Abstract
Twenty-four-hour rhythmicity in physiology and behavior are driven by changes in neurophysiological activity that vary across the light-dark and rest-activity cycle. Although this neural code is most prominent in neurons of the primary circadian pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus, there are many other regions in the brain where region-specific function and behavioral rhythmicity may be encoded by changes in electrical properties of those neurons. In this review, we explore the existing evidence for molecular clocks and/or neurophysiological rhythms (i.e., 24-h) in brain regions outside the SCN. In addition, we highlight the brain regions that are ripe for future investigation into the critical role of circadian rhythmicity for local oscillators. For example, the cerebellum expresses rhythmicity in over 2,000 gene transcripts, and yet we know very little about how circadian regulation drives 24-h changes in the neural coding responsible for motor coordination. Finally, we conclude with a discussion of how our understanding of circadian regulation of electrical properties may yield insight into disease mechanisms which may lead to novel chronotherapeutic strategies in the future.
Keywords: electrophysiology, circadian, review, molecular clock, extra-SCN
Graphical Abstract
In this review, we consolidate the existing evidence for molecular and neurophysiological circadian rhythms throughout the brain, discuss the challenges in investigating these extra-SCN clocks, and describe the importance of circadian regulation of excitability for neuronal function and diseases of the nervous system. In addition, we highlight the brain regions that are ripe for future investigation into the critical role of circadian rhythmicity for local oscillators.
Introduction
The primary mechanism by which the brain processes sensory information, regulates whole body physiology, and balances hormones is electrical signaling. Specifically, the intrinsic excitability of neurons underlies the spiking rate or pattern that encodes the overall output signal of a neuronal network. Membrane properties can be altered by mechanisms that are either passive (e.g., membrane potential and input resistance) or active (e.g., affecting spike waveform dynamics). These mechanisms ultimately influence the all-or-none action potential. Although entirely necessary, firing action potentials are energetically costly and must be tightly regulated and synchronized with function. One evolutionarily conserved mechanism for anticipating daily fluctuations in function is the circadian system. For decades, we have known that neurons of the primary circadian pacemaker in the hypothalamic suprachiasmatic nucleus (SCN) exhibit strong, intrinsic 24-h rhythmicity in spike frequency (Kuhlman & McMahon, 2006; Brown & Piggins, 2007). However, the role of the molecular clock in other neuron types is still largely unexplored, despite the findings that circadian rhythmicity has been demonstrated in numerous other brain regions (Natsubori et al., 2013b; a; 2014; Christiansen et al., 2016; Yoshikawa & Honma, 2016). Thus, the purpose of this review is to: 1) consolidate the existing evidence for circadian regulation of neuronal activity throughout the brain, 2) highlight the challenges in investigating these extra-SCN clocks, pointing areas of future investigation, and 3) describe the importance of circadian regulation of excitability for neuronal function and diseases of the nervous system.
At the cellular level, the molecular machinery that drives 24-hour transcriptional rhythms consists of a transcriptional-translational feedback loop (TTFL; reviewed in (Partch et al., 2014). Briefly, the positive limb of the TTFL begins when the CLOCK-BMAL1 heterodimer activates the E-box elements of Per and Cry genes, inducing transcription. Negative control occurs when the PER-CRY heterodimer interacts with CLOCK-BMAL1 and inhibits the transcription of its own genes. A secondary feedback loop consists of CLOCK-BMAL1 activation of a nuclear orphan receptor Reverbα whose protein product feeds back to transcriptionally repress Bmal1. Phosphorylation of the negative regulators of the molecular clock (by kinases such as casein kinase I) can target the proteins for proteasomal degradation or increase the rate of nuclear translocation. These core clock genes regulate transcription of an abundance of other ‘clock-controlled genes’ (43% of all protein-coding genes); however, which genes are rhythmic and the timing of those rhythms depends on the cell- or tissue-type (Figure 1) (Zhang et al., 2014). Within each brain region below, we will describe the evidence for circadian rhythmicity (~24-h) of these molecular clock components.
Figure 1.
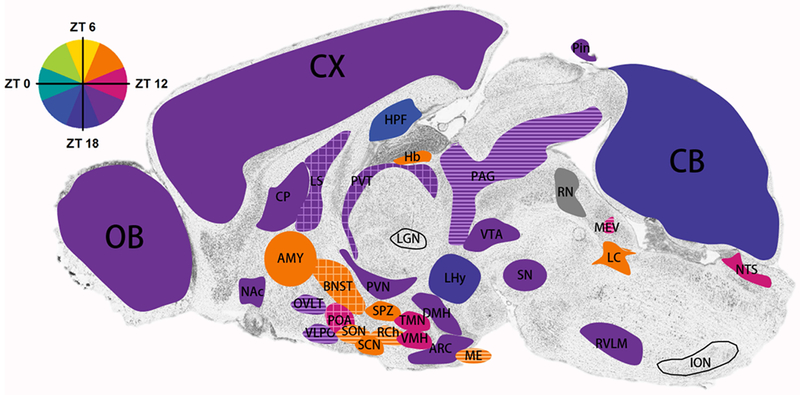
Illustration of timing molecular rhythms in regions throughout the brain of nocturnal rodents. Where data is available, areas are shaded based on the timing of peak Per1/2 mRNA expression (solid) of WT animals under standard conditions (12:12 LD with ad libitum access to food; variations in light intensity were not taken into account). Areas in which only protein levels (grid pattern) or luciferase assays (striped) have been examined are colored according to the predicted timing of peak Per1/2 expression (i.e. 3 hours before peak protein levels). Gray areas are those that express rhythms but timing is still unknown. Unfilled areas are those which express physiological rhythms, but molecular rhythms are not present or have yet to be explored. The amygdala is colored based on Per2 expression in the central nucleus. For details see text. AMY, amygdala; ARC, arcuate nucleus; BNST, bed nucleus of the stria terminalis; CP, caudate putamen; CX, cortex; DMH, dorsomedial hypothalamus; Hb, habenula; HPF, hippocampal formation; ION, inferior olivary nucleus; LC, locus coeruleus; LGN, lateral geniculate nucleus; LHy, lateral hypothalamus; LS, lateral septum; ME, median eminence; MEV, midbrain trigeminal nucleus; NAc, nucleus accumbens; NTS, nucleus of the solitary tract; OVLT, organum vasculosum of the lamina terminalis; OB, olfactory bulb; PAG, periaqueductal gray; POA, medial preoptic area; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular nucleus of the thalamus; RCh, retrochiasmatic area; RN, raphe nuclei; RVLM, rostral ventrolateral medulla; SCN, suprachiasmatic nucleus; SN, substantia nigra; SO, supraoptic nucleus; SPZ, subparaventricular zone; TMN, tuberomammillary nucleus; VMH, ventromedial hypothalamus; VTA, ventral tegmental area.
Suprachiasmatic nucleus of the hypothalamus
Time-of-day information is communicated by the SCN to the rest of the body through characteristic rhythms in spontaneous action potential firing, with high-frequency activity during the day and low activity at night (Inouye & Kawamura, 1979). Additionally, SCN cells exhibit daily changes in membrane properties such as input resistance (Rinput), resting membrane potential, and action potential waveform properties such as after-hyperpolarization amplitude (Kuhlman & McMahon, 2004; Farajnia et al., 2015; Paul et al., 2016). Numerous ionic components underlying SCN neuronal activity rhythms have been identified, many of which express day/night difference in mRNA expression, protein levels, and/or current density (for review, see (Brown & Piggins, 2007; Colwell, 2011; Meijer & Michel, 2015; Allen et al., 2017). Briefly, increased excitatory drive during the day is provided by increased persistent sodium current, sodium leak current, and L-type calcium current (Pennartz et al., 1997; Pennartz et al., 2002; Flourakis et al., 2015; Paul et al., 2016). Moreover, the fast delayed rectifier and A-type potassium currents contribute to decreased spike width during the day (Itri et al., 2005; Itri et al., 2010), whereas increased large-conductance calcium-activated potassium current at results in a larger after-hyperpolarization at night (Meredith et al., 2006; Montgomery & Meredith, 2012).
The daily rhythm in SCN neuronal activity has been replicated in a variety of preparations, including in vivo multiunit recordings in freely moving hamsters (Yamazaki et al., 1998) and in single cell recordings from SCN-containing brain slices in which the retinal input has been severed (Green & Gillette, 1982). Additionally, these circadian firing patterns persist in animals housed under constant conditions (Kuhlman & McMahon, 2004; Nakamura et al., 2011), as well as in dispersed SCN neurons (Herzog et al., 1998). Taken together, these studies show that daily rhythms in SCN neuronal excitability are not dependent on environmental, retinal, or other neuronal input, thus pointing to an endogenous mechanism driving physiological rhythms at a single-cellular level – likely the molecular clock. Evidence that the TTFL drives rhythms in SCN physiology is found in animal models in which the molecular clock machinery has been disrupted. In these models, changes in the molecular rhythms (i.e., period length or arrhythmicity) are reflected in similar changes in SCN neuronal activity (Liu et al., 1997; Albus et al., 2002). Furthermore, recent work has begun to elucidate the possible mechanisms by which the circadian clock affects membrane properties such as rhythmic expression of a protein regulating channel trafficking (Flourakis et al., 2015), phosphorylation of a regulatory kinase (Paul et al., 2016), and expression of an auxiliary subunit (Whitt et al., 2016). Additional putative mechanisms of clock control of neural activity may include daily changes in the distribution (e.g., clustering and declustering) of ion channels within the plasma membrane (Colwell, 2011). Despite the clear association between molecular and neurophysiological rhythms in the SCN, the role of the TTFL on circadian regulation of neuronal excitability in other brain regions is still largely unknown.
Entraining signals are transmitted to the SCN through three main afferent pathways. Light information is projected from the retina directly to the SCN through the retinohypothalamic tract (RHT), while nonphotic information is communicated to the SCN through the release of neuropeptide Y and GABA from the intergeniculate leaflet of the thalamus and serotonin from the dorsal raphe nucleus (Morin et al., 2006). The SCN projects most densely to the nearby subparaventricular zone (SPZ), but innervates multiple hypothalamic targets including: the paraventricular nucleus (PVN), medial preoptic area (POA), anterior hypothalamus, ventromedial hypothalamus (VMH), and dorsomedial hypothalamus (DMH). Outside the hypothalamus, the SCN also projects primarily to the paraventricular nucleus of the thalamus (PVT), the lateral geniculate nucleus (LGN) and the lateral septum (LS) (Watts et al., 1987). However, there is evidence that the SCN does not require direct neural connections to exert influence on all of its targets. As seen in hamsters with SCN lesions, behavioral rhythms can be restored by SCN transplants encapsulated to prevent neural outgrowth (Silver et al., 1996). This study demonstrates that the SCN communicates at least in part through paracrine signaling and raises the possibility that the SCN has many more targets than those found in neuroanatomical studies.
Figure 2 depicts all of the brain regions in the mammalian brain that have evidence for rhythmic clock gene expression and/or day-night differences in electrical properties. Research supporting circadian regulation in these regions is described below in sections ordered by the five primary brain divisions. It is also important to note that an overview of the retina is not included below because the importance of the retinal circadian clock is covered elsewhere in this Special Issue (Ko, 2018).
Figure 2.
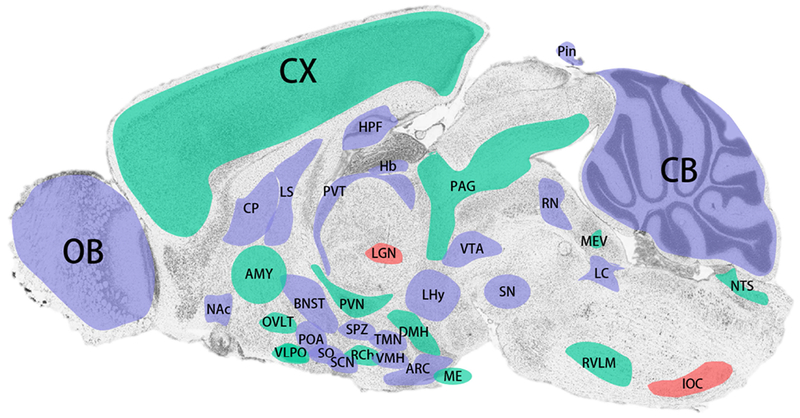
Summary of brain regions expressing molecular and/or physiological rhythms. Areas shaded in red have demonstrated daily rhythms in neuronal excitability. Green shaded areas exhibit oscillations in one or more core molecular clock component. Blue areas are regions which express both molecular and neurophysiological rhythmicity. For details see text. AMY, amygdala; ARC, arcuate nucleus; BNST, bed nucleus of the stria terminalis; CP, caudate putamen; CX, cortex; DMH, dorsomedial hypothalamus; Hb, habenula; HPF, hippocampal formation; IOC, inferior olivary complex; LC, locus coeruleus; LGN, lateral geniculate nucleus; LHy, lateral hypothalamus; LS, lateral septum; ME, median eminence; MEV, midbrain trigeminal nucleus; NAc, nucleus accumbens; NTS, nucleus of the solitary tract; OVLT, organum vasculosum of the lamina terminalis; OB, olfactory bulb; PAG, periaqueductal gray; POA, medial preoptic area; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular nucleus of the thalamus; RCh, retrochiasmatic area; RN, raphe nuclei; RVLM, rostral ventrolateral medulla; SCN, suprachiasmatic nucleus; SN, substantia nigra; SO, supraoptic nucleus; SPZ, subparaventricular zone; TMN, tuberomammillary nucleus; VMH, ventromedial hypothalamus; VTA, ventral tegmental area.
Telencephalon (cerebrum): Olfactory bulb
The olfactory bulb expresses core molecular clock components primarily in the mitral and granular cell layers with higher Per1 and Per2 mRNA levels during the early night and Bmal1 mRNA levels in the late night with a delay for their protein products (Namihira et al., 1999; Shieh, 2003; Hamada et al., 2011). Interestingly, expression of Per1 and Per2 mRNA is increased in response to light exposure (Hamada et al., 2011). In vitro and in vivo bioluminescence recordings of the olfactory bulb from both transgenic Per1-luciferase reporter rats and Per2luc reporter mice demonstrate robust, sustained, and autonomous circadian rhythms of gene expression that peak at night, independent of the SCN (Abe et al., 2002; Abraham et al., 2005; Miller et al., 2014). Odor-induced expression of the immediate early gene, c-Fos, in the olfactory bulb shows circadian variation even under constant dark, with higher expression seen during the subjective night in nocturnal rodents (Amir et al., 1999; Funk & Amir, 2000; Granados-Fuentes et al., 2006). Multi-electrode array recordings of disassociated olfactory bulb neurons reveal a circadian rhythm in neuronal firing rate that oscillates in phase with Per1 mRNA expression rhythms (Granados-Fuentes et al., 2004). Interestingly, olfactory sensitivity also shows circadian rhythmicity that persists in SCN-lesioned mice but is ablated in mice lacking Bmal1 or both Per1 and Per2 (Granados-Fuentes et al., 2011). Whole cell, patch clamp recordings of juxtaglomerular and mitral cells from rat sections show that melatonin decreases an outward potassium currents in a subset of mitral cells only (Corthell et al., 2014). Electrical activity in the olfactory bulb is important for spatial and temporal odor coding (Imai, 2014). Given the strong molecular oscillations in this brain region, coupled with circadian regulation of firing rate, future research is needed to determine how these electrical rhythms correspond to time-of-day dependent changes in temporal and spatial odor coding as well as olfactory learning and plasticity in this complex circuit.
Telencephalon (cerebrum): Cortex
Rhythms in expression of both the positive (Bmal1) and negative (Per1 and Per2) clock gene components have been demonstrated throughout in the medial prefrontal cortex (mPFC) of rats such that Per1/2 expression peaks in the early night and Bmal1 peaks in the late night (Chun et al., 2015). In the piriform cortex of diurnal grass rats, PER1/2-immunoreactivity (-ir) is highest in the late day. This phase difference may be associated with diurnality since clock gene expression of human postmortem PFC (Brodmann’s areas 11 and 47) also exhibits time-of-day variation but with Per1/2/3 expression peaks during the mid-day and Arntl (human Bmal1 homolog) expression peak during the mid-night (Chen et al., 2016). With this human brain dataset, the investigators additionally reported a large number of clock-controlled genes with rhythmic expression specifically in the orbitofrontal cortex. Several of these genes encode voltage-gated ion channels and glutamate receptors (i.e., GRIK2, KCNG1, and KCNH4). Thus, it is not surprising that there is evidence for circadian control of neuronal activity in the cortex of both humans and animal models. For example, cortical excitability in humans (Huber et al., 2013; Ly et al., 2016a) and cortical firing in rodents (Cajochen et al., 2002; Vyazovskiy et al., 2008; Vyazovskiy et al., 2009) increase with time awake over a 24-30 hour period due to a balance between circadian-driven arousal and sleep homeostatic pressure. In fact, 24-hour assessment of cell-to-cell connectivity and excitatory/inhibitory balance (via electroencephalogram; EEG) follows the daily change in visuomotor task performance (Chellappa et al., 2016; Ly et al., 2016b), and sustained attenuation variation over the course of the day is regulated by both circadian and homeostatic processes in a brain-region-specific manner (Muto et al., 2016). While cortical excitation increases with time awake, cortical inhibition starts higher in morning and decreases during day independently of prior sleep history or deprivation (Lang et al., 2011). As a result, cognitive performance and memory vary over the 24-hour day-night cycle with greatest performance during the early wake period and poorest ~12 h later (Burke et al., 2015).
Telencephalon (cerebrum): Amygdala and bed nucleus of the stria terminalis (BNST)
The amygdala (AMY in Fig. 1, 2) is another limbic brain region important for memory (of fear-provoking stimuli, in particular) as well as emotional and motivational regulation (Nieh et al., 2013). Cortical brain regions input to the basolateral amygdala (BLA), and BLA glutamatergic neurons then project to the GABAergic neurons of the central amygdala (CeA) (Nieh et al., 2013). While both brain regions express the core clock components, there is some inconsistency regarding the timing of rhythmic clock gene expression in these areas. Many studies have found that in the BLA and sometimes in the CeA, Period (Per1, Per2, and Per3) mRNA expression peaks in the night with PER2 protein expression lagging behind with a peak at the night-day transition (Lamont et al., 2005; Pantazopoulos et al., 2011; Harbour et al., 2014; Chun et al., 2015; Moriya et al., 2015; Ikeno & Yan, 2016). Rhythmic Bmal1 mRNA expression in the BLA or whole amygdala has been demonstrated in several studies but with variability in circadian phase (Savalli et al., 2014; Chun et al., 2015; Moriya et al., 2015). In the CeA, PER2 expression showed a similar peak as in the BLA in some studies (Pantazopoulos et al., 2011; Chun et al., 2015) but an opposite phase in other studies, with the CeA PER2 peak phase during the early night (Lamont et al., 2005; Perrin et al., 2006; Segall et al., 2006; Segall et al., 2008; Harbour et al., 2013). In addition, Bmal1 mRNA expression rhythms in the CeA peak in the middle of the night with an ~8-hour delay from Per2 (Harbour et al., 2014). The phase of clock gene rhythmicity in the amygdala appears to be influenced by several factors, including input from a rhythmic SCN, environmental fear-provoking stimuli, sex and/or the estrus cycle, and temporal niche. For example, PER2-ir rhythms in the CEA and BLA are shifted in arrhythmic rats with SCN lesions or in mice exposed to fear-inducing stimuli such as fox urine (Lamont et al., 2005; Pantazopoulos et al., 2011). In the oval nucleus of the bed nucleus of the stria terminalis (BNST), a region considered to be part of the ‘extended amygdala,’ PER2-ir peaks during the night in male rats and female rats in diestrus but during the day in pregnant female rats (Perrin et al., 2006; Segall et al., 2006; Schrader et al., 2011; Harbour et al., 2014). Finally, temporal niche may also contribute to molecular clock phase in the amygdala. In diurnal grass rats, PER2-ir peaks in the early day in both the CeA and BNST, while the phase is delayed to the late day in the BLA (Ramanathan et al., 2010a). The functional significance of the phase differences among amygdala regions is yet to be determined.
Interestingly, the acquisition and recall of fear (conditioned by either context or cue) are greater when training occurs during the day (versus night), and these rhythms persist in constant darkness (Chaudhury & Colwell, 2002). Once formed, extinction of fear memory occurs more rapidly for night-trained animals (Chaudhury & Colwell, 2002). Loss of the molecular clock in the forebrain (including the amygdala but not the SCN) reduces daytime enhancement of contextual fear memory to night-time levels (Snider & Obrietan, 2018), suggesting a role of the local clock in the amygdala. In fact, both the amygdala and SCN appear to be necessary for entrainment of foraging behavior by fear. To examine fear entrainment, one recent study investigated circadian regulation of fear memory (fear entrainment) with a “closed economy chamber” in which rat chambers are divided into safe nesting areas and less safe foraging areas due to pairing a signaled or unsignaled foot shock with either the light period or dark period (Pellman et al., 2015). When housed for several weeks in these chambers, rats learn to forage for food at times of the day that are unlikely to result in shock. For example, unsignaled shocks that only occurred during the dark result in day-time foraging for food even though activity stays primarily nocturnal. Day-time foraging behavior appears to be an entrained behavioral rhythm because release into constant darkness (DD) upon cessation of the shocks results in continued foraging at the night-day transition for numerous cycles. SCN-lesioned rats show no light-dark differences in foraging or activity regardless of the time-of-day of unsignaled shocks. Rats with amygdala lesions are unable to shift foraging to the day when shocks only occur during the dark phase. Taken together, both the SCN and amygdala appear to be required for fear entrainment.
One of the most important functions of the amygdala circuit is the formation of synaptic plasticity underlying fear memory (Nieh et al., 2013). For example, repeatedly pairing optogenetic activation of excitatory BLA neurons with a tone conditions the tone to induce freezing behavior (a fear response in rodents)(Johansen et al., 2010). The lateral CeA contains “on” cells which inhibit the inhibitory “off” cells when a conditioned stimulus is present, resulting in disinhibition of the circuit that produces the fear response (Haubensak et al., 2010). To date, no studies have examined whether the formation of synaptic plasticity in amygdala circuit is facilitated during the day versus night and how the molecular clock contributes to the strengthening of synapses. For the extended amygdala, one study examined in vivo multi-unit activity (MUA) of the BNST of hamsters and found that spike frequency is higher during the day, in phase with the SCN (Yamazaki et al., 1998). Given that optogenetic stimulation of BNST neurons can be anxiolytic versus anxiogenic, depending on whether respective inhibitory or excitatory BNST neurons are targeted (Jennings et al., 2013), future studies are necessary to determine whether increased firing during the day corresponds to enhanced fear memory at that time-of-day. It is likely that the neurophysiology of different sub-regions and cell types of the amygdala vary over the time of day, and exactly how these electrical properties are circadian regulated remains an open question. This question is important to address in light of the putative function of the amygdala clock in regulating anxiety as well as anticipating the appropriate time of day to avoid predation or to find food.
Telencephalon (cerebrum): Hippocampal formation
The hippocampal formation is a brain region known to be crucial for learning and memory, particularly spatial learning and memory. It is comprised of four main subregions: Cornu Ammonis regions 1-3 (CA1-3) and the dentate gyrus. The hippocampus expresses all the core components of the molecular clock (Per1/2, Cry1/2, Clock and Bmal1) at the transcript and protein level (Jilg et al., 2010). Several studies have found rhythmic expression of these core clock components (Jilg et al., 2010; Wyse & Coogan, 2010; Harbour et al., 2014; Chun et al., 2015; Besing et al., 2017). Per2 is one of the most well-characterized clock components in the hippocampus. In nocturnal rodents, Per2 mRNA levels peak in the late night, opposite in phase of peak Per2 mRNA expression in the SCN (Wang et al., 2009; Harbour et al., 2014; Chun et al., 2015). Interestingly, hippocampal peak Per2 expression peaks during the day in diurnal degus, in phase with Per2 expression in the SCN (Otalora et al., 2013). Wang et al. (2009) found that rhythmic Per2 expression in nocturnal mice persists even under constant dark conditions, supporting the idea that a functional, rhythmic circadian molecular clock exists in the hippocampus. More recent evidence reported rhythmic expression of Cry1 promoter activity in area CA1 of freely moving mice, which persisted in both a light/dark cycle and constant darkness (Mei et al., 2018). Moreover, organotypic hippocampal slice cultures from mPer2luc transgenic reporter mice show that PER2::LUC expression in the isolated hippocampal circuit oscillates over several circadian cycles, suggesting that the hippocampus possesses an autonomous clock (Wang et al., 2009). Notably, the hippocampus is composed of heterogenous neuronal and cellular populations, and the characterization of clock gene expression has not been cell-type specific for the most part. One study reported snapshots of cell-specific localization of clock proteins in C3H/J mice and found PER1/2, CRY1/2, CLOCK, and BMAL1 expression in hippocampal neurons, including parvalbumin-containing neurons, but not glia (Jilg et al., 2010).
There is clear evidence to support time-of-day regulation of hippocampal-dependent learning and memory processes (for review see (Snider et al., 2018). Impairments in hippocampal-dependent behavioral assays observed in various clock gene knockout (KO) mice suggest that the molecular clock contributes to hippocampal-dependent learning and memory (Sei et al., 2006; Jilg et al., 2010; Kondratova et al., 2010; Wardlaw et al., 2014; Rawashdeh et al., 2016; Snider & Obrietan, 2018). Interestingly, mice lacking Bmal1 exclusively in forebrain structures (but with normal SCN expression) show deficits in novel-object location, novel-object recognition, Barnes maze, and contextual fear conditioning (Shimizu et al., 2016; Snider et al., 2016; Snider & Obrietan, 2018).These findings illustrate the importance of the local hippocampal and forebrain clock for hippocampal-dependent learning and memory.
Given that the local clock can affect circuit function, it is reasonable to assume that the circadian clock would also modulate electrical properties across time of day. However, there is surprisingly little research examining circadian rhythms of electrophysiological phenomenon in the hippocampus and even fewer studies assessing whether the rhythms that do exist are clock-controlled. Barnes et al. (1977) observed diurnal variation in the amplitude of extracellular excitatory postsynaptic potentials (EPSP) and population spikes of granule cells in the dentate gyrus, evoked by perforant pathway stimulation. The study was conducted in awake and moving rats and squirrel monkeys over at least 24 hours and sampled every 30 minutes. In the rats, larger amplitude EPSPs and population spikes were recorded during the dark period compared to the light, with peak amplitude in the middle of the dark phase. This pattern persisted, but with a slight phase advance, in a blinded rat. Interestingly, as opposed to the nocturnal rats, the amplitudes of evoked responses were larger during the light period in diurnal squirrel monkeys. However, West and Deadwyler (1980) used similar methods and found reduced population spike amplitude during the dark period, compared to the light, and no difference in amplitude of evoked EPSPs. These findings were independent of behavioral state as measured by EEG and circulating corticosterone levels. In contrast, another study using similar methods found a robust diurnal rhythm in population EPSP slope, which peaked during the dark phase, yet greater population spike amplitude in the light phase (Cauller et al., 1985). These oscillations persisted in both constant dark and constant light but with a period not equal to 24 hours. Although there are some discrepancies in these three early studies that could be due to differences in experimental protocols, they all suggest time-of-day-dependent variation in electrophysiological properties within the hippocampus. Recently, recordings of spontaneous activity in CA1 of freely moving rats revealed that the firing rates of CA1 pyramidal neurons oscillate over a circadian cycle, but activity was not correlated to the light-dark cycle and was instead entrained by food (Munn & Bilkey, 2012; Munn et al., 2015).
An important and well-studied phenomenon in hippocampal physiology is synaptic plasticity, including long-term synaptic potentiation (LTP) and long-term depression (LTD). Over 30 years ago, the first study to examine circadian regulation of LTP in CA1 and dentate gyrus of rat hippocampal slices found that both the incidence and magnitude of LTP differed across the light-dark cycle (Harris & Teyler, 1983). LTP in the dentate gyrus was more likely to occur and displayed greater magnitude in the dark period. Conversely, LTP in CA1 was more likely to occur and displayed greater magnitude in the light period. Raghavan et al. (1999) found that in Syrian hamster hippocampal slices, LTP was greater during the day, in agreement with previous findings in the rat (Harris & Teyler, 1983). These data would suggest that, in nocturnal rodents, LTP is enhanced during their inactive period. However, more recent studies using hippocampal slices from C57 and C3H mice show that LTP of the Schaffer collateral-CA1 synapse is greater during the night, compared to day (Chaudhury et al., 2005; Besing et al., 2017). This nighttime enhancement is still present in C3H mice released into constant darkness (Chaudhury et al., 2005). Similarly, studies in rats examining LTP in vivo and in slice have also found greater LTP during the night, compared to the day in both CA1 and dentate gyrus (Bowden et al., 2012; Nakatsuka & Natsume, 2014). Discrepancies across studies could be attributed to different stimulation protocols used to induce LTP and outcome metrics used to measure LTP (i.e., population spike versus EPSP). Recently, it has been shown that hippocampal LTP is impaired in mice with disrupted core clock genes (Wang et al., 2009; Wardlaw et al., 2014). LTD has not been as thoroughly examined for circadian variability as LTP in hippocampus. Bowden et al. (2012) observed no diurnal variation of LTD in the dentate gyrus of rats recorded in vivo. However, Yang et al. (2012) found that the ability to induce LTD in vivo in rats is greater during the day compared to night and is dependent upon sleep pressure. In conclusion, the hippocampus appears to express a functional, autonomous clock and displays circadian rhythmicity in some measures of neural activity and plasticity. Given the important role that the hippocampus plays in learning and memory, understanding how the autonomous hippocampal clock modulates physiology and function is an exciting and important area of research.
Telencephalon (cerebrum): Lateral septal complex
The lateral septum (LS) is an area involved in the regulation of mood and motivation which receives inputs from a wide range of brain regions (Sheehan et al., 2004), including sparse innervation from the SCN (Watts et al., 1987). Rhythms in PER1 expression have been reported in the LS of adult rats, with peak expression occurring during the mid-night in ad lib fed animals and during the light-dark transition in animals fed during the mid-day (Angeles-Castellanos et al., 2007). The timing of PER1 expression also appears to respond to the timing of feeding in nursing rabbits. Specifically, rabbits that nurse pups during the day or during the night exhibit PER1 expression rhythms in the LS that peak ~8 hours after nursing in both groups (Meza et al., 2015), suggesting that maternal behavior is a stronger entraining signal in the LS than the light-dark cycle. Surprisingly, in the same study, PER1 expression in the LS showed no change across the 24-hour cycle in non-nursing female rabbits (Meza et al., 2015). Although there is no day/night difference in glucose utilization in rat LS (Room & Tielemans, 1989), in vivo MUA recordings in the LS of freely moving hamsters show a rhythm in neuronal activity that peaks during the night and persists in animals in constant darkness (Yamazaki et al., 1998). However, indirect measures of neuronal activity, such as c-Fos and cytochrome oxidase, suggest that LS activity is highly sensitive to timing of feeding (Angeles-Castellanos et al., 2007; Olivo et al., 2017). Thus, more work is needed to determine if rhythms in LS neuronal activity observed in vivo are a result of rhythmic feeding behavior or due to circadian regulation of excitability in LS neurons.
Telencephalon (striatum): Caudate putamen and nucleus accumbens
Rhythms in both the positive and negative regulators of the TTFL have been reported throughout the striatum. Transcription of Bmal1 peaks during the early day, whereas Per1 and Per2 transcription peaks in antiphase (Cai et al., 2009; Sahar et al., 2010). This early night peak in Per1/2 expression coincides with a nightly increase in striatal dopamine (DA) levels and wheel running activity (Hood et al., 2010), which is followed by a peak in PER1/2 protein levels during the dark to light transition in nocturnal animals (Hood et al., 2010; Ramanathan et al., 2010b). Importantly, in diurnal grass rats, the rhythm in PER1/2 expression instead peaks in the late day in both dorsal and ventral striatum (Ramanathan et al., 2010b). Interestingly, blocking D2 receptor (D2R) activation – through treatment with an antagonist, DA depletion, or genetic D2R knock-out – severely dampens the rhythm of Per1/2 expression in the caudate putamen (CP), located in the dorsal striatum (Hood et al., 2010; Sahar et al., 2010). Although these reports would suggest that the dorsal striatum requires D2R signaling to maintain robust molecular rhythms, CP cultures which lack DAergic input from the substantia nigra (SN) exhibit robust PER2::LUC rhythms. These rhythms persist for multiple cycles, suggesting that the region is capable of expressing self-sustaining oscillations as well (Natsubori et al., 2014). The nucleus accumbens (NAc), an area involved in reward processing located in the ventral striatum, is also capable of maintaining PER2::LUC oscillations in culture. However, rhythmicity in the NAc is associated with the behavioral response to a learned helplessness protocol, with NAc cultures from resilient animals more likely to exhibit PER2::LUC rhythmicity than cultures from helpless mice (Landgraf et al., 2016).
Many studies have reported daily rhythms in electrophysiological activity of the dorsal and ventral striatum. Early work using in vivo MUA recordings in the striatum show rhythmic activity in both the CP and NAc, with peak neuronal activity at night during the animal’s activity phase (Inouye & Kawamura, 1979; Yamazaki et al., 1998). Interestingly, the period of neuronal activity in tau mutant hamsters mirrors the shortened behavioral and molecular rhythms seen in this model (Ralph et al., 1990; Liu et al., 1997; Lowrey et al., 2000), with the MUA cycle lasting as little as 20 hours in DD (Yamazaki et al., 1998). Consistent with the nighttime increase in NAc activity in vivo, NAc medium spiny neurons (MSNs) also exhibit day/night differences in passive and active membrane properties as seen in single cell patch clamp recordings of MSNs in brain slice (Parekh et al., 2017). At night, NAc MSNs from mice show an increase in Rinput and an increased frequency of induced firing in response to current injection. Additionally, night-phased MSNs have reduced rheobase, the minimum amount of current needed to induce spiking, which is likely a result of the increased Rinput (Parekh et al., 2017). Interestingly, MSNs from ClockΔ19 mutant are more hyperpolarized than wildtype (WT) neurons during both times of day and have similar levels of induced spiking as seen in WT neurons (Parekh et al., 2017). Day/night differences MSN excitability could provide an intriguing mechanism for regulating time-of-day changes in reward learning (Webb et al., 2009); however, more work is needed to determine which of the MSN subtypes (i.e. D1 or D2 receptor-expressing) exhibit neurophysiological rhythms before the effect of these rhythms can be fully understood.
Diencephalon (Interbrain): Paraventricular nucleus (PVN), organum vasculosum of the lamina terminalis (OVLT), and supraoptic nucleus (SON) of the hypothalamus
The paraventricular nucleus of the hypothalamus (PVN) is a brain center of great importance in the control of sympathetic tone, neuroendocrine function, body fluid balance, and many other body functions (Ferguson et al., 2008). The PVN contains a mixed and complex integration of various neuronal subtypes and circuits that mediate this center’s control of these body functions. Magnocellular neurons produce and secrete oxytocin (Oxt) and arginine vasopressin (AVP), and parvocellular neurons project to the median eminence, various autonomic control sites in the medulla such as the nucleus tractus solitarius and rostral ventrolateral medulla (each discussed later), or directly to spinal tracts that convey sympathetic nerve activity. Given the robust daily rhythms of AVP and Oxt release along with strong diurnal rhythms in autonomic tone, the PVN plays a very likely role in the circadian control of these factors.
Studies have demonstrated direct projections from the SCN to the PVN in both rat (Cui et al., 2001) and human (Dai et al., 1997). Inhibition of GABAergic signaling from the SCN to the PVN results in an inappropriate time of day release of melatonin indicating SCN inhibitory actions on PVN-mediated melatonin secretion (Kalsbeek et al., 2000). GABAergic and glutamatergic projections from the SCN also mediate PVN autonomic activity in a time-dependent manner relative to insulin and glucose handling by the liver and pancreas (Kalsbeek et al., 2008). AVP mediated connections between the SCN and PVN regulate light sensitive inhibition of feeding behavior in rodents (Nakata et al., 2016; Santoso et al., 2017). As analyzed by in-situ hybridization in rats, the PVN has a very robust clock gene expression rhythm that even rivals the amplitude of the SCN; however, the PVN clock gene expression and activity profile appears to be in antiphase to that of the SCN. Specifically, Bmal1 mRNA expression peaks early in the light phase, and Per1 and Per2 peak in the early dark phase in the PVN (Abe et al., 2002; Girotti et al., 2009; Chun et al., 2015). In both the SCN and PVN, c-fos mRNA expression, an indicator of neuronal activity, corresponds with Per1 and Per2 expression (Girotti et al., 2009). Protein expression of PER1 and PER2 in dopaminergic neurons of the PVN in rat follows the diurnal trend observed in gene expression studies with no observable rhythm in CLOCK protein (Sellix et al., 2006).
Interestingly, in the diurnal, day-active grass rat, Arvicanthis niloticus, the PVN oscillation of clock genes is in phase with that of the SCN. When grass rats are given access to running wheels at night, they become nocturnally active, and the PVN expression of Per1 and Per2 rhythms resembles that of other nocturnal rodents, namely peaking during the dark phase. Neither diurnal nor nocturnal temporal niche changes nocturnal increases in melatonin (Martin-Fairey et al., 2015). This result suggests that PVN oscillations of clock genes may be more closely related to the activity preference of the animals, highlighting potential roles for the purported role of the SPZ as a switch as discussed elsewhere in this review, and indicates that the nocturnal rise in melatonin may not depend on the phase of the local PVN oscillator. These studies suggest there is an intricate relationship between the activity of the SCN and the PVN (Kalsbeek et al., 2011); however, to our knowledge, there are currently no studies that have directly investigated circadian rhythms in neuronal activity of neuronal populations within the PVN.
The supraoptic nucleus (SON) is a collection of magnocellular neurosecretory cells that also release AVP and Oxt. Similar to the PVN, the SON contains GABAergic and glutamatergic projections from the SCN (Cui et al., 1997). The SON also receives projections from the organum vasculosum lamina terminalis (OVLT), which is a circumventricular organ lacking an intact blood-brain barrier and is a vital component of osmosensation and thirst drive (Bourque, 2008). Signaling along networks including the OVLT, SON, and the PVN maintains body fluid status in response to increases in plasma osmolality through promotion of thirst drive and through secretion of AVP from magnocellular neurons that acts via the kidney to reabsorb water (Bourque, 2008). In both the SON and OVLT of rats, Per1 mRNA expression peaks during early-to-mid dark phase hours (Abe et al., 2002).
An impressive body of work by Bourque and colleagues over the years has also demonstrated the influence of circadian factors on these networks. Many of these influences are already elegantly reviewed in detail (Trudel & Bourque, 2012; Gizowski & Bourque, 2018). In brief and of particular interest to this review, Bourque and colleagues have demonstrated that SCN activity blunts SON neuronal response to input from the OVLT. As SCN activity increases during the early to mid-sleep phases (Brown & Piggins, 2007; Okamura, 2007), the sensitivity of the SON to input from the OVLT is inhibited (Trudel & Bourque, 2010; 2012). During the later sleep periods, SCN neuronal activity decreases and the inhibitory drive on the SON is removed. This frees the SON to respond with greater effect to input from the OVLT, thereby increasing AVP secretion (Trudel & Bourque, 2010). Consistent with this idea is the prior report that SON neurons have increased firing frequency in vivo during the late day (Bhumbra et al., 2009). The physiological effect of this pathway is to allow for a late sleep, non-osmotically driven increases in AVP secretion to prevent nocturnal polyuria and interrupted sleep. Additional work from Bourque and colleagues demonstrate that AVP-expressing SCN neurons whose activity increases prior to the sleep phase and that project to the OVLT drive an anticipatory thirst mechanism (Trudel & Bourque, 2010; Gizowski et al., 2016; Gizowski & Bourque, 2018).
Diencephalon: Dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), lateral hypothalamus (LHy), and arcuate nucleus (ARC) of the hypothalamus
The dorsomedial hypothalamus (DMH) is a region of the hypothalamus important for control of feeding, drinking, and stress response. The DMH receives neural inputs from both the SCN and the SPZ and projects to the ventrolateral preoptic area and VMH (for review see (Bernardis & Bellinger, 1998; DiMicco et al., 2002). Studies have provided mixed results regarding the phase of the molecular clock of the DMH. The DMH shows rhythmic PER2::LUC expression in organotypic cultures from both rats and mice albeit with variable periodicities (Guilding et al., 2009; Herichova et al., 2017). Additionally, mRNA collected over a 24-h period from rats indicated peak of Per2 mRNA levels in the early night (Herichova et al., 2017). However, multiple other rodent studies reported very low or a lack of rhythmic Per1 or Per2 expression unless there was also restricted access to food, with peak times varying depending on timing of meals (Mieda et al., 2006; Verwey et al., 2008; Verwey et al., 2009; Verwey & Amir, 2011). Another study investigated the impact of aging on components of the molecular clock and reported that while Bmal1 expression is arrhythmic in the DMH, Clock expression is rhythmic in both young (4 months) and old (16 month) mice and that peak expression shifts from mid-day (~ZT 7) to late night (~ZT 22) with age (Wyse & Coogan, 2010). Complimentarily to PER2::LUC rhythmicity in DMH, multiunit discharge is rhythmic in ~half of the DMH slices; however, these rhythms dampened rapidly (Guilding et al., 2009).
The lateral hypothalamus (LHy) contains the primary orexinergic neurons for the brain that are important for many physiological processes such as feeding and arousal (Yamanaka et al., 2003; Adamantidis & de Lecea, 2009). Rhythmicity in organotypic mPer1-Luc rat cultures containing the LHy is evident in ~75% of the cultures with peak expression at ~ZT 14 (Abe et al., 2002). Population sampling of clock gene transcriptional rhythms in the LHy shows that Per2, Cry1, Cry2, and Per1 peak in the light-dark transition or early-mid night, whereas Bmal1 expression is rhythmic in dark fed animals only, with delayed peak expression in the late night (Opperhuizen et al., 2016). Interestingly, all clock gene rhythmicity is lost when animals are fed exclusively during the light phase (Opperhuizen et al., 2016). Several studies report varying results of electrical rhythms in the LHy. For example, Koizumi and Nishino (1976) reported that the LHy electrical activity is inversely related to activity of the VMH (see below) with higher LHy activity during the day and lower activity at night. In contrast, Ono et al. (1981) reported low day-time activity and high night-time activity in single unit recordings in the rat LHy in vivo. However, very few neurons expressed a rhythm in firing rate, and a majority showed low activity at both times of day (Ono et al., 1981). A more recent study reported higher c-Fos staining in orexinergic neurons in the LHy at night compared to during the day (Ramirez-Plascencia et al., 2017), suggesting that cell-type specificity may be important for measuring physiological rhythms in LHy neurons.
Somewhat less is known regarding the rhythmicity of clock genes and electrophysiology in the ventromedial hypothalamus (VMH). Initial evidence suggested that there were no detectable rhythms in organotypic slice cultures from mPer2Luc mice (Guilding et al., 2009) or in Per1 mRNA expression obtained from diurnal degu brains (Otalora et al., 2013). However, a more recent study reported mRNA rhythms in the VMH for Per1/2 (peaking at the light-dark transition) and for Bmal1 (peaking at night), and these rhythms were lost in mice with Bmal1 knockout specifically in nutrient-sensing neurons of the VMH (Orozco-Solis et al., 2016). Recordings in vivo from the rat VMH region indicated that there is a day/night difference in the frequency of MUA volleys (more volleys during the light phase than the dark) and that these volleys correlate with acute fluctuations in blood pressure and heart rate rhythms (Hirasawa et al., 1996). It may be that VMH neurons rhythmically fire, but only under certain conditions. Specifically, Guilding et al. (2009) found no rhythmicity in isolated VMH firing and predicted that the VMH may be a slave oscillator to the SCN, supporting the finding that VMH is more active at night compared to day when the SCN is intact or with scheduled food access (Inouye, 1983).
The arcuate nucleus (ARC) is the region critical for neuroendocrine secretions and functions as the homeostatic control center for the body. The neurons in the ARC are important for control of rhythmic physiological processes such as food intake and metabolism (Cone et al., 2001; Cowley et al., 2003). As this region is important for rhythmic processes, it has been well investigated in the context of circadian gene expression. Uchida et al. (2016) found that PER2::LUC expression in the ARC had a period of ~24.4. Loss of Cry1 and Cry2 differentially affected period length in PER2::LUC rhythms, with Cry1 knockout (KO) animals having a shortened period (~22 h) and Cry2 KO animals showing a longer period (~26 h). Two other studies using PER2::LUC mice reported ~23-h periods for the ARC or ARC complex (including the median eminence and pars tuberalis; (Piek, 1986; Guilding et al., 2009). Interestingly, Guilding et al. (2009) identified the number of rhythmic cells in both the dorsal and lateral regions of the ARC, with the dorsal region having a higher percentage of rhythmic cells. Follow up experiments using forskolin to resynchronize cultures indicated that the dampened rhythmicity in the ARC was due to cells drifting out of synch with one another over time. Additionally, organotypic ARC cultures from the Per1-luc rat exhibited peak Per1 transcription in the ARC at ~ZT 14 (Abe et al., 2002). This result is consistent with population sampling experiments of Per1/2 mRNA expression in the ARC that showed peaks in the early night ((Wang et al., 2017) but see also (Kriegsfeld et al., 2003). Interestingly, clock gene rhythmicity is lost in the ARC of nocturnal animals with food access only during the day (Wang et al., 2017). In rats, Per1 is expressed in some but not all dopaminergic neurons (Sellix et al., 2006). Surprisingly, Per1 expression does not vary across time-of-day in the ARC of the diurnal degu (Otalora et al., 2013). As for positive regulators of the TTFL in the ARC, BMAL1-ir is not expressed rhythmically in young or old mice but CLOCK-ir does have a diurnal rhythm in both age groups, with a shift in peak expression from ~ZT 6 to ~ZT 23 as the animals age (Wyse & Coogan, 2010). These clock gene rhythms may drive neuronal activity as the immediate early gene c-Fos is expressed in a larger number of ARC cells during the night compared to the day (Ramirez-Plascencia et al., 2017). Although one classic study reported a lack of a circadian rhythm in electrical discharge in ARC slices (Groos & Hendriks, 1982), a more recent study reported that brain slices containing the ARC maintained in vitro show circadian periodicity of both multi- and single-unit activity, although peak activity is not related to time of sacrifice or prior light-dark (LD) cycle (Guilding et al., 2009). It is clear that more research is necessary to determine if electrical rhythms of ARC neurons are driven by the molecular clock.
Diencephalon: Hypothalamic preoptic area (POA) and tuberomammillary nucleus (TMN)
The preoptic area (POA) is a region that controls thermoregulation and is involved in sexual and parental behavior and differs in function between males and females (reviewed in (Paredes, 2003). Female mice exhibit anti-phase expression of PER2 and BMAL1, specifically in neurons expressing gonadotropin-releasing hormone, with peak expression during the night and day, respectively (Hickok & Tischkau, 2010). Circadian expression and neuronal activation in these neurons may be involved in controlling time-of-day specific control of luteinizing hormone (Finn et al., 1998). In addition, nursing induces rhythmic Per1 expression in the POA of female rabbits, with a peak occurring 8 hours after nursing (Meza et al., 2015). While relatively little is known about molecular clock gene rhythmicity in this region, electrical rhythms in MUA measured in the POA is greater at night compared to the day (Inouye, 1983). A more recent examination of light sensitivity of POA neurons reported that despite the numerous light insensitive neurons in this region, the basal firing rate of these light-insensitive neurons varied across the 24-h day with higher rates in the late day and lower rates in the late night (Brown et al., 2011).
The tuberomammillary nucleus (TMN) is a small region of the hypothalamus that is responsible for producing histamine and involved in the sleep/wake system (Eriksson et al., 2001; Huang et al., 2001). Histamine is wake-promoting and has been shown to increase during the dark phase (Fell et al., 2015). The molecular clock in the TMN is somewhat understudied; however, one study reported peak Per1, Cry1, and Rev-erbα mRNA rhythms at the light-to-dark transition (Yu et al., 2014). Importantly, these rhythms are lost in histamine-neuron specific Bmal1 knockout mice (Yu et al., 2014). The number of c-Fos-labelled cells is higher at night compared to day in all three sub-nuclei of the TMN, a pattern that persists in constant darkness (Ko et al., 2003; Ramirez-Plascencia et al., 2017). However, neuronal activation also occurs when animals were awake during the light phase compared to when they are sleeping. Thus, the authors hypothesized that there are acute effects of sleep versus wake as well as circadian effects controlling histaminergic neuronal activity (Ko et al., 2003). Similarly, Takahashi et al. (2006) reported that histaminergic neurons fire only when an animal is awake. Interestingly, c-Fos staining in the TMN of the diurnal grass rat showed a reversal of this pattern, with highest c-Fos expression during the day and lowest levels during the late night (Castillo-Ruiz et al., 2013). More research is necessary to determine whether the TMN molecular clock regulates any of these physiological effects across time-of-day or behavioral states.
Diencephalon: Hypothalamic sub-paraventricular zone (SPZ)
One of the densest efferent synapses from the SCN is to the subparaventricular zone (SPZ) of the hypothalamus (Watts et al., 1987). Here, circadian gene expression exhibits 24-hour variation in animals housed in a light-dark cycle with Per1/2 as well as Cry1 transcription peaking in the mid-late day (Jiang et al., 2012; Mei et al., 2018). In vivo Cry1 transcriptional reporter rhythmicity persists in the absence of an LD cycle (Mei et al., 2018). Surprisingly, PER2-ir peaks during the late night in diestrus female rats (Schrader et al., 2010). While sex may impact the SPZ molecular clock phase, temporal niche does not. Specifically, the phase of the molecular clock in the SPZ is the same in both diurnal and nocturnal rodents, similar to the SCN but unlike the other extra-SCN brain regions. Both PER1-ir and PER2-ir rhythms peak in the late day or day-night transition in grass rats regardless of whether they are naturally diurnal or induced to be nocturnal (Ramanathan et al., 2010b). Like clock gene expression, neuronal activation (as reported by cFos-ir) in nocturnal rats or mice, as well as diurnal grass rats, is high during the early day, declining throughout the light period. During the night, however, c-Fos-ir is higher in diurnal grass rats and lower in nocturnal rats and mice (Schwartz et al., 2004; Todd et al., 2018). Multi-unit activity recordings of mice show an anti-phase relationship between SCN and SPZ with higher SCN spike rates during the day and higher SPZ spike rates during the night, both of which are independent of the LD cycle but dependent upon the molecular clock (Nakamura et al., 2008). Day-night differences in spike rates of SPZ neurons in diurnal animals are yet to be determined. In mice, greater neuronal activation during the early day compared to the early night is functionally associated with reduced aggression at that time of day since chemogenetic inhibition of VMH-projecting SPZ neurons reduces aggression specifically in the early night (Todd et al., 2018). Future studies should examine whether neurophysiological activity of SPZ neurons in diurnal models are associated with diurnal variation in aggressive and social behavior.
Thalamus: paraventricular nucleus (PVT) and lateral geniculate nuclei (LGN)
Daily rhythms in protein levels of PER1 and PER2 have been reported in the paraventricular nucleus of the thalamus of mice and rats (Mendoza et al., 2005; Angeles-Castellanos et al., 2007; Feillet et al., 2008). Interestingly, PER expression, which typically peaks during the mid-to-late night in the PVT, is sensitive to changes in feeding behavior (e.g., time-restricted feeding; (Angeles-Castellanos et al., 2007). Although the PVT molecular clock is sensitive to outside factors, it does not depend on external inputs for its rhythmicity as evidenced by self-sustaining Per1-luc rhythms seen in PVT cultures (Abe et al., 2002). Time-of-day changes in the excitability of PVT neurons have been extensively characterized in rats. As seen in most neurons in the thalamus, PVT cells are capable of displaying two different patterns of action potential firing: tonic firing and bursting (Zhang et al., 2006). Although the proportion of tonic versus bursting cells is similar during the day and night, the vast majority (>90%) of PVT are silent during the day, whereas approximately half of nighttime cells are spontaneously active (Kolaj et al., 2012). This daytime decrease in excitability is paired with changes in intrinsic properties as neurons are more hyperpolarized and with lower Rinput during the day (Kolaj et al., 2012). Furthermore, two currents which contribute to burst firing the PVT neurons – T-type calcium current and hyperpolarization-activated cation current – are enhanced during the night (Kolaj et al., 2012).
The lateral geniculate nuclei (LGN) of the thalamus are recipients of some of the densest inputs from melanopsin-containing intrinsically photoreceptive retinal ganglion cells outside the SCN (Brown et al., 2010). Furthermore, neuropeptide Y containing cells from the ventral LGN and intergeniculate leaflet (IGL) project to the SCN via the geniculohypothalamic tract and contribute to the process of photic and non-photic entrainment (for review see (Harrington, 1997). While the LGN are considered part of the extended circadian network (Morin, 2013), there have been very few studies which have focused on circadian rhythmicity of the cells in these nuclei. One study using in vivo recordings in mice reported diurnal variation in the basal activity levels neurons in the LGN, with neuronal firing peaking during the early to mid-night (Brown et al., 2011). This is consistent with previous studies showing increased glucose utilization during the night in the LGN of mice and rats (Inouye & Kawamura, 1979; Jay et al., 1985; Room & Tielemans, 1989).
Epithalamus: Habenula (medial and lateral)
The habenula, a bilateral structure located in the epithalamus, plays a role in behavioral flexibility (Baker & Mizumori, 2017), emotion, motivation, pain processing, learning and memory, stress, and sleep-wake regulation (for review see (Bano-Otalora & Piggins, 2017). It is divided into two main regions, the lateral habenula (LHb) and the medial habenula (MHb). The LHb receives inputs from the cerebral cortex, lateral hypothalamus, and globus pallidus and has efferent projections to the rostromedial tegmental nucleus, median raphe, caudal dorsal raphe, and pontine central gray (Quina et al., 2015), while the medial habenula receives inputs from the medial septum and sends signals to the interpeduncular nucleus (Herkenham & Nauta, 1979; Omelchenko et al., 2009). Although an early in-situ hybridization study demonstrated the presence of Clock, Per1, and Per2 mRNA in the MHb at one time of day (Shieh, 2003), rhythmicity of clock genes in the LHb and MHb has only recently been investigated. Recordings of bioluminescence in LHb cultures from both mPer2Luc and Per1-luc transgenic mice reveal low amplitude rhythms of expression of these core clock components (Guilding et al., 2010; Sakhi et al., 2014b). In mice deficient for both Cry1 and Cry2, rhythmic expression of Per1-luc is ablated, suggesting it depends on a functional circadian clock (Sakhi et al., 2014b). Both Per2 mRNA and PER2 protein have diurnal rhythmic oscillations in rat LHb, with higher expression during the day compared to night (Zhao et al., 2015). However, this day/night difference is lost in animals fed a free-choice high-fat-high-sucrose diet, implicating diet in the regulation of LHb molecular rhythms (Blancas-Velazquez et al., 2017).
Circadian rhythms in firing frequency in the habenula have been extensively studied, with mixed results across species. In vivo extracellular recordings in rats reveal that baseline firing rates are higher during the subjective day compared to night in the LHb and MHb (Zhao & Rusak, 2005). Additionally, populations of neurons within the LHb and MHb are responsive to photic stimulation, with a greater number of responsive cells in the LHb compared to MHb (Zhao & Rusak, 2005). Baseline firing rates of LHb neurons in vitro also peak during the day, but with no differences in firing rate across time of day in MHb (Zhao & Rusak, 2005). However, recent work in mice shows that the resting membrane potential and spontaneous firing rate of MHb neurons varies across time of day, with more depolarized potentials occurring during the late day when MHb neurons are most active (Sakhi et al., 2014a). Firing rate does not change across time of day in Cry1/2 deficient mice, suggesting that a functional molecular clock contributes to this diurnal variation (Sakhi et al., 2014a). Furthermore, whole-cell recordings in the LHb reveal that the time-of-day changes in LHb firing frequency – which is usually lower in early day compared to the late day – are lost in Cry1/2 deficient mice (Sakhi et al., 2014b). It is important to note that temporal differences in firing frequency (Zhao & Rusak, 2005) may be due to presynaptic input rather than intrinsic LHb activity because Park et al. (2017) reported an increase in mini-excitatory post-synaptic current (mEPSC) frequency and paired-pulse ratio (when stimulating in stria medullaris) in afternoon compared to morning.
In support of the electrophysiology studies described above, Tavakoli-Nezhad and Schwartz (2005) examined immediate early gene activation in the LHb and found that c-Fos expression in hamsters exhibited higher expression during the subjective night compared to subjective day. Interestingly, in hamsters with “split” circadian locomotor activity, c-Fos expression in the LHb was asymmetric across hemispheres during their active phase (Tavakoli-Nezhad & Schwartz, 2005). Paul et al. (2011) found that wheel running during hamsters’ inactive period induces c-Fos expression in both LHb and MHb, suggesting that previous findings of day-night rhythms in c-Fos expression (Tavakoli-Nezhad & Schwartz, 2005) may be dependent upon locomotor activity or arousal. However, Paul et al. (2011) also compared c-Fos expression in hamsters running during the day to hamsters running during the night and found higher c-Fos expression in the night group, indicating that circadian phase may indeed affect c-Fos expression. Disrupting the habenula projections resulted in altered daily activity in hamsters, implicating the habenula as a possible regulator of daily locomotor activity (Paul et al., 2011).
Epithalamus: Pineal gland
The pineal gland is situated in the middle of the brain, tucked between the two hemispheres, and is the primary source of the neurohormone melatonin. Melatonin is often referred to as the “night hormone,” and its nocturnal release signals photoperiod length and season to the rest of the brain and body. The pineal gland has long been recognized as a key component of the circadian system. Circadian clock gene expression is rhythmic with the positive TTFL components peaking at the beginning of the day and the negative TTFL components peaking at night (Wongchitrat et al., 2011; Andrade-Silva et al., 2014). Reverb-α mRNA expression peaks in anti-phase to Bmal1 with highest Reverb-α mRNA expression at the beginning of the night (Wongchitrat et al., 2011; Andrade-Silva et al., 2014). These rhythms persist in constant conditions and are entrained by norepinephrine (Andrade-Silva et al., 2014). Regardless of temporal niche, melatonin is produced at night and suppressed by light. In mammals, rhythmic melatonin production and light-induced melatonin suppression are mediated by a multi-synaptic pathway from the retina to the pineal via the SCN, spinal cord, and superior cervical ganglion (Cassone, 1998). In some vertebrates such as birds and fish, pineal cells are light-sensitive (Vatine et al., 2009; Cassone & Westneat, 2012; Hur et al., 2012). Numerous organs of the zebrafish, including the pineal gland, are light-sensitive, and photostimulation of these organs is critical to induce clock gene rhythmicity in embryos as well as promote maturation of the pineal clock (Vatine et al., 2009). Likewise, the avian pineal gland has photoreceptors, and pinealectomy results in arrhythmic locomotor activity, song, and call (Cassone & Westneat, 2012). Transcriptional profiling of the chick pineal revealed ~400 rhythmic genes, including some encoding ion channels (Bailey et al., 2003). Nocturnal melatonin release in the chick pineal gland appears to rely on depolarization of the membrane and activation of voltage-gated calcium currents (Harrison & Zatz, 1989). Interestingly, one study reported that single unit activity of chick pineal cells maintained in vitro is higher during the day than during the night despite nocturnal release of melatonin (Schenda & Vollrath, 2000). In rodents such as rats, gerbils, hamsters and guinea pigs, however, it has long been known that the pineal neurons spontaneously fire with a higher rate during the night than during the day (Semm et al., 1981; Reuss & Vollrath, 1984; Stehle et al., 1987; Stehle & Reuss, 1988). These electrical rhythms are clearly important for pineal function, the most important of which is melatonin production. Given the importance of melatonin for reducing oxidative stress, amyloid aggregation, and inflammation (Alghamdi, 2018), a better understanding of the mechanisms of clock-controlled electrical firing of pineal neurons is needed.
Mesencephalon (Midbrain): Periaqueductal gray, raphe nuclei, reticular formation, midbrain trigeminal nucleus
The sensory neurons of the midbrain trigeminal nucleus, which contribute to daily rhythms in food intake (Yokoyama et al., 2013), exhibit Per1 transcriptional rhythms ex vivo (Hiler et al., 2008). Although expression of Per1-luc is also detected in the cells of the dorsal and median raphe nuclei (DRN and MRN), these areas do not exhibit sustained rhythms in culture (Abe et al., 2002; Hiler et al., 2008). Interestingly, while PER2::LUC expression is arrhythmic in DRN organotypic cultures under normal conditions, rhythms can be induced in this area by exposure to a learned helplessness paradigm independent of the behavioral outcome (Landgraf et al., 2016). Conversely, in another mood-regulating area, the periaqueductal gray area (PAG), PER2::LUC rhythmicity was less likely to be measured in animals that developed helplessness. In this case, arrhythmicity was due to a loss of synchrony in single cell PER2::LUC rhythms (Landgraf et al., 2016). In vivo MUA recordings from the MRN of freely moving rats show daily rhythms in activity which peak at night, but in the DRN, both rhythmic and arrhythmic activity patterns have been reported (Inouye & Kawamura, 1979). Similarly mixed results have been reported in the midbrain reticular formation, which contains the DRN and MRN (Inouye & Kawamura, 1979). Despite conflicting evidence of activity rhythms in the raphe nuclei, extensive work has demonstrated that release of serotonin (5-HT) in downstream targets – including SCN, cortex and surrounding raphe areas – varies across the 24-hour cycle with peak 5-HT levels found during the day (Quay, 1968; Cagampang et al., 1993; Cagampang & Inouye, 1994); but see also (Crespi & Jouvet, 1983). Furthermore, 5-HT has been shown to play a large role in the process of nonphotic entrainment in the SCN (for review, see (Pontes et al., 2010). Given the importance of neuronal activity of serotonergic neurons in seasonal affective disorder (Green et al., 2015), a better understanding of how the local midbrain clock regulates neuronal firing is needed.
Midbrain: Ventral tegmental area (VTA) and substantia nigra (SN)
Rhythmic mRNA expression of Per2, Bmal1, and Reverbα have been reported in ventral midbrain samples containing ventral tegmental area (VTA) tissue (Chung et al., 2014). However, attempts to measure sustained molecular rhythms in VTA organotypic cultures ex vivo have yielded mixed results – with cultures from Per1-luc rats being arrhythmic (Abe et al., 2002) and less than one third of VTA cultures from mPer2Luc mice showing rhythms (Landgraf et al., 2016) but see also (Logan et al., 2015). Evidence of circadian regulation of VTA neurophysiology has been equally unclear. Early rodent studies of glucose utilization during the day or night report no change across time of day in the VTA (Jay et al., 1985; Room & Tielemans, 1989). Consistent with these findings, multiple groups have reported no differences in neuronal activity across a variety of in vivo conditions. In anaesthetized rats, single unit activity of dopaminergic (DA) neurons does not appear to change when broadly comparing day and night (Luo et al., 2008; Luo & Aston-Jones, 2009; Dominguez-Lopez et al., 2014); however, at a higher resolution (6 time points) the rate of DA neuron firing exhibits a 12-hour rhythm, with peaks in during the early day and early night (Dominguez-Lopez et al., 2014). Yet the rate of DA neuronal activity in freely moving mice during spontaneous REM sleep across the 24hr cycle showed no rhythm (Sidor et al., 2015). Conversely, multiunit activity (MUA) in the VTA of freely moving mice across all vigilance states shows a 24hr rhythm – with peak activity at night – that persists in LD and DD (Fifel et al., 2018); however, when the MUA rhythms for different vigilance states were examined separately, only activity during NREM sleep remained rhythmic (Fifel et al., 2018). Altogether, these issues raise the importance of considering cell-type, vigilance state, and temporal resolution when assessing rhythmic electrical properties within the VTA.
Compared to the VTA, circadian rhythms in the neighboring substantia nigra (SN) have been less well studied. The SN from Per1::luc rats and mice have both been reportedly arrhythmic in culture (Abe et al., 2002; Hiler et al., 2008), but one study in Per2::luc rats reported robust rhythms in SN cultures (Natsubori et al., 2014). Importantly, molecular rhythms were present in SN from animals with intact SCN and those with SCN lesions, suggesting that molecular rhythms in the SN do not depend on external signals from the SCN to drive them. Physiologically, in vivo MUA of freely moving rats showed a daily rhythm – with peak activity at night – that was lost when the SCN outputs were severed (Inouye & Kawamura, 1979; Inouye, 1983). The nightly increase in SN activity is consistent with glucose utilization which is also elevated during the dark phase in the pars compacta and pars reticulata regions in rats (Room & Tielemans, 1989). Interestingly, an earlier study of glucose utilization in mice reported no day/night difference in either SN region (Jay et al., 1985). Similarly, MUA in SN of freely moving mice across all vigilance states does not appear to vary across the 24-hour cycle (Fifel et al., 2018). However, when MUA during NREM sleep was examined separately, lateral SN showed a day/night difference in LD – with peak activity occurring during the night (Fifel et al., 2018). Given that DA release in the dorsal striatum exhibits a clear 24-hour rhythm (Hood et al., 2010) and receives input from DA neurons of the SN, future work should examine if daily rhythms are present in the different nigral cell types (i.e. DA and GABA).
Metencephalon (Hindbrain): Medulla, nucleus of the solitary tract (NTS), rostral ventrolateral medulla (RVLM), inferior olivary nucleus (ION)
The nucleus tractus solitarius (NTS) and rostral ventrolateral medulla (RVLM) along with hypothalamic areas such as the PVN comprise major sites of autonomic and blood pressure control. In general, the RVLM is an integration site for presympathetic pathways and is an important source of vasomotor sympathetic tone and a key player in baroreflex control of blood pressure (Guyenet, 2006). The NTS receives input from a number of visceral centers including cardiopulmonary baroreceptor, chemoreceptor, and gastrointestinal afferents (Andresen & Kunze, 1994; Paton, 1999; Grill & Hayes, 2009). There is a close functional relationship between the NTS and RVLM as baro- and chemoreceptor inputs communicated through the NTS influence RVLM activity and modulate sympathetic tone. Because of the robust blood pressure and sympathetic diurnal rhythms seen in mammals, these areas are of particular interest in the study of circadian control of blood pressure.
Both the NTS and RVLM express clock genes in a cyclic manner. In both the NTS and RVLM of Sprague-Dawley rats, Bmal1 mRNA expression peaks at the transition between dark and light periods, Per2 peaks in the beginning of the dark period, and the clock output gene dbp peaks at the end of the light period. Interestingly, there was no observable rhythm in Clock expression (Herichova et al., 2007). Unlike the PVN, the clock gene expression rhythm in the NTS and RVLM is in phase with that of the SCN. In the NTS of C57BL/6 mice, Bmal1 mRNA expression peaks at the beginning of the light period, Per1 and Per2 peak at the start of the dark period, Rev-erbα peaks in the middle of the light period, and Clock and Cry1 have little rhythmicity (Kaneko et al., 2009).
Unfortunately, there are no studies to our knowledge that have directly investigated diurnal changes in the neuronal activity of these centers. Indirect evidence would suggest that these centers have an important contribution to the diurnal rhythm of blood pressure and autonomic tone. One study overexpressing the angiotensin type II receptor into the RVLM demonstrated a blocking of the nocturnal rise in blood pressure likely due to a continual attenuation of vasomotor sympathetic tone (Gao et al., 2008). This would suggest RVLM driven increases in sympathetic tone contribute to the nocturnal rise in blood pressure in rodents. Future work directly addressing these areas will be invaluable to understanding their contribution towards diurnal autonomic tone and blood pressure control during normal physiological function and during disease states such as hypertension.
The inferior olivary nucleus (ION) comprises the source of climbing fibers into the Purkinje cells of the cerebellum. It has a very high density of synaptic processes and is important in cerebellar learning and motor control (Schweighofer et al., 2013). ION neurons of the hamster express Per1 (Yamamoto et al., 2001), and rat ION express Per1, Per2, and Clock (Shieh, 2003), but the rhythmicity of these clock genes in the ION is currently unexplored.
Metencephalon (hindbrain): Locus Coeruleus
The locus coeruleus (LC) is composed of noradrenergic neurons that receive input from the SCN through the dorsomedial hypothalamus (DMH) and is involved in promoting wakefulness and behavioral arousal (Aston-Jones et al., 2001) and helps to regulate the sleep-wake cycle (Aston-Jones & Bloom, 1981; Gonzalez & Aston-Jones, 2006). Several in vivo studies in rats have shown through EEG and extracellular recordings that the LC has greater tonic firing during the active period compared to the inactive period (Aston-Jones & Bloom, 1981; Aston-Jones et al., 2001; Gonzalez & Aston-Jones, 2006; Gompf & Aston-Jones, 2008). Gonzalez and Aston-Jones (2006) found that amplitude of the sleep-wake rhythm can be reduced by either light deprivation (i.e., DD housing) or via loss of noradrenergic input to the LC, indicating that the functional role of the LC in maintaining sleep-wake amplitude depends on light exposure.
Orexin, a wake regulatory neuropeptide expressed by neurons in the DMH, shows circadian rhythmicity and is increased in expression in the hypothalamus during the rat active period (Gompf & Aston-Jones, 2008). The LC receives inputs from orexin neurons of the DMH, and LC neurons show increased c-fos expression during times when LC impulse activity is high. Extracellular single unit in vivo recordings in rats show that orexin activation of LC neurons causes an increase in firing prior to and during the active period and that blocking orexin receptors prevents the increase in firing during the wake period. These data suggest that orexin signaling may aid in regulation of sleep and waking (Gompf & Aston-Jones, 2008).
Regarding the molecular clock, the expression of Per1 was examined in organotypic slices from mPer1::luc mice for up to five days. Surprisingly, no signal was detected in the LC (Hiler et al., 2008). However, Per1 expression was also examined in Syrian hamsters at ZT 3, 9, 12.5, and 22. In free running hamsters, Per1 expression was found to be rhythmic and higher at ZT 9 and 12.5 than ZT 3. When exposed to constant light, Syrian hamsters adopt a split activity pattern, allowing researchers to examine whether rhythmicity was controlled by the SCN and whether lateralization of signaling occurs. While lateralization of Per1 expression was found in other brain regions, it was not observed in the LC (Mahoney et al., 2013).
Metencephalon (hindbrain): Cerebellum
While the cerebellum is predominantly known for its role in motor coordination, it also plays a role in circadian food anticipatory activity in mice (Mendoza et al., 2010) and exhibits rhythmic expression of the core circadian clock components, including 24-hour rhythmicity in Per1, Per2, Rev-erbα, and dbp mRNA levels in both rats and mice (Rath et al., 2012; Rath et al., 2014). Peak expression of these genes in the cerebellum is delayed by 4-6 hours from SCN clock phase. Arrhythmic animals with SCN lesions, however, no longer exhibit rhythmic clock gene expression, suggesting that the SCN and cerebellum have a hierarchical relationship (Rath et al., 2012). Since there are no direct connections from the SCN to the cerebellum, the mechanism by which circadian rhythmicity in the cerebellum is entrained to the SCN or the LD cycle is unknown; however, several studies have hypothesized some form of hormone signaling (Mendoza et al., 2010; Mordel et al., 2013; Guissoni Campos et al., 2018). Interestingly, clock gene expression is localized to granule cells and Purkinje cells in mice (Rath et al., 2014). In primates, however, PER1/2-ir is expressed in the purkinje cell layer (the primary output of the cerebellum), but not the granule or molecular cell layers, with higher expression during the day (ZT 10) compared to the night (ZT 19) (Guissoni Campos et al., 2018). Bmal1 deletion specifically in granule cells renders expression of the majority of clock genes arrhythmic; however, circadian locomotor behavior is not significantly changed (Bering et al., 2017). Although the cerebellum has >2,000 rhythmic transcripts (detected in animals housed in DD for two cycles; (Pizarro et al., 2013), it is surprising that we know very little about the function of the molecular clock in this important area of the brain.
One possibility is that the molecular clock regulates rhythmic membrane properties of cerebellar neurons. To illustrate this point, we searched the CircaDB database for rhythmic genes in the cerebellum that were also associated with electrical activity (determined by searching the CircaDB database genes with JTK_CYCLE p-value < 0.05 in the cerebellum using a gene list encoding ion channels related to electrical activity using amigo.geneontology.org/amigo (GO:0005244), filtered for Mus musculus; (Pizarro et al., 2013). We found numerous rhythmic gene transcripts (number of transcripts indicated) encoding expression of ion channel subunits such as calcium (3), chloride (3), potassium (13), and sodium (3) as well as receptors for excitatory/inhibitory neurotransmitters (5). Thus, the molecular clock may play a role in physiology in this region. Two studies have empirically tested this possibility (Mordel et al., 2013). First, the investigators performed multi-electrode array recordings from organotypic cerebellar slice cultures lasting 2-5 weeks and found no day-night differences in the firing rate of Purkinje cells (Mordel et al., 2013). However, when the culture medium was replaced roughly every 24 hours, rhythmicity in spiking activity was induced and lasted up to 5 days, suggesting that the Purkinje cells clock phase may have exhibited inter-cellular desynchrony, thus preventing the observation of a whole circuit rhythm. Second, this study measured day-night differences in intrinsic firing properties of Purkinje cells using whole-cell patch clamp recordings. No day-night differences were observed in spike frequencies of neurons firing with either a tonic or a trimodal-like firing pattern. In addition, there were no day-night differences in excitatory or inhibitory synaptic events. As with any study reporting null results, the interpretation can be difficult. Limitations of this study include: possible effects of cerebellar organization (e.g., zebrin II expression banding), use of pups from postnatal days 0-3 prior to cerebellar network development, and potential light contamination during dark phase experiments. The second study (Frederick et al., 2014) examined day-night differences in local field potential (LFP) oscillations in the rat cerebellum and striatum through dopaminergic mechanisms based on evidence that rhythms of dopamine levels (Owasoyo et al., 1979; Hood et al., 2010; Ferris et al., 2014) and expression of D2 receptors are linked with expression of Per2 (Hood et al., 2010; Gravotta et al., 2011). The coherence of oscillations of these regions was explored because of their cooperative role in sensory motor control and locomotor regulation. The investigators observed day-night differences in oscillatory activity, with slow oscillations (0-3 Hz) decreased at the beginning of the active period and 3-8 Hz activity increased during the same period. The influence of the circadian clock on transition of oscillatory activity could aid in establishing optimal neuronal conditions that allow for transitioning between sleep and wake activity. While these efforts are a great start at answering the question of the functional role of the molecular clock in the cerebellum, much more work needs to be done.
Electrical rhythms in invertebrate species
One of the earliest reports of circadian regulation of intrinsic membrane properties in invertebrate species involved experiments in the marine mollusk Bulla gouldiana. Isolated basal retinal neurons of the Bulla have 24-hour variation in membrane potential and potassium conductance, and these rhythms correlate with spike frequency of the intact eye (Michel et al., 1993). Other mollusks such as the crayfish, Procambarus bouvieri, exhibit self-sustained oscillations in electroretinogram (ERG) amplitude across time-of-day. Even when the retinal clocks are uncoupled by splitting the supraesophageal ganglion, ERG rhythms cycle independently but in anti-phase to one another (Barrera-Mera, 1985). The supraesophageal brain is an important circadian center in other sea invertebrates as evidenced by anecdotal observations of in vivo recordings from Octopus vulgarus which exhibits increased spiking during rest and sleep periods (Brown et al., 2006). Even arachnids show spontaneous electrical activity in the suboesophageal ganglion with greater incidence of periodic waves at night compared to the day (Carricaburu & Munoz-Cuevas, 1986). Classic work in the cockroach demonstrated that multi-unit activity rhythms of the cervical connectives and the optic tract are in anti-phase to one another with neural activity peaking in the day in the optic tract versus the night in the cervical connectives (Colwell & Page, 1990). Interestingly, the periodicity of the cockroach optic lobe can drive the period of locomotor rhythms in animals with transplanted lobes acquired from cockroaches reared in altered light cycles (Page, 1982). Taken together, these results suggest that the electrical activity rhythms of the optic lobe are a critical component of the insect circadian clock. More recent work has shown more specifically that the accessory medulla of the optic lobe is critical for nocturnal behavior such that electrical recordings from this region show rapid increases in electrical activity more frequently at night (Schneider & Stengl, 2007). Neurophysiological activity of clock neurons in these invertebrates is inhibited or excited by GABA (see (Giese et al., 2018) in this Special Issue) similar to results reported in vertebrate clocks, where GABA is involved in entrainment and synchronization (Albers et al., 2017). Invertebrate electrical rhythms may be important for learning given that these cockroaches are better at olfactory learning and recall during the night phase compared to during the day (Decker et al., 2007). Like the cockroach, pigment dispersing factor (PDF)-expressing cells in the cricket accessory medulla have higher spike frequencies during the early night compared to the early day (Abdelsalam et al., 2008).
Circadian rhythmicity in Drosophila melanogaster has played a seminal role in chronobiology (see (Helfrich-Forster et al., 2018) in this Special Issue) as exemplified by the recent Nobel Prize in Physiology or Medicine for the discovery of the autonomous molecular clock (Nobelprize.org, 2017). These clock neurons form a network that consists of dorsal neurons and lateral neurons, some of which express PDF, a rhythmically released neuropeptide that is critically important for the fly clock network (Helfrich-Forster et al., 2018). The study of the intact neural circuits in Drosophila using electrophysiology has been notoriously difficult; however, several research groups have been successful. For example, the PDF-expressing, small ventral lateral neurons (s-LNvs) in the fly homologue of the cockroach accessory medulla exhibit changes in electrical membrane properties that feed back to impact the function of the molecular clock. Specifically, when these cells are chronically hyperpolarized, flies display both molecular and behavioral arrhythmicity (Nitabach et al., 2002). To investigate 24-h membrane potential changes of the s-LNvs, one group utilized a recently developed genetically encoded fluorescent voltage indicator called Arclight and found that the s-LNv terminals have greater spontaneous membrane activity during the early morning than the early evening, consistent with the fly’s diurnal circadian rhythm (Cao et al., 2013). In addition to the s-LNvs, other clock neurons in the fly circadian network show 24-h variation in neurophysiology. Electrophysiological recordings of the cell bodies of large LNv clock neurons as well as the posterior dorsal neurons (DN1p) demonstrate responsiveness to light (Sheeba et al., 2008) and neurophysiological rhythms with higher spontaneous firing rate and depolarized membrane potential during the early day compared to the early night (Sheeba et al., 2008; Flourakis et al., 2015; Julienne et al., 2017). At least in the DN1ps, this day-night difference is due to activation of a sodium leak current (encoded by NALCN) (Flourakis et al., 2015). Areas outside the optic lobe also show circadian electrical rhythms. Recordings from the fly antennae revealed robust circadian variation in the electroantennogram response to ethyl acetate in wild-type flies (Krishnan et al., 2005), suggesting that circadian control of electrical activity is important for functional physiological responses during the day and night. In summary, the study of invertebrates has made important contributions to our understanding of chronobiology, and these models will continue to be useful for examining how the circadian clock regulates neuronal activity to drive rhythmic behavior.
Circadian regulation of excitability: Implications for disease
Circadian regulation of neurophysiology is likely to have important implications for diseases of the nervous system, particularly those associated with aging. As animals age, circadian locomotor amplitude declines in parallel with reduced SCN firing rhythmic amplitude (despite normal PER2-ir rhythms) as well as impaired neurophysiological activity rhythms in the SPZ (Nakamura et al., 2011), the most dense projection of the SCN to other brain regions (see above). In addition to this circadian decline associated with the normal aging process, neurodegenerative diseases are also likely to exhibit circadian-related deficits early in the disease progression. As an example of how circadian control of extra-SCN neuronal activity can become dysregulated in disease, we will briefly discuss three neurodegenerative disorders whereby time-of-day variation in neuronal excitability may be significant in prevention or disease progression, recognizing that these examples can be applied to many more disease states. In Parkinson’s disease (PD), dopaminergic neurons of the SN pars compacta provide the main source of dopamine necessary for the initiation of movement (Zhou & Palmiter, 1995; Giros et al., 1996; Gainetdinov et al., 1998). The ability to regeneratively fire action potentials (pacemaking) is a feature of these neurons (Dragicevic et al., 2015) that when dysregulated, may render these neurons selectively vulnerable to toxins, metabolic stress, aging, and neurodegenerative diseases such as PD. In fact, α-synuclein overexpression alters the firing rates of SCN and SN pacemaking neurons (Kudo et al., 2011a; Subramaniam et al., 2014). One theory proposes that triggers of PD narrow the “physiological bandwidth” such that the neurons do not fire quickly enough in some states but have a spike rate that is too high in other states). As a result, this narrow physiological bandwidth reduces flexibility and disrupts calcium homeostasis (Duda et al., 2016). Circadian clock control of membrane properties and pacemaking may allow an adaptable range of firing as the physiological state changes across time-of-day. Interestingly, fly models of PD also exhibit loss of locomotor rhythmicity as well as day-night differences the membrane properties of clock neurons (Julienne et al., 2017). Specifically, two different fly models of PD (expressing mutations in PTEN-induced putative kinase 1/PINK1 or in the E3 ubiquitin ligase parkin) show loss of day-night differences in l-LNv membrane properties of these flies is associated with weakly rhythmic or arrhythmic locomotor activity (Julienne et al., 2017).
Alzheimer’s disease (AD) is also thought to involve dysfunctional neurophysiology early in the disease, prior to neuronal death. Specifically, memory encoding is putatively disrupted by network hypersynchrony in the oscillatory rhythms formed by coordinated activity of excitatory and inhibitory neurons (Palop & Mucke, 2016). Pathologically, Alzheimer’s disease is characterized by aggregation of amyloid-β peptides (Aβ) into oligomers, fibrils, and amyloid plaques, sometimes due to the mutations in amyloid precursor protein or presenilins (as in early-onset, familial AD) (Palop & Mucke, 2016). Levels of Aβ in cerebrospinal fluid and interstitial fluid is molecular clock–dependent, with Aβ levels increasing throughout the active phase in both humans and rodents (Kang et al., 2009; Kress et al., 2018). In animal models of AD as well as people with familial dominant AD, these Aβ rhythms are dampened (Huang et al., 2012; Roh et al., 2012). This rhythm is significant because acute application of Aβ increases firing rate and decreases inhibitory postsynaptic currents in hippocampal pyramidal cells (Kurudenkandy et al., 2014), and Aβ levels are reduced when hyper-excitability is inhibited (Tabuchi et al., 2015). Not only can disrupting the molecular clock accelerate AD pathology (Kress et al., 2018) but this disruption also likely disrupts electrical properties of cortical and hippocampal neurons.
Another neurological disorder known for aggregating proteins and neurodegeneration is Huntington’s disease (HD). Like AD and PD, patients with this disease are known to present with disrupted sleep including advanced sleep phase, insomnia, reduced rapid eye movement (REM) sleep, and REM sleep behavioral disorder (Mattis & Sehgal, 2016). Circadian disruption has been studied in two different mouse models. In the R6/2 HD transgenic mouse model (which expresses the first exon of the HTT gene with 245 CAG repeats), circadian gene expression of Per2 and Bmal1 are disrupted in the SCN (Morton et al., 2005) while SCN firing rate rhythms are unaltered (Pallier et al., 2007). In contrast, the day-night difference in PER2 expression in the SCN of a different HD transgenic mouse model (which expresses the entire human HTT gene with 97 CAA-CAG repeats) is unaltered while peak SCN firing rate during the day is reduced resulting in loss of day-night electrical rhythms (Kudo et al., 2011b). These results suggest that the molecular clock and/or neurophysiological activity of neurons are impaired in this disease. The dorsal striatum is one of the most important motor centers impacted in HD, and future studies should investigate the molecular clock and electrophysiological changes during both day and night in animal models of HD, given the evidence for both rhythmic clock gene expression (Cai et al., 2009; Sahar et al., 2010) and firing rhythms (Inouye & Kawamura, 1979; Yamazaki et al., 1998) as described above. Taken together, all of this work in neurodegenerative diseases suggest that molecular clock dysfunction can disrupt excitability of neurons throughout the brain.
Conclusions: Areas of future investigation
Although rhythmic electrical activity throughout the brain has been reported over the last few decades, we know surprisingly little about how the cellular molecular clock controls membrane properties. Much of the research in this area has been devoted to understanding membrane physiology of SCN neurons, with less focus on extra-SCN brain regions. In the past decade, we have learned more about the role of the molecular clock in controlling neurophysiology and brain-region specific functions, although there are still many questions which need to be addressed. Regarding the molecular clock, are clock genes expressed selectively in certain neuronal populations (e.g., excitatory versus inhibitory neurons) or with population-specific phase differences? The phase relationship between positive and negative molecular clock components may also be important. In the SCN, the length of time for Per1 rhythms to entrain to a 6-h light pulse is much shorter than for Bmal1 rhythms (Ono et al., 2017). Dynamic phase relationships of molecular clock components outside the SCN have yet to be explored but may be important for subsequent physiological changes. For example, acquisition of object location memory induces Per1 expression in hippocampus, and Per1 induction is required for long-term memory formation (Kwapis et al., 2018). Given the complex circuitry in each brain region, the phase and coupling strength of the molecular clock in specific cell types is also likely to affect electrical activity and thus, circuit function. Future studies should also determine whether the SCN is necessary for 24-hour rhythmicity in extra-SCN membrane physiology? When sampling clock gene expression or measuring electrical activity in vivo, it is imperative to consider behavior – feeding/fasting cycles, sleep/wake states, etc. Is the measured change in clock phase or electrical activity a result of the change in behavior or does locomotor behavior induce transcription or alter the neuronal firing rate? How do differences in experimental protocols alter the results across multiple studies? Results can greatly vary depending on whether measurements were made in vivo versus a slice or primary cultures, in the presence or absence of certain pharmacological blockers, or in different species (including diurnal versus nocturnal). However, despite the multitude of factors which could mask physiological rhythms, a remarkable number of extra-SCN oscillators discussed here appear to exhibit daily changes in excitability or membrane properties. As circadian regulation of neuronal activity in different brain regions becomes more apparent, determining how the timing of rhythms in different areas relate to each other becomes more important. Thus, future research must determine the hierarchical nature of the circadian system when it comes to controlling neuronal excitability.
Acknowledgements
This work was supported by NIH grants R01NS082413, R01NS108713, T32HD071866, T32HL105349, T32HL007457, and T32NS061788.
Abbreviations
- 5-HT
serotonin
- AD
Alzheimer’s disease
- AMY
amygdala
- ARC
arcuate nucleus
- AVP
arginine vasopressin
- Aβ
amyloid beta
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- CA1-3
Conus Ammonis regions 1-3
- CeA
central amygdala
- CP
caudate putamen
- CX
cortex
- D2R
D2 receptor
- DA
dopamine
- DD
constant dark
- DMH
dorsomedial hypothalamus
- DN1p
posterior dorsal neurons
- DRN
dorsal raphe nucleus
- EEG
electroencephalogram
- EPSP
excitatory postsynaptic potential
- ERG
electroretinogram
- HD
Huntington’s disease
- HPF
hippocampal formation
- IGL
intergeniculate leaflet
- ION
inferior olivary nucleus
- -ir
immunoreactivity
- KO
knockout
- LC
locus coeruleus
- LD
light-dark cycle
- LFP
local field potential
- LGN
lateral geniculate nucleus
- LHb
lateral habenula
- LHy
lateral hypothalamus
- LS
lateral septum
- LTD
long term depression
- LTP
long term potentiation
- luc
luciferase
- ME
median eminence
- MEV
midbrain trigeminal nucleus
- MHb
medial habenula
- mPFC
medial prefrontal cortex
- MRN
median raphe nucleus
- MSN
medium spiny neuron
- MUA
multi-unit activity
- NAc
nucleus accumbens
- NTS
nucleus of the solitary tract
- OB
olfactory bulb
- OVLT
organum vasculosum of the lamina terminalis
- Oxt
oxytocin
- PAG
periaqueductal gray
- PD
Parkinson’s Disease
- Per1-3
period 1-3
- POA
medial preoptic area
- PFC
prefrontal cortex
- PVN
paraventricular nucleus of the hypothalamus
- PVT
paraventricular nucleus of the thalamus
- RCh
retrochiasmatic area
- RHT
retinohypothalamic tract
- Rinput
input resistance
- RVLM
rostral ventrolateral medulla
- SCN
suprachiasmatic nucleus
- s-LNv
small ventral lateral neuron
- SN
substantia nigra
- SON
supraoptic nucleus
- SPZ
subparaventricular zone
- TMN
tuberomammillary nucleus
- TTFL
transcriptional-translational feedback loop
- VMH
ventromedial hypothalamus
- VTA
ventral tegmental area
- WT
wildtype
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Image credit: modified from Allen Institute
References
- Abdelsalam S, Uemura H, Umezaki Y, Saifullah AS, Shimohigashi M & Tomioka K (2008) Characterization of PDF-immunoreactive neurons in the optic lobe and cerebral lobe of the cricket, Gryllus bimaculatus. J Insect Physiol, 54, 1205–1212. [DOI] [PubMed] [Google Scholar]
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M & Block GD (2002) Circadian rhythms in isolated brain regions. J Neurosci, 22, 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham U, Prior JL, Granados-Fuentes D, Piwnica-Worms DR & Herzog ED (2005) Independent circadian oscillations of Period1 in specific brain areas in vivo and in vitro. J Neurosci, 25, 8620–8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis A & de Lecea L (2009) The hypocretins as sensors for metabolism and arousal. J Physiol, 587, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Walton JC, Gamble KL, McNeill J.K.t. & Hummer DL (2017) The dynamics of GABA signaling: Revelations from the circadian pacemaker in the suprachiasmatic nucleus. Front Neuroendocrinol, 44, 35–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus H, Bonnefont X, Chaves I, Yasui A, Doczy J, van der Horst GT & Meijer JH (2002) Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol, 12, 1130–1133. [DOI] [PubMed] [Google Scholar]
- Alghamdi BS (2018) The neuroprotective role of melatonin in neurological disorders. J Neurosci Res, 96, 1136–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CN, Nitabach MN & Colwell CS (2017) Membrane Currents, Gene Expression, and Circadian Clocks. Cold Spring Harb Perspect Biol, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Cain S, Sullivan J, Robinson B & Stewart J (1999) In rats, odor-induced Fos in the olfactory pathways depends on the phase of the circadian clock. Neurosci Lett, 272, 175–178. [DOI] [PubMed] [Google Scholar]
- Andrade-Silva J, Cipolla-Neto J & Peliciari-Garcia RA (2014) The in vitro maintenance of clock genes expression within the rat pineal gland under standard and norepinephrine-synchronized stimulation. Neurosci Res, 81–82, 1–10. [DOI] [PubMed] [Google Scholar]
- Andresen MC & Kunze DL (1994) Nucleus tractus solitarius--gateway to neural circulatory control. Annu Rev Physiol, 56, 93–116. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J & Escobar C (2007) Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience, 144, 344–355. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G & Bloom FE (1981) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci, 1, 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y & Oshinsky ML (2001) A neural circuit for circadian regulation of arousal. Nat Neurosci, 4, 732–738. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Beremand PD, Hammer R, Bell-Pedersen D, Thomas TL & Cassone VM (2003) Transcriptional profiling of the chick pineal gland, a photoreceptive circadian oscillator and pacemaker. Mol Endocrinol, 17, 2084–2095. [DOI] [PubMed] [Google Scholar]
- Baker PM & Mizumori SJY (2017) Control of behavioral flexibility by the lateral habenula. Pharmacol Biochem Behav, 162, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano-Otalora B & Piggins HD (2017) Contributions of the lateral habenula to circadian timekeeping. Pharmacol Biochem Behav, 162, 46–54. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL, Goddard GV, Douglas RM & Adamec R (1977) Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science, 197, 91–92. [DOI] [PubMed] [Google Scholar]
- Barrera-Mera B (1985) Visual circadian rhythmicity in splitbrain crayfish: a plastic behavioral expression of symmetric circadian pacemakers. Brain Res Bull, 15, 203–208. [DOI] [PubMed] [Google Scholar]
- Bering T, Carstensen MB & Rath MF (2017) Deleting the Arntl clock gene in the granular layer of the mouse cerebellum: impact on the molecular circadian clockwork. J Neurochem. [DOI] [PubMed] [Google Scholar]
- Bernardis LL & Bellinger LL (1998) The dorsomedial hypothalamic nucleus revisited: 1998 update. Proc Soc Exp Biol Med, 218, 284–306. [DOI] [PubMed] [Google Scholar]
- Besing RC, Rogers CO, Paul JR, Hablitz LM, Johnson RL, McMahon LL & Gamble KL (2017) GSK3 activity regulates rhythms in hippocampal clock gene expression and synaptic plasticity. Hippocampus, 27, 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumbra GS, Lombardelli S, Gonzalez JA, Parsy KS & Dyball RE (2009) Daily rhythms of spike coding in the rat supraoptic nucleus. J Neuroendocrinol, 21, 935–945. [DOI] [PubMed] [Google Scholar]
- Blancas-Velazquez A, la Fleur SE & Mendoza J (2017) Effects of a free-choice high-fat high-sugar diet on brain PER2 and BMAL1 protein expression in mice. Appetite, 117, 263–269. [DOI] [PubMed] [Google Scholar]
- Bourque CW (2008) Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci, 9, 519–531. [DOI] [PubMed] [Google Scholar]
- Bowden JB, Abraham WC & Harris KM (2012) Differential effects of strain, circadian cycle, and stimulation pattern on LTP and concurrent LTD in the dentate gyrus of freely moving rats. Hippocampus, 22, 1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ER, Piscopo S, De Stefano R & Giuditta A (2006) Brain and behavioural evidence for rest-activity cycles in Octopus vulgaris. Behav Brain Res, 172, 355–359. [DOI] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S & Lucas RJ (2010) Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol, 8, e1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM & Piggins HD (2007) Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol, 82, 229–255. [DOI] [PubMed] [Google Scholar]
- Brown TM, Wynne J, Piggins HD & Lucas RJ (2011) Multiple hypothalamic cell populations encoding distinct visual information. J Physiol, 589, 1173–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke TM, Scheer FA, Ronda JM, Czeisler CA & Wright KP Jr. (2015) Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res, 24, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagampang FR & Inouye ST (1994) Diurnal and circadian changes of serotonin in the suprachiasmatic nuclei: regulation by light and an endogenous pacemaker. Brain Res, 639, 175–179. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Yamazaki S, Otori Y & Inouye SI (1993) Serotonin in the raphe nuclei: regulation by light and an endogenous pacemaker. Neuroreport, 5, 49–52. [DOI] [PubMed] [Google Scholar]
- Cai Y, Liu S, Li N, Xu S, Zhang Y & Chan P (2009) Postnatal ontogenesis of molecular clock in mouse striatum. Brain Res, 1264, 33–38. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Wyatt JK, Czeisler CA & Dijk DJ (2002) Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience, 114, 1047–1060. [DOI] [PubMed] [Google Scholar]
- Cao G, Platisa J, Pieribone VA, Raccuglia D, Kunst M & Nitabach MN (2013) Genetically targeted optical electrophysiology in intact neural circuits. Cell, 154, 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carricaburu P & Munoz-Cuevas A (1986) Spontaneous electrical activity of the suboesophageal ganglion and circadian rhythms in scorpions. Exp Biol, 45, 301–310. [PubMed] [Google Scholar]
- Cassone VM (1998) Melatonin’s role in vertebrate circadian rhythms. Chronobiol Int, 15, 457–473. [DOI] [PubMed] [Google Scholar]
- Cassone VM & Westneat DF (2012) The bird of time: cognition and the avian biological clock. Front Mol Neurosci, 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Ruiz A, Gall AJ, Smale L & Nunez AA (2013) Day-night differences in neural activation in histaminergic and serotonergic areas with putative projections to the cerebrospinal fluid in a diurnal brain. Neuroscience, 250, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller LJ, Boulos Z & Goddard GV (1985) Circadian rhythms in hippocampal responsiveness to perforant path stimulation and their relation to behavioral state. Brain Res, 329, 117–130. [DOI] [PubMed] [Google Scholar]
- Chaudhury D & Colwell CS (2002) Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res, 133, 95–108. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM & Colwell CS (2005) Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms, 20, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Gaggioni G, Ly JQ, Papachilleos S, Borsu C, Brzozowski A, Rosanova M, Sarasso S, Luxen A, Middleton B, Archer SN, Dijk DJ, Massimini M, Maquet P, Phillips C, Moran RJ & Vandewalle G (2016) Circadian dynamics in measures of cortical excitation and inhibition balance. Sci Rep, 6, 33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E & McClung CA (2016) Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A, 113, 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen SL, Bouzinova EV, Fahrenkrug J & Wiborg O (2016) Altered Expression Pattern of Clock Genes in a Rat Model of Depression. Int J Neuropsychopharmacol, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LE, Woodruff ER, Morton S, Hinds LR & Spencer RL (2015) Variations in Phase and Amplitude of Rhythmic Clock Gene Expression across Prefrontal Cortex, Hippocampus, Amygdala, and Hypothalamic Paraventricular and Suprachiasmatic Nuclei of Male and Female Rats. J Biol Rhythms, 30, 417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, Kim KS, Dluzen DE, Lee I, Hwang O, Son GH & Kim K (2014) Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell, 157, 858–868. [DOI] [PubMed] [Google Scholar]
- Colwell CS (2011) Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci, 12, 553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS & Page TL (1990) A circadian rhythm in neural activity can be recorded from the central nervous system of the cockroach. J Comp Physiol A, 166, 643–649. [DOI] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL & Low MJ (2001) The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord, 25 Suppl 5, S63–67. [DOI] [PubMed] [Google Scholar]
- Corthell JT, Olcese J & Trombley PQ (2014) Melatonin in the mammalian olfactory bulb. Neuroscience, 261, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD & Horvath TL (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron, 37, 649–661. [DOI] [PubMed] [Google Scholar]
- Crespi F & Jouvet M (1983) Differential pulse voltammetry: parallel peak 3 changes with vigilance states in raphe dorsalis and raphe magnus of chronic freely moving rats and evidence for a 5-HT contribution to these peaks after monoamine oxidase inhibitors. Brain Res, 272, 263–268. [DOI] [PubMed] [Google Scholar]
- Cui LN, Coderre E & Renaud LP (2001) Glutamate and GABA mediate suprachiasmatic nucleus inputs to spinal-projecting paraventricular neurons. Am J Physiol Regul Integr Comp Physiol, 281, R1283–1289. [DOI] [PubMed] [Google Scholar]
- Cui LN, Saeb-Parsy K & Dyball RE (1997) Neurones in the supraoptic nucleus of the rat are regulated by a projection from the suprachiasmatic nucleus. J Physiol, 502 (Pt 1), 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Swaab DF & Buijs RM (1997) Distribution of vasopressin and vasoactive intestinal polypeptide (VIP) fibers in the human hypothalamus with special emphasis on suprachiasmatic nucleus efferent projections. J Comp Neurol, 383, 397–414. [DOI] [PubMed] [Google Scholar]
- Decker S, McConnaughey S & Page TL (2007) Circadian regulation of insect olfactory learning. Proc Natl Acad Sci U S A, 104, 15905–15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV & Zaretsky DV (2002) The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav, 71, 469–480. [DOI] [PubMed] [Google Scholar]
- Dominguez-Lopez S, Howell RD, Lopez-Canul MG, Leyton M & Gobbi G (2014) Electrophysiological characterization of dopamine neuronal activity in the ventral tegmental area across the light-dark cycle. Synapse, 68, 454–467. [DOI] [PubMed] [Google Scholar]
- Dragicevic E, Schiemann J & Liss B (2015) Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience, 284, 798–814. [DOI] [PubMed] [Google Scholar]
- Duda J, Potschke C & Liss B (2016) Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson’s disease. J Neurochem, 139 Suppl 1, 156–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE & Haas HL (2001) Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci, 21, 9273–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Meijer JH & Michel S (2015) Age-related changes in large-conductance calcium-activated potassium channels in mammalian circadian clock neurons. Neurobiol Aging, 36, 2176–2183. [DOI] [PubMed] [Google Scholar]
- Feillet CA, Mendoza J, Albrecht U, Pevet P & Challet E (2008) Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci, 37, 209–221. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Flik G, Dijkman U, Folgering JH, Perry KW, Johnson BJ, Westerink BH & Svensson KA (2015) Glutamatergic regulation of brain histamine neurons: In vivo microdialysis and electrophysiology studies in the rat. Neuropharmacology, 99, 1–8. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ & Samson WK (2008) The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets, 12, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Espana RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R & Jones SR (2014) Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci U S A, 111, E2751–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifel K, Meijer JH & Deboer T (2018) Circadian and Homeostatic Modulation of Multi-Unit Activity in Midbrain Dopaminergic Structures. Sci Rep, 8, 7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PD, Steiner RA & Clifton DK (1998) Temporal patterns of gonadotropin-releasing hormone (GnRH), c-fos, and galanin gene expression in GnRH neurons relative to the luteinizing hormone surge in the rat. J Neurosci, 18, 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flourakis M, Kula-Eversole E, Hutchison AL, Han TH, Aranda K, Moose DL, White KP, Dinner AR, Lear BC, Ren D, Diekman CO, Raman IM & Allada R (2015) A Conserved Bicycle Model for Circadian Clock Control of Membrane Excitability. Cell, 162, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick A, Bourget-Murray J, Chapman CA, Amir S & Courtemanche R (2014) Diurnal influences on electrophysiological oscillations and coupling in the dorsal striatum and cerebellar cortex of the anesthetized rat. Front Syst Neurosci, 8, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D & Amir S (2000) Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res, 866, 262–267. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM & Caron MG (1998) Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev, 26, 148–153. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Wang W, Li H, Sumners C & Zucker IH (2008) Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension, 51, 521–527. [DOI] [PubMed] [Google Scholar]
- Giese M, Wei H & Stengl M (2018) Circadian pacemaker neurons of the Madeira cockroach are inhibited and activated by GABAA and GABAB receptors. Eur J Neurosci. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM & Caron MG (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature, 379, 606–612. [DOI] [PubMed] [Google Scholar]
- Girotti M, Weinberg MS & Spencer RL (2009) Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab, 296, E888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizowski C & Bourque CW (2018) The neural basis of homeostatic and anticipatory thirst. Nat Rev Nephrol, 14, 11–25. [DOI] [PubMed] [Google Scholar]
- Gizowski C, Zaelzer C & Bourque CW (2016) Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature, 537, 685–688. [DOI] [PubMed] [Google Scholar]
- Gompf HS & Aston-Jones G (2008) Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity. Brain Res, 1224, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MM & Aston-Jones G (2006) Circadian regulation of arousal: role of the noradrenergic locus coeruleus system and light exposure. Sleep, 29, 1327–1336. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Ben-Josef G, Perry G, Wilson DA, Sullivan-Wilson A & Herzog ED (2011) Daily rhythms in olfactory discrimination depend on clock genes but not the suprachiasmatic nucleus. J Biol Rhythms, 26, 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Saxena MT, Prolo LM, Aton SJ & Herzog ED (2004) Olfactory bulb neurons express functional, entrainable circadian rhythms. Eur J Neurosci, 19, 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A & Herzog ED (2006) A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci, 26, 12219–12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta L, Gavrila AM, Hood S & Amir S (2011) Global depletion of dopamine using intracerebroventricular 6-hydroxydopamine injection disrupts normal circadian wheel-running patterns and PERIOD2 expression in the rat forebrain. J Mol Neurosci, 45, 162–171. [DOI] [PubMed] [Google Scholar]
- Green DJ & Gillette R (1982) Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res, 245, 198–200. [DOI] [PubMed] [Google Scholar]
- Green NH, Jackson CR, Iwamoto H, Tackenberg MC & McMahon DG (2015) Photoperiod programs dorsal raphe serotonergic neurons and affective behaviors. Curr Biol, 25, 1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ & Hayes MR (2009) The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond), 33 Suppl 1, S11–15. [DOI] [PubMed] [Google Scholar]
- Groos G & Hendriks J (1982) Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett, 34, 283–288. [DOI] [PubMed] [Google Scholar]
- Guilding C, Hughes AT, Brown TM, Namvar S & Piggins HD (2009) A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Molecular brain, 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilding C, Hughes AT & Piggins HD (2010) Circadian oscillators in the epithalamus. Neuroscience, 169, 1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guissoni Campos LM, Hataka A, Vieira IZ, Buchaim RL, Robalinho IF, Arantes G, Viegas JS, Bosso H, Bravos RM & Pinato L (2018) Circadian Clock Proteins and Melatonin Receptors in Neurons and Glia of the Sapajus apella Cerebellum. Front Physiol, 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG (2006) The sympathetic control of blood pressure. Nat Rev Neurosci, 7, 335–346. [DOI] [PubMed] [Google Scholar]
- Hamada T, Honma S & Honma K (2011) Light responsiveness of clock genes, Per1 and Per2, in the olfactory bulb of mice. Biochem Biophys Res Commun, 409, 727–731. [DOI] [PubMed] [Google Scholar]
- Harbour VL, Weigl Y, Robinson B & Amir S (2013) Comprehensive mapping of regional expression of the clock protein PERIOD2 in rat forebrain across the 24-h day. PLoS One, 8, e76391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour VL, Weigl Y, Robinson B & Amir S (2014) Phase differences in expression of circadian clock genes in the central nucleus of the amygdala, dentate gyrus, and suprachiasmatic nucleus in the rat. PLoS One, 9, e103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington ME (1997) The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev, 21, 705–727. [DOI] [PubMed] [Google Scholar]
- Harris KM & Teyler TJ (1983) Age differences in a circadian influence on hippocampal LTP. Brain Res, 261, 69–73. [DOI] [PubMed] [Google Scholar]
- Harrison NL & Zatz M (1989) Voltage-dependent calcium channels regulate melatonin output from cultured chick pineal cells. J Neurosci, 9, 2462–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A & Anderson DJ (2010) Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature, 468, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Forster C, Bertolini E & Menegazzi P (2018) Flies as models for circadian clock adaptation to environmental challenges. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herichova I, Hasakova K, Lukacova D, Mravec B, Horvathova L & Kavicka D (2017) Prefrontal cortex and dorsomedial hypothalamus mediate food reward-induced effects via npas2 and egr1 expression in rat. Physiol Res, 66, S501–S510. [DOI] [PubMed] [Google Scholar]
- Herichova I, Mravec B, Stebelova K, Krizanova O, Jurkovicova D, Kvetnansky R & Zeman M (2007) Rhythmic clock gene expression in heart, kidney and some brain nuclei involved in blood pressure control in hypertensive TGR(mREN-2)27 rats. Mol Cell Biochem, 296, 25–34. [DOI] [PubMed] [Google Scholar]
- Herkenham M & Nauta WJ (1979) Efferent connections of the habenular nuclei in the rat. J Comp Neurol, 187, 19–47. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS & Block GD (1998) Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci, 1, 708–713. [DOI] [PubMed] [Google Scholar]
- Hickok JR & Tischkau SA (2010) In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology, 91, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler DJ, Bhattacherjee A, Yamazaki S, Tei H & Geusz ME (2008) Circadian mPer1 gene expression in mesencephalic trigeminal nucleus cultures. Brain Res, 1214, 84–93. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Nishihara M & Takahashi M (1996) Neural activity in the VMH associated with suppression of the circulatory system in rats. Physiol Behav, 59, 1017–1023. [DOI] [PubMed] [Google Scholar]
- Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, Stewart J & Amir S (2010) Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci, 30, 14046–14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Potter R, Sigurdson W, Kasten T, Connors R, Morris JC, Benzinger T, Mintun M, Ashwood T, Ferm M, Budd SL & Bateman RJ (2012) beta-amyloid dynamics in human plasma. Arch Neurol, 69, 1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y & Hayaishi O (2001) Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A, 98, 9965–9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Mäki H, Rosanova M, Casarotto S, Canali P, Casali AG, Tononi G & Massimini M (2013) Human cortical excitability increases with time awake. Cereb. Cortex, 23, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur SP, Takeuchi Y, Itoh H, Uchimura M, Takahashi K, Kang HC, Lee YD, Kim SJ & Takemura A (2012) Fish sleeping under sandy bottom: interplay of melatonin and clock genes. Gen Comp Endocrinol, 177, 37–45. [DOI] [PubMed] [Google Scholar]
- Ikeno T & Yan L (2016) Chronic Light Exposure in the Middle of the Night Disturbs the Circadian System and Emotional Regulation. J Biol Rhythms, 31, 352–364. [DOI] [PubMed] [Google Scholar]
- Imai T (2014) Construction of functional neuronal circuitry in the olfactory bulb. Semin Cell Dev Biol, 35, 180–188. [DOI] [PubMed] [Google Scholar]
- Inouye ST (1983) Does the ventromedial hypothalamic nucleus contain a self-sustained circadian oscillator associated with periodic feedings? Brain Res, 279, 53–63. [DOI] [PubMed] [Google Scholar]
- Inouye ST & Kawamura H (1979) Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A, 76, 5962–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Michel S, Vansteensel MJ, Meijer JH & Colwell CS (2005) Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci, 8, 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S & Colwell CS (2010) Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol, 103, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Jouvet M & des Rosiers MH (1985) Local cerebral glucose utilization in the free moving mouse: a comparison during two stages of the activity-rest cycle. Brain Res, 342, 297–306. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL & Stuber GD (2013) Distinct extended amygdala circuits for divergent motivational states. Nature, 496, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Franklin KM, Duncan MJ, O’Hara BF & Wisor JP (2012) Distinct phase relationships between suprachiasmatic molecular rhythms, cerebral cortex molecular rhythms, and behavioral rhythms in early runner (CAST/EiJ) and nocturnal (C57BL/6J) mice. Sleep, 35, 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilg A, Lesny S, Peruzki N, Schwegler H, Selbach O, Dehghani F & Stehle JH (2010) Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus, 20, 377–388. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT & LeDoux JE (2010) Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci U S A, 107, 12692–12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julienne H, Buhl E, Leslie DS & Hodge JJL (2017) Drosophila PINK1 and parkin loss-of-function mutants display a range of non-motor Parkinson’s disease phenotypes. Neurobiol Dis, 104, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Foppen E, Schalij I, Van Heijningen C, van der Vliet J, Fliers E & Buijs RM (2008) Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS One, 3, e3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pevet P & Buijs RM (2000) Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci, 12, 3146–3154. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Scheer FA, Perreau-Lenz S, La Fleur SE, Yi CX, Fliers E & Buijs RM (2011) Circadian disruption and SCN control of energy metabolism. FEBS Lett, 585, 1412–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Yamada T, Tsukita S, Takahashi K, Ishigaki Y, Oka Y & Katagiri H (2009) Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res, 1263, 58–68. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S & Holtzman DM (2009) Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science, 326, 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EM, Estabrooke IV, McCarthy M & Scammell TE (2003) Wake-related activity of tuberomammillary neurons in rats. Brain Res, 992, 220–226. [DOI] [PubMed] [Google Scholar]
- Ko GY (2018) Circadian regulation in the retina: From molecules to network. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K & Nishino H (1976) Circadian and other rhythmic activity of neurones in the ventromedial nuclei and lateral hypothalamic area. J Physiol, 263, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Zhang L, Ronnekleiv OK & Renaud LP (2012) Midline thalamic paraventricular nucleus neurons display diurnal variation in resting membrane potentials, conductances, and firing patterns in vitro. J Neurophysiol, 107, 1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratova AA, Dubrovsky YV, Antoch MP & Kondratov RV (2010) Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany NY), 2, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM & Musiek ES (2018) Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Korets R & Silver R (2003) Expression of the circadian clock gene Period 1 in neuroendocrine cells: an investigation using mice with a Per1::GFP transgene. Eur J Neurosci, 17, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan P, Dryer SE & Hardin PE (2005) Measuring circadian rhythms in olfaction using electroantennograms. Methods Enzymol, 393, 495–508. [DOI] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Truong D, Wu Y & Colwell CS (2011a) Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol, 232, 66–75. [DOI] [PubMed] [Google Scholar]
- Kudo T, Schroeder A, Loh DH, Kuljis D, Jordan MC, Roos KP & Colwell CS (2011b) Dysfunctions in circadian behavior and physiology in mouse models of Huntington’s disease. Exp Neurol, 228, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ & McMahon DG (2004) Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci, 20, 1113–1117. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ & McMahon DG (2006) Encoding the ins and outs of circadian pacemaking. J Biol Rhythms, 21, 470–481. [DOI] [PubMed] [Google Scholar]
- Kurudenkandy FR, Zilberter M, Biverstal H, Presto J, Honcharenko D, Stromberg R, Johansson J, Winblad B & Fisahn A (2014) Amyloid-beta-induced action potential desynchronization and degradation of hippocampal gamma oscillations is prevented by interference with peptide conformation change and aggregation. J Neurosci, 34, 11416–11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Alaghband Y, Kramar EA, Lopez AJ, Vogel Ciernia A, White AO, Shu G, Rhee D, Michael CM, Montellier E, Liu Y, Magnan CN, Chen S, Sassone-Corsi P, Baldi P, Matheos DP & Wood MA (2018) Epigenetic regulation of the circadian gene Per1 contributes to age-related changes in hippocampal memory. Nat Commun, 9, 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont EW, Robinson B, Stewart J & Amir S (2005) The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A, 102, 4180–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Long JE & Welsh DK (2016) Depression-like behaviour in mice is associated with disrupted circadian rhythms in nucleus accumbens and periaqueductal grey. Eur J Neurosci, 43, 1309–1320. [DOI] [PubMed] [Google Scholar]
- Lang N, Rothkegel H, Reiber H, Hasan A, Sueske E, Tergau F, Ehrenreich H, Wuttke W & Paulus W (2011) Circadian modulation of GABA-mediated cortical inhibition. Cerebral cortex, 21, 2299–2306. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH & Reppert SM (1997) Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell, 91, 855–860. [DOI] [PubMed] [Google Scholar]
- Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X & McClung CA (2015) Chronic Stress Induces Brain Region-Specific Alterations of Molecular Rhythms that Correlate with Depression-like Behavior in Mice. Biol Psychiatry, 78, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M & Takahashi JS (2000) Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science, 288, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH & Aston-Jones G (2009) Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. Eur J Neurosci, 29, 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Georges FE & Aston-Jones GS (2008) Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci, 27, 408–422. [DOI] [PubMed] [Google Scholar]
- Ly JQ, Gaggioni G, Chellappa SL, Papachilleos S, Brzozowski A, Borsu C, Rosanova M, Sarasso S, Middleton B, Luxen A, Archer SN, Phillips C, Dijk DJ, Maquet P, Massimini M & Vandewalle G (2016a) Circadian regulation of human cortical excitability. Nature communications, 7, 11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly JQ, Gaggioni G, Chellappa SL, Papachilleos S, Brzozowski A, Borsu C, Rosanova M, Sarasso S, Middleton B, Luxen A, Archer SN, Phillips C, Dijk DJ, Maquet P, Massimini M & Vandewalle G (2016b) Circadian regulation of human cortical excitability. Nat Commun, 7, 11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CE, Brewer JM & Bittman EL (2013) Central control of circadian phase in arousal-promoting neurons. PLoS One, 8, e67173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fairey CA, Ramanathan C, Stowie A, Walaszczyk E, Smale L & Nunez AA (2015) Plastic oscillators and fixed rhythms: changes in the phase of clock-gene rhythms in the PVN are not reflected in the phase of the melatonin rhythm of grass rats. Neuroscience, 288, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J & Sehgal A (2016) Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol Metab, 27, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Fan Y, Lv X, Welsh DK, Zhan C & Zhang EE (2018) Long-term in vivo recording of circadian rhythms in brains of freely moving mice. Proc Natl Acad Sci U S A, 115, 4276–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH & Michel S (2015) Neurophysiological analysis of the suprachiasmatic nucleus: a challenge at multiple levels. Methods Enzymol, 552, 75–102. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M & Escobar C (2005) A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci, 22, 2855–2862. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Pevet P, Felder-Schmittbuhl MP, Bailly Y & Challet E (2010) The cerebellum harbors a circadian oscillator involved in food anticipation. J Neurosci, 30, 1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF & Aldrich RW (2006) BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci, 9, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza E, Aguirre J, Waliszewski S & Caba M (2015) Suckling induces a daily rhythm in the preoptic area and lateral septum but not in the bed nucleus of the stria terminalis in lactating rabbit does. Eur J Neurosci, 41, 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Geusz ME, Zaritsky JJ & Block GD (1993) Circadian rhythm in membrane conductance expressed in isolated neurons. Science, 259, 239–241. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K & Yanagisawa M (2006) The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A, 103, 12150–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Granados-Fuentes D, Wang T, Marpegan L, Holy TE & Herzog ED (2014) Vasoactive intestinal polypeptide mediates circadian rhythms in mammalian olfactory bulb and olfaction. J Neurosci, 34, 6040–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JR & Meredith AL (2012) Genetic activation of BK currents in vivo generates bidirectional effects on neuronal excitability. Proc Natl Acad Sci U S A, 109, 18997–19002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordel J, Karnas D, Pevet P, Isope P, Challet E & Meissl H (2013) The output signal of Purkinje cells of the cerebellum and circadian rhythmicity. PLoS One, 8, e58457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP (2013) Neuroanatomy of the extended circadian rhythm system. Exp Neurol, 243, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Shivers KY, Blanchard JH & Muscat L (2006) Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience, 137, 1285–1297. [DOI] [PubMed] [Google Scholar]
- Moriya S, Tahara Y, Sasaki H, Ishigooka J & Shibata S (2015) Phase-delay in the light-dark cycle impairs clock gene expression and levels of serotonin, norepinephrine, and their metabolites in the mouse hippocampus and amygdala. Sleep Med, 16, 1352–1359. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA & Maywood ES (2005) Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci, 25, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn RG & Bilkey DK (2012) The firing rate of hippocampal CA1 place cells is modulated with a circadian period. Hippocampus, 22, 1325–1337. [DOI] [PubMed] [Google Scholar]
- Munn RG, Tyree SM, McNaughton N & Bilkey DK (2015) The frequency of hippocampal theta rhythm is modulated on a circadian period and is entrained by food availability. Front Behav Neurosci, 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto V, Jaspar M, Meyer C, Kusse C, Chellappa SL, Degueldre C, Balteau E, Shaffii-Le Bourdiec A, Luxen A, Middleton B, Archer SN, Phillips C, Collette F, Vandewalle G, Dijk DJ & Maquet P (2016) Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science, 353, 687–690. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS & Block GD (2011) Age-related decline in circadian output. J Neurosci, 31, 10201–10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Yamazaki S, Nakamura TJ, Shirakawa T, Block GD & Takumi T (2008) In vivo monitoring of circadian timing in freely moving mice. Curr Biol, 18, 381–385. [DOI] [PubMed] [Google Scholar]
- Nakata M, Gantulga D, Santoso P, Zhang B, Masuda C, Mori M, Okada T & Yada T (2016) Paraventricular NUCB2/Nesfatin-1 Supports Oxytocin and Vasopressin Neurons to Control Feeding Behavior and Fluid Balance in Male Mice. Endocrinology, 157, 2322–2332. [DOI] [PubMed] [Google Scholar]
- Nakatsuka H & Natsume K (2014) Circadian rhythm modulates long-term potentiation induced at CA1 in rat hippocampal slices. Neurosci Res, 80, 1–9. [DOI] [PubMed] [Google Scholar]
- Namihira M, Honma S, Abe H, Tanahashi Y, Ikeda M & Honma K (1999) Daily variation and light responsiveness of mammalian clock gene, Clock and BMAL1, transcripts in the pineal body and different areas of brain in rats. Neurosci Lett, 267, 69–72. [DOI] [PubMed] [Google Scholar]
- Natsubori A, Honma K & Honma S (2013a) Differential responses of circadian Per2 expression rhythms in discrete brain areas to daily injection of methamphetamine and restricted feeding in rats. Eur J Neurosci, 37, 251–258. [DOI] [PubMed] [Google Scholar]
- Natsubori A, Honma K & Honma S (2013b) Differential responses of circadian Per2 rhythms in cultured slices of discrete brain areas from rats showing internal desynchronisation by methamphetamine. Eur J Neurosci, 38, 2566–2571. [DOI] [PubMed] [Google Scholar]
- Natsubori A, Honma K & Honma S (2014) Dual regulation of clock gene Per2 expression in discrete brain areas by the circadian pacemaker and methamphetamine-induced oscillator in rats. Eur J Neurosci, 39, 229–240. [DOI] [PubMed] [Google Scholar]
- Nieh EH, Kim SY, Namburi P & Tye KM (2013) Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res, 1511, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Blau J & Holmes TC (2002) Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell, 109, 485–495. [DOI] [PubMed] [Google Scholar]
- Nobelprize.org (2017) 2017 Nobel Prize in Physiology or Medicine. [Google Scholar]
- Okamura H (2007) Suprachiasmatic nucleus clock time in the mammalian circadian system. Cold Spring Harb Symp Quant Biol, 72, 551–556. [DOI] [PubMed] [Google Scholar]
- Olivo D, Caba M, Gonzalez-Lima F, Rodriguez-Landa JF & Corona-Morales AA (2017) Metabolic activation of amygdala, lateral septum and accumbens circuits during food anticipatory behavior. Behav Brain Res, 316, 261–270. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R & Sesack SR (2009) Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci, 30, 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono D, Honma S, Nakajima Y, Kuroda S, Enoki R & Honma K. i. (2017) Dissociation of Per1 and Bmal1 circadian rhythms in the suprachiasmatic nucleus in parallel with behavioral outputs. Proceedings of the National Academy of Sciences, 114, E3699–E3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Nishino H, Sasaki K, Fukuda M & Muramoto K (1981) Long-term lateral hypothalamic single unit analysis and feeding behavior in freely moving rats. Neurosci Lett, 26, 79–83. [DOI] [PubMed] [Google Scholar]
- Opperhuizen AL, Wang D, Foppen E, Jansen R, Boudzovitch-Surovtseva O, de Vries J, Fliers E & Kalsbeek A (2016) Feeding during the resting phase causes profound changes in physiology and desynchronization between liver and muscle rhythms of rats. Eur J Neurosci, 44, 2795–2806. [DOI] [PubMed] [Google Scholar]
- Orozco-Solis R, Aguilar-Arnal L, Murakami M, Peruquetti R, Ramadori G, Coppari R & Sassone-Corsi P (2016) The Circadian Clock in the Ventromedial Hypothalamus Controls Cyclic Energy Expenditure. Cell Metab, 23, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otalora BB, Hagenauer MH, Rol MA, Madrid JA & Lee TM (2013) Period gene expression in the brain of a dual-phasing rodent, the Octodon degus. J Biol Rhythms, 28, 249–261. [DOI] [PubMed] [Google Scholar]
- Owasoyo JO, Walker CA & Whitworth UG (1979) Diurnal variation in the dopamine level of rat brain areas: effect of sodium phenobarbital. Life Sci, 25, 119–122. [DOI] [PubMed] [Google Scholar]
- Page TL (1982) Transplantation of the cockroach circadian pacemaker. Science, 216, 73–75. [DOI] [PubMed] [Google Scholar]
- Pallier PN, Maywood ES, Zheng Z, Chesham JE, Inyushkin AN, Dyball R, Hastings MH & Morton AJ (2007) Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J Neurosci, 27, 7869–7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ & Mucke L (2016) Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci, 17, 777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Dolatshad H & Davis FC (2011) A fear-inducing odor alters PER2 and c-Fos expression in brain regions involved in fear memory. PLoS One, 6, e20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG (2003) Medial preoptic area/anterior hypothalamus and sexual motivation. Scand J Psychol, 44, 203–212. [DOI] [PubMed] [Google Scholar]
- Parekh PK, Becker-Krail D, Sundaravelu P, Ishigaki S, Okado H, Sobue G, Huang Y & McClung CA (2017) Altered GluA1 (Gria1) Function and Accumbal Synaptic Plasticity in the ClockDelta19 Model of Bipolar Mania. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Cheon M, Kim S & Chung C (2017) Temporal variations in presynaptic release probability in the lateral habenula. Sci Rep, 7, 40866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB & Takahashi JS (2014) Molecular architecture of the mammalian circadian clock. Trends in cell biology, 24, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF (1999) The Sharpey-Schafer prize lecture: nucleus tractus solitarii: integrating structures. Exp Physiol, 84, 815–833. [PubMed] [Google Scholar]
- Paul JR, DeWoskin D, McMeekin LJ, Cowell RM, Forger DB & Gamble KL (2016) Regulation of persistent sodium currents by glycogen synthase kinase 3 encodes daily rhythms of neuronal excitability. Nat Commun, 7, 13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Indic P & Schwartz WJ (2011) A role for the habenula in the regulation of locomotor activity cycles. Eur J Neurosci, 34, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman BA, Kim E, Reilly M, Kashima J, Motch O, de la Iglesia HO & Kim JJ (2015) Time-Specific Fear Acts as a Non-Photic Entraining Stimulus of Circadian Rhythms in Rats. Sci Rep, 5, 14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Bierlaagh MA & Geurtsen AM (1997) Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: involvement of a slowly inactivating component of sodium current. J Neurophysiol, 78, 1811–1825. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J & Geurtsen AM (2002) Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature, 416, 286–290. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Segall LA, Harbour VL, Woodside B & Amir S (2006) The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci U S A, 103, 5591–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek J (1986) Alimentation of head-injured patients. J Neurosurg, 64, 984. [DOI] [PubMed] [Google Scholar]
- Pizarro A, Hayer K, Lahens NF & Hogenesch JB (2013) CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res, 41, D1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes A.L.B.d., Engelberth RCGJ, Nascimento E.d.S., Cavalcante JC, Costa M.S.M.d.O., Pinato L, Toledo C.A.B.d. & Cavalcante J.d.S. (2010) Serotonin and circadian rhythms. Psychology & Neuroscience, 3, 217–228. [Google Scholar]
- Quay WB (1968) Differences in circadian rhythms in 5-hydroxytryptamine according to brain region. Am J Physiol, 215, 1448–1453. [DOI] [PubMed] [Google Scholar]
- Quina LA, Tempest L, Ng L, Harris JA, Ferguson S, Jhou TC & Turner EE (2015) Efferent pathways of the mouse lateral habenula. J Comp Neurol, 523, 32–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan AV, Horowitz JM & Fuller CA (1999) Diurnal modulation of long-term potentiation in the hamster hippocampal slice. Brain Res, 833, 311–314. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC & Menaker M (1990) Transplanted suprachiasmatic nucleus determines circadian period. Science, 247, 975–978. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Stowie A, Smale L & Nunez A (2010a) PER2 rhythms in the amygdala and bed nucleus of the stria terminalis of the diurnal grass rat (Arvicanthis niloticus). Neurosci Lett, 473, 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Stowie A, Smale L & Nunez AA (2010b) Phase preference for the display of activity is associated with the phase of extra-suprachiasmatic nucleus oscillators within and between species. Neuroscience, 170, 758–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Plascencia OD, Saderi N, Escobar C & Salgado-Delgado RC (2017) Feeding during the rest phase promotes circadian conflict in nuclei that control energy homeostasis and sleep-wake cycle in rats. Eur J Neurosci, 45, 1325–1332. [DOI] [PubMed] [Google Scholar]
- Rath MF, Rohde K & Moller M (2012) Circadian oscillations of molecular clock components in the cerebellar cortex of the rat. Chronobiol Int, 29, 1289–1299. [DOI] [PubMed] [Google Scholar]
- Rath MF, Rovsing L & Moller M (2014) Circadian oscillators in the mouse brain: molecular clock components in the neocortex and cerebellar cortex. Cell Tissue Res, 357, 743–755. [DOI] [PubMed] [Google Scholar]
- Rawashdeh O, Jilg A, Maronde E, Fahrenkrug J & Stehle JH (2016) Period1 gates the circadian modulation of memory-relevant signaling in mouse hippocampus by regulating the nuclear shuttling of the CREB kinase pP90RSK. J Neurochem, 138, 731–745. [DOI] [PubMed] [Google Scholar]
- Reuss S & Vollrath L (1984) Electrophysiological properties of rat pinealocytes: evidence for circadian and ultradian rhythms. Exp Brain Res, 55, 455–461. [DOI] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ & Holtzman DM (2012) Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med, 4, 150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room P & Tielemans AJ (1989) Circadian variations in local cerebral glucose utilization in freely moving rats. Brain Res, 505, 321–325. [DOI] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E & Sassone-Corsi P (2010) Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One, 5, e8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi K, Belle MD, Gossan N, Delagrange P & Piggins HD (2014a) Daily variation in the electrophysiological activity of mouse medial habenula neurones. J Physiol, 592, 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi K, Wegner S, Belle MD, Howarth M, Delagrange P, Brown TM & Piggins HD (2014b) Intrinsic and extrinsic cues regulate the daily profile of mouse lateral habenula neuronal activity. J Physiol, 592, 5025–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso P, Nakata M, Ueta Y & Yada T (2017) Suprachiasmatic Vasopressin to Paraventricular Oxytocin Neurocircuit in the Hypothalamus Relays Light Reception to Inhibition of Feeding Behavior. Am J Physiol Endocrinol Metab, ajpendo003382016. [DOI] [PubMed] [Google Scholar]
- Savalli G, Diao W, Schulz S, Todtova K & Pollak DD (2014) Diurnal oscillation of amygdala clock gene expression and loss of synchrony in a mouse model of depression. Int J Neuropsychopharmacol, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenda J & Vollrath L (2000) Single-cell recordings from chick pineal glands in vitro reveal ultradian and circadian oscillations. Cellular and molecular life sciences : CMLS, 57, 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider NL & Stengl M (2007) Extracellular long-term recordings of the isolated accessory medulla, the circadian pacemaker center of the cockroach Leucophaea maderae, reveal ultradian and hint circadian rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol, 193, 35–42. [DOI] [PubMed] [Google Scholar]
- Schrader JA, Nunez AA & Smale L (2010) Changes in and dorsal to the rat suprachiasmatic nucleus during early pregnancy. Neuroscience, 171, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader JA, Nunez AA & Smale L (2011) Site-specific changes in brain extra-SCN oscillators during early pregnancy in the rat. J Biol Rhythms, 26, 363–367. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Nunez AA & Smale L (2004) Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience, 127, 13–23. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Lang EJ & Kawato M (2013) Role of the olivo-cerebellar complex in motor learning and control. Front Neural Circuits, 7, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall LA, Perrin JS, Walker CD, Stewart J & Amir S (2006) Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience, 140, 753–757. [DOI] [PubMed] [Google Scholar]
- Segall LA, Verwey M & Amir S (2008) Timed restricted feeding restores the rhythms of expression of the clock protein, Period2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in adrenalectomized rats. Neuroscience, 157, 52–56. [DOI] [PubMed] [Google Scholar]
- Sei H, Oishi K, Sano A, Seno H, Ohmori T, Morita Y & Ishida N (2006) Clock mutant mice with Jcl/ICR background shows an impaired learning ability in water maze, but not in passive avoidance, at the beginning of dark phase. Congenit Anom (Kyoto), 46, 81–85. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Egli M, Poletini MO, McKee DT, Bosworth MD, Fitch CA & Freeman ME (2006) Anatomical and functional characterization of clock gene expression in neuroendocrine dopaminergic neurons. Am J Physiol Regul Integr Comp Physiol, 290, R1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semm P, Demaine C & Vollrath L (1981) Electrical responses of pineal cells to melatonin and putative transmitters. Evidence for circadian changes in sensitivity. Exp Brain Res, 43, 361–370. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Gu H, Sharma VK, O’Dowd DK & Holmes TC (2008) Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol, 99, 976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA & Russell DS (2004) Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev, 46, 71–117. [DOI] [PubMed] [Google Scholar]
- Shieh KR (2003) Distribution of the rhythm-related genes rPERIOD1, rPERIOD2, and rCLOCK, in the rat brain. Neuroscience, 118, 831–843. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kobayashi Y, Nakatsuji E, Yamazaki M, Shimba S, Sakimura K & Fukada Y (2016) SCOP/PHLPP1beta mediates circadian regulation of long-term recognition memory. Nat Commun, 7, 12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, Arey RN, Enwright JF 3rd, Jacobsen JP, Kumar S, Remillard EM, Caron MG, Deisseroth K & McClung CA (2015) Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry, 20, 1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA & Lehman MN (1996) A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature, 382, 810–813. [DOI] [PubMed] [Google Scholar]
- Snider KH, Dziema H, Aten S, Loeser J, Norona FE, Hoyt K & Obrietan K (2016) Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav Brain Res, 308, 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider KH & Obrietan K (2018) Modulation of learning and memory by the genetic disruption of circadian oscillator populations. Physiol Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider KH, Sullivan KA & Obrietan K (2018) Circadian Regulation of Hippocampal-Dependent Memory: Circuits, Synapses, and Molecular Mechanisms. Neural Plast, 2018, 7292540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle J & Reuss S (1988) The pineal gland of the Mongolian gerbil: nocturnal increase of electrical activity. Neurosci Lett, 86, 173–176. [DOI] [PubMed] [Google Scholar]
- Stehle J, Reuss S & Vollrath L (1987) Electrophysiological characterization of the pineal gland of golden hamsters. Exp Brain Res, 67, 27–32. [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Althof D, Gispert S, Schwenk J, Auburger G, Kulik A, Fakler B & Roeper J (2014) Mutant alpha-synuclein enhances firing frequencies in dopamine substantia nigra neurons by oxidative impairment of A-type potassium channels. J Neurosci, 34, 13586–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi M, Lone SR, Liu S, Liu Q, Zhang J, Spira AP & Wu MN (2015) Sleep interacts with abeta to modulate intrinsic neuronal excitability. Curr Biol, 25, 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Lin JS & Sakai K (2006) Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci, 26, 10292–10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli-Nezhad M & Schwartz WJ (2005) c-Fos expression in the brains of behaviorally “split” hamsters in constant light: calling attention to a dorsolateral region of the suprachiasmatic nucleus and the medial division of the lateral habenula. J Biol Rhythms, 20, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd WD, Fenselau H, Wang JL, Zhang R, Machado NL, Venner A, Broadhurst RY, Kaur S, Lynagh T, Olson DP, Lowell BB, Fuller PM & Saper CB (2018) A hypothalamic circuit for the circadian control of aggression. Nat Neurosci, 21, 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel E & Bourque CW (2010) Central clock excites vasopressin neurons by waking osmosensory afferents during late sleep. Nat Neurosci, 13, 467–474. [DOI] [PubMed] [Google Scholar]
- Trudel E & Bourque CW (2012) Circadian modulation of osmoregulated firing in rat supraoptic nucleus neurones. J Neuroendocrinol, 24, 577–586. [DOI] [PubMed] [Google Scholar]
- Uchida H, Nakamura TJ, Takasu NN, Todo T, Sakai T & Nakamura W (2016) Cryptochrome-dependent circadian periods in the arcuate nucleus. Neurosci Lett, 610, 123–128. [DOI] [PubMed] [Google Scholar]
- Vatine G, Vallone D, Appelbaum L, Mracek P, Ben-Moshe Z, Lahiri K, Gothilf Y & Foulkes NS (2009) Light directs zebrafish period2 expression via conserved D and E boxes. PLoS Biol, 7, e1000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwey M & Amir S (2011) Nucleus-specific effects of meal duration on daily profiles of Period1 and Period2 protein expression in rats housed under restricted feeding. Neuroscience, 192, 304–311. [DOI] [PubMed] [Google Scholar]
- Verwey M, Khoja Z, Stewart J & Amir S (2008) Region-specific modulation of PER2 expression in the limbic forebrain and hypothalamus by nighttime restricted feeding in rats. Neurosci Lett, 440, 54–58. [DOI] [PubMed] [Google Scholar]
- Verwey M, Lam GY & Amir S (2009) Circadian rhythms of PERIOD1 expression in the dorsomedial hypothalamic nucleus in the absence of entrained food-anticipatory activity rhythms in rats. Eur J Neurosci, 29, 2217–2222. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U & Tononi G (2008) Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci, 11, 200–208. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C & Tononi G (2009) Cortical firing and sleep homeostasis. Neuron, 63, 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Opperhuizen AL, Reznick J, Turner N, Su Y, Cooney GJ & Kalsbeek A (2017) Effects of feeding time on daily rhythms of neuropeptide and clock gene expression in the rat hypothalamus. Brain Res, 1671, 93–101. [DOI] [PubMed] [Google Scholar]
- Wang LM, Dragich JM, Kudo T, Odom IH, Welsh DK, O’Dell TJ & Colwell CS (2009) Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw SM, Phan TX, Saraf A, Chen X & Storm DR (2014) Genetic disruption of the core circadian clock impairs hippocampus-dependent memory. Learn Mem, 21, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Swanson LW & Sanchez-Watts G (1987) Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol, 258, 204–229. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM & Lehman MN (2009) Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms, 24, 465–476. [DOI] [PubMed] [Google Scholar]
- West MO & Deadwyler SA (1980) Circadian modulation of granule cell response to perforant path synaptic input in the rat. Neuroscience, 5, 1597–1602. [DOI] [PubMed] [Google Scholar]
- Whitt JP, Montgomery JR & Meredith AL (2016) BK channel inactivation gates daytime excitability in the circadian clock. Nat Commun, 7, 10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongchitrat P, Felder-Schmittbuhl MP, Govitrapong P, Phansuwan-Pujito P & Simonneaux V (2011) A noradrenergic sensitive endogenous clock is present in the rat pineal gland. Neuroendocrinology, 94, 75–83. [DOI] [PubMed] [Google Scholar]
- Wyse CA & Coogan AN (2010) Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res, 1337, 21–31. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N & Okamura H (2001) Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol, 430, 518–532. [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M & Sakurai T (2003) Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron, 38, 701–713. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Kerbeshian MC, Hocker CG, Block GD & Menaker M (1998) Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J Neurosci, 18, 10709–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhang W, Wang M, Ruan D & Chen J (2012) Effects of daytime, night and sleep pressure on long-term depression in the hippocampus in vivo. Neurosci Lett, 511, 106–109. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Kinoshita K, Muroi Y & Ishii T (2013) The effects of bilateral lesions of the mesencephalic trigeminal sensory nucleus on nocturnal feeding and related behaviors in mice. Life Sci, 93, 681–686. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T & Honma S (2016) Lithium lengthens circadian period of cultured brain slices in area specific manner. Behav Brain Res, 314, 30–37. [DOI] [PubMed] [Google Scholar]
- Yu X, Zecharia A, Zhang Z, Yang Q, Yustos R, Jager P, Vyssotski AL, Maywood ES, Chesham JE, Ma Y, Brickley SG, Hastings MH, Franks NP & Wisden W (2014) Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr Biol, 24, 2838–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kolaj M & Renaud LP (2006) Suprachiasmatic nucleus communicates with anterior thalamic paraventricular nucleus neurons via rapid glutamatergic and gabaergic neurotransmission: state-dependent response patterns observed in vitro. Neuroscience, 141, 2059–2066. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME & Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A, 111, 16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H & Rusak B (2005) Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience, 132, 519–528. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Xu H, Liu Y, Mu L, Xiao J & Zhao H (2015) Diurnal Expression of the Per2 Gene and Protein in the Lateral Habenular Nucleus. Int J Mol Sci, 16, 16740–16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY & Palmiter RD (1995) Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell, 83, 1197–1209. [DOI] [PubMed] [Google Scholar]


