Abstract
Despite the numerous lives that have been saved since the first successful procedure in 1954, organ transplant has several shortcomings which prevent it from becoming a more comprehensive solution for medical care than it is today. There is a considerable shortage of organ donors, leading to patient death in many cases. In addition, patients require lifelong immunosuppression to prevent graft rejection postoperatively. With such issues in mind, recent research has focused on possible solutions for the lack of access to donor organs and rejections, with the possibility of using the patient’s own cells and tissues for treatment showing enormous potential. Three-dimensional (3D) bioprinting is a rapidly emerging technology, which holds great promise for fabrication of functional tissues and organs. Bioprinting offers the means of utilizing a patient’s cells to design and fabricate constructs for replacement of diseased tissues and organs. It enables the precise positioning of cells and biologics in an automated and high throughput manner. Several studies have shown the promise of 3D bioprinting. However, many problems must be overcome before the generation of functional tissues with biologically-relevant scale is possible. Specific focus on the functionality of bioprinted tissues is required prior to clinical translation. In this perspective, this paper discusses the challenges of functionalization of bioprinted tissue under eight dimensions: biomimicry, cell density, vascularization, innervation, heterogeneity, engraftment, mechanics, and tissue-specific function, and strives to inform the reader with directions in bioprinting complex and volumetric tissues.
Keywords: Functional tissue bioprinting, transplant, cell density, vascularization, innervation, heterogeneity, engraftment, mechanics
Graphical Abstract
1. BIOPRINTING: FROM A RESEARCH TOOL TOWARDS A TISSUE FABRICATION PROCESS
Three-dimensional (3D) printing has its root in polymer printing, which began with the invention of stereolithography by Charles Hull in 1986 [1]. Polymer printing gave rise to several branches of 3D printing, including metal printing and bioprinting [2]. In 1988, Klebe used cytoscribing technology for two-dimensional (2D) micropositioning of proteins to create 2D patterns [3]. By the late 1990s, scientists began to develop 2D bioprinting techniques with living cells using such micro-positioning techniques, which made complex tissue fabrication possible. A major breakthrough in this regard was seen in 2000 when Thomas Boland modified an inkjet printer to bioprint cells into a Petri dish, giving rise to the first bioprinter [4][5]. Anthony Atala of the Wake Forest Institute for Regenerative Medicine provided the first case of a fully printed organ, with the miniature kidney in 2002 [6]. The concept of tissue spheroids was later discovered in 2008, paving the way for rapid tissue and organ generation by implanting spheroids in scaffolds [7]. In the same year, Objet Geometries Ltd. developed multilateral printing, which further increased the potential developments in bioprinting [8]. In 2009, the first commercial bioprinter was Novogen MMX developed by Organovo [9], and scaffold-free vascular constructs were created [3]. Since then, various different bioprinters have been developed to bioprint a wide array of tissues from cartilage and bone to muscle and liver [10][11]. Such advances are targeted towards a common goal, which is to combine cells and biomaterials to fabricate tissue constructs. This allows for the accurate deposition of cells with high resolution and is, therefore, a promising tool for organ-on-a-chip drug screening systems and tissue fabrication for regenerative medicine [11][12]. Bioprinting can be combined with computer-aided design/computer-aided manufacturing (CAD/CAM) technologies [13][14], which allows the use of patient-specific images to develop anatomically correct tissues or organs with appropriate volumetric measurements. Thus, the wide diversity of knowledge is required in the field of bioprinting, with a variety of concepts pertaining to mechanical design, machine fabrication, and regenerative medicine coming together to find an answer for the current shortage of transplant organs and their side-effects.
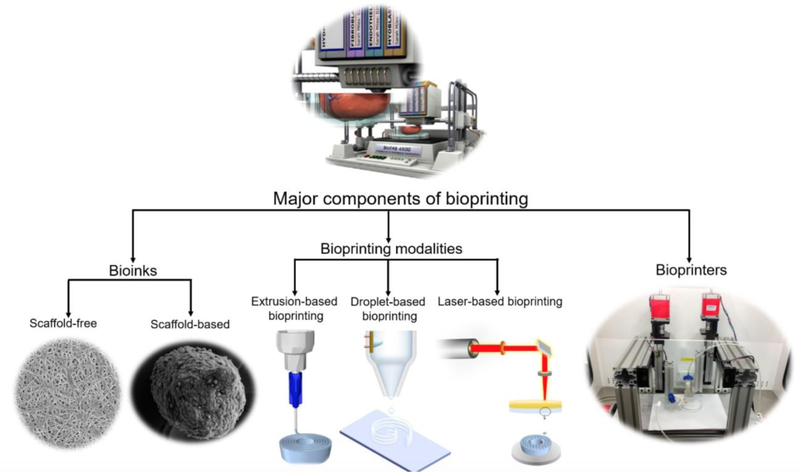
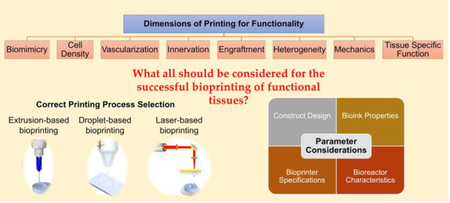
In order to execute bioprinting, three major components are essential, namely a bioink, an appropriate bioprinting process (modality), and a bioprinter (Figure 1). Any of the bioprinting modalities (extrusion, droplet, and laser-based bioprinting), alone or in combination, can be used for tissue fabrication [9].
Figure 1.
A schematic representation of the major components of bioprinting (Bioprinter concept image: courtesy of Christopher Barnatt.
1.1. Extrusion-based Bioprinting
Extrusion-based bioprinting (EBB) deposits bioink from a syringe or nozzle-based system onto a build platform based on a CAD design of the structure being printed. Based on the design, the software is able to determine the locations on the build platform where the bioink must be extruded onto, in order to generate the desired 3D structure. The extrusion is performed in the form of cylindrical depositions of the material, using pneumatic, mechanical, or solenoid driven, as shown in Figure 1 [15]. The deposition system is selected based on the sensitivity of the bioink, with the aim of gradually pushing it down without damaging cells present in the ink. Compared to other processes, it offers greater printing speed, and a large variety of bioinks are able to be used including scaffold-free inks (such as tissue spheroids and strands) or scaffold-based inks (such as hydrogels) [15]. Additionally, it provides better structural integrity due to the continuous deposition of filaments and easily incorporates with CAD software [9]. Post-printing cell viability is usually between 40% to 80% [16][17]. With the optimization of printing parameters such as deposition rate, pressure, and temperature, cell viability can be as high as 97% in the case of materials such as cell-laden gelatin methacrylamide constructs [18]. This technique has been derived from the material extrusion process from conventional additive manufacturing, and research has found EBB to be the most suitable method for the creation of large-scale constructs, primarily due to the aforementioned structural integrity. However, large constructs tend to affect cell viability, as the cells are exposed to dehydration and lack of nutrients during the long printing duration [15]. The technology also has a relatively poor resolution, with 100µm as the optimal [19][20]. EBB also requires the user to understand the rheology of the biomaterial, as shear stress on the wall of the nozzle tip and other areas of the printer may damage the living cells and alter the fluid properties, depending on its shear thinning capabilities [16].
1.2. Droplet-based Bioprinting
Droplet-based bioprinting (DBB) manipulates bioink stored in a cartridge to form droplets utilizing gravity, atmospheric pressure, and fluid mechanics, in order to form constructs by controlling fluid properties such as surface tension and viscosity [21]. DBB originates from traditional paper printing, with Klebe’s research in 1987 exhibiting the possibility of printing cells for the first time [3]. Four different methods for droplet formation can be utilized for this purpose, including inkjet [5][22], electrohydrodynamic jet [23][24][25], acoustic-droplet-ejection [26], and micro-valve [27]. Among these, inkjet bioprinting has been the most common process, due to its simplistic principle of utilizing fluid mechanics, atmospheric pressure and gravity to generate droplets [21]. Additionally, DBB offers many advantages from the perspective of biomaterials that can be used in this process, such as alginate, collagen, gelatin, and fibrin, along with providing the conditions needed for each material’s crosslinking mechanism and cell viability [9][21][28]. The aforementioned issue of resolution seen in EBB is much more complex in the case of DBB, which is dependent on the process being used [29]. However, DBB has attracted interest in researchers due to its simplicity and low cost, and the possibility of rapid deposition using multiple nozzles [21]. DBB has cell viability of greater than 85% in some cases and good control over deposition rate and bioink volume [5][30]. However, there is a tendency of the orifice to clog easily, and the types of bioinks able to be used are very limited compared to EBB, considering the rheological properties needed for this process [21].
1.3. Laser-based Bioprinting
Laser-based bioprinting (LBB) utilizes a laser pulse directed via mirrors onto a bioink layer above the substrate. This induces a high-pressure bubble to be formed by the ensuing heat, displacing some of the ink and spurring cell-laden droplets to be deposited on the substrate. This process is repeated several times in a layer-wise fashion to fabricate the final construct [31][32]. LBB consists of two types, including processes based on photopolymerization (i.e., stereolithography and its modifications) and processes based on cell transfer (i.e., laser-induced forward transfer) [20]. The processes based on cell transfer use a laser pulse to create a bubble at the interface of a donor layer and ribbon layer which propels the bioink to create a jet [33]. They have high initial cell viability at greater than 95%. However, this tends to decrease with time, and become less than extrusion- and droplet-based printing [34]. On the other hand, processes based on photopolymerization use an ultraviolet (UV) laser to cure photocrosslinkable hydrogels in a vat. Compared to EBB and DBB, LBB is a complex process, requiring complex machinery and extensive process control. The complexity associated with the use of a laser affects cell viability and material properties [35]. In addition, the process also requires the biomaterial to be compatible over several criteria, from fluid mechanics to rapid crosslinking after printing, introducing greater limitation for materials than DBB [32]. However, such complexity offers several benefits. For example, LBB has the highest resolution and precision among all three methods [32]. A unique advantage of LBB is to print multiple materials with good cell density, introducing the possibility of printing multiple cell types [36], which may provide the key for printing complex organs and tissues. However, the needs of using LBB to prepare the construct with high resolution (< 50µm) and cell density has to be considered, as LBB requires a highly intricate setup, making it the most expensive modality, and the long-term effect of laser exposure on the cells is still unknown [32][33].
Apart from laser, other light sources have been used as well, such as ultraviolet (UV) lamp and light-emitting diode (LED) sources A common example would be digital light projector (DLP), with the use of selective UV radiation to create layer-wise solid that builds from liquid photopolymer. However, the use of UV radiation induces the creation of free radicals that can cause cell damage and death [37]. Several studies have experimented on utilizing cell suspension in prepolymer solutions, and employed masks for inducing UV exposure only in the desired areas for the purpose of dimensional accuracy and formation of vasculature [38][39]. Several parameters and material combinations have to be controlled here for effective tissue printing and adequate cell viability. Despite the added complexities, porous hydrogel structure fabricated by this process show narrow pore size distributions, good pore interconnectivity, and enhanced mechanical properties, with certain materials showcasing superior cell adhesion and proliferation [37].
Although there have been many bioprinting successes, including but not limited to skin [40][41], bone [42][43][44][45], cartilage [42][46][47][48], liver [27][49][50], and heart [51][52][53][54], many challenges must be overcome before human-scale organs become a reality. It is necessary to understand the advantages and limitations of each method of bioprinting in order to select the correct one for a given tissue and/or application. Table 1 summarizes the different aspects that should be considered for this purpose. A major issue at this stage is functionality, with printed organs only offering a fraction of the functionality of its natural counterpart [17]. Most printed tissues have only one dimension of functionality, but native-like tissue should be bioprinted in a way that they will address most of the dimensions at the same time. In this perspective, the functionality of the bioprinted tissue is categorized into seven dimensions including cell density, vascularization, innervation, heterogeneity, engraftment, mechanics, and tissue-specific function.
Table 1:
Differences between several parameters based on the printing methods: extrusion-, droplet- and laser-based bioprinting.
| Property ╲ Printer | Extrusion-based | Droplet-based | Laser-based | References |
|---|---|---|---|---|
| Material | Large variety of biocompatible materials | Multicell printing is possible, critical for complex organs | High gelation speed | [162] |
| Viscosity | 30 mPa·s to >6 × 107 mPa·s | 3.5–12 mPa·s | 1–300 mPa·s | [17][72][62] |
| Gelation Methods | Chemical, photocrosslinking, shear thinning, temperature | Chemical, photocrosslinking | Chemical, photo crosslinking | [17][72] |
| Mechanical Properties | High with good structure integrity | Low with poor structure integrity | Low | [202][70][73] |
| Preparation Time | Medium | Low | High | [17] |
| Resolution | 5 μm to a few millimeters | <1 pl to >300 pl droplets, 50 μm wide Cell | Single cell manipulation possible | [21][17][32][62] |
| Accuracy | Low | High, with high throughput | High | [21][26] |
| Print Speed | Slow (10–50 μm/s) | Fast (1–10,000 droplets per second) | Medium-fast (200–1,600 mm/s) | [26][17][46][62] |
| Nozzle Dynamics | Shear stress induced by nozzle wall and extrusion pressure | Non-contact Nozzle, but nozzle can clog | Nozzle Free | [21][26] |
| Cell Damage Source | Damage due to shearing in the nozzle | Cell aggregation may occur | Damage due to generation of heat | [21] |
| Cell Viability | 40% – 80% | >85% | >95% | [21][17][73][115][46] |
| Cell Density | High, cell spheroids | Low, <106 cells/ml | Medium, 108 cells/ml | [17][62] |
| Printer Cost | Low | Medium | High | [17][63] |
2. DIMENSIONS OF BIOPRINTED TISSUE FUNCTIONALITY
2.1. Biomimicry
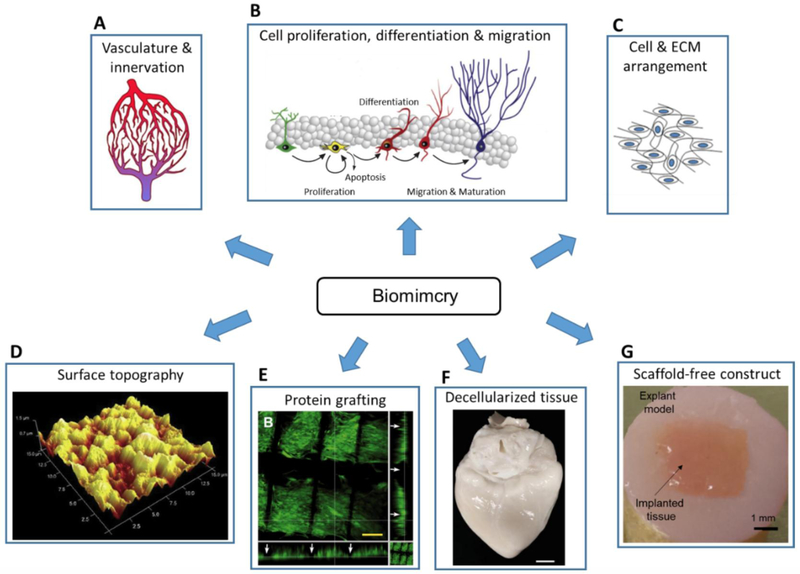
Organisms have evolved over millions of years to be composed of tissues and organs with ideal functional designs, and human beings are no exceptions. Researchers consider such evolutionary changes to the body, which are meant to better adapt to the environment, as critical factors for functional design, giving rise to biologically inspired engineering, or biomimicry. Application of biomimicry can be seen over vastly different fields, such as aeronautical engineering [43], civil engineering [55] and material science [44]. Biomimicry in bioprinting is targeted towards gaining functional accuracy in the printed tissues and organs. This is especially critical for replicating heterogenous tissue with complex internal vasculature, circulatory, and neural structures (Figure 2A). Several other noncellular factors must be considered for tissue sustenance and viability, such as cell arrangement, ECM composition, micronutrient requirements and the surrounding environment [17]. Understanding the properties, advantages, and disadvantages of these factors are critical for successful attachment, and the subsequent functions such as migration, proliferation, and differentiation, culminating in stability and adequate functionality of the tissue or organ (Figure 2B). The surrounding environment and biomaterials being used for the construct do play a vital role in determining the cell size and shape (Figure 2C) [56][57]. Another example of biomimicry would be the addition of surface ligands with the aim of improving cell attachment and proliferation [58]. The surface finish of constructs also plays a major role in attachment and proliferation of cells on polymers and metals, and surface features such as groves and notches should be used to improve attachment (Figure 2D) [59].
Figure 2.
(A-C) Considerations for the biomimicry of bioprinted constructs including but not limited to (A) vasculature, innervation; adapted, with permission, from [189], (B) cell proliferation, differentiation, migration; adapted from [190] and (C) ECM arrangement; adapted, with permission, from [191]. (D-G) Strategies to induce biomimicry of tissue constructs, such as (D) modification of surface finish to promote cell attachment; adapted, with permission, from [192], (E) graphing of bioactive protein on polymer to enhance cell proliferation; adapted from [193], (F) utilization of decellularized tissue to induce cell differentiation; adapted, with permission, from [194], and (G) fabrication of scaffold-free constructs to imitate cell density of native tissue; adapted from [48].
Furthermore, biomimicry factors that can be manipulated to yield better results go beyond the cellular scale, with several proteins (collagens, proteoglycans, and glycoproteins) (Figure 2E) and nanoscale factors influencing cell motility, space filling, intracellular signaling, gene expression and transcriptional activity [60]. Tissue decellularization is also a key feature in studying the composition and functions of extracellular matrix (ECM), enabling researchers to isolate the components needed and eliminate components that may cause toxicity for an improved output (Figure 2F) [17]. Figure 2 highlights several strategies in practice that use biomimicry in tissue engineering. Another aspect of biomimicry being explored is scaffold-free bioprinting, as scaffolds do not occur in nature, and are used for structural purposes in bioprinting (Figure 2G). With research on biomaterials advancing fast in recent years, researches are able to better harness their potential to eliminate scaffold, by fabricating degradable scaffolds, controlling the ECM mechanisms of bioprinted cells, and facilitating better removal of their byproducts [17].
2.2. Cell density
Organs are complex and made up of multiple cell types in varying densities. Each organ has a specific number of cells often in high density. The liver, for example, has 1.3×108 cells per gram of tissue [61]. These densities need to be replicated during bioprinting. However, they are dependent on the bioprinting modality and bioink being used. Extrusion bioprinters can print constructs with high cell density (e.g., cell aggregates) whereas inkjet printers deal with the lowest cell density (<106 cells/mL) [62][63]. Two main categories of bioinks include scaffold-based and scaffold-free bioinks [64]. Scaffold-based bioinks consist of cells within a decellularized ECM components or hydrogels, where both natural and synthetic hydrogels can be used [33]. Scaffold-free bioinks contain a high concentration of cells without a supporting exogenous material, including tissue strands, cell pellets, and spheroids, where the cells generate their own ECM [15][65][66]. Cell density in scaffold-based bioprinting is generally less than 107 cells/mL, whereas density in scaffold-free bioprinting is much higher [67]. For example, tissue spheroids and strands contain tens of thousands of cells per several hundred micrometers [68] and 15 million cells per centimeter length [48], respectively.
Cell content varies in different printing modalities with EBB allowing the highest densities (e.g., tissue spheroids with ~8,000 cells in ~300 µm diameter) and DBB the lowest (<106 cells/mL) [4]. Higher cell content allows for better cell-cell interactions and faster tissue formation but can affect viscosity and printability of the bioink [62], since increased cell concentrations tend to increase the viscosity. Higher viscosity bioinks keep cells in a more precise location, and viscosity can be altered with changes in both temperature and gelation concentration [62]. However, decreased printability may result from high viscosity, which induces an increase in the shear stress experienced during bioprinting when dispensing cells through a nozzle (EBB and DBB) or transferring cells from a donor slide to a receiver slide (LBB).
Printability is influenced by several parameters including viscosity, gelation, shear thinning, yield stress, and shear recovery, with the onus on being able to achieve a balance between these parameters which makes printing with the material feasible. Improvements in one area often lead to a reduction of another making this a complex process. Furthermore, these parameters are directly linked to print fidelity and mechanical strength [69][70][71]. A good biomaterial for printing is one that stays in liquid form during printing in order to avoid jamming in the printer, especially in the nozzle, and becomes solid once it is deposited on the construct in order to maintain its shape. Thus, viscosity is a factor that should be adjustable in biomaterials [72]. Shear stress also negatively affects the cell viability and is dependent on the bioprinting modality. For example, EBB can print viscous bioinks [73] with very high cell density [74] and without affecting viability, whereas DBB requires the lowest viscosity and cell density [75]. Even if a material looks promising printable biomaterial, it is necessary to test the range of values for these parameters to determine whether the properties make it readily printable, repeatable, and economical.
Additionally, cell density will affect cell proliferation and interaction post-bioprinting. Cells require ECM to provide a supportive environment, which is provided by the bioink. Researchers have shown that a higher cell seeding density accelerates ECM remodeling [76][77]. It has been found that utilizing autonomous self-assembly results in higher final cell density, improved cell interactions, and improved long-term function [48][78]. However, it is difficult to control the outcome during this process. Spatial placement of high cell density is also important as organs contain groups of cells in various locations [79]. For example, the pancreas has a high density of beta cells which form islets, and these are surrounded by other cells in a much lower density [80]. Spatial placement can be affected by printer resolution. LBB has the highest resolution (20 µm), whereas EBB has the lowest (> 100 µm), and DBB is in between (50–100 µm) [33]. Cells should be bioprinted in high densities, but considering that each organ has differing densities, they may still need to proliferate after bioprinting to achieve the densities seen in native organs. Temperature, gelation concentration, initial cell density, and time elapsed from the initial cell suspension have all been shown to affect final density and spatiotemporal location of cells [79]. Adjusting these parameters will ultimately allow for the creation of a construct that most closely matches native organs. For example, hydrogels printed at a higher temperature may be more fluid and therefore the initial placement will be more altered after bioprinting. Conversely, a more solid bioink would keep the cells in their initial positions. More research is still needed on the redistribution of cells post-bioprinting and creation of highly viscous hydrogels [62].
2.3. Heterogeneity
3D bioprinted tissues and organs could be designed to mimic the cellular density of native biological structures, with proper consideration given for cellular components, ECM, and 3D spatial orientation. The main constraint in this regard is the heterogeneously complex nature of most organs, with the presence of many different cell types. While the majority of bioprinting research has focused on homogeneous constructs, some groups have printed composite tissues (Figure 3) [81][82][83]. One of the major advantages of some bioprinters is the ability to deposit more than one cell types, making the construct a closer match for the natural architectural arrangement [84]. Recent studies have started focusing on heterogeneous tissue development in bioprinting. For instance, Butcher et al. printed aortic valve heterogeneous cells using EBB (Figure 3A) [19][85], whereas others have used human mesenchymal stem cells combined with endothelial progenitors to bioprint multicellular constructs using EBB [11][86]. In general, the DBB modality is more suitable for generating heterogeneous cellular structures with higher spatial resolution compared to other modalities (EBB and LBB) due to its ability to bioprint multiple cell types in the form of a droplet < 100µm in size [21]. Figure 3B shows droplet-based bioprinted heterogeneous constructs, where cells labeled in different colors were precisely deposited in pre-defined patterns [87].
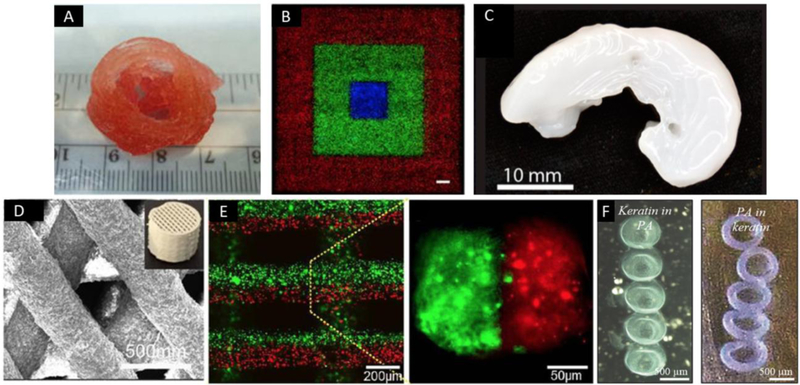
Figure 3.
Examples of heterogeneous constructs generated by bioprinting. (A) 3D printed aortic valve conduit with smooth muscle cells and aortic valve leaflet interstitial cells encapsulated; adapted, with permission, from [19]. (B) Cell patterning with different fluorescence using direct inkjet printing (scale bar=1 mm); adapted from [87]. (C) 3D printed sheep meniscus with bioink of nanofibrillated cellulose and alginate (scale bar=2 mm); adapted, with permission, from [91]. (D) 3D printed scaffolds of calcium sulfate and mesoporous bioactive glass; adapted from [96]. (E) 3D construct with two separated bioinks which simultaneously extruded by a microfluidic system; adapted, with permission, from [97]. (F) Self-assembly of peptide-protein (peptide amphiphiles and keratin) bioinks after bioprinting; adapted, with permission, from [89].
The bioink itself can also be a heterogeneous mixture. Even a small change in component concentration can significantly affect the construct [88]. Researchers have developed complex and biomimetic bioinks for various tissues to provide physical, chemical, and biological cues, as well as structural support [89]. Many biocompatible hydrogels lack structural integrity, and therefore combining components to maximize the advantages of each is a promising strategy. Often, a firm substance is used for its mechanical properties and combined with a softer substance which is biocompatible [34]. Rheological properties and change in state over a temperature range also play a vital role in certain biomaterials, and the advantages they offer are harnessed to reach the desired results. For example, several biomaterials are liquid at a higher temperature, making it more fluid and easier to deposit. However, if the said material is deposited on a platform cooled to below the solidification temperature, good structural integrity can be obtained for the construct being prepared [90]. Several such methods exist that are used to manipulate the properties of biomaterials. New components are always being evaluated to optimize bioink properties. For example, Gao et al. inkjet-bioprinted hydroxyapatite to increase compressive modulus for bone bioprinting [42]. Others have combined nano-fibrillated cellulose with alginate to increase the structural fidelity of bioprinted constructs (Figure 3C) [91]. Synthetic nano-silicates have also been used to increase mechanical properties [92]. Ouyang et al utilized dual-crosslinking to print a hydrogel bioink on hyaluronic acid, which showed no loss of mechanical properties over a one month period [93]. Such studies showcase the potential for altering the desired properties of the biomaterial via changes in material composition. However, this may affect the factors discussed previously, especially printability, and may necessitate additional experimentation to be established if significant changes have been made to such factors [94].
Bioinks should be designed based on their specific application/desired tissue, with several examples seen in literature. Graphene has been used in nerve bioprinting for its excellent electrical and thermal properties [95]. Bioactive glass has been used for bone tissue and showed enhanced bone formation in a study performed by Qi et al. (Figure 3D) [96]. Gold nanorod bioinks have been shown to enhance cardiac cell function due to their bioelectric properties [88]. Additionally, crosslinking bioink with alginate just prior to deposition has been shown to increase resolution and viability (Figure 3E) [97]. ECM-based bioinks use naturally derived materials from human tissue that more closely mimics the native environment, and can help with tissue phenotype regulation and functionality [98]. Self-assembling peptides are a simple biomaterial approach to provide both elements of the ECM [99], which can improve structural complexity and bioactivity. Recently, self-assembling peptides have been used to create a tunable nano-fibrous bioink which allows for manipulation of important gel features (Figure 3F) [89]. Such studies showcase the potential for materials that are not traditionally categorized as biomaterials to be used for Bioprinting applications. Such investigation provides the opportunity to integrate molecular self-assembly and bioprinting and can instigate a huge advancement in the field.
2.4. Vascularization
Vascularization is a critical factor for volumetric tissue biofabrication [100]. A vascular network must be incorporated early to prevent tissue death [34], and thus remains a major impediment, not only in bioprinting but also in tissue engineering and regenerative medicine. Novel techniques in bioprinting will likely solve this problem as vasculature can be printed in tandem with other tissue components to fabricate complex vascularized structures. Additionally, constructs can be printed from patient-specific medical images, which will allow the fabrication of anatomically-correct tissues. The current problem with vasculature is the heterogeneity that it introduces in constructs, the limitations for which have been discussed in the previous section. Constructs thicker than 200 µm require vascularization for transporting oxygen and nutrients to the cells and waste away from them [17]. After implantation, bioprinted tissues can slowly vascularize via angiogenesis. However, they require immediate perfusion [101]. Studies have shown that angiogenesis in gels takes up to 14 days for cells to migrate from perfused vessels and form intervening capillaries [102][103][104]. In these studies, collagen or fibrin gels were created with channels on both sides of the gel. The channels were then lined with endothelial cells in order to create an endothelium. Angiogenesis occurs quicker with shorter distances and the addition of promoting agents. Therefore, obtaining functional vasculature during construct fabrication is essential for tissue survival.
Various methods have been used for the creation of bioprinted vasculature. Channels can be printed by EBB using fugitive inks and later endothelialized [84][105]. Another alternative is using direct bioprinting to print channels. Dolati et al. developed a coaxial nozzle system using EBB to print vascular conduits, which were perfuseable [106]. These conduits were around 600–700 µm which are much larger than native capillaries. The advantage of this technology is that the conduits can be incorporated into larger constructs [107]. Other researchers have directly printed interconnected channels although architectural complexity is limited [108][109]. Several researchers have taken advantage of the high precision of LBB to successfully print microvasculature including bifurcations in vessel constructs [110][111]. Recently, DLP-based printers have also been used for developing vascular structures, exhibiting the versatility in process selection for exhibiting vasculature [38][112]. Figure 4 showcases several cases of fabricated tissues with vasculature which is critical to the development of the constituent cells. DBB can also be used to fabricate micron-sized channels for vascularization [113][114].
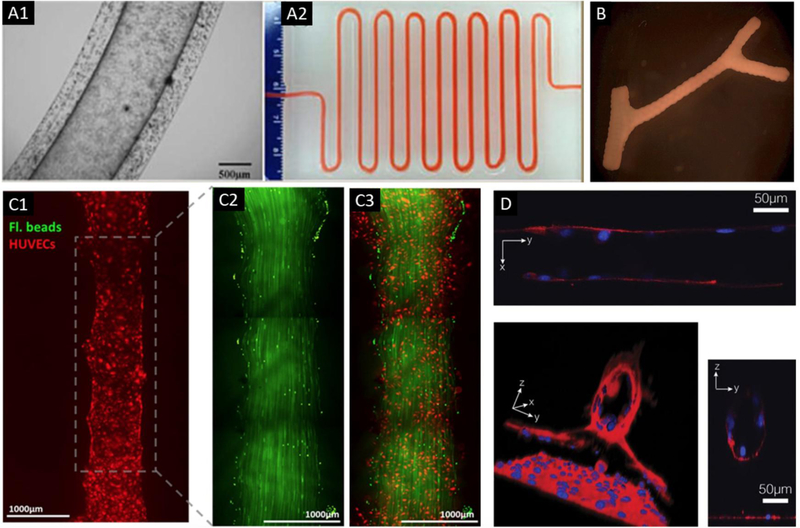
Figure 4.
Bioprinting of vascular constructs. (A) A vascular conduit bioprinted by a coaxial nozzle (A1) with cell media perfused through a meter-long printed conduit (A2); adapted, with permission, from [106]. (B) A scaffold-free tubular construct which was built by printing and fusing multicellular spheroids with a branched construct; adapted, with permission, from [195]. (C) Fluorescent images of a bioprinted vascular channel system, in which HUVECs (red) seeded into a printed channel (C1). Laminar flow in the channel was represented by green fluorescent beads (C2), and a combined image of green fluorescent beads and seeded HUVECs (C3); adapted, with permission, from [196]. (D) Direct cell patterning in collagen hydrogels using a near-infrared femtosecond laser, where confocal microscopy images showed tube formation at day 14; adapted from [110].
Current strategies produce perfusable channels which do not have native vessel anatomy, and these constructs will likely deteriorate in vivo [9]. Large native blood vessels consist of three layers, namely the intima, tunica media, and tunica adventitia. On the other hand, capillaries with a vessel diameter of 5–10 µm consist only of the endothelial intimal layer [115]. Large feeding and draining vessels in the bioprinted constructs must be amenable to surgical anastomosis for in vivo implantation. The media and adventitia layers of blood vessels add strength that allows for a suturable anastomosis, and therefore should be bioprinted when the construct is created, adding an additional challenge. Furthermore, it is not enough to generate perfusable non-branched tubes, as organs contain complex vascular networks ranging from large arteries and veins down to capillaries. It is difficult to bioprint capillaries in vitro, as most techniques are only able to generate vessels > 100 µm in diameter due to limits in bioprinter and bioprinting process resolution [34]. The highest resolution bioprinter, the laser-based printer, utilizes a droplet size of 20 µm [116]. Even if resolution improves, the process of printing capillaries would take a long time which would decrease cell viability [34]. To overcome this limitation, researchers have relied on capillary sprouting via angiogenesis after construct fabrication [117][118]. Angiogenic sprouting occurs at a rate of approximately 1.0 mm per week in vivo [115]. Endothelial cells can be bioprinted within the construct and the microcirculatory system may subsequently develop post-implantation [9]. Angiogenesis promoting agents such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and transforming growth factor (TGF) can also be added to promote new vascular development [119]. Various cell sources can be utilized for vascularization. Traditionally, human umbilical vein endothelial cells (HUVECs) have been utilized for vascular engineering. More recently, vascular progenitor cells have been used to stimulate angiogenesis [98].
2.5. Innervation
Some tissues, such as skeletal muscle and those containing smooth muscle (e.g., esophagus, stomach and bladder), require innervation to function. Without innervation, muscle tissue cannot function effectively and will atrophy. Therefore, they would benefit from pre-innervation. Even in organs where innervation is not critical for function, the nervous system plays important roles in adaptation, regeneration, immune interactions, and sensation [120]. Using 3D printing, custom nerve conduits which bifurcate and/or have multiple channels can be fabricated. Non-biodegradable conduits can lead to chronic inflammation, thus biodegradable materials are advocated [121]. Pateman et al. bioprinted nerve guidance conduits with 1 mm in diameter and 5 mm in length, which supported re-innervation across a 3 mm gap after 21 days (Figure 5A) [122]. Hu et al. created a nerve bio-conduit which supported re-innervation across a 1 cm gap (Figure 5B) [123]. Despite advances in conduit technology, elongation of axons for reinnervation is limited to gaps of < 3 to 4 cm [124]. Acellular conduits serve only as scaffolds, but the addition of Schwann cells to conduits appears to impact regeneration via the release of neurotrophic factors and signals. Schwann cells can be differentiated from stem cells. Figure 5 shows several cases of successful innervation in fabricated tissues.
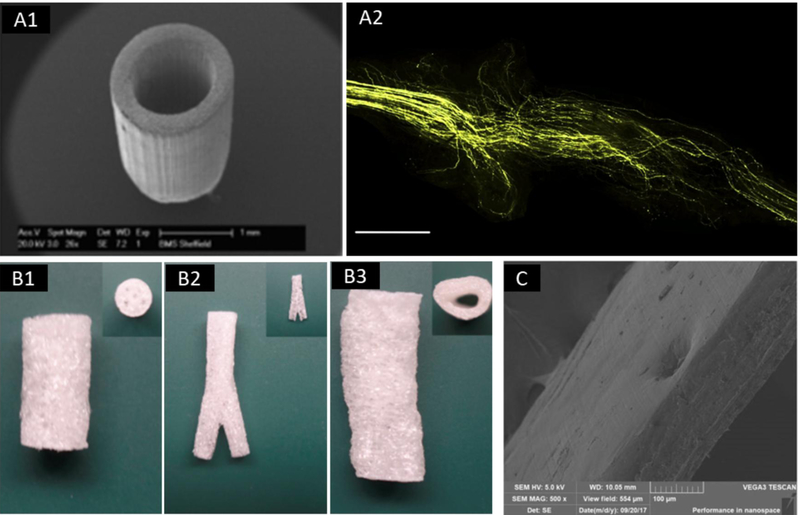
Figure 5.
Bioprinting of constructs for innervation. (A) SEM image of a poly(ethylene glycol) (PEG) nerve guidance conduits (A1) and the implanted nerve guide showing the organization of regenerated axon paths (A2, scale bar = 1.0 mm); adapted, with permission, from [122]. (B) 3D-engineered cellularized conduits for peripheral nerve regeneration with complex geometries, including multichannel (B1), bifurcation (B2) and reconstruction of patient’s sciatic nerve (B3); adapted from [123]. (C) SEM image of a nanoporous and multi-layered 3D structure in graphene-based nanomaterials for peripheral nerve restoration; adapted from [131].
Researchers have also been able to print nervous tissue, but this must be combined with other components of the organ and vascular tree. Owens et al. have successfully bioprinted a fully-cellular nerve graft using a scaffold-free bioink [125], where they combined Schwann cells with stem cells to help promote differentiation. Electrically conductive polymers such as polypyrrole [126] and poly-3,4-ethylene dioxythiophene [127] have been widely studied for neural tissue engineering. Additionally, carbon nanotubules have been used as reinforcement due to their enhanced mechanical properties [128]. Recently, graphene has attracted interest due to its unique electrical properties [129]. As previously mentioned, Wang et al. showed that graphene oxidized grafts facilitated nerve regeneration [130]. Qian et al. created a multi-layered scaffold consisting of graphene and polycaprolactone (PCL) which also promoted axonal regrowth and functional recovery (Figure 5C) [131].
These approaches can likely be integrated with organ fabrication to allow for tissue innervation. As with vessels, nerves have several components/layers which are required for function. In addition, electrical or biochemical stimulation will likely be required for integration of bioprinted nerve and tissue. Based on this, Ghasemi-Mobarakeh et al. developed conductive scaffolds that can accentuate neurite outgrowth and nerve regeneration [132]. Their study focused on different methods for improving cell attachment on conductive scaffolds. However, the mechanisms caused by electrically stimulated cells are still not clear enough to be replicated via bioprinting, and in vivo studies were not performed [133]. Tissue-derived neurotrophic and neural guidance factors, such as nerve growth factor and glial cell line-derived neurotrophic factor, drive axon growth during development and may help with organ innervation post-transplantation. Kang et al. treated differentiated muscle cells with agrin to stimulate acetylcholine receptor expression [134]. Despite such progress, further exploration is needed in order to obtain constructs which permit extensive innervation and allow researchers to venture towards printing complex organs.
2.6. Engraftment
As with allogenic organ transplantation, the immune response will need to be considered for bioprinted organs. Cell sources for bioprinted tissues include embryonic, fetal, amniotic, umbilical cord, induced-pluripotent, and adult stem cells [33]. Embryonic stem cells (ESCs) are isolated from the inner cell mass of embryos during the blastocyst phase of development and cultured on irradiated mouse fibroblasts [135]. The use of viable embryos leads to fierce ethical debate, as fetal stem cells are often harvested from aborted fetuses, and therefore have less ethical concerns than ESCs [136]. The use of amniotic fluid stem cells and umbilical cord stem cells both overcome all of the ethical concerns with the use of fetal tissue and are being used more often in current research. Induced-pluripotent stem cells are derived from adult fibroblasts which are reprogrammed to have pluripotency. The high risk of teratoma formation will limit their clinical translation [137]. Adult stem cells are mesenchymal stem cells which come from many sources including, but not limited to, bone marrow, adipose, heart, liver, dental pulp, and muscle tissues [63]. They have no ethical controversy and when used as an autograft (reinjected into the same person), their risk of rejection is essentially none, and therefore their utilization is increasing.
Cell source has been a major issue as many cells in constructs have led to a heightened immune response post-implantation [9]. A foreign body response can lead to a fibrous capsule around the construct, which leads to constructing failure. Despite this, numerous studies have reported successfully engrafted bioprinted bone constructs in a defect-matched or custom-designed manner to regenerate bone tissue [133]. However, many animal models used for transplant are immunocompromised to prevent rejection. EBB has been used to bioprint autologous cartilage scaffolds for auricular reconstruction [138] and cardiac patches for implantation in the region of myocardial infarction [51][139]. Recently, blood vessels were bioprinted with EBB using a scaffold-free approach and implanted in rat aortae resulting in complete remodeling and endothelialization [140]. Additionally, studies have shown that adult stem cells have low immunogenicity and immunomodulatory properties [141–143]. The use of a patient’s own stem cells (autologous transplant) may avoid the need for immunosuppression. Adipose-derived stem cells, in particular, have been shown to inhibit the immune response and exhibit immunosuppressive capabilities [144][145]. Figure 6 shows several cases of successful implantation of bioprinted constructs in animal models.
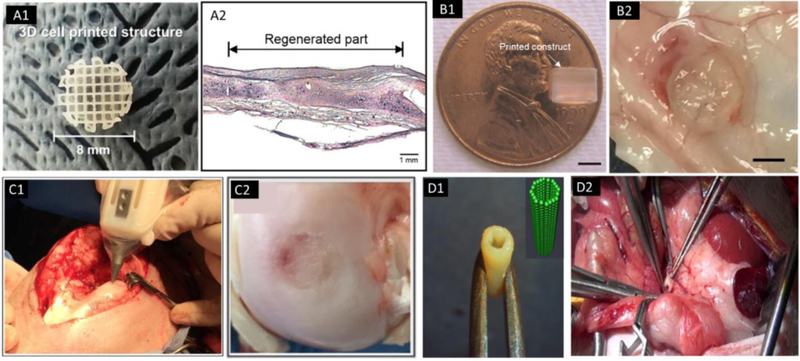
Figure 6.
Implantation of bioprinted constructs. (A) Image of a 3D cell printed structure using polycaprolactone (PCL) and chondrocytes-laden alginate (A1) and the regenerated tissue in a rabbit ear (A2); adapted, with permission, from [138]. (B) A printed PEG hydrogel construct using DBB, with 4 mm in diameter and 4 mm in height (B1, scale bar=2 mm); adapted, with permission, from [197], and gross morphology of implanted scaffold in mice subcutaneous pockets for 21 days (B2); adapted, with permission, from [198]. (C) Intraoperative bioprinting using a “biopen” for treatment of a full-thickness chondral defect in a sheep (C1), and the macroscopic appearance of the treated defect at 8 weeks after implantation (C2); adapted, with permission, from [199]. (D) A scaffold-free vascular graft was generated from multicellular spheroids using a “Bio-3D printer” with the insert showing the computer-designed model (D1), and the graft was used in an end-to-end anastomosis in the abdominal aorta of the nude rat (D2); adapted from [140].
In addition to the complexity of the fabricated tissue, the actual procedural handling and physical attachment of the tissue to native tissue is another major challenge. Handling properties are critical for the possibility of engraftment of bioprinted tissues, and is evaluated based on the tissues’ mechanical properties and resistance to deformation and damage during handling. In the case of the former, testing pertaining to stiffness is common [85][146], with the need for testing seen to be more for tissues using hydrogels, as scaffold-free constructs are prone to deformation and damage [4,38]. Additionally, it may depend on the printing process as well, as LBB has been found to be unsuitable for printing large constructs due to problems in handling [32].
When considering vascular tissues such as blood vessels, the smooth muscles and adventitia those present in native vessels for additional mechanical strength are absent in their fabricated counterparts, which can induce structural deformation after transplantation. It is necessary to strengthen such tissues sufficiently before implantation in order to match the ambient mechanical properties [66]. In the case, stronger tissues with a requisite set of mechanical properties for functionality, in vitro studies are critical for assessing the performance of the tissue after attachment. This can be seen in the study performed by Yu et al. for printed cartilage, after tissue implantation, explants were placed in culture in chondrogenic medium for to four weeks following which histology and immunohistochemistry were used to evaluate cartilage-specific markers expression and ECM formation around the implanted tissue [48].
Degradable biomaterials have different dynamics for acceptable attachment, as the strength, the construct offers can decrease with time. Thus, it is necessary to evaluate the needs of the tissue site and match the properties of the construct for the entire duration of the degradation and on-site tissue growth [94]. For this purpose, simulation-based studies and physical mechanical testing of the constructs at different stages of development are key for successful implantation and functionality [94]. The success of a bioprinted tissue is not evaluated just from its fabrication, but its effectiveness and adaptivity to the environment and application it has been developed for.
Additionally, bioink components must be cytocompatible, but without causing an immune response/inflammation. Some hydrogels such as alginate and polyethylene glycol are semipermeable, which may help mitigate the immune response by shielding passage of the larger immune cells [147]. Encapsulation of cells can also help protect them from the host immune response [148]. Both Vegas et al. [149] and Hiscox et al. [150]. have developed encapsulation devices which showed enhanced survival of beta cells after in vivo implantation. Levato et al. studied microcarriers for bone tissue engineering and showed improved compressive modulus, cell adhesion, and osteogenic differentiation with microcarrier-laden bioinks [151]. There is still much debate regarding whether microcarriers are needed with the use of adult stem cells for the reasons mentioned above.
2.7. Mechanics
Besides the need to be biocompatible and biodegradable, a bioink formulation must possess suitable physicochemical properties in order to fabricate 3D constructs with high resolution and print fidelity [152]. Tissues must be rigid enough to maintain their structure, yet porous enough to allow cell growth and vasculature. Native tissue rigidity ranges from 0.2–5 kPa in soft tissue such as the brain, to 15,000 kPa in bone [152], and construct shear stiffness values range from approximately 100 Pa to 20 kPa [153]. Soft elastic scaffolds (0.1–5 kPa) tend to promote neuronal and adipose tissue differentiation, whereas firm scaffolds (8–30 kPa) promote differentiation into muscle, cartilage, and bone [154][153]. This represents why it is important to recreate the native environment of the desired tissue type. Viscosity, crosslinking strength and speed, and yield stress of a defined bioink will directly affect the mechanical properties of bioprinted tissue constructs. Different bioprinting processes have varied requirements for rheological and mechanical properties, with EBB being used to develop stronger and structurally complex scaffolds and DBB using the fluidic properties of its biomaterials to fabricate constructs. The supported viscosity of EBB ranges from 3 to 6 × 108 mPa·s, DBB from 3.5 to 12 mPa·s, and LBB from 1 to 300 mPa·s [155].
Mechanical properties for constructs can vary with time and temperature. For example, a bioink needs to be malleable enough for successful printing, and at the same time retains its shape post-printing for sufficient mechanical strength and dimensional integrity. However, this transition is not instantaneous, and goes through gradual hardening with a certain green strength, depending on the porosity of the sample. This transition and change in strength are especially critical for load-bearing tissues such as bone, with research showing that the green strength of porous 3D-printed samples is heavily influenced by printing parameters [156]. Farzadi et al. showed that the appropriate printing orientation and layer thickness are necessary for obtaining desired physical and mechanical properties in a bioprinted bone tissue [156]. For more flexible load-bearing tissues such as cartilage, directional loading is a common phenomenon exhibited to match the ambient loading conditions [157]. To replicate this in bioprinted tissues, the introduction of embedded biocompatible fibers in hydrogels in the direction of loading can be a favorable option to meet such requirements of mechanical properties in bioprinted cartilage [158][159][160]. Overcompensation, however, is not an option in such tissues. It can lead to several issues in bone and cartilage repair, such as inadequate mobilization in healing fractures, and the formation of wide, unsightly cutaneous scars. This is further compounded by the difficulty in quantifying a point at which strength becomes excessive [161].
Hydrogels are often unable to display the requisite levels of cell growth and proliferation for the construct [162]. They have weak mechanical properties and are highly permeable as they swell under physiological conditions [163]. Hydrogels such as chitosan, collagen, fibrin, hyaluronic acid, Pluronic, and polyethylene glycol have poor mechanical strength [33], while dECM also has poor mechanical strength. Therefore, some groups have bioprinted a mechanically superior frame using polymers (e.g., PCL and polylactic-co-glycolic acid) to generate strong tissue constructs with a porous architecture utilizing both EBB and DBB [10][164][165], where the frame is then infilled with cell-laden bioink. However, these polymers have a very slow degradation rate, which may interfere with tissue regeneration and integration [9]. Microcarriers can also provide a mechanically strong scaffold [166].
Biochemical and mechanical factors both have important implications in guiding the behavior of cells in vivo, which, however, are rarely considered in the context of tissue biofabrication. Mechanical stimulation may be required before transplantation to improve construct properties and allow more physiologic cell growth [167]. For example, mechanical stretching has been used in the post-printing maturation of cardiac tissue [168]. Additionally, tissues such as bone and cartilage are exposed to high mechanical stress in vivo and are not uniform throughout the body, which means different locations for bones and cartilages showcase different loadbearing capacities [169] and have different nutritional and vascular requirements [170]. Thus, simple control of mechanical properties by adjusting material and printer variables is not enough, and multilateral capabilities, mechanical stimulation for maturity of the tissue and developing tissue designs similar to the native tissue may be necessary to fulfill the requirements of the tissue [171]. For the case of clinical trials with humans, post-operative procedures such as physiotherapy may also be crucial for tissues that are critical for the mobility of the body [172]. This is discussed in further detail for other tissue types in the following section.
2.8. Tissue-Specific Function
Organs are complex structures with distinct functions, including hormonal and protein secretion, filtration, and protection. Thus, despite similarities in properties such as the constituent material(s) and structure, tissues can demonstrate disparate properties. These functions have to be considered during designing, fabrication, and testing of constructs. Several bioprinted tissues have been subcutaneously implanted into animal models and shown some specific functionality. However, most tissues have several different functions. Several studies can be found in the literature that have performed in vivo studies to observe the functionality of bioprinted constructs. Yanez et al. used DBB for fabricating bilayer skin grafts in nude mice, using dermal fibroblasts and epidermal keratinocytes in collagen. It was observed that wound contraction improved by up to 10% compared to controls, and the new skin had similar architecture to normal skin [173]. Moller et al. showed preserved mechanical properties and increased stiffness of bioprinted (LBB) cartilage tissue after 60 days in vivo [146]. Furthermore, Fedorovich et al. encapsulated human chondrocytes and osteogenic progenitors in alginate, bioprinted it using EBB, and showed osteochondral tissue formation after 6 weeks of implantation in immunodeficient mice [86]. Gaebel et al. seeded HUVECs and mesenchymal stem cells on a polyester urethane urea cardiac patch using EBB, which was then transplanted into the infarct zone of rat hearts. These patches showed increased vessel formation and significant functional improvement in the infarcted areas. Additionally, there was enhanced capillary density and integration with the murine vascular system [52].
In vivo studies are critical for substantiating claims made by bioprinting research that involve implantable constructs. A major downside to this is that such studies are time intensive and cannot be sped up, as the primary objective is to observe the reaction of the body to the implant [174]. However, the benefits of animal studies far outweigh its drawbacks and provide one of the final steps for any bioprinting research to be considered a serious contender for human use. This has become especially critical over the last few years, with bioprinting research having shed the “novelty research” tag, and researchers have moved up from just printing constructs and developing printing techniques to study the potential applications and the practicality of their research with relation to human medicine. Table 2 provides a comprehensive summary of the considerations that must be made for each of these features when designing and fabricating a construct.
Table 2:
Considerations about the target organ/tissue that are needed before printing, in order to determine ideal material and printing properties.
| Features | Considerations |
|---|---|
| Biomimicry | Inculcating relevant biomimicry concepts in the design may aid to enhance its properties and success rate |
| Cell Density | The density of the target organ/ tissue and the variation seen in it is critical for fabricating the printed part and will determine several parameters such as printing method, viscosity, and rheological properties. |
| Heterogeneity | The different types of cells that are present and their composition is critical for determining the complexity of fabricating the part, selection of cells and biomaterials, and construct material and design. |
| Vascularization | Studying how vasculature is present in the target organ/tissue is critical for increasing the viability of the construct |
| Construct design should be targeted towards accommodating vasculature, and appropriate layer thickness should be selected. | |
| Innervation | Similar to vasculature, it’s necessary to understand how the nervous system is distributed, especially in tissues where stimulation is critical, such as cardiac tissue. |
| Engraftment | Using the patient’s stem cells are key to the body accepting the printed part without undesirable local or systemic responses |
3. OTHER CRITICAL EXTERNAL FACTORS FOR TISSUE/ORGAN GROWTH AND MATURATION
3.1. Design Considerations for Bioprinting
As mentioned earlier, bioprinting should not only strive towards developing constructs that are similar to the tissue which it is aimed at replicating. A critical factor for completing this objective is the accuracy of the construct’s design. However, researchers should also focus on utilizing design concepts such as design for manufacturing (DfM) and design for additive manufacturing (DfAM). DfAM deals with design considerations that must be made for the printing process being used. The ideal process for fabricating any product is not devoid of limitations, and product design must be able to leverage its advantages while reducing the effects of its limitations. Bioprinting has evolved from additive manufacturing and has subsequently inherited both its pros and cons. Issues that arise from printing which are not seen in conventional processes, such as delamination, reduction in part density, and surface roughness must be considered during construct design [175]. An additional design consideration which controls surface finish and construct strength is slicing and build orientation. Selecting the correct build orientation for a construct is crucial for a successful print, with a balance between the following orientation options – maintaining the height as the smallest possible part dimension [176], orienting the part such that certain desired surface(s) is kept smooth and devoid of any staircase effect due to printing [177], and prevent part failure due to delamination by considering the direction of applicable forces [178]. Furthermore, certain scaffolds may also need careful post-processing, depending on the type of printing process used, part design and constituent material(s) [179]. Thus, scaffold design is a critical factor for successfully bioprinting an organ, and being one of the earlier steps in the process, it is imperative that researchers understand how to design a scaffold in order to achieve the desired structure and functionality from their printed organ.
3.2. Bioink Considerations for Bioprinting
The success of bioprinting and the maturation of a printed construct is dependent on several factors pertaining to the bioink in use. Depending on the printing process selected, several considerations must be made in order for successful fabrication, starting with selecting the right printing method. Extrusion printing usually targets constructs that need considerable mechanical strength. For this, bioink being extruded must have adequate mechanical properties [62], with the aim of the material being soft enough to extrude, but hardening to take shape after deposition. There are several methods employed to achieve this, the most common of which is the gel form of bioink. The target of printing bioinks is to make it printable (i.e., feasible to deposit on the build plate), but make it adhere to the desired shape as soon as the printing is complete. The support material should be soft enough to have adequate fluidity for it to flow through the printer and behave as a printable ink. On the other hand, the gel should subsequently solidify within seconds to allow it to sustain the desired shape. Gelation methods are the key to this property and can be based on either physical or chemical crosslinking. For example, the gel can be extruded into a secondary gel that fulfills the role of a support material [62]. Additionally, the gel construct must also be easy to extract in post processing without any damage. Several other methods, such as photocrosslinking of the material after extrusion, can also be used to enhance its mechanical properties [88]. Unlike chemical crosslinking, the network formation of a physical hydrogel is reversible and is caused by ionic interactions, high molecular chain entanglements, hydrogen bonds and/or hydrophobic interactions [162].
In the case of DBB, fluid mechanics is a critical feature of the process, as the surface tension is inversely proportional to the cell concentration in the bioink, which is primarily due to more cells are adsorbed to the liquid-gas interface. Thus, the surface tension is lowered with the reduction in the total free energy [62]. Additionally, the shear stress characteristic to this process can negatively influence the cell viability. As a result, the bioink must exhibit low viscosities (< 10 mPa·s) and cell densities (< 106 cells ml−1). For the fabrication of the predesigned 3D constructs at high spatial resolution, the bioink must exhibit fast crosslinking. Among the suitable cross-linking mechanisms, ionic crosslinking of sodium alginate containing bioink is frequently used. On the other hand, temperature-dependent gelation of Matrigel or enzymatic driven polymerization of fibrinogen has also been seen [62], showing several potential methods to be chosen for a given case.
The use of nanocomposites is another aspect that has been explored to evaluate its effects on bioinks and applications in bioprinting. Considering their mechanical strength, nanocomposite hydrogels are beginning to be adopted in fields such as optics, sensors, actuators, tissue engineering, etc. Compared to their conventional counterparts they showcase higher physical strength, conductivity, stiffness, and greater stability [180]. The superior nature can be attributed to the combination of hydrogel matrices and nanofillers, with the later opening the door to several future inorganic materials that can be incorporated with hydrogels for enhanced properties. These inorganic particles enhance the mechanical strength, thermal stability, optical properties, magnetic strength, bioactivity, and conductivity of hydrogels [180]. With hydrogel nanocomposites being used for detoxification purposes and drug delivery in modern medicine, they can now perform functions such as attracting, capturing and sensing toxins, using the 3D matrix of the hydrogel as a medium to perform such functions [181]. Though the use of nanocomposites provides several potential applications, toxicity and other side effects remain a deterrent in its use [180]. Considering the growing ambitions of regenerative medicine, such technologies can be the key to the fabrication of complex and heterogeneous functional tissues and improve critical properties in them.
3.3. Bioprinter Considerations for Bioprinting
While the selection of the appropriate bioink is primarily based on the application at hand and the tissue being fabricated, it is necessary to select a complimentary printing process for this biomaterial, with appropriate parameter settings. In the case of an extrusion printer, it is necessary to select the right extrusion mechanism, depending on the sensitivity of the bioink. A piston-driven system may provide more direct control over the flow of the bioink when compared to pneumatic-based systems, which is prone to delays associated with the compressed gas volume. Screw-based deposition provides better spatial control and is capable of dispensing bioinks exhibiting higher viscosities [33]. However, the larger pressure drops generated by this extrusion method can be harmful to the suspended cells due to the possible disruption of the cell membranes, resulting in cell death [62]. In general, the limitations of each bioprinting technique with their key features must be kept in mind for the selection of bioprinting methods (Table 1).
3.4. Bioreactors
Compared with conventional AM, 3D bioprinting involves additional complexities, such as the choice of bioink materials, functional cell types, growth and differentiation factors, and technical challenges related to the sensitivities of living cells and tissue construction. Both adult and stem cells are now being used in printed tissues. Particularly, the bioink selection is highly crucial for tissue-specific function as the bioink can be biologically and chemically functionalized (e.g., use of RGD peptides, nanoparticles, genes, etc.), or structurally modulated for the desired stiffness in order to guide the differentiation of stem cells into tissue-specific cell lineage [162].
Specific bioreactors for various organs will likely be required for organ maturation, establishing the environmental requirements of fabricating such organs (Figure 7). The bioreactor should provide a microenvironment which allows for the development of construct similar to native tissues. For example, the bone may require a bioreactor which provides a stepwise increase in mechanical stress as tissue coherence increases [115]. Cartilage is subject to constant forces [182] and therefore their bioreactors must induce shear stress and compression. Takebe et al. used a rotating wall vessel bioreactor to differentiate cartilage progenitor cells to mature chondrocytes [183]. Chang et al. improved this technology by creating a double chamber bioreactor to fabricate biphasic osteochondral grafts [184]. Nerves and muscle tissue may require electrical stimulation and blood vessels may need pulsatile flow. Bioreactors for vasculature and larger tissue constructs must contain a circulating loop to supply perfusion to the constructs. Song et al. developed an integrated vascular bioreactor system which mimicked the mechanical stimulation and fluid flow on arteries in vivo. Their study showed that dynamic culture condition yielded significantly better results compared to static culture [185]. More specialized bioreactors have been developed for heart valves containing compartments separated by a septum with the tissue engineered valve fixed in the middle of the septum to mimic native anatomy. A pulsatile pump is used to simulate blood flow bursting through a one-way valve [186]. Despite these successes, most bioreactors have low volume output, are extremely time-consuming, and require considerable time for tissue formation [187]. With continued research in this area, it can be expected that a novel bioreactor capable of creating human-scale tissues will soon be developed and available for organ maturation. Thus, numerous factors need to be considered beyond simply having the best material and printer for the application at hand, with Table 3 summarizing the factors discussed in this section and the vital roles they play.
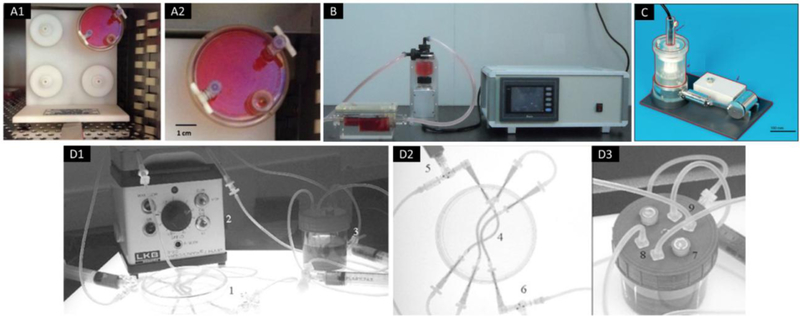
Figure 7.
Examples of bioreactors. (A) A rotating cell culture system with four station rotator base; adapted from [200]. (B) A conventional non-computer-controlled culture system for culture of tissue-engineered vascular vessels; adapted from [185]. (C) A pulsatile conditioning bioreactor which consisted of a core unit with support, an actuation unit, and a monitoring unit; adapted, with permission, from [186]. (D) A bioreactor system for culturing nerve conduits, which consisted of (1) petri dish, (2) peristaltic pump, (3) medium reservoir, (4) silicone tubes containing aligned microfiber scaffold, (5) closable inlets, (6) closable outlets, (7) vent windows with an air permeable film, (8) medium inlets, and (9) medium outlets; adapted from [201].
Table 3:
Considerations in the fabrication process that are critical for successful functionality and appropriate construct growth and maturity
| Factors | Considerations |
|---|---|
| Printing Process | Selection of the appropriate printing process based on the requirements of the construct, with the aim of using a process that complements these requirements with its advantages, while its disadvantages do not create a major hindrance to the fabrication |
| Construct Design | A good design for the construct is needed that fulfills the structural, mechanical and vascular requirements of the tissue/organ while working around the limitations of the printing process selected. |
| Post Processing | Depending on the printing process and the complexity of the design, certain post processing procedures may be necessary |
| Bioreactors | Appropriate environmental conditions must be simulated in a bioreactor to promote cell growth, maturation, and differentiation. |
4. NEXT DECADE: BIOPRINTING OF VOLUMETRIC COMPOSITE TISSUES
With Boland’s seminal study from more than a decade ago, it was shown that cells could be bioprinted in the viable form using a modified thermal inkjet bioprinter [188]. Since then, many researchers have shown exemplary work in transplantation of bioprinted tissues. Recently, scalable tissues have been bioprinted with native tissue-like properties [48] and vascularization [84]. Considering the fast-paced evolution of bioprinting and its promise in tissue engineering and regenerative medicine, it can be envisioned that volumetric composite tissues will become printable in the next decade.
Success in bioprinting functional tissues will emanate from a collaborative team approach including biologists, engineers, and clinicians. The development of faster bioprinters with multiple heads and modalities, as well as the higher resolution will likely enable generation of complex heterocellular tissues with both multi-scale vascularization and innervation. The newly created Integrated Tissue Organ Printer (ITOP) system contains multiple dispensing heads and pressure controllers, addressing the issues of size and stability of bioprinted constructs [10]. New techniques to develop vasculature are required for these large constructs to maintain cell viability [100][84]. The recent development of 4D bioprinting technology which allows shape transformation may also be a useful tool for the development of functional tissues [58]. The development of new bioinks such as self-assembling peptides [99] and the use of components such as graphene [95] will improve mechanical integrity and structural stability. In addition, new bioreactor technologies will allow for rapid post-printing maturation, providing the crucial mechanical and chemical stimulation to assist in tissue remodeling and growth.
Tissues that require minimal or no vascularization (such as skin and cartilage, respectively) are expected to be the first transplanted tissues, while those having more complexity (e.g., liver, pancreas, and heart) will take significantly longer. Several advances in this aspect have already been made, with researchers having bioprinted skin with the characteristics of human skin [45]. Conversely, small liver, pancreas, and heart tissues have been bioprinted but are far from a replicate of native organs. Prior to implantation in humans, standardized regulatory protocols will need to be established [56] including quality assurances, clinical trials, and establishing the requisite safety standards.
5. CONCLUSION
With thousands of patients dying each year waiting for an organ transplant, bioprinted organs are beginning to show the potential to eliminate this ever-increasing organ shortage crisis and save money spent on alternative medical treatments such as dialysis. However, this potential can only be realized by better understanding the functionality of the organs that are to be replaced and by developing the ability to translate this to the bioprinting methodologies. For this purpose, this paper discusses several factors that are critical for printing viable and functional tissues and organs. Accuracy in cellular factors such as cell density and heterogeneity is important for embedding and cell proliferation. Heterogeneity by itself brings additional complexity in tissues and organs, with most of these being volumetric in nature. Volumetric tissues entail the consideration of issues with engraftment, and the inclusion of auxiliary systems such as vascular, neural and circulatory. Each of these systems must be incorporated for the tissue or organ to be viable. Additionally, construct design is a key aspect for obtaining significant functionality, with factors such as mechanics, loading conditions and biomaterial rheology becoming critical aspects for selecting the appropriate materials for the scaffold, and formulating a competent design. Researchers must also consider the significance of characteristics that are specific to the tissue or organ being printed, and not just the cells they are composed of. Cellular interaction with nanoscale factors, enzymes, ECM, and the concerned proteins has to be considered, in order to be consolidated into a cohesive unit and behave as a fully functional tissue or organ. In addition to this, construct design must incorporate the design requirements of the selected printing process, and biomimicry concepts which have enabled the original tissue or organ to perform properly. Integrating the construct and cellular material is another crucial part of the process, with bioreactors controlling several variables needed for successful proliferation, differentiation, and migration.
The true potential of printed tissues and organs can only be realized after successful animal and clinical trials. Although clinical application of bioprinted tissues is still in its early stages, the technology has proved useful for non-biologic surgical implants and guides. Bioprinting research is well on its way to translate this to a biologic platform with the development of advanced bioprinters [4], advances in the design of complex bioinks to allow for better mechanical and structural integrity [50, 54], and new methods to create perfusable vasculature [55, 61]. However, several existing shortcomings in technology and research must be overcome in order to reach this stage. Successful translation of these technologies is expected to have a profound effect on patients suffering from organ and tissue dysfunction in the years to come, based on the expectations discussed in Table 4. Considering the rate at which the field is currently expanding, it is not unreasonable to expect bioprinting to become an integral component of regenerative medicine.
Table 4:
Potential research topics and changes in the future that may be incorporated into the material and printer selection and tissue fabrication
| Topic | Potential Changes |
|---|---|
| Biomaterials | With several new biomaterials being researched upon currently, additional biomaterials will be available in the future, making more tissues printable. |
| Printability should be further enhanced by advancements in multi-material and complex tissue printing for better properties and functionality. | |
| Bioprinters | The current limitations of the different types of bioprinters should diminish further in the future. |
| Bioprinters should be more compatible with new biomaterials, especially those with properties that cause issues currently. | |
| Improvements in structural and mechanical properties will aid in creating complex constructs. | |
| Improvements in accuracy, resolution, and speed with pushing bioprinting towards scalable production, and be lucrative for the industry. | |
| Bioreactors | With bioreactors get closer to mimicking real body conditions, the maturation and differentiation of cells will improve, boosting the success rate of bioprinted tissues and organs. |
Statement of Significance.
With thousands of patients dying each year waiting for an organ transplant, bioprinted tissues and organs show the potential to eliminate this ever-increasing organ shortage crisis. However, this potential can only be realized by better understanding the functionality of the organ and developing the ability to translate this to the bioprinting methodologies. Considering the rate at which the field is currently expanding, it is reasonable to expect bioprinting to become an integral component of regenerative medicine. For this purpose, this paper discusses several factors that are critical for printing functional tissues including cell density, vascularization, innervation, heterogeneity, engraftment, mechanics, and tissue-specific function, and inform the reader with future directions in bioprinting complex and volumetric tissues.
ACKNOWLEDGMENT
This work was supported by the US National Science Foundation CMMI Award 1624515 (I.T.O.) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under BIRCWH award K12HD055882 ‘‘Career Development Program in Women’s Health Research at Penn State’’ (D.J.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the above-mentioned funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Footnote:
Part of the Cell and Tissue Biofabrication Special Issue, edited by Professors Guohao Dai and Kaiming Ye.
REFERENCES
- [1].Hull CW, Apparatus for production of three-dimensional objects by stereolithography, US Pat. 4,575,330 (1986) 1–16. doi: 10.1145/634067.634234. [DOI] [Google Scholar]
- [2].Martins JP, Ferreira MPA, Ezazi NZ, Hirvonen JT, Santos HA, Thrivikraman G, França CM, Athirasala A, Tahayeri A, Bertassoni LE, 3D printing: prospects and challenges, in: Nanotechnologies Prev. Regen. Med, 2018: pp. 299–379. doi: 10.1016/B978-0-323-48063-5.00004-6. [DOI] [Google Scholar]
- [3].Klebe RJ, Cytoscribing: A method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues, Exp. Cell Res 179 (1988) 362–373. doi: 10.1016/0014-4827(88)90275-3. [DOI] [PubMed] [Google Scholar]
- [4].Ozbolat IT, Moncal KK, Gudapati H, Evaluation of bioprinter technologies, Addit. Manuf 13 (2017) 179–200. doi: 10.1016/j.addma.2016.10.003. [DOI] [Google Scholar]
- [5].Xu T, Jin J, Gregory C, Hickman JJ, Boland T, Inkjet printing of viable mammalian cells, Biomaterials 26 (2005) 93–99. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- [6].Lanza RP, Chung HY, Yoo JJ, Wettstein PJ, Blackwell C, Borson N, Hofmeister E, Schuch G, Soker S, Moraes CT, West MD, Atala A, Generation of histocompatible tissues using nuclear transplantation, Nat. Biotechnol 20 (2002) 689–696. doi: 10.1038/nbt703. [DOI] [PubMed] [Google Scholar]
- [7].Olsen TR, Alexis F, Bioprocessing of Tissues using Cellular Spheroids, J. Bioprocess. Biotech 04 (2014) 1–4. doi: 10.4172/2155-9821.1000e112. [DOI] [Google Scholar]
- [8].Wohlers T, Gornet T, History of Additive Manufacturing, in: 2016: pp. 1–24. doi: 10.4018/978-1-5225-2289-8.ch001. [DOI] [Google Scholar]
- [9].Ozbolat IT, 3D Bioprinting: Fundementals, Principles and Applications, Academic Press is an imprint of Elsevier, 2016. [Google Scholar]
- [10].Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity, Nat. Biotechnol 34 (2016) 312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- [11].Ozbolat IT, Peng W, Ozbolat V, Application areas of 3D bioprinting, Drug Discov. Today 21 (2016) 1257–1271. doi: 10.1016/j.drudis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- [12].Lee H, Cho D-W, One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology, Lab Chip 16 (2016) 2618–2625. doi: 10.1039/C6LC00450D. [DOI] [PubMed] [Google Scholar]
- [13].Jung JW, Lee JS, Cho DW, Computer-Aided multiple-head 3D printing system for printing of heterogeneous organ/tissue constructs, Sci. Rep 6 (2016) 21685. doi: 10.1038/srep21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang LG, Fisher JP, Leong KW, Sklare SC, Phamduy TB, Curly JL, Huang Y, Chrisey DB, 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine, 2015. doi: 10.1016/B978-0-12-800547-7.00004-7. [DOI] [Google Scholar]
- [15].Ozbolat IT, Hospodiuk M, Current advances and future perspectives in extrusion-based bioprinting, Biomaterials 76 (2016) 321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- [16].Chang R, Nam J, Sun W, Effects of Dispensing Pressure and Nozzle Diameter on Cell Survival from Solid Freeform Fabrication–Based Direct Cell Writing, Tissue Eng. Part A 14 (2008) 41–48. doi: 10.1089/ten.a.2007.0004. [DOI] [PubMed] [Google Scholar]
- [17].Murphy SV, Atala A, 3D bioprinting of tissues and organs, Nat. Biotechnol 32 (2014) 773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- [18].Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P, The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability, Biomaterials 35 (2014) 49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- [19].Duan B, Hockaday LA, Kang KH, Butcher JT, 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels, J. Biomed. Mater. Res. - Part A 101 A (2013) 1255–1264. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dababneh AB, Ozbolat IT, Bioprinting Technology: A Current State-of-the-Art Review, J. Manuf. Sci. Eng 136 (2014) 061016. doi: 10.1115/1.4028512. [DOI] [Google Scholar]
- [21].Gudapati H, Dey M, Ozbolat I, A comprehensive review on droplet-based bioprinting: Past, present and future, Biomaterials 102 (2016) 20–42. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- [22].Xu C, Chai W, Huang Y, Markwald RR, Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes, Biotechnol. Bioeng 109 (2012) 3152–3160. doi: 10.1002/bit.24591. [DOI] [PubMed] [Google Scholar]
- [23].Jayasinghe SN, Qureshi AN, Eagles PAM, Electrohydrodynamic jet processing: An advanced electric-field-driven jetting phenomenon for processing living cells, Small 2 (2006) 216–219. doi: 10.1002/smll.200500291. [DOI] [PubMed] [Google Scholar]
- [24].Onses MS, Sutanto E, Ferreira PM, Alleyne AG, Rogers JA, Mechanisms, Capabilities, and Applications of High-Resolution Electrohydrodynamic Jet Printing, Small 11 (2015) 4237–4266. doi: 10.1002/smll.201500593. [DOI] [PubMed] [Google Scholar]
- [25].Gasperini L, Maniglio D, Migliaresi C, Microencapsulation of cells in alginate through an electrohydrodynamic process, J. Bioact. Compat. Polym 28 (2013) 413–425. doi: 10.1177/0883911513501599. [DOI] [Google Scholar]
- [26].Tasoglu S, Demirci U, Bioprinting for stem cell research, Trends Biotechnol 31 (2013) 10–19. doi: 10.1016/j.tibtech.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Faulkner-Jones A, Fyfe C, Cornelissen D-J, Gardner J, King J, Courtney A, Shu W, Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D, Biofabrication 7 (2015) 044102. doi: 10.1088/1758-5090/7/4/044102. [DOI] [PubMed] [Google Scholar]
- [28].Wu Y, Wu B, Vijayavenkataraman S, Wong YS, Fuh JYH, Crimped fiber with controllable patterns fabricated via electrohydrodynamic jet printing, Mater. Des 131 (2017) 384–393. doi: 10.1016/j.matdes.2017.06.027. [DOI] [Google Scholar]
- [29].Graham AD, Olof SN, Burke MJ, Armstrong JPK, Mikhailova EA, Nicholson JG, Box SJ, Szele FG, Perriman AW, Bayley H, High-Resolution Patterned Cellular Constructs by Droplet-Based 3D Printing, Sci. Rep 7 (2017). doi: 10.1038/s41598-017-06358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu T, Kincaid H, Atala A, Yoo JJ, High-Throughput Production of Single-Cell Microparticles Using an Inkjet Printing Technology, J. Manuf. Sci. Eng 130 (2008) 021017. doi: 10.1115/1.2903064. [DOI] [Google Scholar]
- [31].Koch L, Gruene M, Unger C, Chichkov B, Laser Assisted Cell Printing, Curr. Pharm. Biotechnol 14 (2013) 91–97. doi: 10.2174/138920113804805368. [DOI] [PubMed] [Google Scholar]
- [32].Guillotin B, Ali M, Ducom A, Catros S, Keriquel V, Souquet A, Remy M, Fricain JC, Guillemot F, Laser-Assisted Bioprinting for Tissue Engineering, in: Biofabrication Micro- Nano-Fabrication, Printing, Patterning Assem, 2013: pp. 95–118. doi: 10.1016/B978-1-4557-2852-7.00006-8. [DOI] [Google Scholar]
- [33].Leberfinger AN, Ravnic DJ, Dhawan A, Ozbolat IT, Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication, Stem Cells Transl. Med 6 (2017) 1940–1948. doi: 10.1002/sctm.17-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bishop ES, Mostafa S, Pakvasa M, Luu HH, Lee MJ, Wolf JM, Ameer GA, He TC, Reid RR, 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends, Genes Dis 4 (2017) 185–195. doi: 10.1016/j.gendis.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hopp B, Smausz T, Kresz N, Barna N, Bor Z, Kolozsvári L, Chrisey DB, Szabó A, Nógrádi A, Survival and Proliferative Ability of Various Living Cell Types after Laser-Induced Forward Transfer, Tissue Eng 11 (2005) 1817–1823. doi: 10.1089/ten.2005.11.1817. [DOI] [PubMed] [Google Scholar]
- [36].Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, Bareille R, Rémy M, Bordenave L, Amédée j J, Guillemot F, Laser assisted bioprinting of engineered tissue with high cell density and microscale organization, Biomaterials 31 (2010) 7250–7256. doi: 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- [37].Gu BK, Choi DJ, Park SJ, Kim MS, Kang CM, Kim CH, 3-dimensional bioprinting for tissue engineering applications, Biomater. Res 20 (2016) 12. doi: 10.1186/s40824-016-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, Wang P, Lai CSE, Gou M, Xu Y, Zhang K, Chen S, Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture, Biomaterials 124 (2017) 106–115. doi: 10.1016/j.biomaterials.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ma X, Qu X, Zhu W, Li Y-S, Yuan S, Zhang H, Liu J, Wang P, Lai CSE, Zanella F, Feng G-S, Sheikh F, Chien S, Chen S, Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting, Proc. Natl. Acad. Sci 113 (2016) 2206–2211. doi: 10.1073/pnas.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S, Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds, Stem Cells Transl. Med 1 (2012) 792–802. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cubo N, Garcia M, Del Cañizo JF, Velasco D, Jorcano JL, 3D bioprinting of functional human skin: Production and in vivo analysis, Biofabrication 9 (2017) 015006. doi: 10.1088/1758-5090/9/1/015006. [DOI] [PubMed] [Google Scholar]
- [42].Gao G, Yonezawa T, Hubbell K, Dai G, Cui X, Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging, Biotechnol. J 10 (2015) 1568–1577. doi: 10.1002/biot.201400635. [DOI] [PubMed] [Google Scholar]
- [43].Jakus AE, Shah RN, Multi and mixed 3D-printing of graphene-hydroxyapatite hybrid materials for complex tissue engineering, J. Biomed. Mater. Res. - Part A 105 (2017) 274–283. doi: 10.1002/jbm.a.35684. [DOI] [PubMed] [Google Scholar]
- [44].Zhang H, Mao X, Du Z, Jiang W, Han X, Zhao D, Han D, Li Q, Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model, Sci. Technol. Adv. Mater 17 (2016) 136–148. doi: 10.1080/14686996.2016.1145532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pourchet LJ, Thepot A, Albouy M, Courtial EJ, Boher A, Blum LJ, Marquette CA, Human Skin 3D Bioprinting Using Scaffold-Free Approach, Adv. Healthc. Mater 6 (2017) 1601101. doi: 10.1002/adhm.201601101. [DOI] [PubMed] [Google Scholar]
- [46].Daly AC, Critchley SE, Rencsok EM, Kelly DJ, A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage, Biofabrication 8 (2016) 045002. doi: 10.1088/1758-5090/8/4/045002. [DOI] [PubMed] [Google Scholar]
- [47].Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD, Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology, Tissue Eng. Part A 18 (2012) 1304–1312. doi: 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, Ozbolat IT, Three-dimensional bioprinting using self-Assembling scalable scaffold-free “tissue strands” as a new bioink, Sci. Rep 6 (2016) 28714. doi: 10.1038/srep28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhong C, Xie HY, Zhou L, Xu X, Sen Zheng S, Human hepatocytes loaded in 3D bioprinting generate mini-liver, Hepatobiliary Pancreat. Dis. Int 15 (2016) 512–518. doi: 10.1016/S1499-3872(16)60119-4. [DOI] [PubMed] [Google Scholar]
- [50].Massa S, Sakr MA, Seo J, Bandaru P, Arneri A, Bersini S, Zare-Eelanjegh E, Jalilian E, Cha BH, Antona S, Enrico A, Gao Y, Hassan S, Acevedo JP, Dokmeci MR, Zhang YS, Khademhosseini A, Shin SR, Bioprinted 3D vascularized tissue model for drug toxicity analysis, Biomicrofluidics 11 (2017) 044109. doi: 10.1063/1.4994708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gaetani R, Doevendans PA, Metz CHG, Alblas J, Messina E, Giacomello A, Sluijter JPG, Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells, Biomaterials 33 (2012) 1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- [52].Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, Gruene M, Toelk A, Wang W, Mark P, Wang F, Chichkov B, Li W, Steinhoff G, Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration, Biomaterials 32 (2011) 9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- [53].Izadifar M, Chapman D, Babyn P, Chen X, Kelly ME, UV-ssisted 3D bioprinting of nano-reinforced hybrid cardiac patch for myocardial tissue engineering, Tissue Eng. Part C Methods 24 (2017) ten.TEC.2017.0346. doi: 10.1089/ten.TEC.2017.0346. [DOI] [PubMed] [Google Scholar]
- [54].Ong CS, Fukunishi T, Nashed A, Blazeski A, Zhang H, Hardy S, DiSilvestre D, Vricella L, Conte J, Tung L, Tomaselli G, Hibino N, Creation of Cardiac Tissue Exhibiting Mechanical Integration of Spheroids Using 3D Bioprinting, J. Vis. Exp (2017) 55438. doi: 10.3791/55438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mohd Sani MSH, Muftah F, Siang TC, Biomimicry engineering: New area of tranformation inspired by the nature, in: BEIAC 2013 – 2013 IEEE Bus. Eng. Ind. Appl. Colloq, IEEE, 2013: pp. 477–482. doi: 10.1109/BEIAC.2013.6560173. [DOI] [Google Scholar]
- [56].Ravnic DJ, Leberfinger AN, Koduru SV, Hospodiuk M, Moncal KK, Datta P, Dey M, Rizk E, Ozbolat IT, Transplantation of Bioprinted Tissues and Organs: Technical and Clinical Challenges and Future Perspectives, Ann. Surg 266 (2017) 48–58. doi: 10.1097/SLA.0000000000002141. [DOI] [PubMed] [Google Scholar]
- [57].Yurie H, Ikeguchi R, Aoyama T, Kaizawa Y, Tajino J, Ito A, Ohta S, Oda H, Takeuchi H, Akieda S, Tsuji M, Nakayama K, Matsuda S, The efficacy of a scaffold-free bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model, PLoS One 12 (2017) e0171448. doi: 10.1371/journal.pone.0171448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li YC, Zhang YS, Akpek A, Shin SR, Khademhosseini A, 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials, Biofabrication 9 (2017) 012001. doi: 10.1088/1758-5090/9/1/012001. [DOI] [PubMed] [Google Scholar]
- [59].Keriquel V, Oliveira H, Rémy M, Ziane S, Delmond S, Rousseau B, Rey S, Catros S, Amédée J, Guillemot JC Fricain, In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications, Sci. Rep 7 (2017) 1778. doi: 10.1038/s41598-017-01914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jones FS, Jones PL, The tenascin family of ECM glycoproteins: Structure, function, and regulation during embryonic development and tissue remodeling, Dev. Dyn 218 (2000) 235–259. doi:. [DOI] [PubMed] [Google Scholar]
- [61].Marcos R, Monteiro RAF, Rocha E, Design-based stereological estimation of hepatocyte number, by combining the smooth optical fractionator and immunocytochemistry with anti-carcinoembryonic antigen polyclonal antibodies, Liver Int 26 (2006) 116–124. doi: 10.1111/j.1478-3231.2005.01201.x. [DOI] [PubMed] [Google Scholar]
- [62].Hotzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A, Bioink properties before, during and after 3D bioprinting, Biofabrication 8 (2016) 032002. doi: 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- [63].Leberfinger AN, Moncal KK, Ravnic DJ, Ozbolat IT, 3D Printing for Cell Therapy Applications, in: Humana Press, Cham, 2017: pp. 227–248. doi: 10.1007/978-3-319-57153-9_11. [DOI] [Google Scholar]
- [64].Ozbolat IT, Scaffold-Based or Scaffold-Free Bioprinting: Competing or Complementing Approaches?, J. Nanotechnol. Eng. Med 6 (2015) 024701. doi: 10.1115/1.4030414. [DOI] [Google Scholar]
- [65].Akkouch A, Yu Y, Ozbolat IT, Microfabrication of scaffold-free tissue strands for three-dimensional tissue engineering, Biofabrication 7 (2015) 031002. doi: 10.1088/1758-5090/7/3/031002. [DOI] [PubMed] [Google Scholar]
- [66].Peng W, Datta P, Wu Y, Dey M, Ayan B, Dababneh A, Ozbolat IT, Challenges in Bio-fabrication of Organoid Cultures, in: Adv Exp Med Biol – Cell Biol. Transl. Med, 2018: pp. 1025–1039. doi: 10.1007/5584_2018_216. [DOI] [PubMed] [Google Scholar]
- [67].Holy CE, Shoichet MS, Davies JE, Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell-seeding density and culture period, J. Biomed. Mater. Res 51 (2000) 376–382. doi:. [DOI] [PubMed] [Google Scholar]
- [68].Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, Disilvestre D, Vricella L, Conte J, Tung L, Tomaselli GF, Hibino N, Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes, Sci. Rep 7 (2017) 4566. doi: 10.1038/s41598-017-05018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ji S, Guvendiren M, Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs, Front. Bioeng. Biotechnol 5 (2017) 23. doi: 10.3389/fbioe.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Courtney JM, Mechanical properties of biomaterials, J. Biomed. Eng 3 (1981) 260–261. doi: 10.1016/0141-5425(81)90094-7. [DOI] [Google Scholar]
- [71].Kyle S, Jessop ZM, Al-Sabah A, Whitaker IS, “Printability” of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art, Adv. Healthc. Mater 6 (2017) 1700264. doi: 10.1002/adhm.201700264. [DOI] [PubMed] [Google Scholar]
- [72].He Y, Yang F, Zhao H, Gao Q, Xia B, Fu J, Research on the printability of hydrogels in 3D bioprinting, Sci. Rep 6 (2016). doi: 10.1038/srep29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chung JHY, Naficy S, Yue Z, Kapsa R, Quigley A, Moulton SE, Wallace GG, Bio-ink properties and printability for extrusion printing living cells, Biomater. Sci 1 (2013) 763–773. doi: 10.1039/c3bm00012e. [DOI] [PubMed] [Google Scholar]
- [74].Peltola SM, Melchels FPW, Grijpma DW, Kellomäki M, A review of rapid prototyping techniques for tissue engineering purposes, Ann. Med 40 (2008) 268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- [75].Heiligenhaus A, Bauer D, Wasmuth S, Steuhl KP, Amniotic membrane transplantation improves herpetic keratitis by local and not by systemic effects, Ophthalmologe 100 (2003) 209–15. doi: 10.1007/s00347-002-0720-z. [DOI] [PubMed] [Google Scholar]
- [76].Mauck RL, Wang CCB, Oswald ES, Ateshian GA, Hung CT, The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading, Osteoarthr. Cartil 11 (2003) 879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- [77].Chang SCN, Rowley JA, Tobias G, Genes NG, Roy AK, Mooney DJ, Vacanti CA, Bonassar LJ, Injection molding of chondrocyte/alginate constructs in the shape of facial implants, J. Biomed. Mater. Res 55 (2001) 503–511. doi:. [DOI] [PubMed] [Google Scholar]
- [78].Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G, Tissue engineering by self-assembly and bio-printing of living cells, Biofabrication 2 (2010) 022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ding H, Tourlomousis F, Chang RC, Bioprinting multidimensional constructs: a quantitative approach to understanding printed cell density and redistribution phenomena, Biomed. Phys. Eng. Express 3 (2017) 035016. doi: 10.1088/2057-1976/aa70f0. [DOI] [Google Scholar]
- [80].Hospodiuk M, Dey M, Ayan B, Sosnoski D, Moncal KK, Wu Y, Ozbolat IT, Sprouting angiogenesis in engineered pseudo islets, 10 (2018) 035003. [DOI] [PubMed] [Google Scholar]
- [81].Merceron TK, Burt M, Seol YJ, Kang HW, Lee SJ, Yoo JJ, Atala A, A 3D bioprinted complex structure for engineering the muscle-tendon unit, Biofabrication 7 (2015) 35003. doi: 10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- [82].Fedorovich NE, Schuurman W, Wijnberg HM, Prins H-J, van Weeren PR, Malda J, Alblas J, Dhert WJA, Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined, Cell-Laden Hydrogel Scaffolds, Tissue Eng. Part C Methods 18 (2012) 33–44. doi: 10.1089/ten.tec.2011.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wu Y, Han Y, Wong YS, Fuh JYH, Fibre-based scaffolding techniques for tendon tissue engineering, J. Tissue Eng. Regen. Med 12 (2018) 1798–1821. doi: 10.1002/term.2701. [DOI] [PubMed] [Google Scholar]
- [84].Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA, 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs, Adv. Mater 26 (2014) 3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- [85].Hockaday LA, Kang KH, Colangelo NW, Cheung PYC, Duan B, Malone E, Wu J, Girardi LN, Bonassar LJ, Lipson H, Chu CC, Butcher JT, Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds, Biofabrication 4 (2012) 035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Fedorovich NE, De Wijn JR, Verbout AJ, Alblas J, Dhert WJA, Three-Dimensional Fiber Deposition of Cell-Laden, Viable, Patterned Constructs for Bone Tissue Printing, Tissue Eng. Part A 14 (2008) 127–133. doi: 10.1089/ten.a.2007.0158. [DOI] [PubMed] [Google Scholar]
- [87].Park JA, Yoon S, Kwon J, Now H, Kim YK, Kim WJ, Yoo JY, Jung S, Freeform micropatterning of living cells into cell culture medium using direct inkjet printing, Sci. Rep 7 (2017) 14610. doi: 10.1038/s41598-017-14726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M, 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances, Bioact. Mater 3 (2018) 144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hedegaard CL, Collin EC, Redondo-Gómez C, Nguyen LTH, Ng KW, Castrejón-Pita AA, Castrejón-Pita JR, Mata A, Hydrodynamically Guided Hierarchical Self-Assembly of Peptide– Protein Bioinks, Adv. Funct. Mater 28 (2018) 1703716. doi: 10.1002/adfm.201703716. [DOI] [Google Scholar]
- [90].Murata H, Polymerization: Rheology - Theory and Application to Biomaterials (Chapter 17), Polymerization (2012) 403–425. doi: 10.1016/S0927-0256(97)00040-2. [DOI] [Google Scholar]
- [91].Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P, 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications, Biomacromolecules 16 (2015) 1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- [92].Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, Kaunas R, Gaharwar AK, Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach, ACS Nano 9 (2015) 3109–3118. doi: 10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- [93].Ouyang L, Highley CB, Rodell CB, Sun W, Burdick JA, 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking, ACS Biomater. Sci. Eng 2 (2016) 1743–1751. doi: 10.1021/acsbiomaterials.6b00158. [DOI] [PubMed] [Google Scholar]
- [94].Badylak S, Kokini K, Tullius B, Whitson B, Strength over time of a resorbable bioscaffold for body wall repair in a dog model, J. Surg. Res 99 (2001) 282–287. doi: 10.1006/jsre.2001.6176. [DOI] [PubMed] [Google Scholar]
- [95].Huang CT, Kumar Shrestha L, Ariga K, Hsu SH, A graphene-polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells, J. Mater. Chem. B 5 (2017) 8854–8864. doi: 10.1039/c7tb01594a. [DOI] [PubMed] [Google Scholar]
- [96].Qi X, Pei P, Zhu M, Du X, Xin C, Zhao S, Li X, Zhu Y, Three dimensional printing of calcium sulfate and mesoporous bioactive glass scaffolds for improving bone regeneration in vitro and in vivo, Sci. Rep 7 (2017) 42556. doi: 10.1038/srep42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, Dokmeci MR, Dentini M, Khademhosseini A, Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink, Adv. Mater 28 (2016) 677–684a. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Romanazzo S, Vedicherla S, Moran C, Kelly DJ, Meniscus ECM-functionalised hydrogels containing infrapatellar fat pad-derived stem cells for bioprinting of regionally defined meniscal tissue, J. Tissue Eng. Regen. Med 12 (2018) e1826–e1835. doi: 10.1002/term.2602. [DOI] [PubMed] [Google Scholar]
- [99].Raphael B, Khalil T, Workman VL, Smith A, Brown CP, Streuli C, Saiani A, Domingos M, 3D cell bioprinting of self-assembling peptide-based hydrogels, Mater. Lett 190 (2017) 103–106. doi: 10.1016/j.matlet.2016.12.127. [DOI] [Google Scholar]
- [100].Datta P, Ayan B, Ozbolat IT, Bioprinting for vascular and vascularized tissue biofabrication, Acta Biomater 51 (2017) 1–20. doi: 10.1016/j.actbio.2017.01.035. [DOI] [PubMed] [Google Scholar]
- [101].Sooppan R, Paulsen SJ, Han J, Ta AH, Dinh P, Gaffey AC, Venkataraman C, Trubelja A, Hung G, Miller JS, Atluri P, In Vivo Anastomosis and Perfusion of a Three-Dimensionally-Printed Construct Containing Microchannel Networks, Tissue Eng. Part C Methods 22 (2016) 1–7. doi: 10.1089/ten.tec.2015.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dew L, English WR, Ortega I, Claeyssens F, Macneil S, Fabrication of biodegradable synthetic vascular networks and their use as a model of angiogenesis, Cells Tissues Organs 202 (2016) 319–328. doi: 10.1159/000446644. [DOI] [PubMed] [Google Scholar]
- [103].Chiu LLY, Montgomery M, Liang Y, Liu H, Radisic M, Perfusable branching microvessel bed for vascularization of engineered tissues, Proc. Natl. Acad. Sci 109 (2012) E3414–E3423. doi: 10.1073/pnas.1210580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yeon JH, Ryu HR, Chung M, Hu QP, Jeon NL, In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices, Lab Chip 12 (2012) 2815–2822. doi: 10.1039/c2lc40131b. [DOI] [PubMed] [Google Scholar]
- [105].Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Khademhosseini A, Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs., Lab Chip 14 (2014) 2202–11. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Dolati F, Yu Y, Zhang Y, De Jesus AM, Sander EA, Ozbolat IT, In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits, Nanotechnology 25 (2014) 145101. doi: 10.1088/0957-4484/25/14/145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang Y, Yu Y, Chen H, Ozbolat IT, Characterization of printable cellular micro-fluidic channels for tissue engineering, Biofabrication 5 (2013) 025004. doi: 10.1088/1758-5082/5/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Cui H, Zhu W, Holmes B, Zhang LG, Biologically Inspired Smart Release System Based on 3D Bioprinted Perfused Scaffold for Vascularized Tissue Regeneration, Adv. Sci 3 (2016) 1600058. doi: 10.1002/advs.201600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wu Y, Hospodiuk M, Peng W, Gudapati H, Neuberger T, Koduru SV, Ravnic DJ, Ozbolat IT, Porous tissue strands: Avascular building blocks for scalable tissue fabrication, Biofabrication (2018). doi: 10.1088/1758-5090/aaec22. [DOI] [PubMed] [Google Scholar]
- [110].Hribar KC, Meggs K, Liu J, Zhu W, Qu X, Chen S, Three-dimensional direct cell patterning in collagen hydrogels with near-infrared femtosecond laser, Sci. Rep 5 (2015) 17203. doi: 10.1038/srep17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Xiong R, Zhang Z, Chai W, Huang Y, Chrisey DB, Freeform drop-on-demand laser printing of 3D alginate and cellular constructs, Biofabrication 7 (2015) 045011. doi: 10.1088/1758-5090/7/4/045011. [DOI] [PubMed] [Google Scholar]
- [112].Hribar KC, Soman P, Warner J, Chung P, Chen S, Light-assisted direct-write of 3D functional biomaterials, Lab Chip 14 (2014) 268–275. doi: 10.1039/c3lc50634g. [DOI] [PubMed] [Google Scholar]
- [113].Cui X, Boland T, Human microvasculature fabrication using thermal inkjet printing technology, Biomaterials 30 (2009) 6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- [114].Pataky K, Braschler T, Negro A, Renaud P, Lutolf MP, Brugger J, Microdrop printing of hydrogel bioinks into 3D tissue-like geometries, Adv. Mater 24 (2012) 391–396. doi: 10.1002/adma.201102800. [DOI] [PubMed] [Google Scholar]
- [115].Cui H, Nowicki M, Fisher JP, Zhang LG, 3D Bioprinting for Organ Regeneration, Adv. Healthc. Mater 6 (2017). doi: 10.1002/adhm.201601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ravnic DJ, Leberfinger AN, Ozbolat IT, Bioprinting and Cellular Therapies for Type 1 Diabetes, Trends Biotechnol 35 (2017) 1025–1034. doi: 10.1016/j.tibtech.2017.07.006. [DOI] [PubMed] [Google Scholar]
- [117].Lee JW, Choi YJ, Yong WJ, Pati F, Shim JH, Kang KS, Kang IH, Park J, Cho DW, Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering, Biofabrication 8 (2016) 015007. doi: 10.1088/1758-5090/8/1/015007. [DOI] [PubMed] [Google Scholar]
- [118].Athirasala A, Lins F, Tahayeri A, Hinds M, Smith AJ, Sedgley C, Ferracane J, Bertassoni LE, A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs, Sci. Rep 7 (2017) 3323. doi: 10.1038/s41598-017-02532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ucuzian AA, Gassman AA, East AT, Greisler HP, Molecular mediators of angiogenesis, J. Burn Care Res 31 (2010) 158–175. doi: 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Keast JR, Innervation of developing and adult organs, Organogenesis 9 (2013) 121. doi: 10.4161/org.25886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Chen Y-S, Chang J-Y, Cheng C-Y, Tsai F-J, Yao C-H, Liu B-S, An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material., Biomaterials (2005) 3911–8. doi: 10.1016/J.BIOMATERIALS.2004.09.060. [DOI] [PubMed] [Google Scholar]
- [122].Pateman CJ, Harding AJ, Glen A, Taylor CS, Christmas CR, Robinson PP, Rimmer S, Boissonade FM, Claeyssens F, Haycock JW, Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair, Biomaterials 49 (2015) 77–89. doi: 10.1016/j.biomaterials.2015.01.055. [DOI] [PubMed] [Google Scholar]
- [123].Hu Y, Wu Y, Gou Z, Tao J, Zhang J, Liu Q, Kang T, Jiang S, Huang S, He J, Chen S, Du Y, Gou M, 3D-engineering of cellularized conduits for peripheral nerve regeneration, Sci. Rep 6 (2016) 32184. doi: 10.1038/srep32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Leberfinger AN, Ravnic DJ, Payne R, Rizk E, Koduru SV, Hazard SW, Adipose-Derived Stem Cells in Peripheral Nerve Regeneration, Curr. Surg. Reports 5 (2017) 5. doi: 10.1007/s40137-017-0169-2. [DOI] [Google Scholar]
- [125].Owens CM, Marga F, Forgacs G, Heesch CM, Biofabrication and testing of a fully cellular nerve graft, Biofabrication 5 (2013) 045007. doi: 10.1088/1758-5082/5/4/045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Shi Z, Gao H, Feng J, Ding B, Cao X, Kuga S, Wang Y, Zhang L, Cai J, In Situ Synthesis of Robust Conductive Cellulose / Polypyrrole Composite Aerogels and Their Potential Application in Nerve Regeneration, Angew. Chemie 126 (2014) 5380–5384. doi: 10.1002/anie.201402751. [DOI] [PubMed] [Google Scholar]
- [127].Abidian MR, Daneshvar ED, Egeland BM, Kipke DR, Cederna PS, Urbanchek MG, Hybrid Conducting Polymer-Hydrogel Conduits for Axonal Growth and Neural Tissue Engineering, Adv. Healthc. Mater 1 (2012) 762–767. doi: 10.1002/adhm.201200182. [DOI] [PubMed] [Google Scholar]
- [128].and KA Cha C, Shin SR, Annabi N, Dokmeci MR, Carbon-Based Nanomaterials: Multi-Functional Materials for Biomedical Engineering, ACS Nano 7 (2013) 2891–2897. doi:0.1021/nn401196a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Neto AHC, Guinea F, Peres NMR, Novoselov KS, Geim AK, The electronic properties of graphene, Rev. Mod. Phys 81 (2007) 109–162. doi: 10.1103/RevModPhys.81.109. [DOI] [Google Scholar]
- [130].Wang Q, Chen J, Niu Q, Fu X, Sun X, Tong X, The application of graphene oxidized combining with decellularized scaffold to repair of sciatic nerve injury in rats, Saudi Pharm. J 25 (2017) 469–doi: 10.1016/j.jsps.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Qian Y, Zhao X, Han Q, Chen W, Li H, Yuan W, An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration, Nat. Commun 9 (2018) 323. doi: 10.1038/s41467-017-02598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S, Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering, J. Tissue Eng. Regen. Med 5 (2011) e17–e35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- [133].Choi YJ, Yi HG, Kim SW, Cho DW, 3D cell printed tissue analogues: A new platform for theranostics, Theranostics 7 (2017) 3118–3137. doi: 10.7150/thno.19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kang H-W, Ko K, Kim JH, Lee J, Yoo JJ, Atala A, 3-D Bioprinting of Innervated Skeletal Muscle Tissue for Accelerated Restoration of Muscle Function, J. Tissue Eng. Regen. Med 36 (2009). [Google Scholar]
- [135].Plusa B, Hadjantonakis A-K, Embryonic stem cell identity grounded in the embryo, Nat Cell Biol 16 (2014) 502–504. doi: 10.1016/j.cogdev.2010.08.003.Personal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Mieth D, Going to the roots of the stem cell debate: The ethical problems of using embryos for research, EMBO Rep 1 (2000) 4–6. doi: 10.1093/embo-reports/kvd020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yoshihara M, Hayashizaki Y, Murakawa Y, Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications, Stem Cell Rev. Reports 13 (2017) 7–16. doi: 10.1007/s12015-016-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Park JY, Choi YJ, Shim JH, Park JH, Cho DW, Development of a 3D cell printed structure as an alternative to autologs cartilage for auricular reconstruction, J. Biomed. Mater. Res. - Part B Appl. Biomater 105 (2017) 1016–1028. doi: 10.1002/jbm.b.33639. [DOI] [PubMed] [Google Scholar]
- [139].Jang J, Park HJ, Kim SW, Kim H, Park JY, Na SJ, Kim HJ, Park MN, Choi SH, Park SH, Kim SW, Kwon SM, Kim PJ, Cho DW, 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair, Biomaterials 112 (2017) 264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- [140].Itoh M, Nakayama K, Noguchi R, Kamohara K, Furukawa K, Uchihashi K, Toda S, Oyama JI, Node K, Morita S, Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae, PLoS One 10 (2015) e0136681. doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW, Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells, Immunol. Cell Biol 91 (2013) 32–39. doi: 10.1038/icb.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gu L-H, Zhang T-T, Li Y, Yan H-J, Qi H, Li F-R, Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats, Cell. Mol. Immunol 12 (2015) 444–455. doi: 10.1038/cmi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Barry FP, Murphy JM, English K, Mahon BP, Immunogenicity of Adult Mesenchymal Stem Cells: Lessons from the Fetal Allograft, Stem Cells Dev 14 (2005) 252–265. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- [144].Ren M-L, Peng W, Yang Z-L, Sun X-J, Zhang S-C, Wang Z-G, Zhang B, Allogeneic Adipose-Derived Stem Cells with Low Immunogenicity Constructing Tissue-Engineered Bone for Repairing Bone Defects in Pigs, Cell Transplant 21 (2012) 2711–2721. doi: 10.3727/096368912X654966. [DOI] [PubMed] [Google Scholar]
- [145].Larocca RA, Moraes-Vieira PM, Bassi EJ, Semedo P, de Almeida DC, da Silva MB, Thornley T, Pacheco-Silva A, Câmara NOS, Adipose tissue-derived mesenchymal stem cells increase skin allograft survival and inhibit Th-17 immune response., PLoS One 8 (2013) e76396. doi: 10.1371/journal.pone.0076396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Möller T, Amoroso M, Hägg D, Brantsing C, Rotter N, Apelgren P, Lindahl A, Kölby L, Gatenholm P, In Vivo Chondrogenesis in 3D Bioprinted Human Cell-laden Hydrogel Constructs, in: Plast. Reconstr. Surg. - Glob. Open, Wolters Kluwer Health, 2017: p. e1227. doi: 10.1097/GOX.0000000000001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zhang XZ, Chu CC, Fabrication and characterization of microgel-impregnated, thermosensitive PNIPAAm hydrogels, Polymer (Guildf) 46 (2005) 9664–9673. doi: 10.1016/j.polymer.2005.08.055. [DOI] [Google Scholar]
- [148].Song S, Roy S, Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: Cells, biomaterials, and devices, Biotechnol. Bioeng 113 (2016) 1381–1402. doi: 10.1002/bit.25895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG, Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice, Nat. Med 22 (2016) 306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK, An Islet-Stabilizing Implant Constructed Using a Preformed Vasculature, Tissue Eng. Part A 14 (2008) 433–440. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- [151].Levato R, Visser J, Planell JA, Engel E, Malda J, Mateos-Timoneda MA, Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers, Biofabrication 6 (2014) 035020. doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- [152].Garreta E, Oria R, Tarantino C, Pla-Roca M, Prado P, Fernández-Avilés F, Campistol JM, Samitier J, Montserrat N, Tissue engineering by decellularization and 3D bioprinting, Mater. Today 20 (2017) 166–178. doi: 10.1016/j.mattod.2016.12.005. [DOI] [Google Scholar]
- [153].Skardal A, Devarasetty M, Kang HW, Mead I, Bishop C, Shupe T, Lee SJ, Jackson J, Yoo J, Soker S, Atala A, A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs, Acta Biomater 25 (2015) 24–34. doi: 10.1016/j.actbio.2015.07.030. [DOI] [PubMed] [Google Scholar]
- [154].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix Elasticity Directs Stem Cell Lineage Specification, Cell 126 (2006) 677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [155].Mandrycky C, Wang Z, Kim K, Kim DH, 3D bioprinting for engineering complex tissues, Biotechnol. Adv 34 (2016) 422–434. doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Farzadi A, Solati-Hashjin M, Asadi-Eydivand M, Osman NAA, Effect of layer thickness and printing orientation on mechanical properties and dimensional accuracy of 3D printed porous samples for bone tissue engineering, PLoS One 9 (2014) e108252. doi: 10.1371/journal.pone.0108252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Coburn JM, Gibson M, Monagle S, Patterson Z, Elisseeff JH, Bioinspired nanofibers support chondrogenesis for articular cartilage repair, Proc. Natl. Acad. Sci 109 (2012) 10012–10017. doi: 10.1073/pnas.1121605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Gasparotto VPO, Landim-Alvarenga FC, Oliveira ALR, Simões GF, Lima-Neto JF, Barraviera B, Ferreira RS, A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells, Stem Cell Res. Ther 5 (2014) 78. doi: 10.1186/scrt467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Gaut C, Sugaya K, Critical review on the physical and mechanical factors involved in tissue engineering of cartilage, Regen. Med 10 (2015) 665–679. doi: 10.2217/rme.15.31. [DOI] [PubMed] [Google Scholar]
- [160].Wu Y, Kennedy P, Bonazza N, Yu Y, Dhawan A, Ozbolat I, Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review, Cartilage (2018) 194760351880941. doi: 10.1177/1947603518809410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Urschel JD, Scott PG, Williams HTG, The effect of mechanical stress on soft and hard tissue repair; a review, Br. J. Plast. Surg 41 (1988) 182–186. doi: 10.1016/0007-1226(88)90049-5. [DOI] [PubMed] [Google Scholar]
- [162].Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT, The bioink: A comprehensive review on bioprintable materials, Biotechnol. Adv 35 (2017) 217–239. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- [163].Zhu J, Marchant RE, Design properties of hydrogel tissue-engineering scaffolds, Expert Rev. Med. Devices 8 (2011) 607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW, Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink, Nat. Commun 5 (2014) 3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Sawkins MJ, Mistry P, Brown BN, Shakesheff KM, Bonassar LJ, Yang J, Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair, Biofabrication 7 (2015) 035004. doi: 10.1088/1758-5090/7/3/035004. [DOI] [PubMed] [Google Scholar]
- [166].Tan YJ, Tan X, Yeong WY, Tor SB, Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy, Sci. Rep 6 (2016) 39140. doi: 10.1038/srep39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Mayer-Wagner S, Passberger A, Sievers B, Aigner J, Summer B, Schiergens TS, Jansson V, Müller PE, Effects of low frequency electromagnetic fields on the chondrogenic differentiation of human mesenchymal stem cells, Bioelectromagnetics 32 (2011) 283–290. doi: 10.1002/bem.20633. [DOI] [PubMed] [Google Scholar]
- [168].Jang J, 3D Bioprinting and In Vitro Cardiovascular Tissue Modeling, Bioengineering 4 (2017) 71. doi: 10.3390/bioengineering4030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Pal S, Design of artificial human joints & organs, Des. Artif. Hum. Joints Organs 9781461462 (2014) 1–419. doi: 10.1007/978-1-4614-6255-2. [DOI] [Google Scholar]
- [170].Sharma C, Gautam S, Dinda AK, Mishra NC, Cartilage tissue engineering: Current scenario and challenges, Adv. Mater. Lett 2 (2011) 90–99. doi: 10.5185/amlett.2011.1211. [DOI] [Google Scholar]
- [171].Baker BM, Shah RP, Huang AH, Mauck RL, Dynamic Tensile Loading Improves the Functional Properties of Mesenchymal Stem Cell-Laden Nanofiber-Based Fibrocartilage, Tissue Eng. Part A 17 (2011) 1445–1455. doi: 10.1089/ten.tea.2010.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Hayhurst C, The Role of PTs in REGENERATIVE MEDICINE., PT Motion 8 (2016) 18–25. http://www.apta.org/PTinMotion/2016/3/Feature/RegenerativeMedicine/ (accessed September 28, 2018). [Google Scholar]
- [173].Yanez M, Rincon J, Dones A, De Maria C, Gonzales R, Boland T, In Vivo Assessment of Printed Microvasculature in a Bilayer Skin Graft to Treat Full-Thickness Wounds, Tissue Eng. Part A 21 (2015) 224–233. doi: 10.1089/ten.tea.2013.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Saeidnia S, Manayi A, Abdollahi M, From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons., Curr. Drug Discov. Technol 12 (2015) 218–24. doi: 10.2174/1570163813666160114093140. [DOI] [PubMed] [Google Scholar]
- [175].Dizon JRC, Espera AH, Chen Q, Advincula RC, Mechanical characterization of 3D-printed polymers, Addit. Manuf 20 (2018) 44–67. doi: 10.1016/j.addma.2017.12.002. [DOI] [Google Scholar]
- [176].Ozbolat IT, Koc B, Modeling of spatially controlled biomolecules in three-dimensional porous alginate structures, Journal of Medical Devices 4 (2010) 041003. [Google Scholar]
- [177].Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM, Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences, Anal. Chem 86 (2014) 3240–3253. doi: 10.1021/ac403397r. [DOI] [PubMed] [Google Scholar]
- [178].Berman B, 3-D printing: The new industrial revolution, Bus. Horiz 55 (2012) 155–162. doi: 10.1016/j.bushor.2011.11.003. [DOI] [Google Scholar]
- [179].Mellor S, Hao L, Zhang D, Additive manufacturing: A framework for implementation, in: Int. J. Prod. Econ., Elsevier, 2014: pp. 194–201. doi: 10.1016/j.ijpe.2013.07.008. [DOI] [Google Scholar]
- [180].Vashist A, Kaushik A, Ghosal A, Bala J, Nikkhah-Moshaie R, Wani WA, Manickam P, Nair M, Nanocomposite Hydrogels: Advances in Nanofillers Used for Nanomedicine, Gels 4 (2018) 75. doi: 10.3390/gels4030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Gou M, Qu X, Zhu W, Xiang M, Yang J, Zhang K, Wei Y, Chen S, Bio-inspired detoxification using 3d-printed hydrogel nanocomposites, Nat. Commun 5 (2014) 1–9. doi: 10.1038/ncomms4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Griffin DJ, Vicari J, Buckley MR, Silverberg JL, Cohen I, Bonassar LJ, Effects of enzymatic treatments on the depth-dependent viscoelastic shear properties of articular cartilage, J. Orthop. Res 32 (2014) 1652–1657. doi: 10.1002/jor.22713. [DOI] [PubMed] [Google Scholar]
- [183].Takebe T, Kobayashi S, Kan H, Suzuki H, Yabuki Y, Mizuno M, Adegawa T, Yoshioka T, Tanaka J, Maegawa J, Taniguchi H, Human elastic cartilage engineering from cartilage progenitor cells using rotating wall vessel bioreactor, in: Transplant. Proc, 2012: pp. 1158–1161. doi: 10.1016/j.transproceed.2012.03.038. [DOI] [PubMed] [Google Scholar]
- [184].Chang CH, Lin FH, Lin CC, Chou CH, Liu HC, Cartilage tissue engineering on the surface of a novel gelatin-calcium- phosphate biphasic scaffold in a double-chamber bioreactor, J. Biomed. Mater. Res. - Part B Appl. Biomater 71 (2004) 313–321. doi: 10.1002/jbm.b.30090. [DOI] [PubMed] [Google Scholar]
- [185].Song L, Zhou Q, Duan P, Guo P, Li D, Xu Y, Li S, Luo F, Zhang Z, Successful development of small diameter tissue-engineering vascular vessels by our novel integrally designed pulsatile perfusion-based bioreactor, PLoS One 7 (2012) e42569. doi: 10.1371/journal.pone.0042569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].König F, Hollweck T, Pfeifer S, Reichart B, Wintermantel E, Hagl C, Akra B, A Pulsatile Bioreactor for Conditioning of Tissue-Engineered Cardiovascular Constructs under Endoscopic Visualization, J. Funct. Biomater 3 (2012) 480–496. doi: 10.3390/jfb3030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Moffat KL, Neal RA, Freed LE, Guilak F, Engineering Functional Tissues: In Vitro Culture Parameters, in: Princ. Tissue Eng Fourth Ed., Academic Press, 2013: pp. 237–259. doi: 10.1016/B978-0-12-398358-9.00013-6. [DOI] [Google Scholar]
- [188].Wilson WC, Boland T, Cell and organ printing 1: Protein and cell printers, Anat. Rec. - Part A Discov. Mol. Cell. Evol. Biol 272 (2003) 491–496. doi: 10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- [189].Paulsen SJ, Miller JS, Tissue vascularization through 3D printing: Will technology bring us flow?, Dev. Dyn 244 (2015) 629–640. doi: 10.1002/dvdy.24254. [DOI] [PubMed] [Google Scholar]
- [190].Bolijn S, Lucassen PJ, How the Body Talks to the Brain; Peripheral Mediators of Physical Activity-Induced Proliferation in the Adult Hippocampus, Brain Plast 1 (2015) 5–27. doi: 10.3233/BPL-150020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Nielsen MJ, Leeming DJ, Karsdal MA, Krag A, Biomarkers of Extracellular Matrix Remodeling in Liver Diseases, in: Springer, Dordrecht, 2017: pp. 221–246. doi: 10.1007/978-94-007-7675-3_14.L. [DOI] [Google Scholar]
- [192].Nayyer L Birchall M Seifalian AM Jell G, Design and development of nanocomposite scaffolds for auricular reconstruction., Nanomedicine 10 (2014) 235–46. doi: 10.1016/j.nano.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [193].Wu Y, Sriram G, Fawzy AS, Fuh JY, Rosa V, Cao T, Wong YS, Fabrication and evaluation of electrohydrodynamic jet 3D printed polycaprolactone/chitosan cell carriers using human embryonic stem cell-derived fibroblasts., J. Biomater. Appl 31 (2016) 181–92. doi: 10.1177/0885328216652537. [DOI] [PubMed] [Google Scholar]
- [194].Hodgson MJ, Knutson CC, Momtahan N, Cook AD, Extracellular Matrix from Whole Porcine Heart Decellularization for Cardiac Tissue Engineering, in: Methods Mol. Biol, 2017. doi: 10.1007/7651_2017_31. [DOI] [PubMed] [Google Scholar]
- [195].Norotte C, Marga FS, Niklason LE, Forgacs G, Scaffold-free vascular tissue engineering using bioprinting, Biomaterials 30 (2009) 5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo SS, Vincent PA, Dai G, Creating perfused functional vascular channels using 3D bio-printing technology, Biomaterials 35 (2014) 8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Cui X, Breitenkamp K, Lotz M, D’Lima D, Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation, Biotechnol. Bioeng 109 (2012) 2357–2368. doi: 10.1002/bit.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Gao G, Zhang XF, Hubbell K, Cui X, NR2F2 regulates chondrogenesis of human mesenchymal stem cells in bioprinted cartilage, Biotechnol. Bioeng 114 (2017) 208–216. doi: 10.1002/bit.26042. [DOI] [PubMed] [Google Scholar]
- [199].Di Bella C, Duchi S, O’Connell CD, Blanchard R, Augustine C, Yue Z, Thompson F, Richards C, Beirne S, Onofrillo C, Bauquier SH, Ryan SD, Pivonka P, Wallace GG, Choong PF, In situ handheld three-dimensional bioprinting for cartilage regeneration, J. Tissue Eng. Regen. Med 12 (2018) 611–621. doi: 10.1002/term.2476. [DOI] [PubMed] [Google Scholar]
- [200].Anton D, Burckel H, Josset E, Noel G, Three-dimensional cell culture: A breakthrough in vivo, Int. J. Mol. Sci 16 (2015) 5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Sun T, Norton D, Vickers N, McArthur SL, Mac Neil S, Ryan AJ, Haycock JW, Development of a bioreactor for evaluating novel nerve conduits, Biotechnol. Bioeng 99 (2008) 1250–1260. doi: 10.1002/bit.21669. [DOI] [PubMed] [Google Scholar]
- [202].Boland T, Tao X, Damon BJ, Manley B, Kesari P, Jalota S, Bhaduri S, Drop-on-demand printing of cells and materials for designer tissue constructs, Mater. Sci. Eng. C 27 (2007) 372–376. doi: 10.1016/j.msec.2006.05.047. [DOI] [Google Scholar]