccarelli C, Berishaj M, Chang Q, Rajasekhar VK, Perna F, Bowman RL, Vidone M, Daly L, Nnoli J, et al. 2016. Selfrenewal of CD133(hi) cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nature Communications 7: 10442. doi: 10.1038/Ncomms10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Kaneda A, Tsuji S, Isagawa T, Yamamoto S, Fujita T, Yamanaka R, Tanaka Y, Nukiwa T, Marquez VE, et al. 2013. PRC2 overexpression and PRC2-target gene repression relating to poorer prognosis in small cell lung cancer. Scientific Reports 3:1911–1920. doi: 10.1038/srep01911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau M, and Sauvageau G. 2008. Polycomb group genes: Keeping stem cell activity in balance. PLOS Biology 6 (4):678–681. doi: 10.1371/journal.pbio.0060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YA, Wang CY, Hsieh YT, Chen YJ, and Wei YH. 2015. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle 14 (1):86–98. doi: 10.4161/15384101.2014.974419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian DL, Diehn M, Liu HP, Panula SP, Chiao E, et al. 2009. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 138 (3):592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo T, Nishiyama N, Niinuma T, Kitajima H, Kai M, Tokino T, Shinkai N, Suzuki H, and Masumori N. 2017. Downregulation of mir-200b is associated with cisplatin-resistance in bladder cancer cells. The Journal of Urology 197 (Suppl 4):568–569. doi: 10.1016/j.juro.2017.02.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui IA, Sanna V, Adhami VM, Shabana SM, Sechi M, and Mukhtar H. 2013. Prostate specific membrane antigen (PSMA) targeting nano-EGCG for prostate cancer prevention and treatment. Cancer Research 73 (8):3663. doi: 10.1158/1538-7445.AM2013-3663. [DOI] [Google Scholar]
- Siu PM, and Alway SE. 2005. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. The Journal of Physiology 565 (1):309–323. doi: 10.1113/jphysiol.2004.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, and Achen MG. 2014. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nature Reviews Cancer 14 (3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- Su J, Wu SF, Wu HY, Le LI, and Guo T. 2016. CD44 is functionally crucial for driving lung cancer stem cells metastasis through wnt/β-catenin-FoxM1-Twist signaling. Molecular Carcinogenesis 55 (12):1962–1973. doi: 10.1002/mc.22443. [DOI] [PubMed] [Google Scholar]
- Suh J, Kim DH, and Surh YJ. 2018. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Archives of Biochemistry and Biophysics 643:62–71. doi: 10.1016/j.abb.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Sun MJ, Zhang N, Wang XL, Li YM, Qi WW, Zhang HW, Li ZJ, and Yang QF. 2016. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells. Cell & Bioscience 6:44. doi:ARTN4410.1186/s13578-016-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Xu C, Xiao G, Meng J, Wang J, Tang SC, Qin S, Du N, Li G, Ren H, and Liu D. 2018. Breast cancer stem-like cells are sensitized to tamoxifen induction of self-renewal inhibition with enforced let-7c dependent on wnt blocking. International Journal of Molecular Medicine 41 (4):1967–1975. doi: 10.3892/ijmm.2018.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino D, Cironi L, et al. 2009. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Research 69(24):9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Miyagi S, Sashida G, Chiba T, Yuan J, Mochizuki-Kashio M, Suzuki Y, Sugano S, Nakaseko C, Yokote K, et al. 2012. Ezh2 augments leukemogenicity by reinforcing differentiation blockage in acute myeloid leukemia. Blood 120 (5):1107–1117. doi: 10.1182/blood-2011-11-394932. [DOI] [PubMed] [Google Scholar]
- Tang BW, Raviv A, Esposito D, Daniel C, Flanders KC, Yang YA, and Wakefield LM. 2015. Transforming growth factor-beta (TGF-beta) directly regulates breast cancer stem cell dynamics in vitro and in vivo. Cancer Research 75(15 Suppl):2221. doi: 10.1158/1538-7445.AM2015-2221. [DOI] [Google Scholar]
- Tay Y, Zhang JQ, Thomson AM, Lim B, and Rigoutsos I. 2008. MicroRNAs to nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 455 (7216):1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- To K, Fotovati A, Reipas KM, Law JH, Hu KJ, Wang J, Astanehe A, Davies AH, Lee L, Stratford AL., et al. 2010. Y-Box binding protein-1 induces the expression of CD44 and CD49f leading to enhanced self-renewal, mammosphere growth, and drug resistance. Cancer Research 70 (7):2840–2851. doi: 10.1158/0008-5472.CAN-09-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP 2014. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nature Reviews Drug Discovery 13 (11): 813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way TD, and Chen WJ. 2013. 3,5,4’-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and wnt/beta-catenin signaling Cascades and reversal of epithelial-mesenchymal transition. Toxicology and Applied Pharmacology 272 (3):746–756. doi: 10.1016/j.taap.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Tsang JYS, Huang YH, Luo MH, Ni YB, Chan SK, Lui PCW, Yu AMC, Tan PH, and Tse GM. 2012. Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Research and Treatment 136 (2):407–417. doi: 10.1007/s10549-012-2271-6. [DOI] [PubMed] [Google Scholar]
- Tuna M, Machado AS, and Calin GA. 2016. Genetic and epigenetic alterations of MicroRNAs and implications for human cancers and other diseases. Genes, Chromosomes and Cancer 55 (3):193–214. doi: 10.1002/gcc.22332. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W 2012. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacological Research 65(6):565–576. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Vlashi E, Kim K, Lagadec C, Donna LD, McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W, and Pajonk F. 2009. In vivo imaging, Tracking, and targeting of cancer stem cells. JNCI: Journal of the National Cancer Institute 101 (5):350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong T, Mallet JF, Ouzounova M, Rahbar S, Hernandez-Vargas H, Herceg Z, and Matar C. 2016. Role of a polyphenol-enriched preparation on chemoprevention of mammary carcinoma through cancer stem cells and inflammatory pathways modulation. Journal of Translational Medicin 14:1–12. doi: 10.1186/s12967-016-0770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright EN, and Scaffidi P. 2017. Epigenetics and cancer stem cells: Unleashing, hijacking, and restricting cellular plasticity. Trends in Cancer 3 (5):372–386. doi: 10.1016/j.trecan.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Cai C, Dong X, Yu QC, Zhang XO, Yang L, and Zeng YA. 2015. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 517 (7532):81–84. doi: 10.1038/nature13851. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang L, Song Y, Wang S, Huang X, Xuan Q, Kang X, and Zhang Q. 2017. CD44(+)/CD24(−) phenotype predicts a poor prognosis in triple-negative breast cancer. Oncology Letters 14 (5): 5890–5898. doi: 10.3892/ol.2017.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Fan H, Chen QS, Ma GJ, Zhu M, Zhang XM, Zhang YY, and Yu J. 2015. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 26 (1): 15–24. doi: 10.1097/CAD.0000000000000132. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang ZY, Peng C, You JS, Shen JG, Han SW, and Chen JP. 2014. Dietary compound isoliquiritigenin targets GRP78 to chemosensitize breast cancer stem cells via beta-catenin/ABCG2 signaling. Carcinogenesis 35 (11):2544–2554. doi: 10.1093/carcin/bgu187. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang ZY, Wang Y, Xie XM, Shen JG, Peng C, You JS, Peng F, Tang HL, Guan XY, and Chen JP. 2015. Dietary compound isoliquiritigenin prevents mammary carcinogenesis by inhibiting breast cancer stem cells through WIF1 demethylation. Oncotarget 6 (12):9854–9876. doi: 10.18632/oncotarget.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RA, Li ZS, Zhang HZ, Zheng PJ, Li QL, Shi JG, Yan QG, Ye J, Wang JB, Guo Y, et al. 2013. Invasive cancers are not necessarily from preformed in situ tumours - an alternative way of carcinogenesis from misplaced stem cells. Journal of Cellular and Molecular Medicine 17(7):921–926. doi: 10.1111/jcmm.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chen R, Zhong Z, Shi Z, Chen M, and Wang Y. 2014. Epigallocatechin-3-gallate potentiates the effect of curcumin in inducing growth inhibition and apoptosis of resistant breast cancer cells. The American Journal of Chinese Medicine 42 (05):1279–1300. doi: 10.1142/S0192415X14500803. [DOI] [PubMed] [Google Scholar]
- Wang T, and Xu ZY. 2010. miR-27 promotes osteoblast differentiation by modulating wnt signaling. Biochemical and Biophysical Research Communications 402 (2):186–189. doi: 10.1016/j.bbrc.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Wang XZ, Hang YK, Liu JB, Hou YQ, Wang N, and Wang MJ. 2017. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncology Letters 13 (6):4825–4831. doi: 10.3892/ol.2017.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YW, Yu JY, Cui R, Lin JJ, and Ding XT. 2016. Curcumin in treating breast cancer: A review. Journal of Laboratory Automation 21 (6):723–731. doi: 10.1177/2211068216655524. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Du WJ, Piazza GA, and Xi Y. 2013. MicroRNAs are involved in the self-renewal and differentiation of cancer stem cells. Acta Pharmacologica Sinica 34 (11):1374–1380. doi: 10.1038/aps.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel NA, and El-Deiry WS. 2014. HIF-1 signaling in drug resistance to chemotherapy. Current Medicinal Chemistry 21 (26): 3021–3028. doi: 10.2174/0929867321666140414101056. [DOI] [PubMed] [Google Scholar]
- Warin RF, Chen HD, Soroka DN, Zhu YD, and Sang SM. 2014. Induction of lung cancer cell apoptosis through a p53 pathway by [6]-shogaol and its cysteine-conjugated metabolite M2. Journal of Agricultural and Food Chemistry 62 (6):1352–1362. doi: 10.1021/jf405573e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Senanayake TH, Warren G, and Vinogradov SV. 2013. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjugate Chemistry 24 (4):658–668. doi: 10.1021/bc300632w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Hong BH, Ho CT, and Yen GC. 2015. Targeting cancer stem cells in breast cancer: potential anticancer properties of 6-shogaol and pterostilbene. Journal of Agricultural and Food Chemistry 63 (9):2432–2441. doi: 10.1021/acs.jafc.5b00002. [DOI] [PubMed] [Google Scholar]
- Wu LC, Guo LX, Liang YH, Liu X, Jiang LH, and Wang LS. 2015. Curcumin suppresses stem-like traits of lung cancer cells via inhibiting the JAK2/STAT3 signaling pathway. Oncology Reports 34 (6):3311–3317. doi: 10.3892/or.2015.4279. [DOI] [PubMed] [Google Scholar]
- Xi G, Hayes E, Lewis R, Ichi S, Mania-Farnell B, Shim K, Takao T, Allender E, Mayanil CS, and Tomita T. 2016. CD133 and DNA-PK regulate MDR1 via the PI3K- or Akt-NF-kappa B pathway in multidrug-resistant glioblastoma cells in vitro. Oncogene 35 (42): 5576–5576. doi: 10.1038/onc.2016.64. [DOI] [PubMed] [Google Scholar]
- Xiao W, Gao ZY, Duan YX, Yuan WX, and Ke Y. 2017. Notch signaling plays a crucial role in cancer stem-like cells maintaining stemness and mediating chemotaxis in renal cell carcinoma. Journal of Experimental & Clinical Cancer Research 36 (1):41. doi: 10.1186/S13046-017-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Wang S, Zhao Y, Zhang ZC, Qin C, and Yang XJ. 2017. MiR-519d impedes cisplatin-resistance in breast cancer stem cells by down-regulating the expression of MCL-1. Oncotarget 8 (13): 22003–22013. doi: 10.18632/oncotarget.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhang L, Hu C, Liang S, Fei X, Yan N, Zhang Y, and Zhang F. 2016. WNT pathway inhibitor pyrvinium pamoate inhibits the self-renewal and metastasis of breast cancer stem cells. International Journal of Oncology 48 (3):1175–1186. doi: 10.3892/ijo.2016.3337. [DOI] [PubMed] [Google Scholar]
- Yang F, Xu J, Tang L, and Guan X. 2017. Breast cancer stem cell: the roles and therapeutic implications. Cellular and Molecular Life Sciences 74 (6):951–966. doi: 10.1007/s00018-016-2334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JZ, Wang CL, Zhang ZJ, Chen XJ, Jia YS, Wang B, and Kong T. 2017. Curcumin inhibits the survival and metastasis of prostate cancer cells via the notch-1 signaling pathway. APMIS 125 (2):134–140. doi: 10.1111/apm.12650. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sun N, Cheng R, Zhao C, Liu Z, Li X, Liu J, and Tian Z. 2017. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials 147:53–67. doi: 10.1016/j.biomaterials.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Hirohashi Y, Mariya T, Murai A, Tabuchi Y, Kuroda T, Kusumoto H, Takaya A, Yamamoto E, Kubo T, et al. 2017. Phosphorylation of HSF1 at serine 326 residue is related to the maintenance of gynecologic cancer stem cells through expression of HSP27. Oncotarget 8 (19):31540–31553. doi: 10.18632/oncotarget.16361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, AlmeidaPorada G, Ogawa M, Leary AG, Olweus J, Kearney J, and Buck DW. 1997. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90 (12):5002–5012. [PubMed] [Google Scholar]
- Yin T, Wei HJ, Gou SM, Shi PF, Yang ZY, Zhao G, and Wang CY. 2011. Cancer Stem-Like cells enriched in panc-1 spheres possess increased migration ability and resistance to gemcitabine. International Journal of Molecular Sciences 12 (3):1595–1604. doi: 10.3390/ijms12031595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Shin HS, Lee YS, Lee D, Kim S, and Lee YC. 2014. Genistein attenuates cancer stem cell characteristics in gastric cancer through the downregulation of Gli1. Oncology Reports 31 (2): 673–678. doi: 10.3892/or.2013.2893. [DOI] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, and Song E. 2007. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 131 (6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, and Wang ZY. 2011. A positive feedback loop of ER-alpha36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene 30 (7):770–780. doi: 10.1038/onc.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Ma J, Fan YL, Wang ZY, Tian R, Ji W, Zhang F, and Niu RF. 2018. TGF-beta transactivates EGFR and facilitates breast cancer migration and invasion through canonical Smad3 and ERK/Sp1 signaling pathways. Molecular Oncology 12 (3):305–321. doi: 10.1002/1878-0261.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Shen SJ, Zhou YD, Mao F, Guan JH, Lin Y, Xu YL, and Sun Q. 2014. ALDH1 is a better clinical indicator for relapse of invasive ductal breast cancer than the CD44(+)/CD24(−) phenotype. Medical Oncology 31 (3):1–8. doi: 10.1007/s12032-014-0864-0. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhao LC, Jiang N, Wang XL, Zhou XN, Luo XL, and Ren J. 2017. MicroRNA miR-590-5p inhibits breast cancer cell stemness and metastasis by targeting SOX2. European Review for Medical and Pharmacological Sciences 21 (1):87–94. [PubMed] [Google Scholar]
- Zhou ML, Hou YX, Yang GL, Zhang HL, Tu G, Du YE, Wen SY, Xu LY, Tang X, Tang SF, et al. 2016. LncRNA-Hh strengthen cancer stem cells generation in twist-positive breast cancer via activation of hedgehog signaling pathway. Stem Cells 34 (1): 55–66. doi: 10.1002/stem.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Ye M, Lu Y, Zhang H, Chen Q, Huang S, and Su S. 2015. Curcumin improves the tumoricidal effect of mitomycin C by suppressing ABCG2 expression in stem Cell-Like breast cancer cells. PLoS One 10 (8):e0136694. doi: 10.1371/journal.pone.0136694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Jiang Y, Yang X, Wang S, Xie C, Li X, Li Y, Chen Y, Wang X, Meng Y, et al. 2017. Wnt/beta-catenin pathway mediates (−)-epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochemical and Biophysical Research Communications 482 (1): 15–21. doi: 10.1016/j.bbrc.2016.11.038. [DOI] [PubMed] [Google Scholar]
- Zhu JY, Yang X, Chen Y, Jiang Y, Wang SJ, Li Y, Wang XQ, Meng Y, Zhu MM, Ma X, et al. 2017. Curcumin suppresses lung cancer stem cells via inhibiting wnt/beta-catenin and sonic hedgehog pathways. Phytotherapy Research 31(4):680–688.doi:0.1002/ptr.5791. [DOI] [PubMed] [Google Scholar]


